
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 12 February 2020
Sec. Women's Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00120
There are many risk factors associated with breast cancer (BC) such as the familial history of BC, using hormone replacement therapy, obesity, personal habits, and other clinical factors; however, not all BC cases are attributed to these risk factors. Recent researches show a correlation between patient microbiome and BC suggested as a new risk factor. The present review article aimed at evaluating the role of the microbiome as a risk factor in the occurrence of BC, investigating the proposed mechanisms of interaction between the microbiome and human genes involved in BC, and assessing the impact of the altered composition of breast, gut, and milk microbiome in the physiological status of normal breast as well as cancerous or non-cancerous breast lesions. The study also evaluated the growing evidence that these altered populations may hinder chemotherapeutic treatment. The role of microbiome in the development and maintenance of inflammation, estrogen metabolism, and epigenetic alterations was properly investigated. Finally, clinical and therapeutic applications of the microbiome- e.g., probiotics, microbiome genome modulation, and engineered microbiome enzymes in the management of BC were reviewed.
Breast cancer (BC) is the most common cancer among women worldwide. It includes Luminal A, Luminal B, Her2-enriched, and triple-negative subtypes based on the expression of estrogen, progesterone and Her2 receptors, and Ki67 protein (1). Genetic factors, hormone replacement therapy, lifestyle, eating habits, and age are among the BC risk factors (2); however, they cannot explain all the BC cases and other possible risk factors should be considered. In past decades, microbial composition of human body (microbiota) raised so much attention in different areas, including cancer biology. There is a dynamic and complex relationship between the human host and microbiota. Bacteria and their metabolites can manipulate different signaling pathways- e.g., E-Cadherin/β-catenin (3), cause DNA double-strand breaks (4), promote apoptosis, alter cell differentiation (5), and interact with toll-like receptors (TLRs) of the innate immune system to trigger inflammatory signaling pathways and help maintain the hemostasis of the body (6). The interaction between the human microbiome and cancer is referred to as “oncobiome” (7). Moreover, the human host can also affect the microbiota and their mechanisms (8).
Based on recent studies, the microbiome is a risk factor for BC and an explanation for different responses to therapy (9). The question that arises is “How can microbiome affect BC risk; in which mechanisms they detain or improve different therapeutic approaches; and what are the effects of probiotics on breast cancer management? “The disruption of commensal bacteria communities results in dysbiosis and may contribute to the development of carcinomas (10). For instance, it is observed that exposure to antibiotics (e.g., clarithromycin, metronidazole, and ciprofloxacin) decreases the biodiversity and abundance of some bacterial communities and disrupts the balance of the gut microbiome (11, 12) associated with a higher risk of BC (13).
Although it is not proved yet that dysbiosis can cause BC, the comparison of breast tissue samples show differences in the composition and abundance of some specific bacterial taxa between patients and healthy individuals (8). Therefore, there might be a relationship between breast microbiome and BC occurrence, which should be investigated.
There are fewer studies on microbiome and BC than other cancers. Particular composition of gut, breast tissue, and milk microbiome and the critical evidence of interaction with the inflammatory system, estrogen metabolism, and genetic and epigenetic alterations were also discussed in the current review. Moreover, the effects and mechanisms of microbiome on different therapeutic approaches such as hormone, chemo-, radio-, and immuno-therapy of BC were scrutinized. Although there are some reviews on microbiome and breast cancer (14–18), little is known about the clinical applications of the microbiome such as probiotics, microbiome genome modulation, and engineered microbiome enzymes in BC therapy, which also were discussed at the end.
It was believed that breast tissue is sterile, but now it is known that breast tissue has its specific microbiome, which is different from those of other tissue such as gut (8). Since breast is made up of fatty tissue with extensive vasculature and lymphatic drainage, it can be a favorable environment for the growth of bacteria (19). Early studies focusing on pathogenic viruses such as human papillomavirus (HPV) showed a correlation between HPV infection and BC (20, 21). In a study, about 32% of mammary tumors had an association with Epstein–Barr virus (EBV) or human herpesvirus-4 (HHV-4) infections (22). These results were controversial since no other studies confirmed them (23, 24). Investigations show a trilateral relationship between HPV infection, and signal transducer and activator of transcription 3 (STAT3) activity, and interleukin 17 (IL-17) level. HPV infection activates STAT3 signaling, which in turn raises IL-17 level. Therefore, HPV can be a factor in the development of pro-inflammatory responses in breast tissue and, hence, contribute to BC progression (25). Further studies are needed to prove the possible role of breast virome, the viral community in breast tissue, in maintaining the physiological status of breast tissue.
Next-generation sequencing services paved the way for finding the microbiome composition of the breast (26). The breast microbiome may be accumulated through different routes either during breastfeeding from the skin via the nipple-areolar by nipple-mouth contact, intercourse, or even through bacterial translocation from the gut (27). Local dysbiosis is observed in BC tissue compared to non-BC tissue. The analysis of published data sets with bioinformatics platform for microbial genomics revealed that the composition of microbiome community varies in patients with BC in different ethnicities; it also varies in nipple aspirate of survivors compared to those of the healthy individuals, is different in benign and malignant BC tissue, and varies in BC subtypes (17). To explain it, Urbaniak et al. identified eight new species and seven bacterial phyla in breast tissue samples. Accordingly, Proteobacteria and Firmicutes have a higher frequency that may be due to the adaptation to a higher amount of breast tissue fatty acid in comparison with other tissue (8). Some species in these groups of bacteria show a significant increase in BC samples. The results of clinical studies on the relationship between BC and microbiome are summarized in Table 1.
Although some studies showed no evidence of bacterial abundance in BC (30), other investigations indicated that diversity (40) and abundance (41) of associated taxa are reduced in BC. The analysis of 16S rRNA showed a higher relative abundance of Enterobacteriaceae, Bacillus and Staphylococcus spp. in patients with BC. Interestingly, Escherichia coli (Enterobacteriaceae family) and Staphylococcus epidermidis induce double-stranded DNA break in BC cells (28).
There is a significant difference in the microbiome combination of malignant and benign breast tissue specimens (27) (Figure 1). An overview of taxonomic profiles showed that the overall microbiome of breast tissue was similar in benign and invasive BC, dominated by Bacteroidetes and Firmicutes. Assessing differential taxa between these two groups demonstrated malignancy correlated with enrichment in taxa of lower abundance, including the genera Fusobacterium, Atopobium, Gluconacetobacter, Hydrogenophaga, and Lactobacillus (27). The carcinogenicity of 10 infectious pathogens is proven (46). Therefore, a greater understanding of the effects of microbial agents within BC can expand the ability to prevent, diagnose, and treat it in the future. In this regard, studying viral, bacterial, fungal, and parasitic genomic sequences led to the discovery of two distinct microbial signatures in patients with triple-negative BC (TNBC) (29). However, further studies are needed to identify how these signatures affect BC development. Nevertheless, there is a large amount of evidence that microorganisms regulate the tumor microenvironment. Finding a unique microbial signature for TNBC may have diagnostic and targeted therapeutic applications in the future (29).
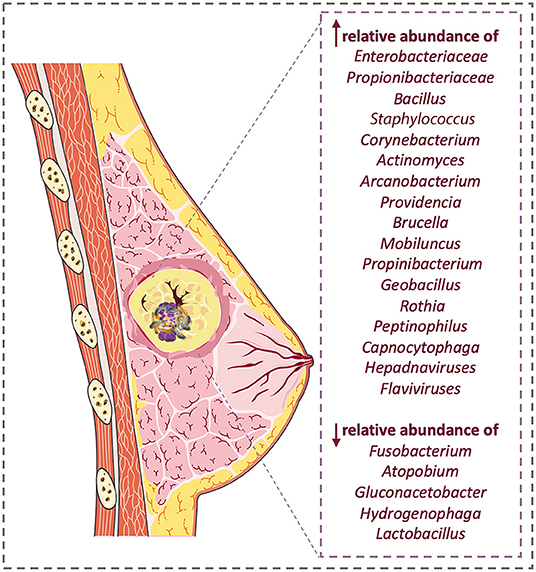
Figure 1. Breast cancer microbiome change; there are significant differences in microbiome population in patients compared with healthy samples.
Quantification of bacterial DNA in BC showed that Methylobacterium radiotolerance was abundant in tumor tissue, while Sphingomonas yanoikuyae (S. yanoikuyae) was dominant in the normal adjacent tissue samples (30). Additionally, the amount of bacterial DNA significantly reduced in tumor tissue compared to the adjacent tissue. The association of S. yanoikuyae with healthy tissue and its significant reduction in the tumor tissue might be a confirmation of the probiotic function of this microorganism in the breast. The lower amount of S. yanoikuyae in tumor areas led to reducing one-third of antibacterial gene expression responses. Innate immune system receptors such as TLRs 2, 5, 9, and the factors responsible for anti-microbial responses such as IL-12 subunit alpha (IL-12A), bactericidal/permeability-increasing protein (BPI), and myeloperoxidase (MPO) were expressed minor in the tumor in comparison with healthy tissue.
The difference in breast tissue microbiome profile between healthy individuals and patients with BC was also confirmed in another study (20). This difference was also observed in patients with BC at various clinical stages (39). There was a clinical trial (MICROMA) (NCT03885648) that evaluated contribution of bacteria, archaea, viruses, and fungi in breast tissue, stool, and urine samples with their alteration by environmental contaminants to the risk of BC. The results could contribute to elucidate risk factors, improve the prognosis, and propose new intervention studies in BC.
This evidence suggests that bacteria may maintain the healthy status of breast tissue by stimulating host inflammatory responses. Reduction of bacterial load in a healthy individual may exacerbate the risk of BC. These findings demonstrated an unknown link between dysbiosis and BC, and the potential diagnostic and therapeutic implications of these discoveries should be investigated in further investigations. Although many studies were performed on the body-wide impacts of microbiota, there is still no clear pattern about the direct effect of these microorganisms on the risk of BC.
Findings of the bacterial biodiversity in human breast milk and its changes over time are limited. However, culture-independent molecular techniques such as qPCR and NGS approaches allow valuable complementary assessments of the human milk microbiota.
Pregnancy, childbirth, postpartum period, diet, and consumption of antibiotics are key factors that affect the bacterial biodiversity of human milk. The bacteria of human milk originate from the gastrointestinal tract and are transferred to the breast through the entero-mammary path. They can also be transmitted from the infant's mouth via maternal skin contact during breastfeeding (47). Breast and milk microbiomes are almost similar. The most abundant phyla of both breast tissue and breast milk are Firmicutes, Actinobacteria, and Bacteroidetes (36). It is observed that frequent bacterial strains, including Staphylococcus, Serratia, Corynebacteria, and Streptococcus are the most abundant bacteria in milk (48). In the studies on human milk, various species of Bifidobacterium are reported (49).
Furthermore, several cohort studies are conducted based on geographical variations (50–52) demonstrating that the microbial community is significantly variable in different geographic locations. For instance, the highest relative abundance of Proteobacteria, Firmicutes, Streptococcus, Propionibacterium, and Pseudomonas were in South African, Finnish, Chinese, and Spanish human breast milk samples, respectively. It is assumed that geographical distinction creates remarkable changes in the composition of the microbiome (50).
Milk contains more than 360 genera of prokaryotes including phyla of Proteobacteria (65%), Firmicutes (34%), and the genera of Pseudomonas (61.1%), Aureus (33.4%), and Streptococcus (0.5%). Milk has less biodiversity in comparison with infants' and mothers' stools at the level of phylum. Researches show that enriched immune-modulatory DNA motifs of Lactobacillus contribute to immune development by modulating the immune responses (53). Moreover, studies by Ward et al. on the functional capacity of milk metagenome showed that these sequences along with open reading frames associated with nitrogen metabolism, membrane transport, and stress response in the human intestine resulted in the colonization of the newborn's gut and immunity. Given the presence of immune-modulatory motifs in the milk metagenome, further investigations on this biological fluid are warranted (54). Some of these motifs in commensal bacteria can hypothetically be used for therapeutic purposes.
Understanding the functions and composition of the human milk microbiota has critical implications in terms of the infant gut microbiome establishment and the mammary health since dysbiosis in the milk microbiome may prime to mastitis (55). Metagenomic analysis indicated that Firmicutes, Proteobacteria, and Bacteroidetes were not found in milk samples of patients with mastitis when compared to samples from healthy individuals (56). However, S. aureus and S. epidermidis were abundant in patients with acute and subacute mastitis, respectively.
In contrast, Staphylococcus, Streptococcus, Bacterioides, Faecalibacterium, Ruminococcus, Lactobacillus, and Propionibacterium species were isolated from samples obtained from healthy individuals (56). Lower microbial diversity, depletion of commensal obligate anaerobes, and increased abundance of opportunistic pathogens in patients with mastitis were confirmed in another study. Functional metagenomics identified several gene pathways in bacterial secretion system and motility proteins related to bacterial proliferation and colonization in sub-acute and acute mastitis samples. It was reported that ~45% of genes belonged to metabolism, 18% to environmental information and processing, 14% to genetic information processing, and 1% to human diseases (57).
A significant difference in the microbiome composition of nipple aspirate fluid between healthy individuals and patients with BC suggested the potential role of the ductal microbiome in BC incidence. The analysis of nipple aspirate fluid in patients with BC revealed a relatively high proportion of Alistipes genus, while an unclassified strain of the Sphingomonadaceae family was more abundant in healthy individuals. Moreover, BC-related microbes increase beta-glucuronidase activity that may increase cancer risk (31). It is also proved that chemotherapy results in significant deviations of healthy microbial populations and their metabolomic profiles. A decrease in Bifidobacterium, Eubacterium, Aureus, and Clobacterium species and an increase in Acinetobacter, Xanthomonadaceae, and Stenotropomonas species were observed in the milk of healthy females and those of the ones undergoing chemotherapeutic treatment for Hodgkin's lymphoma (58).
The human gastrointestinal tract supports the growth of beneficial microbiota owing to their ability to protect the body against pathogens (59). Their contribution to immune system development and maintenance (60), the fermentation of indigestible fibers into short-chain fatty acids (SCFAs) (61), production of essential amino acids (62) and vitamins (63), absorption of minerals (64), and deactivation of toxins (65) and carcinogens (66) are among their benefits. Animal model studies showed associations between the microbiome and the development of many diseases, including cancer (67, 68). Diet can contribute to the development of various diseases, including cancer (69), since it has a direct role in controlling the composition of the microbial community. Accordingly, a plant-based diet stimulates bacterial diversity (70), while animal-based regimen decreases the Firmicutes population (the common bacterial phylum in breast tissue) in digestive system. Shifting to a plant-based diet would increase Firmicutes population (71). Thus, changes to the diet might contribute to the development of diseases through alternation in microbial metabolism and production of toxic metabolites.
Some evidence shows that the microbiome metabolite influences the occurrence of BC; this hypothesis may help estimate the cancer risk and prevention. For instance, cadaverine as a biogenic amine is formed through the direct decarboxylation of L-lysine (45). It is reported that cadaverine biosynthesis is reduced in the gut in early-stage BC, resulting in lower production of an anti-cancer bacterial metabolite and reduced BC invasion (45). It is shown that bacterial metabolites can stimulate oxidative and nitrosative stress that inhibit BC progression. These metabolites such as lithocholic acid can inhibit BC progression, epithelial-mesenchymal transition, and metastasis via activation of nuclear factor erythroid 2-related factor 2 (NRF2) and other proteins involved in the antioxidant defense system. Therefore, decreased microbiome diversity and quantity in gut microbiota affects these anti-proliferative metabolites that may result in BC or its progression (43). As previously mentioned, SCFAs are produced by microbiome when dietary fiber is fermented in the colon (72). Propionate, acetate, and butyrate are the three most predominant SCFAs. They are well-known modulators for cell invasion and apoptosis in BC (73). SCFAs can positively or negatively affect BC (74). The abundance of Akkermansia muciniphila, as a key player of propionate production, is associated with the richness of the gut microbiota in patients with BC (44).
It was shown that intestinal bacteria can turn some plant lignans such as flaxseed, sunflower, caraway, pumpkin, legumes, and soybean (75), into mammalian lignans with protective effects against BC (76–78). Lignans of edible plants are converted to enterolignans, enterolactone, and enterodiol by the intestinal microbiome. It is suggested that enterolactone may act as a selective modulator of estrogen signaling and may be associated with lowering the risk of BC (79, 80).
Additionally, lignans consumption may both enhance the survival of postmenopausal female patients with BC (81) and reduce the risk of BC before menopause (82). A significant inverse correlation was observed with BC risk in premenopausal females daily receiving ≥30 g of fiber, fruits, or seeds (83). Also, high consumption of raw vegetables showed a significant protective effect against BC risk; being dropped by 34% (84). It was observed that high levels of plant dietary fibers in the gut resulted in proliferation of Bifidobacterium and Faecalibacterium prausnitzii (85) with anti-inflammatory (86, 87) and anti-tumor effects (88). Studies on twins represent that obesity alters the balance of Firmicutes in non-obese individuals to Bacteroidetes phyla in obese ones (89). This shift may result in the increase of estrogen levels in the blood and contribute to higher risk of BC. Diet is the main element of gut microbial diversity. But, recent studies on non-human primates show the effect of Mediterranean diet on increasing mammary gland Lactobacillus abundance and upper levels of bile acid metabolites (90).
Therefore, it can be concluded that modifying dietary patterns affects the microbiome population and indirectly affects disease occurrence (91).
Using antibiotics can affect the target pathogen and the commensal inhabitants of the human host. The extent of the impact on non-target microbial populations depends on the particular antibiotic used, its mode of action, and the degree of resistance in the community. Bhatt et al. found that irregular use of antibiotics increases the probability of dysbiosis and lower bacterial diversity (92). Knekt et al. showed that the overuse of antibiotics might reduce the plasma level of lignan enterolactone; therefore, it might directly affect the microbiome populations and increase the BC risk (93). However, it is acknowledged that selection of antibiotics leads to antimicrobial resistance, but it is believed that the commensal microbiota is normalized a few weeks after treatment secession (11).
In comparison with other human organs, the microbial load and its variety are increased in the digestive system, especially in the large intestine (94). This complex intestinal microbiome plays a significant role in both local and distal areas of the body through the production of metabolites, hormonal intermediates, and immunologic cytokines (95). It was shown that higher phylogenetic diversity in the intestinal microbiome raises hydroxylated estrogen metabolites in the urine of a healthy female (96). In postmenopausal females, an increased level of circulating estrogen is associated with increased risk of BC (97). Intestinal microbiome is one of the major regulators of circulating estrogens (98). Therefore, dysbiosis in the gut microbiome potentially disrupts homeostasis through the disruption of estrogen metabolism (98). It is suggested that estrobolome, the bacterial gene mass in the human intestine, the products of which take part in estrogens metabolism, may increase the risk of estrogen receptor-positive BC in postmenopausal females (99, 100). Additionally, it was shown that a decrease in stool bacterial biodiversity leads to estrogen excretion and finally elevation of the BC risk (101). In contrast, an increase in the levels of estrogen metabolites, compared to parent estrogens, estrone, and estradiol, decreases the risk of BC (102).
Postmenopausal females recently received BC diagnosis, had higher levels of urinary estrogen, which does not correlate with their bacterial biodiversity (40). Compared to controls, postmenopausal patients with BC had significant estrogen-independent associations with the IgA+/IgA− gut microbiota, suggesting that the gut microbiome may influence the BC risk by altered metabolism, estrogen recycling, and immune pathway (42).
Interestingly, intestinal bacteria are capable of converting plant lignans into enterolignans (103), which can induce estrogenic effect (104). Furthermore, phytoestrogen can start signaling through estrogen receptors when present at extremely high levels, reducing the activity of natural estrogen hormones in the body and blocking the effect of estrogen in specific tissue (105, 106). Therefore, changes in gut microbiome composition may lead to the estrogen metabolism alternation and affect the BC risk.
The microbiome might also diminish the risk of BC by modulating the functional estrogen. The current hormone replacement therapies might be further enhanced by combining the microbiota, which acts individually or synergistically to improve a more comprehensive therapeutic approach for metabolic diseases after menopause (107).
Notably, it is found that gut microbiome diversity might contribute to better psychosocial and cardiorespiratory fitness outcomes in the post-primary treatment of BC survivors. Microbial profiling of fecal samples showed that prospective alteration in the gut microbiome was significantly associated with depression, anxiety, fatigue, and cardiorespiratory fitness outcomes related to the quality of life of the patients. The extent of longitudinal changes in fatigue, anxiety, and cardiorespiratory fitness was correlated with the frequency of the Prevotella, Faecalibacterium, Bacteroides, and Coprococcus genera, a subset of the Clostridiaceae family, and SMB53 and Roseburia genera (108).
Bacterial populations employ molecular signals to communicate with each other (109, 110). Bacteria can communicate with ecological conditions, detect environmental changes, or sense the abundance and type of living bacterial species via chemical communications called quorum sensing (111). De Spiegeleer et al. proved that many pathogenic and commensal bacterial species produce quorum sensing compounds in the human intestine. These endogenous compounds including phosphatase RapG inhibitor (PhrG) produced by Bacillus subtilis, competence stimulating peptide (CSP) produced by Streptococcus mitis, and extracellular death factor (EDF) produced by E. coli, cause in vitro angiogenesis and BC cells invasion (112).
Dysbiosis of the microbial population often results in the disruption of the host immune system. Changes in the microbial community lead to lymphocytes decrease, and neutrophils increase, both of which can contribute to a reduction in survival of patients with BC (107). Studies on mice specimens showed that gut microbiome alterations lead to breast tumorigenesis (113). There is an ongoing clinical trial (NCT02696759) that investigates whether the gut microbiome plays a role in fighting advanced BC by affecting the efficacy of immune cells.
According to another study, the proportions of Blautia and F. Prausnitzii and absolute numbers of Blautia and Bifidobacterium species in the gut microbiome are directly correlated with the clinical stage of BC. For instance, patients with stage 1 BC had a lower number of gut Blautia sp. in comparison with the ones with stage grade 3 (41). The presented shreds of evidence showed that the gut microbiome plays a fundamental role in the development of various diseases, including cancer. Therefore, therapeutic targeting of the gut microbiome should be explored as part of the preventative and therapeutic approaches.
Correlations between the human microbiome and BC open up new horizons for the prognosis and treatment of cancer. Hence, researchers focus on the therapeutic application of microbiome (Figure 2).
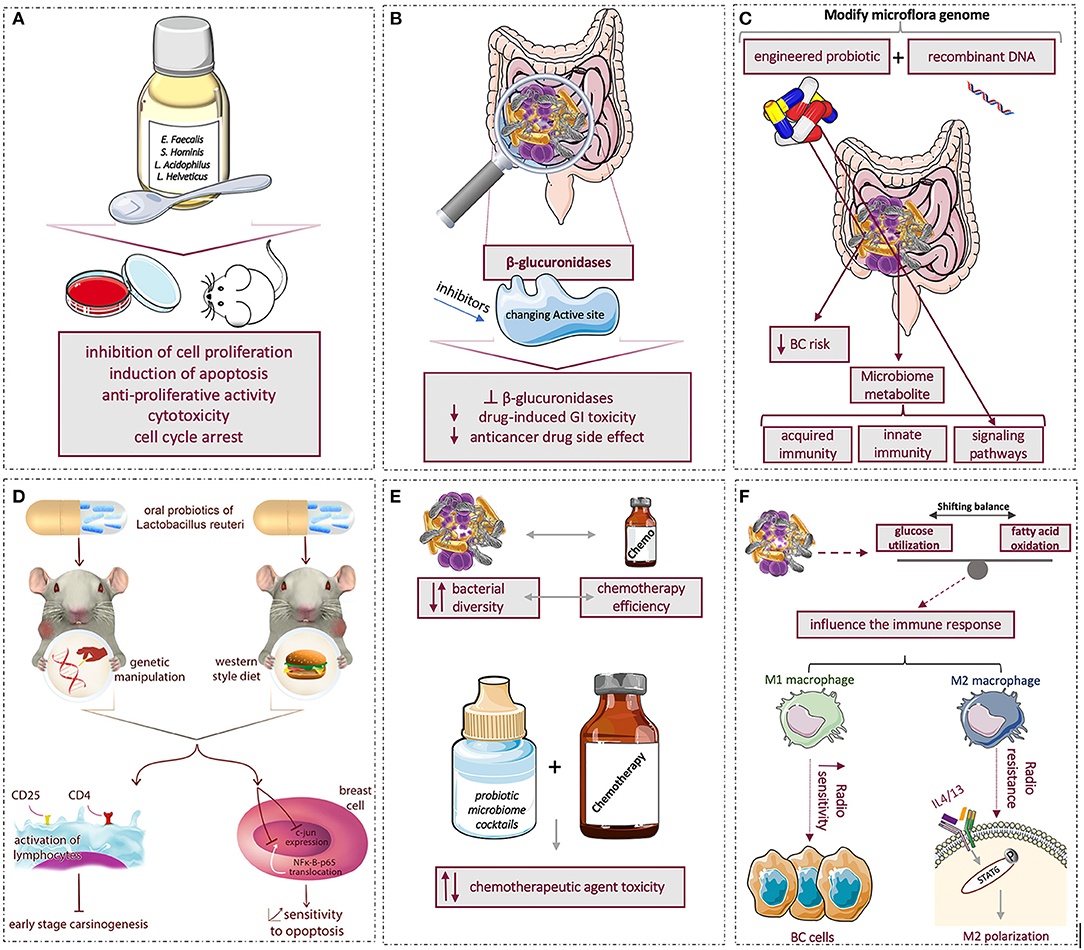
Figure 2. Summary of microbiome effects and applications in breast cancer: (A) using probiotics to affect tumor progression by inhibiting cell proliferation and inducing apoptosis (B) conformational changes in β-glucoronidaze enzyme active site and different catalytic activities with genetic engineering might serve as targets to decrease the anticancer drug-induced toxicity, (C) using novel engineered recombinant probiotic to modify and target gut microbiome to reduce breast cancer risk, (D) The left-side mouse model was genetically manipulated to develop human breast tumor while right-side mouse model was fed by the Western-style diet to develop mammary tumors. Both models were treated with oral intake of probiotic lactic acid microbe, Lactobacillus reutri. The investigation showed that oral probiotic activates CD4+ and CD25+ lymphocytes and inhibits early-stage breast carcinogenesis. Moreover, oral probiotic prevented c-jun expression and NFκ-B-p65 translocation in the nucleus of breast cells and raised breast cell sensitivity to apoptosis, (E) reciprocal interaction of chemotherapy with bacterial diversity; using combination of chemotherapy and probiotics microbiome cocktails showed no effect, decreased and in some cases increased the chemotherapy agent toxicity, (F) microbiome by shifting balance of glucose utilization and fatty acid oxidation can indirectly affect immune system; hence, during radiotherapy, M1 macrophages increase the radio-sensitivity of BC cells, but M2 macrophages trigger radio-resistance via IL-4/IL-13-mediated STAT6 phosphorylation and M2 polarization.
Several in vitro and in vivo studies investigated the effect of probiotics on BC; for instance, significant inhibition of cell proliferation, induction of apoptosis, and cell cycle arrest of Enterococcus faecalis and Staphylococcus hominis are proved (114). Lakritz et al. studied two groups of mice: a group manipulated to develop human breast tumors and the other group fed by a Western-style diet (high fat and sugar, low vitamin D3, vitamin C, and fiber) to develop mammary tumors. The two groups were treated with oral intake of probiotic lactic acid microbes. The results showed that the probiotic Lactobacillus reuteri inhibited early-stage carcinogenesis and raised breast cell sensitivity to apoptosis (115).
Additionally, it was confirmed that oral administration of L. acidophilus represents anticancer activity in mice bearing breast tumors (116). Another in vivo study showed that drinking milk fermented with Lactobacillus helveticus R389 elevated IL-10 and decreased IL-6 levels both in serum and mammary cells of mice, which lead to breast tumor cell inhibition (117). Moreover, anticancer effects of probiotics on cancer cell lines are well gathered in the review by Mendoza et al. They showed anti-proliferative activity, apoptosis, cytotoxicity, and cell cycle arrest of probiotics (118). Long-term exposure to probiotics such as L. casei Shirota and soy isoflavones in Japanese females demonstrated their chemopreventive effect on cancer development (119).
Although the mentioned studies provided the evidence that probiotics display activity against BC, there are still essential questions on the use of probiotics in BC. The strains, dosage, and regimen of probiotics should be determined based on the clinical feature of BC and probiotics interaction with the conventional treatment. Probiotics are already used in the treatment of a wide range of diseases; however, their application to BC is in its infancy. There are also some clinical trials on probiotics and BC. A study demonstrated that two species of Lactobacillus can treat mastitis (120). Thus, probiotics might be good alternatives for antibiotics to treat breast infections during breastfeeding (120).
In the (NCT03358511) clinical trial, the role of probiotics on the number of CD8+ T-cells at stages 1–3 BC in post-menopausal patients is under investigation. Twenty post-menopausal females with BC took probiotics (15 billion colony-forming units of 13 beneficial bacterial species) for 2–4 weeks, three times a day. Another trial (NCT03760653) determined the effect of probiotics supplementation (Lactobacillus rhamnosus, Lactobacillus paracasei, Lactobacillus acidophilus, and Bifidobacterium bifidum) and physical exercise on the bacterial balance of gut, immune system, and the quality of life in BC survivors.
Although using probiotics inhibited tumor growth, induced apoptosis, and also enhanced the immune system in in vitro and in vivo studies, using them as a promised treatment in the clinical practice is not established yet. Although not confirmed, it seems that the tissue-specific microbiome cannot be changed even with long-term applications of probiotics; hence, the aforementioned positive effects might be obtained by modulating the gut microbiome and subsequently preventing tumorigenesis in the breast.
TLRs are deregulated in some types of cancers and can drive a pathological immune activation in response to the normal microbial mass (121). For instance, it is suggested that activation of TLR5 by S. typhimurium flagellin in BC cells mediates the pro-inflammatory responses to obtain an effective anti-tumor activity, which may serve as a novel therapeutic target for BC therapy (122). Successful inhibition of this pathway would be a unique therapeutic avenue worth exploring.
Another suitable therapeutic approach is the potential of the bacterial β-glucuronidases enzyme (123, 124) released by the gut microbiome, and is required for the healthy digestive system and xenobiotic metabolism (125, 126). Small changes in the structure of inhibitors can cause particular conformational changes in the enzyme active site and different catalytic activities, which might serve as targets to decrease the anticancer drug-induced toxicity in the intestinal tract; in other words, they can reduce the side effects of anti-breast cancer drugs (123). As this enzyme has substantial roles, beneficial engineered bacteria with modified proteins might be therapeutically used to minimize drug side effects. This area needs more detailed information at basic and clinical levels to confirm the positive effects of the microbiome and its metabolites on gene-based therapy in patients with cancer.
The microbiome affects chemo-, immuno-, and radio-therapy for BC. In the absence of commensal microbes, the activity of platinum-based drugs is diminished, although they enter the cells. DNA damage and double-strand breaks are essential in platins. The production of ROS promotes these mechanisms via microbes (127). For example, Lactobacillus acidophilus can restore the cisplatin antitumor activity in germ-free mice (9).
There is clinical evidence of immunotherapy showing better survival in triple-negative BC with anti-PD1 antibody (128). Previous studies show that antibiotic therapy for lung cancer and, accordingly, reduction of Akkermansia muciniphila would diminish the effect of anti-PD1 antibody and reduce the survival time in patients. It is hypothesized that T-cell mediated response is stimulated by IL-12, and the increase of anti-tumor activity of cytotoxic T-cells in response to A. muciniphila may improve the clinical response to this antibody (129). The same mechanism can be extended in BC, while antibiotics are often prescribed to females with BC during or after surgery.
There are limited studies on microbiome and radio-response in BC. Previous studies showed that cancer radiotherapy has less effect on germ-free mice compared to intact mice (130). It can be concluded that antibiotic therapy may reduce the effect of cancer radiotherapy. Intestinal bacteria and fungi can alter the immune system in determining the response to radiation. It was shown that during macrophage polarization, the metabolic situation of the cell would be changed (131). Radiotherapy response or resistance is highly dependent on tumor microenvironment (TME). The byproducts of the microbiome alter the metabolic situation by shifting the balance between glucose utilization and fatty acid oxidation, influencing the immune response in the TME (132). This alteration may change radio-sensitivity of the cancer cells. M1 macrophages enhance the radio-sensitivity of BC cells, but M2 macrophages trigger radio-resistance via IL-4/IL-13-mediated STAT6 phosphorylation and M2 polarization (132, 133).
While chemotherapy can change the bacterial diversity, specific microbiome composition can, in turn, modify the efficacy of chemotherapy. Therefore, it is feasible that specific probiotic microbiome cocktails can be administered in combination with chemotherapeutic agents to enhance their functionality (92). Lehouritis et al. examined the effects of bacteria through their enzymatic regulation on 30 common chemotherapy medications in vitro. They revealed that wild-type bacteria might increase the toxicity of six chemotherapeutic agents, decrease the toxicity of nine others (including doxorubicin), and not affect the toxicity of the rest 15 drugs. Thus, the response to therapy in BC tumors may be improved by microbiome modulation (134). Local and systemic bacterial infections act as in situ bio-transforming reservoirs. They influence treatment efficacy and increase the toxicity outside the targeted area that may complicate the cancer treatment process (134).
To date, few studies addressed the link between the gut microbiome and BC chemotherapy. NCT03586297 demonstrates the associated dominance of specific gut and intratumoral microbiome with the pathologic response in newly diagnosed patients with TNBC receiving AC-T neoadjuvant chemotherapy. Another trial (NCT04138979) recruited 80 participants to explore more information about the intestinal microbiome of patients with BC undergoing chemotherapy. These studies can prove that gut microbiome analysis holds the potential to predict patient response to chemotherapy before treatment and personalize medicine. However, further studies are required to understand the biochemical interactions between therapeutic agents and bacteria. In addition, new combinations of chemotherapy with probiotic treatments should be taken into consideration.
Microbiome engineering may open new horizons in prevention, diagnosis, and treatment of cancer. As mentioned above, alterations in gut bacterial populations may increase the risk of cancer. Therefore, designing antibiotics that target a particular spectrum of the microbiome might help regulate the gastrointestinal microbiome as a possible way to reduce the BC risk (135). Since cancer occurrence is affected by suppression of the immune system and many proinflammatory pathways (136), it is not surprising that the microbiome and bacterial metabolites might have a direct effect on the incidence or progression of cancer (137, 138). It may occur through changes in the activation of signaling pathways (139) as well as the innate and acquired immune responses (60, 140).
Consequently, engineered probiotics might be useful in targeting these signaling pathways. The combination of microbiome engineering and recombinant DNA technology can be utilized to modify the genome of vital microflora compartments and reprogram microbial mechanisms (141). The future discovery of dominant or unique members of the microbial population has the potential to drive new ideas in bacteriotherapy (142–145).
Microbiome diversity across different people caused by pathological, physiological, and environmental differences is a crucial challenge when trying to define beneficial or pathological microbial signatures. The complex diversity of these microorganisms makes it challenging to identify specific cancer signatures that are stable over time.
The microbiota plays a crucial role in preserving the health status of the human body, and their impairment causes pathobiological changes, including BC. Although the evidence of the correlation of microbiome with BC is undeniable, there are essential questions to unlock the exact role of the microbiome in the development and treatment of BC. Strains, dosage, and regimen of probiotics based on the clinical feature of BC and probiotics interaction with the conventional treatment are not determined entirely yet. Further studies are needed to find the exact relationship between microbiome and cancer. In other words, it needs to be clarified whether microbiome alteration leads to cancer or cancer occurrence leads to microbiome alteration, whether dysbiosis is carcinogenic or if there is a way to regulate dysbiosis. To answer these questions, large-scale studies including animal models, retrospective and prospective ones, as well as clinical trials should be designed. Engineered bacteria such as probiotic products might be new modalities to develop a therapeutic approach on clinical scale.
This manuscript was originally submitted to the Cancer Metabolism section of Frontiers in Oncology.
RE and KM-A: conceptualization. RE, ZE-S, SH, and FB: drafting of the manuscript. RE, SH, and ZE-S: designing figures and tables. RE, SH, ZE-S, and KM-A: review and editing of the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors hereby wish to acknowledge their gratitude to Dr. Stephanie Ana Conos for proof-reading and suggestion.
1. Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surgical Oncol Clin North America. (2018) 27:95–120. doi: 10.1016/j.soc.2017.08.005
2. Lacey JV, Kreimer AR, Buys SS, Marcus PM, Chang S-C, Leitzmann MF, et al. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer. (2009) 9:84. doi: 10.1186/1471-2407-9-84
3. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. (2013) 14:195–206. doi: 10.1016/j.chom.2013.07.012
4. Nougayrède J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. (2006) 313:848–51. doi: 10.1126/science.1127059
5. Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, et al. Mice lacking secretory phospholipase A 2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. (1998) 114:675–89. doi: 10.1016/S0016-5085(98)70581-5
6. Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. (2009) 9:57. doi: 10.1038/nrc2541
7. Thomas RM, Jobin C. The microbiome and cancer: is the 'oncobiome'mirage real? Trends Cancer. (2015) 1:24–35. doi: 10.1016/j.trecan.2015.07.005
8. Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, et al. Microbiota of human breast tissue. Appl Environ Microbiol. (2014) 80:3007–14. doi: 10.1128/AEM.00242-14
9. Armstrong H, Bording-Jorgensen M, Dijk S, Wine E. The complex interplay between chronic inflammation, the microbiome, and cancer: understanding disease progression and what we can do to prevent it. Cancers. (2018) 10:e83. doi: 10.3390/cancers10030083
10. Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep. (2014) 16:406. doi: 10.1007/s11912-014-0406-0
11. Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE. (2010) 5:e9836. doi: 10.1371/journal.pone.0009836
12. Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. (2008) 6:e280. doi: 10.1371/journal.pbio.0060280
13. Kilkkinen A, Rissanen H, Klaukka T, Pukkala E, Heliövaara M, Huovinen P, et al. Antibiotic use predicts an increased risk of cancer. Int J Cancer. (2008) 123:2152–5. doi: 10.1002/ijc.23622
14. Baxevanis CN, Fortis SP, Perez SA. The balance between breast cancer and the immune system: Challenges for prognosis and clinical benefit from immunotherapies. Semin Cancer Biol. (2019) pii: S1044-579X(19)30418-3. doi: 10.1016/j.semcancer.2019.12.018. [Epub ahead of print].
15. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan B, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 25:377–88. doi: 10.1038/s41591-019-0377-7
16. Mani S1. Microbiota and breast cancer. Prog Mol Biol Transl Sci. (2017) 151:217–29. doi: 10.1016/bs.pmbts.2017.07.004
17. Parida S, Sharma D. The power of small changes: comprehensive analyses of microbial dysbiosis in breast cancer. Biochim Biophys Acta Rev Cancer. (2019) 1871:392–405. doi: 10.1016/j.bbcan.2019.04.001
18. Picardo SL, Coburn B, Hansen AR. The microbiome and cancer for clinicians. Crit Rev Oncol Hematol. (2019) 141:1–12. doi: 10.1016/j.critrevonc.2019.06.004
19. O'Connor H, MacSharry J, Bueso YF, Lindsay S, Kavanagh EL, Tangney M, et al. Resident bacteria in breast cancer tissue: pathogenic agents or harmless commensals? Disc Med. (2018) 26:93–102.
20. Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel A, Al Moustafa A. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer. (2008) 99:404–7. doi: 10.1038/sj.bjc.6604503
21. Heng B, Glenn W, Ye Y, Tran B, Delprado W, Lutze-Mann L, et al. Human papilloma virus is associated with breast cancer. Br J Cancer. (2009) 101:1345–50. doi: 10.1038/sj.bjc.6605282
22. Fina F, Romain S, Ouafik LH, Palmari J, Ayed FB, Benharkat S, et al. Frequency and genome load of Epstein-Barr virus in 509 breast cancers from different geographical areas. Br J Cancer. (2001) 84:783. doi: 10.1054/bjoc.2000.1672
23. Glaser SL, Ambinder RF, DiGiuseppe JA, Horn-Ross PL, Hsu JL. Absence of Epstein-Barr virus EBER-1 transcripts in an epidemiologically diverse group of breast cancers. Int J Cancer. (1998) 75:555–8. doi: 10.1002/(SICI)1097-0215(19980209)75:4<555::AID-IJC10>3.0.CO;2-8
24. Herrmann K, Niedobitek G. Lack of evidence for an association of Epstein-Barr virus infection with breast carcinoma. Breast Cancer Res. (2003) 5:R13–7. doi: 10.1186/bcr604
25. Zhang N, Ma ZP, Wang J, Bai HL, Li YX, Sun Q, et al. Human papillomavirus infection correlates with inflammatory Stat3 signaling activity and IL-17 expression in patients with breast cancer. Am J Transl Res. (2016) 8:3214.
26. Urbaniak C, Burton JP, Reid G. Breast, milk and microbes: a complex relationship that does not end with lactation. Women's Health. (2012) 8:385–98. doi: 10.2217/WHE.12.23
27. Hieken TJ, Chen J, Hoskin TL, Walther-Antonio M, Johnson S, Ramaker S, et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. (2016) 6:30751. doi: 10.1038/srep30751
28. Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. (2016) 82:5039–48. doi: 10.1128/AEM.01235-16
29. Banerjee S, Wei Z, Tan F, Peck KN, Shih N, Feldman M, et al. Distinct microbiological signatures associated with triple negative breast cancer. Sci Rep. (2015) 5:15162. doi: 10.1038/srep15162
30. Xuan C, Shamonki JM, Chung A, Dinome ML, Chung M, Sieling PA, et al. Microbial dysbiosis is associated with human breast cancer. PLoS ONE. (2014) 9:e83744. doi: 10.1371/journal.pone.0083744
31. Chan AA, Bashir M, Rivas MN, Duvall K, Sieling PA, Pieber TR, et al. Characterization of the microbiome of nipple aspirate fluid of breast cancer survivors. Sci Rep. (2016) 6:28061. doi: 10.1038/srep28061
32. Wang H, Altemus J, Niazi F, Green H, Calhoun BC, Sturgis C, et al. Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget. (2017) 8:88122–38. doi: 10.18632/oncotarget.21490
33. Yazdi HR, Movafagh A, Fallah F, Alizadeh Shargh S, Mansouri N, Heidary Pour A, et al. Evaluation of Methylobacterium radiotolerance and Sphyngomonas yanoikoaie in sentinel lymph nodes of breast cancer cases. Asian Pacific J Cancer Prev. (2016) 17:279–85. doi: 10.7314/APJCP.2016.17.S3.279
34. Thompson KJ, Ingle JN, Tang X, Chia N, Jeraldo PR, Walther-Antonio MR, et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS ONE. (2017) 12:e0188873. doi: 10.1371/journal.pone.0188873
35. Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Peck KN, et al. Distinct microbial signatures associated with different breast cancer types. Front Microbiol. (2018) 9:951. doi: 10.3389/fmicb.2018.00951
36. Costantini L, Magno S, Albanese D, Donati C, Molinari R, Filippone A, et al. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci Rep. (2018) 8:16893. doi: 10.1038/s41598-018-35329-z
37. Meng S, Chen B, Yang J, Wang J, Zhu D, Meng Q, et al. Study of microbiomes in aseptically collected samples of human breast tissue using needle biopsy and the potential role of in situ tissue microbiomes for promoting malignancy. Front Oncol. (2018) 8:318. doi: 10.3389/fonc.2018.00318
38. Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. (2014) 99:4632–40. doi: 10.1210/jc.2014-2222
39. Bard J-M, Luu HT, Dravet F, Michel C, Moyon T, Pagniez A, et al. Relationship between intestinal microbiota and clinical characteristics of patients with early stage breast cancer. FASEB J. (2015) 29(Suppl.1):914.2. doi: 10.3390/ijerph15081747
40. Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst. (2015) 107:djv147. doi: 10.1093/jnci/djv147
41. Luu TH, Michel C, Bard J-M, Dravet F, Nazih H, Bobin-Dubigeon C. Intestinal proportion of Blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr Cancer. (2017) 69:267–75. doi: 10.1080/01635581.2017.1263750
42. Goedert JJ, Hua X, Bielecka A, Okayasu I, Milne GL, Jones GS, et al. Postmenopausal breast cancer and oestrogen associations with the IgA-coated and IgA-non-coated faecal microbiota. Br J Cancer. (2018) 118:471–9. doi: 10.1038/bjc.2017.435
43. Mikó E, Vida A, Kovács T, Ujlaki G, Trencsényi G, Márton J, et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim Biophys Acta. (2018) 1859:958–74. doi: 10.1016/j.bbabio.2018.04.002
44. Fruge AD, Van der Pol W, Rogers LQ, Morrow CD, Tsuruta Y, Demark-Wahnefried W. Fecal Akkermansia muciniphila is associated with body composition and microbiota diversity in overweight and obese women with breast cancer participating in a presurgical weight loss trial. J Acad Nutr Diet. (2018). doi: 10.1016/j.jand.2018.08.164. [Epub ahead of print].
45. Kovács T, Mikó E, Vida A, Sebo É, Toth J, Csonka T, et al. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci Rep. (2019) 9:1300. doi: 10.1038/s41598-018-37664-7
46. De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. (2012) 13:607–15. doi: 10.1016/S1470-2045(12)70137-7
47. McGuire MK, McGuire MA. Got bacteria? The astounding, yet not-so-surprising, microbiome of human milk. Curr Opin Biotechnol. (2017) 44:63–8. doi: 10.1016/j.copbio.2016.11.013
48. Hunt KM, Foster JA, Forney LJ, Schütte UM, Beck DL, Abdo Z, et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE. (2011) 6:e21313. doi: 10.1371/journal.pone.0021313
49. Martín R, Jiménez E, Heilig H, Fernández L, Marín ML, Zoetendal EG, et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol. (2009) 75:965–9. doi: 10.1128/AEM.02063-08
50. Kumar H, du Toit E, Kulkarni A, Aakko J, Linderborg KM, Zhang Y, et al. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front Microbiol. (2016) 7:1619. doi: 10.3389/fmicb.2016.01619
51. Lackey KA, Williams JE, Meehan CL, Zachek JA, Benda ED, Price WJ, et al. What's normal? microbiomes in human milk and infant feces are related to each other but vary geographically: the INSPIRE study. Front Nutr. (2019) 6:45. doi: 10.3389/fnut.2019.00045
52. Gomez-Gallego C, Morales JM. Human breast milk NMR metabolomic profile across specific geographical locations and its association with the milk microbiota. (2018) 10:e1355. doi: 10.3390/nu10101355
53. Bouladoux N, Hall J, Grainger J, Dos Santos L, Kann M, Nagarajan V, et al. Regulatory role of suppressive motifs from commensal DNA. Mucosal Immunol. (2012) 5:623–34. doi: 10.1038/mi.2012.36
54. Ward TL, Hosid S, Ioshikhes I, Altosaar I. Human milk metagenome: a functional capacity analysis. BMC Microbiol. (2013) 13:116. doi: 10.1186/1471-2180-13-116
55. Ruiz L, García-Carral C, Rodriguez JM. Unfolding the human milk microbiome landscape in the omics era. Front Microbiol. (2019) 10:1378. doi: 10.3389/fmicb.2019.01378
56. Jiménez E, de Andrés J, Manrique M, Pareja-Tobes P, Tobes R, Martínez-Blanch JF, et al. Metagenomic analysis of milk of healthy and mastitis-suffering women. J Human Lactation. (2015) 31:406–15. doi: 10.1177/0890334415585078
57. Patel SH, Vaidya YH, Patel RJ, Pandit RJ, Joshi CG. Culture independent assessment of human milk microbial community in lactational mastitis. Sci Rep. (2017) 7:7804. doi: 10.1038/s41598-017-08451-7
58. Urbaniak C, McMillan A, Angelini M, Gloor GB, Sumarah M, Burton JP, et al. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome. (2014) 2:24. doi: 10.1186/2049-2618-2-24
59. Ubeda C, Djukovic A, Isaac S. Roles of the intestinal microbiota in pathogen protection. Clin Transl Immunol. (2017) 6:e128. doi: 10.1038/cti.2017.2
60. Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. (2004) 4:478–85. doi: 10.1038/nri1373
61. Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. (2017) 52:1–8. doi: 10.1007/s00535-016-1242-9
62. Neis EP, Dejong CH, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. (2015) 7:2930–46. doi: 10.3390/nu7042930
63. Hill M. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. (1997) 6:S43–5. doi: 10.1097/00008469-199703001-00009
64. Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporosis Rep. (2015) 13:125–30. doi: 10.1007/s11914-015-0257-0
65. Swann J, Wang Y, Abecia L, Costabile A, Tuohy K, Gibson G, et al. Gut microbiome modulates the toxicity of hydrazine: a metabonomic study. Mol BioSystems. (2009) 5:351–5. doi: 10.1039/b811468d
66. Lee NK, Park JS, Park E, Paik HD. Adherence and anticarcinogenic effects of Bacillus polyfermenticus SCD in the large intestine. Lett Appl Microbiol. (2007) 44:274–8. doi: 10.1111/j.1472-765X.2006.02078.x
67. Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. (2011) 8:110–20. doi: 10.1038/cmi.2010.67
68. Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal microbiota promote lung cancer development via gammadelta T cells. Cell. (2019) 176:998–1013 e16. doi: 10.1016/j.cell.2018.12.040
69. McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene. (2004) 23:6349–64. doi: 10.1038/sj.onc.1207716
70. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. (2014) 7:17–44. doi: 10.3390/nu7010017
71. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505:559–63. doi: 10.1038/nature12820
72. Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. (2006) 40:235–43. doi: 10.1097/00004836-200603000-00015
73. Salimi V, Shahsavari Z, Safizadeh B, Hosseini A, Khademian N, Tavakoli-Yaraki M. Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis. (2017) 16:208. doi: 10.1186/s12944-017-0593-4
74. Thirunavukkarasan M, Wang C, Rao A, Hind T, Teo YR, Siddiquee AA-M, et al. Short-chain fatty acid receptors inhibit invasive phenotypes in breast cancer cells. PLoS ONE. (2017) 12:e0186334. doi: 10.1371/journal.pone.0186334
75. Patel D, Vaghasiya J, Pancholi S, Paul A. Therapeutic potential of secoisolariciresinol diglucoside: a plant lignan. Int J Pharm Sci Drug Res. (2012) 4:15–8. doi: 10.3389/fgene.2018.00641
76. Wang L-Q. Mammalian phytoestrogens: enterodiol and enterolactone. J Chromatogr B. (2002) 777:289–309. doi: 10.1016/S1570-0232(02)00281-7
77. Webb AL, McCullough ML. Dietary lignans: potential role in cancer prevention. Nutr Cancer. (2005) 51:117–31. doi: 10.1207/s15327914nc5102_1
78. Ingram D, Sanders K, Kolybaba M, Lopez D. Case-control study of phyto-oestrogens and breast cancer. Lancet. (1997) 350:990–4. doi: 10.1016/S0140-6736(97)01339-1
79. Pietinen P, Stumpf K, Männistö S, Kataja V, Uusitupa M, Adlercreutz H. Serum enterolactone and risk of breast cancer: a case-control study in eastern Finland. Cancer Epidemiol Prev Biomark. (2001) 10:339–44.
80. Saarinen NM, Wärri A, Airio M, Smeds A, Mäkelä S. Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Food Res. (2007) 51:857–66. doi: 10.1002/mnfr.200600240
81. McCann SE, Thompson LU, Nie J, Dorn J, Trevisan M, Shields PG, et al. Dietary lignan intakes in relation to survival among women with breast cancer: the Western New York Exposures and Breast Cancer (WEB) Study. Breast Cancer Res Treat. (2010) 122:229–35. doi: 10.1007/s10549-009-0681-x
82. Cotterchio M, Boucher BA, Kreiger N, Mills CA, Thompson LU. Dietary phytoestrogen intake-lignans and isoflavones-and breast cancer risk (Canada). Cancer Causes Control. (2008) 19:259–72. doi: 10.1007/s10552-007-9089-2
83. Cade JE, Burley VJ, Greenwood DC. Dietary fibre and risk of breast cancer in the UK Women's Cohort Study. Int J Epidemiol. (2007) 36:431–8. doi: 10.1093/ije/dyl295
84. Sieri S, Krogh V, Pala V, Muti P, Micheli A, Evangelista A, et al. Dietary patterns and risk of breast cancer in the ORDET cohort. Cancer Epidemiol Prev Biomark. (2004) 13:567–72.
85. Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. (2008) 101:541–50. doi: 10.1017/S0007114508019880
86. Khokhlova EV, Smeianov VV, Efimov BA, Kafarskaia LI, Pavlova SI, Shkoporov AN. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol. (2012) 56:27–39. doi: 10.1111/j.1348-0421.2011.00398.x
87. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux J-J, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. (2008) 105:16731–6. doi: 10.1073/pnas.0804812105
88. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
89. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. (2009) 457:480–4. doi: 10.1038/nature07540
90. Shively CA, Register TC, Appt SE, Clarkson TB, Uberseder B, Clear KYJ, et al. Consumption of mediterranean versus western diet leads to distinct mammary gland microbiome populations. Cell Rep. (2018) 25:47–56 e3. doi: 10.1016/j.celrep.2018.08.078
91. Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15:73. doi: 10.1186/s12967-017-1175-y
92. Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. (2017) 67:326–44. doi: 10.3322/caac.21398
93. Knekt P, Adlercreutz H, Rissanen H, Aromaa A, Teppo L, Heliövaara M. Does antibacterial treatment for urinary tract infection contribute to the risk of breast cancer? Br J Cancer. (2000) 82:1107. doi: 10.1054/bjoc.1999.1047
94. Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. (2014) 14:667–85. doi: 10.1038/nri3738
95. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41. doi: 10.1016/j.cell.2014.03.011
96. Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. (2014) 99:4632–40. doi: 10.1210/jc.2014-2222
97. Kaaks R, Rinaldi S, Key T, Berrino F, Peeters P, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Related Cancer. (2005) 12:1071–82. doi: 10.1677/erc.1.01038
98. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. (2017) 103:45–53. doi: 10.1016/j.maturitas.2017.06.025
99. Plottel CS, Blaser MJ. Microbiome and malignancy. Cell host & microbe. (2011) 10:324–35. doi: 10.1016/j.chom.2011.10.003
100. Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. (2016) 108:djw029. doi: 10.1093/jnci/djw029
101. Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. (2012) 10:253. doi: 10.1186/1479-5876-10-253
102. Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. (2013) 15:R34. doi: 10.1186/bcr3416
103. Clavel T, Borrmann D, Braune A, Doré J, Blaut M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe. (2006) 12:140–7. doi: 10.1016/j.anaerobe.2005.11.002
104. Power KA, Saarinen NM, Chen JM, Thompson LU. Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. Int J Cancer. (2006) 118:1316–20. doi: 10.1002/ijc.21464
105. Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr. (2010) 92:141–53. doi: 10.3945/ajcn.2009.28573
106. Zaineddin AK, Vrieling A, Buck K, Becker S, Linseisen J, Flesch-Janys D, et al. Serum enterolactone and postmenopausal breast cancer risk by estrogen, progesterone and herceptin 2 receptor status. Int J Cancer. (2012) 130:1401–10. doi: 10.1002/ijc.26157
107. Chace D. Turning Off Breast Cancer: A Personalized Approach to Nutrition and Detoxification in Prevention and Healing. Skyhorse Publishing, Inc. (2015).
108. Paulsen JA, Ptacek TS, Carter SJ, Liu N, Kumar R, Hyndman L, et al. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support Care Cancer. (2017) 25:1563–70. doi: 10.1007/s00520-016-3568-5
109. Bassler BL. Small talk: cell-to-cell communication in bacteria. Cell. (2002) 109:421–4. doi: 10.1016/S0092-8674(02)00749-3
110. Sureshchandra B. Quorum sensing-cell to cell communication in bacteria. J Endodontol. (2010) 22:97–101.
111. Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. (2005) 21:319–46. doi: 10.1146/annurev.cellbio.21.012704.131001
112. De Spiegeleer B, Verbeke F, D'Hondt M, Hendrix A, Van De Wiele C, Burvenich C, et al. The quorum sensing peptides PhrG, CSP, and EDF promote angiogenesis and invasion of breast cancer cells in vitro. PLoS ONE. (2015) 10:e0119471. doi: 10.1371/journal.pone.0119471
113. Lakritz JR, Poutahidis T, Mirabal S, Varian BJ, Levkovich T, Ibrahim YM, et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. (2015) 6:9387–96. doi: 10.18632/oncotarget.3328
114. Hassan Z, Mustafa S, Rahim RA, Isa NM. Anti-breast cancer effects of live, heat-killed and cytoplasmic fractions of Enterococcus faecalis and Staphylococcus hominis isolated from human breast milk. In vitro Cell Dev Biol Anim. (2016) 52:337–48. doi: 10.1007/s11626-015-9978-8
115. Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, et al. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. (2014) 135:529–40. doi: 10.1002/ijc.28702
116. Yazdi MH, Soltan Dallal MM, Hassan ZM, Holakuyee M, Agha Amiri S, Abolhassani M, et al. Oral administration of Lactobacillus acidophilus induces IL-12 production in spleen cell culture of BALB/c mice bearing transplanted breast tumour. Br J Nutr. (2010) 104:227–32. doi: 10.1017/S0007114510000516
117. de Moreno de LeBlanc A, Matar C, Theriault C, Perdigon G. Effects of milk fermented by Lactobacillus helveticus R389 on immune cells associated to mammary glands in normal and a breast cancer model. Immunobiology. (2005) 210:349–58. doi: 10.1016/j.imbio.2005.05.024
118. Mendoza L. Potential effect of probiotics in the treatment of breast cancer. Oncol Rev. (2019) 13:422. doi: 10.4081/oncol.2019.422
119. Toi M, Hirota S, Tomotaki A, Sato N, Hozumi Y, Anan K, et al. Probiotic beverage with soy isoflavone consumption for breast cancer prevention: a case-control study. Curr Nutr Food Sci. (2013) 9:194–200. doi: 10.2174/15734013113099990001
120. Arroyo R, Martín V, Maldonado A, Jiménez E, Fernández L, Rodríguez JM. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin Infect Dis. (2010) 50:1551–8. doi: 10.1086/652763
121. Moossavi S, Rezaei N. Toll-like receptor signalling and their therapeutic targeting in colorectal cancer. Int Immunopharmacol. (2013) 16:199–209. doi: 10.1016/j.intimp.2013.03.017
122. Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. (2011) 71:2466–75. doi: 10.1158/0008-5472.CAN-10-1993
123. Wallace BD, Roberts AB, Pollet RM, Ingle JD, Biernat KA, Pellock SJ, et al. Structure and inhibition of microbiome β-glucuronidases essential to the alleviation of cancer drug toxicity. Chem Biol. (2015) 22:1238–49. doi: 10.1016/j.chembiol.2015.08.005
124. Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. (2010) 330:831–5. doi: 10.1126/science.1191175
125. McBain A, Macfarlane G. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J Med Microbiol. (1998) 47:407–16. doi: 10.1099/00222615-47-5-407
126. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. (2004) 79:727–47. doi: 10.1093/ajcn/79.5.727
127. Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. (2013) 342:967–70. doi: 10.1126/science.1240527
128. Planes-Laine G, Rochigneux P, Bertucci F, Chretien AS, Viens P, Sabatier R, et al. PD-1/PD-L1 targeting in breast cancer: the first clinical evidences are emerging. A literature review. Cancers. (2019) 11:1033. doi: 10.3390/cancers11071033
129. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
130. McLaughlin MM, Dacquisto MP, Jacobus DP, Horowitz RE. Effects of the germfree state on responses of mice to whole-body irradiation. Radiat Res. (1964) 23:333–49. doi: 10.2307/3571614
131. Stunault MI, Bories G, Guinamard RR, Ivanov S. Metabolism plays a key role during macrophage activation. Med Inflamm. (2018) 2018:2426138. doi: 10.1155/2018/2426138
132. McGee HM, Jiang D, Soto-Pantoja DR, Nevler A, Giaccia AJ, Woodward WA. Targeting the tumor microenvironment in radiation oncology: proceedings from the 2018 ASTRO-AACR research workshop. Clin Cancer Res. (2019) 25:2969–74. doi: 10.1158/1078-0432.CCR-18-3781
133. Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S, Jimenez C, et al. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. (2018) 100:1034–43. doi: 10.1016/j.ijrobp.2017.11.043
134. Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G, et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. (2015) 5:14554. doi: 10.1038/srep14554
135. Yang J, Tan Q, Fu Q, Zhou Y, Hu Y, Tang S, et al. Gastrointestinal microbiome and breast cancer: correlations, mechanisms and potential clinical implications. Breast Cancer. (2017) 24:220–8. doi: 10.1007/s12282-016-0734-z
136. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
137. Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. (2013) 98:111–20. doi: 10.3945/ajcn.112.056689
138. Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. (2014) 12:661–72. doi: 10.1038/nrmicro3344
139. Yang L, Francois F, Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. (2012) 18:2138–44. doi: 10.1158/1078-0432.CCR-11-0934
140. Ivanov II, de Llanos Frutos R, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. (2008) 4:337–49. doi: 10.1016/j.chom.2008.09.009
141. Kali A. Human microbiome engineering: the future and beyond. J Clin Diagnost Res. (2015) 9:DE01. doi: 10.7860/JCDR/2015/14946.6570
142. Seekatz AM, Aas J, Gessert CE, Rubin TA, Saman DM, Bakken JS, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. (2014) 5:e00893–14. doi: 10.1128/mBio.00893-14
143. Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. (2010) 44:551–61. doi: 10.1097/MCG.0b013e3181e5d06b
144. Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. (2010) 44:354–60. doi: 10.1097/MCG.0b013e3181c87e02
Keywords: estrogen metabolism, milk microbiome, microbiome chemotherapies, probiotic therapy, gene-based therapy, microbiome immunotherapy, microbiome radiotherapy
Citation: Eslami-S Z, Majidzadeh-A K, Halvaei S, Babapirali F and Esmaeili R (2020) Microbiome and Breast Cancer: New Role for an Ancient Population. Front. Oncol. 10:120. doi: 10.3389/fonc.2020.00120
Received: 01 October 2019; Accepted: 22 January 2020;
Published: 12 February 2020.
Edited by:
Saori Furuta, University of Toledo, United StatesReviewed by:
Peter Bai, University of Debrecen, HungaryCopyright © 2020 Eslami-S, Majidzadeh-A, Halvaei, Babapirali and Esmaeili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rezvan Esmaeili, ZXNtYWVpbGkucmV6dmFuQGdtYWlsLmNvbQ==; Keivan Majidzadeh-A, a21hamlkemFkZWhAYWNlY3IuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.