- 1Department of Orthopaedics, Tongji Medical College, Union Hospital, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Otorhinolaryngology, The Third Hospital of Wuhan City, Wuhan, China
- 3Department of Orthopaedics, The Qichun People's Hospital, Qichun, China
- 4Department of Orthopaedics, Tongji Medical College, Union Jiangbei Hospital, Huazhong University of Science and Technology, Wuhan, China
Purpose: There have been many attempts to preoperatively evaluate the chemotherapy response of osteosarcoma patients using 99mTc-MIBI scintigraphy. However, the evaluations were lacking in consistency. We performed this systematic review and meta-analysis to systematically evaluate the ability of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
Methods: For this systematic review and meta-analysis, PubMed, Web of Science, OVID, the Cochrane Library, and CNKI were searched. Eligible studies were included based on the defined criteria. The index test was 99mTc-MIBI scintigraphy, the reference standard was tumor necrosis rate. Quality Assessment of Diagnostic Accuracy Studies-2 was adopted for quality assessment of included studies. The statistical pooling analysis, meta-regression analysis, subgroup analysis, sensitivity analysis, and publication bias of our research were performed using STATA 15.
Results: Eight articles with 189 osteosarcoma patients were included in this systematic review and meta-analysis. Our results demonstrated that the threshold effect of our meta-analysis was significant. The uptake change ratio of 99mTc-MIBI scintigraphy had a pooled sensitivity, specificity, positive and negative likelihood ratio, diagnostic odds ratio, and the area under curve of 0.98 (0.58–1.00), 0.68 (0.47–0.84), 3.1 (1.7–5.5), 0.03 (0.00–0.90), 103 (4–3,003), and 0.91 (0.88–0.93) in preoperative assessment of response of osteosarcoma patients to neoadjuvant chemotherapy. Meta-regression analysis and subgroup analysis indicated the factors of method and cut off value may introduce the heterogeneity. The pooled sensitivity, specificity, positive and negative likelihood ratio, diagnostic odds ratio, and the area under curve of washout rate of 99mTc-MIBI were 0.87 (0.69–0.95), 0.91 (0.75–0.97), 9.3 (3.2–27.0), 0.15 (0.06–0.37), 64 (14–301), and 0.89 (0.86–0.92), respectively. Sensitivity analysis and publication bias demonstrated our meta-analysis was reliable.
Conclusion: Both the ΔUR and WR derived from 99mTc-MIBI scintigraphy were valuable in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy, and ΔUR may possess a more outstanding diagnostic accuracy than WR.
Introduction
Osteosarcoma is the most commonly seen primary malignant bone tumor that originates from mesenchymal tissue. It usually occurs in the metaphysis of long bones, especially around the knee, of adolescents and young adults (1). It is characterized by its high degree of malignancy, namely high aggressive ability, and early systematic metastasis; 80% of osteosarcoma patients are present with imaging-detectable lung metastatic disease or micro-metastatic disease at their first diagnosis, besides, many patients without metastasis at diagnosis will progress to metastasis during their stages of treatment, and 80% of metastatic lesions arise in the lungs (2, 3). Over past decades, the modality of combining neoadjuvant chemotherapy, surgical resection, and postoperative chemotherapy has been clinically regarded as the standard treatment modality for osteosarcoma patients, and it has remarkably ameliorated patients' survival rate and avoided the necessity of amputation (4, 5). The 5-year survival rate of osteosarcoma patients was around 20–30% in the past with surgery alone, whilst after the advent of multiagent chemotherapy regimens, the long-term survival rate has improved to 65–70% for patients with localized osteosarcoma (2, 6). However, the survival for osteosarcoma has reached a plateau in the last 30–40 years, and patients with tumor recurrence after primary tumor resection or lung metastases continued to have very poor outcomes, which may closely relate to their poor response to chemotherapy (7–9). Thus, more effective therapeutic strategies and helpful and convenient prognostic evaluation methods are highly needed for patients with osteosarcoma.
Response to chemotherapy has been regarded as the most important prognostic factor for osteosarcoma patients (10). Currently, the standard of chemotherapy response assessment is to histologically assess the necrosis rate of the resected tumor after neoadjuvant chemotherapy (11). Osteosarcoma patients with a necrosis rate ≥90% had been defined as good responders, as an indication that they had received an effective preoperative treatment and that might have led to a good outcome (12, 13). The tumor necrosis rate (TNR) also was clinically used to identify patients with a high risk for metastasis and recurrence. However, TNR of the resected lesions is available only postoperatively. Furthermore, this method is complicated and cannot be timely monitoring the tumor response to chemotherapy and applied in non-surgical patients. Using a more convenient method at an early point preferably before treatment or operation to assess whether preoperative chemotherapy was effective was of great significance for the selection and modification of the therapeutic strategy and the evaluation of prognosis. The treatment strategy of suspected poor responders could be intensified and modified earlier, potentially ameliorating the effect of treatment and their prognosis.
There have been many attempts to evaluate the chemotherapy response of osteosarcoma patients before treatment or surgical resection, by various imaging techniques including 18F-FDG PET/CT, MRI, ultrasound, angiography, and radionuclide imaging (14–16). Tc-99mhexakis-2-methoxyisobutylisonitrile (99mTc-MIBI), commonly used as a myocardial perfusion imaging agent, has also been used for assessing the chemotherapy response with high sensitivity and specificity in types of cancers, such as breast cancer, lung cancer, and osteosarcoma (17–20). As far as we know, the evaluation of chemotherapy response can be achieved by analysis of the uptake change ratio (ΔUR) of 99mTc-MIBI in osteosarcoma before and after chemotherapy (21). In addition, the washout rate (WR) of 99mTc-MIBI before chemotherapy, which correlates with the level of P-glycoprotein (P-gp) and multidrug resistance-associated protein (MRP), also has the power to evaluate the chemotherapeutic effect of osteosarcoma patients (22, 23). However, the evaluations were limited in sample size and inconsistent outcome, making the results of low reliability. The aim of this systematic review and meta-analysis was to collect all relevant studies through searching electronic databases to systematically evaluate the performance of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
Materials and Methods
This systematic review and bivariate meta-analysis was performed and organized according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses–Diagnostic Test Accuracy, namely the PRISMA-DTA Statement (24).
Search Strategy
We systematically searched the up-to-date electronic databases which included PubMed, Web of Science, OVID, the Cochrane Library, and CNKI to obtain the articles of using ΔUR or WR of 99mTc-MIBI scintigraphy to preoperatively predict the response of osteosarcoma patients to neoadjuvant chemotherapy. We limited the literature search language to English in the databases of PubMed, Web of Science, OVID, and the Cochrane Library, and to Chinese in CNKI. The last search date was December 20, 2019. To avoid article omission and promote search sensitivity, both MeSH terms and free words were used in our research strategy, which included the terms “99mTc-MIBI” and “Osteosarcoma.” In CNKI, the search strategy was correspondingly translated into Chinese. We also checked and screened the reference lists of all identified primary studies and topic relevant reviews for other potential publications to avoid omitting any potential original articles.
Inclusion and Exclusion Criteria
An article was considered eligible for inclusion in the systematic review and meta-analysis if it met the following inclusion criteria: (1) It was an original research article on osteosarcoma patients, who could be divided into good responders and poor responders by the TNR of more than or <90% according to Salzer-Kuntschik's grading systems (25). (2) Both 99mTc-MIBI scintigraphy and TNR were used to evaluate the response of osteosarcoma patients to neoadjuvant chemotherapy. (3) Availability of data including ΔUR or WR of 99mTc-MIBI scintigraphy and TNR, which can be obtained directly from the study or indirectly from the curves. (4) Availability of constructing a 2 * 2 contingency table which contained cases of true and false positives and negatives for performing the meta-analysis with 95% confidence interval (95% CI). However, article types of expert opinions, meeting papers, case reports, editorials, reviews, systematic reviews, and research studies unavailability of useful data or full-text were all excluded. If there were repeated researches, or relevant data was contained and reported in a previous study, we chose the most complete study or the most recent one. All the potential studies were carefully identified and assessed by two independent researchers (F.W. and X.H.) and the decision to include or exclude a study was initially made basing on the study title and abstract, and then the whole article. If met with discrepancies and controversies, decisions were made upon group discussion.
Data Extraction and Quality Assessment
The two researchers (F.W. and Y.H.) independently performed the data extraction process from eligible studies. To rule out discrepancy, extracted data was then cross-checked by the two researchers, and consensus was reached for all items through discussion. The following items of each eligible study were collected, which included first author, year of publication, countries, sample size, gender, age, study design, methods of retrieving data, dose of 99mTc-MIBI, region of interest, scan time, interested index, criteria to define cut off value, data of cases of true positive, false positive, false negative and true negative, and so on. In the circumstance that data was not presented directly in the publication, we utilized the Engauge Digitizer version 9.8 to obtain useful data from the curves. Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was implemented in RevMan 5.3 to assess the quality of eligible studies, which was independently performed by two researchers (F.W. and Y.H.) (26). Discrepancies were resolved through group discussion. QUADAS-2 contained four domains: Patient Selection, Index Test, Reference Standard, and Flow and Timing. All four domains were assessed in terms of risk of bias; the first three domains were also assessed in terms of concerns regarding applicability.
Statistical Analysis
The statistical analysis of our research was performed using STATA 15 (Stata Corporation, College Station, TX, USA). Midas module used an exact binomial rendition for synthesis of the diagnostic test data (27). Pooled sensitivity, pooled specificity, the corresponding positive likelihood (PLR), negative likelihood (NLR), and pooled diagnostic odds ratio (DOR) were calculated. The summary receiver operating characteristic (SROC) curve was constructed. Chi-square (χ2) based Cochran Q test and Higgins I2 statistic test were performed to evaluate the between-study heterogeneity. In our analysis, a P-value of <0.05 or an I2 value of >50% would be regarded as significant heterogeneity. The value of proportion of heterogeneity likely due to threshold effect provided information about the influence of a diagnostic threshold effect on heterogeneity. To detect other potential source of heterogeneity, meta-regression analysis, subgroup analysis, and sensitivity analysis were performed in our analysis. Graphical model of sensitivity analysis consisted of four plots: One, quantile plot of residual-based goodness-of fit. Two, chi-squared probability plot of squared Mahalanobis distances for assessment of the bivariate normality assumption. Three, spikeplot for checking for particularly influential observations using Cook's distance. And four, the scatterplot for checking for outliers using standardized predicted random effects (standardized level-2 residuals). To quantitatively evaluate the publication bias, Deeks' Funnel Plot Asymmetry Test was applied. A P < 0.05 hinted a statistically significant publication bias. Fagan nomogram was created to provide information about the likelihood that an osteosarcoma patient with a positive or negative 99mTc-MIBI scintigraphy result was a good responder or not.
Results
Search Results
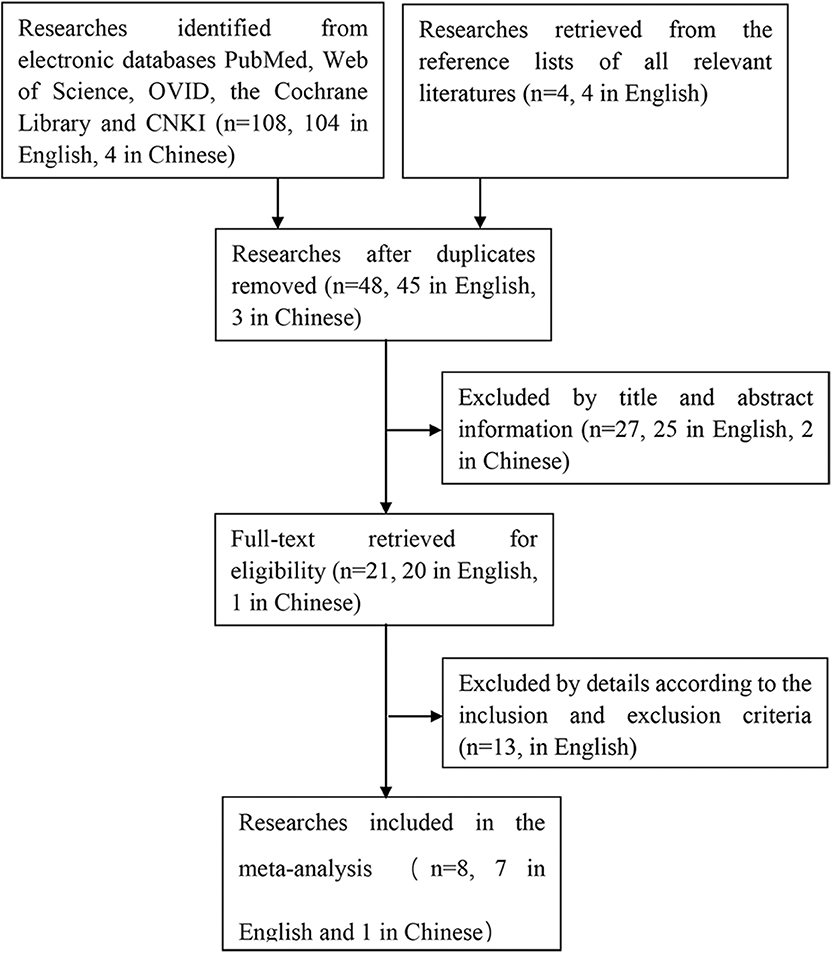
The process of searching and retrieving articles for this analysis was shown in Figure 1. A total of 108 potential articles (104 in English and 4 in Chinese) were identified from the electronic databases through our search process. We also screened the reference lists of all relevant literatures to identify other potential publications relating to the topic (n = 4, in English). After duplicates were eliminated, there were 48 articles (45 in English, 3 in Chinese) left, of which 27 articles (25 in English, 2 in Chinese) were excluded based on the title and abstract. Thirteen articles in English were excluded after reviewing the full article for the reasons as follows: 1 was eliminated because of full-text unavailable, 8 studies were eliminated for unavailability of getting the cases of true and false positives and negatives to construct a 2 * 2 contingency table, 2 articles were meeting papers, 2 articles were repeated researches, of which data was reported in previous studies. In the end, 8 articles (7 in English, 1 in Chinese) with a total of 189 osteosarcoma patients fulfilled all the eligible criteria and were evaluated in this meta-analysis (20, 23, 28–33).
Study Selection and Characteristics
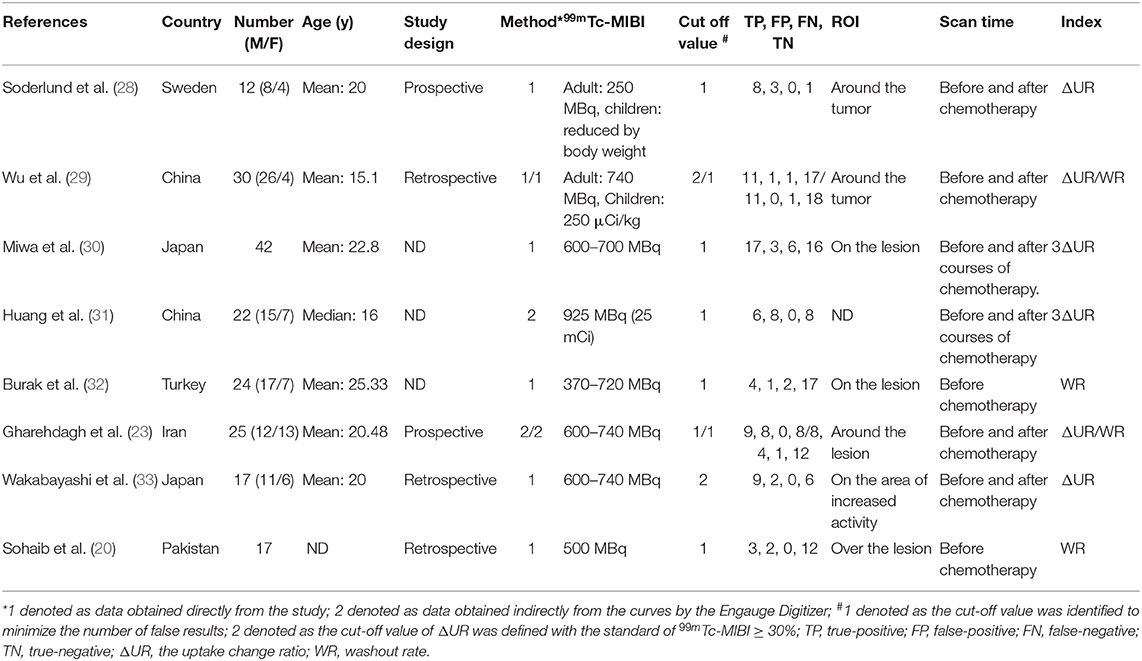
Table 1 shows the main characteristics of all the included studies, among which 6 studies with a total of 148 osteosarcoma patients were about the ΔUR of 99mTc-MIBI between pre- and post-chemotherapy and the histological response of osteosarcoma patients to neoadjuvant chemotherapy. Four studies with 96 osteosarcoma patients were about the WR of 99mTc-MIBI before chemotherapy and the response of osteosarcoma patients to neoadjuvant chemotherapy. The publication year of these eligible studies was from 1997 to 2019. Data of cases of true and false positives and negatives was obtained directly from 6 studies. In the other studies, it was obtained indirectly from the curves by the Engauge Digitizer. The number of patients in every study varied from 12 to 42. The optimal cut-off value was identified for WR to minimize the number of false results. So was ΔUR in 2 studies. In the other 4 studies, osteosarcoma patients with ΔUR of 99mTc-MIBI ≥30% were defined as positive responders (29). ΔUR of 99mTc-MIBI was received by comparing the activities of the osteosarcoma site before and after the chemotherapy in 4 studies or after 3 courses of chemotherapy in 2 studies. WR was measured on pre-chemotherapy 99mTc-MIBI imaging. All original studies of this meta-analysis fulfilled six or more “low risk” answers in the QUADAS-2 tool for methodological quality except for Shinji Miwa's research (Supplementary Figure 1). There was no “high risk” response to any domains. In the domain of applicability concerns, there were all “low risk” responses. In several researches, authors did not mention the sampling methods or inappropriate exclusions in the patient selection part.
Meta-Analysis of the Alteration Ratio of 99mTc-MIBI for the Preoperative Assessment of Histological Response to Neoadjuvant Chemotherapy in Osteosarcoma Patients
The meta-analysis of the ΔUR of 99mTc-MIBI for the preoperative assessment of the histological response of osteosarcoma patients to neoadjuvant chemotherapy included 6 studies with a total of 148 patients. Overall, the pooled sensitivity and pooled specificity were 0.98 (95% CI 0.58–1.00, I2 = 50.51%, P =0.07), and 0.68 (95% CI 0.47–0.84, I2 = 70.31%, P < 0.01), respectively (Figure 2). Besides, the pooled PLR was 3.1 (95% CI 1.7–5.5, I2 = 44.55%, P = 0.01) and the NLR was 0.03 (95% CI 0.00–0.90, I2 = 0.00, P = 0.42) (Supplementary Figure 2). There was a significant difference between the good and poor responders in the DOR (pooled DOR: 103, 95% CI 4–3,003, I2 = 68.49%, P = 0.01) (Supplementary Figure 3). The proportion of heterogeneity was likely due to threshold effect = 1.00, demonstrating that threshold effect of the meta-analysis existed, and the differences in the cut-off values of ΔUR of 99mTc-MIBI scintigraphy were related to the heterogeneity. The SROC curve showed the AUC was 0.91 (95% CI 0.88–0.93) (Figure 3).
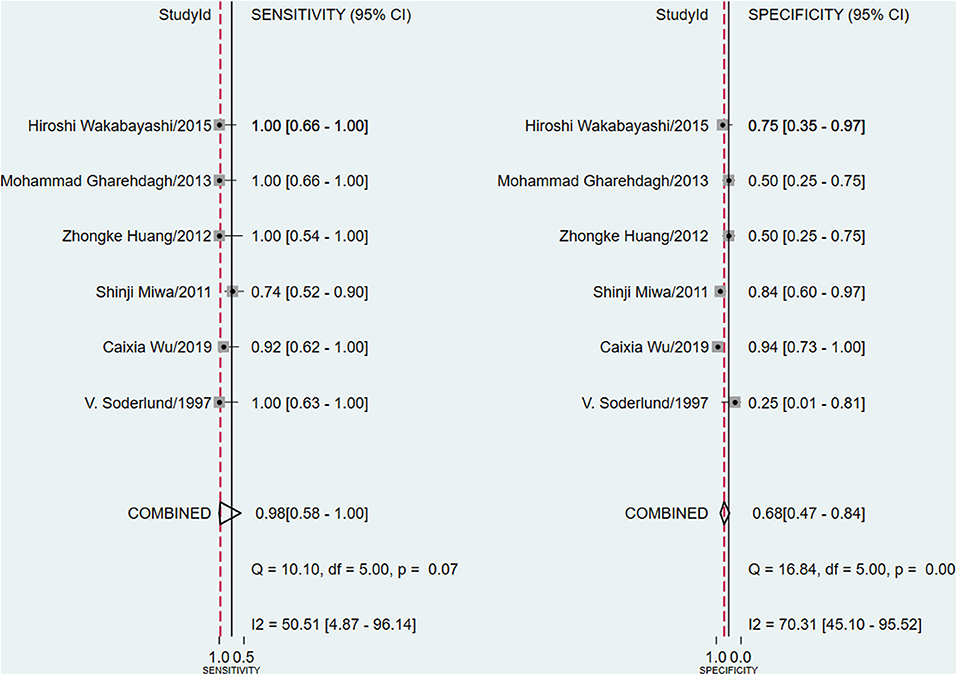
Figure 2. Forest plots of the pooled sensitivity and specificity for the uptake change ratio of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
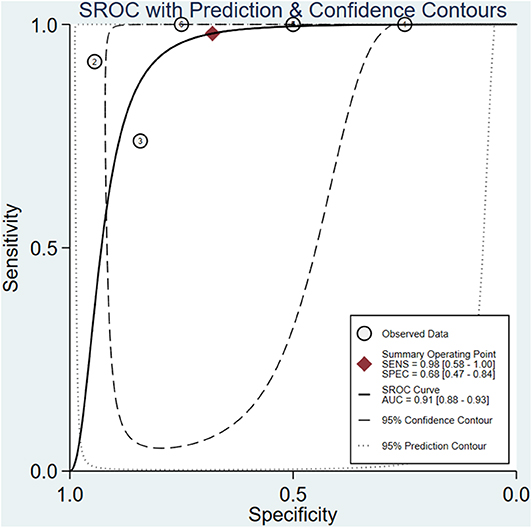
Figure 3. Summary receiver operating characteristic plot for the uptake change ratio of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
Except for the parameter of NLR (I2 = 0.00, P = 0.42), significant heterogeneity existed among the included studies, and the overall I2 for bivariate model was 81% (95% CI 58–100%), P = 0.003. The heterogeneity of parameters including sensitivity, specificity, PLR, and DOR among the 6 studies was significant. Due to the substantial heterogeneity caused by threshold effect and non-threshold effect, univariate meta-regression analyses and subgroup analyses were performed. The results of univariate meta-regression analyses revealed that the method of retrieving data of cases of true and false positives and negatives and cut off value may be the source of heterogeneity (Figure 4). In stratified analysis based on the factors of method and cut-off value, we found that the pooled sensitivity, specificity, PLR, NLR, DOR, and AUC were 0.94 (95% CI 0.53–1.00), 0.78 (95% CI 0.50–0.92), 4.2 (95% CI 1.7–10.6), 0.08 (95% CI 0.00–0.82), 55 (95% CI 5–612), and 0.93 (95% CI 0.90–0.95), respectively in the group of obtaining data directly from the study. And the heterogeneity was I2 = 36%, P = 0.105, which was attenuated compared with overall heterogeneity (I2 = 81%, P = 0.003). In the subgroup of identifying cut-off value to minimize the number of false results, the pooled sensitivity, specificity, PLR, NLR, DOR, and AUC were 0.98 (95% CI 0.37–1.00), 0.57 (95% CI 0.34–0.77), 2.3 (95% CI 1.4–3.8), 0.04 (95% CI 0.00–2.39), 65 (95% CI 1–4327), and 0.83 (95% CI 0.80–0.86), respectively. The heterogeneity was still significant with I2 = 74% and P = 0.011. Owing to the limited amount of included studies in the groups of method of obtaining data indirectly from the curves and identifying 99mTc-MIBI ≥30% as the cut-off value to define positive responders, pooled results could not be received in these subgroups. As mentioned above, the factors of method and cut-off value may introduce the heterogeneity, and works in consideration of the same method to retrieve data, the same criteria to identify positive responders, and the same protocol to perform 99mTc-MIBI scintigraphy may help us to get an ideal conclusion in the future.
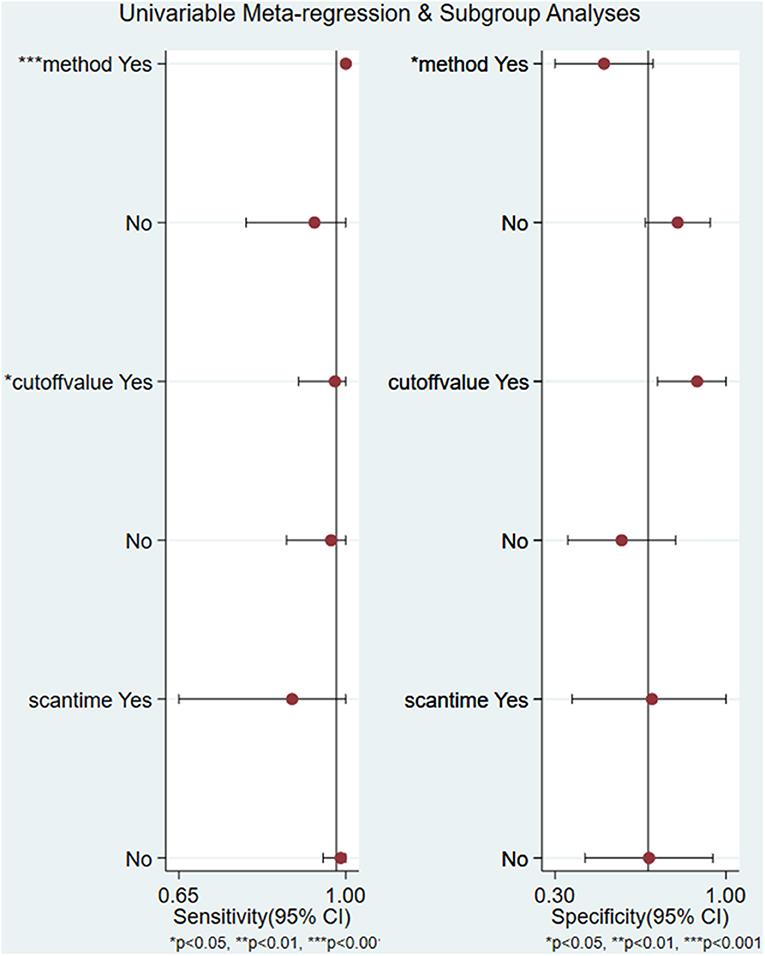
Figure 4. Univariate regression analysis and subgroup analysis for the uptake change ratio of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
Meta-Analysis of the Washout Rate of 99mTc-MIBI for the Preoperative Assessment of Histological Response to Neoadjuvant Chemotherapy in Osteosarcoma Patients
A total of 4 studies with 96 patients were about the WR of 99mTc-MIBI for the preoperative assessment of the histological response of osteosarcoma patients to neoadjuvant chemotherapy. The pooled sensitivity, specificity, PLR, NLR, and DOR were 0.87 (95% CI 0.69–0.95, I2 = 0.00, P =0.42), 0.91 (95% CI 0.75–0.97, I2 = 52.97%, P = 0.09) (Figure 5), 9.3 (95% CI 3.2–27.0, I2 = 0.00, P = 0.16), 0.15 (95% CI 0.06–0.37, I2 = 0.00, P = 0.51) (Supplementary Figure 4), and 64 (95% CI 14–301, I2 = 53.28%, P = 0.09) (Supplementary Figure 5), respectively. The SROC curve showed the AUC was 0.89 (95% CI 0.86–0.92) (Figure 6).
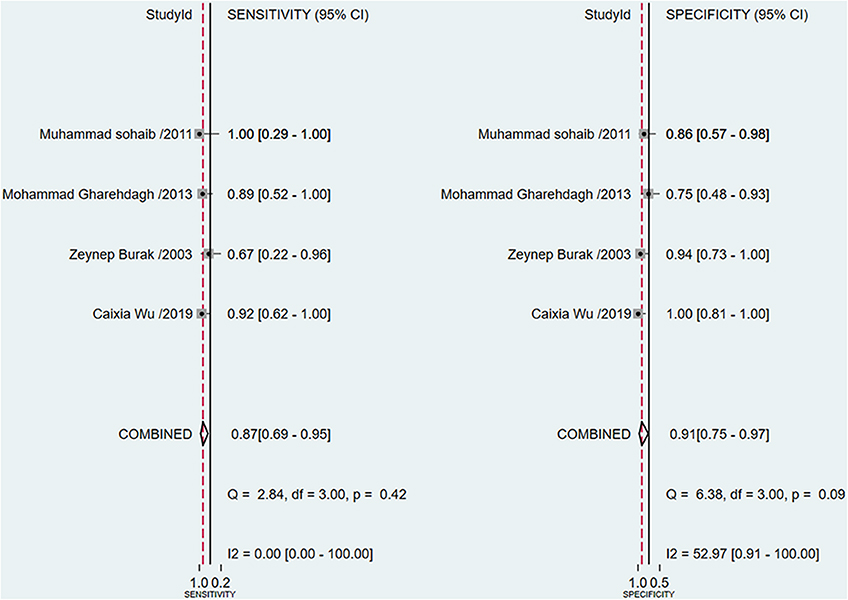
Figure 5. Forest plots of the pooled sensitivity and specificity for the washout rate of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
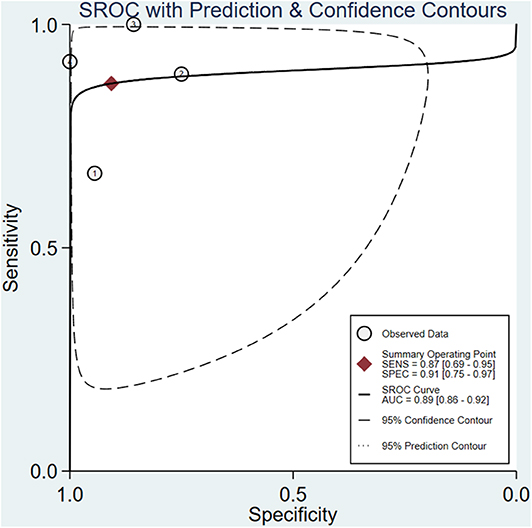
Figure 6. Summary receiver operating characteristic plot for the washout rate of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
The proportion of heterogeneity was likely due to threshold effect =1.00, demonstrating that different cut-off values to define positive responders among studies may play an important role in the heterogeneity. Except for threshold effect, the overall heterogeneity for bivariate model was I2 = 0.00, P = 0.377. There was not significant heterogeneity among the 4 studies under the pooled sensitivity, PLR, and NLR. In terms of the pooled specificity and DOR, the heterogeneity was present with I2 = 52.97%, P = 0.09 and I2 = 53.28%, P = 0.09. However, univariate meta-regression and subgroup analyses were not performed in this part, because the number of included studies was limited, and the WR measured on pre-chemotherapy took the same criteria to define positive responders. To attenuate threshold effect and heterogeneity, more researches on the topic with a more homogenous protocol to perform 99mTc-MIBI scintigraphy and criteria to define positive responders were highly needed.
Publication Bias and Sensitivity Analysis
The Deeks' Funnel Plot hinted no publication bias in the meta-analysis of using ΔUR or WR of 99mTc-MIBI to preoperatively assess the histological response of osteosarcoma patients to neoadjuvant chemotherapy (P = 0.74 and P = 0.32, respectively) (Supplementary Figure 6). The sensitivity analysis was performed, the quantile plot of residual-based goodness-of-fit and chi-squared probability plot for bivariate normality demonstrated that each included study had only minimal influence on the overall estimates, spikeplot for influence analysis and the scatterplot for checking for outliers identified no outlier studies, indicating that our meta-analysis were full of stability (Figure 7).
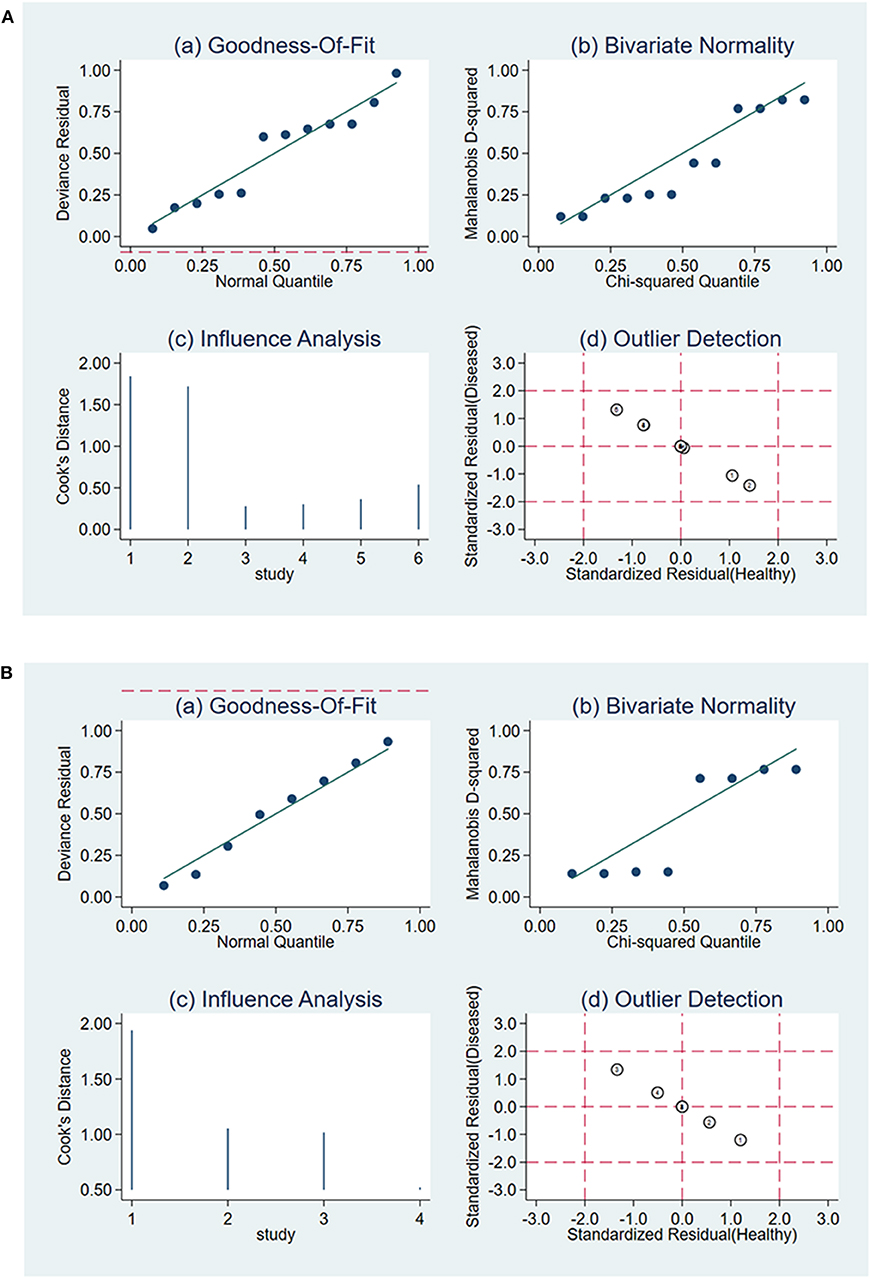
Figure 7. Sensitivity analysis for the uptake change ratio (A) and washout rate (B) of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
Fagan Nomogram
Likelihood ratios and post-test probabilities presented in Fagan nomogram were of great value for clinicians, which provided the possibility that an osteosarcoma patient with a positive or negative 99mTc-MIBI scintigraphy result was a good responder or not. In our analysis, the positive likelihood ratio of 3 implied that osteosarcoma patients defined as positive responders according to ΔUR of 99mTc-MIBI were 3 times more likely to have TNR ≥ 90% than those with negative ΔUR result. Given a pretest probability of 50%, the probability for osteosarcoma patients with positive ΔUR result having TNR ≥ 90% was 75%. The negative likelihood ratio of 0.03 decreased the post-test probability to 3% for patients with negative ΔUR result, implying that the probability for an osteosarcoma patient with a negative ΔUR result having a TNR ≥ 90% was 3% (Figure 8A). According to Figure 8B, we found that the positive likelihood ratio of WR was 9, which was higher than that of ΔUR. Given a pretest probability of 50%, the post-test probability of WR was 90%. The negative likelihood ratio of 0.15 decreased the post-test probability to 13% for an osteosarcoma patient with a negative WR result, namely, the probability for osteosarcoma patients with negative WR result having TNR ≥ 90% was 13%.
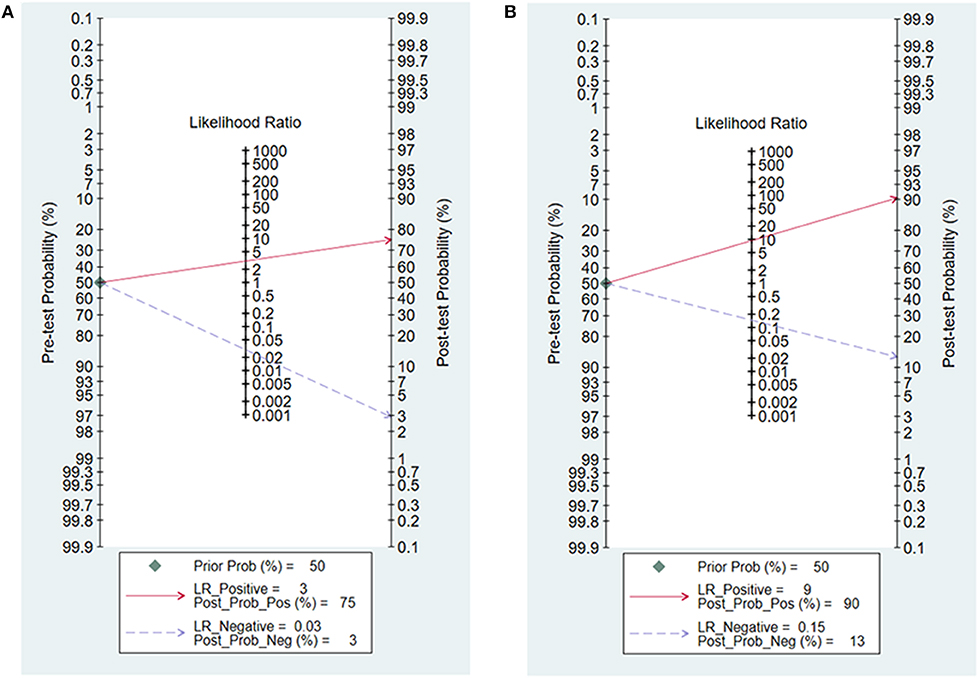
Figure 8. Fagan nomogram for the uptake change ratio (A) and washout rate (B) of 99mTc-MIBI scintigraphy in preoperatively assessing the response of osteosarcoma patients to neoadjuvant chemotherapy.
Discussion
Imaging techniques including 18F-FDG PET/CT, MRI, radionuclide imaging, etc. could be easily conducted at any treatment stage and they have played an important role in assessing the response of osteosarcoma patients to preoperative chemotherapy (14, 15). However, there has not been consensus on which imaging method is the best. Many researchers have reported that radionuclide imaging, such as thallium-201 and 99mTc-MIBI scintigraphy, possessed acceptable performance in preoperatively evaluating efficacy of osteosarcoma patients to preoperative chemotherapy (29, 34). Besides, thallium-201 and 99mTc-MIBI scintigraphy were relatively economical and convenient to conduct. In the aspect of physical properties, 99mTc-MIBI is comparatively better than thallium-201, and the half-value periods of thallium-201 are much longer than that of 99mTc-MIBI, which limits the dose and results of thallium-201 scintigraphy in poor image quality (30). 99mTc-MIBI could passively diffuse through the cell membrane and accumulate in mitochondria under the effect of transmembrane potential. Metabolically active tissues containing more mitochondria will take up and retain more 99mTc-MIBI (35). In osteosarcoma patients, the uptake and accumulation of 99mTc-MIBI indirectly represents the presence of metabolically active osteosarcoma cells, and 99mTc-MIBI scintigraphy evaluation for response to preoperative chemotherapy can be achieved by analyzing the ΔUR of tumor 99mTc-MIBI before and after neoadjuvant chemotherapy. Response of osteosarcoma patients to chemotherapeutics is often significantly correlated with the level of P-gp and MRP, which can efflux chemotherapeutics out of tumor cells and thus impair the chemotherapy effect (36). 99mTc-MIBI is a transport substrate of both P-gp and MRP, which would transport 99mTc-MIBI out of tumor cells (37). A rapid WR of 99mTc-MIBI is positively related to a high level of P-gp and MRP in tumor patients. Theoretically, response of osteosarcoma patients to chemotherapy also could be predicted by the WR of 99mTc-MIBI from the early and delayed images. There have been many researches assessing the 99mTc-MIBI scintigraphy for the preoperative prediction of neoadjuvant chemotherapy response in osteosarcoma patients, but the researches were limited in sample size and lack of reliability. In some researches, the conclusions were even inconsistent (32, 38). Thus, to derive more robust and reliable estimates of the evaluating performance of 99mTc-MIBI scintigraphy for the response of osteosarcoma patients to neoadjuvant chemotherapy, we collected and pooled all relevant researches in this meta-analysis, which, as far as we know, had not previously been published.
The result of our analysis revealed that the proportion of heterogeneity likely due to threshold effect = 1.00, namely, threshold effect of the meta-analysis was significant, and statistical pooling of sensitivity, specificity, PLR, NLR, and DOR should be cautious. Then, the AUC serving as a global measure of test performance could be a more useful index to estimate the performance of 99mTc-MIBI scintigraphy for the response of osteosarcoma patients to neoadjuvant chemotherapy. The following guidelines were accepted for interpretation of intermediate AUC values: low accuracy (0.5 ≤ AUC ≤ 0.7), moderate accuracy (0.7 ≤ AUC ≤ 0.9), and high accuracy (0.9 ≤ AUC ≤ 1) (39). According to the value of AUC, we concluded the index of ΔUR of 99mTc-MIBI uptake possessed a high diagnostic accuracy. Due to the substantial heterogeneity of the meta-analysis of ΔUR of 99mTc-MIBI for the preoperative assessment of the histological response of osteosarcoma patients to neoadjuvant chemotherapy, meta-regression and subgroup analyses were performed. Their results suggested that the method of retrieving data of cases of true and false positives and negatives and cut-off value may be the source of heterogeneity. As for the WR of 99mTc-MIBI before chemotherapy, threshold effect existed and the heterogeneity of several parameters was insignificant except for the specificity and DOR. The index of WR of 99mTc-MIBI possessed a moderate diagnostic accuracy with the value of AUC = 0.89. Owing to the limited number of included studies, meta-regression and subgroup analyses were not performed in this part. To attenuate the threshold effect and heterogeneity of our analysis, more researches on the topic with the same method to retrieve data and a more homogenous protocol to perform 99mTc-MIBI scintigraphy and criteria to define positive responders were highly needed in the future. According to the value of AUC and pooled DOR, both the indexes of ΔUR and WR derived from 99mTc-MIBI scintigraphy were valuable in preoperatively assessing the histological response of osteosarcoma patients to neoadjuvant chemotherapy, and ΔUR possessed a more outstanding diagnostic accuracy than that of WR. The result of sensitivity analysis identified no outlier studies and the included studies had only minimal influence on the overall estimates. Assessment of publication bias was performed through the Deeks' Funnel Plot Asymmetry Test, which did not hint any evidence of publication bias in the meta-analysis. Both the sensitivity analysis and the publication bias analysis demonstrated the result of our study was stable, reliable, and believable.
Our research was the first meta-analysis on this topic. It was based on thorough literature searches upon different electronic databases. Data extraction, study selection with well-defined inclusion and exclusion criteria, and the quality assessment of included studies using QUADAS-2 implemented in RevMan 5.3 were all performed independently by two researchers, and discrepancies were resolved through group discussion. However, several limitations of our study still should be considered. Firstly, the number of included studies and patients included in the analysis was limited, because several studies were eliminated for unavailability of getting the cases of true and false positives and negatives. More studies relevant to the topic were necessary to make our results more reliable and credible. Secondly, the threshold effect of our analysis was obvious, which would somewhat impair the credibility of our conclusions. Osteosarcoma patients with ΔUR ≥ 30% were defined as positive responders in 2 studies. However, in the other 4 studies on ΔUR, the cut-off value was identified to minimize the number of false results. Thus, original researches with a more homogenous protocol to perform 99mTc-MIBI scintigraphy and criteria to define positive responders were highly needed in the future. Thirdly, heterogeneity of the meta-analysis of ΔUR of 99mTc-MIBI for the preoperative assessment of histological response of osteosarcoma patients to neoadjuvant chemotherapy was significant. However, subgroup analysis was unavailable in some cases, owing to the limited number of included studies. More studies on the topic were necessary, and meta-regression and subgroup analysis based on other factors could be considered to vanish the heterogeneity. Fourthly, we did not compare the diagnostic accuracy of 99mTc-MIBI scintigraphy with other imaging techniques, and we still did not reach consensus on which imaging method was most suitable to be applied in daily clinical work. Fifthly, the study design of most included studies was not clearly mentioned in text, which made the subgroup analysis based on the factor of study design could not be performed. In the end, we only included studies written in English and Chinese, which might affect our findings.
In this meta-analysis, we aimed to semi-quantitatively assess the histological response of osteosarcoma patients to neoadjuvant chemotherapy before surgery or treatment by using ΔUR or WR of 99mTc-MIBI scintigraphy. According to the value of AUC and pooled DOR, both the ΔUR and WR derived from 99mTc-MIBI scintigraphy were valuable in preoperatively assessing the histological response of osteosarcoma patients to neoadjuvant chemotherapy, and ΔUR possessed a more outstanding diagnostic accuracy than WR. More researches on the topic of this analysis with larger sample, the same method to retrieve data, and a more homogenous protocol to perform 99mTc-MIBI scintigraphy and criteria to define positive responders were highly required to verify the outcomes. Moreover, other more accurate and convenient methods of assessing the response of osteosarcoma patients to neoadjuvant chemotherapy still need to be developed and investigated in the future.
Data Availability Statement
All datasets generated and analyzed for this study are included in the article/Supplementary Material.
Author Contributions
FW, ZZ, and ZS: conception and design. FW and XinH (3rd Author): literature search and identification. FW and YH: data extraction and quality assessment. FW and ZZ: data analysis. FW, SF, XiaH, XinH (6th Author), and ZS: original draft prepararion. FW and ZS: writing-review, editing and supervision. ZZ and ZS: funding acquisition.
Funding
Our research was supported by the National Key Research and Development Program of China (2016YFC1100100), the Major Research Plan of National Natural Science Foundation of China (No. 91649204), and the Natural Science Foundation of Hubei Province, China (No. 2018CFB118).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00762/full#supplementary-material
Abbreviations
TNR, tumor necrosis rate; ΔUR, uptake change ratios; WR, washout rate; P-gp, P-glycoprotein; MRP, multidrug resistance-associated protein; 95% CI, 95% confidence interval; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies-2; PLR, positive likelihood; NLR, negative likelihood; DOR, diagnostic odds ratio; SROC, summary receiver operating characteristic; AUC, the area under curve.
References
1. Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma overview. Rheumatol Ther. (2017) 4:25–43. doi: 10.1007/s40744-016-0050-2
2. Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. (2014) 162:65–92. doi: 10.1007/978-3-319-07323-1_4
3. PosthumaDeBoer J, Witlox MA, Kaspers GJ, van Royen BJ. Molecular alterations as target for therapy in metastatic osteosarcoma: a review of literature. Clin Exp Metast. (2011) 28:493–503. doi: 10.1007/s10585-011-9384-x
4. Picci P, Ferrari S, Bacci G, Gherlinzoni F. Treatment recommendations for osteosarcoma and adult soft tissue sarcomas. Drugs. (1994) 47:82–92. doi: 10.2165/00003495-199447010-00006
5. Ferrari S, Meazza C, Palmerini E, Tamburini A, Fagioli F, Cozza R, et al. Picci: nonmetastatic osteosarcoma of the extremity. Neoadjuvant chemotherapy with methotrexate, cisplatin, doxorubicin and ifosfamide. An Italian Sarcoma Group study (ISG/OS-Oss). Tumor. (2014) 100:612–9. doi: 10.1700/1778.19262
6. Isakoff MS, Bielack SS, Meltzer P R. Gorlick: osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. (2015) 33:3029–35. doi: 10.1200/JCO.2014.59.4895
7. Palmerini E, Torricelli E, Cascinu S, Pierini M, De Paolis M, Donati D, et al. Ferrari: is there a role for chemotherapy after local relapse in high-grade osteosarcoma? Pediatr Blood Cancer. (2019) 66:e27792. doi: 10.1002/pbc.27792
8. Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, Marec-Berard P, et al. Hartmann: metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome–the French pediatric experience. Cancer Am Cancer Soc. (2005) 104:1100–9. doi: 10.1002/cncr.21263
9. Bacci G, Briccoli A, Longhi A, Ferrari S, Mercuri M, Faggioli F, et al. Picci: treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol. (2005) 44:748–55. doi: 10.1080/02841860500327503
10. Bramer JA, van Linge JH, Grimer RJ, Scholten RJ. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol. (2009) 35:1030–6. doi: 10.1016/j.ejso.2009.01.011
11. Sami SH, Rafati AH, Hodjat P. Tissue necrosis after chemotherapy in osteosarcoma as the important prognostic factor. Saudi Med J. (2008) 29:1124–9.
12. Bacci G, Mercuri M, Longhi A, Ferrari S, Bertoni F, Versari M, et al. Grade of chemotherapy-induced necrosis as a predictor of local and systemic control in 881 patients with non-metastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy in a single institution. Eur Cancer J. (2005) 41:2079–85. doi: 10.1016/j.ejca.2005.03.036
13. Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. (2002) 20:776–90. doi: 10.1200/JCO.2002.20.3.776
14. Eftekhari F. Imaging assessment of osteosarcoma in childhood and adolescence: diagnosis, staging, and evaluating response to chemotherapy. Cancer Treat Res. (2009) 152:33–62. doi: 10.1007/978-1-4419-0284-9_3
15. Taki J, Inaki A, Wakabayashi H, Sumiya H, Tsuchiya H, Zen Y, et al. Early prediction of histopathological tumor response to preoperative chemotherapy by Tc-99m MIBI imaging in bone and soft tissue sarcomas. Clin Nucl Med. (2010) 35:154–9. doi: 10.1097/RLU.0b013e3181cc637d
16. Connolly LP, Drubach LA Ted TS. Applications of nuclear medicine in pediatric oncology. Clin Nucl Med. (2002) 27:117–25. doi: 10.1097/00003072-200202000-00009
17. Sobic-Saranovic DP, Pavlovic SV, Jovanovic IV, Stefanovic ID, Artiko VM, Djukic MM, et al. Evaluation of myocardial perfusion and function by gated single-photon emission computed tomography technetium-99m methoxyisobutylisonitrile in children and adolescents with severe congenital heart disease. Nucl Med Commun. (2010) 31:12–21. doi: 10.1097/MNM.0b013e3283295622
18. Novikov SN, Kanaev SV, Petr KV, Tatyana SY, Elena TA, Ludmila JA, et al. Technetium-99m methoxyisobutylisonitrile scintimammography for monitoring and early prediction of breast cancer response to neoadjuvant chemotherapy. Nucl Med Commun. (2015) 36:795–801. doi: 10.1097/MNM.0000000000000331
19. Yang Xue J, Li X, Yu Y, Deng H, Hu G, Meng X, et al. Experimental and clinical observations of 99mTc-MIBI uptake correlate with P-glycoprotein expression in lung cancer. Nucl Med Commun. (2007) 28:696–703. doi: 10.1097/MNM.0b013e3281f74d97
20. Sohaib M, Wiqar MA, Ali MK, Hussain F, Umer-i-Farooq. A single (99m)Tc-MIBI study to predict response to neoadjuvant treatment in sarcoma patients. Hell J Nucl Med. (2011) 14:140–5.
21. Huang Z, Lou C. Application of the alteration uptake ratio of (99m)Tc-MIBI scintigraphy for evaluating the efficacy of neoadjuvant chemotherapy in osteosarcoma patients. Hell J Nucl Med. (2018) 21:55−9.
22. Burak Z, Ersoy O, Moretti JL, Erinc R, Ozcan Z, Dirlik A, et al. The role of 99mTc-MIBI scintigraphy in the assessment of MDR1 overexpression in patients with musculoskeletal sarcomas: comparison with therapy response. Eur J Nucl Med. (2001) 28:1341–50. doi: 10.1007/s002590100588
23. Gharehdaghi M, Dabbagh KV, Khooei A, Novferesti G, Hootkani A, Farzadnia M, et al. Is (99m)Tc-MIBI scintigraphy a predictor of response to pre-operative neoadjuvant chemotherapy in Osteosarcoma? Asia Ocean J Nucl Med Biol. (2013) 1:22–7.
24. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, And TPG, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. (2018) 319:388–96. doi: 10.1001/jama.2017.19163
25. Salzer-Kuntschik M, Delling G, Beron G, Sigmund R. Morphological grades of regression in osteosarcoma after polychemotherapy - study COSS 80. J Cancer Res Clin Oncol. (1983) 106(Suppl):21–4. doi: 10.1007/bf00625047
26. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
27. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. (2005) 58:982–90. doi: 10.1016/j.jclinepi.2005.02.022
28. Soderlund V, Larsson SA, Bauer HC, Brosjo O, Larsson O, Jacobsson H. Use of 99mTc-MIBI scintigraphy in the evaluation of the response of osteosarcoma to chemotherapy. Eur J Nucl Med. (1997) 24:511–5. doi: 10.1007/bf01267682
29. Wu C, Wang Q, Li Y. Prediction and evaluation of neoadjuvant chemotherapy using the dual mechanisms of (99m)Tc-MIBI scintigraphy in patients with osteosarcoma. J Bone Oncol. (2019) 17:100250. doi: 10.1016/j.jbo.2019.100250
30. Miwa S, Shirai T, Taki J, Sumiya H, Nishida H, Hayashi K, et al. Tsuchiya: use of 99mTc-MIBI scintigraphy in the evaluation of the response to chemotherapy for osteosarcoma: comparison with 201Tl scintigraphy and angiography. Int J Clin Oncol. (2011) 16:373–8. doi: 10.1007/s10147-011-0194-6
31. Huang ZK, Lou C, Shi GH. TI and (99m)Tc-MIBI scintigraphy in evaluation of neoadjuvant chemotherapy for osteosarcoma. Zhejiang Da Xue Xue Bao Yi Xue Ban. (2012) 41:183–7; 191.
32. Burak Z, Moretti JL, Ersoy O, Sanli U, Kantar M, Tamgac F, et al. 99mTc-MIBI imaging as a predictor of therapy response in osteosarcoma compared with multidrug resistance-associated protein and P-glycoprotein expression. J Nucl Med. (2003) 44:1394–401.
33. Wakabayashi H, Saito J, Taki J, Hashimoto N, Tsuchiya H, Gabata T, Kinuya S. Triple-phase contrast-enhanced MRI for the prediction of preoperative chemotherapeutic effect in patients with osteosarcoma: comparison with (99m)Tc-MIBI scintigraphy. Skeletal Radiol. (2016) 45:87–95. doi: 10.1007/s00256-015-2250-1
34. Kubo T, Shimose S, Fujimori J, Furuta T, Ochi M. Quantitative (201)thallium scintigraphy for prediction of histological response to neoadjuvant chemotherapy in osteosarcoma; systematic review and meta-analysis. Surg Oncol. (2015) 24:194–9. doi: 10.1016/j.suronc.2015.06.009
35. Chiu ML, Kronauge JF, Piwnica-Worms D. Effect of mitochondrial and plasma membrane potentials on accumulation of hexakis (2-methoxyisobutylisonitrile) technetium(I) in cultured mouse fibroblasts. J Nuclear Med. (1990) 31:1646–53.
36. Okada T, Tanaka K, Nakatani F, Sakimura R, Matsunobu T, Li X, et al. Involvement of P-glycoprotein and MRP1 in resistance to cyclic tetrapeptide subfamily of histone deacetylase inhibitors in the drug-resistant osteosarcoma and Ewing's sarcoma cells. Int. Cancer J. (2006) 118:90–7. doi: 10.1002/ijc.21297
37. Zhou J, Higashi K, Ueda Y, Kodama Y, Guo D, Jisaki F, et al. Expression of multidrug resistance protein and messenger RNA correlate with (99m)Tc-MIBI imaging in patients with lung cancer. J Nuclear Med. (2001) 42:1476–83.
38. Gorlick R, Liao AC, Antonescu C, Huvos AG, Healey JH, Sowers R, et al. Lack of correlation of functional scintigraphy with (99m)technetium-methoxyisobutylisonitrile with histological necrosis following induction chemotherapy or measures of P-glycoprotein expression in high-grade osteosarcoma. Clin Cancer Res. (2001) 7:3065–70.
Keywords: 99mTc-MIBI scintigraphy, uptake change ratios, washout rate, chemotherapy response, osteosarcoma, meta-analysis
Citation: Wu F, Huang Y, Huang X, Fang S, Huang X, Huang X, Zhang Z and Shao Z (2020) 99mTc-MIBI Scintigraphy for the Preoperative Assessment of Histological Response to Neoadjuvant Chemotherapy in Patients With Osteosarcoma: A Systematic Review and a Bivariate Meta-Analysis. Front. Oncol. 10:762. doi: 10.3389/fonc.2020.00762
Received: 10 February 2020; Accepted: 21 April 2020;
Published: 22 May 2020.
Edited by:
Georgios S. Limouris, National and Kapodistrian University of Athens, GreeceReviewed by:
Feng Chen, Peking University, ChinaAthanasios G. Zafeirakis, Army Share Fund Hospital (NIMTS), Greece
Copyright © 2020 Wu, Huang, Huang, Fang, Huang, Huang, Zhang and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fashuai Wu, MTg3NzEwMzYzNDcmI3gwMDA0MDsxNjMuY29t; Zhicai Zhang, emhpY2FpemhhbmcmI3gwMDA0MDsxMjYuY29t; Zengwu Shao, c3p3cHJvJiN4MDAwNDA7MTYzLmNvbQ==
 Fashuai Wu
Fashuai Wu Yu Huang2
Yu Huang2 Xin Huang
Xin Huang Zengwu Shao
Zengwu Shao