
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 21 February 2020
Sec. Cancer Genetics
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00184
A correction has been applied to this article in:
Corrigendum: A Dual-Circular RNA Signature as a Non-invasive Diagnostic Biomarker for Gastric Cancer
Gastric cancer (GC) is the TOP3 leading cause of human mortality in malignant tumors. Notwithstanding, the association between GC and circRNAs is not clear. The purpose of this research was to determine the association between GC progression and circRNAs. The data of circRNAs was obtained from the Gene Expression Omnibus (GEO) database to identify gene, which differentially expressed circRNAs in GC tissues and paired normal tissues. The expression of circRNAs in cancer tissues and normal tissues were tested, and the target circRNA was verified before and after surgery in the plasma. A circRNA-micro(mi)RNA-mRNA competing endogenous RNAs (ceRNAs) network was established, and GO and KEGG analysis are performed. Five candidate circRNAs were identified through bioinformatics analysis. Hsa_circ_0021087 and hsa_circ_0005051 were both downregulated in GC tissues, cells and plasma by RTq-PCR. Additionally, there was a significant difference in the expression of plasma hsa_circ_0021087 in patients with GC at the preoperative and postoperative stages (P < 0.001). Hsa_circ_0021087 also promoted the proliferation of GC cells in vitro. Next, the circRNA-miRNA-mRNA network of hsa_circ_0021087 was predicted, which may be associated with the development of GC by bioinformatics analysis. In summary, the aforementioned dual-circular RNAs may have important implications on the potential, novel and non-invasive diagnostic method for patients with GC.
Gastric cancer (GC) has become the third leading cause of cancer-associated death and is the 6th common cancer in the world (1). Although much progress has been made in the diagnosis of GC and survival after radiotherapy, chemotherapy and surgery, the 5-year survival rate in most countries is still <30% (2). Hence, in order to improve the early diagnosis, treatment and prevention of GC, the discovery of new molecular biomarkers and therapeutic targets is significant.
Circular RNAs (circRNAs), which is a novel class of non-coding RNAs, have a covalently closed loop, derived by pre-mRNA back-splicing of genes (3). CircRNAs usually express tissues/developmental stage-specificity and most are evolutionarily conserved in different organisms, including fruit flies, mammals and plants (4). With the discovery of more and more circRNAs, the function of circRNAs has been gradually reported, especially to promote the physiological and pathological progression of disease, such as cell proliferation, apoptosis, migration and carcinogenesis (5). In the past few years, studies have demonstrated that circRNAs are involved in many malignancies, such as gastric cancer (6), hepatocellular carcinoma (7), colon cancer (8), and pancreatic carcinoma (9), by acting as miRNA sponges and inhibiting target gene expression.
Furthermore, circRNAs could act as new signatures because of their conservatism, specificity, and stability (3, 10). In addition, circRNA can exist in exosomes and even the blood (11, 12). Liquid biopsies are more convenient and less invasive than traditional biopsies, and can be used to analyze biomarkers in specific tumor tissues. Therefore, circulating tumor circRNAs (ct circRNAs) can be applied as potential signatures for the diagnosis of cancer. Tang et al. (13) had demonstrated that plasma circ-KIAA1244 might be a potential signature in patients with GC. As far as we know, however, there are few studies on circRNAs in the plasma of patients with GC.
The aim of this research was the identification and verification of specific circRNAs in the blood for the diagnosis of early GC. Bioinformatics was used to analyze differentially expressed circRNAs and RTq-PCR (reverse-transcription-quantitative) was used to verify the aforementioned circRNAs. The expression of circRNAs in cells and blood was verified at the same time. One of the most important components of competitive endogenous RNAs (ceRNAs) was demonstrated to be circRNAs (14). Micro (mi)RNAs can be bind to their binding sites through miRNA sponge, which regulates the miRNA of the target gene (3). Therefore, in order to predict the hypothetical mechanism and functions of circRNA in GC, a ceRNA network was established and its function was analyzed.
Tissues and peripheral blood of 70 patients were obtained from the Department of general surgery of the Affiliated Hospital of Qingdao University between March and June 2018. GC tissues and their matching paracancerous tissues were obtained from 70 patients undergoing surgery without radiotherapy or chemotherapy. All collected tissues were frozen in a −80°C refrigerator until further use. Matched blood samples were collected from 70 patients before operation and 6 days after operation. The 70 normal controls without any history of cancer were matched with GC cases by sex and age. The clinical information of samples is summarized in Table S1. The Institutional Review Board of Affiliated Hospital of Qingdao University approved this study.
CircRNA expression profiles of GC were obtained from GEO (Gene Expression Omnibus) database at https://www.ncbi.nlm.nih.gov/geo/, including GSE83521, GSE89143, and GSE93541. The Limma package was used to analyze the differently expressed circRNAs (DEcircRNAs), for which the cutoff standard was |logFC| >1 and P < 0.05 (15). Five circRNAs were selected from the intersection of DEcircRNAs of the aforementioned three datasets. The CircBank (http://www.circbank.cn/) was used to find the original gene associated with the circRNA (16). Furthermore, the expression levels of original genes were analyzed by the Kaplan–Meier plotter (http://kmplot.com/analysis/index.php?p=service&cancer=gastric) to evaluate their prognostic value (17).
Normal human gastric epithelium cell (GES1), MGC-803, and BGC-823 cell lines were obtained from Institutes for Biological Sciences. These cell lines were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and grew at 5% CO2 of 37°C. Lipofectamine® 3,000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect cells with siRNA, as following the manufacturer's instructions.
Total RNA was extracted by TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) from tissues and blood. After dissolution, NanoDrop Lite spectrophotometer was used to evaluate the concentration and purity of total RNA. Subsequently, the total RNA was reverse transcribed for the synthesis of cDNA and used to conduct qPCR assays for circRNAs. GAPDH was used as the internal control, and all procedures were repeated three times. Fold change (2−ΔΔCT) was used to represent relative gene expression levels. Table S2 lists all primer sequences and small interference sequences, and the divergent primers designed for each circRNA.
The CCK-8 (Cell Counting Kit-8) assay was performed to examine whether hsa_circ_0020187 was associated with cell proliferation. MGC-803 and BGC-823 cells, after transfection and culture for a day, were seeded into 96–well plates (5 × 103 cells for each well). Each group was replicated in three independent wells. Cell proliferation was determined at 0, 24, 48, and 72 h by optical density (OD) at 450 nm using a spectrophotometer.
The transfected cells were inoculated into a six-well plate and were allowed to reach 80% confluence. The single-cell layer was scratched with the tip of a 10-μl pipette and then washed with 1X PBS three times to clear the cell debris, and replaced with fresh serum-containing medium. The wound was allowed to heal for 24 h, and the images (Nikon Corporation) were obtained at 0 and 24 h, respectively. The wound width was calculated by ImageJ software (version 1.8.0).
Adding 10% FBS to the bottom chamber as a chemotactic agent. Approximately 5 × 104 transfecting cells were added to 200 μl serum-free medium and inoculated in the upper chamber, at 5% CO2 of 37°C. Following 16 h, the cells in the upper chamber were gently wiped with cotton swabs. The contralateral cells of the filter were fixed with 70% ethanol for 30 min, and then stained with 0.1% crystal violet for 10 min. The images were captured under a microscope (Nikon Corporation) and the migrated cells were counted by ImageJ software.
CircInteractome (18) and circBank (16) were used to predict the miRNAs of candidate circRNAs. Furthermore, the Starbase (19) and TargetScan (20) were applied to predict the miRNA-target. The circRNA-miRNA-mRNA network was calculated and constructed by GDCRNATools packages and visualized with Cytoscape (21). In addition, in order to further understand the function of ceRNAs, GO and KEGG pathway analyses were performed through the clusterProfiler (22).
All the data were analyzed by GraphPad Prism v7.0, R software 3.6.1 and the SPSS v23.0. The paired t-test was used to evaluate the differences in the circRNA expression levels before and after operating groups. The associations between circRNA expression in patients and the clinicopathological factors with GC were analyzed by Chi-square test. The diagnostic value of these specific circRNAs was assessed by ROC (receiver operating characteristic) curve analysis and the AUC (area under the ROC curve). P < 0.05 was considered statistically significant.
By using the Limma package, the datasets GSE83521, GSE89143, and GSE93541 from the GEO database were re-analyzed, which identified DEcircRNAs in GC tissues and paired non-tumor tissues (Figure 1A). Following the intersection, a total of five downregulated circRNAs were obtained (hsa_circ_0000554, hsa_circ_0021087, hsa_circ_0005051, hsa_circ_0000332, and hsa_circ_0007518) (Figure 1B). Subsequently, in order to identify the above candidate circRNAs, the prognostic value of their host gene in gastric samples were analyzed using the Kaplan–Meier plotter (Figure 1C). The potential prognostic value of the aforementioned five circRNAs was demonstrated.
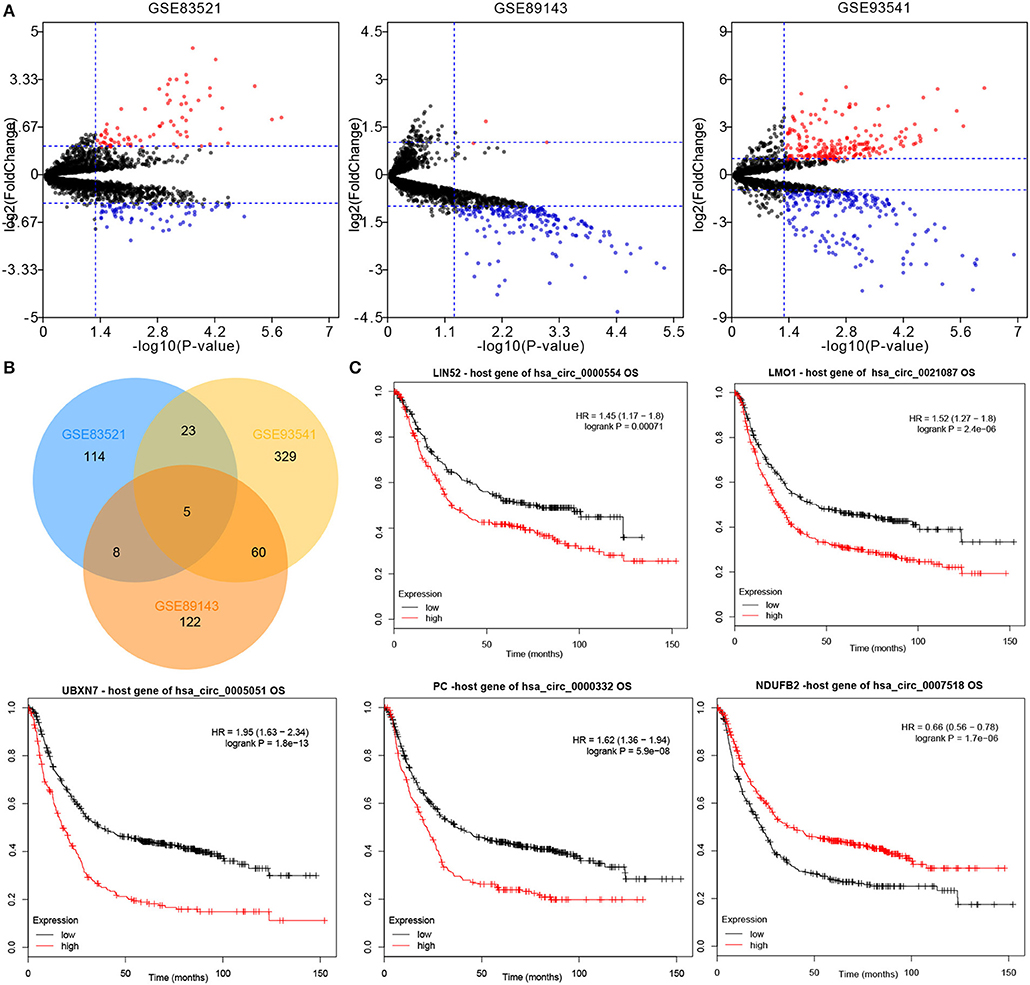
Figure 1. CircRNA expression profiles of GC and matched normal tissues. (A) Differentially expressed circRNAs between the tumor and non-tumor groups were shown by volcano plots. (B) Intersection of differentially expressed circRNAs in the datasets: GSE83521, GSE89143, and GSE93541. (C) The overall survival of five circRNAs host genes in GC patients was analyzed by Kaplan–Meier plotter. Log-rank test was used to determine the statistical significance. CircRNA, circular RNA; GC, gastric cancer.
The expression levels of five candidate circRNAs were verified by RTq-PCR in 70 GC samples and 19 normal blood samples. The results indicated that both hsa_circ_0021087 and hsa_circ_0005051 were downregulated in GC (P < 0.01; Figures 2A,B), which was consistent with the results of the microarray analysis. Nonetheless, the expression levels of hsa_circ_0000332, hsa_circ_0007518 and hsa_circ_0000554 showed no significant difference in the plasma of patients with GC compared with the healthy controls (P = 0.9794, Figure 2C; P = 0.8312, Figure 2D; P = 0.319, Figure 2E). The above two circRNAs sources are shown in Figure S1.
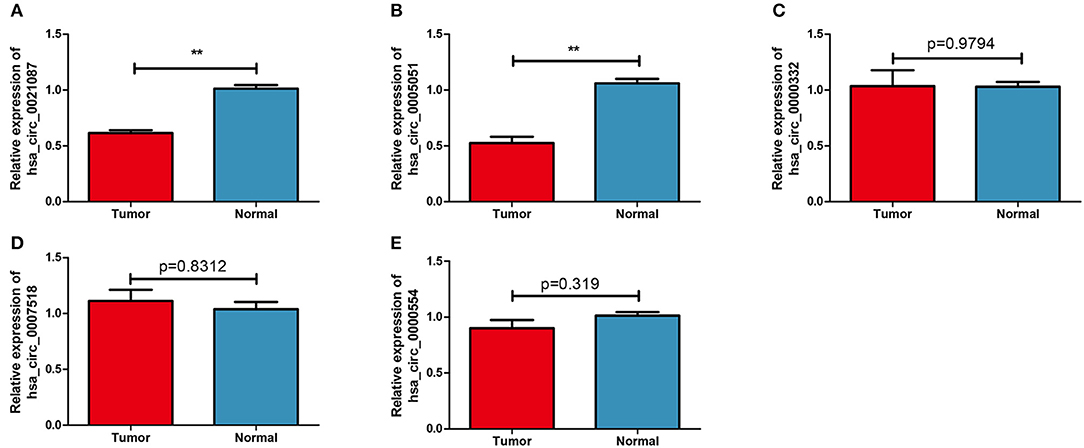
Figure 2. Candidate circular RNAs in patients with GC and normal groups were analyzed by reverse transcription-quantitative PCR. (A) Compared with the normal group, the expression of plasma Hsa_circ_0021087 in patients with GC was down-regulated. (B) Hsa_circ_0005051 was downregulated in GC patients. Hsa_circ_0000332 (C), hsa_circ_0007518 (D), and hsa_circ_0000554 (E) were determined with no differential expression between the two groups. **P < 0.01. GC, gastric cancer.
The diagnostic value of hsa_circ_0021087 and hsa_circ_0005051 from the Figure 3 shows the potential of ROC analysis and CEA to distinguish GC patients from normal controls. Hsa_circ_0021087 and hsa_circ_0005051's AUC was 0.7056 and 0.73, respectively. The expression values of the above dual-circRNA signature and CEA combination that provide the best distinction was analyzed, which resulted in an AUC of 0.7988, P < 0.0001.
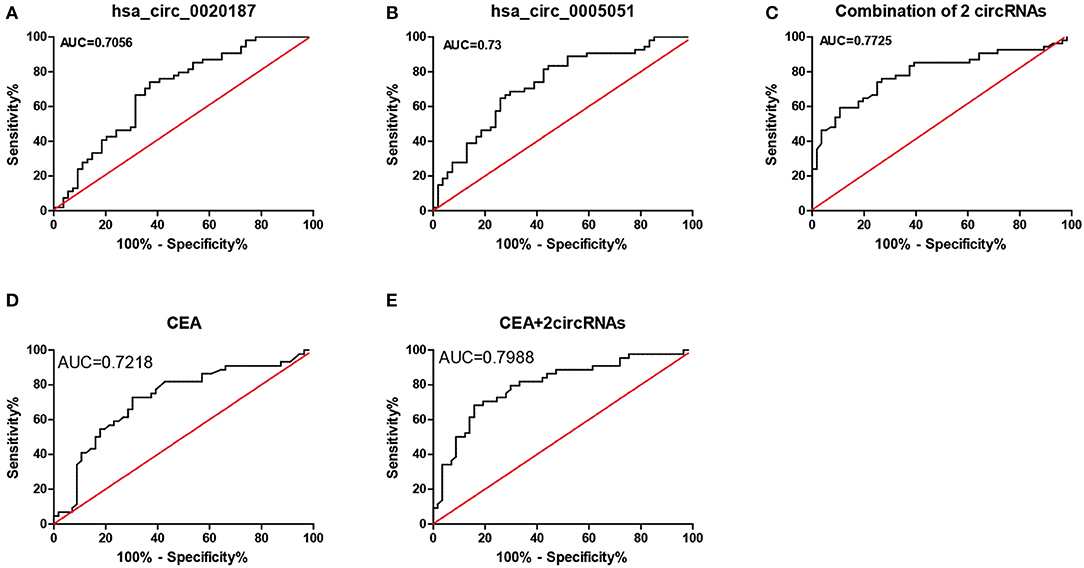
Figure 3. ROC analysis of hsa_circ_0021087, hsa_circ_0005051, and the combination of the two molecular biomarkers were used in the diagnosis of GC. (A) The hsa_circ_0021087 area under the ROC curve (AUC) 0.7056, P < 0.001. (B) Hsa_circ_0005051: 0.73, P < 0.001. (C) Combination of 2 circRNAs: 0.7725, P < 0.001. (D) CEA: 0.7218, P < 0.001. (E) CEA and 2 circular RNAs: 0.7988, P < 0.001. ROC, receiver operating characteristic; AUC, area under the curve; CEA, carcinoembryonic antigen.
The correlation between the clinical characteristics of patients with GC and the expression levels of the two kinds of circRNAs was performed to evaluate by additional analysis. As shown in Table 1, hsa_circ_0021087 and hsa_circ_0005051 expression was associated with tumor size and TNM stage, respectively (P = 0.017; P = 0.032). However, the results showed no association between circRNAs and the patients' age, sex, and lymph node metastasis.
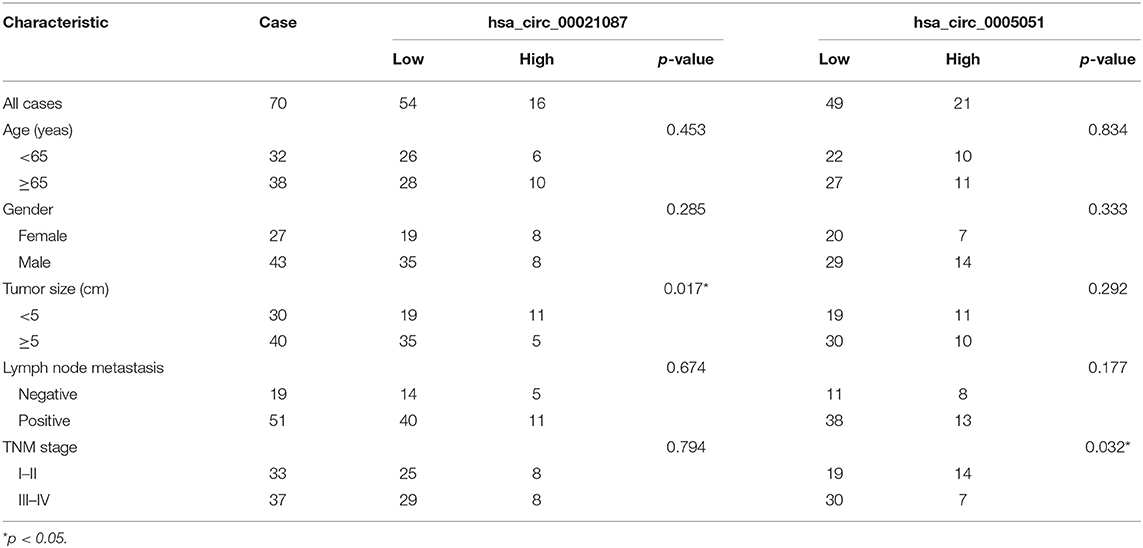
Table 1. Correlations between hsa_circ_00021087 and hsa_circ_0005051 expression and clinical characteristics in GC patients.
The expression of hsa_circ_0021087 and hsa_circ_0005051 was detected from the blood of patients with GC before and after operation. The findings revealed that the expression of hsa_circ_0021087 was significantly increased in 24 of the 31 (77.42%) patients after surgery (P < 0.001; Figure 4A). However, it was found that no obvious sense in expression of hsa_circ_0005051 was discovered before and after operation (P = 0.11; Figure 4B).
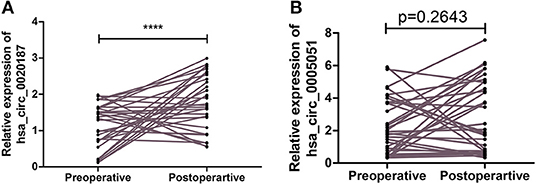
Figure 4. Comparison the expression of hsa_circ_0021087 and hsa_circ_0005051 in patients' plasma with gastric cancer before and after operation. (A) Downregulated hsa_circ_0021087 was detected at a higher level in plasma samples after surgical resection. (B) Hsa_circ_0005051 was detected at preoperative and postoperative stages with no significant differential expression. ****P < 0.0001.
Furthermore, in order to explore the roles of circRNAs in GC, preliminary experiments were carried out in vitro. Firstly, the expression levels of hsa_circ_0021087 and hsa_circ_0005051 were examined in the two GC cell lines. As Figures 5A,B showed that compared with human GES1, hsa_circ_0005051 in GC cell line was significantly down-regulated. Furthermore, as hsa_circ_0021087 was significantly downregulated in the blood and cells of GC, it could be used as a target to study the role of circRNA in the carcinogenesis of GC. A negative control siRNA (si-NC) or si-hsa_circ_0021087 were transfected into BGC-823 and MGC-803 cells. The RT-qPCR assay showed that siRNA downregulated the expression of hsa_circ_0021087 in GC cells compared with cells treated with si-NC and OE-circ (Figure 5C). CCK8 assay was performed to assess whether hsa_circ_0021087 was associated with cell proliferation. It was observed that proliferation was notably changed in BGC-823 and MGC-803 cells, following the expression of hsa_circ_0021087 (Figures 5D,E). These experiments indicated that hsa_circ_0021087 could promote GC cell proliferation in vitro. Functionally, compared with the control, cells with hsa_circ_0020187 depletion showed accelerated wound healing (Figures 5F–H). Consistently, transwell migration assays demonstrated that the inhibition of hsa_circ_0021087 led to increasing cell migration in MGC-803 and BGC-823 cells (Figures 5I–K).
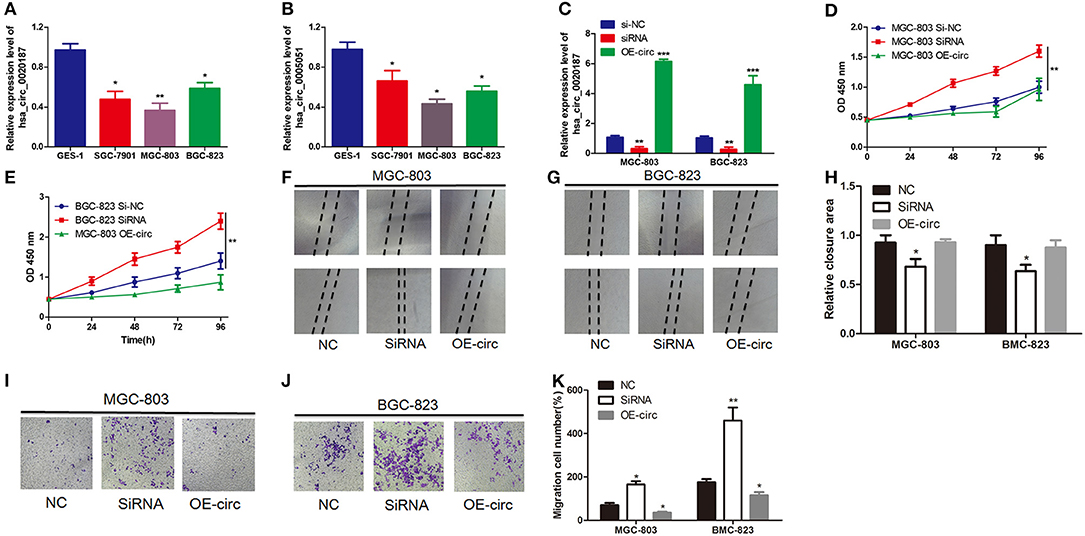
Figure 5. Hsa_circ_0021087 promotes GC cell proliferation in vitro experiments. (A) Hsa_circ_0021087 expression level was downregulated in GC cells (SGC-7901, MGC-803 and BGC-823) compared with the normal cell GES1. (B) Hsa_circ_0005051 was also downregulated in the GC cell lines. (C) Following the transfection of MGC-803 and BGC-823 cells with specifically synthesized small interference RNA and OE-hsa_circ_0021087, the expression of hsa_circ_0021087 in cells was analyzed by reverse transcription-quantitative PCR. (D,E) The Cell Counting Kit-8 assay indicated that hsa_circ_0021087 promoted MGC-803 and BGC-823 cell proliferation. (F–H) Wound healing assays were performed on MGC-803 and BGC-823 cells treated with si-NC, si-circ_0021087 and OE-circ_0021087. (I–K) Transwell chambers were used to perform cell migration assays in MGC-803 and BGC-823 cells. *P < 0.05, **P < 0.01, and ***P < 0.001. GC, gastric cancer.
The CircInteractome and circBank predicted three miRNAs (hsa-miR-184, hsa-miR-1276, hsa-miR-450b-3p) after intersection that were targeted by hsa_circ_0021087 (Figure S2). Next, the target mRNAs of these miRNAs were predicted using the StarBase and TargetScan databases. Pearson correlation analysis and hypergeometric test were used to assess the ceRNA network. Finally, the interaction of three miRNAs and 211 target mRNAs are indicated in Figure 6. Additionally, the ceRNA network regulated by hsa_circ_0005051 is also illustrated in Figure S3.
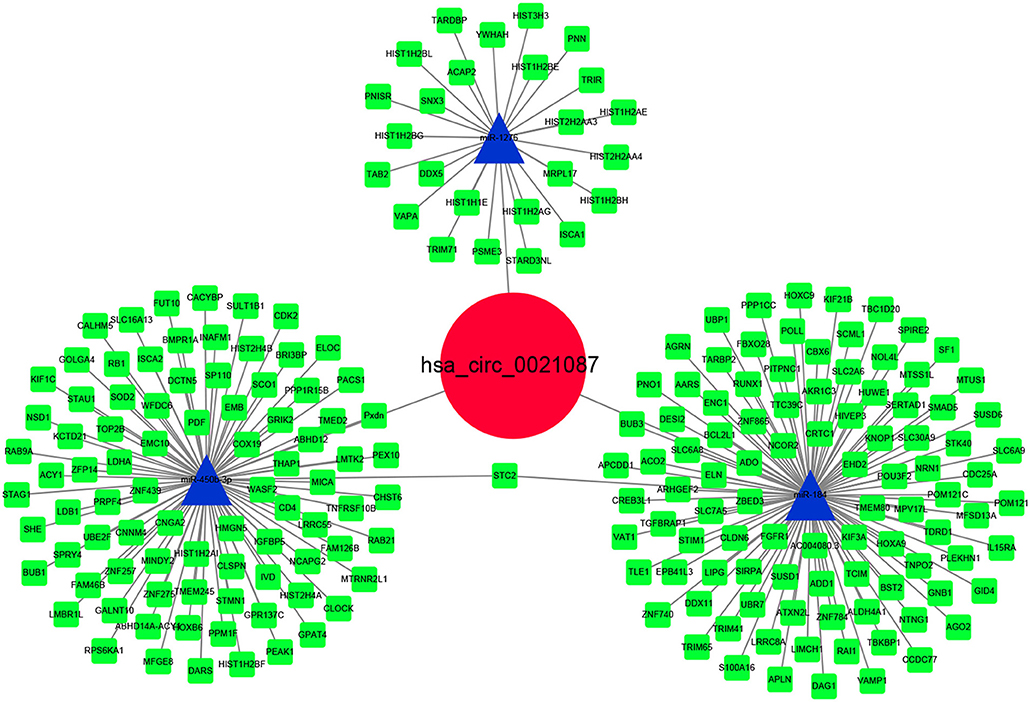
Figure 6. Hsa_circ_0021087–microRNA–mRNA interactions were constructed and visualized by Cytoscape software.
GO analysis was performed for hsa_circ_0021087 regulatory target genes. The top ten significantly enriched molecular functions (MFs) and biological processes (BPs), cellular components (CCs) are visualized in Figures 7A–C. In the BPs ontology, transcription, DNA-templated and positive regulation of transcription from RNA polymerase II promoter were the most enriched terms. In MFs ontology, the most enriched terms were catalytic activities and binding. In CCs ontology, nucleosome, nucleoplasm and extracellular exosome were the most enriched terms. The genes in the KEGG pathway database were also mapped, which showed that the top five most annotated genes were enriched in alcoholism, systemic lupus erythematosus, viral carcinogenesis, pathways in cancer and cell cycle (Figure 7D). In addition, the functional analysis of hsa_circ_0005051 is shown in Figure S4.
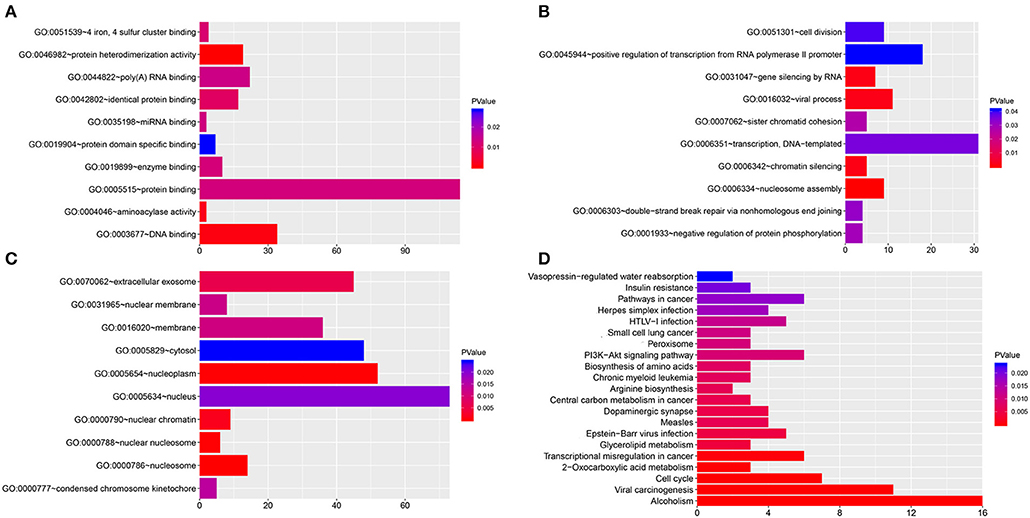
Figure 7. Functional analysis of hsa_circ_0021087. (A–C) GO analysis of hsa_circ_0021087 based on the ceRNA network. The MFs (A), BPs (B) and CCs (C) of top 10 significantly enriched are listed. (D) KEGG pathway analysis of hsa_circ_0021087 based on the ceRNA network. GO, Gene Ontology. BPs, biological processes. MFs, molecular functions. CCs, cellular components; KEGG, Kyoto Encyclopedia of Genes and Genomes; ceRNA, competing endogenous RNA.
GC still has difficulty with early diagnosis among malignant tumors worldwide. Because of its late clinical manifestations and not enough sensitivity to radiotherapy and chemotherapy, the overall survival rate of patients with gastric cancer is still unsatisfactory. Therefore, it is very important to increase the sensitivity of early diagnosis, to develop effective prognostic biomarkers and to find new targets for GC molecular therapy.
CircRNAs have gained increasing attention for candidate biomarkers detection in the past years, due to their abundance and stability in body fluids, such as exosomes, plasma, and saliva (11, 23, 24). Several studies have published interesting results that reveal the biogenesis of circRNAs and its possible mechanisms (3, 12). In the present study, three GEO datasets were analyzed to screen for DEcircRNAs in GC, as candidate circRNAs, which was differentially expressed in TCGA-STAD data and was correlated with prognosis. It is speculated that these candidate DEcircRNAs may be associated with the pathogenesis of GC.
Subsequently, the candidate circRNAs expression levels were verified in tissues and the plasma of patients with GC. As far as we know, this is the first research to report the expression of hsa_circ_0021087 and hsa_circ_0005051 in plasma of cancer patients. Because of the advantages of availability and non-invasive, plasma samples were used in this research. Zhang et al. (25) demonstrated a difference in the expression of plasma ciRS-133 levels between GC patients and healthy controls. Furthermore, Tang et al. (13) showed lower expression of circ-KIAA1244 in GC tissues, cells and plasma compared with normal controls. The present research examined the expression level of both hsa_circ_0021087 and hsa_circ_0005051 to be differentially expressed in plasma of GC patients, and that the combination of these two types of circRNA can improve the diagnostic accuracy of GC. Additionally, the other three candidate circRNAs displayed no significant difference in the plasma of GC samples and normal samples, but this difference was attributed to the detecting method (RT-qPCR and microarray analysis), sample type (tissue and plasma) and sample size. These findings revealed that the dual circular RNA signature can be applied as a potential non-invasive biomarker for the diagnosis of GC.
Li et al. (26) reported that a number of differentially expressed circRNAs were detected in plasma of patients with cervical cancer before and after operation, some of which were considered to be prognostic biomarkers, suggesting that these circRNAs in plasma may be involved in tumor progression. Li et al. (27) demonstrated that the expression levels of hsa_circ_0061276 and hsa_circ_0001017 before and after operation in plasma can be used as independent monitoring indexes for the recurrence of GC. In this research, the expression of a specific circRNA, hsa_circ_0021087, was lower in preoperative patients with GC compared with postoperative patients, demonstrating that it may be involved in the progression and occurrence of GC. This increase may be due to a decrease in the release of nucleic acids from tumor sources that inhibit the circRNA following tumor removal, leading to convincing changes in plasma hsa_circ_0021087 levels at the preoperative and postoperative stages. It was reported that the increase of circRNA abundance in exosome may be a possible mechanism for the increase in circRNA expression (3). Yet, there was no significantly different expression of hsa_circ_0005051 before and after surgery. Therefore, this result could be attributed to the fact that this circRNA may not be increased through exosomes.
It was reported that circRNA regulates mRNA expression through competing for miRNA (3). For example, circPDSS1 can sponge miR-186-5p to promote GC cell cycle and inhibit apoptosis (28). The present study demonstrated that hsa_circ_0021087 might be associated with cell proliferation in GC, determined by the CCK-8 assay. In order to further explore this mechanism, the hsa_circ_0021087-miRNA-mRNA network was predicted and analyzed the functional enrichment of the target genes. Hence, three kinds of miRNA (miR-1286, miR-184, and miR-450b-3p) and their 205 target genes were identified. Previous findings indicated that miR-184 can inhibit the expression of SND1, MMP-2/9, CD44 in breast cancer and glioma, and act as a tumor inhibitor miRNA by inhibiting the expression of p53 and p21 and activity of caspase-3/8 in glioblastomas (29). Additionally, it was reported that hsa-miR-184 can regulate apoptosis and invasion of GC (30). A previous study has shown that miR-450b-3p was downregulated in breast cancer and could inhibit HER3 expression through the PI3K/Akt pathway (31). LIM domain only 1 (LMO1), which as a hsa_circ_0021087 host gene, has been reported as an oncogene in multiple tumors, such as lung cancer (32), neuroblastoma (33) and gastric cancer (34). Pathway analysis revealed that it is also involved in several cancer-associated pathways, such as cell cycle, viral carcinogenesis and pathways in cancer. Above results revealed that hsa_circ_0021087 may promote the development of GC as an miRNA sponge, and its mechanism is worthy of further study.
Finally, the term “circRNAs” was used on the https://clinicaltrials.gov/ online website to search for clinical trials, in order to better understand the clinical use of circRNAs. CircRNAs were recruited in clinical trials as a biomarker of acute myocardial infarction (NCT03170830) in the year 2017 and neurological outcome after cardiac arrest (NCT02297776) in the year 2014. However, there are no clinical trials on circRNA biomarkers for GC. The current study provides evidence for circulating circRNAs as a non-invasive biomarker of GC.
This research first demonstrated significant downregulation of hsa_circ_0021087 and hsa_circ_0005051 in GC tissues, cells and plasma, indicating that the dual-circular RNA signature can be applied as a novel non-invasive biomarker in the diagnosis of GC. Next, hsa_circ_0021087 was demonstrated to have different expression in the plasma of GC patients at preoperative and postoperative stages and suggested that it might be involved in the occurrence and development of GC in vitro experiments.
Publicly available datasets were analyzed in this study. This data can be found here: Data is available at NCBI GEO, accession numbers: GSE83521, GSE89143, and GSE93541 and tcga database https://cancergenome.nih.gov/.
The studies involving human participants were reviewed and approved by The Institutional Review Board of Affiliated Hospital of Qingdao University. The patients/participants provided their written informed consent to participate in this study.
We have obtained consents to publish this paper from all the participants of this study.
LH, HH, and LY conceived and designed the experiments. LH, XZ, AW, YJ, XC, QQ, TY, LY, and HH collected and analyzed data. LH and HH wrote this manuscript. All authors read and approved the final manuscript.
The Department of Emergency Internal Medicine, Affiliated Hospital of Qingdao University supported this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful for the invaluable support and useful discussions with other members of the Department of Emergency and Cardiology.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00184/full#supplementary-material
Table S1. Detailed clinical information for QRT-PCR detection of circRNAs.
Table S2. Primers of circRNAs.
Figure S1. Hsa_circ_0021087 is derived from the second and third exons of the LIM domain only 1 gene on chromosome 21, and hsa_circ_0005051 is produced at the UBXN7 gene locus containing exon 4, 5 on chromosome 3.
Figure S2. The number of circular RNAs that can interact with microRNAs comes from the intersection of the circBank and circinteractome databases.
Figure S3. Hsa_circ_0005051–microRNA–mRNA interactions were constructed and visualized by Cytoscape software.
Figure S4. Functional analysis of hsa_circ_0005051. (a) GO analysis of hsa_circ_0005051 based on the ceRNA network. The BPs, MFs and CCs of top ten significantly enriched are listed. (b) KEGG pathway analysis of hsa_circ_0005051 based on the ceRNA network. GO, Gene Ontology. BPs, biological processes. MFs, molecular functions. CCs, cellular components. KEGG, Kyoto Encyclopedia of Genes and Genomes. ceRNA, competing endogenous RNA.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. (2015) 385:977–1010. doi: 10.1016/S0140-6736(14)62038-9
3. Wang Y, Lu T, Wang Q, Liu J, Jiao W. Circular RNAs: crucial regulators in the human body (Review). Oncol Rep. (2018) 40:3119–35. doi: 10.3892/or.2018.6733
4. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. (2013) 495:333–8. doi: 10.1038/nature11928
5. Wang KW, Dong M. Role of circular RNAs in gastric cancer: recent advances and prospects. World J Gastrointest Oncol. (2019) 11:459–69. doi: 10.4251/wjgo.v11.i6.459
6. Zhang Y, Liu H, Li W, Yu J, Li J, Shen Z, et al. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging. (2017) 9:1585–94. doi: 10.18632/aging.101254
7. Zhang X, Xu Y, Qian Z, Zheng W, Wu Q, Chen Y, et al. circRNA_104075 stimulates YAP-dependent tumorigenesis through the regulation of HNF4a and may serve as a diagnostic marker in hepatocellular carcinoma. Cell Death Dis. (2018) 9:1091. doi: 10.1038/s41419-018-1132-6
8. He JH, Li YG, Han ZP, Zhou JB, Chen WM, Lv YB, et al. The CircRNA-ACAP2/Hsa-miR-21–5p/ Tiam1 regulatory feedback circuit affects the proliferation, migration, and invasion of colon cancer SW480 cells. Cell Physiol Biochem. (2018) 49:1539–50. doi: 10.1159/000493457
9. Chen G, Shi Y, Zhang Y, Sun J. CircRNA_100782 regulates pancreatic carcinoma proliferation through the IL6-STAT3 pathway. Onco Targets Ther. (2017) 10:5783–94. doi: 10.2147/OTT.S150678
10. Guarnerio J, Zhang Y, Cheloni G, Panella R, Mae Katon J, Simpson M, et al. Intragenic antagonistic roles of protein and circRNA in tumorigenesis. Cell Res. (2019) 29:628–40. doi: 10.1038/s41422-019-0192-1
11. Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. (2015) 25:981–4. doi: 10.1038/cr.2015.82
12. Bonizzato A, Gaffo E, Te Kronnie G, Bortoluzzi S. CircRNAs in hematopoiesis and hematological malignancies. Blood Cancer J. (2016) 6:e483. doi: 10.1038/bcj.2016.81
13. Tang W, Fu K, Sun H, Rong D, Wang H, Cao H. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer. (2018) 17:137. doi: 10.1186/s12943-018-0888-8
14. Bai N, Peng E, Qiu X, Lyu N, Zhang Z, Tao Y, et al. circFBLIM1 act as a ceRNA to promote hepatocellular cancer progression by sponging miR-346. J Exp Clin Cancer Res. (2018) 37:172. doi: 10.1186/s13046-018-0838-8
15. Diboun I, Wernisch L, Orengo CA, Koltzenburg M. Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma. BMC Genomics. (2006) 7:252. doi: 10.1186/1471-2164-7-252
16. Liu M, Wang Q, Shen J, Yang BB, Ding X. Circbank: a comprehensive database for circRNA with standard nomenclature. RNA Biol. (2019) 16:899–905 doi: 10.1080/15476286.2019.1600395
17. Gyorffy B, Surowiak P, Budczies J, Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE. (2013) 8:e82241. doi: 10.1371/journal.pone.0082241
18. Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, Gorospe M. CircInteractome: a web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. (2016) 13:34–42. doi: 10.1080/15476286.2015.1128065
19. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. (2014) 42:D92–7. doi: 10.1093/nar/gkt1248
20. Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. (2015) 4:e05005. doi: 10.7554/eLife.05005
21. Li R, Qu H, Wang S, Wei J, Zhang L, Ma R, et al. GDCRNATools: an R/Bioconductor package for integrative analysis of lncRNA, miRNA and mRNA data in GD. Bioinformatics. (2018) 34:2515–17. doi: 10.1093/bioinformatics/bty124
22. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. (2012) 16:284–7. doi: 10.1089/omi.2011.0118
23. Koh W, Pan W, Gawad C, Fan HC, Kerchner GA, Wyss-Coray T, et al. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc Natl Acad Sci USA. (2014) 111:7361–6. doi: 10.1073/pnas.1405528111
24. Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, et al. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. (2015) 61:221–30. doi: 10.1373/clinchem.2014.230433
25. Zhang H, Zhu L, Bai M, Liu Y, Zhan Y, Deng T, et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer. (2019) 144:2501–15. doi: 10.1002/ijc.31977
26. Li S, Teng S, Xu J, Su G, Zhang Y, Zhao J, et al. Microarray is an efficient tool for circRNA profiling. Brief Bioinform. (2018) 20:1420–33. doi: 10.1093/bib/bby006
27. Li T, Shao Y, Fu L, Xie Y, Zhu L, Sun W, et al. Plasma circular RNA profiling of patients with gastric cancer and their droplet digital RT-PCR detection. J Mol Med. (2018) 96:85–96. doi: 10.1007/s00109-017-1600-y
28. Ouyang Y, Li Y, Huang Y, Li X, Zhu Y, Long Y, et al. CircRNA circPDSS1 promotes the gastric cancer progression by sponging miR-186–5p and modulating NEK2. J Cell Physiol. (2019) 234:10458–69. doi: 10.1002/jcp.27714
29. Feng R, Dong L. Inhibitory effect of miR-184 on the potential of proliferation and invasion in human glioma and breast cancer cells in vitro. Int J Clin Exp Pathol. (2015) 8:9376–82.
30. Xu Y, Ma H, Yu H, Liu Z, Wang LE, Tan D, et al. The miR-184 binding-site rs8126 T>C polymorphism in TNFAIP2 is associated with risk of gastric cancer. PLoS ONE. (2013) 8:e64973. doi: 10.1371/journal.pone.0064973
31. Zhao Z, Li R, Sha S, Wang Q, Mao W, Liu T. Targeting HER3 with miR-450b-3p suppresses breast cancer cells proliferation. Cancer Biol Ther. (2014) 15:1404–12. doi: 10.4161/cbt.29923
32. Du L, Zhao Z, Suraokar M, Shelton SS, Ma X, Hsiao TH, et al. LMO1 functions as an oncogene by regulating TTK expression and correlates with neuroendocrine differentiation of lung cancer. Oncotarget. (2018) 9:29601–18. doi: 10.18632/oncotarget.25642
33. Saeki N, Saito A, Sugaya Y, Amemiya M, Ono H, Komatsuzaki R, et al. Chromatin Immunoprecipitation and DNA Sequencing Identified a LIMS1/ILK Pathway Regulated by LMO1 in Neuroblastoma. Cancer Genomics Proteomics. (2018) 15:165–174. doi: 10.21873/cgp.20074
Keywords: gastric cancer, circRNAs, plasma, diagnosis, signature, Hsa_circ_0020187, Hsa_circ_0005051
Citation: Han L, Zhang X, Wang A, Ji Y, Cao X, Qin Q, Yu T, Huang H and Yin L (2020) A Dual-Circular RNA Signature as a Non-invasive Diagnostic Biomarker for Gastric Cancer. Front. Oncol. 10:184. doi: 10.3389/fonc.2020.00184
Received: 20 November 2019; Accepted: 03 February 2020;
Published: 21 February 2020.
Edited by:
Xiao Zhu, Guangdong Medical University, ChinaReviewed by:
Xinying Xue, People's Liberation Army General Hospital, ChinaCopyright © 2020 Han, Zhang, Wang, Ji, Cao, Qin, Yu, Huang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Huang, ZG9jaGFwcHlAMTI2LmNvbQ==; Lei Yin, cHVtYXlsQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.