- 1Department of Radiation Oncology, Peking University Third Hospital, Beijing, China
- 2Clinical Epidemiology Department, Peking University Third Hospital, Beijing, China
- 3Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, China
- 4Stanford University School of Medicine, Stanford, CA, United States
- 5Tianjin Key Laboratory of Cancer Prevention and Therapy, Department of Radiotherapy, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
- 6Division of Radiation Oncology, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Objective: This study aimed to investigate the relationship between the timing of stereotactic radiosurgery (SRS) intervention and the complications of cerebral radiation necrosis (CRN) in patients with brain metastases of lung adenocarcinoma who received tyrosine kinase inhibitor (TKI) treatment.
Methods: A total of 361 targets from 257 patients with brain oligometastases of lung adenocarcinoma who received CyberKnife treatment between 2010 and 2017 were retrospectively collected from three CyberKnife centers. The difference in brain necrosis between patients with or without TKI application was statistically counted. Logistic regression analysis was used to analyze the effect of applying TKI on the occurrence of CRN in patients and the effect of SRS before and after TKI resistance on CRN.
Results: The rate of CRN in the TKI group was significantly higher than that in the non-TKI group. The incidence of brain necrosis in patients undergoing SRS after drug resistance was significantly higher than that in patients undergoing SRS before drug resistance. Regression analysis showed that combination of TKI with SRS, and SRS after TKI resistance were important influencing factors for CRN.
Conclusion: Performing the SRS for brain metastases after TKI resistance worsened the occurrence of CRN of patients treated with TKI.
Clinical Trial Registration: Chinese clinical trial registry, http://www.chictr.org.cn/edit.aspx?pid=38395&htm=4, Registration number: ChiCTR1900022750.
Introduction
Whether radiotherapy or targeted therapy should be performed first for brain metastases of non-small-cell lung cancer (NSCLC) has remained controversial. The recent research mainly focused on the survival (1–3). There were no reports concerning the increased occurrence of radiotherapy complications such as cerebral radiation necrosis (CRN), which further affected the quality of life in patients receiving stereotactic radiosurgery (SRS) after tyrosine kinase inhibitor (TKI) resistance. This study retrospectively analyzed the real-world case data on whether the TKI was applied when performing SRS, as well as the occurrence of CRN when performing SRS before and after TKI resistance, in patients with brain metastases of NSCLC from multiple centers. We investigated the TKI medication combined with SRS, and the occurrence of CRN and its impact on cognitive function and quality of life when performing SRS before and after drug resistance. This study was expected to provide evidence and reference for the treatment of such patients in clinical practice.
Materials and Methods
Case Data and Study Design
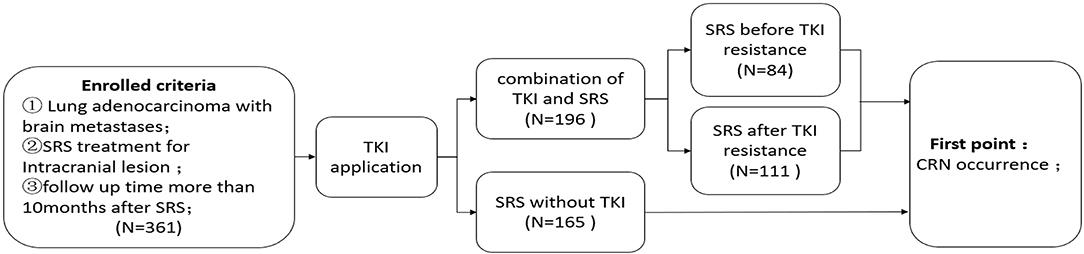
A total of 361 lesions from 257 patients with brain metastases of lung adenocarcinoma were collected from the Peking University Third Hospital, Tianjin Cancer Hospital, Huashan Hospital affiliated to Fudan University between January 2010 and December 2017. The inclusion criteria were as follows: (1) intracranial metastases of lung adenocarcinoma (number of metastatic lesions ≤ 4); (2) SRS was performed for the intracranial metastases or whole-brain radiotherapy combined with SRS; and (3) the follow-up time after SRS was >10 months (4). The exclusion criteria were as follows: (1) the follow-up time was <10 months for patients with no brain necrosis after stereotactic radiotherapy; and (2) the local lesions could not be further characterized during their surgery after SRS. The study was approved by the Ethics Committee of the Peking University Third Hospital and completed under its supervision. It was in accordance with all the ethical requirements (Figure 1).
Treatment and Examination Methods
All intracranial metastasis SRS was treated with stereotactic radiotherapy by CyberKnife (Table 1). The epidermal growth factor receptor (EGFR) mutations were detected by PCR, digital PCR, or second-generation sequencing; and the results were independently determined by more than three specialists. Patients with EGFR mutations (19 or 21 exon mutation) taking TKI medication were treated with the first generation of TKI (gefitinib, erlotinib, or icotinib). Patients without EGFR mutations were treated with chemotherapy-based comprehensive treatment. The patients in this study received routine enhanced magnetic resonance imaging (MRI) 2 months after treatment, and then the reexamination interval was determined according to the local lesions, which was at least four times in the first year and at least three times per year from the second year for patients with stable lesions, but the longest interval did not exceed 6 months. If there were intracranial symptoms, reexamination was immediately performed. CRN was diagnosed by comprehensive medical history, symptoms, and signs combined with dynamic observation of intracranial lesions and MRI examination. For patients with doubtful diagnosis of CRN by enhanced brain MRI, routine spectral analysis or PET-CT and other means were further performed for final diagnosis. Imaging diagnosis required three doctors to simultaneously diagnose the brain necrosis (4–6).
Statistical Analysis
Statistical analysis was performed using the SPSS 25.0 software. The measurement data in accordance with the normal distribution were expressed as mean ± standard deviation. The comparison between groups was performed by independent sample t-test. The measurement data that did not conform to the normal distribution was expressed as median (25% quantile, 75% quantile). The comparison between groups was performed using the non-parametric Mann-Whitney U-test. The enumeration data were expressed as the number of cases (percentage), and the comparison between groups was performed using the chi-square test. Logistic regression was used to analyze the effect of applying TKI on CRN in patients. Meanwhile, logistic regression was used to analyze the effect of performing SRS before and after drug resistance on difference in CRN in patients taking TKI medication. The statistically significant differences were accepted as bilateral p < 0.05 for all tests.
Results
The General Data of Cerebral Radiation Necrosis
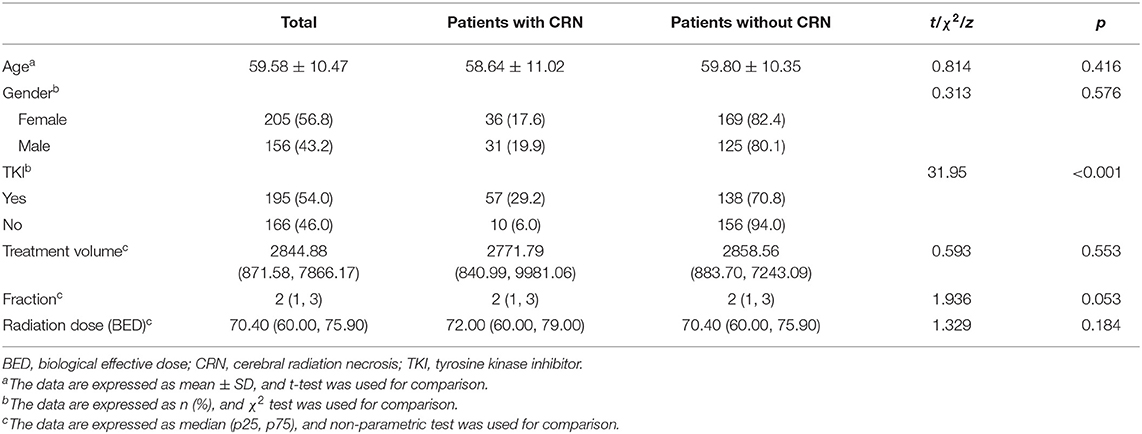
For the 361 targets, a total of 67 targets (18.6%) had CRN. Among them, 57 (29.2%) cases had CRN in the TKI combination treatment group, and 10 (6.0%) cases had CRN in the non-TKI combination treatment group. The difference between the two groups was statistically significant (χ2 = 31.95, p < 0.001) (Table 1).
Patients With Tyrosine Kinase Inhibitor Medication Were More Likely to Have Cerebral Radiation Necrosis Than Those Without Tyrosine Kinase Inhibitor
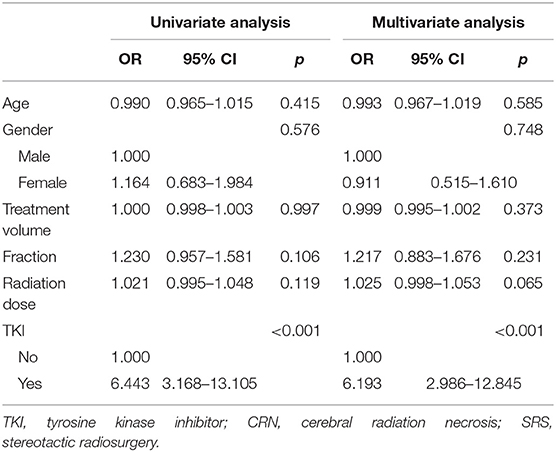
Logistic regression analysis was performed on factors such as age, gender, therapeutic dose, target volume, number of divisions, and whether patients used TKI. The results showed that TKI medication was an important prognostic factor for CRN. The risk of CRN in patients using TKI was six times higher than those who did not use drugs (Table 2).
Comparison of Performing Stereotactic Radiosurgery Before and After Tyrosine Kinase Inhibitor Resistance
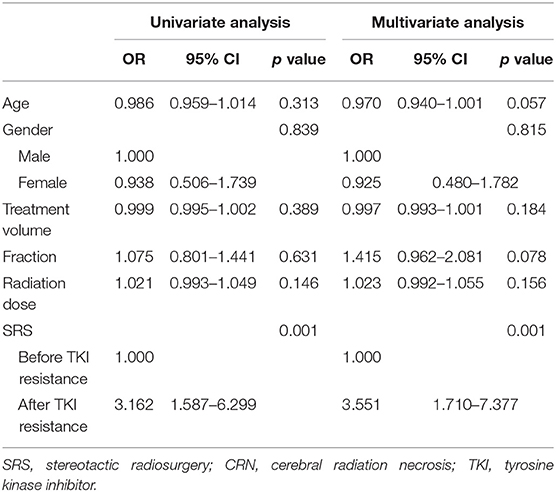
When TKI was applied in clinical practice, some patients did not undergo local radiotherapy owing to absence of symptoms after medical treatment. Radiotherapy was performed after the progression of drug resistance. However, other patients underwent brain radiotherapy at the initial period of drug use or before drug resistance. Statistical analysis of CRN occurrence before and after drug resistance in this group was performed by t-test, and the results showed a statistically significant difference between the two groups (p = 0.001). Regression analysis also showed that performing SRS after drug resistance was an important factor for the occurrence of CRN (Table 3). The incidence of CRN was significantly increased when the SRS was performed after drug resistance.
Discussion
For brain metastases of EGFR-mutated NSCLC, TKI treatment and the timing of SRS during the TKI treatment had a direct impact on the occurrence of CRN. Performing SRS after TKI resistance increased the incidence of CRN. This was a useful analysis of the current clinical routine treatment mode of local radiotherapy after drug resistance for NSCLC patients with brain metastasis receiving TKI treatment.
Histological changes of intracranial tumor metastases after TKI treatment may be an important mechanism for the increase of CRN after SRS. First, in clinical practice, most of the intracranial metastases were small lesions and massive edema before TKI resistance, whereas most of the intracranial metastases were micro-edema or no edema after drug resistance, which was considered as a change in vascular status of the tumor. Preclinical studies have also shown that the blood vessels in the tumor tissues were significantly decreased after erlotinib treatment, as compared with pretreatment, and the tumor tissues showed significant ischemia and hypoxia. In contrast to pre-TKI resistance, tissue preparation for the occurrence of brain necrosis after SRS was indicated (7). Second, CRN occurred mainly owing to vascular injury (8–11). After TKI resistance, the vascular components in the tumor were significantly reduced (7, 12), and the local ischemia and hypoxia were more severe from the radiation-induced vascular injury, which was more likely to cause CRN.
This study had innovative significance for the clinical treatment mode of brain metastasis of EGFR-mutated NSCLC. The treatment mode of NSCLC with brain metastases in the TKI era was controversial. Professor Wu Yilong proposed a treatment mode of performing radiotherapy for local progression after TKI resistance (12, 13). However, studies published in the Journal of Clinical Oncology (JCO) (1) and International Journal of Radiation Oncology, Biology, Physics (2) suggested that the survival of patients with early radiotherapy was better in patients receiving TKI treatment. Nevertheless, all these studies did not mention the effects of performing radiotherapy after drug resistance or the long-term complications of radiotherapy. This study showed that it may worsen damage in the late period after drug resistance, which further affected the cognitive function and quality of life. Therefore, for patients with advanced EGFR-mutated NSCLC, if brain radiotherapy was not performed early on, it affected their survival, was more likely to cause CRN, and affected their quality of life. This was another exploration of the treatment mode for brain metastases of EGFR-mutated NSCLC patients.
This was a retrospective study. Given the difficulty of clinical research on radiotherapy in the TKI-targeted era and only few prospective studies on TKI combined with radiotherapy for brain metastasis (14), this retrospective data also had good reference value. Prospective studies can be conducted in the future to further confirm the results of this retrospective study (15).
Conclusion
In summary, for brain metastases of NSCLC, TKI treatment and the timing of SRS intervention during TKI treatment had a direct impact on the occurrence of CRN. Performing the SRS after TKI resistance worsened the occurrence of CRN of patients treated with TKI. This study provided a good reference for the timing of TKI combined with radiotherapy in patients with brain metastases of EGFR-mutated NSCLC, clinical data for the development of treatment mode in such patients, and a useful exploration for improving the quality of life of the patients.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Peking University Third Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This paper was supported by Clinical Key Project of Peking University Third Hospital (BYSY2017030).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the CyberKnife Center.
References
1. Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. (2017) 35:1070–7. doi: 10.1200/JCO.2016.69.7144
2. Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys. (2016) 95:673–9. doi: 10.1016/j.ijrobp.2016.01.037
3. Jiang T, Su C, Li X, Zhao C, Zhou F, Ren S, et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of tkis alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol. (2016) 11:1718–28. doi: 10.1016/j.jtho.2016.05.013
4. Zhuang H, Yuan X, Zheng Y, Li X, Chang JY, Wang J, et al. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep. (2016) 6:24364. doi: 10.1038/srep24364
5. Vellayappan B, Tan CL, Yong C, Khor LK, Koh WY, Yeo TT, et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. (2018) 8:395. doi: 10.3389/fonc.2018.00395
6. Loganadane G, Dhermain F, Louvel G, Kauv P, Deutsch E, Le Péchoux C, et al. Brain radiation necrosis: current management with a focus on non-small cell lung cancer patients. Front Oncol. (2018) 8:336. doi: 10.3389/fonc.2018.00336
7. Masuda C, Yanagisawa M, Yorozu K, Kurasawa M, Furugaki K, Ishikura N, et al. Bevacizumab counteracts VEGF-dependent resistance to erlotinib in an EGFR-mutated NSCLC xenograft model. Int J Oncol. (2017) 51:425–34. doi: 10.3892/ijo.2017.4036
8. Lo EH, Frankel KA, Steinberg GK, DeLaPaz RL, Fabrikant JI. High-dose single-fraction brain irradiation: MRI, cerebral blood flow, electrophysiological, and histological studies. Int J Radiat Oncol Biol Phys. (1992) 22:47–55. doi: 10.1016/0360-3016(92)90981-M
9. Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma–a review of the literature and current understanding. Acta Neurochir. (2012) 154:191–201; discussion 201. doi: 10.1007/s00701-011-1228-6
10. Tallet AV, Dhermain F, Le Rhun E, Noël G, Kirova YM. Combined irradiation and targeted therapy or immune checkpoint blockade in brain metastases: toxicities and efficacy. Ann Oncol. (2017) 28:2962–76. doi: 10.1093/annonc/mdx408
11. Hirata H, Nakamura K, Kunitake N, Shioyama Y, Sasaki T, Ohga S, et al. Association between EGFR-TKI resistance and efficacy of radiotherapy for brain metastases from EGFR-mutant lung adenocarcinoma. Anticancer Res. (2013) 33:1649–55.
12. Wu YL, Zhou C, Cheng Y, Lu S, Chen GY, Huang C, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol. (2013) 24:993–9. doi: 10.1093/annonc/mds529
13. Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou Q, Su J, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer. (2013) 79:33–9. doi: 10.1016/j.lungcan.2012.09.016
14. Liu X, Zhang Y, Tang LL, Le QT, Chua MLK, Wee JTS, et al. Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years. JAMA Oncol. (2018) 4:1073–9. doi: 10.1001/jamaoncol.2018.0887
15. Zhuang H. Management of brain metastases in tyrosine kinase inhibitortreated epidermal growth factor receptor-mutant non-small-cell lung cancer from the perspective of long-term radiation brain damage: a multiinstitutional retrospective analysis. In: 2019 ASCO meeting, Journal of Clinical Oncology (JCO). Chicago, CL (2019). doi: 10.1200/JCO.2019.37.15_suppl.e13587
Keywords: cerebral radiation necrosis, tyrosine kinase inhibitor, brain metastasis, epidermal growth factor receptor, stereotactic radiosurgery
Citation: Zhuang H, Tao L, Wang X, Shi S, Yuan Z, Wang E and Chang JY (2020) Tyrosine Kinase Inhibitor Resistance Increased the Risk of Cerebral Radiation Necrosis After Stereotactic Radiosurgery in Brain Metastases of Non-small-Cell Lung Cancer: A Multi-Institutional Retrospective Case-Control Study. Front. Oncol. 10:12. doi: 10.3389/fonc.2020.00012
Received: 03 September 2019; Accepted: 07 January 2020;
Published: 11 February 2020.
Edited by:
John Varlotto, University of Massachusetts Medical School, United StatesReviewed by:
Sabareesh Natarajan, UMass Memorial Health Care, United StatesSamuel Chao, Case Western Reserve University, United States
Copyright © 2020 Zhuang, Tao, Wang, Shi, Yuan, Wang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqing Zhuang, aG9uZ3Fpbmd6aHVhbmcmI3gwMDA0MDsxNjMuY29t; Zhiyong Yuan, MTAwX3BpYW4mI3gwMDA0MDsxNjMuY29t; Enmin Wang, dGlhbmppbmdtYXNoaSYjeDAwMDQwOzE2My5jb20=
†These authors have contributed equally to this work
 Hongqing Zhuang
Hongqing Zhuang Liyuan Tao2†
Liyuan Tao2†