
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Oncol., 14 July 2020
Sec. Cancer Molecular Targets and Therapeutics
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00922
This article is part of the Research TopicNew Insights in the Landscape of Rare Tumors: Translational and Clinical Research PerspectiveView all 17 articles
 Enrico Melis1†
Enrico Melis1† Enzo Gallo2†
Enzo Gallo2† Simona di Martino2†
Simona di Martino2† Filippo Tommaso Gallina1
Filippo Tommaso Gallina1 Valentina Laquintana2
Valentina Laquintana2 Beatrice Casini2
Beatrice Casini2 Paolo Visca2
Paolo Visca2 Federica Ganci3
Federica Ganci3 Gabriele Alessandrini1
Gabriele Alessandrini1 Mauro Caterino4
Mauro Caterino4 Fabiana Letizia Cecere5
Fabiana Letizia Cecere5 Chiara Mandoj6
Chiara Mandoj6 Arianna Papadantonakis2
Arianna Papadantonakis2 Nicoletta De Bello1
Nicoletta De Bello1 Rossano Lattanzio7
Rossano Lattanzio7 Giovannella Palmieri8
Giovannella Palmieri8 Marina Chiara Garassino9
Marina Chiara Garassino9 Nicolas Girard10
Nicolas Girard10 Laura Conti6
Laura Conti6 Giovanni Blandino3
Giovanni Blandino3 Francesco Fazi11
Francesco Fazi11 Francesco Facciolo1
Francesco Facciolo1 Edoardo Pescarmona2
Edoardo Pescarmona2 Gennaro Ciliberto12
Gennaro Ciliberto12 Mirella Marino2*
Mirella Marino2*Among the group of thymic epithelial tumors (TET), thymomas often show either uncertain or explicit malignant biological behavior, local invasiveness, and intrathoracic relapse and are often difficult to manage. From the initial stages, thymic carcinomas tend to show aggressive behavior and extrathoracic spread. Moreover, the interplay of epithelial cells and thymocytes in thymomas causes complex immune derangement and related systemic autoimmune diseases. Due to their rare occurrence and to the limited funding opportunities available for rare tumors, it is challenging to make advances in clinical and translational research in TET. The authors of this paper are all members of a multidisciplinary clinical and research thoracic tumor team. Strong input was given to the team by long-standing expertise in TET in the Pathology Department. In addition, thanks to the collaboration between research units at our Institute as well as to national collaborations, over the last 10 years we were able to perform several tissue-based research studies. The most recent studies focused on microRNA and on functional studies on the thymic carcinoma cell line 1889c. The recent implementation of our biobank now provides us with a new tool for networking collaborative research activities. Moreover, the participation in a worldwide community such as ITMIG (International Thymic Malignancy Interest Group) has allowed us to significantly contribute toward fundamental projects/research both in tissue-based studies (The Cancer Genome Atlas) and in clinical studies (TNM staging of TET). Our achievements derive from constant commitment and long-standing experience in diagnosis and research in TET. New perspectives opened up due to the establishment of national [the Italian Collaborative Group for ThYmic MalignanciEs (TYME)] and European reference networks such as EURACAN, for an empowered joint clinical action in adult solid rare tumors. The challenge we face still lies in the advancement of clinical and basic science in thymic epithelial malignancies.
Thymic epithelial tumors (TET) are a rare group of tumors, comprising thymoma (THY) and thymic carcinoma (TC), that have an incidence rate of 0.13/100,000 per population in the United States according to the National Cancer Institute's (NCI) Surveillance, Epidemiology, and End Results (SEER) program/SEER database (DB) (1). Population-based data were provided by the European cancer registries (CRs) participating in the RARECARE project: compared to that in the United States, TET showed a slightly higher incidence rate of 0.17/100,000 per population, and “malignant” thymomas accounted for 0.14/100,000. TC, a much rarer disease than THY, occurs with an incidence rate of 0.2–0.5 per million individuals (2). Data on the epidemiology of two families of rare thoracic neoplasias (epithelial tumors of thymus and mesothelioma of pleura and pericardium) for 27 European countries have been recently reported in more detail by the RARECARENet working group (www.rarecarenet.eu) Malignant TET showed (in the period 2000–2007) a 5-year survival of 64%, on average (3). Recent advances in tumor biology and pathology reveal that TET constitute a unique group of neoplasias deriving from the epithelial cell network of the thymus (TEC). The extraordinary properties and characteristics of this primary lymphatic organ have been firmly established in the last 60 years, after the discovery by Miller (4) and Good (5) of the unique thymic immunological functions. Due to its central role in the homeostasis of the immune system, it is not surprising that the tumors deriving from TEC are associated with derangement of the immune system (6). In 2015, the World Health Organization (WHO) changed the International Classification for Disease of Oncology (ICD-O) code associated with thymoma from the suffix /1 applied to the third edition classification (7) to the suffix /3 for the fourth edition (8). This change reflects our increased knowledge in the biology of TET and contributes to forming the statement that “all thymomas can behave in a clinically aggressive fashion” irrespective of tumor stage and should be considered malignant (9). In recent years, significant interest in TET has been shown all around the world, and much progress has been made in the last few years due to the activity of the International Thymic Malignancy Interest Group (ITMIG) scientific society (10, 11) (www.itmig.org) and to the International Association for the Study of Lung Cancer (IASLC). Due to the joint effort of ITMIG and of IASLC and to the contribution of several important DBs (12), TET for the first time were included in the TNM staging system (13). The new staging system relies on retrospective data from more than 10,000 TET cases observed all around the world (14). Specific interest raised toward these unique tumors was also due to the US NCI's inclusion of TET in The Cancer Genome Atlas (TCGA) project (15), only one of the few families of rare tumors considered. Moreover, due to the inclusion of TET in the rare cancers included in the G8 group (rare thoracic tumors) of EURACAN, the network of rare adult solid cancers in the European reference networks (ERNs) (http://euracan.ern-net.eu), significant progress in their management has to be expected over the next few years (16). Recently, we also joined the Italian Collaborative Group for ThYmic MalignanciEs (TYME) as a reference center for the diagnosis and treatment of TET (17).
We wish to point out that the driving force behind bringing new opportunities in rare tumor research and international collaborations to our local setting was the renewed commitment and long-standing expertise of the Pathology Department. Pathology now plays a major role in bridging the gap between tumor research and clinical management in every field of tumor research. This also applies to our Institute in relation to the TET family of rare tumors. We describe here our own developing workup within the clinical and scientific contexts of TET, focusing mainly on the surgical approach, on the pathological workup, and on the ongoing research activities in different fields. Recently, a renewed opportunity was offered by progressing from a “sample collection”-based biobank to an institutionally certified ISO9001:2015 biobank. We discuss here specificities, critical issues, and challenges, focusing on our surgical, pathological, and biobank activities, as these are the main players of translational research. We also briefly mention the research projects accomplished to date and discuss how we will implement and improve our model/strategy for making progress in the future.
The surgical procedures cited for both open-access and mini-invasive approaches for TET were performed with the standard surgical instruments of a thoracic surgery operating room. Robotic thymectomy was performed by the da Vinci® surgical system (Intuitive Surgical Inc.).
Fixation of tumor specimens in 10% buffered formalin and routine laboratory techniques and equipment of a pathology laboratory were adopted to fix and to process tumor samples. Hematoxylin–eosin (H&E) was the standard routine used for staining. The Aperio system AT2 (Aperio Leica Biosystems) (CE IVD) whole-slide scanner (400-slide capacity) was used to scan slides for digital pathology.
We mention here only the main equipment available at the Pathology Department; other platforms/equipment found in the collaborating laboratories are described in detail elsewhere (18, 19): Immunohistochemistry (IHC) at our Pathology Department is performed on BOND-III, the fully automated IHC platform (LEICA BIOSYSTEMS). Our molecular biological/genetic equipment includes (1) the Ion Gene Studio™ S5 series for next-generation sequencing (NGS, Thermo Fisher); (2) the Applied Biosystems 3130 Genetic Analyzer (Thermo Fisher). The Platforms for MicroRNA study (Agilent 2,100 Bioanalyzer and “Affymetrix® Human Gene 2.0 ST Arrays 2.0,” both from Affymetrix, Santa Clara, California) are of routine research use at our Oncogenomic and Epigenetic Research Unit.
Biobanking instruments include cryogenic systems, labeling machines, and barcode readers. Systems for cryopreservation include electric freezers (−80°C); liquid nitrogen storage systems; a dedicated biobanking software, EasyTrack2D® and instruments used for quality control of biological samples in measuring various cellular components (DNA, RNA, and protein) (Bioanalyzer, Agilent Biotechnologies). All these sets of equipment are available within the dedicated spaces with controlled access. Our biobank is ISO certified (ISO9001:2015) (20).
Between 2000 and 2019, 196 patients were recorded in our DB at the IRCCS Regina Elena National Cancer Institute (IRE), including demographic data, histologic type updated to 2015 WHO classification (8), surgical procedures, and the main outcome indicators. Cases evaluated for pathological diagnosis as a second opinion were recorded together with internal cases.
At our Institute, patients who have been identified with an anterior mediastinal mass all undergo physical examination and routine biochemical tests, an electrocardiogram (echocardiogram when indicated), chest X-ray, arterial blood gas analyses, and pulmonary function tests. A neurological protocol to exclude autoimmune diseases, particularly myasthenia gravis (MG) (21), is applied. After multi-slice computerized tomography (CT) scans (128 slices) are performed, the case is then discussed during multidisciplinary thoracic tumor board meetings together with a thoracic surgeon, pathologist, oncologist, anesthesiologist, radiotherapist, pneumologist, and chest radiologist. In case of indication to radical surgery, patients undergo cardiological, and pneumological evaluation of preoperative risk. Surgical indications are mainly based on patient clinical conditions and on the CT findings. Positron emission tomography (PET)–CT with fluorine-18 fluorodeoxyglucose (18F-FDG), magnetic resonance imaging (MRI), and octreotide scan are not part of the routine preoperative workup but are additional exams (22). When a complete resection is possible, preoperative biopsy is not indicated (23, 24). In case of invasion of adjoining structures such as the anonymous vein, pericardium, superior vena cava, phrenic nerves, and pleural cavities, a diagnostic biopsy is required; after the diagnosis by surgical biopsy or by fine needle biopsy aspiration (FNAB), the patient is usually referred to induction chemotherapy (25, 26) or to surgical treatment in combination with radiotherapy.
Sternotomy and, in selected cases, thoracotomy represent the first surgical options because they allow an open extended resection of mediastinal masses and surrounding tissues, including mediastinal fat around the great vessels (27). However, in the last two decades, minimally invasive techniques took progressive place into clinical practice by a growing number of surgeons (28, 29). Minimally invasive techniques include video-assisted thoracoscopic surgery (VATS) (30) and robotic-assisted thoracoscopic surgery (RATS) (31). According to TYME, minimally invasive surgery is recommended for a tumor dimension smaller than 5 cm (17); however, also in case of invasion of neighboring organs (the pericardium, lungs, mediastinal pleura, or phrenic nerve), this procedure is not a contraindication in expert hands (23). The objective is quite similar for both RATS and VATS approaches: to perform standard extended thymectomy, including the thymus and the surrounding mediastinal fatty tissue, en bloc.
The process of biobanking starts once a patient suspected of having mediastinal masses for thymic malignancy is identified and gives his/her institutional review board (IRB)-approved informed consent to preserve samples in our biobank. The consent is signed by both the patient and surgeon. A request for banking biological fluids is prepared prior to the surgical intervention by the surgeon through the creation of a computerized order entry to the Biological Fluids Biobank in the Clinical Pathology Laboratory. Blood samples (whole blood, serum, and plasma) are withdrawn by research nurses in the surgical ward (prior to operation and during follow-up to outpatients). The sterile tissue specimen is immediately collected from the operating room and taken to the Tissue Biobank in the Pathology Department upon removal. After checking and testing for biomaterial conformity and adequacy for diagnosis, sampling is performed by a “dedicated” pathologist (32). Each specimen is sampled depending on size and quality of the tissue; consecutive samples are prepared. The selected samples are immediately snap-frozen in liquid nitrogen or are frozen in optimal cutting temperature (OCT) and stored at −80°C. The procedure applies to both resected surgical specimens and biopsies (when sufficient material is available). Representative corresponding samples like morphological controls from either the tumor or the peritumoral thymus—when available—are fixed in formalin overnight (at 4°C) (minimum 24 h) and embedded in paraffin [formalin-fixed, paraffin-embedded (FFPE) material] (33) in a specific biobank archive. Tissue specimens are processed and stored in our tissue biobank by our “biobankers” according to the biobank standard operating procedures (SOPs) compliant with ISO9001/2015 certification (20). For sample collection and storage, clinical and biological data are recorded and managed by a dedicated software EasyTrack2D® according to the specific biobank SOPs. The quality of different fractions/samples (snap-frozen/OCT frozen/FFPE) is periodically evaluated for the preservation and yield of the cellular components by checking RNA/DNA extracted with RNA integrity number (RIN) (34). Figure 1 shows the RIN value of some of our sample RNAs. Recently, our biobank group has introduced the collection and isolation of tumor cells from fresh tumor specimens/neoplastic effusions (35). As for TET, we are setting up primary tumor cell cultures (preliminary data, not shown).
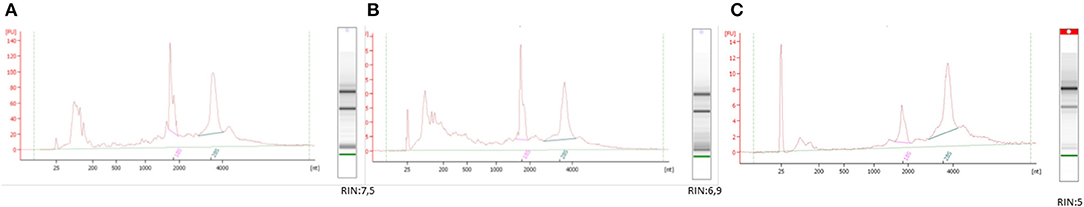
Figure 1. Methods in biobank: ID 528090BIOSPECIMEN QUALITY CONTROL. The image shows the quality of the different TET samples after RNA extraction using the different processing protocols (A) snap-frozen, (B) OCT, and (C) FFPE. The panel shows representative electropherograms of each sample type. The RIN values from each category were different between the groups (protocols A and B produced RIN values of ≥5, protocol C produced RIN values of ≤ 5—only moderately degraded RNA). All three methods guarantee the integrity of the RNA, rendering it suitable for most types of downstream applications.
The recommendations of C.A. Moran and S. Suster (36) and of the International Collaboration on Cancer Reporting (ICCR) (37) in tumor sampling are followed (one tissue sample per centimeter of tumor or a minimum of 10 blocks for very large tumors). The peritumoral thymus is investigated by a “dedicated” pathologist who accurately and thoroughly examines the specimen and performs multiple sample embedding of peritumoral thymic fat tissue. In regard to pathological reporting, the 2015 WHO classification (8, 38) together with the ICCR recommendations (37) are followed. IHC plays a role in the diagnostic workup for diagnosis of thymomas with ambiguous histology and for the distinction between thymomas and thymic carcinomas (38). Pathological staging is performed by the pathologist on the basis of the tumor extent according to the eighth TNM (14, 39–41) published in its final and official version in 2017 (13). The Pathology Department, equipped with the Leica digital pathology platform Aperio AT2 (Aperio Leica Biosystems), performs most routine scans of representative slides of TET cases. Each H&E or significant IHC slide is scanned at a magnification of ×40. The scanning parameter settings are the default instrument settings. Digital images are analyzed by using the ImageScope® software. The image management system is the eSlide Manager® (12.3.3.5049) (Aperio Leica Biosystems).
For molecular pathology, the methods applied in our tissue-based studies are only briefly mentioned here; the reader is referred to the original publications (18, 19, 42–44). We used sequencing and egfr fluorescence in situ hybridization (FISH) to genotype our series of thymomas: (I) for polymorphisms and somatic loss of heterozygosity of the non-coding egfr CA-SSR-1 microsatellite and (II) for egfr gene copy number changes. More recently, for our NGS study, we used the Ion AmpliSeq Cancer Hotspot Panel v2 targets 50, which is the most commonly used cancer panel adopted for solid tumors in order to identify mutations indicating sensitivity and resistance to targeted therapies. The panel is able to identify more than 2,800 COSMIC hot spots of 50 genes, as described in several studies (45, 46). For the microRNA study, microRNA expression profiling of FFPE tumor tissue and peritumoral thymus was performed by microarray analysis; mRNA expression profiling of fresh frozen TET and peritumoral thymus was performed by microarray analysis. The role of miR-145-5p in TETs was evaluated in vitro, modulating its expression in a thymic carcinoma (1889c) cell line. The epigenetic transcriptional regulation of miR-145-5p was examined by treating the cell line with the HDAC inhibitor valproic acid (VPA) (19).
Between 2001 and 2019, 196 cases of TET were recorded, excluding non-neoplastic thymic disease cases, in adult patients. The data reported in Table 1A exclude lymphoid neoplasias occurring or involving the thymus, such as Hodgkin lymphomas as well as non-Hodgkin lymphomas and the relatively common metastatic disease to the thymus/anterior mediastinum. Primary non-epithelial as well as non-lymphoid tumors were rarely diagnosed in the thymus (47). Table 1A briefly reports basic demographical data and subtype distribution of 188 TET cases seen at our institution. A slight increase in cases per year was recorded from 2016 (Table 1B). Most of the TET cases were surgically treated at our Institute. Cases involving second opinions were also included. Most of them derived from regions of Central or Southern Italy and were shared for second opinion diagnostic purposes from the Rare Cancer Center of the Regione Campania (CRTR). However, recently, cases referred to the NCI in Milan (INT), within the TYME network, were also shared with us and examined for a second opinion. Our Institute is a participating reference center both for diagnostic activity on TET in Italy within the TYME network (17) and for the pathological assessment of cases within a biological translational study (BIOTET) designed by the NCI in Milan (48).

TABLE 1A. Distribution by sex and histotypes of TET cases according to the 2015 WHO classification in the period 2001–2019—TET PATIENTS tot 196; TET, not further classifiable: 8 cases; Male: 100 (51%); Female:96 (49%).
Most cases, including those referred for a second diagnostic opinion and treated at IRE, are evaluated and discussed at the multidisciplinary thoracic tumor board (49). At our Institute, we apply consolidated surgical procedures, thymectomy being the cornerstone surgical approach used for treating patients with TET. According to international guidelines, the open approach is the first choice (23, 27); however, VATS and RATS (Table 2) also play a relevant role in our approach to thymic surgery. In our clinical practice, we routinely perform the RATS left approach for left-sided and central mediastinal lesions and reserve the right approach for right-sided tumors. The main advantages of this type of technique include the three-port access through 1-cm incisions, CO2 inflation in the mediastinum that radically increases operating space, accuracy of instrument movement under mechanical control, and 2D stereoscopic full-HD vision. Moreover, in the last few years, we have moved on from using the three-port VATS to the uniportal VATS. In comparison to RATS, the uniportal VATS approach, used only for small lesions with no invasion to adjacent structures (50, 51), even though slightly less accurate, has direct control over surgical instruments, returning to the tactile feedback of the surgeon's hand. Moreover, the uniportal access technique shows relevant post-operative pain reduction and better aesthetic results in comparison to the open approach.
For tumor diagnosis, classification, and digital imaging, in all cases, surgical specimens as well as bioptic material are classified according to the 2015 WHO classification, and the B2 subtype was the most represented histotype (33% of recorded cases) (Table 1A). Tumor tissue is routinely extensively sampled, and even though the amount of lymphocytes and/or thymocytes might vary in different areas of THY, the histological variation does not affect the main TET subtyping, performed according to the criteria set out in the 2015 WHO classification (8, 38). Moreover, extensive sampling allows the availability of FFPE material not only from the tumor itself but also from the peritumoral thymus, whenever remnant tissue is available. We provide blocks with “key-blocks” in order to evaluate the tumor and its surrounding tissue for accurate staging (37). Anterior mediastinal lymph nodes are also included in the sampling, because they are usually removed by the surgeons together with the fat tissue of the anterior mediastinum (52). In surgically treated THY cases at IRE, we found a metastasis in only one case, in a laterocervical lymph node (53), which developed 9 years from the original diagnosis. Recently, the use of the digital pathology is growing at an exponential rate, and we have been scanning most of the representative slides.
Tissue-based research activity in TET at IRE was first based on a tissue microarray (TMA)-based immunohistochemical study of vascular endothelial growth factor receptors (VEGFR family) in 200 cases from different Italian institutions. The TMA study provided evidence that tissue receptors of the VEGFR family are distributed among TET subtypes, reaching the maximum expression in TC (18). Subsequently, in a pilot study carried out on the egfr microsatellite CA-SSR-1 performed by the first genetic analyzer available in pathology, Thermo Fisher's 3130 genetic analyzer, we were able to show that CA-SSR-1 allelic imbalance with short allele relative prevalence significantly correlated with EGFR 3+ immunohistochemical scores, increased egfr gene copy numbers, and advanced stage with relapsing/metastatic behavior in thymomas (44). More recently, we have established further collaborations with other in-house research units (43) and national (19, 42, 54) and international institutes (55, 56). Thanks to frequent participation in meetings and interfacing with members of the scientific community at major conferences on thymic tumors, as well as holding structured workgroups supported by the scientific society ITMIG, our boundaries have changed and widened. The TCGA-THYM study participation is an example of a major cornerstone. This study, among other results, demonstrated the existence of four molecular subtypes in TET, which corresponded to the morphological subtypes in the WHO classification (57). In-house, we started an NGS study in order to map the genomic alterations of our TC series; preliminary data were presented at the most important conferences held on TET or at IASLC WCLC (58, 59).
For biobanking and TET frozen tissue-based research (60), the Thoracic Surgery Unit and the Pathology Department between September 2017 and May 2019 provided our biobank with tumor tissues from over 241 patients with thoracic tumors, including the most common lung carcinoma; TET; mesothelioma; and thoracic lymphoma (Table 3). At present (02/2020), we have 263 stored tumor samples from 31 patients affected with TET. The tumor samples preserved as morphological control and fixed in formalin at 4°C provided better morphological results than routine specimens (Figures 2, 3) (61). In the same period, at the Biological Fluid Biobank, we started to collect peripheral blood (PB) and serum/plasma from TET patients, thus preserving in the biobank complete samples (tumors and germline tissue) from 26 TET patients. Moreover, in the last few years, also before establishing our institutional biobank, we provided high-quality material from our “frozen collection of cases” to a gene expression profile carried out in our national scientific collaboration on microRNA. By analysis of a series of TET samples and peritumoral thymus, we identified a 69-gene signature of miR-145-5p putative target mRNAs. These mRNAs are differentially expressed between tumor and peritumoral thymus, and their expression is inversely correlated to that of miR-145-5p. Moreover, we evidenced that the epigenetic treatment of TC cell line 1889c with VPA, a histone acetylation inhibitor, resulted in the induction of miR-145-5p expression and downregulation of its target genes, showing antitumor effects in TET (cell cycle arrest and reduction of cell viability, colony-forming ability, and migration capability) (19).
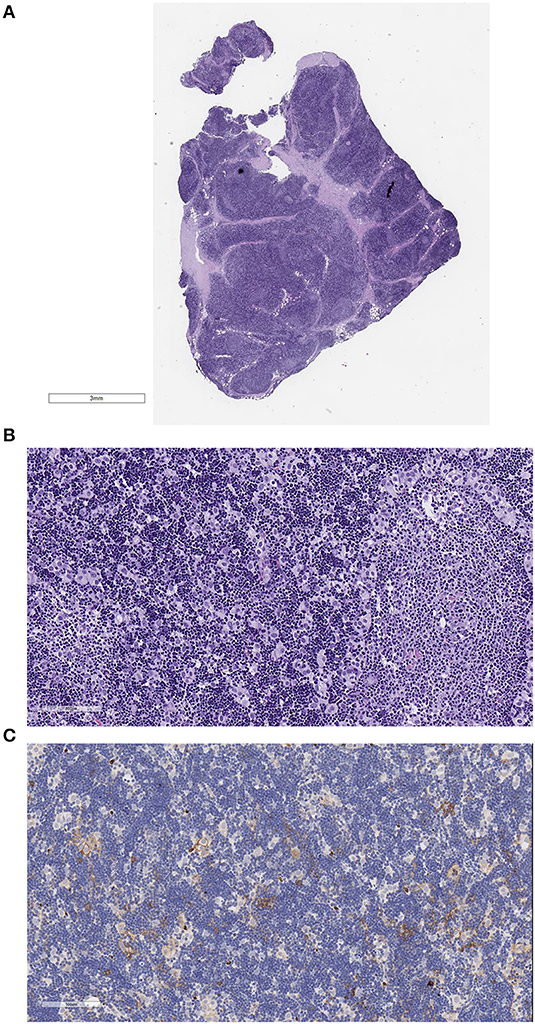
Figure 2. Example of a B2 thymoma fixed in formalin at 4°C and embedded in paraffin. The slides were scanned with the Aperio system 40×. Good morphological details are observed. (A) Hematoxylin–eosin (HE) stain, low magnification to show the whole section present on the scanned slide. (B) HE stain, 200×, showing the cortex-like tumor rich in epithelial cells (EC) and in thymocytes and a medullary island mostly containing lymphocytes. (C) Glut-1 stain of the B2 thymoma. Only few epithelial cells react.
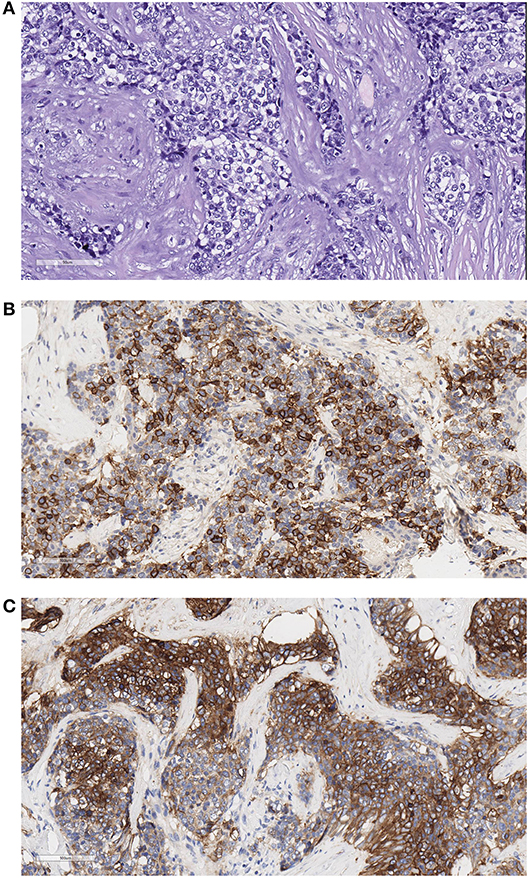
Figure 3. Example of a thymic carcinoma fixed in formalin at 4°C and embedded in paraffin. The slides were scanned with the Aperio system 40×. Good morphological details are observed. (A) Hematoxylin–eosin (HE) stain, 200×, showing the atypical epithelial cells forming ribbons infiltrating sclerotic tissue. (B) CD117 stain, 200×. Most cells are stained with this thymic carcinoma marker. (C) Glut-1 stains in thymic carcinoma ribbons and networks of epithelial cells.
The thymus is a primary lymphatic organ which sees the beginning of thymic involution at puberty (62), yet seeding, in an adult age, epithelial tumors deriving from highly specialized cells (63) of fundamental biological and pathological relevance. Our Institute has a long-standing interest in the diagnosis of thymic and mediastinal lesions (8, 38, 47) and management of TET patients (26, 42). Moreover, our Institute is well-known as an Italian expert center for the surgical and multimodality approach applied for the removal of mediastinal masses (25). At present, we play an active leading role in TYME, the main Italian network for thymic tumor management (17, 64), and we will be contributing to the ongoing ninth TNM staging project of thymic tumors and lung carcinoma, expected in 2024 (65, 66). In EURACAN, the G8 network, we contribute to ongoing activities in the clinical patient management system (CPMS), a web-based complex clinical software, and to the Digital Pathology Task force, and research projects are moving forward (16); currently, EURACAN in conjunction with the European Organization for Research on Cancer (EORTC) are moving ahead. EORTC, through SPECTA, an academic translational research infrastructure for biomaterial collection, aims to promote a comprehensive molecular profiling and virtual central pathology review also in the field of rare thoracic tumors.
In our experienced clinical setting, over the past few years, we have applied multiple approaches toward TET tissue-based research studies. The TET biological system requires particular attention due to the occurrence of strictly intermingled epithelial and lymphoid cells in tumors. Therefore, IHC shows advantages because cells labeled with biomarkers are singularly identified. In our multicenter study on a series of 200 TET cases collected in the larger TET-TMA series built up, an extensive immunohistochemical angiogenesis-related investigation showed that VEGFR expression was associated with invasiveness and advanced stage (18). These data could provide biological support for the use of anti-angiogenetic drugs in TET treatment (67, 68). An Italian clinical trial exploring the role of angiogenetic receptors in TET is currently in progress (48).
Molecular and genomic studies, on the other hand, require attention in using TEC-enriched samples. In our pilot study focusing on the egfr relevance in the pathogenesis of TET, we provided statistically significant insight on the possible role that the length of the egfr microsatellite CA-SSR-1 and the egfr gene copy number could play in TET growth (44).
Subsequently, we established a successful collaboration with our Oncogenomic and Epigenetic Unit together with the Sapienza University of Rome, where we approached the epigenetic control of TET by microRNA-focused studies. First, we approached this field by using FFPE materials (43); then circulating microRNAs were investigated (54); subsequently, we contributed high-quality biobank-derived frozen material, allowing the gene expression profile of the mRNA putative target of miR-145-5p (19). We also started to perform the functional characterization of the 1889c cell line (60) by investigating the epigenetic regulation of miR-145-5p, as well as the modulation of its functional target mRNAs in our system. Of note, we are now engaged in the characterization of the contribution of the long non coding RNA (lncRNA) function in TET. Very few reports so far investigated lncRNA in TET (69). We are focusing our attention on the sponge activity of lncRNAs, which are able to inhibit the microRNA function generating molecular networks relevant for tumor establishment and progression. Our preliminary data (not shown) highlight the relevance of the epigenetic deregulation of ncRNA in TET for the identification of novel molecular targets of therapy.
The quality of our biobank material was also confirmed by the inclusion of our samples among the cases included in the TCGA-THYM study (57). Recently, we have focused on implementing our biobanking activities. These were supported by a strategy based on a positive feedback cycle between the thoracic surgeon and the “dedicated” pathologist, by the development of an efficient and certified biobanking system, and by the implementation of laboratory cell culture facilities. In fact, our purpose now is to set up a procedure for the isolation of stem cells from fresh TET specimens, based on our previous experience in different tumor systems (70). Preliminary data on primary cultures of TET appear to be promising (data not shown). In the field of imaging analysis, digital pathology is a rapidly evolving and increasingly utilized tool in histology. It enables high throughput and precise analysis of a large number of samples and facilitates easier interactive consensus in remote diagnostic discussions, as we achieved in the TCGA-THYM study (57). TCGA deriving image archives—otherwise underutilized—recently provided insight into the tumor-immune microenvironment in 13 TCGA tumor types (71). All the studies reported a major role played by the “dedicated” pathologist. The role of pathologist evolved from giving microscopic description to adhering to internationally validated classification criteria (38) and to adopting structured pathology reports (37) in order to provide standardized and relevant information for prognostic stratification of patients. The pathologist also plays a major role in identifying new biomarkers by IHC; digitized slides provide quantitative as well as qualitative observations. Moreover, the morphological evaluation of tumor samples for molecular analyses prevents inadequate sampling and inappropriate molecular analyses on necrotic or fibrotic tissue. Bridging the gap between molecular data and the knowledge of the biological/tumoral systems, the pathologists contribute to integrating morphology with molecular findings. Based on our examples above, it is evident that solid commitment from the Pathology Department is critical for translational research and in all aspects of clinical care, especially in rare tumor types.
The challenging points of our well-established study on TET and of tissue-based translational studies range from the limited availability of cases and funding to the difficulties in clinical data collection. Moreover, given the specific biology of TET, outcome indicators are difficult to collect due to the long natural history of thymomas and to the possibilities of patients migrating or returning to their place of origin, being lost to follow-up. Clinical trials for TET (48) are difficult to promote and to find collaborative support from pharmaceutical companies, as these tumors are orphan diseases (10). Currently, at our institute, new TET cases are discussed at our multidisciplinary thoracic tumor Board meetings (49, 72) as they are an important tool in achieving the best approach to patient management. Our Institute routinely performs second opinion pathological review for the majority of patients who seek oncologic consultations. A second look in specialized centers for rare tumors can result in major prognostic and therapeutic modifications (73). Despite the limited funding for our translational research projects on TET, we have received free support from our research collaborating units who have contributed in providing reagents, human resources, and the use of their platforms. This type of eager collaborative support happens when there is a deep-seated belief in a type of rare tumor that is deserving of attention and interest. At the same time, health networks such as EURACAN provided improvement in patient assistance (74) and are expected to promote translational research in rare tumor.
Therefore, although our clinical responsibilities have been greatly burdened over the last few years, we, as a team, have set the grounds for significantly contributing scientifically to TET research. We hope to implement our translational research activity by improving our networking with other research centers in both Italy/Europe and abroad. In the future, translational research will offer precision medicine data and targeted therapies to the clinical management of TET patients.
The datasets generated for this study are available on request to the corresponding author.
This study was carried out in accordance with the recommendations of our Ethical Committee. The protocol was reviewed and approved by the Comitato Etico Centrale IRCCS Lazio -Found. Bietti. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
MM designed the manuscript and drafted it. EM, EG, SM, FG, VL, GA, and FFaz participated in the designing and drafting up of the manuscript. GB and NG critically revised it. EP, FFac, LC, and GC coordinated the manuscript. Administrative support was given by EP. All the authors contributed to the work during the years by their clinical or experimental activity. All authors contributed to the article and approved the submitted version.
The TMA study was supported by a grant from the Italian National Health Ministry to MM, the other studies by current research grants IRE to MM or to EP, or from other Research Units (Oncogenomic and Epigenetic Unit) at IRE. Other institutions (FFaz at Sapienza University) contributed with their own grants to microRNA studies. The biobank was fully supported by the Scientific Direction IRE. Funds for open-access publication fees were received from the IRCCS Regina Elena National Cancer Institute. This work was partially funded by EURACAN EC 739521.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors want to thank our Biobank IRCCS Regina Elena National Cancer Institute (BBIRE), Rome, Italy, for sample and data preservation and the International Thymic Malignancy Interest Group (ITMIG) and the ERN-EURACAN G8 Rare Thoracic Tumors network for fruitful discussion and for the several projects on TET realized or ongoing. We thank Dr. Tania Merlino for English language editing.
1. Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. (2010) 5(10 Suppl. 4):S260–5. doi: 10.1097/JTO.0b013e3181f1f62d
2. Siesling S, van der Zwan JM, Izarzugaza I, Jaal J, Treasure T, Foschi R, et al. Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer. (2012) 48:949–60. doi: 10.1016/j.ejca.2012.02.047
3. Gatta G, Trama A, Capocaccia R. Epidemiology of rare cancers and inequalities in oncologic outcomes. Eur J SurgOncol. (2019) 45:3–11. doi: 10.1016/j.ejso.2017.08.018
4. Miller J. Immunological function of the thymus. Lancet. (1961) 278:748–9. doi: 10.1016/S0140-6736(61)90693-6
5. Good R, Maclean L, Varco R, Zak S. Thymic tumor and acquired agammaglobulinemia, a clinical and experimental study of the immune response. Surgery. (1956) 40:1010–17.
6. Bernard C, Frih H, Pasquet F, Kerever S, Jamilloux Y, Tronc F, et al.Thymoma associated with autoimmune diseases, 85 cases and literature review. Autoimmun Rev. (2016) 15:82–92. doi: 10.1016/j.autrev.2015.09.005
7. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARCPress (2004).
8. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer-IARC- press (2015).
9. Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL, et al. The 2015 world health organization classification of tumors of the thymus, continuity and changes. J Thorac Oncol. (2015) 10:1383–95. doi: 10.1097/JTO.0000000000000654
10. Detterbeck FC. The creation of the international thymic malignancies interest group as a model for rare diseases. Am Soc Clin Oncol Educ Book. (2012) 2012:471–4. doi: 10.14694/EdBook_AM.2012.32.471
11. Detterbeck F, Korst R. The international thymic malignancy interest group thymicinitiative, a state-of-the-art study of thymic malignancies. Semin Thorac Cardiovasc Surg. (2014) 26:317–22. doi: 10.1053/j.semtcvs.2015.02.002
12. Huang J, Ahmad U, Antonicelli A, Catlin AC, Fang W, Gomez D, et al. Contributors, development of the international thymic malignancy interest group international database, an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol. (2014) 9:1573–8. doi: 10.1097/JTO.0000000000000269
13. Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Hoboken, NJ: John Wiley and Sons, Inc. (2017).
14. Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project, proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. (2014) 9(9 Suppl. 2) :S65–72. doi: 10.1097/JTO.0000000000000290
15. Chang K, Creighton C, Davis C, Donehower L, Drummond J, Wheeler D, et al. The cancer genome atlas pan-cancer analysis project. Nat Gene. (2013) 45:1113–20. doi: 10.1038/ng.2764
16. Imbimbo M, Maury JM, Garassino M, Girard N. Mesothelioma and thymic tumors, treatment challenges in (outside) a network setting. Eur J Surg Oncol. (2019) 45:75–80. doi: 10.1016/j.ejso.2018.01.078
17. Imbimbo M, Ottaviano M, Vitali M, Fabbri A, Leuzzi G, Fiore M, et al. Best practices for the management of thymic epithelial tumors, a position paper by the Italian collaborative group for Th Ymic Malignanci Es (TYME) . Cancer Treat Rev. (2018) 71:76–87. doi: 10.1016/j.ctrv.2018.10.001
18. Lattanzio R, La Sorda R, Facciolo F, Sioletic S, Lauriola L, Martucci R, et al. Thymic epithelial tumors express vascular endothelial growth factors and their receptors as potential targets of antiangiogenic therapy, a tissue micro array-based multicenter study. Lung Cancer. (2014) 85:191–6. doi: 10.1016/j.lungcan.2014.05.010
19. Bellissimo T, Ganci F, Gallo E, Sacconi A, Tito C, De Angelis L, et al. Thymicepithelial tumors phenotype relies on miR-145-5p epigenetic regulation. Mol Cancer. (2017) 16:88. doi: 10.1186/s12943-017-0655-2
20. Minegishi N, Nishijima I, Nobukuni T, Kudo H, Ishida N, Terakawa T, et al. Tohoku medical megabank project study and yamamotoM, biobank establishment and sample management in the tohoku medical megabank project. Tohoku J Exp Med. (2019) 248:45–55. doi: 10.1620/tjem.248.45
21. Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol. (2014) 9(9 Suppl. 2):S143–7. doi: 10.1097/jto.0000000000000300
22. Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass, a guide for clinicians. J Thorac Oncol. (2014) 9(9 Suppl. 2):S102–9. doi: 10.1097/JTO.0000000000000294
23. Ruffini E, Van Raemdonck D, Detterbeck F, Rocco G, Thomas P, Venuta F, et al. Management of thymic tumors, a survey of current practice among members of the European society of thoracic surgeons. J Thorac Oncol. (2011) 6:614–23. doi: 10.1097/JTO.0b013e318207cd74
24. Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S, Committee EG. Thymic epithelial tumours, ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2015) 26(Suppl. 5):v40–55. doi: 10.1093/annonc/mdv277
25. Leuzzi G, Rocco G, Ruffini E, Sperduti I, Detterbeck F, Weder W, et al. Multimodality therapy for locally advanced thymomas, a propensity score-matched cohort study from the European society of thoracic surgeons database. J Thor Cardiovasc Surg. (2016) 151:47–57.e1. doi: 10.1016/j.jtcvs.2015.08.034
26. Leuzzi G, Alessandrini G, Sperduti I, Forcella D, Marino M, Ceribelli A, et al. Induction therapy versus initial surgery in advanced thymic tumors, perioperative and oncological outcome. Thor Cardiovasc Surg. (2017) 65:234–43. doi: 10.1055/s-0035-1564890
27. Falkson CB, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak DE, et al. The management of thymoma, a systematic review and practice guideline. J Thorac Oncol. (2009) 4:911–9. doi: 10.1097/jto.0b013e3181a4b8e0
28. Fok M, Bashir M, Harky A, Sladden D, DiMartino M, Elsyed H, et al. Video-assisted thoracoscopic versus robotic-assisted thoracoscopicthymectomy, systematic review and meta-analysis. Innovations. (2017) 12:259–64. doi: 10.1097/IMI.0000000000000382
29. O'Sullivan KE, Kreaden US, Hebert AE, Eaton D, Redmond KC. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothor Surg. (2019) 8:174–93. doi: 10.21037/acs.2019.02.04
30. Odaka M, Shibasaki T, Asano H, Marushima H, Yamashita M, Morikawa T. Feasibility of thoracoscopicthymectomy for treatment of early-stage thymoma. Asian J Endoscopic Surg. (2015) 8:439–44. doi: 10.1111/ases.12202
31. Gkouma A. Robotically assisted thymectomy, a review of the literature. J. Robotic Surg. (2018) 12:3–10. doi: 10.1007/s11701-017-0748-3
32. Andry C, Duffy E, Moskaluk CA, McCall S, Roehrl MH A, Remick D. Biobanking-budgets and the role of pathology biobanks in precision medicine. Acad Pathol. (2017) 4:2374289517702924. doi: 10.1177/2374289517702924
33. Gündisch S, Annaratone L, Beese C, Drecol E, Marchiò C, Quaglino E, et al. Critical roles of specimen type and temperature before and during fixation in the detection of phosphoproteins in breast cancer tissues. Lab Invest. (2015) 95:561–71. doi: 10.1038/labinvest.2015.37
34. Gaignaux A, Ashton G, Coppola D, De Souza Y, De Wilde A, Eliason J, et al. Van den eynden and betsou f, abiospecimen proficiency testing program for biobank accreditation, four years of experience. Biopreserv Bio Bank. (2016) 14:429–39. doi: 10.1089/bio.2015.0108
35. Bruschini S, di Martino S, Pisanu ME, Fattore L, De Vitis C, Laquintana V, et al. CytoMatrix for a reliable and simple characterization of lung cancer stem cells from malignant pleural effusions. J Cell Physiol. (2020) 235:1877–87. doi: 10.1002/jcp.29121
36. Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms. Impact of sampling in subtyping and classification of thymomas. Am J Clin Pathol. (2000) 114:760–6. doi: 10.1309/CYJH-9RXM-P2PK-120J
37. Nicholson AG, Detterbeck F, Marx A, Roden AC, Marchevsky AM, Mukai K, et al. Dataset for reporting of thymic epithelial tumours, recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology. (2017) 70:522–38. doi: 10.1111/his.13099
38. Marx A, Ströbel P, Badve SS, Chalabreysse L, Chan JK, Chen G, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma, refined definitions, histological criteria, and reporting. J Thorac Oncol. (2014) 9:596–611. doi: 10.1097/JTO.0000000000000154
39. Rami-Porta R. Staging Manual in Thoracic Oncology. North Fort Myers, FL; Editorial Rx Press (2016).
40. Kondo K, Van Schil P, Detterbeck FC, Okumura M, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project, proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. (2014) 9(9 Suppl. 2):S81–7. doi: 10.1097/JTO.0000000000000291
41. Bhora FY, Chen DJ, Detterbeck FC, Asamura H, Falkson C, Filosso PL, et al. The ITMIG/IASLC thymic epithelial tumors staging project, a proposed lymph node map for thymic epithelial tumors in the forthcoming 8th edition of the tnm classification of malignant tumors. J Thorac Oncol. (2014) 9(9 Suppl. 2):S88–96. doi: 10.1097/JTO.0000000000000293
42. Palmieri G, Marino M, Buonerba C, Federico P, Conti S, Milella M, et al. Imatinibmesylate in thymic epithelial malignancies. Cancer Chemother Pharmacol. (2012) 69:309–15. doi: 10.1007/s00280-011-1690-0
43. Ganci F, Vico C, Korita E, Sacconi A, Gallo E, Mori F, et al. MicroRNA expression profiling of thymic epithelial tumors. Lung Cancer. (2014) 85:197–204. doi: 10.1016/j.lungcan.2014.04.008
44. Conti S, Gallo E, Sioletic S, Facciolo F, Palmieri G, Lauriola L, et al. Molecular genetic alterations in egfr CA-SSR-1 microsatellite and egfr copy number changes are associated with aggressiveness in thymoma. J Thor Dis. (2016) 8:386–95. doi: 10.21037/jtd.2016.02.40
45. de Leng WW, Gadellaa-van Hooijdonk CG, Barendregt-Smouter FA, Koudijs MJ, Nijman I, Hinrichs JW, et al. Targeted next generation sequencing as a reliable diagnostic assay for the detection of somatic mutations in tumours using minimal DNA amounts from formalin fixed paraffin embedded material. PLoS ONE. (2016) 11:e0149405. doi: 10.1371/journal.pone.0149405
46. Lee A, Lee SH, Jung CK, Park G, Lee KY, Choi HJ, et al. Use of the ion ampliseq cancer hotspot panel in clinical molecular pathology laboratories for analysis of solid tumours, with emphasis on validation with relevant single molecular pathology tests and the oncomine focus assay. Pathol Res Practice. (2018) 214:713–9. doi: 10.1016/j.prp.2018.03.009
47. Marino M, Ascani S. An overview on the differential diagnostics of tumors of the anterior-superior mediastinum, the pathologist's perspective. Mediastinum. (2019) 3:1–31. doi: 10.21037/med.2018.12.01
48. Imbimbo M, Vitali M, Fabbri A, Ottaviano M, Pasello G, Petrini I, et al. RELEVENT trial, phase II trial of ramucirumab, carboplatin, and paclitaxel in previously untreated thymic carcinoma/B3 thymoma with area of carcinoma. Clin Lung Cancer. (2018) 19:e811–4. doi: 10.1016/j.cllc.2018.06.005
49. Chalabreysse L, Thomas De Montpreville V, De Muret A, Hofman V, Lantuejoul S, Parrens M, et al. Rythmic-pathology, the French national pathology network for thymic epithelial tumours. Ann Pathol. (2014) 34:87–91. doi: 10.1016/j.annpat.2014.01.010
50. Pennathur A, Qureshi I, Schuchert MJ, Dhupar R, Ferson PF, Gooding WE, et al. Comparison of surgical techniques for early-stage thymoma, feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg. (2011) 141:694–701. doi: 10.1016/j.jtcvs.2010.09.003
51. Marulli G, Rea F, Melfi F, Schmid TA, Ismail M, Fanucchi O, et al. Robot-aided thoracoscopicthymectomy for early-stage thymoma, a multicenter European study. J Thorac Cardiovasc Surg. (2012) 144:1125–30. doi: 10.1016/j.jtcvs.2012.07.082
52. Ruffini E, Fang W, Guerrera F, Huang J, Okumura M, Kim DK, et al. The international association for the study of lung cancer thymic tumors staging project, the impact of the eighth edition of the union for international cancer control and american joint committee on cancer tnm stage classification of thymic tumors. J Thoracic Oncol. (2020) 15:436–47. doi: 10.1016/j.jtho.2019.11.013
53. Sioletic S, Lauriola L, Gallo E, Martucci R, Evoli A, Palmieri G, et al. Diagnostic features and subtyping of thymoma lymph node metastases. Biomed Res Int. (2014) 2014:546149. doi: 10.1155/2014/546149
54. Bellissimo T, Russo E, Ganci F, Vico C, Sacconi A, Longo F, et al. Circulating miR-21-5p and miR-148a-3p as emerging non-invasive biomarkers in thymic epithelial tumors. Cancer Biol Ther. (2016) 17:79–82. doi: 10.1080/15384047.2015.1108493
55. Ströbel P, Zettl A, Shilo K, Chuang WY, Nicholson AG, Matsuno Y, et al. Tumor genetics and survival of thymic neuroendocrine neoplasms, a multi-institutional clinicopathologic study. Genes Chromosomes Cancer. (2014) 53:738–49. doi: 10.1002/gcc.22183
56. Dinter H, Bohnenberger H, Beck J, Bornemann-Kolatzki K, Schütz E, Küffer S, et al. Molecular classification of neuroendocrine tumors of the thymus. J Thorac Oncol. (2019) 14:1472–83. doi: 10.1016/j.jtho.2019.04.015
57. Radovich M, Pickering CR, Felau I, Ha G, Zhang H, Jo H, et al. The integrated genomic landscape of thymic epithelial tumors. Cancer Cell. (2018) 33:244–58.e10. doi: 10.1016/j.ccell.2018.01.003
58. Casini B, Gallo E, Melis E, Cecere FL, Laquintana V, Cerasoli V, et al. MS08.04 novel biomarkers for thymic carcinoma. In: CellPress, Editor. 2019 World Conference on Lung Cancer. Barcelona: Ed Elsevier (2019) 244–259. https://doi.org/10.1016/j.jtho.2019.08.338
59. Casini B, Sarti D, Gallo E, Alessandrini G, Cecere F, Pescarmona E, et al. Thymic carcinoma, preliminary data of next generation sequencing MS08.04 Novel Biomarkers for Thymic Carcinoma. In: Elsevier, Editor. World Conference on Lung cancer; Barcelona, Spain: IASLC - Journal of Thorac Oncol. Seul: Ed Mediastinum (2019), p.s169–s70. doi: 10.21037/med.2018.AB008
60. Ehemann V, Kern MA, Breinig M, Schnabel PA, Gunawan B, Schulten HJ, et al. Establishment, characterization and drug sensitivity testing in primary cultures of human thymoma and thymic carcinoma. Int J Cancer. (2008) 122:2719–25. doi: 10.1002/ijc.23335
61. Bussolati G, Annaratone L, Medico E, D'Armento G, Sapino A. Formalin fixation at low temperature better preserves nucleic acid integrity. PLoS ONE. (2011) 6:e21043. doi: 10.1371/journal.pone.0021043
62. Rezzani R, Nardo L, Favero G, Peroni M, Rodella LF. Thymus and aging, morphological, radiological, and functional overview. Age. (2014) 36:313–51. doi: 10.1007/s11357-013-9564-5
63. Kadouri N, Nevo S, Goldfarb Y, Abramson J. Thymic epithelial cell heterogeneity, TEC by TEC. Nat Rev Immunol. (2019) 20:1–15. doi: 10.1038/s41577-019-0238-0
64. Serpico D, Trama A, Haspinger ER, Agustoni F, Botta L, Berardi R, et al. Available evidence and new biological perspectives on medical treatment of advanced thymic epithelial tumors. Ann Oncol. (2015) 26:838–47. doi: 10.1093/annonc/mdu527
65. Ruffini E, Guerrera F, Brunelli A, Passani S, Pellicano D, Thomas P, et al. Report from the European society of thoracic surgeons prospective thymic database 2017, a powerful resource for a collaborative global effort to manage thymictumours. Eur J Cardiothorac Surg. (2019) 55:601–9. doi: 10.1093/ejcts/ezy448
66. Edwards JG, Chansky K, Van Schil P, Nicholson AG, Boubia S, Brambilla E, et al. The IASLC lung cancer staging project, analysis of resection margin status and proposals for residual tumor descriptors for non-small cell lung cancer. J Thorac Oncol. (2020) 15:344–59. doi: 10.1016/j.jtho.2019.10.019
67. Drevet G, Collaud S, Tronc F, Girard N, Maury JM. Optimal management of thymic malignancies, current perspectives. Cancer Manag Res. (2019) 11:6803–14. doi: 10.2147/cmar.s171683
68. Krishnan M, Ganti AK. The role of targeted therapy in thymic carcinoma. J Oncol Pharm Pract. (2019) 25:1712–8. doi: 10.1177/1078155219852758
69. Gong J, Jin S, Pan X, Wang G, Ye L, Tao H, et al. Identification of long non-coding RNAs for predicting prognosis among patients with thymoma. Clin Lab. (2018) 64:1193–8. doi: 10.7754/Clin.Lab.2018.180136
70. di Martino S, De Luca G, Grassi L, Federici G, Alfonsi R, Signore M, et al. Renal cancer, new models and approach for personalizing therapy. J Exp Clin Cancer Res. (2018) 37:217. doi: 10.1186/s13046-018-0874-4
71. Saltz J, Gupta R, Hou L, Kurc T, Singh P, Nguyen V, et al. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep. (2018) 23:181–93.e7. doi: 10.1016/j.celrep.2018.03.086
72. Basse C, Thureau S, Bota S, Dansin E, Thomas PA, Pichon E, et al. Multidisciplinary tumor board decision making for postoperative radiotherapy in thymic epithelial tumors, insights from the RYTHMIC prospective cohort. J Thorac Oncol. (2017) 12:1715–22. doi: 10.1016/j.jtho.2017.07.023
73. Lopez-Beltran A, Canas-Marques R, Cheng L, Montironi R. Histopathologic challenges, the second OPINION issue. Eur J Surg Oncol. (2019) 45:12–5. doi: 10.1016/j.ejso.2018.09.003
Keywords: thymic epithelial tumors, thymoma, thymic carcinoma, biobank, microRNA, TCGA, ITMIG, EURACAN
Citation: Melis E, Gallo E, di Martino S, Gallina FT, Laquintana V, Casini B, Visca P, Ganci F, Alessandrini G, Caterino M, Cecere FL, Mandoj C, Papadantonakis A, De Bello N, Lattanzio R, Palmieri G, Garassino MC, Girard N, Conti L, Blandino G, Fazi F, Facciolo F, Pescarmona E, Ciliberto G and Marino M (2020) Thymic Epithelial Tumors as a Model of Networking: Development of a Synergistic Strategy for Clinical and Translational Research Purposes. Front. Oncol. 10:922. doi: 10.3389/fonc.2020.00922
Received: 06 February 2020; Accepted: 11 May 2020;
Published: 14 July 2020.
Edited by:
Giuseppe Giaccone, Weill Cornell Medicine, United StatesReviewed by:
Christine Fillmore Brainson, University of Kentucky, United StatesCopyright © 2020 Melis, Gallo, di Martino, Gallina, Laquintana, Casini, Visca, Ganci, Alessandrini, Caterino, Cecere, Mandoj, Papadantonakis, De Bello, Lattanzio, Palmieri, Garassino, Girard, Conti, Blandino, Fazi, Facciolo, Pescarmona, Ciliberto and Marino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirella Marino, bWlyZWxsYS5tYXJpbm9AaWZvLmdvdi5pdA==; bWlyZWxsYW1hcmlub0BpbndpbmQuaXQ=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.