
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 23 April 2020
Sec. Gastrointestinal Cancers
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00513
Background: Increasing evidences from phase II or III trials have proved that salvage systematic therapy, including chemotherapy, target therapy, or checkpoint inhibitor therapy can prolong survival in patients who do not succeed with second line therapy, yet there are no guidelines for the optimum third-line treatments. To compare the effectiveness and safety of current third-line therapies for metastatic Gastric Cancer (mGC), we conducted this network analysis.
Methods: Literature up to Sep 30, 2019 were systematically searched and analyzed by a Bayesian fixed-effect model.
Results: This study included seven randomized clinical trails which involved 2,655 patients. It turns out that for overall survival, nivolumab has the highest probability to be the optimal choice for overall survival (OS). For patients with no peritoneal metastases, the network meta-analysis showed that Nivolumab (HR:0.64; 95% CI: 0.48–0.85) and Trifluridine/tipiacil (HR:0.66; 95% CI: 0.51–0.86) were associated with significantly higher improvement in OS than placebo. However, patients with peritoneal metastases could not benefit from nivolumab, ramucirumab, or Trifluridine/tipiacil, when compared with a placebo. For progression-free survival, apatinib (850 mg) was the most likely candidate, followed by ramucirumab. Statistically, Apatinib (850 mg), Trifluridine/tipiacil, and SLC had higher incidences of high-grade adverse events (AEs) than placebo.
Conclusion: Our findings demonstrate that nivolumab has the best balance between acceptability and effectiveness in the third line therapy for mGC.
Gastric cancer (GC) has a high incidence rate all over the world, especially in eastern Asia. About 40% of GC patients were diagnosed with an advanced stage in China (1, 2). Several guidelines have recommended platinum plus fluropyimidine as first-line therapy for those patients who were advanced or had metastatic gastric cancer (3). For human epidermal growth factor receptor 2 (Her-2) positive advanced gastric cancer, adding trastuzumab to platinum-based chemotherapy has been considered as standard first line therapy (4). Both irinotecan and taxane have been accepted as second line therapy (5). Increasing evidence from phase II or III trials have proved that salvage systematic therapy, including chemotherapy, target therapy, or checkpoint inhibitor therapy can prolong survival in patients who do not succeed with second line therapy (6–10), when compared with a placebo. But it is hard for clinicians to determine the best therapy, because there are no head-to-head randomized clinical trials (RCTs) to evaluate the efficacy and safety among the available third line therapies. Besides, JAVELIN Gastric 300 study failed to demonstrate avelumab in third line setting was superior in OS improvement, compared with the physician's choice of chemotherapy (11). What if it were compared with a placebo? Will it be a possible treatment because of its similar mechanism to Nivolumab? In the present study, to answer these questions, we will use Bayesian network analysis to compare and summarize the clinical results and adverse events (AEs) of available third-line regimens for metastatic GC (mGC).
Our research was conducted under the guidance of PRISMA extension statement for network meta-analysis. We performed a valid literature search on Cochrane Library, PubMed,Web of Science, and ClinicalTrials.gov for RCTs comparing at least two agents in third-line treatment of mGC in Sep, 2019. All literature was screened to ensure eligibility. Search terms such as chemotherapy, nivolumab, avelumab, apatinib etc., and search strategies are shown in Appendix 1 in Supplementary Information.
Only randomized controlled trials were included. Target mGC patients must have received at least two anti-tumor systemic therapies. Interventions included, but were not limited to: chemotherapy, nivolumab, avelumab, apatinib, ramucirumab, and Trifluridine/tipiracil (TAS102). Articles were excluded if the patient received first-line or second-line systemic therapy.
First, the main exclusion criteria for title and abstract screen of the studies obtained through systematic search were as follows: (1) non-RCT designs including preclinical experiments, case-control studies, and case reports (2) duplicates; (3) trials without comparisons; (4) commentaries and editorials; and (5) pooled analyses. After identification by title and abstract, the remaining studies were then reviewed in full text, and the exclusion criteria for the full text were as follows: (1) preliminary or repeated reports, (2) non-randomized clinical trials, (3) second-line treatment clinical trials. After the full-text review, we included 7 eligible RCTs and performed further analysis.
The primary outcome of the study was to measure overall survival (OS). Progression free survival (PFS) and high-grade (grade ≥ 3) drug related adverse events (AEs) (National Cancer Institute Common Toxicity Criteria version 3.0) were assessed as secondary outcomes.
The two reviewers (Wentao Pan and Miaozhen Qiu) searched and screened for studies with potential research at the level of title and abstract levels. Full texts were reviewed when abstracts were insufficient to assess trial eligibility. Then data on patient characteristics, treatment strategies, outcome definitions, and so forth were extracted into a standardized form by one reviewer (Wentao Pan), and validated by the other (Miaozhen Qiu). Disagreements arose and issues was resolved in consultation with a third reviewer (Suna Zhou). For example, should we include a single arm phase II study? After careful discussion, we decided not to include these studies. The Cochrane Handbook's Risk of Bias Assessment Tool for randomized trials was used to evaluate the quality of studies included.
In our analysis, we first tried Bayesian methods for random-effect model and fixed-effect model, but due to the absence of heterogeneities, only fixed-effect model could be run. Published hazard ratios (HRs) with 95% confidence intervals (CIs) were used as relevant parameters for OS and PFS assessment. Odds ratios (ORs) analyzed using available data attained from the trials were used to assess drug-related AEs. The results were assessed using contrast-based approach with 50,000 iterations in the training phase, for a total of three chains, for a total of 100,000. Operation code was offered in Appendix 2 in Supplementary Material. Based on the analysis of OS, PFS, and high-grade AEs, the distribution of ranking probabilities to rank treatments was used and exported directly from OpenBUGS version 3.2.2.
Apart from the calculation of OR using Stata v.13 (StataCorp, College Station, TX, USA), most of the data analyses were performed using OpenBUGS version 3.2.2. Under Stata v.13, the heterogeneity between studies was assessed by the Q-test and I2 statistic, and heterogeneity was considered when P < 0.10 and I2 > 50%.
Through our literature search, we reviewed 949 potential studies, of which 912 were excluded after screening titles and abstracts (Figure 1). Through a thoughtful review of the remaining 24 studies, 7 phase III RCTs (2,655 patients) were included in this network meta-analysis (Table 1), with an average of 189 (range 69–337) per group and at least 100 cases per group.
The RCT features were shown in Table 1, and no significant differences were observed in study characteristics, with a median age of 60 years and 72.4% of male patients (Supplementery Figure 1). The comparison network was represented as a network diagram with eight and six treatments for those with OS data (Figure 2A) and those with PFS data (Figure 2B), respectively. We firstly tried a Bayesian approach with random-effect model and fixed-effect model, but there was no heterogeneities due to the included studies. Therefore, only analysis with fixed-effect model could be attained, and in each analysis, the three chains showed good fusion (Supplementery Figures 2, 3).
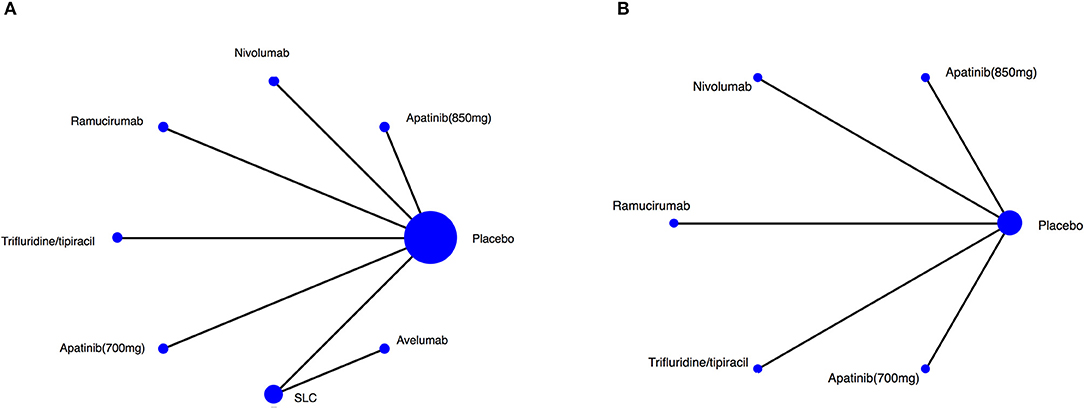
Figure 2. Network of the comparisons for the Bayesian network meta-analysis. Network plot for (A) OS, (B) PFS. The size of every treatment node corresponds to the patient's number of randomly assigned treatment. SLC, salvage chemotherapy.
OS was analyzed in 7 studies (2,655 patients) with 8 third-line systemic therapies. Network meta-analysis indicated that Apatinib (850 mg), Nivolumab, Trifluridine/tipiacil, and SLC were significantly associated with improved OS compared with a placebo (Figure 3A). According to the ranking results, Nivolumab was most likely to be the best choice for OS, and a placebo was considered the least likely (Figure 3B). In patients with ECOG = 0, Nivolumab, Trifluridine/tipiacil, and SLC improved significantly more than the placebo with OS (Figure 4A), juxtaposed first with Apatinib, Nivolumab, and SLC (Figure 4B). In patients with ECOG = 1, Nivolumab, Ramucirumab, and Trifluridine/tipiacil improved OS significantly compared with placebo (Figure 4C), with Nivolumab, Ramucirumab, and Trifluridine/tipiacil juxtaposed first (Figure 4D).
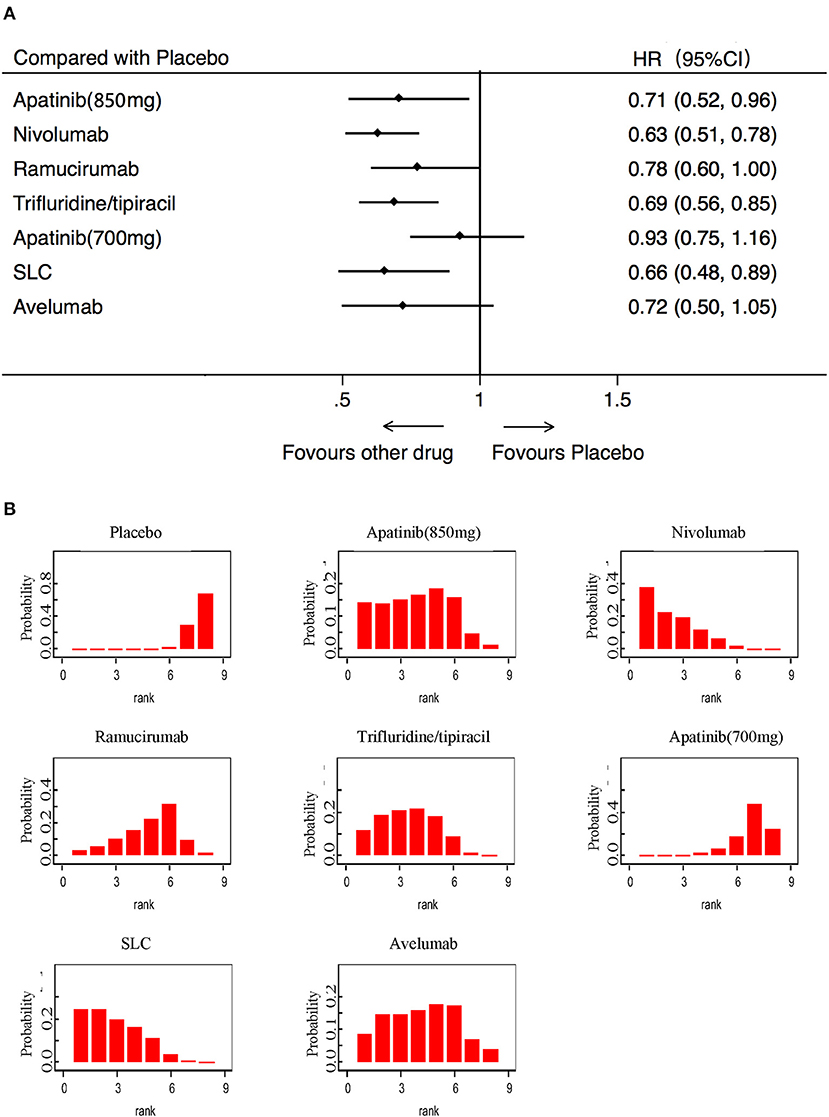
Figure 3. Pooled hazard ratios for overall survival. (A) Forest plot, with placebo as the comparator; A fixed effect model was adopted due to non-significant heterogeneity of publications (I2 = 7.1%, p = 0.374). (B) Ranking of treatments in terms of overall survival. Rankograms were drawn according to distribution of the ranking probabilities. HR, hazard ratio; CI, credible interval; Numbers in parentheses indicate 95% credible intervals. SLC, salvage chemotherapy.
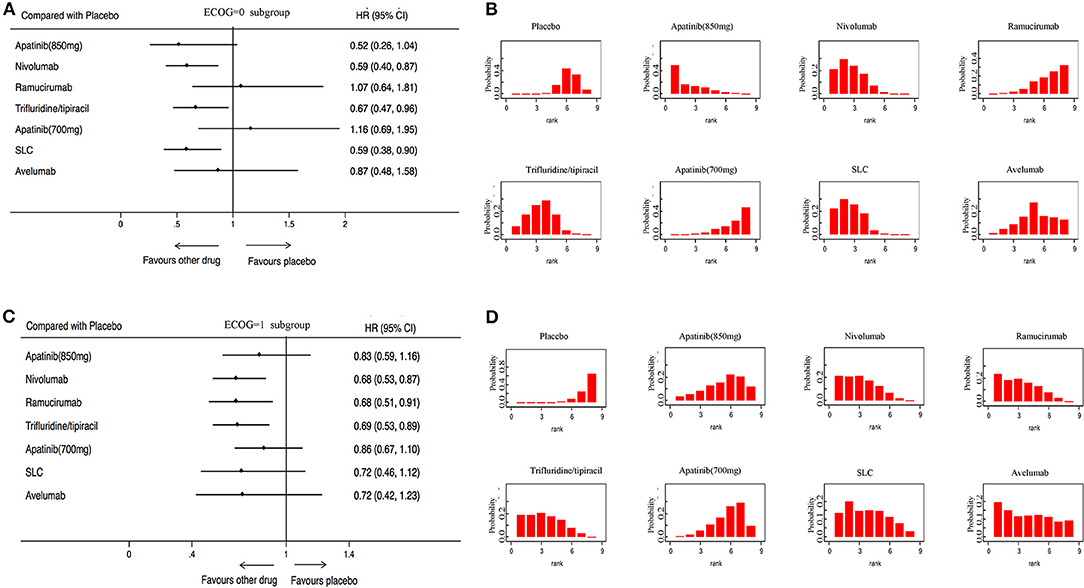
Figure 4. Pooled hazard ratios for overall survival in subgroup patients. (A) Forest plot, with placebo as the comparator in patients with ECOG = 0; A fixed effect model was adopted due to non-significant heterogeneity of publications (I2 = 1.0%, p = 0.417). (B) Ranking of treatments in terms of overall survival in patients with ECOG = 0. (C) Forest plot, with placebo as the comparator in patients with ECOG = 1; A fixed effect model was adopted due to non-significant heterogeneity of publications (I2 = 0.0%, p = 0.854). (D) Ranking of treatments in terms of overall survival in patients with ECOG = 1. Rankograms were drawn according to distribution of the ranking probabilities. HR, hazard ratio; CI, credible interval. Numbers in parentheses indicate 95% credible intervals. SLC, salvage chemotherapy.
Further, we analyze patients with or without more than 2 metastatic sites in 6 studies for OS. The network meta-analysis showed that Apatinib (850 mg) (HR:0.70; 95% CI:0.50–0.99), Trifluridine/tipiacil (HR:0.68; 95% CI: 0.49–0.95), and SLC (HR:0.55; 95% CI: 0.33–0.93) were associated with significantly higher improvement in OS than a placebo (Supplementery Figure 4A), with SLC ranking the first (Supplementery Figure 4B). For those with more than 2 metastatic sites, we found that Nivolumab (HR:0.62; 95% CI:0.49–0.79), Trifluridine/tipiacil (HR:0.71; 95% CI: 0.54–0.94), and SLC(HR:0.63; 95% CI: 0.42–0.94) were associated with significantly higher improvements in OS than a placebo (Supplementery Figure 4C), with Nivolumab and SLC ranking the highest (Supplementery Figure 4D).
Meanwhile, 309 patients with no measurable disease were used in 5 studies for OS. The network meta-analysis showed that Trifluridine/tipiacil (HR:0.21; 95% CI: 0.09–0.50) and SLC(HR:0.36; 95% CI: 0.20–0.67) were associated with significantly higher improvements in OS than a placebo (Supplementery Figure 5A), with Trifluridine/tipiacil ranking the highest (Supplementery Figure 5B). For those with a measurable disease, we found that Nivolumab (HR:0.64; 95% CI:0.49–0.83) and TAS102 (HR:0.74; 95% CI: 0.59–0.93) were associated with significantly higher improvements in OS than a placebo (Supplementery Figure 5C), with Nivolumab ranking the highest (Supplementery Figure 5D).
Lastly, we found that patients with or without peritoneal metastases have different responses to treatment. Three studies with corresponding data were analyzed. For patients with no peritoneal metastases, the network meta-analysis showed that Nivolumab (HR:0.64; 95% CI: 0.48–0.85) and Trifluridine/tipiacil (HR:0.66; 95% CI: 0.51–0.86) were associated with significantly higher improvements in OS than a placebo (Supplementery Figure 6A), with Nivolumab ranking the highest (Supplementery Figure 6B). Patients with peritoneal metastases could not benefit from Nivolumab, Ramucirumab, or Trifluridine/tipiacil, when compared with a placebo (Supplementery Figures 6C,D).
In terms of PFS, 5 trials including 2,035 patients were available for evaluation. The results showed that Apatinib (850 mg), Nivolumab, Ramucirumab, Trifluridine/tipiacil, and Apatinib (700 mg) were statistically superior to a placebo (Figure 5A). PFS rankings showed that Apatinib (850 mg) was the most likely to be preferred. In second place was ramucirumab, followed closely by Nivolumab, Trifluridine/tipiacil, and Apatinib (700 mg) (Figure 5B).
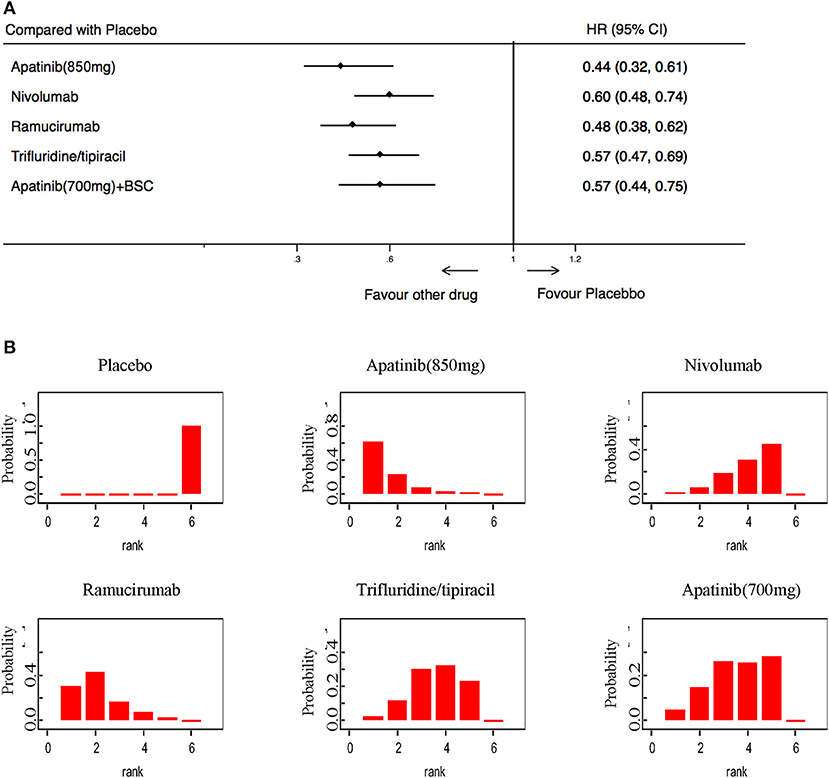
Figure 5. Pooled hazard ratios for progression-free survival. (A) Forest plot, with placebo as the comparator; A fixed effect model was adopted due to non-significant heterogeneity of publications (I2 = 0.0%, p = 0.437). (B) Ranking of treatments in terms of progression-free survival. Rankograms were drawn according to distribution of the ranking probabilities. HR, hazard ratio; CI, credible interval. Numbers in parentheses indicate 95% credible intervals.
High-grade treatment-related toxicities were analyzed in 2,608 patients in 7 RCTs. Compared with a placebo, only the Avelumab (OR: 0.38; 95% CI: 015–099) was associated with a lower incidence of high-grade AEs (Figure 6A). Apatinib (850 mg), Trifluridine/tipiacil, and SLC showed statistically higher rates of high-grade AEs than a placebo (Figure 6A). Avelumab was the most tolerable of all treatments, while Apatinib (850 mg) was the most toxic (Figure 6B).
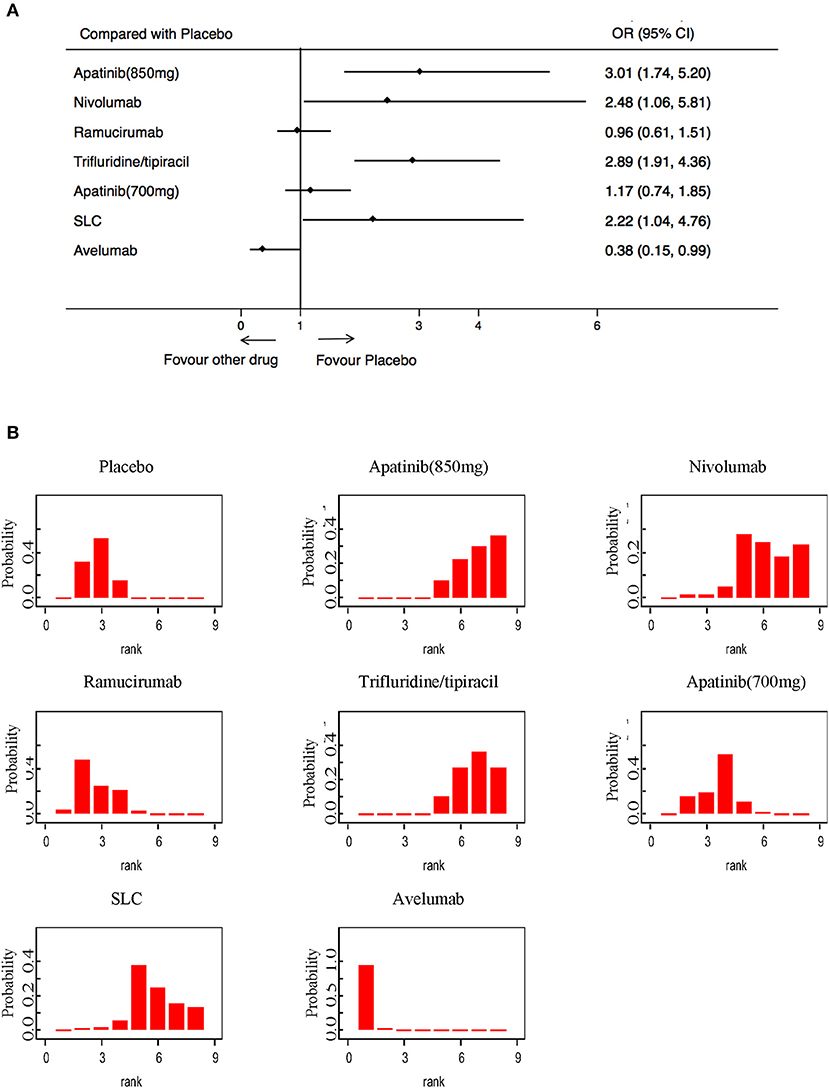
Figure 6. Pooled hazard ratios for high-grade adverse events. (A) Forest plot, with placebo as the comparator; significant heterogeneity of publications was seen (I2 = 77.1%, p = 0.000). (B) Ranking of treatments in terms of high-grade adverse events. Rankograms were drawn according to distribution of the ranking probabilities. HR, hazard ratio; CI, credible interval. Numbers in parentheses indicate 95% credible intervals.
To test the robustness of the significant results, we performed a network heterogeneity test, only to find homogeneity. By comparing the adjusted funnel plots of OS and PFS, we reported a symmetric distribution (Supplementery Figure 7), indicating the absence of small study effects and publication bias. Of the included studies, the methodological quality was good, with only one trial verbally reported and not accurately assessing its risk bias. Overall, all remaining studies had no significant high risk of bias with respect to random sequence generation, allocation concealment, incomplete outcome data, and selective reporting of outcomes (Supplementery Figure 8).
The network meta-analysis was based on 7 trials involving 2,655 patients, and 7 placebo-controlled mGC third-line treatments. There are several major findings. First of all, nivolumab may be the optimal choice for OS improvement. Secondly, in terms of PFS, Apatinib (850 mg) may be the first choice followed by ramucirumab, Nivolumab, Trifluridine/tipiacil, and Apatinib (700 mg). Finally, Avelumab had the best safety profile followed by ramucirumab, Apatinib (700 mg), Nivolumab, SLC, Trifluridine/tipiacil, and Apatinib (850 mg).
A systematic review and meta-analysis of third-line chemotherapy in advanced GC concluded that patients with advanced gastric cancer who underwent third line chemotherapy had superior results to those who received optimal supportive care (10), but there is no comparison among different third line treatment, such as apatinib, nivolumab, and trifluridine/tipiacil. Based on the present analysis, nivolumab appeared to be the best option for OS. Except for nivolumab, another immune checkpoint inhibitor, pembrolizumab, was approved for patients with recurrent locally advanced or metastatic PD-L1 positive GC. The results were based on KEYNOTE-059 study (7). Since KEYNOTE-059 was an open label, phase 2 trial, it was not included in our meta-analysis. As we know, Avelumab is an anti-PD-L1 antibody, blocking the combination of PD-1 and PD-L1, and sharing a similar MOA with Nivolumab. Compared with chemotherapy, it failed to improve OS. In our study, although compared with a placebo, it still could not get OS benefit. These indicated that anti-PD-L1 antibody and anti-PD-1 antibody played different roles in anti-tumor.
Peritoneal metastasis is related to poor prognosis in mGC patients (16). PHOENIX-GC Trial showed that intraperitoneal paclitaxel plus systemic chemotherapy did not improve the prognosis of GC patients with peritoneal metastasis (17). In the subgroup analysis of the present study, we found that patients with peritoneal metastases could not benefit from available third line therapy including Nivolumab, Ramucirumab, or Trifluridine/tipiacil, when compared with a placebo. Though it is disappointing, our results remind us that best supportive care is enough for GC patients with peritoneal metastases when they do not respond to second line therapy.
In terms of PFS, apatinib (850 mg), ramucirumab, Nivolumab, Trifluridine/tipiacil, and apatinib (700 mg) were statistically superior to a placebo. Though we observed the OS benefit for Nivolumab over apatinib, the different effectiveness of these treatments relative to OS and PFS were worth noting. Though immune checkpoint inhibitors can bring survival benefit, the response rate and PFS of immune checkpoint inhibitors are not high. This phenomenon has also been observed in other phase 3 randomized clinical trial in gastric cancer (18) or in esophageal carcinoma (8, 19). Though apatinib (850 mg) prolongs PFS and OS, it has the poorest safety profile among the available third line therapies. When the dose of apatinib decreased to be 700 mg, it is well-tolerated, but the OS was not significantly different with a placebo.
Our research advantages are as follows. Firstly, this research is by far the most comprehensive network analysis for evaluating the effectiveness and safety of currently available third-line systemic therapies for mGC. In addition, we could integrate the available information from RCTs, indirectly evaluate multiple treatments without head-to-head trials, and provide a hierarchical order for treatments based on OS, PFS, and high-grade AEs in mGC by using Bayesian network meta-analysis. Although theoretically it may have resulted in the expansion of type I error in the Bayesian network meta-analysis, the type I error rate proved to be controllable (20). Finally, the evaluation of effectiveness and safety provides a new perspective for different systemic therapies, and probably provides guidance for patients and clinicians to make treatment decisions and design future comparative trials. Nevertheless, we should also consider the limitations of our research. The main limitation of our study was research design, because 6 out of 7 studies took placebo as reference treatment. Only fixed-effect model could be used, excluding random-effect model, meta-regression analysis, and sensitivity analysis. Fortunately, these 7 high-quality phase III studies included were not heterogeneous in publication, and there were no significant differences in research features. In addition, there were no confounding factors such as prognostic risk categories and peritoneal metastasis that might affect the benefits of systemic therapy.
Our results indicated that nivolumab could provide the best OS benefit for mGC. Apatinib (850 mg) is the best choice for PFS. Nivolumab might also be a potential option for mGC, as it had the most favorable balance between effectiveness and safety. Given the limitations of this study, more head-to-head comparative RCTs are needed to verify our conclusions.
MQ, D-JY, and LZ: conception and design of this study. W-TP, S-NZ, and MQ: data extraction. M-XP and Q-YL: data analysis and statistical analysis. W-TP and MQ: manuscript writing. All authors: reviewed and approved.
This study was supported by the National Natural Science Foundation of China (NSFC: 81602066 and 81772587), Guangdong Esophageal Cancer Institute Science and Technology Program (Grant Number M201809), CSCO-HengRui Oncology Research Fund (Grant Number Y-HR2018-184), and the third outstanding young talents training plan and Medical Scientist program of Sun Yat-sen University cancer center, Medical Science and Technology Research Fund Project of Guangdong Province (Grant Number A2015003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00513/full#supplementary-material
1. Qiu MZ, Wang ZQ, Zhang DS, Liu Q, Luo HY, Zhou ZW, et al. Comparison of 6th and 7th AJCC TNM staging classification for carcinoma of the stomach in China. Ann Surg Oncol. (2011) 18:1869–76. doi: 10.1245/s10434-010-1542-x
2. Qiu MZ, Wang ZX, Zhou YX, Yang DJ, Wang FH, Xu RH, et al. Proposal for a New TNM Stage based on the 7(th) and 8(th) American Joint Committee on Cancer pTNM staging classification for gastric cancer. J Cancer. (2018) 9:3570–6. doi: 10.7150/jca.26351
3. Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). (2019) 39:10. doi: 10.1186/s40880-019-0349-9
4. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
5. Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. (2013) 31:4438–44. doi: 10.1200/JCO.2012.48.5805
6. Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. (2013) 31:3219–25. doi: 10.1200/JCO.2013.48.8585
7. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
8. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
9. Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. (2012) 30:1513–8. doi: 10.1200/JCO.2011.39.4585
10. Zheng Y, Zhu XQ, Ren XG. Third-line chemotherapy in advanced gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore). (2017) 96:e6884. doi: 10.1097/MD.0000000000006884
11. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. (2018) 29:2052–60. doi: 10.1093/annonc/mdy264
12. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. (2016) 34:1448–54. doi: 10.1200/JCO.2015.63.5995
13. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. (2014) 383:31–9. doi: 10.1016/S0140-6736(13)61719-5
14. Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2018) 19:1437–48. doi: 10.1016/S1470-2045(18)30739-3
15. Kang Y, Kang WK, Di Bartolomeo M, Chau I, Yoon HH, Cascinu S, et al. Randomized Phase 3 Angel Study of Rivoceranib (APATINIB) + Best Supportive Care (BSC) vs Placebo + BSC in Patients with Advanced/Metastatic Gastric Cancer Who Failed _2 Prior Chemotherapy Regimens. Ann Oncol. (2019) 30(Suppl. 5):v851–934. doi: 10.1093/annonc/mdz394
16. De Andrade JP, Mezhir JJ. The critical role of peritoneal cytology in the staging of gastric cancer: an evidence-based review. J Surg Oncol. (2014) 110:291–7. doi: 10.1002/jso.23632
17. Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III Trial Comparing Intraperitoneal and Intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. (2018) 36:1922–9. doi: 10.1200/JCO.2018.77.8613
18. Shitara K, Özgüroglu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. (2018) 392:123–33. doi: 10.1016/S0140-6736(18)31257-1
19. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
Keywords: gastric cancer, systematic therapy, Bayesian network analysis, overall survival, progression free survival, safety
Citation: Pan W-T, Zhou S-N, Pan M-X, Luo Q-Y, Zhang L, Yang D-J and Qiu M (2020) Role of Systemic Treatment for Advanced/Metastatic Gastric Carcinoma in the Third-Line Setting: A Bayesian Network Analysis. Front. Oncol. 10:513. doi: 10.3389/fonc.2020.00513
Received: 01 November 2019; Accepted: 23 March 2020;
Published: 23 April 2020.
Edited by:
Pashtoon Murtaza Kasi, The University of Iowa, United StatesReviewed by:
Jinping Liu, University of Pennsylvania, United StatesCopyright © 2020 Pan, Zhou, Pan, Luo, Zhang, Yang and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhang, emhhbmdsaW5Ac3lzdWNjLm9yZy5jbg==; Da-Jun Yang, eWFuZ2RqQHN5c3VjYy5vcmcuY24=; Miaozhen Qiu, cWl1bXpoQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.