
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 April 2020
Sec. Cancer Genetics
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00385
This article is part of the Research Topic Next Generation Sequencing Based Diagnostic Approaches in Clinical Oncology View all 19 articles
 Ekaterina A. Zhigalova1†
Ekaterina A. Zhigalova1† Anna I. Izosimova2†
Anna I. Izosimova2† Diana V. Yuzhakova2†
Diana V. Yuzhakova2† Lilia N. Volchkova2
Lilia N. Volchkova2 Irina A. Shagina3,4
Irina A. Shagina3,4 Maria A. Turchaninova2,3,4
Maria A. Turchaninova2,3,4 Ekaterina O. Serebrovskaya2,3,4
Ekaterina O. Serebrovskaya2,3,4 Elena V. Zagaynova2
Elena V. Zagaynova2 Dmitriy M. Chudakov1,2,3,4*
Dmitriy M. Chudakov1,2,3,4* George V. Sharonov2,3,4
George V. Sharonov2,3,4Substantial effort is being invested in the search for peripheral or intratumoral T cell receptor (TCR) repertoire features that could predict the response to immunotherapy. Here we demonstrate the utility of MiXCR software for TCR and immunoglobulin repertoire extraction from RNA-Seq data obtained from sorted tumor-infiltrating T and B cells. We use this approach to extract TCR repertoires from RNA-Seq data obtained from sorted tumor-infiltrating CD4+ and CD8+ T cells in an HKP1 (KrasG12Dp53−/−) syngeneic mouse model of lung cancer after anti-PD-1 treatment. For both subsets, we demonstrate decreased TCR diversity in response to therapy. At a later time point, repertoire diversity is restored in progressing disease but remains decreased in responders to therapy in both CD4+ and CD8+ subsets. These observations complement previous studies and suggest that stably increased intratumoral CD4+ and CD8+ T cell clonality after anti-PD-1/PD-L1 therapy could serve as a predictor of long-term response.
Active tumor infiltration by CD8+ and Th1 T cells has repeatedly been shown to correlate with improved clinical outcomes in a variety of cancers (1–4). At the same time, it remains a matter of debate which proportion of these infiltrating T cells is actually tumor-reactive and could participate in an antitumor response (5), and this proportion may differ between different cancer types and individual patients.
T cell receptor (TCR) repertoire analysis can reveal the clonal content of tumor-infiltrating T cells, the presence of large clonal expansions (6), and the presence of clusters of convergent TCR variants that potentially respond to the same antigen (7–10). However, the prognostic and predictive value of TCR repertoire profiling in cancer immunotherapy remains a matter of investigation.
In a seminal work by Tumeh et al. (6), it was shown that high intratumoral T cell clonality—indicating the presence of large clonal expansions—may be associated with clinical response to anti-PD-1 therapy in patients with advanced melanoma. Furthermore, responders demonstrated a tendency toward increased clonal expansion during therapy. Tamura et al. (11) likewise observed increased intratumoral T cell clonality in response to peptide vaccines and oxaliplatin-based chemotherapy in colorectal cancer patients who exhibited long periods of progression-free survival. A combination of neoadjuvant ipilimumab with high-dose IFNα2b in advanced melanoma showed higher efficiency for patients exhibiting increased T cell clonality in the primary tumor at 6–8 weeks following neoadjuvant therapy (12).
Several studies have also shown that the analysis of peripheral blood TCR repertoire clonality could assist in predicting therapeutic outcomes. In particular, response to anti-PD-1 therapy has been associated with the initial presence of clonal peripheral blood T cell expansions in metastatic melanoma (13), although the opposite was reported for PD-L1 blockade in urothelial cancer (14). In another study of metastatic urothelial cancer patients treated with anti-PD-L1, clinical response was associated with high intratumoral T cell clonality and induced peripheral blood expansion of major tumor-resident T cell clones (15).
Response to anti-CTLA-4 therapy has been linked with initially low peripheral blood TCR clonality in melanoma (13) and pancreatic ductal adenocarcinoma (16), with the latter study also observing increased presence of clonal expansions over the course of therapy (16). These results are in line with the logic of anti-CTLA-4 action via blocking regulatory T cell (Treg)-mediated suppression of antigen-presenting cells and interclonal competition between CD4+ T cells (17–20). This allows multiple novel expansions to arise, thereby broadening the peripheral TCR repertoire (21). Although anti-CTLA-4 therapy has been associated with essential remodeling and diversification of peripheral TCR repertoires, it has also been reported that improved clinical outcomes may be associated with the persistence of initially high-frequency clones during therapy (22). Using the ALICE algorithm on the data described in Robert et al. (21) and Subudhi et al. (23), we have also recently shown that the number of TCR sequences actively involved in current immune response—as judged by the number of clusters of non-randomly met (non-public) homologous TCR variants—increases after anti-CTLA4 therapy (10), suggesting reactivation of immune response to diverse antigens.
Notably, an increase in intratumoral T cell clonality was also observed in response to targeted therapy with a BRAF inhibitor, and persistence of initially detected dominant T cell clones was associated with therapy response (24). In a B16 mouse melanoma model, expansion of CD8+ T cells within the tumor—but not in the periphery—was associated with antitumor effects (25). In FGFR2K660N/p53mut lung cancer mouse model, reduced TCR clonality was found in responders receiving anti-PD-1 therapy in combination with an FGFR inhibitor (26). Thus, the current data on the dependence of response to different immunotherapies on the clonal composition of T cell repertoires remain incomplete and somewhat contradictory.
A recent study on the HKP1 (KrasG12Dp53−/−) immunocompetent, syngeneic mouse lung cancer model, which is histologically similar to human adenocarcinoma (27), used RNA-Seq analysis of fluorescence-activated cell sorting (FACS)-sorted tumor-infiltrating CD4+ and CD8+ T cells in order to reveal the intrinsic features of T cell behavior associated with early immune response to anti-PD-1 therapy (28). This work showed that response to anti-PD-1 treatment was correlated with T cell subset-specific alterations, although the clonality of T cells was not specifically analyzed. However, TCR transcripts are present in bulk RNA-Seq data and enriched in sorted T cell RNA-Seq data, and MiXCR software allows one to extract TCR CDR3 repertoires with near-maximal efficiency and accuracy (29–31).
Here, we show that MiXCR efficiently extracts TCR and immunoglobulin repertoires from RNA-Seq data obtained from sorted tumor-infiltrating T or B cells. We applied this approach to extract TCRα and β CDR3 repertoires from the tumor-infiltrating T cell RNA-Seq data reported by Mittal and colleagues (28), and compared clonality and diversity parameters in responders to anti-PD-1 therapy vs. progressors and untreated control mice. At 1 week after the start of therapy, we find that TCR diversity goes down in both CD4+ and CD8+ T cells, reflecting clonal expansion. At a later time point, about 2 weeks after the start of the therapy, diversity remains low for responders, but reverts back to high diversity in progressors, reflecting reduced clonal expansion. These data demonstrate that the primary response to anti-PD-1 immunotherapy, as expressed by clonal expansion of T cells, is insufficient to provide sustained response to therapy, and that stability of the intratumoral clonal T cell expansions acquired in the course of the treatment is associated with longer-term response.
RNA-Seq based immune repertoire profiling may work well for sorted T or B cells, where the percentage of CDR3-covering reads is relatively high (30). This approach—preferably using relatively long (e.g., 100+100-nt) paired-end sequencing—makes it possible to combine transcriptomic profiling of tumor-infiltrating lymphocyte populations of interest with relatively deep profiling of immune repertoires.
To illustrate and validate this approach, we performed RNA-Seq analysis on sorted T and B cells from mouse B16F0 melanoma tumors. Tumors were excised and carefully cleaned from the outer fibrous capsule, which contains a large amount of immune cells, in order to focus on lymphocytes that have truly infiltrated the tumor tissue. CD4+ T cells or CD19+ B cells were sorted from the isolated tumor tissues, and all extracted RNA materials and all obtained cDNA were used to generate cDNA libraries using the Clontech Smart-Seq v4 Ultra Low Input RNA Kit. These were sequenced on an Illumina HiSeq (100+100-nt paired-end reads), and TCRβ or IGH CDR3 repertoires were extracted using the MiXCR software. Table 1 shows typical counts of cells, obtained RNA-Seq reads, CDR3 reads, and CDR3 clonotypes extracted from RNA-Seq data for the sorted T and B cells.
To verify if the RNA-Seq-based repertoire analysis can be informative in terms of repertoire diversity and clonality, we extracted TCRα and TCRβ CDR3 repertoires from transcriptomes obtained for the human naïve and effector CD8+ T cells reported in Simoni et al. (32). For each sample, we extracted from 399 to 1,255 distinct TCRα and from 795 to 1,579 TCRβ CDR3 clonotypes. Due to the limited repertoire information extracted from some of the samples, we opted to analyze joint TCRα and β repertoires in order to get better averaged statistics for diversity metrics. For normalization (33), we down-sampled our clonesets by extracting an equal number of 1,500 randomly chosen CDR3-covering reads from each set of clones. Diversity and clonality metrics were analyzed using the VDJtools software (34); results are shown in Figure 1. This analysis clearly distinguished naïve and effector CD8+ subsets, as expected.
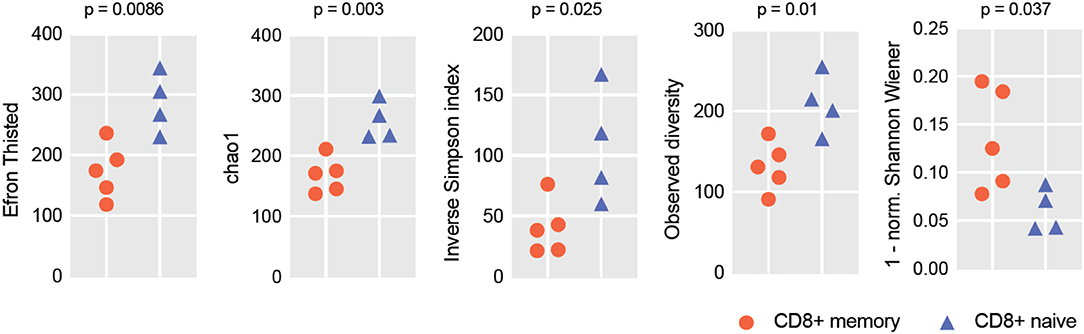
Figure 1. RNA-Seq-based TCR repertoire diversity in naïve and memory CD8+ T cells. Diversity metrics calculated for naïve and effector CD8+ TCR α + β (combined) CDR3 repertoires are shown. Note that each sample is represented by 1,500 randomly chosen CDR3-covering sequencing reads for normalization. Observed diversity is a total number of unique clonotypes in a sample, so it takes into account all clonotypes. Chao1 depends mostly on the representation of singletons and doubletons—clonotypes represented by one and two reads, respectively. Both Chao1 and Efron-Thisted indices estimate relative total TCR diversity, similar to the estimation of species richness. These numbers should not be, of course, understood as true total diversity, but only as a lower bound calculated based on a given sample size. Inverse Simpson's Diversity Index takes into account both richness and evenness. [1-normalized Shannon Wiener] represents “clonality” metrics used in Tumeh et al. (6) and largely reflects the presence of large clonal expansions.
We next applied the same approach to the CD4+ and CD8+ sorted T cell RNA-Seq data obtained by Mittal and colleagues from their syngeneic mouse model of lung cancer (28). We extracted joint TCRα and β CDR3 repertoires from these data using the MiXCR RNA-Seq mode. These samples were divided into four groups: (1) controls treated with IgG2a; (2) early response to anti-PD-1 therapy, with therapy on days 7, 10, and 13 and tumors excised at day 14 post-implantation; (3) late response with regression, with therapy on days 7, 10, 13, and 16 and tumors excised at day 17 or 24 post-implantation; and (4) late response with progression, with the same treatment and excision regimen. Note that the early response group was not split into regression and progression due to the insufficient number of usable samples that would have sufficient depth of TCR repertoire analysis in terms of CDR3-covering reads.
Similar to the one described above, we analyzed joint TCRα and β repertoires, down-sampled by extracting an equal number of 500 randomly chosen CDR3-covering reads from each set of clones. Datasets with fewer than 500 reads were discarded.
Our comparison of the resulting diversity and clonality metrics yielded several findings (Figure 2, Supplementary Figure 1). First, the diversity of TCR repertoires in anti-PD-1-treated animals decreased at early time points compared to controls treated by IgG2a, both in the CD4+ and CD8+ subsets. Additionally, although the limited number of samples did not allow us to estimate statistically meaningful differences between responders and progressors at the early time point (day 14), there was a trend toward more reduced CD4+ diversity among the responders. At the later time points (day 17 or 24), repertoire diversity was restored in both progressors and responders, and in both CD4+ and CD8+ subsets. However, the latter effect was much stronger for progressors, leading to statistically significant differences in repertoire diversity metrics between responders and progressors at the late time point for both T cell subsets.
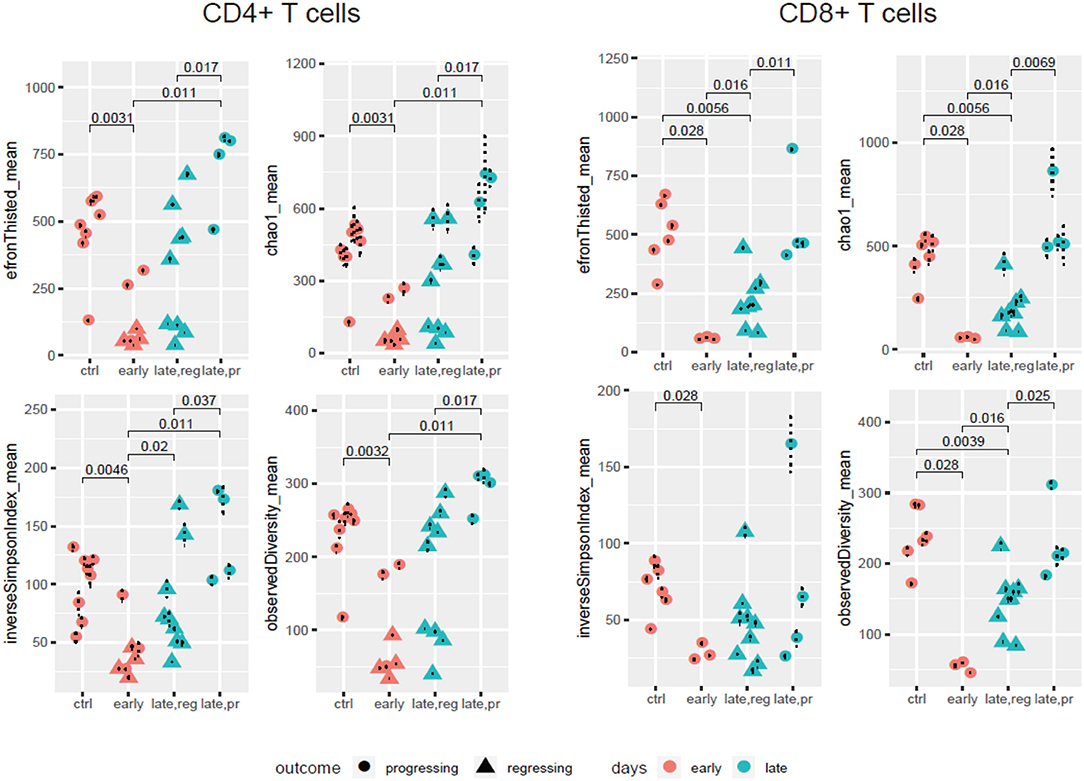
Figure 2. TCR repertoire diversity in the course of anti-PD-1 therapy. Diversity metrics calculated for CD4+ and CD8+ TCR α + β (combined) CDR3 repertoires extracted from the four groups of samples: control; early response (day 14); late response, regressing (days 17 and 24); and late response, progressing (days 17 and 24). Each sample is represented by 500 randomly chosen CDR3-covering sequencing reads, for normalization.
These data complement the results obtained by Mittal and colleagues as well as the current knowledge on the association between TCR clonality and response to anti-PD-1 therapy. Sustained response is associated with stably increased clonality and decreased diversity in both CD4+ and CD8+ subsets over the course of therapy, while restoration of initial diversity seems to be associated with disease progression.
One possible interpretation is that initial proliferation of previously suppressed PD-1+ T cell clones specific to locally present immunogenic antigens—including both tumor and non-tumor (e.g., viral) antigens (32, 35)—in response to anti-PD1 therapy leads to temporarily increased clonality and decreased diversity. Notably, it has been shown that increased intratumoral TCR clonality in adenocarcinoma is uniquely correlated with CD8+PD-1+ T cell subsets, but not with bulk CD8+ T cells (36). The same observation was noted in targeted analysis of CD8+PD1+ T cell subsets in NSCLC after anti-PD-1 treatment. Only intratumoral CD8+ T lymphocyte populations with high PD-1 expression levels were characterized as having significantly increased clonality (37). Thus, the pool of oligoclonal PD-1+ T cells could serve as a source for rapidly growing clonality in response to anti-PD-1 therapy.
On the other hand, some studies show that PD-1 blockade may unleash a novel tumor-specific TCR repertoire that was not previously observed in the tumor (38). Experiments tracking tumor-infiltrating TCR clones from patients with basal cell carcinoma and squamous cell carcinoma have demonstrated that clonal expansions of memory CD8+ T cells with an exhausted phenotype referred to chronical activation before and after anti-PD-1 therapy are distinct from each other. The authors called this phenomenon “clonal replacement” and suggested that exhaustion of tumor-infiltrating T cells limits their renewal following checkpoint blockade. They further proposed that the T cell response relies on the expansion of novel tumor-specific T cell clones originating from non-tumor sites such as lymphoid organs or rare, unexpanded clones present within the tumor (38).
The association of a prolonged response to treatment with the stability of clonal expansions acquired during initial response, as we have observed in this work, makes intuitive sense and may be interpreted as evidence that a sustained immune response results in the stable presence and proliferation of tumor-specific CD4+ and CD8+ T cells.
Our results support the concept that monitoring intratumoral T cell clonality—for example, by measuring T cell clonality in excised tumor samples after neoadjuvant anti-PD-1/PD-L1 therapy (6, 11, 12, 39)—is a rational strategy for predicting long-term response.
Technically, we advocate for performing RNA-Seq on sorted T and B cell subsets as a means to simultaneously evaluate both functional behavior and repertoire features in response to immunotherapy. MiXCR currently provides the most efficient computational solution for such analyses, either as an offline tool (https://mixcr.milaboratory.com) or as an Illumina BaseSpace cloud application (https://www.illumina.com/products/by-type/informatics-products/basespace-sequence-hub/apps/milaboratory-mixcr-immune-repertoire-analyzer.html).
Experiments were carried out on C57BL/6-Foxp3eGFP mice kindly provided by Prof. A. Rudensky (Memorial Sloan-Kettering Cancer Center) (40). Tumors were generated by subcutaneous (s.c.) injection of 105 B16F0 cancer cells in 300 μl PBS into the left flank of 5–7-month-old female mice. These tumor cells were initially grown in a DMEM medium supplemented with 10% fetal bovine serum (FBS), 0.06% L-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. Cells were cultured in an incubator at 37°C and 5% CO2 and passaged two or three times per week. Before injection, cells were detached by trypsin, then counted and resuspended in PBS to a final concentration of 106 cells in 3 ml. Mice with tumor diameters ranging from 0.5 to 1.2 cm were sacrificed with isoflurane (Esteve, Italy), after which tumors were excised and prepared for further analysis. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). The experimental protocol was approved by the Ethical Committee of the Privolzhsky Research Medical University Academy, Russia (EC #6, granted April 17, 2019).
Tumors were excised and cleaned from the outer tumor capsule. For lymphocyte isolation, excised tumor nodules or tumor parts were homogenized with a gentleMACS dissociator (Miltenyi Biotec, Germany) and incubated in 1–2 ml dissociation solution [RPMI medium supplemented with 417 μg/ml Liberase TL (Roche, Germany) and 10 μg/ml DNase I (Roche, Germany) for 30 min at 37°C in a shaker]. After dissociation, cell suspensions were passed through a 70-μm cell strainer and washed twice with 5 ml of an incubation buffer (PBS, pH 7.2 containing 0.5% bovine serum albumin and 2 mM EDTA).
Cell pellets were resuspended in 100 μl of an incubation buffer with the following antibodies (2 μl each): CD45-PerCP/Cy5.5 (Clone 30-F11, BioLegend), CD3-APC (Clone 145-2C11, BioLegend), CD4-V450 (Clone RM4-5, BD Biosciences), and CD19-PE/Cy7 (Clone 6D5, BioLegend); 400 μl of incubation buffer was added after 45–120 min staining at 4°C. CD3+CD4+ (excluding eGFP-positive Treg cells) and CD19+ subsets were sorted with a FACSAria III cell sorter (BD Biosciences) using the 85-μm nozzle directly into 200 μl of an RLT cell lysis buffer (Qiagen). After sorting, the samples were vortexed and then left at room temperature for 10 min to ensure cell lysis, and finally stored at −20°C.
Mittal and colleagues utilized an immunocompetent, syngeneic preclinical model of early-stage non-small-cell lung cancer (NSCLC), which has been shown to exhibit histological similarities to human adenocarcinoma (28). Mice were injected with 250 μg of a rat monoclonal blocking anti–PD-1 antibody or IgG2a intraperitoneally on days 7, 10, 13, and 16 after tumor implantation. Mice were sacrificed on day 14, day 17, or day 24 depending on the group. RNA was extracted from sorted tumor-infiltrating CD4+ and CD8+ T cells. RNA-Seq libraries were prepared with an Illumina TruSeq RNA Sample Preparation kit and sequenced on an Illumina HiSeq4000 with single-end 50-bp reads, 8 samples per lane. Samples were grouped based on the day of tumor excision (day 14—early tumors, days 17 and 24—late tumors) and tumor growth (progressing, regressing, or partially regressing). For reference, see Figure 7A in Markowitz et al. (28). There were 31 samples of intratumoral CD8+ and CD4+ T cells in total (Supplementary Table 1). Joint TCRα and β CDR3 repertoires were extracted from raw fastq files using MiXCR v2.1.11 (https://github.com/milaboratory/mixcr/releases/tag/v2.1.11).
Publicly available datasets were analyzed in this study. This data can be found here: The RNA sequencing data are available in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSE114300.
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). The experimental protocol was approved by the Ethical Committee of the Privolzhsky Research Medical University Academy, Russia (EC #6, granted April 17, 2019).
EZh, MT, and DC worked on data analysis. AI, DY, LV, IS, and GS worked with mouse tumors and library preparation. ES, MT, EZa, DC, and GS wrote the manuscript. EZa and DC supervised the study.
The work was supported by grant of the Ministry of Education and Science of the Russian Federation (14.W03.31.0005).
DC is a co-founder of MiLaboratory LLC, which develops MiXCR software and has exclusive rights for its commercial distribution.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00385/full#supplementary-material
1. Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. (2009) 27:5944–51. doi: 10.1200/JCO.2008.19.6147
2. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. (2011) 29:1949–55. doi: 10.1200/JCO.2010.30.5037
3. Talebian Yazdi M, Van Riet S, Van Schadewijk A, Fiocco M, Van Hall T, Taube C, et al. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget. (2016) 7:3477–88. doi: 10.18632/oncotarget.6506
4. Ovarian Tumor Tissue Analysis C, Goode EL, Block MS, Kalli KR, Vierkant RA, Chen W, et al. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. (2017) 3:e173290. doi: 10.1001/jamaoncol.2017.3290
5. Scheper W, Kelderman S, Fanchi LF, Linnemann C, Bendle G, De Rooij M, et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med. (2019) 25:89–94. doi: 10.1038/s41591-018-0266-5
6. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. (2014) 515:568–71. doi: 10.1038/nature13954
7. Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. (2017) 547:89–93. doi: 10.1038/nature22383
8. Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, et al. Identifying specificity groups in the T cell receptor repertoire. Nature. (2017) 547:94–8. doi: 10.1038/nature22976
9. Sidhom JW, Bessell CA, Havel JJ, Kosmides A, Chan TA, Schneck JP. ImmunoMap: a Bioinformatics Tool for T-cell Repertoire Analysis. Cancer Immunol Res. (2018) 6:151–62. doi: 10.1158/2326-6066.CIR-17-0114
10. Pogorelyy MV, Minervina AA, Shugay M, Chudakov DM, Lebedev YB, Mora T, et al. Detecting T cell receptors involved in immune responses from single repertoire snapshots. PLoS Biol. (2019) 17:e3000314. doi: 10.1371/journal.pbio.3000314
11. Tamura K, Hazama S, Yamaguchi R, Imoto S, Takenouchi H, Inoue Y, et al. Characterization of the T cell repertoire by deep T cell receptor sequencing in tissues and blood from patients with advanced colorectal cancer. Oncol Lett. (2016) 11:3643–9. doi: 10.3892/ol.2016.4465
12. Tarhini A, Lin Y, Lin H, Rahman Z, Vallabhaneni P, Mendiratta P, et al. Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-alpha2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire. J Immunother Cancer. (2018) 6:112. doi: 10.1186/s40425-018-0428-5
13. Hogan SA, Courtier A, Cheng PF, Jaberg-Bentele NF, Goldinger SM, Manuel M, et al. Peripheral blood TCR repertoire profiling may facilitate patient stratification for immunotherapy against melanoma. Cancer Immunol Res. (2019) 7:77–85. doi: 10.1158/2326-6066.CIR-18-0136
14. Snyder A, Nathanson T, Funt SA, Ahuja A, Buros Novik J, Hellmann MD, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med. (2017) 14:e1002309. doi: 10.1371/journal.pmed.1002309
15. Funt S, Charen AS, Yusko E, Vignali M, Benzeno S, Boyd ME, et al. Correlation of peripheral and intratumoral T-cell receptor (TCR) clonality with clinical outcomes in patients with metastatic urothelial cancer (mUC) treated with atezolizumab. J Clin Oncol. (2016) 34:3005. doi: 10.1200/JCO.2016.34.15_suppl.3005
16. Hopkins AC, Yarchoan M, Durham JN, Yusko EC, Rytlewski JA, Robins HS, et al. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight. (2018) 3:e122092. doi: 10.1172/jci.insight.122092
17. Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. (2002) 3:27–32. doi: 10.1038/ni742
18. Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. (2011) 332:600–3. doi: 10.1126/science.1202947
19. Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol. (2019) 20:218. doi: 10.1038/s41590-018-0280-2
20. Ovcinnikovs V, Ross EM, Petersone L, Edner NM, Heuts F, Ntavli E, et al. CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells. Sci Immunol. (2019) 4:eaaw0902. doi: 10.1126/sciimmunol.aaw0902
21. Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. (2014) 20:2424–32. doi: 10.1158/1078-0432.CCR-13-2648
22. Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. (2014) 6:238ra270. doi: 10.1126/scitranslmed.3008211
23. Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci USA. (2016) 113:11919–24. doi: 10.1073/pnas.1611421113
24. Cooper ZA, Frederick DT, Juneja VR, Sullivan RJ, Lawrence DP, Piris A. BRAF inhibition is associated with increased clonality in tumor-infiltrating lymphocytes. Oncoimmunology. (2013) 2:e26615. doi: 10.4161/onci.26615
25. Hosoi A, Takeda K, Nagaoka K, Iino T, Matsushita H, Ueha S, et al. Increased diversity with reduced “diversity evenness” of tumor infiltrating T-cells for the successful cancer immunotherapy. Sci Rep. (2018) 8:1058. doi: 10.1038/s41598-018-19548-y
26. Palakurthi S, Kuraguchi M, Zacharek SJ, Zudaire E, Huang W, Bonal DM, et al. The combined effect of FGFR inhibition and PD-1 blockade promotes tumor-intrinsic induction of antitumor immunity. Cancer Immunol Res. (2019) 7:1457–71. doi: 10.1158/2326-6066.CIR-18-0595
27. Choi H, Sheng J, Gao D, Li F, Durrans A, Ryu S, et al. Transcriptome analysis of individual stromal cell populations identifies stroma-tumor crosstalk in mouse lung cancer model. Cell Rep. (2015) 10:1187–201. doi: 10.1016/j.celrep.2015.01.040
28. Markowitz GJ, Havel LS, Crowley MJ, Ban Y, Lee SB, Thalappillil JS, et al. Immune reprogramming via PD-1 inhibition enhances early-stage lung cancer survival. JCI Insight. (2018) 3:e96836. doi: 10.1172/jci.insight.96836
29. Bolotin DA, Poslavsky S, Mitrophanov I, Shugay M, Mamedov IZ, Putintseva EV, et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat Methods. (2015) 12:380–1. doi: 10.1038/nmeth.3364
30. Bolotin DA, Poslavsky S, Davydov AN, Frenkel FE, Fanchi L, Zolotareva OI, et al. Antigen receptor repertoire profiling from RNA-seq data. Nat Biotechnol. (2017) 35:908–11. doi: 10.1038/nbt.3979
31. Bolotin DA, Poslavsky S, Davydov AN, Chudakov DM. Reply to “Evaluation of immune repertoire inference methods from RNA-seq data”. Nat Biotechnol. (2018) 36:1035–6. doi: 10.1038/nbt.4296
32. Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. (2018) 557:575–9. doi: 10.1038/s41586-018-0130-2
33. Izraelson M, Nakonechnaya TO, Moltedo B, Egorov ES, Kasatskaya SA, Putintseva EV, et al. Comparative analysis of murine T-cell receptor repertoires. Immunology. (2018) 153:133–44. doi: 10.1111/imm.12857
34. Shugay M, Bagaev DV, Turchaninova MA, Bolotin DA, Britanova OV, Putintseva EV. VDJtools: unifying post-analysis of T cell receptor repertoires. PLoS Comput Biol. (2015) 11:e1004503. doi: 10.1371/journal.pcbi.1004503
35. Kvistborg P, Shu CJ, Heemskerk B, Fankhauser M, Thrue CA, Toebes M, et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology. (2012) 1:409–18. doi: 10.4161/onci.18851
36. Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. (2017) 169:750–65 e717. doi: 10.1016/j.cell.2017.04.014
37. Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. (2018) 24:994–1004. doi: 10.1038/s41591-018-0057-z
38. Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. (2019) 25:1251–9. doi: 10.1038/s41591-019-0522-3
39. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. (2018) 378:1976–86. doi: 10.1056/NEJMoa1716078
Keywords: tumor-infiltrating lymphocytes, TCR repertoire, RNA-Seq, anti-PD-1, T cell clonality, MiXCR
Citation: Zhigalova EA, Izosimova AI, Yuzhakova DV, Volchkova LN, Shagina IA, Turchaninova MA, Serebrovskaya EO, Zagaynova EV, Chudakov DM and Sharonov GV (2020) RNA-Seq-Based TCR Profiling Reveals Persistently Increased Intratumoral Clonality in Responders to Anti-PD-1 Therapy. Front. Oncol. 10:385. doi: 10.3389/fonc.2020.00385
Received: 21 November 2019; Accepted: 04 March 2020;
Published: 28 April 2020.
Edited by:
Ira Ida Skvortsova, Innsbruck Medical University, AustriaReviewed by:
Alvaro Teijeira, ETH Zürich, SwitzerlandCopyright © 2020 Zhigalova, Izosimova, Yuzhakova, Volchkova, Shagina, Turchaninova, Serebrovskaya, Zagaynova, Chudakov and Sharonov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dmitriy M. Chudakov, Y2h1ZGFrb3ZkbUBtYWlsLnJ1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.