- Department of Urology, Affiliated Jiang-yin Hospital of the Southeast University Medical College, Jiangyin, China
Background and Purpose: Although the prognostic value of lymphovascular invasion (LVI) for upper tract urinary carcinoma (UTUC) has been reported, there is a lack of consensus regarding the prognostic factor of LVI in UTUC after radical nephroureterectomy (RNU). The aim of the present study was to evaluate the contemporary role of LVI using systematic review and meta-analysis.
Materials and Methods: Using Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, we performed a systematic search of Web of Science, PubMed, and EMBASE for all reports published up to July 2019. Cumulative analyses of hazard ratios (HRs)/odds ratios (ORs) and their corresponding 95% confidence intervals were conducted to assess the association between LVI and oncological outcomes and clinicopathological features.
Results: Our meta-analysis included 31 eligible studies containing 14,653 patients with UTUC (81–1,363 per study). Our results indicated a significant correlation of LVI with worse cancer-specific survival (HR = 1.59, p < 0.001), overall survival (HR = 1.55, p < 0.001), recurrence-free survival (HR = 1.46, p < 0.001), cancer-specific mortality (HR = 1.25, p = 0.047), and recurrence (HR = 1.23, p = 0.026). LVI was also correlated with advanced tumor stage (III/IV vs. I/II: OR = 7.63, p < 0.001), higher tumor grade (3 vs. 1/2: OR = 5.61, p < 0.001), lymph node metastasis (yes vs. no: OR = 4.95, p < 0.001), carcinoma in situ (yes vs. no: OR = 1.92, p < 0.001), and positive surgical margin (yes vs. no: OR = 4.38, p < 0.001), but not related to gender (male vs. female: OR = 0.98, p = 0.825), and multifocality (multifocal vs. unifocal: OR = 1.09, p = 0.555). The funnel plot test indicated no significant publication bias.
Conclusions: This study demonstrated that LVI was associated with aggressive clinicopathological features. LVI may serve as a poor prognostic factor for patients with UTUC after RNU.
Introduction
The upper tract urothelial carcinoma (UTUC), which accounts for ~5% of all urothelial carcinoma, develops from the urothelium that lines the renal pelvis and the ureter (1). Although UTUCs share many similarities with bladder cancer, little is known about their pathogenesis, given the rarity of the disease. Radical nephroureterectomy (RNU) with bladder cuff excision is the gold standard curative therapy for localized UTUC; however, about 33% of patients with RNU will experience early tumor recurrence within 5 years (2), and the 5-years cancer-specific survival (CSS) is <50% for patients with early-stage UTUC (3). The current predictive nomograms based on preoperative parameters may guide surgeons for decision-making regarding RNU with or without lymphadenectomy (4). However, predicting oncologic outcomes is another major concern in patients with UTUC. Because there are aggressive features characteristic to UTUC, a comprehensive recognition of the potential prognostic factors for survival is critical.
Lymphovascular invasion (LVI), which is defined as the presence of tumor cells within lymphatic or vascular channels, is a significant step in tumor distant metastasis (5, 6). According to the recommended European urology guidelines, LVI is an independent prognostic factor for bladder cancer using cystectomy specimens (7). In 2013, Ku et al. (8) performed a meta-analysis of 17 studies and confirmed the significant prognostic role of LVI in RNU specimens. However, much of the raw data in the included literature were lost in their paper. No additional study was conducted to determine the relationship between LVI and other clinicopathological features. In recent years, many studies have contributed relevant information toward the clinicopathological implications of LVI. The purpose of this study was to investigate the relationship between LVI and the clinical outcomes in patients with UTUC to enhance our understanding of prognostic values of LVI and facilitate efficient and prompt clinical decision-making for the patient.
Materials and Methods
Literature Search Strategy
Using Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (9), we (Z.L.Z. and H.Z.) conducted a computerized search using PubMed, EMBASE, and Web of Science in July 2019 to identify studies that documented the incidence of LVI in patients with UTUC undergoing RNU. The combination of the following keywords were used: (“upper urinary tract tumor” OR “renal pelvis” OR “ureter”) AND (“radical nephroureterectomy”) AND (“lymphovascular invasion”) AND (“prognosis” OR “clinical outcome” OR “survival”). The language was restricted to English. At the same time, we manually screened the reference lists of the selected papers, including all of the relevant studies and reviews. For the data obtained from the published studies, no ethical approval and informed consent were required.
Study Inclusion and Exclusion Criteria
The following inclusion criteria were used to select eligible studies: (a) the diagnoses of UTUC and LVI were pathologically confirmed; (b) treatment was limited to RNU; (c) the prognostic values [hazard ratios (HRs) and 95% confidence intervals (95% CIs)] of LVI for overall survival (OS), CSS, recurrence-free survival (RFS), cancer-specific mortality (CSM), and recurrence risk were reported. Accordingly, the exclusion criteria of the meta-analysis were as follows: (a) studies that were not written in English; (b) meeting abstracts, reviews, review papers, or case reports; and (c) no sufficient data to estimate the HRs and 95% CIs. If more than one article from one patient cohort was identified, the most complete article was selected.
Data Extraction
Two authors (J.Y. and Y.J.F.) independently extracted data from the included studies using a predefined data extraction form. Discrepancies were resolved through discussion by a third author (B.W.). The following variables were recorded: patients' characteristics (first author's name, year of publication, geographical region, number of patients, ages, gender, study period, and follow-up duration), tumor characteristics (TNM stage, tumor grade, LVI, lymph node metastasis, tumor multifocality, tumor necrosis, and positive surgical margin), and outcomes of interest. Our primary outcomes included OS, CSS, RFS, CSM, and recurrence. When multivariate analysis and univariate analysis results were both presented in one study, we chose the multivariate analysis results because they account for confounding factors and are more accurate.
Quality Assessment
The Newcastle Ottawa Scale (NOS) (10), which was recommended for evaluating non-randomized studies, was used to assess the quality of the selected studies. This scale assesses risk in three domains: patient selection, comparability of control and intervention groups, and assessment of outcomes. A score of 0–9 stars was allocated to each study. We defined high quality as a score of 6–9 and low quality as a score of <6.
Statistical Analysis
Effect measures for the outcomes of OS, CSS, RFS, CSM, and recurrence were HRs and 95% CIs extracted from the published studies. We studied the associations between LVI and clinicopathological parameters of UTUC. The numbers of events were obtained from the original studies, and the odds ratios (ORs) and the corresponding 95% CIs were calculated. The heterogeneity across studies was tested by using Cochran's Q test and Higgins I-squared statistic. There was marked heterogeneity if P ≤ 0.10 and/or I2 was >50%. A random-effects (RE) model was applied to pool results under significant heterogeneity; otherwise, a fixed-effects (FE) model was applied. A pooled HR ≥ 1 indicated poor survival for patients with an LVI expression. The source for interstudy heterogeneity was explored using subgroup analysis. Publication bias was evaluated by assessing the asymmetry of the funnel plot. Furthermore, Egger's test for funnel plots, which provides quantitative evidence, was employed to search for publication bias between the studies. To examine the stability and the reliability of the overall meta-analysis results, we performed the sensitivity analysis by excluding one study in turn. The statistical analyses were performed using Stata 12.0 software (Stat Corp, College Station, TX, USA). All P-values were two-sided, and P < 0.05 was considered to be statistically significant.
Results
Search Results
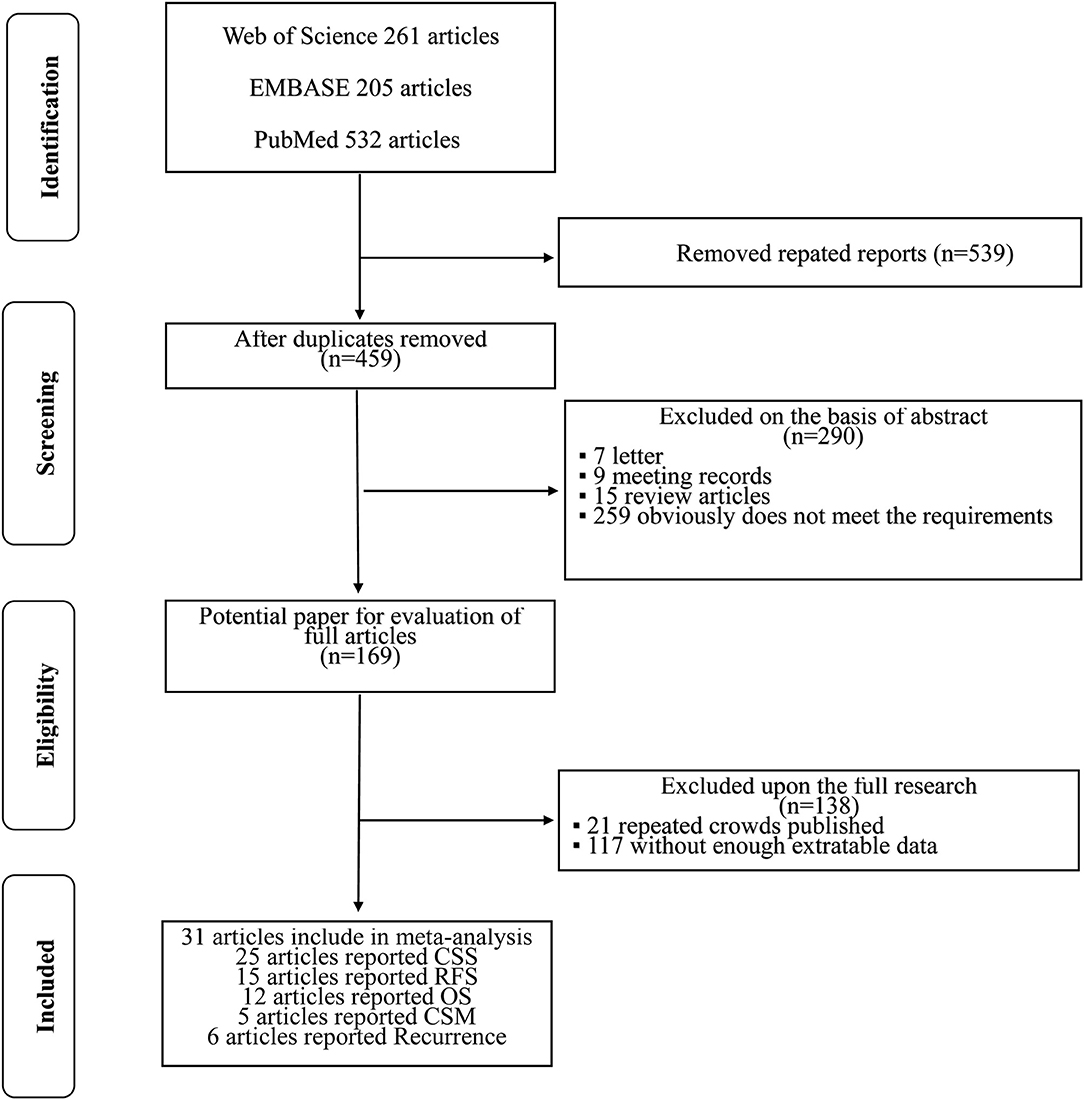
The initial search yielded 998 references, and 539 studies were excluded because of duplication. After title and/or abstracts were screened, 169 articles remained for full-text assessment, and 290 articles were excluded, including reviews, letters, meeting abstracts, and other articles irrelevant to our study. In accordance with the study inclusion criteria, 138 articles were excluded for repeated crowds or without enough extractable data. Finally, 31 studies, which were retrospective in design, were included in this meta-analysis. A flow diagram about the literature search and study selection process is presented in Figure 1.
Features of Included Studies
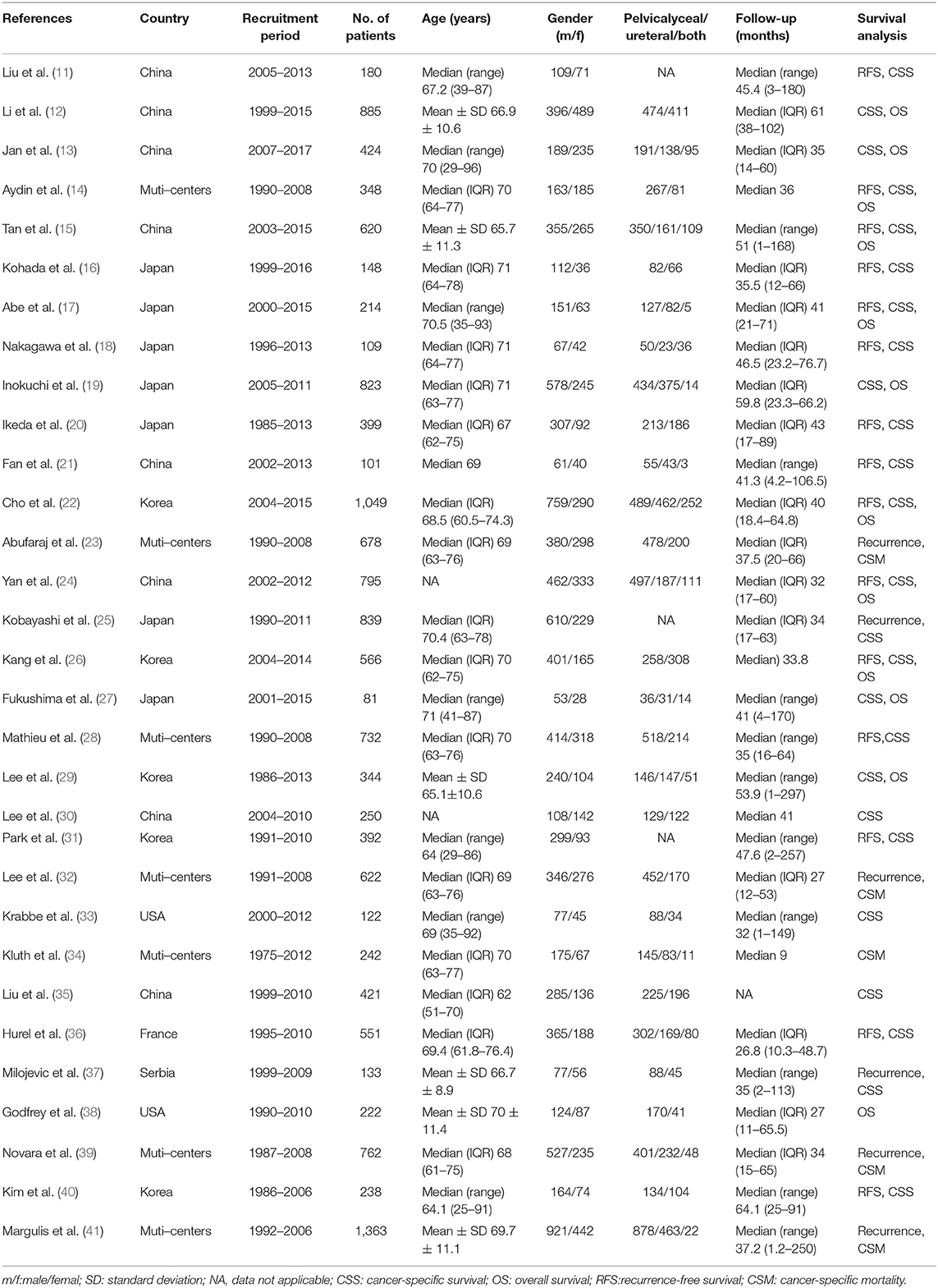
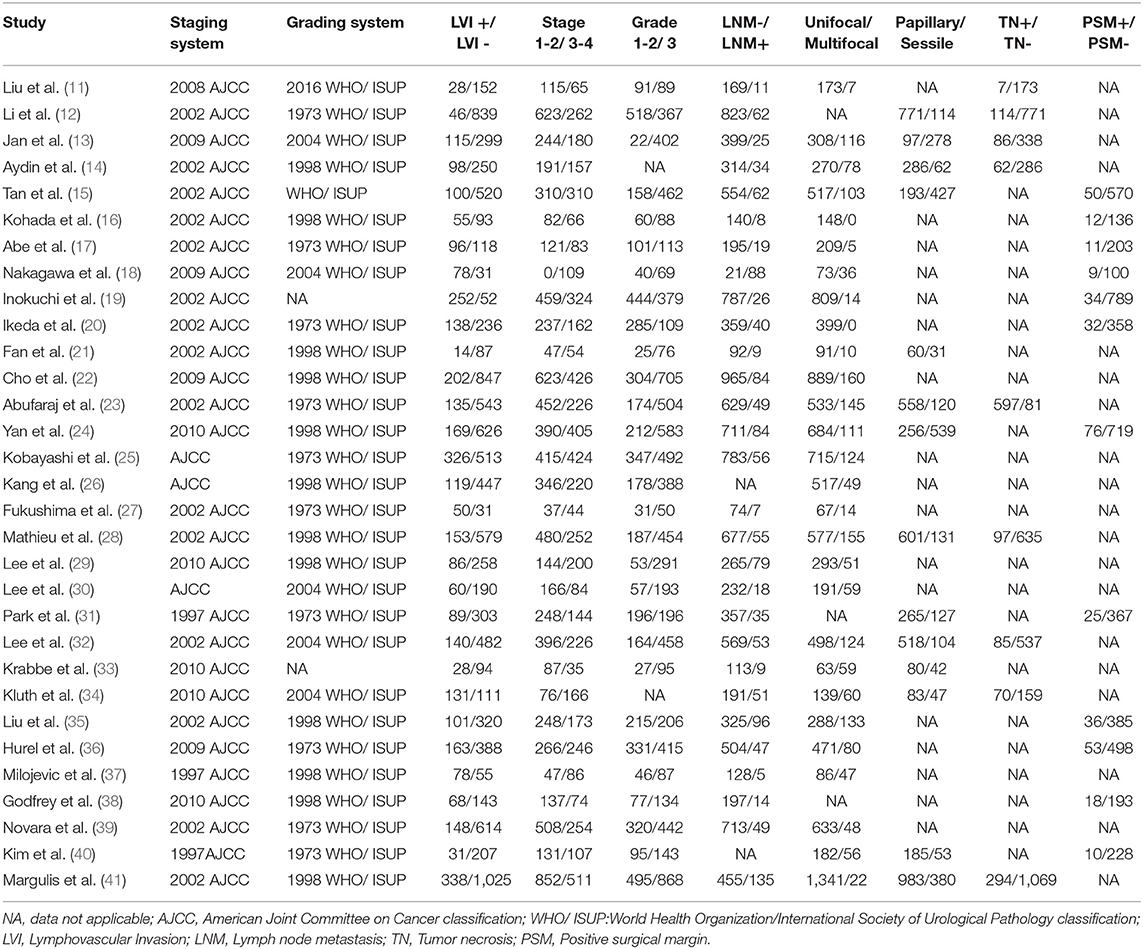
The summary characteristics of these studies are shown in Table 1. A total of 14,653 patients with UTUC (range, 81–1,363) were included in the study. The median or mean age of patients ranged from 62 years to 71 years. The 31 included articles were published from 2009 to 2019. Geographically, 20 studies were conducted in Asia, 7 in multicenters worldwide, 2 in the USA, 1 in France, and 1 in Serbia. All of the patients had received RNU as their primary treatment for UTUC. Of these studies, 12 studies reported OS, 25 studies reported CSS, 15 studies reported RFS, 5 studies reported CSM, and 6 studies reported recurrence. The characteristics of tumor features and pathologic outcomes are summarized in Table 2. LVI was detected in 24.8% (3,635/14,653) of pathological specimens of the included patients. According to the NOS, we assessed the quality of the 31 eligible studies (11–41). The quality scores of the studies varied from 7 to 9, with a mean of 8.7; therefore, all of the studies were of high quality (Supplementary Table 1).
Meta-Analysis Results
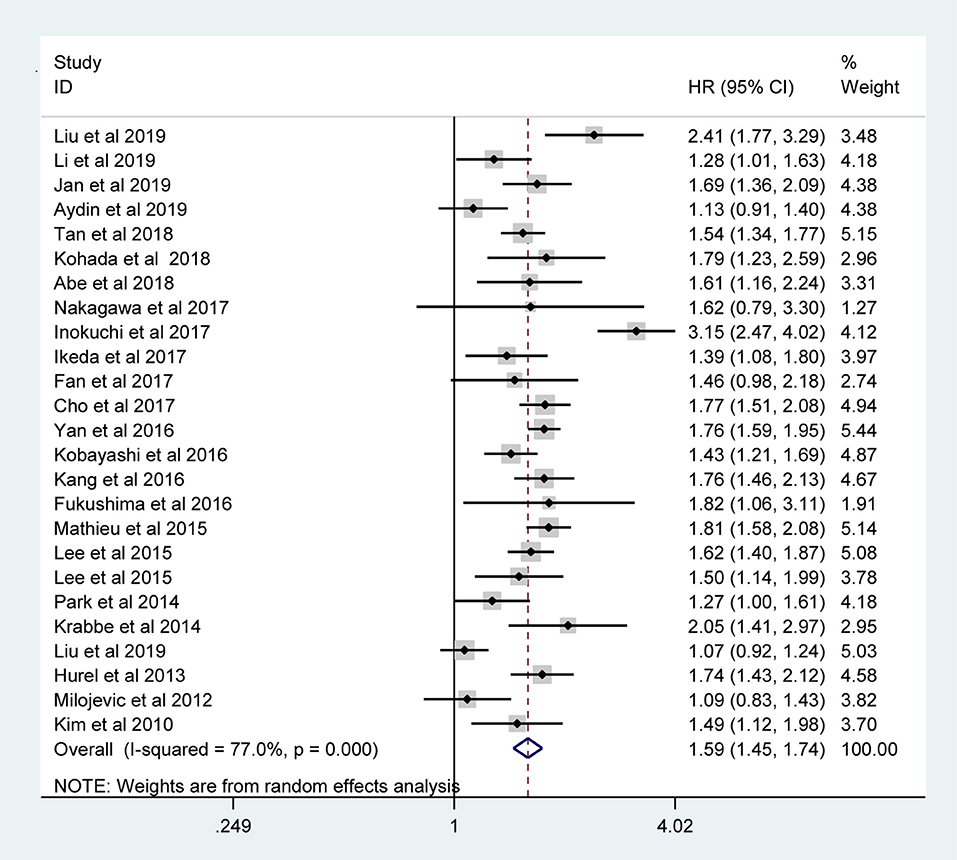
The pooled results indicated that the presence of LVI in UTUC specimens was associated with poor CSS (RE model, HR = 1.59, 95% CI: 1.45–1.74, p < 0.001; I2 = 77%) (Figure 2), OS (RE model, HR = 1.55, 95 % CI: 1.41–1.71, p < 0.001; I2= 73.2%) (Figure 3A), RFS (RE model, HR = 1.46, 95 % CI: 1.32–1.61, p < 0.001; I2 = 78.6%) (Figure 3B), CSM (RE model, HR=1.25, 95 % CI: 1.00–1.56, p = 0.047; I2 = 91.6%) (Figure 3C), and recurrence (RE model, HR=1.23, 95 % CI: 1.03–1.48, p = 0.026; I2 = 89%) (Figure 3D). To explore the heterogeneity for CSS, OS, and RFS, the prognostic value of LVI was evaluated using subgroup analysis under the geographical region (Asia vs. non-Asian), year of publication (≥2014 vs. <2014), TNM stage (T3+T4 %) (≥40 vs. <40), tumor grade (G2+G3 %) (≥60 vs. <60), number of patients (≥500 vs. <500), and median follow-up (≥ 40 months vs. <40 months) (Table 3). Because of the few cohorts in the CSM and recurrence groups, no subgroup analysis was conducted. The results in the subgroup analysis were consistent with the primary findings, which suggested that LVI was a prognostic factor despite heterogeneity among some groups. Although no significant changes for the interstudy heterogeneity were detected, the observed heterogeneity dropped significantly in some subgroup models, such as the number of patients <500 and Grade (G3+G4 %) ≥60.

Figure 3. Forest plots assessing the correlation of LVI and (A) OS, (B) RFS, (C) CSM, and (D) recurrence in studies considering patients with UTUC.
The risk estimate with pooled ORs was used to assess the associations between the LVI and the clinicopathological parameters in patients with UTUC. As shown in Table 4, LVI was significantly related to TNM stage (III/IV vs. I/II: OR = 7.63, 95% CI: 5.60–10.39, p < 0.001) (Supplementary Figure 1A), higher tumor grade (3 vs. 1/2: OR = 5.61, 95% CI: 3.71–8.48, p < 0.001) (Supplementary Figure 1B), lymph node metastasis (LNM) (yes vs. no: OR = 4.95, 95% CI: 3.66–6.71, p < 0.001) (Supplementary Figure 1C), concomitant carcinoma in situ (CIS) (yes vs. no: OR = 1.92, 95% CI: 1.36–2.70, p < 0.001) (Supplementary Figure 1D), and positive surgical margin (PSM) (yes vs. no: OR = 4.38, 95% CI: 2.71–7.07, p < 0.001) (Supplementary Figure 1E), but not related to gender (male vs. female: OR = 0.98, 95% CI: 0.80–1.19, p = 0.825) (Supplementary Figure 2A) and multifocality (multifocal vs. unifocal: OR = 1.09, 95% CI: 0.82–1.46, p = 0.555) (Supplementary Figure 2B). No significant heterogeneity was observed in those groups. In sensitivity analyses omitting enrolled studies in turn, the results showed that the pooled HRs did not alter significantly, which suggested that the findings were reliable and robust (Supplementary Figure 3).
Publication Bias
We conducted the publication bias assessment of the studies using funnel plots and Egger's test. As shown in Figure 4, no obvious asymmetry was observed in all of the groups. The P values of the Egger's test were all >0.05 in CSS (p-Egger = 0.977) (Figure 4A), OS (p-Egger = 0.330) (Figure 4B), RFS (p-Egger = 0.811) (Figure 4C), CSM (p-Egger = 0.984) (Figure 4D), and recurrence (p-Egger = 0.843) (Figure 4E).
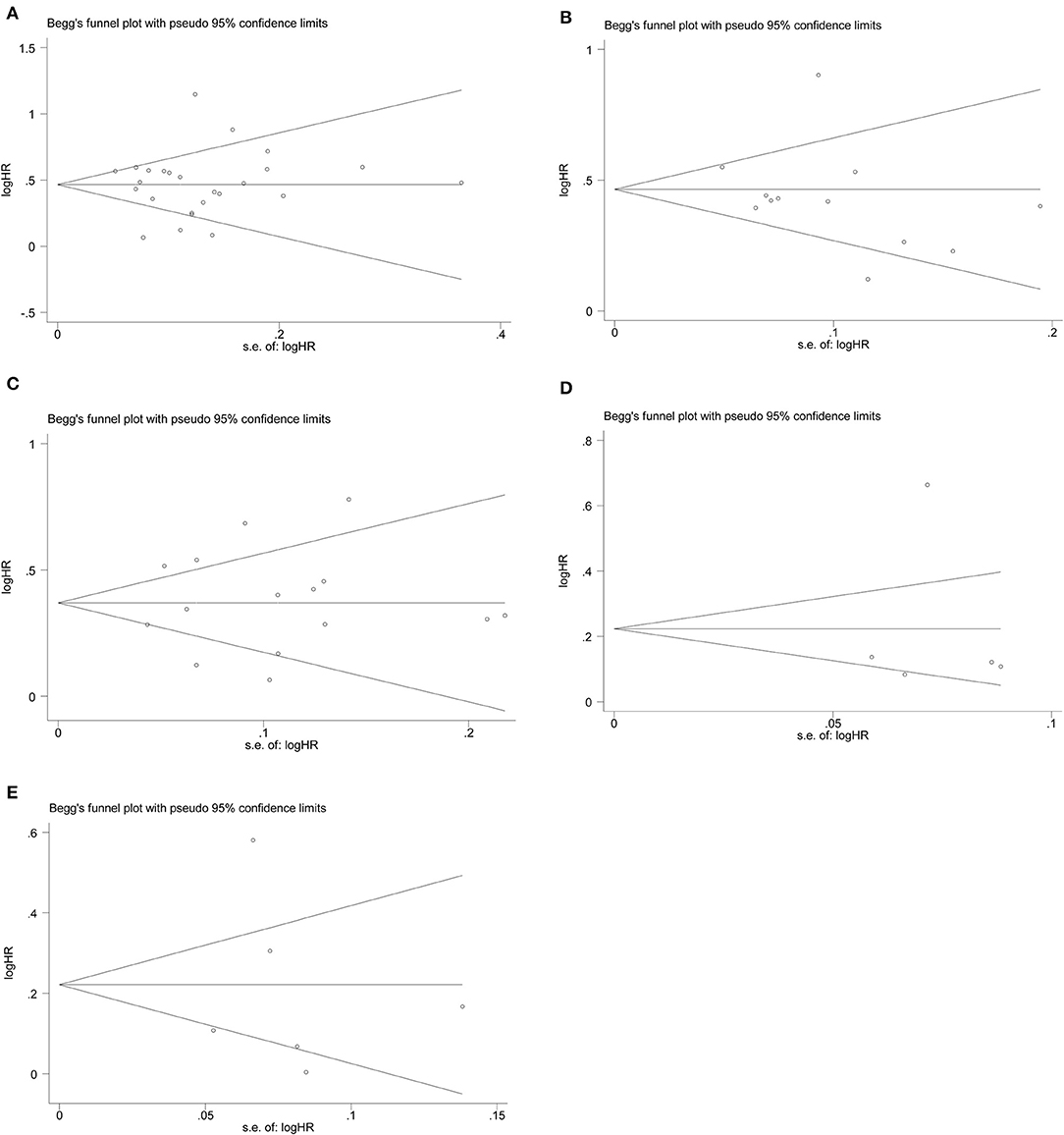
Figure 4. Funnel plots evaluating possible publication bias regarding (A) CSS, (B) OS, (C) RFS, (D) CSM, and (E) recurrence.
Discussion
UTUC is a rare urothelial carcinoma compared with bladder cancer; however, the incidence of UTUC has increased significantly in the last decade (42). Although we have made great strides to understand UTUC, its management remains challenging. Even after the standard RNU surgery was performed in the majority of patients with UTUC, there were still some patients with poor postoperative outcomes. Therefore, it is very important to accurately predict the clinical prognosis of patients with UTUC after RNU. To date, many studies have been conducted to identify the significant prognostic factors of UTUC. Several traditional prognostic predictors, such as pathologic characteristics of RNU specimens including tumor stage and grade (40), tumor architecture (21), tumor size (24), and CIS (43), have been identified as significant prognostic variables for CSS and RFS in different studies.
LVI was considered to be the first step in the metastasis of tumor cells and LNM (30). Recently, mounting evidence has indicated that LVI is associated with poor prognosis for many types of tumors, such as liver, bladder, and prostate cancer (7, 44). Jiang et al. (6) reported that LVI is an independent prognostic factor for predicting worse progression in prostate cancer, and they recommended that LVI should be reported in the final pathological diagnosis after radical prostatectomy. Similarly, Canter et al. (45) found that the presence of LVI in the final pathological reports for bladder cancer delivers significant risks for worse CSS and OS. Several studies have suggested that LVI can be used as an independent prognostic factor in patients with UTUC after RNU (29, 36). However, some studies have suggested that the prognostic value of LVI in assessing survival outcome is meaningless (28, 46). The possible outcomes of these few negative papers may have been related to the heterogeneity of UTUC biology and different clinicopathological features.
Based on the findings of previous research, ~15%−30% of patients with UTUC after surgery have a positive rate of LVI in the final pathology report (8, 30, 39). Consistent with results in previous reports, we found that LVI appeared in ~24.8% of patients. LVI is an easily accessible pathological parameter, which can be accurately measured among observers. Hurel et al. (36), in their study involving 551 patients, concluded that the presence of LVI was an independent risk factor for UTUC. Likewise, Lee et al. (30) reported a significant association between LVI and tumor grade, tumor stage, and LNM. Although it has been proposed that LVI should be accurately recorded in the pathological reports for UTUC specimens, there are still controversial data regarding the impact of LVI on patient prognosis and survival. For example, Jan et al. (13) recently reported that LVI was not associated with OS and CSS in a multivariate analysis. Eich et al. (47) found that LVI was not associated with tumor progression, total mortality, and CSM.
Although the previous studies had largely enhanced our knowledge of UTUC, they were limited to small sample sizes and heterogeneous populations. To overcome these shortcomings and better understand the clinical value of LVI, we assessed LVI and 14,653 patients treated with RNU for UTUC using a meta-analysis. In this study, we demonstrated that LVI was an independent prognostic factor for CSS, RFS, OS, CSM, and recurrence among patients with UTUC treated with RNU. In several studies, LVI has been related to worse tumor differentiation, higher stage and grade, LNM, and PSMs. Consistent with findings in previous outcomes (11, 30, 36), our results indicated that LVI was associated with clinicopathological features, which are all independent poor prognostic factors. All of the results strongly supported the prognostic value of LVI with regard to poor outcomes in UTUC and its role in tumor progression. Furthermore, the results of the study may provide a postoperative follow-up protocol for patients with UTUC to evaluate the prognostic value of LVI. Interestingly, no obvious association between LVI, and multifocality was found in our study. Although multifocal tumors had a worse oncological outcome than renal pelvic tumors, the role of tumor location has not yet been confirmed (39, 48). Hence, multifocal tumors may develop from a more aggressive carcinogenesis pathway.
The results obtained in this study are mainly consistent with the outcomes in a previous system review by Ku et al. (8). However, our study presented a series of advancements. At first, the search time by Ku et al. ended in 2013. However, we added 23 extra studies including 10,963 patients, thereby allowing us to perform a subgroup analysis with more exact evaluation for LVI. Besides, the quality as assessed by NOS in the present meta-analysis was greater, which strengthened the persuasiveness of this research. With the stupendous prognostic value of LVI, we suggest that LVI should always be presented in the pathologic report of RNU specimens. Moreover, patients with LVI expression may be given intensive treatment after RNU. Currently, there is insufficient evidence to recommend adjuvant chemotherapy (AC) as a treatment strategy for patients with UTUC (49). Lee et al. (29) showed that AC does not reduce mortality in patients with UTUC. However, in the subgroup of patients with LVI, AC could significantly improve CSS and OS. Unfortunately, we are unable to further explore the relationship between LVI and AC due to the insufficient data in this study.
Our study has several limitations that should be acknowledged. First, the literature was mainly retrospective, with an obvious heterogeneity in our study. A subgroup analysis that aimed to identify the source of heterogeneity was conducted in the present study. Although considerable heterogeneity among studies had no effect on the pooled results, heterogeneity should not be completely neglected. Thus, the conclusions yielded in this study must be interpreted with caution. Second, the studies in our paper were mainly conducted in four regions. The observed differences in the statistical results might reflect regional ethnic differences. Third, other potential risk factors involved in this report may have affected the final results. For example, the surgical methods were different. Most RNUs were laparoscopic approaches, but some were performed by open surgeries. Thus, there may be performance bias. Fourth, reporting bias may exist in our research, as some studies with negative results may not have been published. However, no significant bias was observed in this research, which indicates that our results were stable and reliable.
Conclusion
In summary, LVI is associated with unfavorable prognosis and clinicopathological features in patients with UTUC. Given its convenience and inexpensiveness in clinical application, LVI could be a useful tool for predicting prognosis and outcomes of patients with UTUC. However, additional prospective, multicenter studies should be conducted to confirm our findings and address the limitations observed in our meta-analysis.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Author Contributions
LZ project development and manuscript writing. BW data management and manuscript editing. JY and YF data collection. ZZ and HZ data analysis and data management.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00487/full#supplementary-material
Supplementary Figure 1. Forest plots of meta-analyses of the association between LVI and clinicopathological features in UTUC: (A) TNM stage, (B) tumor grade, (C) LNM, (D) CIS, and (E) PSM.
Supplementary Figure 2. Forest plots of meta-analyses of the association between LVI and clinicopathological features in UTUC: (A) gender and (B) multifocality.
Supplementary Figure 3. Sensitivity analysis in this meta-analysis. (A) Sensitivity analysis for CSS; (B) sensitivity analysis for OS; (C) sensitivity analysis for RFS; (D) sensitivity analysis for CSM; and (E) sensitivity analysis for recurrence.
Abbreviations
LVI, lymphovascular invasion; UTUC, upper tract urinary carcinoma; RNU, radical nephroureterectomy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle-Ottawa scale; HRs, hazard ratios; ORs, odds ratio; CIs, confidence intervals; CSS, cancer-specific survival; OS, overall survival; RFS, recurrence-free survival; CSM, cancer-specific mortality; CIS, concomitant carcinoma in situ; LNM, lymph node metastasis; PSM, positive surgical margin; AC, adjuvant chemotherapy.
References
2. Cha EK, Shariat SF, Kormaksson M, Novara G, Chromecki TF, Scherr DS, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. European urology. (2012) 61:818–25. doi: 10.1016/j.eururo.2012.01.021
3. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. European urology. (2015) 68:868–79. doi: 10.1016/j.eururo.2015.06.044
4. Duquesne I, Ouzaid I, Loriot Y, Moschini M, Xylinas E. Lymphadenectomy for upper tract urothelial carcinoma: a systematic review. J Clin Med. (2019) 8:1190. doi: 10.3390/jcm8081190
5. Ukai R, Hashimoto K, Nakayama H, Iwamoto T. Lymphovascular invasion predicts poor prognosis in high-grade pT1 bladder cancer patients who underwent transurethral resection in one piece. Jpn J Clin Oncol. (2017) 47:447–52. doi: 10.1093/jjco/hyx012
6. Jiang W, Zhang L, Wu B, Zha Z, Zhao H, Jun Y, et al. The impact of lymphovascular invasion in patients with prostate cancer following radical prostatectomy and its association with their clinicopathological features: an updated PRISMA-compliant systematic review and meta-analysis. Medicine. (2018) 97:e13537. doi: 10.1097/MD.0000000000013537
7. Mari A, Kimura S, Foerster B, Abufaraj M, D'Andrea D, Gust KM, et al. A systematic review and meta-analysis of lymphovascular invasion in patients treated with radical cystectomy for bladder cancer. Urol Oncol. (2018) 36:293–305. doi: 10.1016/j.urolonc.2018.03.018
8. Ku JH, Byun SS, Jeong H, Kwak C, Kim HH, Lee SE. Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: a systematic review and meta-analysis. Eur J Cancer. (2013) 49:2665–80. doi: 10.1016/j.ejca.2013.04.016
9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Europ J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
11. Liu W, Zhou Z. Prognostic value of lymphovascular invasion in node-negative upper urinary tract urothelial carcinoma patients undergoing radical nephroureterectomy. Yonsei Med J. (2019) 60:174–81. doi: 10.3349/ymj.2019.60.2.174
12. Li Y, Fang D, Bao Z, He A, Guan B, He S, et al. High aspartate transaminase/alanine transaminase ratio predicts poor prognosis in patients with localized upper tract urothelial cancer: a propensity score-matched study in a large Chinese center. OncoTargets Ther. (2019) 12:2635–48. doi: 10.2147/OTT.S193771
13. Jan HC, Yang WH, Ou CH. Combination of the preoperative systemic immune-inflammation index and monocyte-lymphocyte ratio as a novel prognostic factor in patients with upper-tract urothelial carcinoma. Ann Surg Oncol. (2019) 26:669–84. doi: 10.1245/s10434-018-6942-3
14. Aydin AM, Singla N, Panwar V, Woldu SL, Freifeld Y, Wood CG, et al. Prognostic significance of BAP1 expression in high-grade upper tract urothelial carcinoma: a multi-institutional study. World J Urol. (2019) 37:2419–27. doi: 10.1007/s00345-019-02678-x
15. Tan P, Xie N, Liao H, Zou L, Xu H, Yang L, et al. Prognostic impact of preoperative anemia on upper tract urothelial carcinoma. Medicine. (2018) 97:e12300. doi: 10.1097/MD.0000000000012300
16. Kohada Y, Hayashi T, Goto K, Kobatake K, Abdi H, Honda Y, et al. Preoperative risk classification using neutrophil-lymphocyte ratio and hydronephrosis for upper tract urothelial carcinoma. Jpn J Clin Oncol. (2018) 48:841–50. doi: 10.1093/jjco/hyy084
17. Abe T, Kondo T, Harabayashi T, Takada N, Matsumoto R, Osawa T, et al. Comparative study of lymph node dissection, and oncological outcomes of laparoscopic and open radical nephroureterectomy for patients with urothelial carcinoma of the upper urinary tract undergoing regional lymph node dissection. Jpn J Clin Oncol. (2018) 48:1001–11. doi: 10.1093/jjco/hyy128
18. Nakagawa T, Komemushi Y, Kawai T, Otsuka M, Miyakawa J, Uemura Y, et al. Efficacy of post-nephroureterectomy cisplatin-based adjuvant chemotherapy for locally advanced upper tract urothelial carcinoma: a multi-institutional retrospective study. World J Urol. (2017) 35:1569–75. doi: 10.1007/s00345-017-2032-6
19. Inokuchi J, Eto M, Hara T, Fujimoto H, Nishiyama H, Miyazaki J, et al. Impact of lymph node dissection on clinical outcomes during nephroureterectomy in patients with clinically node-negative upper urinary tract urothelial cancer: subanalysis of a multi-institutional nationwide case series of the Japanese Urological Association. Jpn J Clin Oncol. (2017) 47:652–9. doi: 10.1093/jjco/hyx051
20. Ikeda M, Matsumoto K, Sakaguchi K, Ishii D, Tabata KI, Kurosawa K, et al. Effect of lymphadenectomy during radical nephroureterectomy in locally advanced upper tract urothelial carcinoma. Clin Genitour Cancer. (2017) 15:556–62. doi: 10.1016/j.clgc.2017.04.004
21. Fan B, Hu B, Yuan Q, Wen S, Liu T, Bai S, et al. Impact of tumor architecture on disease recurrence and cancer-specific mortality of upper tract urothelial carcinoma treated with radical nephroureterectomy. Tum Biol. (2017) 39:1010428317710822. doi: 10.1177/1010428317710822
22. Cho YH, Hwang JE, Chung HS, Kim MS, Hwang EC. The De Ritis (aspartate transaminase/alanine transaminase) ratio as a predictor of oncological outcomes in patients after surgery for upper urinary tract urothelial carcinoma. Int Urol Nephrol. (2017) 49:1383–90. doi: 10.1007/s11255-017-1613-z
23. Abufaraj M, Moschini M, Soria F, Gust K, Ozsoy M, Mathieu R, et al. Prognostic role of expression of N-cadherin in patients with upper tract urothelial carcinoma: a multi-institutional study. World J Urol. (2017) 35:1073–80. doi: 10.1007/s00345-016-1968-2
24. Shibing Y, Liangren L, Qiang W, Hong L, Turun S, Junhao L, et al. Impact of tumour size on prognosis of upper urinary tract urothelial carcinoma after radical nephroureterectomy: a multi-institutional analysis of 795 cases. BJU Int. (2016) 118:902–10. doi: 10.1111/bju.13463
25. Kobayashi H, Kikuchi E, Tanaka N, Shirotake S, Miyazaki Y, Ide H, et al. Patient age was an independent predictor of cancer-specific survival in male patients with upper tract urothelial carcinoma treated by radical nephroureterectomy. Jpn J Clin Oncol. (2016) 46:554–559. doi: 10.1093/jjco/hyw028
26. Kang SG, Hwang EC, Jung SI, Yu HS, Chung HS, Kang TW, et al. Poor preoperative glycemic control is associated with dismal prognosis after radical nephroureterectomy for upper tract urothelial carcinoma: a korean multicenter study. Cancer Res Treat. (2016) 48:1293–301. doi: 10.4143/crt.2016.021
27. Fukushima H, Nakanishi Y, Kataoka M, Tobisu K, Koga F. Prognostic significance of sarcopenia in upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Cancer Med. (2016) 5:2213–20. doi: 10.1002/cam4.795
28. Mathieu R, Klatte T, Margulis V, Karam JA, Roupret M, Seitz C, et al. Survivin is not an independent prognostic factor for patients with upper tract urothelial carcinoma: a multi-institutional study. Urol Oncol. (2015) 33:495.e415–22. doi: 10.1016/j.urolonc.2015.06.016
29. Lee KS, Kim KH, Yoon YE, Choi KH, Yang SC, Han WK. Impact of adjuvant chemotherapy in patients with upper tract urothelial carcinoma and lymphovascular invasion after radical nephroureterectomy. Kor J Urol. (2015) 56:41–7. doi: 10.4111/kju.2015.56.1.41
30. Lee HY, Li CC, Huang CN, Ke HL, Li WM, Liang PI, et al. Prognostic significance of lymphovascular invasion in upper urinary tract urothelial carcinoma is influenced by tumor location. Ann Surg Oncol. (2015) 22:1392–400. doi: 10.1245/s10434-014-4103-x
31. Park J, Park S, Song C, Hong JH, Kim CS, et al. Peripelvic/periureteral fat invasion is independently associated with worse prognosis in pT3 upper tract urothelial carcinoma. World J Urol. (2014) 32:157–63. doi: 10.1007/s00345-013-1073-8
32. Lee DJ, Xylinas E, Rieken M, Khani F, Klatte T, Wood CG, et al. Insulin-like growth factor messenger RNA-binding protein 3 expression helps prognostication in patients with upper tract urothelial carcinoma. Europ Urol. (2014) 66:379–85. doi: 10.1016/j.eururo.2013.12.008
33. Krabbe LM, Westerman ME, Bagrodia A, Gayed BA, Khalil D, Kapur P, et al. Surgical management of the distal ureter during radical nephroureterectomy is an independent predictor of oncological outcomes: results of a current series and a review of the literature. Urol Oncol. (2014) 32:54.e19–26. doi: 10.1016/j.urolonc.2013.08.032
34. Kluth LA, Xylinas E, Kent M, Hagiwara M, Kikuchi E, Ikeda M, et al. Predictors of survival in patients with disease recurrence after radical nephroureterectomy. BJU Int. (2014) 113:911–7. doi: 10.1111/bju.12369
35. Liu JY, Li YH, Zhang ZL, Ye YL, Liu ZW, Yao K, et al. Age-specific effect of gender on upper tract urothelial carcinoma outcomes. Med Oncol. (2013) 30:640. doi: 10.1007/s12032-013-0640-6
36. Hurel S, Roupret M, Ouzzane A, Rozet F, Xylinas E, Zerbib M, et al. Impact of lymphovascular invasion on oncological outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int. (2013) 111:1199–207. doi: 10.1111/bju.12116
37. Milojevic B, Djokic M, Sipetic-Grujicic S, Milenkovic-Petronic D, Vuksanovic A, Bumbasirevic U, et al. Upper urinary tract transitional cell carcinoma: location is not correlated with prognosis. BJU Int. (2012) 109:1037–42. doi: 10.1111/j.1464-410X.2011.10461.x
38. Godfrey MS, Badalato GM, Hruby GW, Razmjoo M, McKiernan JM. Prognostic indicators for upper tract urothelial carcinoma after radical nephroureterectomy: the impact of lymphovascular invasion. BJU Int. (2012) 110:798–803. doi: 10.1111/j.1464-410X.2011.10893.x
39. Novara G, Matsumoto K, Kassouf W, Walton TJ, Fritsche HM, Bastian PJ, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Europ Urol. (2010) 57:1064–71. doi: 10.1016/j.eururo.2009.12.029
40. Kim DS, Lee YH, Cho KS, Cho NH, Chung BH, Hong SJ. Lymphovascular invasion and pT stage are prognostic factors in patients treated with radical nephroureterectomy for localized upper urinary tract transitional cell carcinoma. Urology. (2010) 75:328–32. doi: 10.1016/j.urology.2009.07.1350
41. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. (2009) 115:1224–33. doi: 10.1002/cncr.24135
42. Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. (2011) 107:1059–64. doi: 10.1111/j.1464-410X.2010.09675.x
43. Redrow GP, Guo CC, Brausi MA, Coleman JA, Fernandez MI, Kassouf W, et al. Upper urinary tract carcinoma in situ: current knowledge, future direction. J Urol. (2017) 197:287–95. doi: 10.1016/j.juro.2016.03.194
44. McCleskey BC, Epstein JI, Albany C, Hashemi-Sadraei N, Idrees MT, Jorns JM, et al. The significance of lymphovascular invasion of the spermatic cord in the absence of cord soft tissue invasion. Arch Pathol Lab Med. (2017) 141:824–9. doi: 10.5858/arpa.2016-0226-OA
45. Canter D, Guzzo T, Resnick M, Magerfleisch L, Sonnad S, Bergey M, et al. The presence of lymphovascular invasion in radical cystectomy specimens from patients with urothelial carcinoma portends a poor clinical prognosis. BJU Int. (2008) 102:952–7. doi: 10.1111/j.1464-410X.2008.07732.x
46. Yoshida T, Kinoshita H, Shimada S, Sugi M, Matsuda T. Preoperative pyuria is a poor prognostic factor in patients with urothelial carcinoma of the upper urinary tract after surgery. Clin Genitour Cancer. (2017) 15:e543–50. doi: 10.1016/j.clgc.2016.12.021
47. Eich ML, Tregnago AC, Faraj SF, Palsgrove DN, Fujita K, Bezerra SM, et al. Insulin-like growth factor-1 receptor expression in upper tract urothelial carcinoma. Urology. (2019) 474:21–27. doi: 10.1007/s00428-018-2468-0
48. Ouzzane A, Colin P, Xylinas E, Pignot G, Ariane MM, Saint F, et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Euro Urol. (2011) 60:1258–65. doi: 10.1016/j.eururo.2011.05.049
49. Necchi A, Lo Vullo S, Mariani L, Moschini M, Hendricksen K, Rink M, et al. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. BJU Int. (2018) 121:252–9. doi: 10.1111/bju.14020
Keywords: lymphovascular invasion, upper tract urinary carcinoma, radical nephroureterectomy, prognosis, meta-analysis
Citation: Zhang L, Wu B, Zha Z, Zhao H, Yuan J and Feng Y (2020) The Prognostic Value of Lymphovascular Invasion in Patients With Upper Tract Urinary Carcinoma After Surgery: An Updated Systematic Review and Meta-Analysis. Front. Oncol. 10:487. doi: 10.3389/fonc.2020.00487
Received: 23 July 2019; Accepted: 18 March 2020;
Published: 22 April 2020.
Edited by:
Ian Pearce, Manchester University NHS Foundation Trust (MFT), United KingdomReviewed by:
Marco Roscigno, Ospedale Papa Giovanni XXIII, ItalyZiv Radisavljevic, Brigham and Woman's Hospital and Harvard Medical School, United States
Copyright © 2020 Zhang, Wu, Zha, Zhao, Yuan and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijin Zhang, stzlj913729553@163.com
†These authors have contributed equally to this work
 Lijin Zhang
Lijin Zhang Bin Wu
Bin Wu Hu Zhao
Hu Zhao Jun Yuan
Jun Yuan