
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 06 March 2020
Sec. Genitourinary Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00286
Background and Objectives: Published data from individual studies present conflicting evidence about the relationship between clinicopathological risk factors and oncological outcomes in renal cell cancer (RCC) following nephron-sparing surgery (NSS). This study was conducted to explore the potential risk factors for RCC progress after NSS.
Methods: Studies published in PubMed, Web of Science, and EMBASE were systematically reviewed from inception to March 2019 to determine risk factors for RCC following NSS. The predictive ability of identified predictors was assessed by hazard ratios (HRs) with 95% confidence intervals (CIs). A fixed-effect or random-effect was used to pool the estimates. Subgroup analyses were performed to explore the source of heterogeneity.
Results: Seventeen studies including 38,522 patients with RCC were analyzed. The meta-analysis indicated that positive surgical margin (pooled HR = 1.47; 95% CI:1.24–1.73; P < 0.001), higher Fuhrman grade (pooled HR = 1.58; 95% CI:1.10–2.28; P = 0.013), higher pathological stage (pooled HR = 1.72; 95% CI:1.40–2.12; P < 0.001) and large tumor size (pooled HR = 1.09; 95% CI:1.03–1.16; P = 0.003) were significantly associated with recurrence risk. However, age (pooled HR = 1.00; 95% CI: 1.00–1.01; P = 0.257), sex (male vs. female) (pooled HR = 1.04; 95% CI: 0.89–1.21; P = 0.605) and surgical approach (laparoscope vs. open) (pooled HR = 0.80; 95% CI: 0.59–1.07; P = 0.129) had no effect on recurrence after NSS. In addition, we found that positive surgical margin was significantly associated with recurrence-free survival (pooled HR = 1.87; 95% CI: 1.32–2.66; P < 0.001) and overall mortality (pooled HR = 1.15; 95% CI: 1.07–1.23; P < 0.001), as well as large tumor size for recurrence-free survival (pooled HR = 1.18; 95% CI: 1.06–1.30; P = 0.002)and overall mortality (pooled HR = 1.01; 95% CI: 1.00–1.02; P = 0.004).
Conclusions: Unfavorable pathological characteristics were distinctly related to worse oncological outcomes in RCC patients following NSS. These results may contribute to proposed prediction models for RCC patients to aid in counseling and risk stratification.
Renal cell cancer (RCC) is the third most fatal genitourinary malignancy, accounting for 2–3% of all adult malignancies in humans (1). With the widespread use of cross-sectional imaging in the last decades, more renal tumors are expected to be detected as local lesions. Although the conventional treatment for RCC is radical nephrectomy (RN), patients with RN have substantial risk of progression to future renal insufficiency (2). Hence, nephron-sparing surgery (NSS), involving preservation of normal kidney parenchyma, has become widely accepted as the standard treatment of small renal masses (3).
Traditionally, nephron-sparing surgery includes partial nephrectomy and simple enucleation. Current evidence demonstrates that NSS leads to equal oncological outcomes compared to RN for pathologically staged T1- T2 tumors (4, 5). However, 20–40% of patients experience local recurrence or distant metastasis after NSS (6, 7). This has led to attempts to identify clinicopathological factors to assist clinical decision-making and patient counseling. Nowadays, some prognostic parameters, such as TNM staging system (8), tumor size (9), histological subtype (10) and Fuhrman grade (11)have been developed to predict disease recurrence or survival outcomes. However, the prognostic value of these parameters are controversial according to published studies, and there is no consensus about which patients are at greater risk to develop recurrence or distant metastasis.
These controversies could be a result of differences in limited sample sizes and (or) individual variations. To date, there is no comprehensive study containing the clinicopathological variables for RCC. In this setting, we searched the relevant studies and conducted this meta-analysis in order to assess the potential prognostic factors for oncologic outcomes of recurrence, recurrence-free survival (RFS) and overall mortality (OM) after NSS in RCC patients.
Following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) protocols (12), relevant studies were searched through the electronic databases of PubMed, EMBAS, and Web of Science up to March, 2019.The following MeSH terms and text words were used in combination: “renal cell cancer,” “nephron-sparing surgery,” “partial nephrectomy,” “simple enucleation,” “prognosis,” “clinicopathological,” and “survival.” We also searched relevant studies and reviews by manually screening the references list. The search was restricted to studies of human subjects written in English. Institutional Review Board approval was not required for this study.
The criteria for inclusion were listed as follows: (1) patients with a diagnosis of RCC was pathologically confirmed; (2) the study considered NSS as a primary treatment; (3) the study included clinicopathological factors for oncological outcomes in RCC; and (4) the authors offered the hazard ratios (HRs) and their 95% confidence intervals (CIs) in the paper. The exclusion criteria for the primary studies included following criteria: (1) duplicate publications; (2) studies which did not provide sufficient data to acquire HRs and 95%CIs; (3) non-original articles (e.g., reviews, letters, case reports, and author's reply). When multiple articles were published about the same population, the article reporting the most complete data would be used.
Two authors (ZlZ and HZ) independently extracted data from each included paper. Any disagreement was resolved by discussing with the senior author (BW). The following extracted information was collected and recorded in standardized form: first author, year of publication, ethnicity, number of patients, recruitment period, age, sex, type of NSS, histopathological information, follow-up time, and HRs for survival outcome (recurrence, RFS, and OM) with its 95% CIs. If one study reported results using both multivariate and univariate analysis, we choose the results from multivariate analysis, as it accounts for confounding factors and is more precise.
The Newcastle–Ottawa Quality Assessment Scale (NOS) (13) was applied to assess the methodological quality of each included study. Selection of cohorts, comparability, and ascertainment of outcomes were involved in this assessment scale. Only studies with an NOS score >6 were defined as high quality and finally included.
The software Stata version 12.0 (STATA Corporation, College Station, TX) was used to perform the meta-analysis of the included articles. HRs with their 95% CIs extracted from each publication were applied to calculate pooled HRs. Pooled HRs were used to identify the correlation between clinicopathological risk factors and patient survival. A pooled HR >1 indicated a poorer survival for the patients with a certain clinicopathological feature. The Chi2 and I2 statistic were performed to evaluate inter-study heterogeneity. The value of Pheterogeneity > 0.1 and I2 < 50% represents low-level heterogeneity. If Pheterogeneity > 0.1 and I2 < 50%, a fixed-effects model was applied; otherwise, a random-effects model was applied.
Subgroup and meta-regression analysis based on geographical region, publication year, sample size, and follow-up duration were performed for recurrence analysis to identify the source of heterogeneity. Funnel plots and Begg's test were applied to assess potential publication bias. Sensitivity analysis was also conducted by deleting one single study to measure the reliability of the pooled results. Two-sided value of p < 0.05 was considered statistically significant.
The process of searching articles is presented as a flow diagram in Figure 1. A total of 3,653 potentially relevant studies were identified through the primary study searching. Of all identified records, 1,947 were excluded due to duplicate studies. After title and/or abstracts scanning, 559 articles remained for full-text assessment. Then 543 articles were further excluded by the inclusion criteria. Ultimately, 17 published retrospective studies that met our inclusion criteria were included in this meta-analysis (14–30), containing a total of 38,522 patients (ranging from 69 to 20,762).
The main clinicopathological characteristic of included studies are presented in Table 1 and Table 2. Totally, all the studies were written in English and published from 2002 to 2018. The median follow-up intervals were from 19 to 102 months. In these studies, nine studies were reported from American countries (USA, Canada, Mexico, Argentina), four from Asian countries (Korea, Japan, Indian), three from European countries (Italy, France), and one being a multi-center study. Thirteen studies reported the correlation between recurrence and clinicopathological features; the remaining three studies reported RFS and/or OM and clinicopathological details. The results of the NOS assessment are summarized in Supplementary Table S1; the quality scores of the studies varied from 6 to 8, with a mean of 7.5.
Fourteen studies with 10,106 patients was enrolled to disclose the clinicopathological factors in patients with NSS.As shown in Figure 2, positive surgical margin(PSM) (pooled HR = 1.47; 95% CI:1.24–1.73; P < 0.001, Figure 2A), higher Fuhrman grade (pooled HR = 1.58, 95% CI:1.10–2.28, P = 0.013, Figure 2B), higher pathological stage (pooled HR = 1.72; 95% CI:1.40–2.12; P < 0.001, Figure 2C) and large tumor size (pooled HR = 1.09; 95% CI:1.03–1.16; P = 0.003, Figure 2D) were significantly associated with higher recurrence risk. However, age (pooled HR = 1.00; 95% CI: 1.00–1.01; P = 0.257, Figure 3A), sex (male vs. female) (pooled HR = 1.04, 95% CI: 0.89–1.21, P = 0.605, Figure 3B) and surgical approach (laparoscope vs. open) (pooled HR = 0.80, 95% CI: 0.59–1.07, P = 0.129, Figure 3C) had no effect on recurrence.
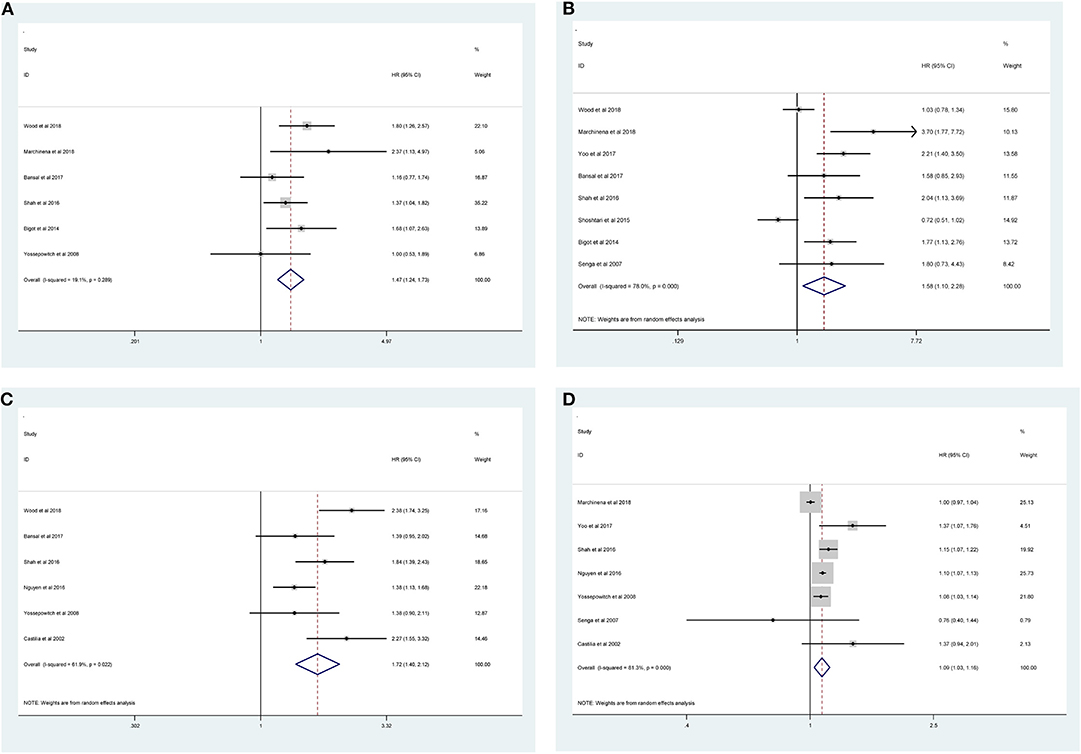
Figure 2. Forest plot of hazard ratio for the association between clinicopathological features and recurrence risk in RCC patients following NSS: (A) positive surgical margin; (B) higher Fuhrman grade; (C) higher pathological stage; (D) large tumor size.
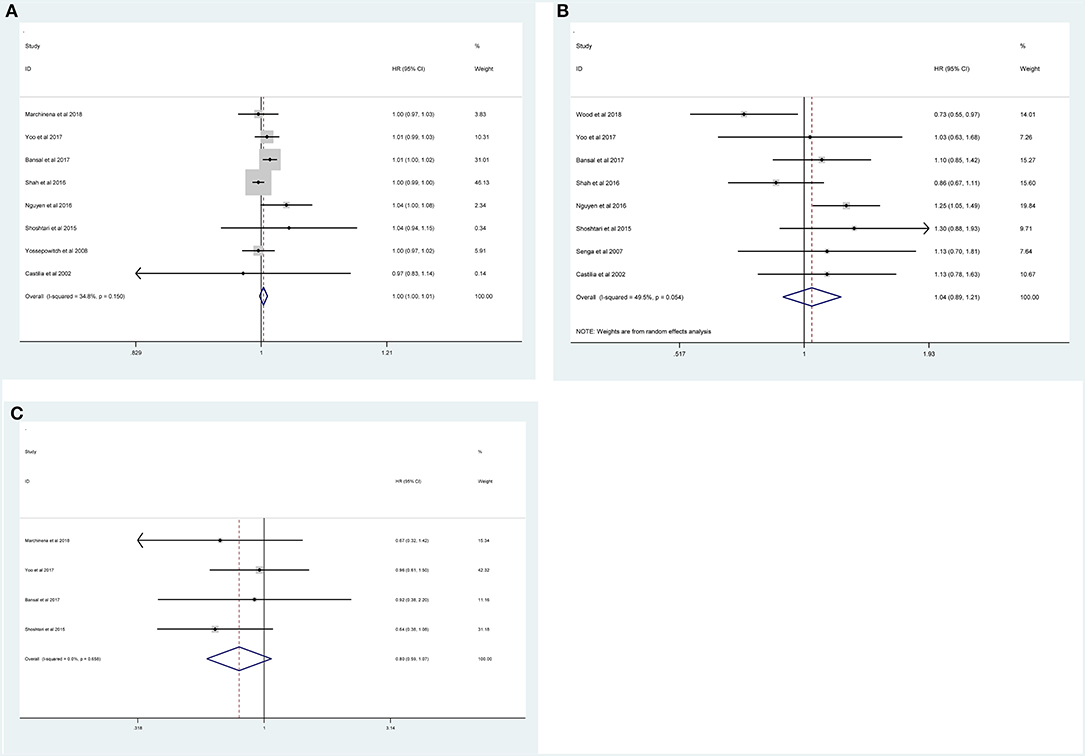
Figure 3. Forest plot depiction of the association between clinicopathological factors and recurrence risk in RCC patients following NSS: (A) age; (B) sex; (C) surgical approach.
We also investigated the potential clinicopathological risk factors for RFS and OM in three studies with 28,416 patients. As shown in Table 3, PSM (pooled HR = 1.87,95% CI:1.32–2.66, P < 0.001, Supplementary Figure S1a), higher Fuhrman grade (pooled HR = 1.75, 95% CI:1.30–2.37, P < 0.001, Supplementary Figure S1b), higher pathological stage (pooled HR = 2.21, 95% CI:1.64–2.97, P < 0.001, Supplementary Figure S1c) and large tumor size (pooled HR = 1.18, 95% CI:1.06–1.30, P = 0.002, Supplementary Figure S1d) were significantly associated with poor RFS. However, age (pooled HR = 1.01, 95% CI: 0.98–1.04, P = 0.488, Supplementary Figure S1e) and sex (male vs. female) (pooled HR = 1.04, 95% CI: 0.90–1.21, P = 0.592, Supplementary Figure S1f) had no relationship with RFS. Also, PSM (pooled HR = 1.15, 95% CI: 1.07–1.23, P < 0.001, Supplementary Figure S2a) and large tumor size (pooled HR = 1.01, 95% CI: 1.00–1.02, P = 0.004, Supplementary Figure S2b) indicated a strong association with OM. Meanwhile, OM has no association with sex (male vs. female) (pooled HR = 1.08, 95% CI: 0.96–1.23, P = 0.193, Supplementary Figure S2c). Taken together, the results demonstrated that worse pathological features may be considered as significant biomarkers for prognosis of patients following NSS.
Because the number of studies that evaluated RFS and OM was relatively small, we only conducted subgroup analysis for recurrence. The results of this subgroup analysis again suggested that PSM, bigger tumor size, higher Fuhrman grade and pathological stage were prognostic factors for RCC, despite certain heterogeneity among some groups (Table 4). Notably, heterogeneity for recurrence was significantly decreased in some models, such as geographical region in Asia, number of patients ≥800 cases, and year of publication before 2016.
Sensitivity analysis was performed to examine the stability of the current meta-analysis. As shown in Supplementary Figure S3, the overall HRs for recurrence were found to be stable and not altered by removal of any single study. Funnel plots and Begg's test were used to assess the publication bias in this meta-analysis. Funnel plots for clinicopathological risk factors and recurrence are shown in Figure 4. Using Begg's test, no obvious publication bias was found regarding PSM (p = 0.807, Figure 4A), higher pathological stage (p = 0.483, Figure 4B), large tumor size (p = 0.543, Figure 4C), age (p = 0.524, Figure 4D), sex (p = 0.728, Figure 4E) and surgical approach (p = 0.781, Figure 4F). However, a slight publication bias existed in higher Fuhrman grade (p = 0.043). Because the number of included studies was limited, the publication bias for RFS and OM were not assessed.
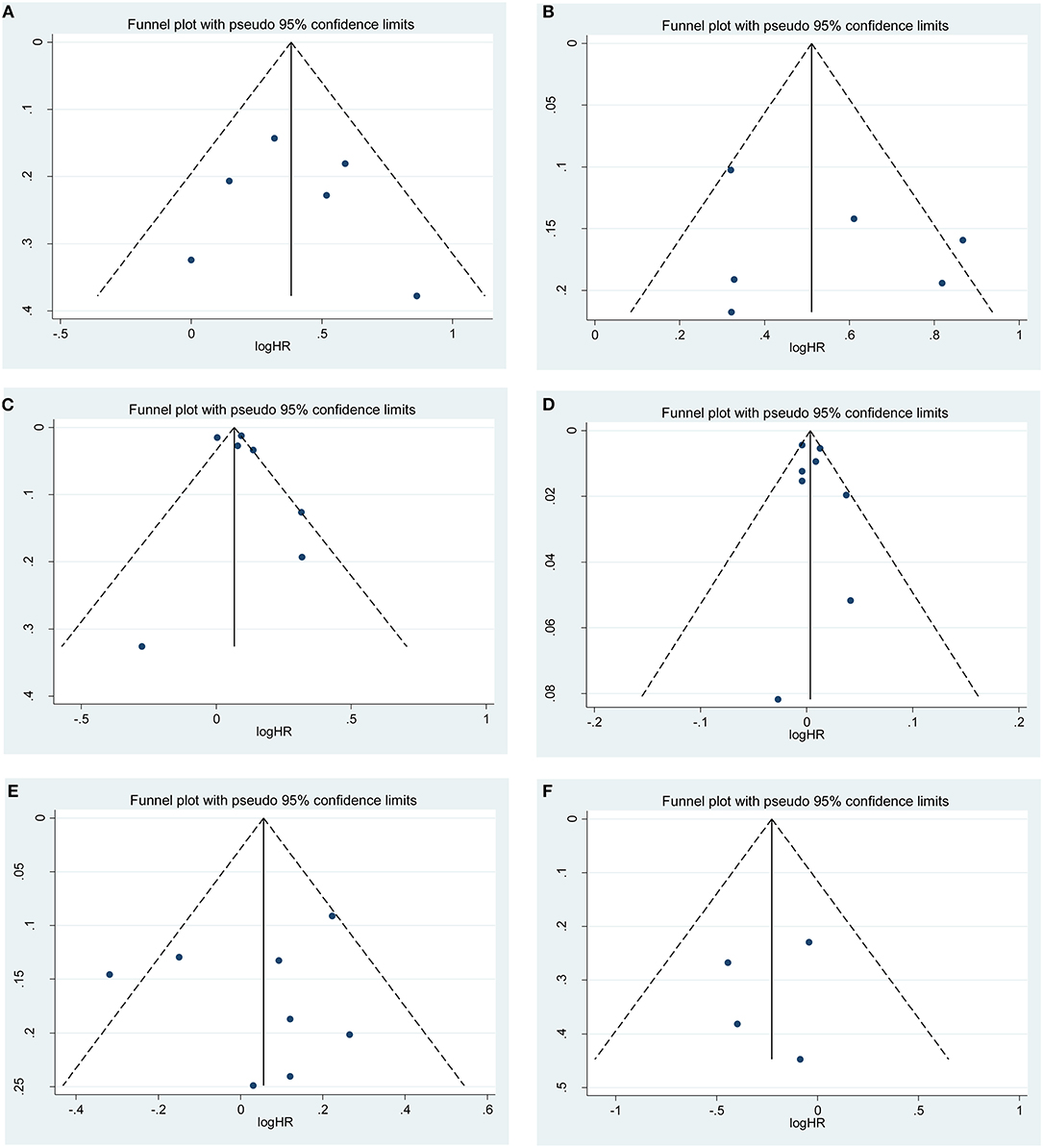
Figure 4. Funnel plots of publication bias on the correlation between clinicopathological features and recurrence risk in this meta-analysis: (A) positive surgical margin; (B) higher pathological stage; (C) large tumor size; (D) age; (E) sex; (F) surgical approach.
RCC is one of the most common cancers, with a major worldwide clinical and public health burden. Improved diagnostics have resulted in the increasing discovery of low stage renal tumors. Although the application of standardized NSS treatment has significantly improved the prognosis of early-stage RCC (31), tumor recurrence and metastasis is still a serious challenge for doctors and patients. RCC have different clinical and biological characteristics, with an extremely heterogeneous oncological outcome (32). Therefore, it is necessary to find prognostic predictors to distinguish high-risk patients and improve the overall clinical outcome in RCC.
Several stratification nomograms have been developed to predict the progression and prognosis after NSS in the postoperative setting. Clinicopathological features that are associated with survival outcome have been intensively studied in the past few years. The Kattan nomogram (33), which contain both clinical and pathological parameters, was the first reported classification system to predict the probability of recurrence in RCC patients. Similarly, the University of California, Los Angeles, Integrated Staging System (UISS) (34) and TNM stage, tumor size, Fuhrman grade, and tumor necrosis (SSIGN) score (35) developed from the Mayo Clinic were conducted to predicting the oncological outcome.
However, most studies investigating the prognosis predictors for RCC following NSS are restricted by single-center design, small sample size, or ethnic differences. For example, the Kattan nomogram has low predictive accuracy in French (36) and Japanese patients (37). Therefore, the precision of the models may be unsatisfactory, and the proposed nomograms still need to be externally validated before clinical application. Moreover, some researchers have reported that other clinicopathological factors such as sex, age, and race may also influence the RCC patients' outcomes (38, 39). Neglecting these prognostic parameters may reduce the accuracy of survival predictions. Hence, we aimed to evaluate the significant clinicopathological variables of oncologic outcomes after NSS from a meta-analysis based on all the available data.
To the best of our knowledge, the present meta-analysis is the first comprehensive study to analyze the prognostic value of clinicopathological parameters in RCC patients after NSS. The pooled results indicated that large tumor size, high Fuhrman grade, higher pathological stage, and PSM at surgery were unfavorable predictors for both recurrence and RFS. Similarly, there was a significant correlation of PSM and large tumor size with worse OM in RCC patients. Generally, in the subgroup analysis for recurrence, these adverse features were still an important prognostic marker in RCC patients, regardless of race, publication year, sample size and follow-up duration. Since these clinicopathologic factors are easily obtained, they can be used to guide patient counseling and risk stratification after NSS.
Several limitations in this meta-analysis should be acknowledged. First, all included studies were retrospectively performed, and data extracted from those studies may have led to inherent bias. Although we extract the results from multivariate models, there still confounding factors can't be controlled. Second, significant heterogeneity was found among studies. However, subgroup analyses showed that the heterogeneity diminished in some groups. Moreover, the stability of our results was confirmed by sensitivity analysis. Third, only published articles were included, and they were all written in English. Therefore, potential selection bias may existed in our study. Fourth, the number of included studies of RFS and OM were smaller, and relevant data could not be obtained for further analysis. Additionally, publication bias was detected in higher Fuhrman grade for recurrence. It is well known that papers with positive results are more likely to be published.
The present study demonstrated that PSM, large tumor size, higher pathological stage and Fuhrman grade were closely associated with poorer prognosis in patients following NSS. However, age, sex and surgical approach were not associated with recurrence, RFS and OM. The findings in our study may help clinicians to identify high-risk patients and formulate treatment decisions. Patients with those with risk factors should be subjected to closer surveillance. Due to the limitations in this meta-analysis, further well-designed studies are required to clarify our results.
All datasets generated for this study are included in the article/Supplementary Material.
LZ and BW: conceptualization. ZZ, WQ, and BW: literature search. JY and HZ: data analysis. LZ: writing—original draft. BW: writing—review and editing. All authors approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00286/full#supplementary-material
Supplementary Figure S1. Meta-analysis for the pooled HRs of RFS: (a) positive surgical margin; (b) higher Fuhrman grade; (c) higher pathological stage; (d) large tumor size; (e) age; (f) sex.
Supplementary Figure S2. Meta-analysis for the pooled HRs of OM: (a) positive surgical margin; (b) large tumor size; (c) sex.
Supplementary Figure S3. Sensitivity analysis on the relationship between clinicopathological features and recurrence risk: (a) positive surgical margin; (b) higher Fuhrman grade; (c) higher pathological stage; (d) large tumor size; (e) age; (f) sex; (g) surgical approach.
RCC, renal cell cancer; NSS, nephron-sparing surgery; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle Ottawa scale; HRs, Hazard ratios; CIs, corresponding 95% confidence intervals; RFS, recurrence-free survival; OM, overall mortality; PSM, positive surgical margin; PN, partial nephrectomy; SE, simple enucleation.
1. Siegel RL, Miller KD. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. (2015) 67:913–24. doi: 10.1016/j.eururo.2015.01.005
3. Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 182 (2009) 1271–9. doi: 10.1016/j.juro.2009.07.004
4. Ghandour RA, Danzig MR, McKiernan JM. Renal cell carcinoma: risks and benefits of nephron-sparing surgery for T1 tumors. Adv Chronic Kidney Dis. (2015) 22:258–65. doi: 10.1053/j.ackd.2015.03.006
5. Rinott Mizrahi G, Freifeld Y, Klein I, Boyarsky L, Zreik R, Orlin I, et al. Comparison of partial and radical laparascopic nephrectomy: perioperative and oncologic outcomes for clinical T2 renal cell carcinoma. J Endourol. (2018) 32:950–4. doi: 10.1089/end.2018.0199
6. Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep. (2005) 6:7–18. doi: 10.1007/s11934-005-0062-x
7. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. (2007) 109:1763–8. doi: 10.1002/cncr.22600
8. Ficarra V, Novara G, Iafrate M, Cappellaro L, Bratti E, Zattoni F, et al. Proposal for reclassification of the TNM staging system in patients with locally advanced (pT3-4) renal cell carcinoma according to the cancer-related outcome. Eur Urol. (2007) 51:722–9; discussion 729–31. doi: 10.1016/j.eururo.2006.07.010
9. Zhang N, Wu Y, Wang J, Xu J, Na R, Wang X. The effect of discrepancy between radiologic size and pathologic tumor size in renal cell cancer. SpringerPlus. (2016) 5:899. doi: 10.1186/s40064-016-2645-z
10. Volpe A, Novara G, Antonelli A, Bertini R, Billia M, Carmignani G, et al. Chromophobe renal cell carcinoma (RCC): oncological outcomes and prognostic factors in a large multicentre series. BJU Int. (2012) 110:76–83. doi: 10.1111/j.1464-410X.2011.10690.x
11. Borgmann H, Musquera M, Haferkamp A, Vilaseca A, Klatte T, Shariat SF, et al. Prognostic significance of Fuhrman grade and age for cancer-specific and overall survival in patients with papillary renal cell carcinoma: results of an international multi-institutional study on 2189 patients. World J Urol. (2017) 35:1891–7. doi: 10.1007/s00345-017-2078-5
12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
14. Wood EL, Adibi M, Qiao W, Brandt J, Zhang M, Tamboli P, et al. Local tumor bed recurrence following partial nephrectomy in patients with small renal masses. J Urol. (2018) 199:393–400. doi: 10.1016/j.juro.2017.09.072
15. Tellini R, Antonelli A, Tardanico R, Fisogni S, Veccia A, Furlan MC, et al. Positive surgical margins predict progression-free survival after nephron-sparing surgery for renal cell carcinoma: results from a single center cohort of 459 cases with a minimum follow-up of 5 years. Clin Genitourin Cancer. (2018) 17:e26–31. doi: 10.1016/j.clgc.2018.08.004
16. Shum CF, Bahler CD, Sundaram CP. Impact of positive surgical margins on overall survival after partial nephrectomy-a matched comparison based on the national cancer database. Urol Oncol. (2018) 36:90.e15–21. doi: 10.1016/j.urolonc.2017.11.009
17. Marchinena PG, Tirapegui S, Gonzalez IT, Jurado A, Gueglio G. Positive surgical margins are predictors of local recurrence in conservative kidney surgery for pT1 tumors. Int Braz J Urol. (2018) 44:475–82. doi: 10.1590/s1677-5538.ibju.2017.0039
18. Yoo S, You D, Jeong IG, Song C, Hong B, Hong JH, et al. Histologic subtype needs to be considered after partial nephrectomy in patients with pathologic T1a renal cell carcinoma: papillary vs. clear cell renal cell carcinoma. J Cancer Res Clin Oncol. (2017) 143:1845–51. doi: 10.1007/s00432-017-2430-6
19. Bansal RK, Tanguay S, Finelli A, Rendon R, Moore RB, Breau RH, et al. Positive surgical margins during partial nephrectomy for renal cell carcinoma: results from Canadian Kidney Cancer information system (CKCis) collaborative. Can Urol Assoc J. (2017) 11:182–7. doi: 10.5489/cuaj.4264
20. Shah PH, Moreira DM, Okhunov Z, Patel VR, Chopra S, Razmaria AA, et al. Positive surgical margins increase risk of recurrence after partial nephrectomy for high risk renal tumors. J Urol. (2016) 196:327–34. doi: 10.1016/j.juro.2016.02.075
21. Nguyen DP, Vertosick EA, Corradi RB, Vilaseca A, Benfante NE, Touijer KA, et al. Histological subtype of renal cell carcinoma significantly affects survival in the era of partial nephrectomy. Urol Oncol. (2016) 34:259.e1–8. doi: 10.1016/j.urolonc.2016.01.005
22. Maurice MJ, Zhu H, Kim SP, Abouassaly R. Reexamining the association between positive surgical margins and survival after partial nephrectomy in a large American cohort. J Endourol. (2016) 30:698–703. doi: 10.1089/end.2016.0031
23. Lee C, You D, Yoo S, Song C, Hong B, Hong JH, et al. Oncological outcomes of patients with incidental pathological T3a stage small renal cell carcinoma after partial nephrectomy. J Cancer Res Clin Oncol. (2016) 142:1651–7. doi: 10.1007/s00432-016-2172-x
24. Zargar-Shoshtari K, Kim T, Simon R, Lin HY, Yue B, Sharma P, et al. Surveillance following nephron-sparing surgery: an assessment of recurrence patterns and surveillance costs. Urology. (2015) 86:321–6. doi: 10.1016/j.urology.2015.05.013
25. Minervini A, Rosaria Raspollini M, Tuccio A, Di Cristofano C, Siena G, Salvi M, et al. Pathological characteristics and prognostic effect of peritumoral capsule penetration in renal cell carcinoma after tumor enucleation. Urol Oncol. (2014) 32:50.e15–22. doi: 10.1016/j.urolonc.2013.07.018
26. Bigot P, Hetet JF, Bernhard JC, Fardoun T, Audenet F, Xylinas E, et al. Nephron-sparing surgery for renal tumors measuring more than 7 cm: morbidity, and functional and oncological outcomes. Clin Genitourin Cancer. (2014) 12:e19–27. doi: 10.1016/j.clgc.2013.09.004
27. Lane BR, Gill IS. 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol. (2010) 183:473–9. doi: 10.1016/j.juro.2009.10.023
28. Yossepowitch O, Thompson RH, Leibovich BC, Eggener SE, Pettus JA, Kwon ED, et al. Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol. (2008) 179:2158–63. doi: 10.1016/j.juro.2008.01.100
29. Senga Y, Ozono S, Nakazawa H, Nagamori S, Marumo K, Horie S, et al. Surgical outcomes of partial nephrectomy for renal cell carcinoma: a joint study by the Japanese society of renal cancer. Int J Urol. (2007) 14:284–8. doi: 10.1111/j.1442-2042.2006.01725.x
30. Castilla EA, Liou LS, Abrahams NA, Fergany A, Rybicki LA, Myles J, et al. Prognostic importance of resection margin width after nephron-sparing surgery for renal cell carcinoma. Urology. (2002) 60:993–7. doi: 10.1016/S0090-4295(02)01983-0
31. Hadjipavlou M, Khan F, Fowler S, Joyce A, Keeley FX, Sriprasad S. Partial vs radical nephrectomy for T1 renal tumours: an analysis from the British Association of Urological Surgeons Nephrectomy Audit. BJU Int. (2016) 117:62–71. doi: 10.1111/bju.13114
32. Beksac AT, Paulucci DJ, Blum KA, Yadav SS, Sfakianos JP, Badani KK. Heterogeneity in renal cell carcinoma. Urol Oncol. (2017) 35:507–15. doi: 10.1016/j.urolonc.2017.05.006
33. Veeratterapillay R, Rakhra S, El-Sherif A, Johnson M, Soomro N, Heer R. Can the Kattan nomogram still accurately predict prognosis in renal cell carcinoma using the revised 2010 tumor-nodes-metastasis reclassification? Int J Urol. (2012) 19:773–6. doi: 10.1111/j.1442-2042.2012.03008.x
34. Ficarra V, Novara G, Galfano A, Brunelli M, Cavalleri S, Martignoni G, et al. The 'stage, size, grade and necrosis' score is more accurate than the university of California Los Angeles integrated staging system for predicting cancer-specific survival in patients with clear cell renal cell carcinoma. BJU Int. (2009) 103:165–70. doi: 10.1111/j.1464-410X.2008.07901.x
35. Ficarra V, Martignoni G, Lohse C, Novara G, Pea M, Cavalleri S, et al. External validation of the Mayo Clinic Stage, Size, Grade and Necrosis (SSIGN) score to predict cancer specific survival using a European series of conventional renal cell carcinoma. J Urol. (2006) 175:1235–9. doi: 10.1016/S0022-5347(05)00684-1
36. Hupertan V, Roupret M, Poisson JF, Chretien Y, Dufour B, Thiounn N, et al. Low predictive accuracy of the Kattan postoperative nomogram for renal cell carcinoma recurrence in a population of French patients. Cancer. (2006) 107:2604–8. doi: 10.1002/cncr.22313
37. Suzuki K, Nishiyama T, Hara N, Akazawa K, Takahashi K. Kattan postoperative nomogram for renal cell carcinoma: predictive accuracy in a Japanese population. Int J Urol. (2011) 18:194–9. doi: 10.1111/j.1442-2042.2010.02693.x
38. Wu J, Zhang P, Zhang G, Wang H, Gu W, Dai B, et al. Renal cell carcinoma histological subtype distribution differs by age, gender, and tumor size in coastal Chinese patients. Oncotarget. (2017) 8:71797–804. doi: 10.18632/oncotarget.17894
39. Chen VS, Abouassaly R, Gonzalez CM, Kutikov A, Smaldone MC, Meropol NJ, et al. Association of race and margin status among patients undergoing robotic partial nephrectomy for T1 renal cell carcinoma: results from a population-based cohort. Urol Oncol. (2017) 35:662.e17–21. doi: 10.1016/j.urolonc.2017.07.011
Keywords: renal cell cancer, nephron-sparing surgery, clinicopathological, oncological outcome, meta-analysis
Citation: Zhang L, Wu B, Zha Z, Qu W, Zhao H and Yuan J (2020) The Clinicopathological Risk Factors in Renal Cell Cancer for the Oncological Outcomes Following Nephron-Sparing Surgery: A PRISMA Systematic Review and Meta-Analysis. Front. Oncol. 10:286. doi: 10.3389/fonc.2020.00286
Received: 29 May 2019; Accepted: 18 February 2020;
Published: 06 March 2020.
Edited by:
Ronald M. Bukowski, Cleveland Clinic, United StatesReviewed by:
Jonas Busch, Charité Medical University of Berlin, GermanyCopyright © 2020 Zhang, Wu, Zha, Qu, Zhao and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijin Zhang, c3R6bGo5MTM3Mjk1NTNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.