
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 May 2020
Sec. Radiation Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00417
 John M. Varlotto1,2*
John M. Varlotto1,2* Isabel Emmerick2,3
Isabel Emmerick2,3 Rick Voland4
Rick Voland4 Malcom M. DeCamp5
Malcom M. DeCamp5 John C. Flickinger6
John C. Flickinger6 Debra J. Maddox7
Debra J. Maddox7 Christine Herbert2
Christine Herbert2 Molly Griffin2
Molly Griffin2 Paul Rava1,2
Paul Rava1,2 Thomas J. Fitzgerald1,2
Thomas J. Fitzgerald1,2 Paulo Oliveira2,8
Paulo Oliveira2,8 Jennifer Baima9
Jennifer Baima9 Rahul Sood8
Rahul Sood8 William Walsh2,7
William Walsh2,7 Lacey J. McIntosh2,10
Lacey J. McIntosh2,10 Feiran Lou2,3
Feiran Lou2,3 Mark Maxfield2,3
Mark Maxfield2,3 Negar Rassaei11
Negar Rassaei11 Karl Uy2,3
Karl Uy2,3Purpose: To identify the incidence, preoperative risk factors, and prognosis associated with pathologically positive lymph node (pN+) in patients undergoing a sub-lobar resection (SLR).
Methods: This is a retrospective study using the National Cancer Database (NCDB) from 2004 to 2014 analyzing SLR excluding those with any preoperative chemotherapy and/or radiation, follow-up <3 months, stage IV disease, or >1 tumor nodule. Multivariable modeling (MVA) was used to determine factors associated with overall survival (OS). Propensity score matching (PSM) was used to determine preoperative risk factors for pN+ in patients having at least one node examined to assess radiation's effect on OS in those patients with pN+ and to determine whether SLR was associated with inferior OS as compared to lobectomy for each nodal stage.
Results: A total of 40,202 patients underwent SLR, but only 58.3% had one lymph node examined. Then, 2,615 individuals had pN+ which decreased progressively from 15.1% in 2004 to 8.9% in 2014 (N1, from 6.3 to 3.0%, and N2, from 8.4 to 5.9%). A lower risk of pN+ was noted for squamous cell carcinomas, bronchioloalveolar adenocarcinoma (BAC), adenocarcinomas, and right upper lobe locations. In the pN+ group, OS was worse without chemotherapy or radiation. Radiation was associated with a strong trend for OS in the entire pN+ group (p = 0.0647) which was largely due to the effects on those having N2 disease (p = 0.009) or R1 resections (p = 0.03), but not N1 involvement (p = 0.87). PSM noted that SLR was associated with an inferior OS as compared to lobectomy by nodal stage in the overall patient population and even for those with tumors <2 cm.
Conclusion: pN+ incidence in SLRs has decreased over time. SLR was associated with inferior OS as compared to lobectomy by nodal stage. Radiation appears to improve the OS in patients undergoing SLR with pN+, especially in those with N2 nodal involvement and/or positive margins.
In 1995, a landmark investigation by the Lung Cancer Study Group demonstrated that sub-lobar resection (SLR) was inferior to lobectomy (tumor size <3 cm), reporting an increase in recurrence and trend toward decreased survival in patients undergoing SLR for non-small-cell lung cancer (NSCLC) (1). Subsequently, SLRs are often reserved for patients who cannot tolerate larger pulmonary resection (e.g., lobectomy) due to marginal pulmonary function tests and other comorbidities. Although there are several different definitions of adequate lymph node dissection (2), none pertains particularly to patients undergoing SLR.
Even with clinical stage I NSCLC in the era of PET-CT staging, past reports have indicated that as many as 10–20% of patients may have microscopic nodal involvement (3–6). Unfortunately, as many as 40–70% of patients undergoing SLR have not had a single lymph node removed or examined (7–10). Nevertheless, one recent retrospective review using the SEER database noted a lung cancer-specific survival (LCSS) and overall survival (OS) benefit with more extensive lymph node dissection in patients who received a sub-lobar excision (11).
The purpose of our study is to identify preoperative factors associated with lymph node involvement so that it could be better understood which patients would preferentially benefit from a lymphadenectomy and to assess whether patients with pathologically positive lymph nodes (pN+) may benefit from radiation therapy. We investigate the incidence of pN+ in patients undergoing SLR and whether SLR was associated with inferior OS in patients with pN+.
Data for this study were derived from the National Cancer Database (NCDB) registry for patients diagnosed between 2004 and 2014. The NCDB currently captures 70% of all newly diagnosed malignancies in the United States annually. The patient information was de-identified. Thus, this investigation was exempted from institutional review board (IRB) approval. Individuals included were those undergoing SLR (sub-lobar NOS, wedge resection, segmentectomy) for stages I–III NSCLC. We also included patients undergoing (bi)lobectomy (N = 107,193) for the propensity score matching (PSM) to patients undergoing SLR so that the OS could be compared for each nodal level depending upon surgical resection. Exclusion criteria were any preoperative chemotherapy and/or radiation, follow-up <3 months, stage IV disease, >1 tumor nodule, or missing data on the timing of adjuvant therapy. To ensure that patients were receiving adjuvant and not salvage radiation, chemotherapy or postoperative radiation (PORT) had to be initiated within 120 days of surgery. PORT was permitted to start within 240 days after surgery if chemotherapy was started within the 120-day interval of surgery. Full selection criteria can be seen in Figure 4 in the Supplementary Material.
The outcomes were (a) pN+ involvement and (b) OS in those with lymph node involvement.
To identify the factors associated with pathologic lymph node involvement (among those who had any lymph node examined), it was performed as a bivariate and multivariate analysis considering the following variables: age, sex, race (non-Hispanic white, white Hispanic, black, other), location [right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), left upper lobe (LUL), left lower lobe (LLL), other, NOS], histology [adenocarcinoma, bronchioloalveolar adenocarcinoma (BAC), adenosquamous cell carcinoma, large cell carcinoma, NSCLC NOS, squamous cell carcinoma], facility type (community program, academic research institute, comprehensive community cancer program, integrated), facility location (New England, East North Central, East South Central, Middle Atlantic, Mountain, Pacific, South Atlantic, West North Central, West South Central), insurance (unknown, private, Medicaid, Medicare, no insurance, government), income (<$38,000; $38,000–$48,000; >48,000–$63,000; >$63,000), area (rural near or not near metropolitan area, metropolitan area >1,000,000 population, metropolitan area > or <250,000, urban > or <20,000 near metropolitan area, urban > or <20,000 not near metropolitan area), type of resection (segmentectomy, wedge, sub-lobar NOS), Charlson comorbidity status as adapted by Deyo et al. (12) and tumor size.
To assess the effect of radiation on OS in those with pN+, all of the preoperative factors were considered. Additionally, the following factors were included: surgical margins (R0 clear, R1 microscopically positive, R2 grossly positive), lymphatic vascular invasion, tumor grade (well, moderate, poor, undifferentiated/anaplastic), number of nodes examined, number of pN+, radiation dose, chemotherapy, hospital readmission, length of stay, and T/N stage. These same factors for OS were also used in the PSM comparing lobectomy vs. SLR per N stage.
1. pN+ or node involvement or positive nodes refers to pathologic node involvement regardless of clinical enlargement, i.e., enlarged CT and/or fluorodeoxyglucose (FDG) avid.
2. cN+ refers to clinically enlarged nodes regardless of pathologic involvement. cN+ is the only term for clinical involvement. Of note, some patients could have had biopsy-positive nodes prior to surgical resection and be classified as clinical node involvement as per NCDB staging rules.
The propensity match for the evaluation of factors predicting pN+ was performed only in the patients who had at least one node examined and clinically negative nodes. These propensity scores were created through logistic regression [area under the receiver operating characteristic (AUROC) = 0.8552] and matched for type of surgical resection and number of nodes examined.
The propensity match for comparison of the SLR and lobectomy groups by node stage were matched by age, sex, pathologic t-stage, number of nodes examined, number of positive nodes, tumor size, histology group (squamous, adenocarcinoma, and other), lymphovascular invasion (LVI), and Charlson comorbidity status.
Multivariate analysis for OS was performed in the pN+ population using proportional hazard Cox regression.
The propensity scores to assess the effects of radiation on the OS and the OS by surgical type by node stage were both performed only on patients with pN+ and matched by factors that were significant in the MVA for OS (age, sex, race, tumor location, histology, facility type, facility location, insurance status, income, urban/rural location, type of surgical resection, N stage, and tumor size). The Toolkit for Weighting and Analysis of Non-equivalent Groups (TWANG) macro was run in SAS to create the propensity scores for this pathologically node-positive group (13).
A total of 40,202 patients received SLR during the period analyzed. Then, 23,440 patients (58.3%) had at least one lymph node examined, and 2,615 of them were found to have pN+.
Of those having at least one node examined, the median number of nodes examined was 4 (1–83), and 11.2% of patients had positive nodes. Table 1 presents the percentage of patients with pN+ per analyzed variable for patients having at least one node examined. Facility type and location were significantly associated with having positive nodes. Academic research programs had a lower incidence of node involvement (10.28%) as compared to community hospitals and integrated network cancer programs (11.36–12.86%). Patients in New England had the lowest incidence of node involvement (9.14%), while the west south central area had the highest incidence of node involvement (15.08%). Personal characteristics (age, sex, and race) were all associated with node involvement. Patients who were younger than 65 years had a higher rate of nodal positivity than older (13.83 vs. 10.17%), while white non-Hispanic patients (10.82%) had the lowest incidence of all races, and women had a lower rate than men (10.48 vs. 12.01%). Both T stage and resection type (R0, R1, R2) were associated with a significant rise in node positivity with stage (T1, 8.93%; T2–T4, 18.45–18.27%), (R0, 10.09%; R1, 24.32%; R2, 34.11%). As differentiation decreased, so did the percentage of patients with nodal positivity (3.87–18.15%). Tumor size and lymphatic vascular invasion were also significantly associated with nodal positivity. Histology was significantly associated with nodal positivity with BAC (4.75%) and squamous cell carcinoma (8.53%) having the lowest risk, and large cell (16.16%) and NSCLC-NOS (18.44%) having the highest risk. Charles Mayo score was inversely associated with nodal positivity. Patients receiving their care through Medicare had the lowest rate of positive nodes (10.02%). SLR-NOS had a much greater rate of nodal positivity (19.1%) than segmentectomy (10.62%) and wedge resection (11.02%). There was no significant association with nodal positivity based upon geographic location or income.
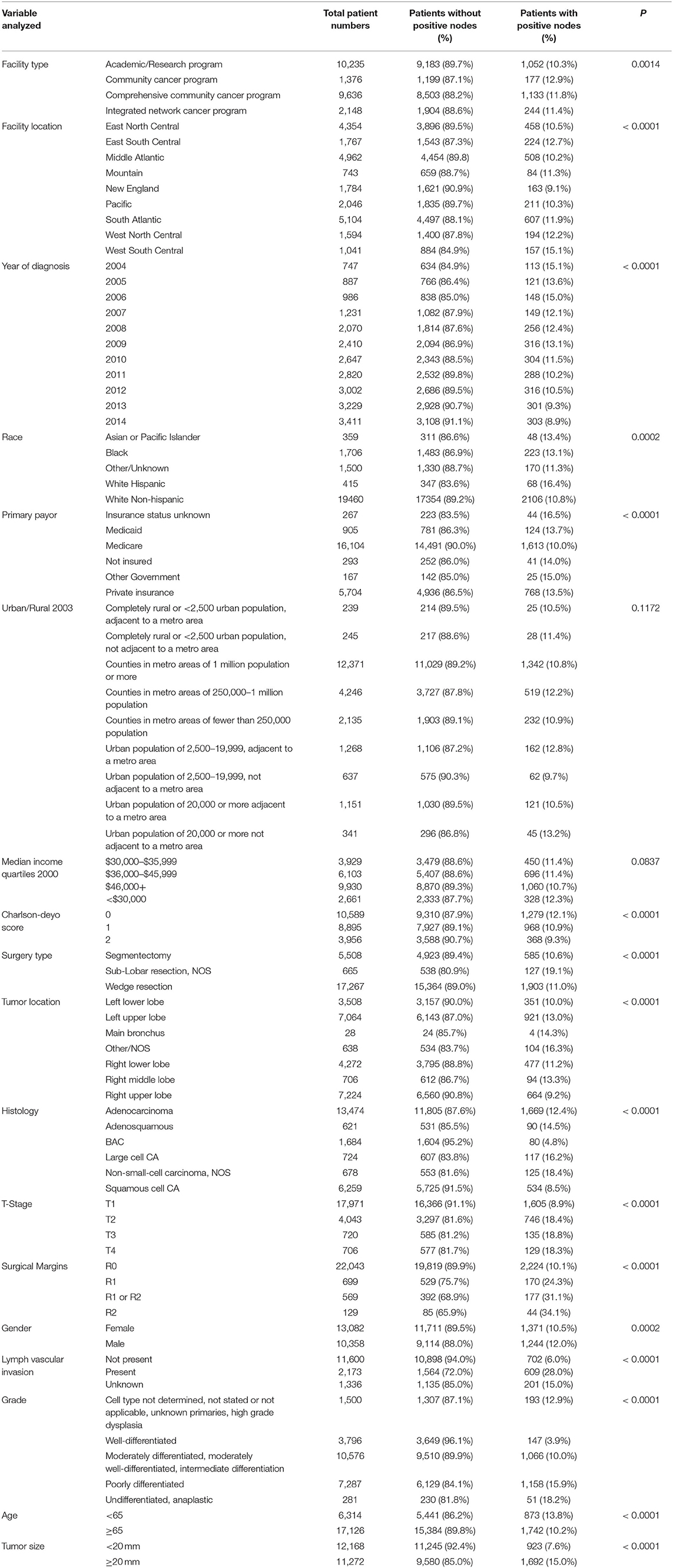
Table 1. Percentage of patients having pathological nodal positivity by analyzed factor for patients having at least one node examined.
When clinically node-negative patients with (5.2% of these patients) and without pathologic node involvement were matched by PSM for type of resection and number of nodes examined, the factors that were associated with pN+ are listed by the factor and its associated odds ratio. Non-RUL locations remained significantly associated with node involvement: LLL, 1.59 (1.28–1.97); LUL, 1.71 (1.42–2.06); RLL, 1.77 (1.44–2.17); RML, 1.78 (1.20–2.64); and other locations, 2.50 (1.70–3.70). Likewise, squamous cell carcinoma 0.51 (0.43–0.62) and BAC 0.34 (0.23–0.49) histologies were significantly less likely to have node involvement, and tumor size was positively associated with node involvement 1.02 (1.014–1.031). However, non-traditional factors associated with nodal involvement included comprehensive community cancer center vs. academic research program, 1.18 (1.012–1.374), and white Hispanic vs. white non-Hispanic ethnicity, 1.68 (1.11–2.54). A total of 762 patients had clinical N1, 930 had clinical N2, and 82 had clinical N3 involvement, and the percentage of patients with pathologic lymph node involvement was 64, 71, and 38%, respectively.
The OS of patients receiving SLR and at least one node examined by node stage can be seen in Figure 1A. There are 15,875, 793, 1,173, and 29 patients with N0, N1, N2, and N3 nodes. Despite the inadequate surgical treatment of the primary tumor, the OS still sharply decreased by node stage. Figure 1B shows that OS by node stage is significantly less for the SLR group compared to the lobectomy group without propensity matching. After propensity matching, the SLR group still has lower OS for the entire surgical population (Figure 1C) and for the subset of patients with tumors < = 2 cm (Figure 1D). See Supplementary Material for Figures S1B–D including N3 nodes.
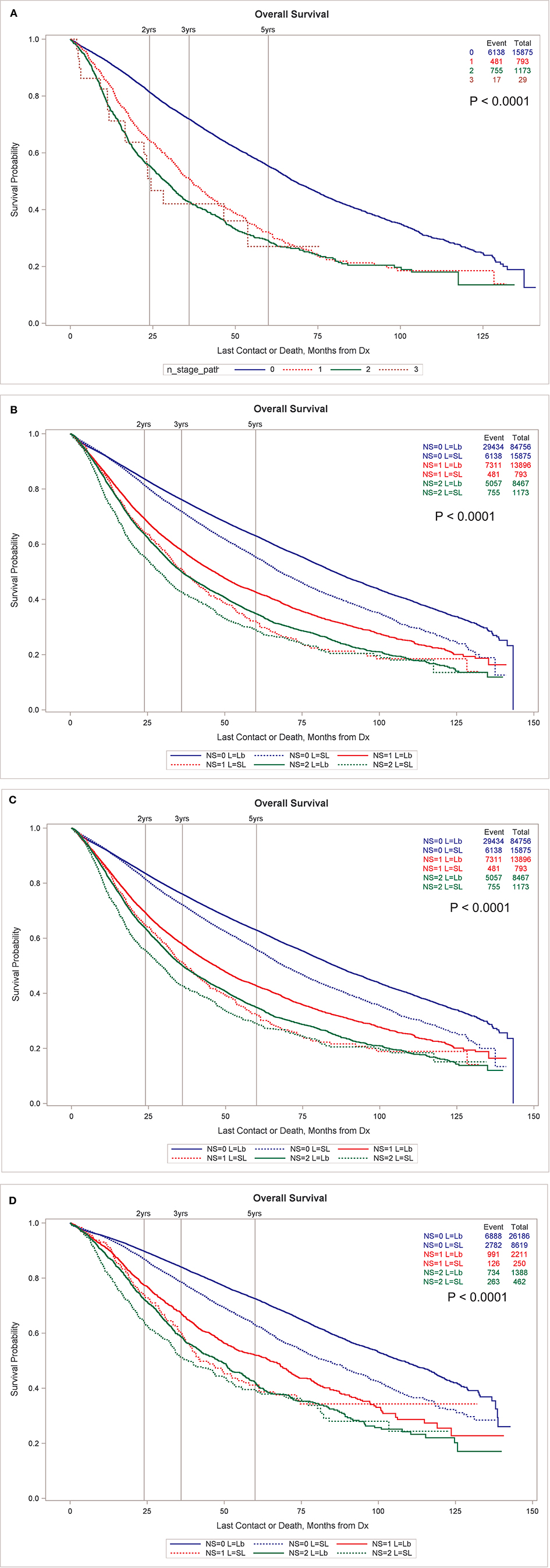
Figure 1. (A) OS by node stage in patients undergoing SLR. (B) OS by node stage by type of resection, sub-lobar vs. lobectomy, unmatched. Propensity match for OS by node stage by type of resection for all resected tumors (C) and only for those <2 cm in size (D). All node stages are pathologic in this figure. (B–D) exclude N3 nodes for clarity. (B–D) Including N3 nodes are available in Supplementary Material.
Table 2 demonstrates that the risk of patients having pN+ decreased during the years of our study from 2004 to 2014 in all node stages. The risk of any positive nodes (15.13–8.88%) as well as N1 nodes (6.29–2.96%), N2 (8.43–5.86%), and N3 (0.4–0.06%) all decreased during the years of our study. Supplemental Table 2 notes the incidence of pN+ in patients who are not cN+.
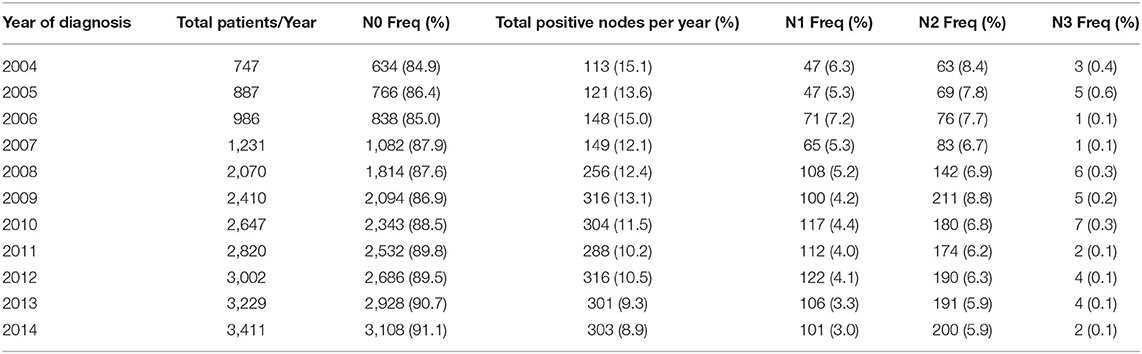
Table 2. Percentage of patients with pathologically N1, N2, and N3 node involvement during years 2004–2014.
Multivariate analysis for OS for patients having pN+ can be seen in Table 3. Supplemental Table 3 includes the MVA for OS in patients having only pN+ without cN+. Personal characteristics associated positively with OS include younger age, female sex, Asian/Pacific Islander vs. non-Hispanic white, and having a 0 for Charlson comorbidity status. Histologic factors associated with survival included tumor size, number of positive nodes, number of nodes examined, higher T stage, poor differentiation, and lymphatic vascular invasion. Treatment factors associated with OS include not having radiation or chemotherapy and length of stay.
A propensity match to assess the effects of radiation on OS was performed only on patients with pN+ and matched by factors that were significant in the MVA for OS (age, sex, race, tumor location, histology, facility type, facility location, insurance status, income, urban/rural location, type of surgical resection, N stage, and tumor size). The standardized differences between the patients receiving and not receiving radiation before and after the match can be seen in Figure 2, demonstrating the successful alignment of prognostic factors after the match. After propensity matching, OS curves were generated for the entire population and the subgroups with negative margins and positive margins, as can be seen in Figures 3A–E. When an adequate radiation dose of >44 Gy was given, OS was significantly better in the entire node-positive group (p = 0.0047; Figure 3A), those with either N1 or N2 nodal involvement and R0 resection (0.0092; Figure 3B), those with N2 nodal involvement and R0 resection (p = 0.0030; Figure 3D), and those with N1 or N2 node involvement and an R1 resection (p = 0.0034; Figure 3E), but not in those with N1 nodal involvement and R0 resection (p = 0.707; Figure 3C).
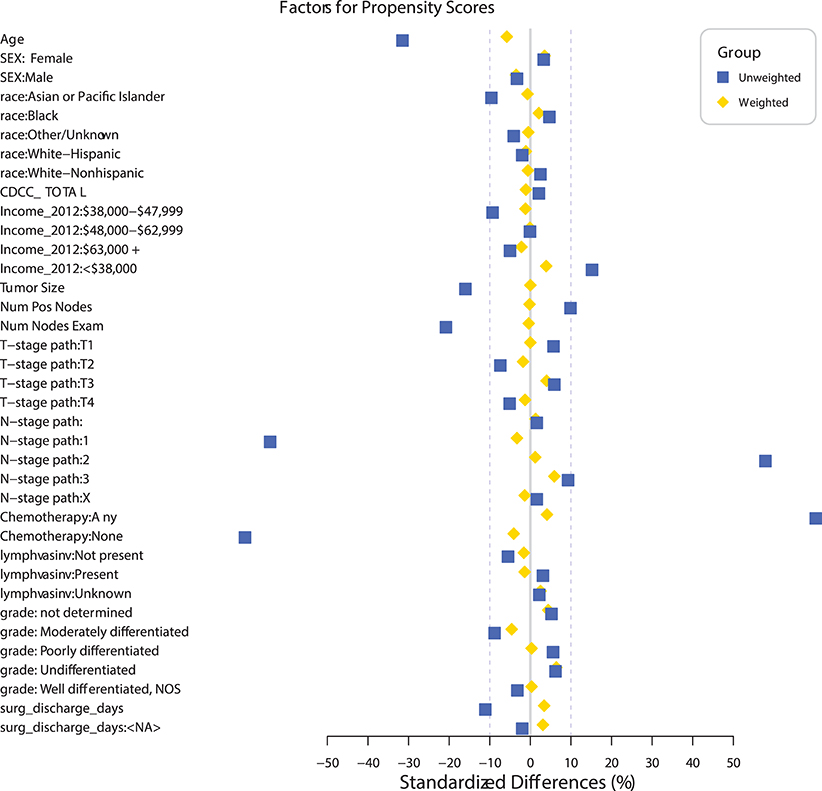
Figure 2. Standardized differences before and after propensity matching between the groups receiving and not receiving radiation in patients undergoing sub-lobar resection with at least one pathologically positive lymph node (pN+).
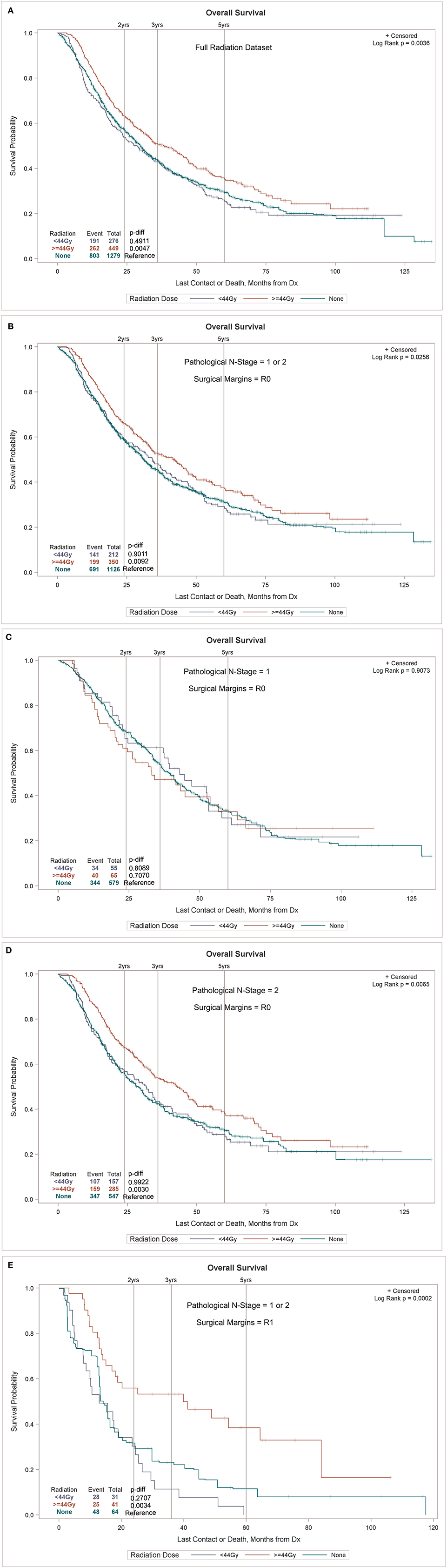
Figure 3. Overall survival (OS) of the sub-lobar resection (SLR) group with pathologically positive lymph node (pN+) with or without radiation in the entire node-positive population (A) and in the subgroups with involvement of both N1 and N2 node involvement (B), N1 node involvement (C), N2 node involvement (D), and with positive surgical margins (E).
During the years of our study 2004–2014, 40,202 patients underwent SLR, but similar to another recent series of patients receiving SLR, only a small majority of patients had at least one lymph node examined, 58.3% in our population and 55.4% in the past series (11). Then, 2,615 patients or 11.2% of the 23,440 patients having one node examined had at least one pN+. The percentage of patients with pN+ decreased steadily during the years of our study from 15.1 to 8.9%. Likewise, the percentage of pN+ decreased in patients with both N1 and N2 disease.
The propensity match for preoperative factors for pN+ did not include differentiation because this information is often not present with biopsies and can be inaccurate. Small biopsies often contain scant tumor cells, crush artifact, and/or necrosis, so differentiation is often difficult to obtain (14). We did not include cN+ in our models because we feel that all clinically enlarged nodes should be assessed for nodal involvement prior to consideration of any planned surgical procedure. Our investigation noted that of the patients presenting with clinically enlarged nodes, 66.5% had positive nodes. Central location is a known risk factor for lymph node involvement, but this risk factor was not readily available for all populations in this large database, and it should be noted that this definition does vary in the literature (15). Furthermore, one recent large series of 938 patients noted that those with centrally located tumors (defined the inner two thirds of the lung on CT) were not at increased risk of lymph node involvement (16). Our propensity match noted that white Hispanic patients have an increased risk of node involvement. There is conflicting literature in regard to white Hispanic patients' risk of node involvement compared to other races (17, 18). We do not know of any other series that demonstrates that the RUL location has a lower risk of node involvement except for one series using the SEER database that demonstrated a significantly lower risk of node involvement with right upper as compared to right lower lobe locations (18). Since central location is associated with a higher chance of lymph node involvement (19) and this factor is unavailable in NCDB, we speculate that the RUL location possibly had more tumors located peripherally within the lung. Of course, smaller tumor size (20) and BAC have been associated with a lower risk of lymph node involvement (21). However, it must be acknowledged that BAC as classified in this database is a heterogeneous category since a multi-society committee reclassifed BAC into new categories of adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), invasive lepidic adenocarcinoma, and invasive mucinous adenocarcinoma (22), so we expect tumors classified as BAC in our project to represent a heterogeneous group with a relatively low risk of pN+. Squamous cell carcinoma was also found to have a lower risk of node involvement as noted previously (23–25). Comprehensive community cancer centers had a higher risk of lymph node involvement [odds ratio (OR) = 1.18] as compared to those undergoing SLRs at an academic research institution.
In our population of patients with SLR having at least one node examined, it was noted that the nodal stage was still predictive of OS, with OS decreasing as pathologic nodal stage increased. Even after propensity matching the patients with SLR to those with lobectomy, it was noted that OS with SLR was significantly less for all pathologic node stages. This indicates that suboptimal surgery is a major prognostic factor that is associated with a survival decrement regardless of the extent of node involvement.
Our PSM for radiation was well-balanced for patients receiving and not receiving radiation and was matched for all major prognostic factors including LVI, age, gender, chemotherapy use, tumor size, T stage, N stage, Charlson comorbidity status, tumor grade, number of positive nodes, etc. When analyzed by an adequate radiation dose of >44 Gy, radiation was associated with a significant survival increase in all patients with pathologically involved nodes, particularly those with positive margins and N2 involvement. Radiation had no effect on OS in the patients with margin-negative disease with pathologic N1 involvement.
The role of postoperative radiation has not been definitely proven in patients with or without optimal surgical resection. We know of no other studies assessing the role of radiation therapy in patients treated with SLR with pN+. There are currently no recommendations for this situation. Because of the limited number of patients with SLR who are found to have positive nodes, the role of radiation in this setting will likely never be proven. Our investigation has noted that similar to patients undergoing lobectomy/pneumonectomy, OS decreases with higher pathologic nodal stage. Because our results echo the current National Comprehensive Cancer Network (NCCN) guideline recommendations concerning PORT in patients receiving optimal surgery, we believe SLR does not change the current indications for PORT despite the known inferior OS and local recurrence risk in this situation. The NCCN guidelines note that PORT appears to improve OS in patients with N2 disease and/or positive margins but states that radiation is not recommended for N0 or N1 disease (26). The PORT meta-analysis (27) of prospective trials using outdated radiation techniques demonstrated a possible survival benefit noted in the N2 population (26) in the era prior to the proven role of adjuvant chemotherapy. Similarly, a retrospective study-assembled data prior to the proven role of adjuvant chemotherapy also noted the beneficial effects of radiation on survival in those having N2 nodal involvement (28). However, in the setting of adjuvant chemotherapy and better staging, the beneficial role of radiation in patients having N2 involvement have been mixed in more recent retrospective studies (29–31). We speculate that these more modern studies did not assess patients by factors associated with local recurrence which caused the variable results. Indeed, one recent retrospective series noted that node stage was related to distal recurrence and OS, but not local recurrence (32). In regard to positive margins, radiation therapy has been suggested to improve OS whether it is given concurrently or after chemotherapy (33, 34). Of interest, a recent randomized phase II study assessed the benefits of postoperative concurrent chemo/radiation vs. post-operative chemotherapy only in patients undergoing complete resection [>90% (bi)lobectomy] of N2 involved NSCLC and noted no OS or disease-free survival benefit to radiation therapy (35). Due to small patient numbers in our study, the question of sequencing could not be entertained in our investigation.
There are many shortcomings with our investigation. The data used in this study are retrospective, but they cover ~70% of all newly diagnosed malignancies in the United States annually. Important information is missing such as the rationale for treating these patients with SLR and the presurgical performance status. Furthermore, it is not known why some patients received suboptimal doses of radiation. Suboptimal radiation may have been given due to normal tissue constraints, tumor progression, or other unknown reasons. Other important information was missing including pulmonary function tests, tumor location (central vs. peripheral), pack years of smoking, extent of preoperative and postoperative workup, and type of chemotherapy. However, despite the limitations of the data, there is no other study that we know that assesses the role of radiation in patients with pathologically positive nodes receiving SLR, demonstrates the incidence of pN+ in patients undergoing SLR, and shows the inferior OS of patients with pN+ undergoing SLR as compared to (bi)lobectomy.
It should be noted that the authors of this manuscript are not endorsing SLR in patients with pN+. The purpose of our investigation is to provide guidance when patients fall under these unique, less than optimal circumstances. Furthermore, because of the inferior OS associated with SLR as compared to (bi)lobectomy when pN+, we feel that aggressive surgical staging prior to surgery can spare this suboptimal outcome, especially with improving OS noted when consolidative durvalumab follows chemo/radiation in patients with N2 involvement (36).
Although the incidence of pathologically positive nodes has been falling during the years of our study, positive nodes were present in close to 9% of patients undergoing SLR during the last year of our study in the small majority of patients who had one or more nodes examined. Over 40% of patients undergoing SLR had no nodes examined. Radiation, when given with adequate doses, appears to improve the OS in patients undergoing SLR with pathologic nodal positivity, especially in those with N2 nodal involvement and/or positive margins.
The datasets generated for this study are available on request to the corresponding author.
Data for this study were derived from the National Cancer Database (NCDB) registry, the patient information was de-identified, thus this investigation was exempted from IRB approval.
KU, JV, IE, RV, and JF contributed to conceptualization. IE and JV contributed to data curation and contributed to the methodology and project administration. MD, KU, IE, RV, JF, DM, TF, PO, JB, RS, WW, LM, FL, and NR performed formal analysis. TF and JV contributed to funding acquisition. TF, JV, and IE contributed to procuring the resources. IE handled the software and performed validation. JV, MD, and RV supervised. All authors performed the investigation and contributed to writing the original draft. All authors contributed to writing, reviewing, and editing the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer TK declared a past co-authorship with one of the author MD to the handling Editor.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00417/full#supplementary-material
1. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer: lung cancer study group. Ann Thorac Surg. (1995) 60:615–22. doi: 10.1016/0003-4975(95)00537-U
2. Smeltzer MP, Faris NR, Ray MA. Association of pathologic nodal staging quality with survival among patients with non-small cell lung cancer after resection with curative intent. JAMA Oncol. (2017) 4:80–8. doi: 10.1001/jamaoncol.2017.2993
3. Licht PB, Jørgensen OD, Ladegaard L. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg. (2013) 96:943–9; discussion 949–50. doi: 10.1016/j.athoracsur.2013.04.011
4. Krantz SB, Lutfi W, Kuchta K. Improved lymph node staging in early-stage lung cancer in the National Cancer Database. Ann Thorac Surg. (2017) 104:1805–14. doi: 10.1016/j.athoracsur.2017.06.066
5. Heineman DJ, Ten Berge MG, Daniels JM. Clinical staging of stage I non-small cell lung cancer in the netherlands-need for improvement in an Era with expanding nonsurgical treatment options: data from the dutch lung surgery audit. Ann Thorac Surg. (2016) 102:1615–21. doi: 10.1016/j.athoracsur.2016.07.054
6. Xiong J, Wang R, Sun Y. Clinical analysis of sixty-four sixty-four patients with T1aN2M0 stage non-small cell lung cancer who had undergone resection. Thorac Cancer. (2016) 7:215–21. doi: 10.1111/1759-7714.12314
7. Kent M, Landreneau R, Mandrekar S. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. Ann Thorac Surg. (2013) 96:1754–5. doi: 10.1016/j.athoracsur.2013.05.104
8. Khullar OV, Gillespie T, Nickleach DC. Socioeconomic risk factors for long-term mortality after pulmonary resection for lung cancer: an analysis of more than 90,000 patients from the National Cancer Data Base. J Am Coll Surg. (2015) 220:156–68.e4. doi: 10.1016/j.jamcollsurg.2014.10.009
9. Razi SS, John MM, Sainathan S. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res. (2016) 200:683–9. doi: 10.1016/j.jss.2015.08.045
10. Thomas PA. Lymph node dissection during sublobar resection: why, when, and how? J Thor Dis. (2018) 10:1145–50. doi: 10.21037/jtd.2018.01.30
11. Cao J, Xu J, He Z. Prognostic impact of lymphadenectomy on outcomes of sublobar resection for stage IA non-small cell lung cancer <2cm. J Thorac Cardiovasc Surg. (2018) 156:796–805.e4. doi: 10.1016/j.jtcvs.2018.03.122
12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. (1992) 45:613–9. doi: 10.1016/0895-4356(92)90133-8
13. Griffin BA, Ridgeway G, Morral AR. Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG). Santa Monica, CA, RAND Corporation (2014).
14. Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol. (2012) 25(Suppl 1):S18–30. doi: 10.1038/modpathol.2011.150
15. Fiorelli A, Sagan D, Mackiewicz L Incidence risk factors and analysis of survival of unexpected N2 disease in stage I non-small cell lung cancer. Thorac Cardiovasc Surg. (2015) 63:558–67. doi: 10.1055/s-0034-1399764
16. Farjah F, Lou F, Sima C. A prediction model for pathologic N2 disease in lung cancer patients with a negative mediastinum by positron emission tomography. J Thorac Oncol. (2013) 8:1170–80. doi: 10.1097/JTO.0b013e3182992421
17. Weksler B, Kosinski AS, Burfeind WR. Racial and ethnic differences in lung cancer surgical stage: an STS Database Study. Thorac Cardiovasc Surg. (2015) 63:538–43. doi: 10.1055/s-0035-1546295
18. Varlotto JM, McKie K, Voland RP. The role of race and economic characteristics in the presentation and survival of patients with surgically-resected non-small cell lung cancer. Front Oncol. (2018) 8:146. doi: 10.3389/fonc.2018.00146
19. Boada M, Guzman R, Montesinos M. Centrality and survival in early-stage non-small cell lung cancer video-assisted surgery. Lung Cancer. (2019). 134:254–8. doi: 10.1016/j.lungcan.2019.06.030
20. Veeramacnaneni NK, Battafarano RJ, Meyers BF. Risk factors for occult nodal metastasis in clinical T1N0 lung cancer: a negative impact on survival. Eur J Cardiothorac Surg. (2008) 33:466–9. doi: 10.1016/j.ejcts.2007.12.015
21. Sakurai H, Dobashi Y, Mizuntani E. Bronchioloalveolar carcinoma of the lung 3cm or less in diameter: a prognostic assessment. Ann Thorac Surg. (2004) 78:1728–33. doi: 10.1016/j.athoracsur.2004.05.017
22. Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European Respiratory Society International Multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2011) 6:244–85.
23. Cho S, Song IH, Yang HC. Predictive factors for node metastasis in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg. (2013) 96:239–45. doi: 10.1016/j.athoracsur.2013.03.050
24. Deng HY, Zeng M, Li G. Lung adenocarcinoma has a higher risk of lymph node metastasis than squamous cell carcinoma: a propensity score-match analysis. World J Surg. (2019) 43:955–62. doi: 10.1007/s00268-018-4848-7
25. Wang J, Welch K, Wang L. Negative predictive value of positron emission tomography and computed tomography for stage I T1-T2N0 non-small cell lung cancer: a meta-analysis. Clin Lung Cancer. (2012) 13:81–9. doi: 10.1016/j.cllc.2011.08.002
26. NCCN Guidelines. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
27. PORT. Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-Anal Trialists Group Lancet. (1998) 352:257–63. doi: 10.1016/S0140-6736(98)06341-7
28. Lally BE, Zeterman D, Colasanto JM. Post-operative radiotherapy for stage II or III non-small cell lung cancer using the surveillance epidemiology, and end results database. J Clin Oncol. (2006) 24:2998–3006. doi: 10.1200/JCO.2005.04.6110
29. Douillard JY, Rosell R, De Lena M. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant. Navelbine Int Trialist Assoc ANITA randomized trial. Int J Radiat Oncol Biol Phys. (2008) 72:695–701. doi: 10.1016/j.ijrobp.2008.01.044
30. Robinson CG, Patel AP, Bradley JD. Postoperative radiotherapy for pathologic N2 non-small cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Database. J Clin Oncol. (2015) 33:870–6. doi: 10.1200/JCO.2014.58.5380
31. Wisnivesky JP, Halm EA, Bonomi M, Smith C, Mhango G, Bagiella E. Postoperative Radiotherapy for Elderly Patients with Stage III Lung Cancer. Cancer. (2012) 118:4478–85. doi: 10.1002/cncr.26585
32. Varlotto JM, Yao AN, DeCamp MM. Nodal stage of surgically-resected non-small cell lung cancer and its effect on recurrence patterns and overall survival. Int J Radiat Oncol Biol Phys. (2015) 91:765–73. doi: 10.1016/j.ijrobp.2014.12.028
33. Wang EH, Corso CD, Ruttler CE. Postoperative radiation therapy is associated with improved overall survival in incompletely-resected stage II and III non-small cell lung cancer. J Clin Onc. (2015) 33:2727–34. doi: 10.1200/JCO.2015.61.1517
34. Francis S, Orton A, Stoddard G. Sequencing of postoperative radiotherapy and chemotherapy for locally advanced or incompletely resected non-small-cell lung cancer. J Clin Oncol. (2018) 36:333–41. doi: 10.1200/JCO.2017.74.4771
35. Sun JM, Noh JM, Kim HK, Lee SH, Choi YS, Pyo H, et al. Randomized phase II trial comparing chemoradiotherapy with chemotherapy for completely resected unsuspected N2-positive non–small cell lung cancer. J Thorac Oncol. (2017) 12:1806–13. doi: 10.1016/j.jtho.2017.09.1954
Keywords: sub-lobar resection (SLR), radiation, node positive, incidence, lung cancer
Citation: Varlotto JM, Emmerick I, Voland R, DeCamp MM, Flickinger JC, Maddox DJ, Herbert C, Griffin M, Rava P, Fitzgerald TJ, Oliveira P, Baima J, Sood R, Walsh W, McIntosh LJ, Lou F, Maxfield M, Rassaei N and Uy K (2020) The Incidence of Node-Positive Non-small-Cell Lung Cancer Undergoing Sublobar Resection and the Role of Radiation in Its Management. Front. Oncol. 10:417. doi: 10.3389/fonc.2020.00417
Received: 09 January 2020; Accepted: 10 March 2020;
Published: 26 May 2020.
Edited by:
Rupesh Kotecha, Baptist Hospital of Miami, United StatesReviewed by:
Martin Tom, Cleveland Clinic, United StatesCopyright © 2020 Varlotto, Emmerick, Voland, DeCamp, Flickinger, Maddox, Herbert, Griffin, Rava, Fitzgerald, Oliveira, Baima, Sood, Walsh, McIntosh, Lou, Maxfield, Rassaei and Uy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John M. Varlotto, am9obi52YXJsb3R0b0B1bWFzc21lbW9yaWFsLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.