- 1Department of Pathology, Shantou University Medical College, Shantou, China
- 2Department of Abdominal Surgery, The Tumor Hospital of Shantou University Medical College, Shantou, China
- 3Department of Gastrointestinal Surgery, The First Affiliated Hospital of Shantou University Medical College, Shantou, China
Background: Gastric cardia cancer (GCC) arises in the area of the stomach adjoining the esophageal–gastric junction and has unique risk factors. It was suggested that the involvement of Helicobacter pylori is associated with GCC from high-risk population. Myeloid differentiation factor 88 (MyD88) is a crucial adaptor molecule in Toll-like signaling pathway recognizing H. pylori. Its role in GCC has not been elucidated yet. In this study, our purpose is to investigate the expression and significance of MyD88 in GCC tissue.
Methods: Expression of MyD88 and nuclear factor κB (NF-κB) p105/p50 and infection of H. pylori were detected by immunohistochemistry in gastric cardia tissue. The correlation of MyD88 expression to NF-κB p105/p50 expression, H. pylori infection, and clinicopathologic characteristics in gastric cardia tissue was analyzed. The involvement of MyD88 in patient prognosis was also analyzed.
Results: Our data showed that the expression of MyD88 elevated from normal mucosa to inflammation (p = 0.071). The expression of MyD88 was enhanced in GCC tissues by contrast to non-malignant cardia mucosa (p = 0.025). What's more, overexpression of MyD88 was detected in intestinal-type adenocarcinoma with inflammation. Patients with high MyD88 staining revealed a better differentiation (p = 0.02). MyD88 also positively correlated with NF-κB p105/p50 expression (p = 0.012) in cancer tissue. Expression of MyD88 was increased but not significantly in biopsies with H. pylori infection compared with non-infected biopsies. Multivariate analyses revealed lymph node metastasis but not MyD88 expression was an independent predictor for patient survival.
Conclusion: These findings provide pathological evidence that upregulating MyD88 and inducing inflammation might be involved in gastric cardia carcinogenesis in high-risk population. MyD88 plays a role in gastric cardia carcinogenesis with NF-κB pathway activation. Higher MyD88 expression is not a major prognostic determinant in GCC, but it may relate to the tumor cell differentiation.
Gastric cancer is a significant global health problem. It is one of the five most common malignancies and ranks after lung, breast, colorectal, and prostate cancer in 2012 (1). Geographically, 43% of total global cases occur in China (1). Gastric cancer is generally divided into two topographical categories: gastric cardia cancer (GCC) arising in the upper part of the stomach, where it connects to the esophageal, and non-GCC (NGCC) arising from rest part of the stomach. Gastric cardia cancer has unique epidemiology and risk factors different from NGCC. The incidence of GCC has been stable or increased, and the NGCC incidence decreased since the mid-1970s (1).
In China, the incidence of GCC differs on the basis of geographical situation and populations. Gastric cardia cancer has epidemiologic features of population and familial aggregation. The regions in China with high incidence rate of esophageal cancer also have high incidence of GCC, such as Linzhou (2) and Chaoshan area (3). Different from GCC, the incidence of NGCC is low in these areas. Risk factors of GCC are unclear and controversial. Studies of Caucasian populations suggested risk factors for GCC are similar to those for esophageal adenocarcinoma, including obesity, gastroesophageal reflux disease, and Barrett esophagus (1). Helicobacter pylori with positive test associated with NGCC is suggested inversely associated with GCC in Western countries. However, in high-risk settings, a positive association between H. pylori infection and gastric cancer was observed both for cardia and non-cardia cancers (4). Reports showed that the influence of gender, socioeconomic status, presence of intestinal metaplasia, and past alcohol intake also differ in GCC and NGCC (1). Considering the differences, more and more researches are addressing GCC and NGCC as separate diseases.
The Chaoshan GCC high-incidence area of east Guangdong province is the only coastal high-incidence area in China. From 1995 to 2004, previous epidemiological data revealed that the incidence of GCC was unusually high (34.81/100,000) on Nan'ao Island in the Chaoshan area (3). Our previous researches found that H. pylori infection accompanied with chronic inflammation may result in the carcinogenesis of GCC in Chaoshan region (5, 6).
Toll-like receptors (TLRs) may acquire oncogenic potential by initiating inflammatory pathways, which are essential for H. pylori recognition (5–7). The TLRs transmit signals through adaptor proteins. The first adaptor molecule of TLRs to be discovered is myeloid differentiation factor 88 (MyD88) (8). MyD88 is essential in regulating innate immune signals from members of the TLR and interleukin families. Toll-like receptors and interleukin 1 receptors can recognize microbes or endogenous ligands and then recruit MyD88, which can induce nuclear factor κB (NF-κB) activation (8–12). Previous study suggested that abnormal expression of MyD88 was closely associated with the development of tumor and resistance of drugs. In stomach, lung, liver, ovary cancer tissues, the expression of MyD88 was enhanced (8). However, the research data are contradictory. The effects of MyD88 in the development and progression of cancers are controversial (13, 14). MyD88-deficient mice models have shown MyD88 may either promote (10, 15–17) or suppress (18–20) tumor development. In colon cancer models, MyD88 showed contradictory roles even in the same cancer (21, 22).
Our previous study suggested that TLR4 plays a role in carcinogenesis of Chaoshan GCC (7). However, the expression of MyD88 in GCC and its effects on GCC development remain unknown. In the present study, we investigate the expression of MyD88 in gastric cardia tissue of different lesions from Chaoshan high-risk area and evaluate its correlation with H. pylori infection and NF-κB pathway activation.
Materials and Methods
Study Patients
One hundred two gastric cardia carcinoma samples and 95 non-malignant gastric cardia mucosa were obtained from the Tumor Hospital and the First Affiliated Hospital of Shantou University Medical College in Chaoshan area. The inclusion criterion for GCC is the center of cancer within 2 cm below the gastroesophageal junction defined by the World Health Organization (23). Follow-up survey was conducted for survival status of 71 patients by mobile phone or personal interview. Table 3 shows the clinicopathological features of the GCC patients. The median age was 62 years with range 40–78 years. Mean tumor diameter was 6 cm (range, 3–15 cm). In this study, we obtained all patients' informed consent and approval from the ethical review committees of the Medical College of Shantou University.
Immunohistochemistry
Formalin-fixed paraffin-embedded samples were sectioned at 4 μm and deparaffinized with xylene and rehydrated with graded ethanol, and then 3% hydrogen peroxide was used for preincubating for 10 min. Antigen retrieval was performed by heating for 20 min in microwave oven. Then, the sections were incubated with 10% normal goat serum to block/eliminate non-specific staining. Next, the tissues were incubated overnight at 4°C with the following antibodies: anti-MyD88 rabbit monoclonal antibody (ab133739; Abcam; Cambridge, MA, United States), anti–NF-κB p105/p50 rabbit monoclonal antibody (ab32360; Abcam; Cambridge, MA, United States), or anti-H. pylori rabbit polyclonal antibody (RAB-0064; Fuzhou Maixin Biotechnology; Fuzhou, Fujian Province, China). The tissues were incubated with the secondary antibody conjugated with horseradish peroxidase at 37°C for 30 min. Sections were counterstained with hematoxylin and mounted with glycerol gelatin. We used Olympus BX43 microscope (Olympus, Japan) and Olympus DP21 image management system (Olympus, Japan) to capture images.
Immunohistochemistry (IHC) staining score was evaluated by two experienced researchers in a blinded manner. The expression of MyD88 and NF-κB p105/p50 was rated (0–3) semiquantitatively according to the signal intensity (0 = no immunostaining, 1 = weak positive staining, 2 = moderate positive staining, 3 = strong positive staining) (24). We found intensity in different areas of the specimen was different. We observed the whole specimen and counted all positive and negative cells. Most sections with total number of cells varied from 5,000 to 8,000. The value was calculated by multiplying the scores of staining intensity by the proportion of positive cells (0–100%).
All values were added to generate a final score ranging from 0 to 300 (25). Helicobacter pylori IHC–positive test showed H. pylori are brownish yellow, thick, and rod-like, and some of them are clumps (26).
Chronic Inflammation Grading
According to the updated Sydney System, chronic inflammation was measured by the presence of polymorphonuclear leukocytes alongside the mononuclear inflammatory infiltration. The normal gastric mucosa has fewer than 5 inflammatory cells in the lamina propria. Mild inflammation shows 5–30 inflammatory cells in the lamina propria per high-power field or the foveolae. More than 30 inflammatory cells per high-power field infiltrating mucosal layer was considered severe inflammation (27).
Statistical Analysis
Independent non-parametric t-tests for trend were used to evaluate increased or decreased MyD88 expression among groups. Spearman correlation was used to determine the correlation between MyD88 and NF-κB p105/p50. The risk factors for overall survival were tested by a Cox proportional hazards model with a stepwise forward procedure. All statistical analyses were performed by using SPSS v19 (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
MyD88 Expression in Gastric Cardia Tissue
To detect the expression of MyD88 in nonmalignant tissue and GCC tissue, we performed MyD88 immunohistochemical staining in this retrospective cohort study. In Table 1 and Figure 1, MyD88 expression in the different gastric cardia lesions is shown. Immunostaining of MyD88 protein was mainly found in the cytoplasm, which was consistent with published result (28). Among the non-malignant gastric cardia tissues, MyD88 expression was higher in inflamed epithelia than that in normal gastric cardia mucosa (Figures 1A,B), and the p-value was close to significant level, but did not differ between mild and severe inflammation. In 102 GCC cases, MyD88 expression was detectable in most of the cases 72/102 (70.59%). MyD88 expression was significantly higher in tumor tissue than that in non-malignant gastric cardia tissues (p = 0.025) (Table 1). Moreover, the stronger MyD88 staining was found in intestinal-type adenocarcinoma with severe inflammation than in diffuse-type cancer (Figures 1C,D).
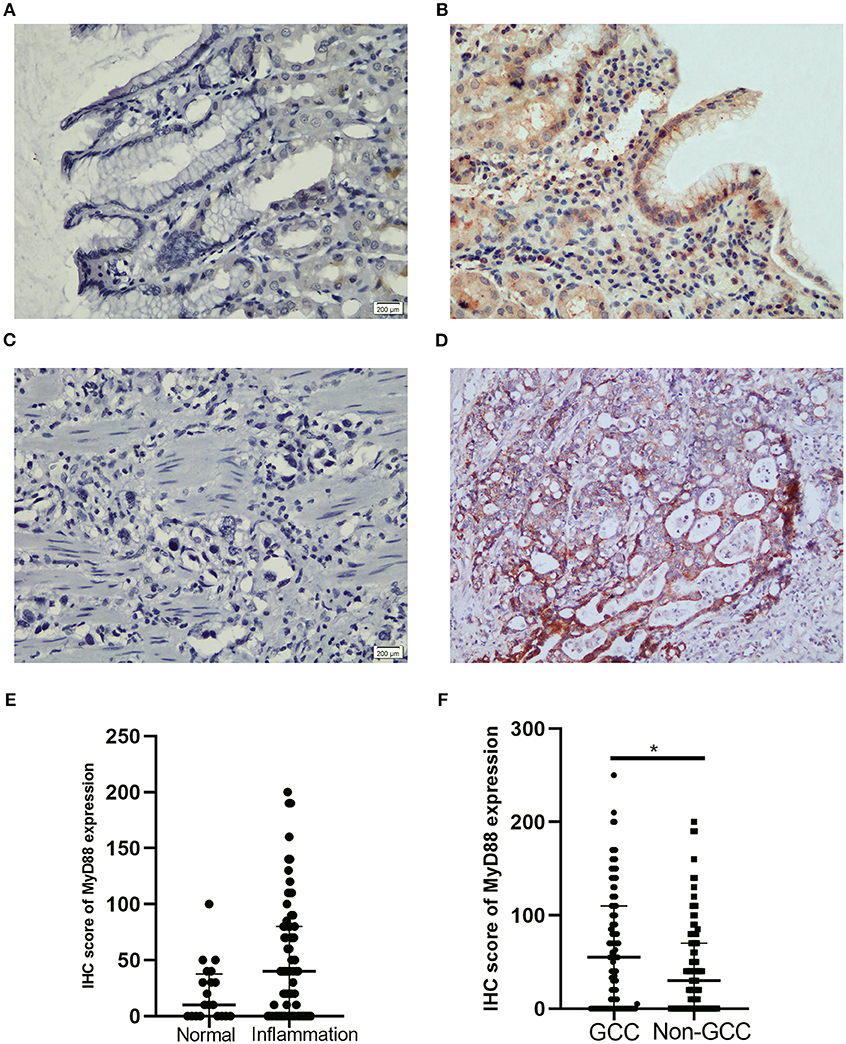
Figure 1. Representative IHC staining for MyD88 in gastric cardia mucosae and gastric cardia cancer tissue. (A) No immunostaining in normal mucosae, (B) moderate positive staining in mucosae with inflammation, (C) negative MyD88 staining in poorly differentiated tumor, and (D) strong positive staining in intestinal-type adenocarcinoma. (E) Boxplot shows MyD88 expression was higher in the inflammation cases than normal tissues. (F) Boxplot shows MyD88 expression was significantly higher in the GCC cases compared to the non-malignant cases (*p < 0.05).
Correlation Between MyD88 Expression and H. pylori Infection
MyD88 may play a role in gastric immunologic response to H. pylori (29, 30). We hypothesized that MyD88 expression correlates with H. pylori infection. At the beginning we tried to compare the MyD88 expression correlates with H. pylori infection in GCC tissue. However we found that most of the tumor tissue had necrosis and very few samples could found H. pylori by IHC. So we just used the non-GCC tissue to analyze H. pylori infection. We think that the results from non-malignant gastric cardia tissues can reflect the relationship between MyD88 expression and H. pylori infection. Thus, we use immunohistochemical staining to detect H. pylori infection in the non-malignant gastric cardia tissues. Helicobacter pylori was seen in the mucosa and gland epithelium tissues (Figure 2A). Expression of MyD88 was increased in biopsies with H. pylori infection compared with non-infected biopsies; however, the difference was not significant (Table 1, Figure 2B).
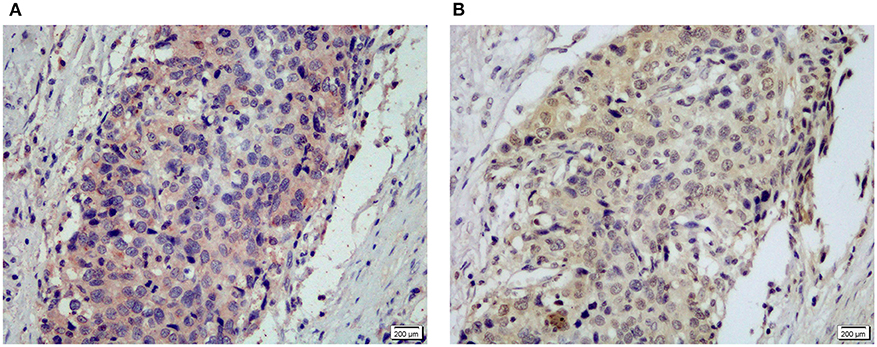
Figure 2. Representative IHC staining for MyD88 (A) and NF-κB p50/105 (B) in the same gastric cardia cancer tissue.
Correlation Between MyD88 Expression and NF-κB in GCC
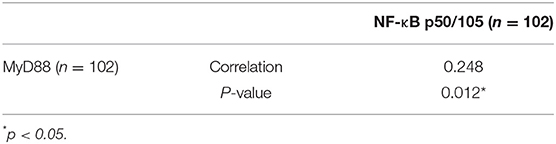
MyD88 plays an important role in tumor immunity by regulating NF-κB–mediated functions (8, 31). We used an antibody that can recognize both p105 and p50 proteins to quantify NF-κB p105/p50 protein in the same cohort of samples. Immunohistochemical staining detected NF-κB p105/p50 in all non-malignant and malignant samples. Expression of NF-κB p105/p50 was higher in GCC (n = 104) than in non-malignant tissues (n = 94) (p = 0.000). Moreover, increased NF-κB p105/p50 staining in gastric cardia tissues was positively associated with overexpression of MyD88 expression (p = 0.012) (Table 2). The strongest immunostaining of NF-κB p105/p50 and MyD88 coexisted in tumors (Figure 3).
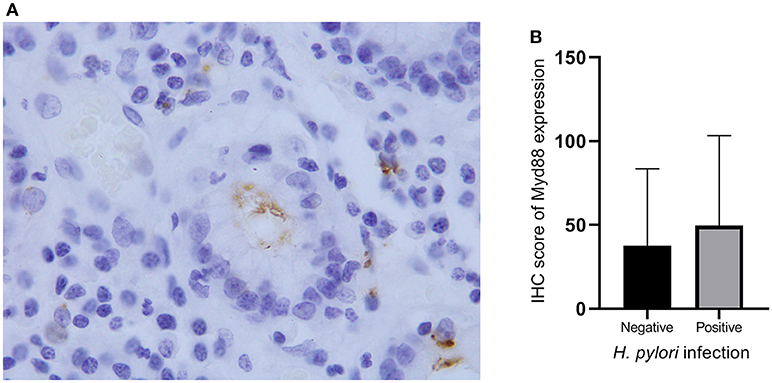
Figure 3. Helicobacter pylori infection and MyD88 expression. (A) Helicobacter pylori infection was identified by IHC in gastric cardia glands. (B) Boxplot shows that MyD88 expression was higher in the severe H. pylori infection cases than the cases without H. pylori infection but not significantly.
Clinical Significance of MyD88 Expression in Gastric Cardia Cancer Patients
We then analyze the relationship between MyD88 expression and clinicopathologic features of GCC patients including gender, size of the tumor, lymph node metastasis, histological grade, depth of tumor invasion, and TNM stage (Table 3).
Table 3. The associations of MyD88 expression with clinicopathologic characteristics concerning 71 of the 102 GCC patients.
Gastric cardia cancer tumors with higher MyD88 expression had higher histological grade (p = 0.041). There was no significant relationship between the expression of MyD88 and other clinical and pathological parameters in GCC.
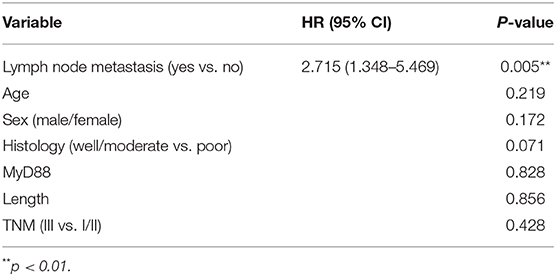
All 70 patients followed were involved for survival analysis. On multivariable analysis, MyD88 did not correlate with survival in the GCC patients (overall survival, p = 0.828). Lymph node metastasis [hazard ratio (HR), 2.715; 95% confidence interval (CI), 1.348–5.468; p = 0.005] was independently associated with GCC patients' survival (Table 4).
Discussion
The etiology of GCC is unclear. Previous reports showed that GCC is different from adenocarcinomas located in the lower esophagus or distal stomach in both epidemiology and biology (7). Gastric cardia cancer is defined as carcinoma in which the epicenter is ≤2 cm below the esophageal–gastric junction (32) in China. The highest regional rate of GCC was in Eastern/Southeastern Asia (1). The reason for a higher incidence of GCC in Chaoshan area in China is unknown. Given the differences between GCC and NGCC, in the present study, we considered GCC as a separate disease and reported for the first time in gastric cardia tissues the expression of MyD88 and its relationship with H. pylori infection and NF-κB p105/p50 expression. We observed that MyD88 expression gradually increased from normal tissue, gastric cardia inflammation, and carcinoma. A positive correlation between MyD88 and p105/p50 expression was detected. Thereby we provide pathological evidence that MyD88 expression is involved in gastric cardia tissue inflammation and carcinogenesis.
Lipopolysaccharide (LPS) is found in the outer membrane of Helicobacter, and it was reported that LPS could upregulate MyD88 expression. Few studies showed correlation between H. pylori and MyD88 expression in gastric cardia tissue. Here, we showed that MyD88 expression is higher in H. pylori–positive cases in comparison with H. pylori–negative cases, but the result was not significant. Several factors may contribute to lack of significant relationship between MyD88 expression and the H. pylori infection. One is the induction of endotoxin tolerance (33, 34). Lipopolysaccharide is the major component of H. pylori. Studies showed that after repeated challenge by LPS a reduced inflammatory response was observed, which is termed LPS tolerance. Lipopolysaccharide-induced tolerance can down-regulate the surface expression of the TLR4-MD2 complex, which might block MyD88-dependent pathways (35). We supposed that some of the patients with repeated H. pylori infection might reduce MyD88 expression. Second is that evidences suggest host genetics, environmental factors, and bacterial virulence factors might affect the ability of H. pylori to manipulate the immune response (33). These factors may contribute that some positive infection individuals show higher expression of MyD88 but not reach significant level. Third, pattern of MyD88 expression might not significantly change after the bacteria were eradicated. Michalkiewicz et al. (34) found that the involvement of H. pylori did not result in a significant upregulation of MyD88 mRNA expression when analyzing the expression of innate immunity components in the gastric mucosa among H. pylori–infected and uninfected children, which was consistent with our result.
Evidence showed that MyD88 can induce proinflammatory response and inflammation, which is regarded as the most important factor contributing to tumorigenesis (8, 21, 36). In the present study, MyD88 expression was evaluated from normal mucosa to inflammation and carcinoma restricted to gastric cardia tissue. MyD88 expression in normal cells differs in different tissue. Similar to the studies in gastric tissue (8, 37), our data indicated that the expression of MyD88 is low in normal gastric cardia tissue. MyD88 expression was increased during chronic inflammation. Echizen et al. (38) reported that depletion of MyD88 results in suppression of the inflammatory microenvironment in gastric tumors. These evidences indicated that MyD88 plays a role in increasing inflammation and changes innate immune activation between normal and mild inflammation. Gastric cardia cancer has the highest MyD88 expression and mainly in intestinal-type adenocarcinoma with inflammation. Gastric adenocarcinomas can be classified as the intestinal type and diffuse type according to the Lauren classification (39). Intestinal-type adenocarcinoma cells tend to form glands. Diffuse-type adenocarcinoma cells are poorly differentiated and tend to scatter throughout the stomach rather than form glands (40). Inflammatory cell infiltration was common in the intestinal-type adenocarcinoma in this study. We also showed that MyD88 expression was significantly higher in the well- and moderately differentiated tumors than in the poorly differentiated tumors, and most of the intestinal-type adenocarcinoma are well-differentiated. Studies reported that diffuse- and intestinal-type gastric carcinomas differ in risk factors, epidemiology, and distinct causal pathways (41–46). Our observation suggested that MyD88 pathway plays more important role in intestinal-type adenocarcinoma and might be responsible for the inflammatory response and carcinogenesis of this type of adenocarcinoma. The higher MyD88 expression in well-/moderately differentiated tumors comparing to poorly differentiated tumors might suggest that the MyD88 expression level is changing with the differentiation of cells. With the constant accumulation of different gene mutation and expression during tumor differentiation, MyD88 expression might be changing. We speculated that during tumor progression the antitumor role of MyD88 affects tumor differentiation to a certain degree resulting in well-/moderately differentiated tumors with higher MyD88 expression.
Reports have shown that MyD88 coupled with NF-κB contributes to carcinogenesis. Nuclear factor κB is the important signaling molecule downstream of MyD88 and data on how MyD88 deficiency affects carcinogenesis involved the role of NF-κB in cancer (21, 47). MyD88 is thought to mediate NF-κB activation and cytokine production (48, 49). Nuclear factor κB is able to regulate inflammation, cell differentiation, and apoptosis and plays a role in tumorigenesis (50–54). Nuclear factor κB p105/p50 usually locates in the cytoplasm. Adverse stimuli can activate NF-κB pathway, and p50 translocates into the nucleus then changes cell signaling (55). In the present study, we demonstrated positive expression of p105/p50 both in the cytoplasm and nucleus of GCC cells. We found that MyD88 had significantly positive relationship with NF-κB p105/p50, suggesting that p105/p50 and MyD88 are both involved in GCC tumorigenesis.
Different from the study results in hepatocellular carcinoma and epithelial ovarian cancer in which recurrence rate was higher and recurrence-free survival and overall survival were poorer in patients with MyD88 overexpression (8), we showed that the expression of MyD88 did not correlate with survival of GCC patients. The role of MyD88 in cancer prognosis might differ in different cancers. Lymph node metastasis was independently associated with GCC patient survival.
Although some previous studies proved that MyD88 has protective effects in gastric carcinogenesis (56). Our results provide evidences about the contribution of MyD88 in the regulation of inflammation, carcinogenesis, and tumor differentiation in gastric cardia tissue. Enhanced MyD88 expression was closely related with the intestinal-type carcinomas with inflammatory cell infiltration. Furthermore, NF-κB p105/p50 showed positive relationship with MyD88 expression in GCC tissue. The lack of significance of MyD88 as a prognostic factor in GCC might be due to the complex role of MyD88 in cancer tissue, and we need further studies to provide evidences.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical review committees of the Medical College of Shantou University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SL and GC designed the research study. JC, DX, WL, and DG conducted the experiments. GC and MX collected clinical data. SL, GC, and RS analyzed the data. SL wrote the manuscript with contribution from all authors. All authors read and approved the final version of the paper.
Funding
This work was supported by the Natural Science Foundation of China (Grant no. 81702717), the Natural Science Foundation of Guangdong Province (Grant no. 2014A030310139), and the Medical Science Foundation of Guangdong Province (Grant number A2019312).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation, though no other collaboration, with the authors.
References
1. Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. (2015) 64:1881–8. doi: 10.1136/gutjnl-2014-308915
2. Wang LD, Zheng S, Zheng ZY, Casson AG. Primary adenocarcinomas of lower esophagus, esophagogastric junction and gastric cardia: in special reference to China. World J Gastroenterol. (2003) 9:1156–64. doi: 10.3748/wjg.v9.i6.1156
3. Su M, Liu M, Tian DP, Li XY, Zhang GH, Yang HL, et al. Temporal trends of esophageal cancer during 1995-2004 in Nanao Island, an extremely high-risk area in China. Eur J Epidemiol. (2007) 22:43–8. doi: 10.1007/s10654-006-9086-x
4. Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. (2011) 22:375–87. doi: 10.1007/s10552-010-9707-2
5. Melit LE, Marginean CO, Marginean CD, Marginean MO. The relationship between toll-like receptors and Helicobacter pylori-related gastropathies: still a controversial topic. J Immunol Res. (2019) 2019:8197048. doi: 10.1155/2019/8197048
6. Nagashima H, Yamaoka Y. Importance of toll-like receptors in pro-inflammatory and anti-inflammatory responses by Helicobacter pylori infection. Curr Top Microbiol Immunol. (2019) 421:139–58. doi: 10.1007/978-3-030-15138-6_6
7. Chen G, Xu M, Chen J, Hong L, Lin W, Zhao S, et al. Clinicopathological features and increased expression of toll-like receptor 4 of gastric cardia cancer in a high-risk Chinese population. J Immunol Res. (2018) 2018:7132868. doi: 10.1155/2018/7132868
8. Liang B, Chen R, Wang T, Cao L, Liu Y, Yin F, et al. Myeloid differentiation factor 88 promotes growth and metastasis of human hepatocellular carcinoma. Clin Cancer Res. (2013) 19:2905–16. doi: 10.1158/1078-0432.CCR-12-1245
9. Anthoney N, Foldi I, Hidalgo A. Toll and toll-like receptor signalling in development. Development. (2018) 145:dev156018. doi: 10.1242/dev.156018
10. Maeda Y, Echizen K, Oshima H, Yu L, Sakulsak N, Hirose O, et al. Myeloid differentiation factor 88 signaling in bone marrow-derived cells promotes gastric tumorigenesis by generation of inflammatory microenvironment. Cancer Prev Res. (2016) 9:253–63. doi: 10.1158/1940-6207.CAPR-15-0315
11. Bajo M, Patel RR, Hedges DM, Varodayan FP, Vlkolinsky R, Davis TD, et al. Role of MyD88 in IL-1beta and ethanol modulation of GABAergic transmission in the central amygdala. Brain Sci. (2019) 9:361. doi: 10.3390/brainsci9120361
12. Wang L, Yu K, Zhang X, Yu S. Dual functional roles of the MyD88 signaling in colorectal cancer development. Biomed Pharmacother. (2018) 107:177–84. doi: 10.1016/j.biopha.2018.07.139
13. Pradere JP, Dapito DH, Schwabe RF. The yin and yang of toll-like receptors in cancer. Oncogene. (2014) 33:3485–95. doi: 10.1038/onc.2013.302
14. Lozano-Pope I, Sharma A, Matthias M, Doran KS, Obonyo M. Effect of myeloid differentiation primary response gene 88 on expression profiles of genes during the development and progression of helicobacter-induced gastric cancer. BMC Cancer. (2017) 17:133. doi: 10.1186/s12885-017-3114-y
15. Lee SH, Hu LL, Gonzalez-Navajas J, Seo GS, Shen C, Brick J, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. (2010) 16:665–70. doi: 10.1038/nm.2143
16. Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. (2007) 317:121–4. doi: 10.1126/science.1140485
17. Kennedy CL, Najdovska M, Tye H, McLeod L, Yu L, Jarnicki A, et al. Differential role of MyD88 and Mal/TIRAP in TLR2-mediated gastric tumourigenesis. Oncogene. (2014) 33:2540–6. doi: 10.1038/onc.2013.205
18. Banerjee A, Thamphiwatana S, Carmona EM, Rickman B, Doran KS, Obonyo M. Deficiency of the myeloid differentiation primary response molecule MyD88 leads to an early and rapid development of helicobacter-induced gastric malignancy. Infect Immun. (2014) 82:356–63. doi: 10.1128/IAI.01344-13
19. Garrett WS, Punit S, Gallini CA, Michaud M, Zhang D, Sigrist KS, et al. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. (2009) 16:208–19. doi: 10.1016/j.ccr.2009.07.015
20. Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. (2012) 209:1671–87. doi: 10.1084/jem.20111706
21. Salcedo R, Cataisson C, Hasan U, Yuspa SH, Trinchieri G. MyD88 and its divergent toll in carcinogenesis. Trends Immunol. (2013) 34:379–89. doi: 10.1016/j.it.2013.03.008
22. Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. (2010) 207:1625–36. doi: 10.1084/jem.20100199
23. Wen D, Shan B, Wang S, Zhang L, Wei L, Zhou W, et al. A positive family history of esophageal/gastric cardia cancer with gastric cardia adenocarcinoma is associated with a younger age at onset and more likely with another synchronous esophageal/gastric cardia cancer in a Chinese high-risk area. Eur J Med Genet. (2010) 53:250–5. doi: 10.1016/j.ejmg.2010.06.011
24. Jee H, Nam KT, Kwon HJ, Han SU, Kim DY. Altered expression and localization of connexin32 in human and murine gastric carcinogenesis. Dig Dis Sci. (2011) 56:1323–32. doi: 10.1007/s10620-010-1467-z
25. Xu Q, Xu Y, Pan B, Wu L, Ren X, Zhou Y, et al. TTK is a favorable prognostic biomarker for triple-negative breast cancer survival. Oncotarget. (2016) 7:81815–29. doi: 10.18632/oncotarget.13245
26. Liu LLD, Li C, Chen ZY. Comparison of three staining methods for detection of Helicobacter pylori in gastric biopsy specimens. West China Medical J. (2010) 25:80–1.
27. Zhang Y, Wang H, Bi C, Xiao Y, Liu Z. Expression of CDX2 in gastric cardia adenocarcinoma and its correlation with H. pylori and cell proliferation. Oncotarget. (2016) 7:54973–82. doi: 10.18632/oncotarget.10362
28. Yue Y, Zhou T, Gao Y, Zhang Z, Li L, Liu L, et al. High mobility group box 1/toll-like receptor 4/myeloid differentiation factor 88 signaling promotes progression of gastric cancer. Tumour Biol. (2017) 39:1010428317694312. doi: 10.1177/1010428317694312
29. Fulgione A, Di Matteo A, Contaldi F, Manco R, Ianniello F, Incerti G, et al. Epistatic interaction between MyD88 and TIRAP against Helicobacter pylori. FEBS Lett. (2016) 590:2127–37. doi: 10.1002/1873-3468.12252
30. Wang F, Mao Z, Liu D, Yu J, Wang Y, Ye W, et al. Overexpression of Tim-3 reduces Helicobacter pylori-associated inflammation through TLR4/NFkappaB signaling in vitro. Mol Med Rep. (2017) 15:3252–8. doi: 10.3892/mmr.2017.6346
31. Sakai J, Cammarota E, Wright JA, Cicuta P, Gottschalk RA, Li N, et al. Lipopolysaccharide-induced NF-κB nuclear translocation is primarily dependent on MyD88, but TNFα expression requires TRIF and MyD88. Sci Rep. (2017) 7:1428. doi: 10.1038/s41598-017-01600-y
32. Peng XB, Zhang CW, Chen ZF, Liang ZH, Ou ZX, Wei KR. Epidemiology of gastric cardia cancer in China. Chin Arch Gen Surg. (2014) 8:156–9.
33. Zaric SS, Coulter WA, Shelburne CE, Fulton CR, Zaric MS, Scott A, et al. Altered toll-like receptor 2-mediated endotoxin tolerance is related to diminished interferon beta production. J Biol Chem. (2011) 286:29492–500. doi: 10.1074/jbc.M111.252791
34. Michalkiewicz J, Helmin-Basa A, Grzywa R, Czerwionka-Szaflarska M, Szaflarska-Poplawska A, Mierzwa G, et al. Innate immunity components and cytokines in gastric mucosa in children with Helicobacter pylori infection. Mediators Inflamm. (2015) 2015:176726. doi: 10.1155/2015/176726
35. Sato S, Takeuchi O, Fujita T, Tomizawa H, Takeda K, Akira S. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int Immunol. (2002) 14:783–91. doi: 10.1093/intimm/dxf046
36. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
37. Je EM, Kim SS, Yoo NJ, Lee SH. Mutational and expressional analyses of MYD88 gene in common solid cancers. Tumor. (2012) 98:663–9. doi: 10.1700/1190.13209
38. Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. (2016) 107:391–7. doi: 10.1111/cas.12901
39. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. (1965) 64:31–49. doi: 10.1111/apm.1965.64.1.31
40. van der Woude CJ, Kleibeuker JH, Tiebosch ATGM, Homan M, Beuving A, Jansen PLM, et al. Diffuse and intestinal type gastric carcinomas differ in their expression of apoptosis related proteins. J Clin Pathol. (2003) 56:699–702. doi: 10.1136/jcp.56.9.699
41. Waldum HL, Fossmark R. Types of gastric carcinomas. Int J Mol Sci. (2018) 19:4109. doi: 10.3390/ijms19124109
42. Chen Y-C, Fang W-L, Wang R-F, Liu C-A, Yang M-H, Lo S-S, et al. Clinicopathological variation of lauren classification in gastric cancer. Pathol Oncol Res. (2016) 22:197–202. doi: 10.1007/s12253-015-9996-6
43. Yamazawa S, Ushiku T, Shinozaki-Ushiku A, Hayashi A, Iwasaki A, Abe H, et al. Gastric cancer with primitive enterocyte phenotype: an aggressive subgroup of intestinal-type adenocarcinoma. Am J Surg Pathol. (2017) 41:989–97. doi: 10.1097/PAS.0000000000000869
44. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. (1992) 52:6735–40.
45. Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. (2004) 128:765–70. doi: 10.1043/1543-2165(2004)128<765:DTITIA>2.0.CO;2
46. Shibata A, Longacre TA, Puligandla B, Parsonnet J, Habel LA. Histological classification of gastric adenocarcinoma for epidemiological research: concordance between pathologists. Cancer Epidemiol Biomarkers Prev. (2001) 10:75–8.
47. DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. (2012) 246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x
48. Shang L, Wang T, Tong D, Kang W, Liang Q, Ge S. Prolyl hydroxylases positively regulated LPS-induced inflammation in human gingival fibroblasts via TLR4/MyD88-mediated AKT/NF-κB and MAPK pathways. Cell Prolif. (2018) 51:e12516. doi: 10.1111/cpr.12516
49. Hirano H, Yoshioka T, Yunoue S, Fujio S, Yonezawa H, Niiro T, et al. TLR4, IL-6, IL-18, MyD88 and HMGB1 are highly expressed in intracranial inflammatory lesions and the IgG4/IgG ratio correlates with TLR4 and IL-6. Neuropathology. (2012) 32:628–37. doi: 10.1111/j.1440-1789.2012.01310.x
50. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. (2018) 18:309–24. doi: 10.1038/nri.2017.142
51. Markopoulos GS, Roupakia E, Tokamani M, Alabasi G, Sandaltzopoulos R, Marcu KB, et al. Roles of NF-κB Signaling in the regulation of miRNAs impacting on inflammation in cancer. Biomedicines. (2018) 6:40. doi: 10.3390/biomedicines6020040
52. Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. (2011) 12:695–708. doi: 10.1038/ni.2065
53. Siggers T, Chang AB, Teixeira A, Wong D, Williams KJ, Ahmed B, et al. Principles of dimer-specific gene regulation revealed by a comprehensive characterization of NF-κB family DNA binding. Nat Immunol. (2011) 13:95–102. doi: 10.1038/ni.2151
54. Weng H, Deng Y, Xie Y, Liu H, Gong F. Expression and significance of HMGB1, TLR4 and NF-κB p65 in human epidermal tumors. BMC cancer. (2013) 13:311. doi: 10.1186/1471-2407-13-311
55. Xia S, Ji R, Zhan W. Long noncoding RNA papillary thyroid carcinoma susceptibility candidate 3 (PTCSC3) inhibits proliferation and invasion of glioma cells by suppressing the Wnt/β-catenin signaling pathway. BMC Neurol. (2017) 17:30. doi: 10.1186/s12883-017-0813-6
Keywords: MyD88, Helicobacter pylori, gastric cardia cancer, cancer and inflammation, prognosis
Citation: Chen J, Xia D, Xu M, Su R, Lin W, Guo D, Chen G and Liu S (2020) Expression and Significance of MyD88 in Patients With Gastric Cardia Cancer in a High-Incidence Area of China. Front. Oncol. 10:559. doi: 10.3389/fonc.2020.00559
Received: 18 January 2020; Accepted: 27 March 2020;
Published: 14 May 2020.
Edited by:
Jianjun Xie, Shantou University, ChinaReviewed by:
Johan Nicolay Wiig, Oslo University Hospital, NorwayChangting Meng, Institute for Systems Biology (ISB), United States
Copyright © 2020 Chen, Xia, Xu, Su, Lin, Guo, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangcan Chen, NTE1Nzc2NzE4JiN4MDAwNDA7cXEuY29t; Shuhui Liu, bGl1c2h1aHVpc3R1JiN4MDAwNDA7MTI2LmNvbQ==
†These authors have contributed equally to this work
 Jingyao Chen1†
Jingyao Chen1† Di Xia
Di Xia Shuhui Liu
Shuhui Liu