
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 January 2020
Sec. Cancer Molecular Targets and Therapeutics
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.01439
This article is part of the Research Topic New Insights in the Landscape of Rare Tumors: Translational and Clinical Research Perspective View all 17 articles
Background: Adolescents and young adults (AYAs) diagnosed with cancer between ages 15 and 45 years may exhibit unique biologic and genomic characteristics as well as clinical features, resulting in differences in clinical characters and drug resistance. However, compared to other solid cancers, relatively few studies have been conducted in this age group in cholangiocarcinoma (CCA). This study is performed to investigate the clinical and molecular features of AYAs with CCA.
Methods: Three cohorts, including the external dataset (TCGA and MSKCC) and the perihilar CCA databank of Chinese tertiary hospitals, were contained in this study. Pathway and process enrichment analysis had been carried out with the following ontology sources: KEGG Pathway, GO Biological Processes, Reactome Gene Sets, Canonical Pathways, and CORUM. Metascape and GEPIA datasets were used for bioinformatic analysis. P < 0.05 was considered statistically significant. All statistical analyses were performed with GraphPad Prism (version 7.0; GraphPad Software, La Jolla, California) and R studio (version 3.6.1; R studio, Boston, Massachusetts).
Results: Compared to older adults, AYAs with CCA presented with worse overall survival, although the difference was not significant. Specific to patients with stage IV CCAs who underwent chemotherapy, AYAs were associated with significantly poorer overall survival (OS) (p = 0.03, hazards ratio (HR) 3.01, 95% confidence interval (CI) 1.14-4.91). From the anatomical perspective, more extrahepatic CCA was detected in the AYA group. Microsatellite instability (MSI) occurred in 3% of older patients in the present study. Nevertheless, none of the AYAs had MSI status. In this study, AYAs gained an enhanced frequency of additional sex combs like 1 (ASXL1) (p = 0.02) and KMT2C (p = 0.02) mutation than their older counterparts. Besides ASXL1 and KMT2C, the genes enriched in AYAs with CCA were analyzed by pathway and process enrichment analysis. And those genes were found to be associated with poorer differentiation, deubiquitination, and WNT signal pathway. Moreover, AYAs were relevant to poor differentiation and advanced tumor stage.
Conclusion: This study offered a preliminary landscape of the clinical and molecular features of early-onset biliary cancers. Further studies including more samples are essential to investigate whether ASXL1 and KMT2C could be considered as potentially targetable genomic signatures for young patients.
Cholangiocarcinoma (CCA) is a highly fatal malignant tumor with rising incidence. It accounts for ~10–25% of all hepatobiliary malignancies and <1% of all types of cancers (1). The incidence of adolescents and young adults (AYAs) with CCA was even less. Despite recognition of the importance of AYAs with cancers, the biologic and genomic characteristics of AYAs with CCA remain largely unknown.
AYAs diagnosed with cancer between ages 15 and 45 years may exhibit unique biologic and genomic features, resulting in differences in clinical behaviors and chemotherapy/targeted therapy resistance (2). These features could also be clinically exploited to develop companion diagnostics and novel therapies for treating AYAs with cancers (3). For instance, AYAs with solid tumors, such as colorectal carcinomas, are more likely to exhibit signet-ring histology, synchronous or metachronous metastasis, and present at a late stage (4, 5). From the mutational perspective, most early-onset (age <50 years) patients present with lower prevalence of KRAS, BRAF, and NRAS mutations in comparison with late-onset patients (6).
To date, AYAs with other solid tumors have been extensively described in the literature. However, few studies have been conducted for patients with CCA at this age group. Despite, most recently, genomic analysis of patients with CCA being performed by the Cancer Genome Atlas (TCGA) and Memorial Sloan Kettering Cancer Center (MSKCC) (7), the genomic underpinnings of these AYAs with this rare cancer remain largely unknown. Therefore, in this study, the clinical and molecular features of AYA CCA patients were investigated by analyzing the external dataset (8, 9) and internal hilar CCA databank to shed light on early-onset biliary malignancy.
Three cohorts were included in the present study. The first cohort included 155 consecutive patients with perihilar CCA (pCCA) from three hepatobiliary surgery centers affiliated to tertiary hospitals in China between January 2013 and November 2018. Eighteen patients (12%) in this cohort were AYA (aged 15–45 years) and were set as AYA group. The rest (age >45) was set as the group “Others.” This retrospective study was approved by the institutional review board (IRB) of the Renji Hospital and the Study Group of Biliary Surgery of the surgical branch of the Chinese Medical Association.
In the second cohort, the genomic data (e.g., mutation frequency) of AYAs and the elderly with CCAs extracted from the TCGA database were compared. This cohort included five AYA (10%) and 46 elder patients.
The third cohort contained the data of age-associated gene mutation of 192 patients with CCA extracted from the MSKCC dataset, including 26 (14%) AYAs. cBioPortal platform (www.cbioportal.org) was used for analyzing (8, 9) (Table 1).
In the present study, progression-free survival (PFS) was defined as the time after the treatment with the disease not getting worse. Disease-free survival (DFS) was the time for any recurrence. If the postoperative margin was negative, the operation was considered as R0 resection. Follow-up consisted of serum tumor marker measurements every 1–3 months and computed tomography (CT) every 6 months. Complete follow-up was conducted for the entire cohort of patients.
Tumor specimens were sent for pathological evaluation about the quality, grading, tumor stage according to AJCC 7th edition, risk factor (perineuronal invasion, etc.), and lymph node status. CCAs are a heterogeneous group of tumors that can be classified into three clinically distinct types of cancers, intrahepatic CCA (iCCA), pCCA, and distal CCA (dCCA) basing on its anatomical location. pCCA and dCCA were also grouped as extrahepatic CCA (eCCA). Specifically, pCCA in the present study was defined as the CCA that developed at the point where the left and right hepatic ducts joined to form the common hepatic duct by imaging (CT or magnetic resonance cholangiopancreatography).
Programmed cell death protein 1 (PD-1) blockade provides a therapeutic opportunity for patients with high tumor mutation burden (TMB), high microsatellite instability (10) (MSI-H), and deficient mismatch repair (dMMR). Therefore, the MSI score, microsatellite instability (MSI)/microsatellite stability (MSS) status, and TMB were also analyzed between the two groups by using cBioPortal platform.
The intraoperative evaluation included the length of operation, intraoperative hemorrhage, intraoperative blood transfusion, and vascular anastomosis. Additionally, blood routine examination, biochemical test, total bilirubin (Blood) (TBil), aspartate transaminase (AST), alanine transaminase (ALT), and so on, and other hepatic and renal function examinations were performed perioperatively.
Metascape (http://metascape.org/gp/index.html) is an effective and efficient tool for experimental biologists to comprehensively analyze and interpret OMICs-based studies in the big data era (19). The database was used to perform the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, which is used to predict the potential biological functions of the overlapping genes of the DEGs and target genes. Then, verification was performed by the GEPIA database (http://gepia.cancer-pku.cn) to identify hub genes (11–19).
Pearson's Chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables were used to compare various parameters in AYA and the other group. The Kaplan-Meier method was used to estimate overall survival (OS), DFS, or PFS. Differences in survival outcomes were assessed by the log-rank test. Results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). P < 0.05 was considered statistically significant. All statistical analyses were performed with GraphPad Prism (version 7.0; GraphPad Software, La Jolla, California) and R studio (version 3.6.1; R studio, Boston, Massachusetts).
From the prognosis perspective, the length of OS in AYAs with CCA was worse (36 vs. 44 months) than the older patients. However, the difference was not significant (Figure 1A; p = 0.26, HR 1.39, 95% CI 0.78–2.47). Specific to patients with stage IV CCAs who underwent chemotherapy, AYAs were associated with significantly poorer OS (Figure 1B; p = 0.03, HR 3.01, 95% CI 1.14–4.91), and the survival period was almost half of their older counterparts (18 vs. 34 months). From the anatomical perspective, more eCCA was detected in the AYA group (29 vs. 17%, Figure 1C).
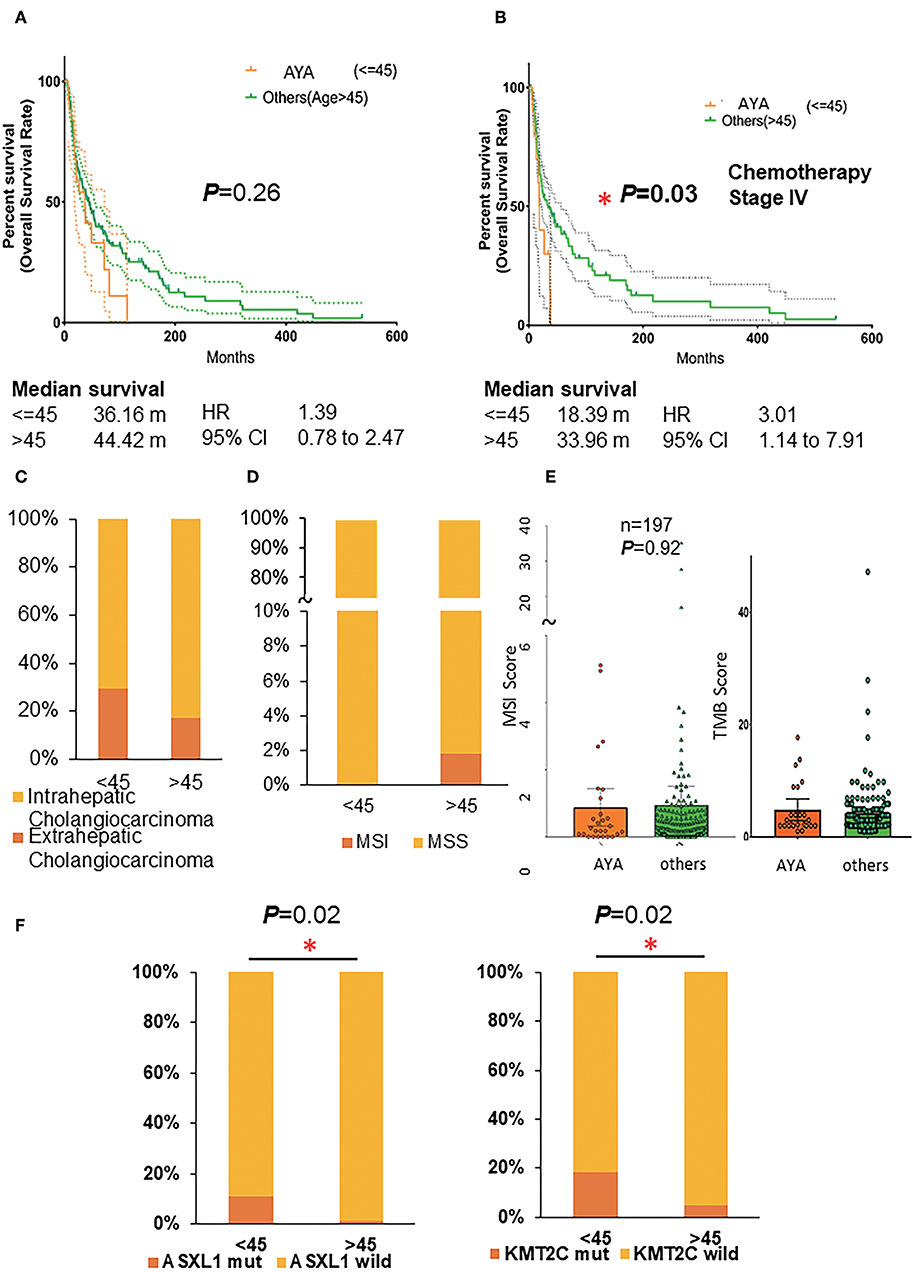
Figure 1. (A) Overall survival rate of AYA patients and others (age >45). (B) Overall survival rate of AYA patients and others (age >45) with stage IV cholangiocarcinoma and underwent the treatment of chemotherapy. (C) The proportion of intrahepatic and extrahepatic cholangiocarcinoma in AYA (<=45) and other (>45) groups. (D) The MSI/MSS status of patients in AYA (<=45) and other(>45) groups. (E) The MSI score and TMB score of patients in AYA (<=45) and other (>45) groups. (F) The mutation frequency of ASXL1 and KMT2C of patients in AYA (<=45) and other (>45) groups basing on cohort 3 (MSKCC). AYA, adolescents and young adults; MSI, microsatellite instability; TMB, tumor mutation burden.
PD-1 blockade provides a therapeutic opportunity for patients with high TMB, MSI-H, and dMMR. Therefore, the MSI score, MSI/MSS status, and TMB (Figure 1D) were also analyzed between the two groups. It has been reported that MSI status occurred in 3–10% of CCA; consistently, MSI occurred in 3% of older patients (>45 years old) in the present study. Intriguingly, none of the AYA patients had MSI status, although the average MSI score was similar (Figure 1E; AYA group: 0.8785 ± 0.2727, Others group: 0.944 ± 0.2831) between the two groups. Additionally, AYA patients had similar TMB compared to their counterparts (AYA group: 4.258 ± 0.3885, Others group: 4.452 ± 0.8883).
Additional sex combs like 1 (ASXL1) is the obligate regulatory subunit of a deubiquitinase complex. Heterozygous mutations of ASXL1 are frequent in myeloid leukemias and other malignancies. Here we demonstrated in the first cohort that AYAs with CCAs gained a higher frequency of ASXL1 mutation than their older counterparts [Figure 1F; p = 0.02, 11% (3/27) vs. 1% (2/167)].
KMT2C mutates frequently and is considered crucial for the occurrence and development of numerous cancers. In the present study, significantly higher KMT2C (histone lysine methyltransferase 2C) mutation rate was in the AYA group [Figure 1F; p = 0.02, 19% (5/27) vs. 4.7% (8/169)]. Specifically, 40% of the patients who had mutated ASXL1 also harbored a mutated KMT2C (also known as MLL3), KMT2D, or ARID1A. And 38.5% of the KMT2C mutated synergistically with ARID1A mutation. Additionally, although the difference was not significant, AYAs were likely to harbor more frequent mutated FGFR2 (18.5 vs. 9.5%) or PBRM1 (18.5 vs. 9.5%) or ERBB3 (11.1 vs. 2.4%) genes and less BAP1, KRAS, and SMAD4 (Supplemental Figures 1A,B; Table 2).
In the second cohort extracted from the TCGA dataset, the MCM8 gene mutation (p < 0.05) was significantly enriched in AYAs with CCA. Besides KMT2C, mutations of LAMA4, AGAP6, AKAP13, ARMC12, MAP1A, NAV3, ADAMTS7, FTH1, and ITPR2 were also observed in AYAs with CCA (Figure 2A). From the protein expression aspect, BCL2L11 was significantly downregulated in AYAs (Figure 2B; q = 0.0383). From the RNA expression perspective, PIK3C3, IQCH, RGP1, and LPP were upregulated in the AYA group (Supplemental Figure 1C).
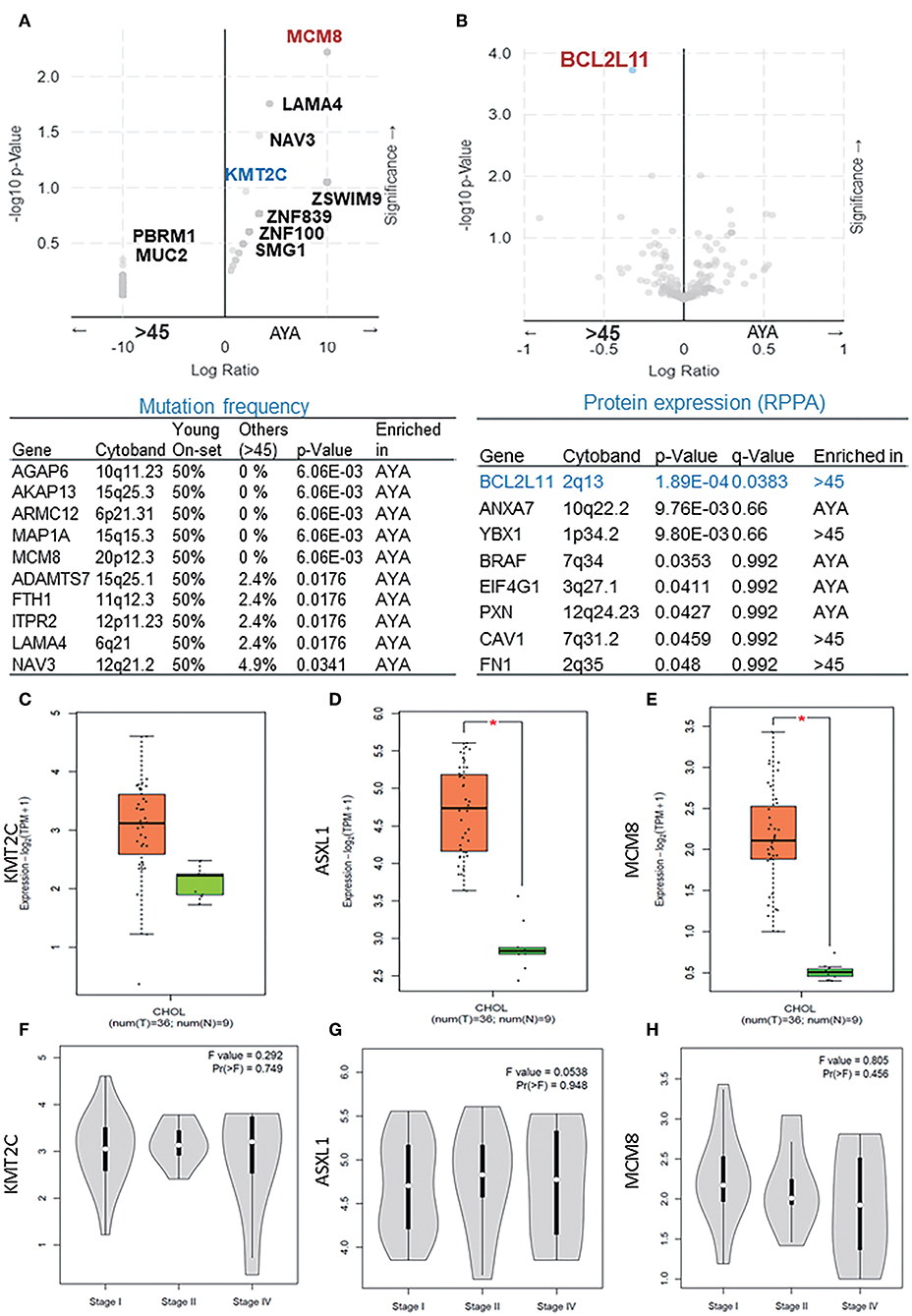
Figure 2. (A) The mutation frequency of presentative genes (p < 0.05) in AYA and other groups basing on cohort 2. (B) The difference of protein expression between the two groups basing on cohort 2. (C–E) The expression level of ASXL1, KMT2C, and MCM8 in tumor vs. paired normal samples in CCA. (F–H) Expression level of ASXL1, KMT2C, and MCM8 in different tumor stages. AYA, adolescents and young adults; ASXL1, additional sex combs like 1. *P < 0.05.
We then verified the expression level of KMT2C, ASXL1, and MCM8 in CCA using the GEPIA database and found that all of the three genes, especially ASXL1 (p < 0.05) and MCM8 (p < 0.05), were overexpressed in tumor tissues (Figures 2C–E). However, the expression level of the three genes was associated with neither tumor stages nor OS rate, respectively (Figures 2F–H, 3A,B). Pearson's correlation coefficient of ASXL1 and KMT2C was 0.83 (Figure 3C).
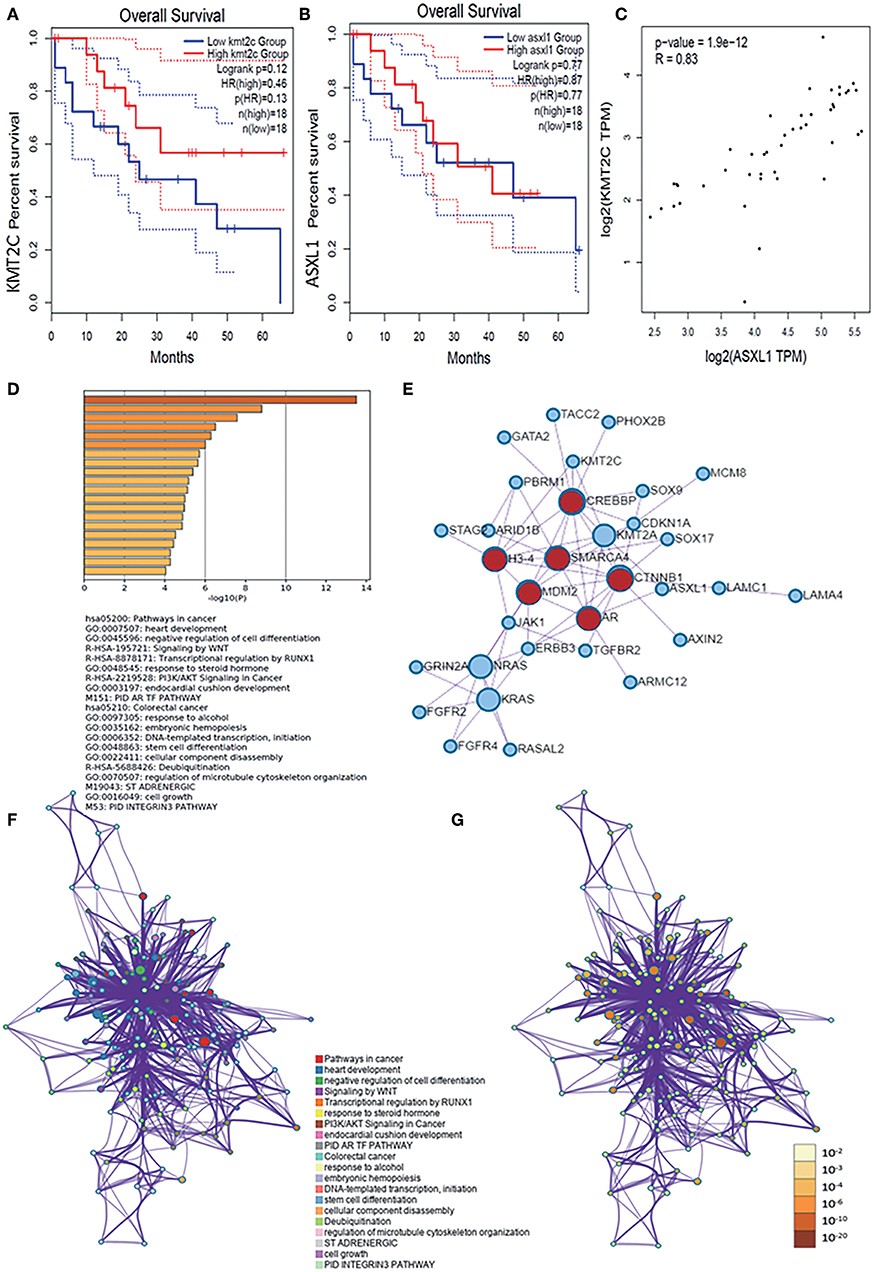
Figure 3. (A,B) Survival analysis based on the expression status of KMT2C and ASXL1 and a Kaplan-Meier curve was plotted. (C) Correlations of KMT2C and ASXL1 in CCA. (D) Bar graph of enriched terms across these enriched genes in AYAs with CCA, colored by p-values. (E) Protein–protein interaction network and MCODE components identified in the genes enriched in AYAs with CCA. (F,G) Network of enriched terms: (F) colored by cluster-ID, where nodes that share the same cluster ID are typically close to each other; (G) colored by p-value, where terms containing more genes tend to have a more significant p-value. ASXL1, additional sex combs like 1; AYA, adolescents and young adults; CCA, cholangiocarcinoma.
For these enriched genes in AYAs with CCA, pathway and process enrichment analysis had been carried out with the following ontology sources: KEGG Pathway, GO Biological Processes, Reactome Gene Sets, Canonical Pathways, and CORUM. Top 20 clusters with their enriched representative terms were shown in Figure 3D. To further capture the relationships between the terms, a subset of enriched terms had been selected and rendered as a network plot, where terms with a similarity >0.3 were connected by edges. The network was visualized using Cytoscape, where each node represented an enriched term and was colored first by its cluster ID (Figure 3F) and then by its p-value (Figure 3G). Specifically, the genes enriched in AYAs with CCA were associated with several pathways, such as cancer-associated pathways, negative regulation of cell differentiation, deubiquitination, WNT signal pathway, and so on.
Then, for these enriched genes in AYAs with CCA, protein–protein interaction enrichment analysis had also been carried out. Densely connected network components, including MDM2, SMARCA4, CTNNB1, AR, CREBBP, H3-4, were identified in Figure 3E.
External genomic profiles (cohort 2, cohort 3) were analyzed, and it was found that iCCA presented significant better OS than eCCA (p = 0.04, 44 vs. 35 months) and slightly better than pCCA, too (p = 0.09, 40 vs. 18 months) (Figures 4A,B).
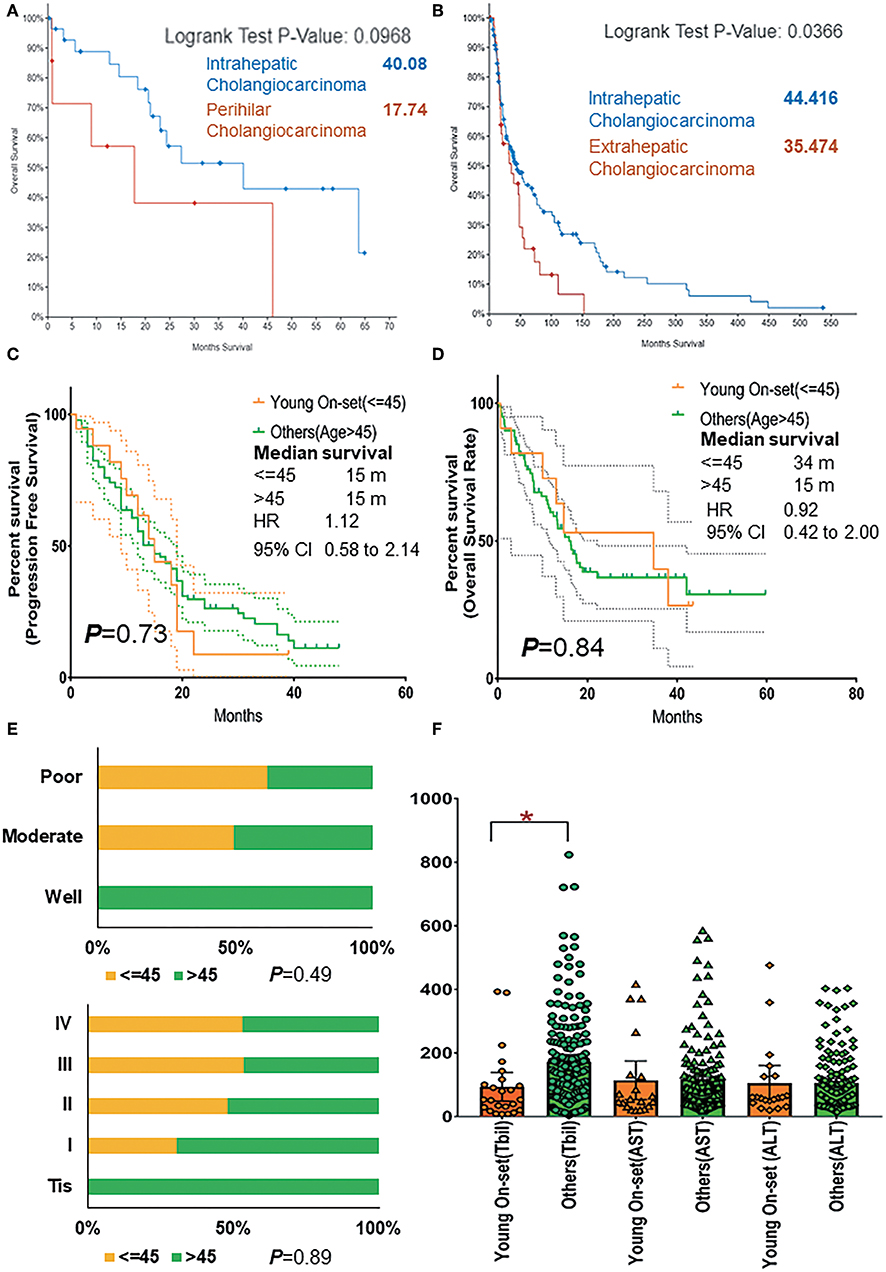
Figure 4. (A) Comparison of overall survival rate of patients with intrahepatic and perihilar CCA basing on cohorts 2 and 3. (B) Comparison of overall survival rate of patients with intrahepatic and extrahepatic CCA basing on cohorts 2 and 3. (C) The progression-free survival rate of AYAs and others (age >45) with pCCA basing on cohort 1. (D) The overall survival rate of AYAs and others (age >45) with pCCA basing on cohort 1. (E) The proportion and ratio of different grades of differentiation and different pathological stages in AYA and others (age >45) group. (F) The comparison of TBil, AST, and ALT expression in AYA (<=45, young-onset) group and other (>45) group. ALT, alanine transaminase; AST, aspartate transaminase; AYA, adolescents and young adults; CCA, cholangiocarcinoma; pCCA, perihilar CCA; TBil, total bilirubin (blood). *P < 0.05.
As is known, for patients in the intrahepatic, perihilar, and distal groups, the 5-year survival was 40, 10, and 23%, respectively (20). The prognosis of pCCA was the worst. Thus, by using our pCCA dataset containing 245 patients, we further investigated the prognosis between AYAs (cohort 1) and older patients (>45). Intriguingly, these patients had similar PFS (Figure 4C; p = 0.73, 15 vs. 15 months, HR 1.12, 95% CI 0.58–2.14) and OS rate (Figure 4D; p = 0.84, 34 vs. 15 months, HR 0.92, 95% CI 0.42–2.00).
Moreover, it was shown that AYAs were relevant to poor differentiation (Figure 4E) and advanced tumor stage (III and IV, 67%, Figure 4E). All AYAs in the current study presented with moderate and poor differentiation (Table 1). The comparison of chemical examinations showed that TBil value of older patients (>45 years old) were significantly elevated (Figure 4F).
Recognition of the clinical and genomic characters of AYAs with CCA is crucial for treatment strategy design. The treatments, especially targeted therapy and immunotherapy of AYAs, may differ from those best suited to older patients. It was reported that solid cancers (21), such as colorectal carcinoma, in AYAs were more aggressive and associated with a poorer prognosis as well as enriched MSI-H status compared to older patients (22, 23). In contrast, no MSI status was detected in AYAs with CCA in the present study. In the older patients' group, MSI occurred in 3~10% of the patients, similar to the reported general probability in all CCAs. The length of survival of AYAs (1.5 years) was almost half of the older patients (3 years); however, owing to the small sample size, no statistical significance was achieved. This was also the limitation of the present study.
The present study provided an initial landscape of genes that displays a greater mutational frequency in AYAs with CCA. Specifically, ASXL1 and KMT2C were found more frequent in AYAs compared with older patients with CCA.
ASXL1 mutations were known to be upregulated in solid cancers with metastasis (24) and in castration-resistant prostate cancer (CRPC) (25). Intriguingly, the significantly greater mutation frequency of ASXL1 combined with lower KRAS mutation was reported in kinase rearrangements (KRE). And lower KRAS mutation frequency was also detected in AYA patients as reported. Moreover, the high mutation rate of ASXL1 rates was also associated with MSI status enrichment (26). In the present study, the mutation frequency of ASXL1 was significantly higher in AYAs. KRAS mutation also tended to decrease but without statistical significance owing to the inadequate sample size. The only inconsistency was that all AYAs with CCA had MSS status instead of MSI. Patients with MSI-H status and KRE could benefit from both tyrosine kinase inhibitor (TKI) and checkpoint inhibitor treatment. However, this advantage seems to attenuate in AYAs with CCA. In contrast, it was reported that the transcription regulator ASXL1 mutation was associated with poorer outcomes as well as drug resistance (27), which might explain why AYAs with stage IV CCAs who underwent chemotherapy had worse prognosis in the present study.
Similar to ASXL1, KMT2C mutation was also enriched in late-stage or metastatic status of iCCA (28), breast cancer (29), and prostate cancer (30) and was associated to poor prognosis (31). Especially in AYAs with late-stage CCA, greater ASXL1 and KMT2C mutation rates were detected, which might suggest that CCAs in AYA patients is more aggressive.
Besides ASXL and KMT2C, the genes enriched in AYAs with CCA were analyzed by pathway and process enrichment analysis. And those genes were found to be associated with poorer differentiation, deubiquitination, and WNT signal pathway. Surgical resection remains the mainstay of potentially curative treatment for CCA. However, the probability of radical curative resection is low, and the prognosis is insufficient. Molecular profiling has delineated the genomic and transcriptomic characters of each CCA subtype. However, the genomic signature of AYA patients was not reported before. This study offered a preliminary landscape of the clinical and molecular features of early-onset biliary cancers. Further studies including more samples are essential to investigate whether ASXL1 and KMT2C could be considered potentially targetable genomic signatures for young patients.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of the Renji Hospital and Study Group of Biliary Surgery of the surgical branch of the Chinese Medical Association. The patients/participants provided their written informed consent to participate in this study.
HF and JW conceived the project, designed the study, and drafted the manuscript. HF and HT designed the study, wrote and revised the manuscript, and approved the final submission. HF, HT, and JW revised the manuscript and approved the final submission. JY, WC, and MH were involved in the design of the study. All authors read and approved the manuscript. All authors qualify as per ICJME criteria for authorship.
This study was supported by the Funding Program from Shanghai Jiao Tong University (SJTU) Cross-disciplinary project (HF, YG2017QN54), Shanghai Science and Technology Committee (STCSM) (HF, 18ZR1424200), National Natural Science Foundation, China (HF, 81902388 and HT, 81702545), Shanghai Shenkang Hospital Development Center (16CR2002A), 2016 Shanghai Leader Program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01439/full#supplementary-material
Supplemental Figure 1. (A) The mutation frequency of FGFR2, PBRM1, ERBB3 elevated in patients of AYA (< = 45) group; The mutation frequency of BAP1, KRAS, SMAD4 elevated in patients of others (>45) groups basing on cohort 3 (MSKCC); (B) A summary of presentative mutation in AYA and other groups basing on cohort 3 (MSKCC). AYA, Adolescents, and young adults; mut, mutation. (C) The difference of RNA expression between the two groups basing on cohort 2.
1. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. (2013) 145:1215–29. doi: 10.1053/j.gastro.2013.10.013
2. Hughes N, Stark D. The management of adolescents and young adults with cancer. Cancer Treat Rev. (2018) 67:45–53. doi: 10.1016/j.ctrv.2018.05.001
3. Mcveigh TP, Sundar R, Diamantis N, Kaye SB, Banerji U, Lopez J, et al. The role of genomic profiling in adolescents and young adults (AYAs) with advanced cancer participating in phase I clinical trials. Eur J Cancer. (2018) 95:20–9. doi: 10.1016/j.ejca.2018.02.028
4. Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. (editors). SEER Cancer Statistics Review, 1975-2010 [based on the November 2012 SEER data submission, posted in the SEER web site, April 2013].
5. Liang JT, Huang KC, Cheng AL, Jeng YM, Wu MS, Wang SM. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg. (2003) 90:205–14. doi: 10.1002/bjs.4015
6. Willauer AN, Liu Y, Pereira AA, Lam M, Morris JS, Raghav KP, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. (2019) 125:2002–10. doi: 10.1002/cncr.31994
7. Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. (2018) 24:4154–61. doi: 10.1158/1078-0432.CCR-18-0078
8. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. (2012) 2:401–4. doi: 10.1158/2159-8290.CD-12-0095
9. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross BE, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. (2013) 6:pl1. doi: 10.1126/scisignal.2004088
10. Silva VW, Askan G, Daniel TD, Lowery M, Klimstra DS, Abou-Alfa GK, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. (2016) 5:62. doi: 10.21037/cco.2016.10.04
11. Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. (2019) 10:1523. doi: 10.1038/s41467-019-09234-6
13. Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. (1990) 9:811–8. doi: 10.1002/sim.4780090710
14. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. (1960) 20:27–46. doi: 10.1177/001316446002000104
15. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. (2003) 11:2498–504. doi: 10.1101/gr.1239303
16. Stark C, Breitkreutz BJ, Reguly T, Boucher L, Breitkreutz A, Tyers M. BioGRID: a general repository for interaction datasets. Nucleic Acids Res. (2006) 34:D535–9. doi: 10.1093/nar/gkj109
17. Li T, Wernersson R, Hansen RB, Horn H, Mercer J, Slodkowicz G, et al. A scored human protein-protein interaction network to catalyze genomic interpretation. Nat Methods. (2017) 14:61–4. doi: 10.1038/nmeth.4083
18. Türei D, Korcsmáros T, Saez-Rodriguez J. OmniPath: guidelines and gateway for literature-curated signaling pathway resources[J]. Nat Methods. (2016) 13:966–7. doi: 10.1038/nmeth.4077
19. Bader GD, Hogue CWV. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. (2003) 4:2. doi: 10.1186/1471-2105-4-2
20. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. (2007) 245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3
21. Tricoli JV, Blair DG, Anders CK, Bleyer A, Boardman LA, et al. Biological and clinical characteristics of adolescent and young adult cancers: acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma and sarcoma. Cancer. (2016) 122:1017–1028. doi: 10.1002/cncr.29871
22. Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. (2014) 89:216–24. doi: 10.1016/j.mayocp.2013.09.006
23. Kirzin S, Marisa L, Guimbaud R, De Reynies A, Legrain M, Laurent-Puig P, et al. Sporadic early-onset colorectal cancer is a specific sub-type of cancer: a morphological, molecular and genetics study. PLoS ONE. (2014) 9:e103159. doi: 10.1371/journal.pone.0103159
24. Lee J, Ahn BK, Baik SS, Lee KH. Comprehensive analysis of somatic mutations in colorectal cancer with peritoneal metastasis. In vivo. (2019) 33:447–52. doi: 10.21873/invivo.11493
25. Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. (2012) 487:239–43. doi: 10.1038/nature11125
26. Madison R, Pietrantonio F, Juckett L, Cremolini C, Chung J, Albacker LA, et al. 457PD Kinase fusions in colorectal cancers: a unique biologic subset. Ann Oncol. 29: mdy281.005. doi: 10.1093/annonc/mdy281.005
27. Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. (2018) 562:526–31. doi: 10.1038/s41586-018-0623-z
28. Loffler M, Chandran PA, Laske K, Schroeder C, Bonzheim I, Walzer M, et al. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol. (2016) 65:849–5. doi: 10.1016/j.jhep.2016.06.027
29. Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, André F. Genomic characterization of metastatic breast cancers. Nature. (2019) 569:560–4. doi: 10.1038/s41586-019-1056-z
30. Armenia J, Wankowicz SA, Liu DR, Gao J, Kundra R, Reznik E, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. (2018) 50:645–51. doi: 10.1038/s41588-018-0078-z
Keywords: adolescents and young adults (AYAs), mutation, cholangiocarcinoma, early-onset, ASXL1
Citation: Feng H, Tong H, Yan J, He M, Chen W and Wang J (2019) Genomic Features and Clinical Characteristics of Adolescents and Young Adults With Cholangiocarcinoma. Front. Oncol. 9:1439. doi: 10.3389/fonc.2019.01439
Received: 06 August 2019; Accepted: 03 December 2019;
Published: 14 January 2020.
Edited by:
Toni Ibrahim, Romagnolo Scientific Institute for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Antonio Rozzi, Centre Hospitalier Régional Metz, Thionville, FranceCopyright © 2020 Feng, Tong, Yan, He, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wang, c3VyZ193YW5namlhbkAxMjYuY29t; Wei Chen, Y2hlbnN1cmdAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.