- 1Center of Interventional Radiology and Vascular Surgery, Department of Radiology, Zhongda Hospital, Medical School, Southeast University, Nanjing, China
- 2Center of Oncology, Tianchang City Hospital of Chinese Medicine, Chuzhou, China
- 3Department of Intervention and Vascular Surgery, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, China
Purpose: To compare the efficacy and safety of computed tomography (CT)–guided 125I seed implantation with second-line chemotherapy in treatment of oligorecurrence non–small cell lung cancer after failure of first-line chemotherapy.
Methods: Data of oligorecurrence non–small cell lung cancer patients after failure of first-line chemotherapy at two institutions were retrospectively reviewed from January 2013 to July 2018. A total of 53 patients who received the treatment of 125I seed implantation or second-line chemotherapy were eligible for this study. In group A, 25 patients, 84 lesions, received CT-guided permanent 125I seed implantation, whereas in group B, 28 patients, 96 lesions, received second-line chemotherapy. The outcomes were measured in terms of disease control rate, overall survival, quality of life, and complications.
Results: The median follow-up period was 13 months (range, 5–42 months). Disease control rate in group A was higher than that in group B (70.8 vs. 42.3%, P = 0.042) at 6 months after treatment. The median overall survival was 12.8 months (95% confidence interval, 10.5–15.1 months) in group A and 15.2 months (95% confidence interval, 12.2–18.2 months) in group B, with no significant difference (P = 0.847). Since the fourth month, the number of patients in group A with a non-decreasing Karnofsky Performance Scale score was more than that in group B (P < 0.05). The incidence of grade 3 or higher complications especially hematologic toxicity in group A was significantly lower than that in group B (P < 0.05).
Conclusion: Radioactive 125I seed implantation is safe and feasible in selected non–small cell lung cancer patients with oligorecurrence after failure of first-line chemotherapy and seems to provide a better long-term quality of life in these patients compared with second-line chemotherapy.
Introduction
Lung cancer is currently the most common malignant carcinoma with the highest mortality rate in China (1). Approximately 75–80% of the pathological types of lung cancer are non–small cell lung cancer (NSCLC). In NSCLC, 50% of patients who are newly diagnosed are found to have metastases (2). Lung cancer with distant metastases was treated as advanced stage disease and indicated for systemic treatment. Platinum-based chemotherapy has been the standard treatment for metastatic NSCLC. And recently, immunotherapy has become an important treatment strategy for NSCLC (3–6). Despite of recent progress in pharmacotherapy, prognosis of metastatic cancer remains unsatisfactory.
However, not all NSCLC patients with metastasis have a poor prognosis. Hellman and Weichselbaum (7) reported the existence of “oligometastatic state.” These states may be noted at the time of diagnosis (i.e., oligometastasis) or as failure after definitive therapy (i.e., oligorecurrence). Oligometastasis and oligorecurrence refer to patients with metastases limited in number and location, which represents an intermediate state between locally confined and widely metastatic cancer. There is currently no clear definition of oligometastatic disease. Most clinical trials and clinicians accept a definition of fewer than five metastatic lesions (8–11). Many studies indicated that patients with oligometastases might benefit from local treatment, such as surgical resection, radiotherapy, and radiofrequency ablation (12–14). Stereotactic body radiotherapy (SBRT) is a specialized form of radiotherapy, which is characterized by higher doses of radiation, shorter time period, and more precise targeting system. Stereotactic body radiotherapy has become a preferred treatment strategy for oligometastatic disease (13, 15).
Radioactive 125I seed (RIS) brachytherapy recently has been a standard treatment in early low- to intermediate-risk prostate cancer (16, 17). It is a type of low-dose-rate brachytherapy, which could deliver high radiation dose to target tumor while safely sparing the adjacent normal tissue. Previous studies indicated that 125I brachytherapy is a feasible salvage treatment method for many cancers, including prostate cancer, lung cancer, pancreatic cancer, and head and neck cancer (18). To a certain extent, RIS implantation brachytherapy is similar to a single dose of stereotactic ablative radiotherapy and can also be called “stereotactic ablative brachytherapy” (16). However, whether RIS implantation could provide benefits in terms of local control and survival to oligorecurrence NSCLC patients after failure of first-line chemotherapy remains unclear.
The purpose of this study was to compare the efficacy and safety of computed tomography (CT)–guided 125I seed implantation with second-line chemotherapy in treatment of oligorecurrence NSCLC after failure of first-line chemotherapy.
Materials and Methods
This study was approved by the institutional review board. From January 2013 to July 2018, data of consecutive case of stage IV NSCLC who experienced failure of first-line chemotherapy were queried from electronic medical record. Those patients did not have history of polymetastatic disease before oligometastatic NSCLC diagnosis, while they had history of oligometastatic disease. And oligometastatic disease is diagnosed within 6 months after diagnosis of the primary tumor. Those patients had newly developed metastases after receiving first-line chemotherapy (paclitaxel + cisplatin, gemcitabine + cisplatin) four to six cycles. According to the characterization and classification of oligometastatic disease consensus recommendation, those patients were defined as repeat oligoprogression (19). Those patients who underwent CT-guided percutaneous RIS implantation (group A) or second-line chemotherapy (group B) were included in this study. Patients who received RIS implantation signed an informed consent form. It is important to point out that both primary tumor and metastatic sites received RIS implantation. The inclusion criteria were (1) histologically proven NSCLC, (2) negative driver mutation, (3) intolerant to surgical resection and external beam radiotherapy, (4) fewer than 5 metastatic nodules, (5) absence of severe coagulation dysfunction, and (6) Karnofsky Performance Scale (KPS) score ≥70. The exclusion criteria were as follows: (1) with other types of primary tumors except NSCLC and (2) with incomplete data. After removing patients who were lost to follow-up or with missing data, a total of 53 patients were finally eligible for this study.
Treatment
125I Radioactive Seed Implantation
Instruments
Brachytherapy treatment planning system (Qilin Co., Ltd., Peking, China) was applied to calculate 125I seed dose distribution, according to the American Association of Physicists in Medicine TG43 brachytherapy formalism (20). The parameters of 125I seed (XinKe Pharmaceutical Ltd., Shanghai, China) were 0.8 mm in diameter, 4.5 mm in length, and 0.05 mm in thickness of the wall of the titanium capsule. The gamma rays delivered by 125I (5% of 35 keV, 95% of 28 keV) was with a half-life of 59.6 days, penetration of 17 mm, incipient rate of 7 cGy/h, and activities of 0.5–0.8 mCi. Eighteen-gauge implantation needles and turntable implantation gun (XinKe Pharmaceutical Ltd.) were applied for the RIS implantation.
CT-guided implantation protocol
All patients received CT (Siemens, Munich, Germany) scan with 5-mm slice thickness and spacing 2–3 days before RIS implantation (Figure 1A). Then CT images were uploaded into a treatment planning system. Radiation physicians outlined the gross tumor volume (GTV) on each transverse image and determined the prescription dose [according to international standards for prostate cancer and expert consensus on RIS brachytherapy (18, 21)]. And the total number and activity of seed to be implanted were calculated via the modified level formula (22) (Figure 1B). The dosimetric goal was that the dose received by 90% of the GTV should reach the prescription dose as much as possible, and the doses delivered to the adjacent normal organs were as low as possible (16).
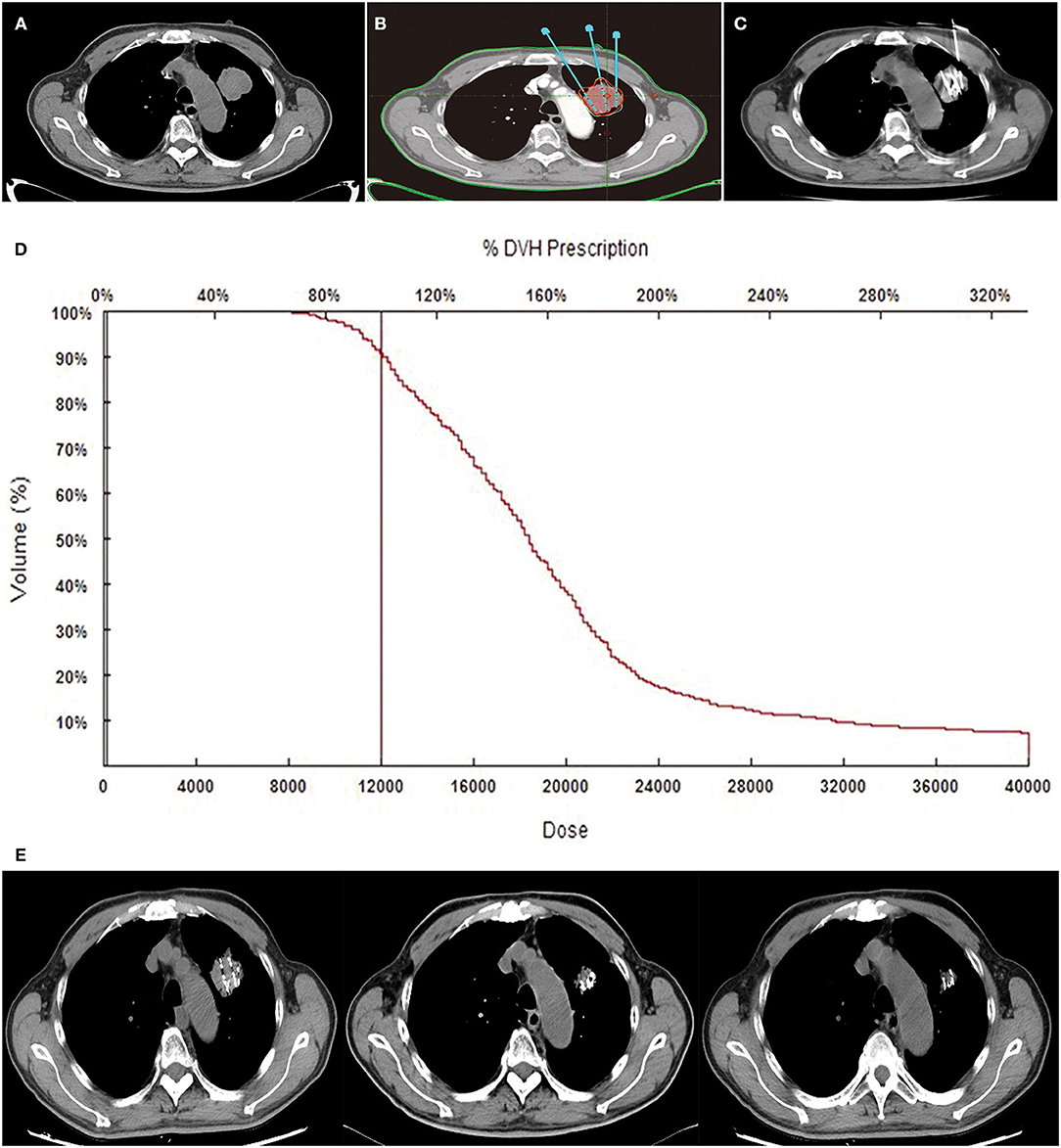
Figure 1. Tumor, treatments, and follow-up CT. An 81-year-old male patient with NSCLC received RIS implantation. Preoperative CT images showed the oligometastatic lesion was located in the upper lobe of the left lung (A). Isodose curves and planning target volume plotted by TPS. The red line shows the planning target volume, and the green line shows the 120-Gy isodose curves (B). A total of 30 125I seeds were implanted into the tumor under CT guidance, and the radioactivity of the seed was 0.6 mCi (C). Dose–volume histograms (DVH). The prescription dose is 120 Gy during planning. A total of 90% of the tumor target received 120.0 Gy, and 95.4% of the tumor received 100% of the prescribed dose (D). Gradually, the mass shrunk at the second, fourth, and sixth months on the following CT images (E).
As radiation sources, 125I seeds were implanted into primary and metastatic lesions under CT guidance, at a spacing of 1 cm. Prescription dose was 100–140 Gy. Activity of 125I seeds was 0.4–0.8 mCi. All the RIS implantation procedures were performed by interventional radiologists in a standard CT room under local anesthesia. The space between the adjacent implantation needles was ~1 cm each. Repeated CT allowed adjustment of depth and angle of needles to avoid adjacent vessels and organs. Seeds were released every 0.5–1.0 cm apart upon withdrawing the needles (Figure 1C). After RIS implantation, patients were observed in the interventional radiology wards for 1–2 days.
Postimplantation dosimetry
After the completion of RIS implantation, immediately CT scan should be performed to validate the actual post-operative distribution of RIS. Then CT images would be uploaded into TPS software, a redundancy check was performed to prevent seed duplication. Dosimetric parameters, including D90 and V100, were used to evaluate the dosimetry. D90 represents the dose delivered to the 90% of GTV, and V100 means the percentage of GTV receiving 100% of prescription dose. Actual isodose distributions for each slice and dose volume histograms for the target were generated (Figures 1D,E).
Second-line chemotherapies
The chemotherapy regimen for squamous cell carcinoma patients was docetaxel 75 mg/m2 per 3 weeks, and that for non-squamous cell carcinoma was pemetrexed 500 mg/m2 per 3 weeks. The drug dose was modified on the basis of blood cell counts and renal function on the day of therapy. During chemotherapy, routine blood test and coagulation function plus liver and kidney function tests were performed.
Follow-up
Patients who underwent 125I radioactive seed implantation were closely observed for their vital signs within 24 h, with electrocardiogram monitoring performed and all post-operative complications recorded in detail. Follow-up of all patients was carried out at an interval of 2 months, including disease control rate (DCR), quality of life (QOL), complications, and death time. Dynamic enhanced CT, brain magnetic resonance, and laboratory test (routine blood test and coagulation function plus liver and kidney function) were performed routinely.
Outcomes and definitions
The primary endpoint is DCR. In this study, DCR refers to disease control of both primary tumor and metastatic sites. It is overall response assessment, which is a result of the combined assessment of current lesions and new lesions. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.0): complete response (CR): all target lesions disappear, confirmed at 4 weeks; partial response (PR): baseline lesion total diameter reduction ≥30%, confirmed at 4 weeks; stable disease (SD): between PR and PD; progression disease (PD): total length of lesion increased ≥20% or new lesions. Disease control rate was calculated as (CR + PR +S D)/total number of patients × 100%. The result of tumor response was confirmed by two to three senior radiologists. Quality of life was evaluated according to KPS score. The improvement of QOL was defined as an increase of at least 10 points of KPS after treatment, whereas the worsening of QOL referred to a decrease of 10 points or more, and the stability of QOL meant that the fluctuation of KPS was <10 points. The overall survival (OS) was defined as the time interval from initial treatment to death or the last follow-up. The Common Terminology Criteria for Adverse Events 4.0 was used to assess treatment-related adverse effects.
Statistical analysis
Numerical data with normal distribution were expressed as mean ± SD, whereas data with non-normal distribution were expressed as median (interquartile range). Continuous variables were compared using the t-test or Mann-Whitney U test for variables with a normal or non-normal distribution. Categorical variables were compared using the χ2 test or Fisher exact test. Overall survival time analyses were performed with the Kaplan–Meier method and log-rank test. P < 0.05 was considered statistically significant. All data analyses were performed using SPSS 18.0 software (IBM, Armonk, NY, USA).
Results
Patient Characteristics
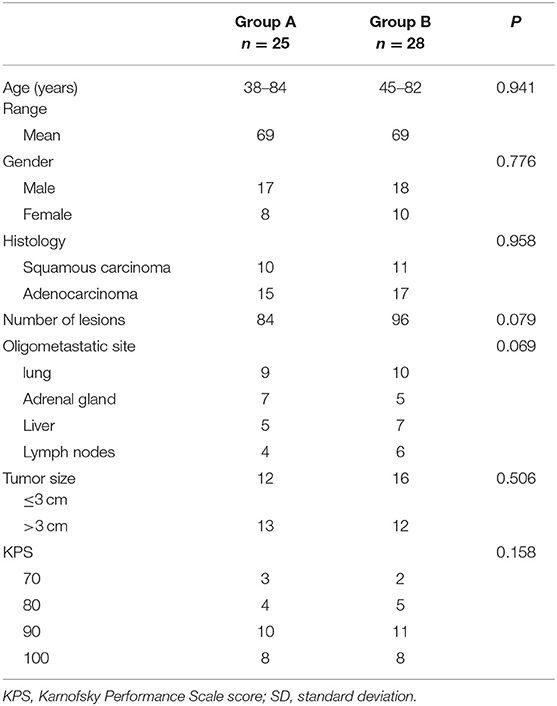
In group A, 25 patients received CT-guided percutaneous RIS implantation. As shown in Table 1, 17 male and 8 female patients, with a median age of 68 years (range, 38–84 years), were evaluated. Fifteen cases were adenocarcinomas, and 10 were squamous cell carcinomas. Oligometastatic sites were located in lung (n = 9), adrenal gland (n = 7), liver (n = 5), and lymph nodes (n = 4). Metastatic lymph nodes were located in supraclavicular and mediastinal lymph nodes. In group A, 25 patients, 84 lesions [lung (31), adrenal gland (24), liver (16), and lymph nodes (13)], received CT-guided 125I seed implantation. In group B, 28 patients, 96 lesions [lung (36), adrenal gland (23), liver (21), and lymph nodes (16)], underwent second-line chemotherapy with docetaxel or pemetrexed. The baseline characteristics of these patients are summarized in Table 1.
Disease Control Rate
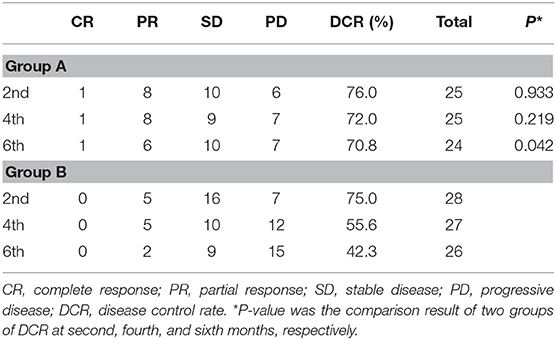
At the second, fourth, and sixth months after treatment, DCR in group A vs. group B was 76.0 vs. 75.0%, 72.0 vs. 55.6%, and 70.8 vs. 42.3% (p2 = 0.933, p4 = 0.219, p6 = 0.042), respectively (Table 2). Disease control rate of group A was statistically higher than that of group B at the sixth month.
Quality of Life
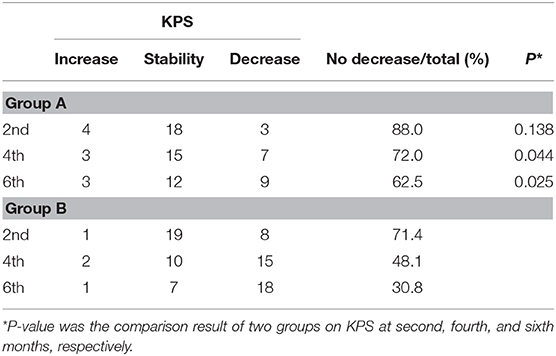
At the second, fourth, and sixth months after treatment, the numbers of patients with non-decreasing KPS (increase and stable) in group A vs. group B were 22 (88.0%) vs. 20 (71.4%), 18 (72.0%) vs. 12 (48.1%), and 15 (62.5%) vs. 8 (30.8%) (p2 = 0.138, p4 = 0.044, p6 = 0.025). No significant differences were observed between two groups at the second month after the treatment, whereas at the fourth and sixth follow-up, the difference in QOL between two groups turned out to be significant (Table 3).
Complication
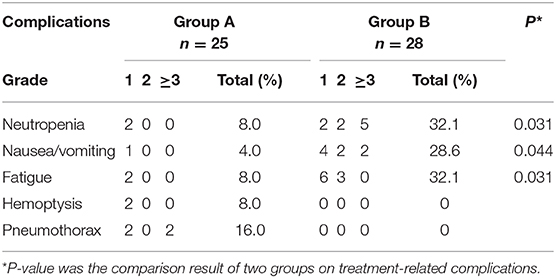
Treatment-related deaths were not observed in the two groups. The incidence rate of nausea/vomiting, neutropenia, and fatigue in group A was statistically lower than that in group B (Table 4). In group B, 5 patients (17.9%) had grade ≥3 neutropenia, and 2 (7.1%) had grade ≥3 nausea/vomiting. In group A, CT images showed four patients had pneumothorax, and two of them had pulmonary tissue compression of more than 30%, which needed catheter drainage, and recovered in 2–3 days. Since pulmonary tissue compression was <30% and no significant symptoms appeared, the other two pneumothorax patients did not receive any clinical treatment and recovered spontaneously in a week. In group A, one patient manifested self-limited hemoptysis for ~20 mL, and another one presented bloody sputum. The symptoms disappeared soon without any treatment.
Overall Survival
The median follow-up time was 13 months (range, 5–42 months). By the end of the follow-up period, 22 patients died in group A, and 25 patients died in group B. The 1-year OS and 2-year OS for group A vs. group B were 59.5vs. 62.6% and 23.9 vs. 22.3%, respectively. The median OS was 12.8 months (95% confidence interval, 10.5–15.1 months) in group A and 15.2 months (95% confidence interval, 12.2–18.2 months) in group B, with no significant difference (P = 0.847) (Figure 2).
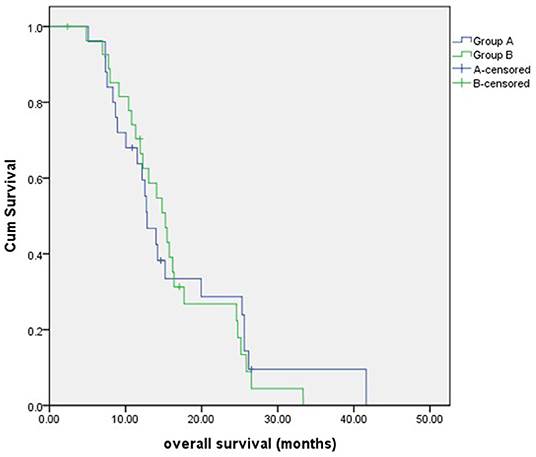
Figure 2. The overall survival of patients in two groups. There was no significant difference between groups A and B.
Discussion
The present study indicates that CT-guided RIS implantation is safe and feasible in NSCLC with oligorecurrence after failure of first-line chemotherapy and seems to provide a better long-term QOL in these patients compared with second-line chemotherapy.
At present, many patients with oligometastatic cancer receive systemic therapy, while their physical strength is usually impaired. In most situations, long-term survival could not be always achievable; thus, in order to allow them to live their remaining lives with a better QOL, less invasive local treatment strategies are desirable (13). Stereotactic body radiotherapy can provide a high level of local control with less associated adverse events, which has become one of the preferred modalities for local ablation of oligometastatic disease. 125I brachytherapy has been applied for primary treatment of cancer for decades and can also be called “stereotactic ablative brachytherapy.” 125I brachytherapy and SBRT have some similarities as a highly precise local therapy. 125I brachytherapy can deliver high radiation dose, because of accumulated radiation dose delivered continuously by 125I seeds and localized in the target tumor. Therefore, the adjacent normal tissues could be spared (16). However, a few complications associated with needle puncture should be noticed.
Recently, RIS implantation brachytherapy has been successfully used to treat diverse kinds of malignant tumors (17, 23–26). A large quantity of studies on RIS implantation for NSCLC have been reported with promising results. A small sample study suggests that 125I seed implantation for lung cancer patients was safe, and no complications were observed (27). A meta-analysis on 1,188 cases from 15 clinical studies suggests that 125I seed implantation combined with chemotherapy could improve the efficacy without increasing the incidence of adverse effects for advanced NSCLC (28).
In group A, the total incidence rate of treatment-related complications was lower than that in group B. Although pemetrexed and docetaxel are considered the standard second-line chemotherapy regimens (29), drug toxicity cannot be ignored. In a randomized controlled trial (30), patients who received second-line chemotherapy with docetaxel presented grade ≥3 adverse effects, including neutropenia (24%), leukopenia (11%), and febrile neutrophils reduction (7%), and those who cannot tolerate drug toxicity had to cease the treatment.
In this study, the incidence of myelosuppression in group A was significantly lower than that in group B (8.0 vs. 32.1%, P = 0.031). Actually, because the bone marrow is highly radiosensitive, external beam radiotherapy can cause suppression on bone marrow system (31). A study shows that for advanced NSCLC patients who received external beam radiotherapy, the incidence of grade 2 myelosuppression was 16.7% (32). In our study, the incidence of myelosuppression was 8.0%, which was lower than external beam radiotherapy caused. We speculated that it may be attributed to the short irradiation distance and low dose rate of 125I radioactive seeds, which reduce irradiation damage on hematopoietic cells in bone marrow. In addition, the toxicity induced by 125I seed, such as gastrointestinal reaction, radiation esophagitis, radiation pneumonia, and radiation dermatitis, is rarely observed, which is similar to previous studies (28).
Patients who received percutaneous RIS implantation have a high relative risk of pneumothorax. A study reports that seven patients with NSCLC were treated with percutaneous 103Pd or 125 I seed implantation, and two patients developed pneumothorax (33). In this study, four patients (16.0%) had pneumothorax in group A. Usually, the occurrence of pneumothorax may be related to tumor location, lung function, puncture needle diameter, rib obstruction, and respiratory movement. In this study, four patients all received repeated punctures, owing to rib blockage and respiratory movement.
Radioactive 125I seed implantation has advantages of relieving tumor-related symptoms and improving QOL (34). A study shows that, for advanced NSCLC patients after failure of first-line chemotherapy, 125I seed implantation could improve DCR and relieve chest pain, cough, and shortness of breath without increasing the incidence of treatment-related adverse effects (35). In this study, the difference in QOL between two groups manifested significant since the fourth-month follow-up. In the long run, 125I brachytherapy seems to provide a better long-term QOL than second-line chemotherapy. Computed tomography–guided 125I implantation is a minimally invasive treatment with a low incidence of complications, which may help improve the long-term QOL of patients. Meanwhile, we noted that the difference in QOL between two groups manifested significant since the fourth-month follow-up. And the accumulated dose that tumor absorbed increases as irradiation time moves along. Therefore, RIS implantation gradually showed an advantage over disease control since the fourth month.
Interestingly, similar phenomena were observed in terms of DCR. 125I brachytherapy is a type of low-dose-rate therapy because the dose delivered by RIS implanted permanently in place is <1 Gy per h. Meanwhile, 125I brachytherapy can also be treated as high-dose-rate therapies, in which the radiation is delivered over several courses (36, 37). The accumulated dose of tumor and efficacy increase as irradiation time moves along. Radioactive 125I seed has a half-life of nearly 2 months, meaning half of the energy is released in 2 months. The feature of 125I radioactive seed may be associated with the above result.
There are some limitations to our research. The sample size was small, and the duration of follow-up was short. Also, the retrospective aspect of this study could not provide sufficient evidence. Therefore, multicenter randomized controlled trails should be carried out in the future. One limitation that has to be pointed out is that RIS implantation is considered a local treatment, whereas second-line chemotherapy is a systemic therapy. However, when it comes to clinical significance, a treatment that is more effective for patients who failed first-line chemotherapy is the one that should win the most concern.
In summary, the present study indicates that RIS implantation is safe and feasible in NSCLC with oligorecurrence after failure of first-line chemotherapy and seems to provide a better long-term QOL in these patients compared with second-line chemotherapy.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HW, JL, and J-HG contributed to study concept and design. HW, X-TZ, and W-DJ contributed to acquisition of data. HW, J-HZ, C-HZ, and JL contributed to drafting of the manuscript. J-HG, YW, LC, and G-YZ contributed to analysis and interpretation of data. HW contributed to statistical analysis. J-HG and G-YZ supervised and oversaw the study. The corresponding authors had full access to all of the data and take full responsibility for the veracity of the data and the statistical analyses. All authors contributed to review and critical revision of the manuscript and approved the final version of the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (81520108015, 81827805, 81971716) and the Jiangsu Provincial Special Program of Social Development (BE2016783, SBK20190350). The funding sources had no role in the design, writing of the report, or decision to submit the paper for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank all the patients and the families in this study for their collaboration and support; Zhongda Hospital, Medical School, Southeast University, for his technical assistance and guidance for this study.
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
2. Mehta N, Mauer AM, Hellman S, Haraf DJ, Cohen EE, Vokes EE, et al. Analysis of further disease progression in metastatic non-small cell lung cancer: implications for locoregional treatment. Int J Oncol. (2004) 25:1677–83. doi: 10.3892/ijo.25.6.1677
3. Lasinska I, Kolenda T, Teresiak A, Lamperska KM, Galus L, Mackiewicz J. Immunotherapy in patients with recurrent and metastatic squamous cell carcinoma of the head and neck. Anticancer Agents Med Chem. (2019) 19:290–303. doi: 10.2174/1871520618666180910092356
4. Assi HI, Kamphorst AO, Moukalled NM, Ramalingam SS. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer Am Cancer Soc. (2018) 124:248–61. doi: 10.1002/cncr.31105
5. Iijima Y, Hirotsu Y, Amemiya K, Ooka Y, Mochizuki H, Oyama T, et al. Very early response of circulating tumour-derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non-small cell lung cancer. Eur J Cancer. (2017) 86:349–57. doi: 10.1016/j.ejca.2017.09.004
6. Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res. (2017) 6:196–211. doi: 10.21037/tlcr.2017.03.01
7. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. (1995) 13:8–10. doi: 10.1200/JCO.1995.13.1.8
8. Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. (2012) 83:878–86. doi: 10.1016/j.ijrobp.2011.08.036
9. Palma DA, Salama JK, Lo SS, Senan S, Treasure T, Govindan R, et al. The oligometastatic state - separating truth from wishful thinking. Nat Rev Clin Oncol. (2014) 11:549–57. doi: 10.1038/nrclinonc.2014.96
10. Gomez DR, Blumenschein GJ, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. (2016) 17:1672–82. doi: 10.1016/S1470-2045(16)30532-0
11. Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. (2018) 4:e173501. doi: 10.1001/jamaoncol.2017.3501
12. Wang Z, Gao SG, Xue Q, Guo XT, Wang LX, Yu X, et al. Surgery of primary non-small cell lung cancer with oligometastasis: analysis of 172 cases. J Thorac Dis. (2018) 10:6540–6. doi: 10.21037/jtd.2018.11.125
13. Otake S, Goto T. Stereotactic radiotherapy for oligometastasis. Cancers. (2019) 11:E133. doi: 10.3390/cancers11020133
14. Hiraki T, Kanazawa S. Lung radiofrequency ablation: potential as a therapy to oligometastasis and oligorecurrence. Pulm Med. (2012) 2012:196173. doi: 10.1155/2012/196173
15. Suh YG, Cho J. Local ablative radiotherapy for oligometastatic non-small cell lung cancer. Radiat Oncol J. (2019) 37:149–55. doi: 10.3857/roj.2019.00514
16. Chen Y, Jiang Y, Ji Z, Jiang P, Xu F, Zhang Y, et al. Efficacy and safety of CT-guided (125)I seed implantation as a salvage treatment for locally recurrent head and neck soft tissue sarcoma after surgery and external beam radiotherapy: a 12-year study at a single institution. Brachytherapy. (2019) 19:81–9. doi: 10.1016/j.brachy.2019.09.006
17. Zuber S, Weiss S, Baaske D, Schope M, Stevens S, Bodis S, et al. Iodine-125 seed brachytherapy for early stage prostate cancer: a single-institution review. Radiat Oncol. (2015) 10:49. doi: 10.1186/s13014-015-0349-0
18. Wang J, Chai S, Zheng G, Jiang Y, Ji Z, Guo F, et al. Expert consensus statement on computed tomography-guided (125)I radioactive seeds permanent interstitial brachytherapy. J Cancer Res Ther. (2018) 14:12–7. doi: 10.4103/jcrt.JCRT_888_17
19. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, DeSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. (2020) 21:e18–28. doi: 10.1016/S1470-2045(19)30718-1
20. Chen HH, Jia RF, Yu L, Zhao MJ, Shao CL, Cheng WY. Bystander effects induced by continuous low-dose-rate 125I seeds potentiate the killing action of irradiation on human lung cancer cells in vitro. Int J Radiat Oncol Biol Phys. (2008) 72:1560–6. doi: 10.1016/j.ijrobp.2008.07.038
21. Davis BJ, Horwitz EM, Lee WR, Crook JM, Stock RG, Merrick GS, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. (2012) 11:6–19. doi: 10.1016/j.brachy.2011.07.005
22. Monk BJ, Tewari KS, Puthawala AA, Syed AM, Haugen JA, Burger RA. Treatment of recurrent gynecologic malignancies with iodine-125 permanent interstitial irradiation. Int J Radiat Oncol Biol Phys. (2002) 52:806–15. doi: 10.1016/S0360-3016(01)02728-6
23. Raleigh DR, Seymour ZA, Tomlin B, Theodosopoulos PV, Berger MS, Aghi MK, et al. Resection and brain brachytherapy with permanent iodine-125 sources for brain metastasis. J Neurosurg. (2017) 126:1749–55. doi: 10.3171/2016.4.JNS152530
24. Han Q, Deng M, Lv Y, Dai G. Survival of patients with advanced pancreatic cancer after iodine125 seeds implantation brachytherapy: a meta-analysis. Medicine. (2017) 96:e5719. doi: 10.1097/MD.0000000000005719
25. Stewart A, Parashar B, Patel M, O'Farrell D, Biagioli M, Devlin P, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. (2016) 15:1–11. doi: 10.1016/j.brachy.2015.09.006
26. Han L, Li C, Wang J, He X, Zhang X, Yang J, et al. Iodine-125 radioactive seed tissue implantation as a remedy treatment for recurrent cervical cancer. J Cancer Res Ther. (2016) 12:C176–80. doi: 10.4103/0973-1482.200611
27. Heelan RT, Hilaris BS, Anderson LL, Nori D, Martini N, Watson RC, et al. Lung tumors: percutaneous implantation of I-125 sources with CT treatment planning. Radiology. (1987) 164:735–40. doi: 10.1148/radiology.164.3.3615870
28. Qiu H, Ji J, Shao Z, Wang J, Ma G, Zhang L, et al. The Efficacy and safety of Iodine-125 brachytherapy combined with chemotherapy in treatment of advanced lung cancer: a meta-analysis. J Coll Physicians Surg Pak. (2017) 27:237–45.
29. Durm G, Hanna N. Second-Line chemotherapy and beyond for non-small cell lung cancer. Hematol Oncol Clin North Am. (2017) 31:71–81. doi: 10.1016/j.hoc.2016.08.002
30. Herbst RS, Sun Y, Eberhardt WE, Germonpre P, Saijo N, Zhou C, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. (2010) 11:619–26. doi: 10.1016/S1470-2045(10)70132-7
31. Abravan A, Eide HA, Londalen AM, Helland A, Malinen E. Mapping bone marrow response in the vertebral column by positron emission tomography following radiotherapy and erlotinib therapy of lung cancer. Mol Imaging Biol. (2019) 21:391–8. doi: 10.1007/s11307-018-1226-7
32. Li W, Guan J, Yang L, Zheng X, Yu Y, Jiang J. Iodine-125 brachytherapy improved overall survival of patients with inoperable stage III/IV non-small cell lung cancer versus the conventional radiotherapy. Med Oncol. (2015) 32:395. doi: 10.1007/s12032-014-0395-8
33. Martinez-Monge R, Pagola M, Vivas I, Lopez-Picazo JM. CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC). Lung Cancer. (2008) 61:209–13. doi: 10.1016/j.lungcan.2007.12.016
34. Xiang Z, Wang L, Yan H, Zhong Z, Liu W, Mo Z, et al. (125)I seed brachytherapy versus external beam radiation therapy for the palliation of painful bone metastases of lung cancer after one cycle of chemotherapy progression. Onco Targets Ther. (2018) 11:5183–93. doi: 10.2147/OTT.S154973
35. Zhang T, Lu M, Peng S, Zhang W, Yang G, Liu Z, et al. CT-guided implantation of radioactive 125I seed in advanced non-small-cell lung cancer after failure of first-line chemotherapy. J Cancer Res Clin Oncol. (2014) 140:1383–90. doi: 10.1007/s00432-014-1655-x
36. Wang Y, Guo JH, Zhu GY, Zhu HD, Chen L, Lu J, et al. A Novel self-expandable, radioactive airway stent loaded with (125)I seeds: a feasibility and safety study in healthy beagle dog. Cardiovasc Intervent Radiol. (2017) 40:1086–93. doi: 10.1007/s00270-017-1639-8
Keywords: non–small cell lung cancer, oligometastases, oligorecurrence, 125I seed implantation, brachytherapy, chemotherapy
Citation: Wang H, Lu J, Zheng X-T, Zha J-H, Jing W-D, Wang Y, Zhu G-Y, Zeng C-H, Chen L and Guo J-H (2020) Oligorecurrence Non–small Cell Lung Cancer After Failure of First-Line Chemotherapy: Computed Tomography–Guided 125I Seed Implantation vs. Second-Line Chemotherapy. Front. Oncol. 10:470. doi: 10.3389/fonc.2020.00470
Received: 03 December 2019; Accepted: 16 March 2020;
Published: 15 April 2020.
Edited by:
Rupesh Kotecha, Baptist Hospital of Miami, United StatesReviewed by:
Martin Tom, Cleveland Clinic, United StatesMinesh P. Mehta, Baptist Health South Florida, United States
Copyright © 2020 Wang, Lu, Zheng, Zha, Jing, Wang, Zhu, Zeng, Chen and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-He Guo, amluaGVndW9Ac2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
 Hao Wang1†
Hao Wang1† Jian Lu
Jian Lu Chu-Hui Zeng
Chu-Hui Zeng Jin-He Guo
Jin-He Guo