
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 29 November 2019
Sec. Head and Neck Cancer
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.01343
This article is part of the Research TopicAdvances in the Pathogenesis and Therapeutic Strategies for Nasopharyngeal CarcinomaView all 18 articles
Background and Purpose: Evidence for induction chemotherapy plus concurrent chemoradiotherapy (IC+CCRT) in nasopharyngeal carcinoma (NPC) was derived from landmark clinical trials excluding the T3N0, T3N1, T4N0 subgroups. This study used Epstein-Barr virus (EBV) DNA to select IC beneficiaries from the three subgroups.
Materials and Methods: Significant predictors of overall survival (OS) were identified using multivariate Cox analyses. Risk stratification was generated using recursive partitioning analysis (RPA). IC+CCRT was compared with CCRT in each risk stratification and in different subgroups. Individual-level data from a clinical trial (NCT01245959) was used for validation.
Results: Gender and EBV DNA were included in RPA-generated risk stratification, categorizing patients into low-risk (EBV DNA <2,000 copies/mL; female and EBV DNA ≥2,000 copies/mL) and high-risk groups (male and EBV DNA ≥2,000 copies/mL). The OS superiority of IC+CCRT over CCRT was only observed in the high-risk group (HR = 0.64, 95% CI = 0.43–0.97; P = 0.032). Subgroup analysis indicated the OS benefit was exclusively from the docetaxel–cisplatin−5-fluorouracil regimen (HR = 0.41, 95% CI = 0.22–0.78; P = 0.005). The status of the T3N1 subgroup as an IC beneficiary is more explicit than the T3N0 and T4N0 subgroups. IC+CCRT showed improved OS in the validation cohort combining high-risk cases of real-world data with clinical trial data (HR = 0.62, 95% CI = 0.42–0.94; P = 0.023).
Conclusion: Patients with high-risk T3N1 NPC is the definite target population for receiving IC+CCRT in real-world practice. T3N0 and T4N0 subgroups need further investigations in future IC-related studies.
As nasopharyngeal carcinoma (NPC) has the highest incidence in endemic areas such as Southern China, randomized controlled trials (RCTs) conducted in this region are incredibly important in optimizing clinical decision-making (1, 2). In excess of 70% of new cases are defined as locoregionally advanced NPC (LANPC; stage III–IVA), which is prone to distant metastasis and therefore requires intensive treatments over and above radiotherapy alone (3).
Since the INT 0099 trial successfully introduced chemotherapy for improved management of LANPC in 1998, various chemoradiotherapy schedules have been investigated using clinical trials (4–8). In the past two decades, concurrent chemoradiotherapy (CCRT) followed by adjuvant chemotherapy (AC) has been recommended by the National Comprehensive Cancer Network (NCCN) clinical guidelines as the standard treatment for LANPC due to its strong therapeutic intensity (9). However, a clinical trial from endemic area by Chen et al. reported that the additional AC induced severe gastrointestinal toxicities and low patient compliance (63%), which greatly restricted its broad practical application (5). Induction chemotherapy (IC) is used before radiotherapy and thought to be less toxic, improve tumor shrinkage, and lead to early eradication of micrometastases (10). The 2018 NCCN guidelines increased the recommendation of IC+CCRT from category III to IIA as one of the most appropriate treatments for LANPC, rendering it superior to CCRT (IIB) and equivalent to CCRT+AC (IIA) (11).
Three phase III RCTs from endemic areas provided supporting evidences for IC+CCRT (7, 8, 12). Cao et al. (7) investigated the cisplatin−5-fluorouracil (PF) IC regimen in LANPC excluding T3N0–1 subgroup and found IC+CCRT achieved higher 3-year disease-free survival than CCRT alone (82.0 vs. 74.1%; P = 0.028). Sun et al. (8) and Zhang et al. (12) individually explored docetaxel–cisplatin−5-fluorouracil (TPF) and gemcitabine–cisplatin (GP) IC regimens in LANPC excluding T3–4N0 subgroups. Both trials suggested that the additional IC can significantly improve 3-year overall survival (OS) compared with CCRT alone. Notably, target population of the three RCTs covered all LANPC but not T3N0–1 (7) or T3–4N0 (8, 12) subgroups, since these patients were crudely considered to have low risk of distant metastasis and not warranting additional IC. Although this inclusion criterion enhanced the power to detect survival benefits of IC+CCRT, it raised clinical questions that whether patients with T3N0, T3N1, and T4N0 NPC could benefit from IC, in that data on a relatively favorable subgroup is scarce and these patients are not always included in clinical trials. A phase III trial including all subgroups of LANPC patients in a non-endemic area reported non-significantly different OS between IC+CCRT and CCRT (P = 0.059) (13). Thus, the three subgroups (i.e., T3N0, T3N1, and T4N0) has become a potentially confounding factor that may exert effects on trial results, yet it has not been thoroughly investigated.
As the Tumor-Node-Metastasis (TNM) staging system only utilizes anatomical information, it solely may fail to identify IC beneficiaries from the three excluded subgroups. Epstein-Barr virus (EBV) DNA has been demonstrated to better refine risk stratification and guide individualized treatment in NPC (14). In this retrospective, joint analysis based on real-world and clinical trial data, we used pre-treatment EBV DNA and other critical predictors to select and validate the IC beneficiaries from these excluded T3N0, T3N1, and T4N0 NPC cases, with the purpose of providing real-world evidences to inform choices between treatment strategies in patients with T3N0, T3N1, and T4N0 NPC.
A flow diagram depicting the study design and inclusion/exclusion criteria is presented as Supplementary Figure 1. Given the reliance on a big-data intelligence platform (YiduCloud Technology Ltd., Beijing, China), we generated a NPC-specific real-world dataset that was adopted to identify all untreated, non-metastatic cases that were initially diagnosed at Sun Yat-sen University Cancer Center (SYSUCC) between April 2009 to December 2015. All patients received radical treatments based on intensity-modulated radiotherapy (IMRT) and complete basic data were obtained for each patient. A detailed description of the intelligence platform is presented in Supplementary Materials and has been published in a previous study (15).
This study was approved by the Institutional Review Board and the Ethics Committee with the approval ID YB2018-71; the need for informed consent was waived. To ensure study integrity, original raw data have been uploaded to a public platform named Research Data Deposit (http://www.researchdata.org.cn) with the identifier RDDA2018000782.
Clinical staging was guided by the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) manual. Pre-treatment examinations included complete medical history, physical examination, blood profile, nasopharyngoscopy, head-to-neck magnetic resonance imaging (MRI), chest radiography/computed tomography (CT), abdominal ultrasound, and skeletal scintigraphy; 18F-fluorodeoxyglucose positron emission tomography-CT was used to replace the latter three items for detection of possible metastases in the lung, liver, and bones. Moreover, circulating cell-free EBV DNA was quantified using a real-time quantitative polymerase chain-reaction (PCR) assay; the detailed method for this has been described in a previous study (14).
All patients underwent IMRT using the simultaneous integrated boost technique on 5 consecutive days every week. IC regimens consisted of PF regimen (80 mg/m2 and 4,000 mg/m2, respectively), docetaxel–cisplatin (TP; 75 and 75 mg/m2, respectively), and TPF (60, 60, and 3,000 mg/m2, respectively), every 3 weeks for 2–3 cycles. Concurrent chemotherapy was weekly (30–40 mg/m2) or 3-weekly cisplatin (80–100 mg/m2) treatment. Detailed information is shown in the Supplementary Materials.
Follow-up duration was measured from the day of diagnosis to the last visit or death. During the visits, head-to-neck MRI, chest radiography/CT, abdominal ultrasound, and skeletal scintigraphy were routinely performed, every 3 months during the first 2 years, then every 6 months for 3 years thereafter. Clinical suspicion of recurrence and distant metastasis were confirmed using cytological biopsies and imaging. The main endpoint was OS, measured from day of diagnosis until death due to any cause or the latest known date alive. Secondary endpoints were failure-free survival (FFS; from the date of diagnosis to failure, death, or last follow-up), locoregional relapse-free survival (LRRFS; to local/regional relapse), and distant metastasis-free survival (DMFS; to distant metastasis).
Continuous variables were converted into categorical variables based on the interquartile range (IQR; age at diagnosis) and clinical cut-off values [hemoglobin (Hb), albumin, lactate dehydrogenase (LDH), and C-reactive protein (CRP)]. Robust evidence has indicated that pre-treatment EBV DNA can refine the TNM staging for NPC at the cut-off of 2,000 copies/mL (16), which was also supported by this study using the receiver-operating characteristic (ROC) curve analysis (Supplementary Figure 2). Actuarial survival rates were calculated using the Kaplan-Meier curve and compared using the log-rank test (17). Univariate and multivariate Cox regression models were performed to quantify the effect of variables on OS. Univariate Cox analysis was performed a priori via a hypothesis-driven method. Predictors with P < 0.05 in the univariate analysis were entered into the multivariate analysis to validate their significance by backward stepwise algorithm (18). Hazard ratios (HRs) and 95% confidence intervals (CIs) were used as the summary statistics.
In accordance with the optimized binary partition algorithm, we included all validated predictors for 5-year OS to perform recursive partitioning analysis (RPA) using the rpart package in R, with the purpose of distinctly categorizing heterogeneous patients into purified risk stratifications (19). The prune package in R was used to remove the excessive branches of RPA-generated risk stratification for realistic application (19).
Individual-level, 5-year follow-up data of the TPF trial (NCT01245959), which discarded the T3–4N0 NPC cases was used to establish validation cohorts (20). Essentially, we aim to use the real-world dataset of T3–4N0 NPC patients to establish pseudo-trial cohorts (basic characteristics of patients should be consistent with their counterparts in a clinical trial), combine these data with the trial data, and determine whether the original trial results have changed significantly. Consequently, we validate the importance of additional IC to patients with T3–4N0 NPC. A three-step method was used to achieve this. Firstly, patients with T3–4N0 NPC at different risks were individually selected from the real-world dataset to produce two pseudo-trial cohorts. The sample size of T3–4N0 NPC was estimated according to its proportion, relative to the whole NPC population. Secondly, pseudo-trial cohorts of T3–4N0 NPC were processed to have similar baseline characteristics to the TPF trial, by using propensity score matching (PSM) to balance potential differences, since PSM can excellently mimic features of clinical trials and reduce selection bias caused by observed confounders (21). PSM was used in accordance with the nearest-neighbor algorithm without replacement. Thirdly, two validation cohorts were generated by combining pseudo-trial cohorts with the TPF trial data and verified by Kaplan-Meier survival analysis. C-index was used to measure the discriminatory performance of treatment via the Hemins package in R. All statistical analyses and figures were generated using SPSS, version 23.0 (SPSS Inc., Chicago, IL, USA) and R software, version 3.3.2 (http://www.r-project.org/). All tests were two-sided; P < 0.05 was significant.
The baseline characteristics of 2,692 patients with T3N0, T3N1, and T4N0 NPC are shown in Table 1. The median age was 45 (IQR = 37–52) years, with a male-to-female ratio of 2.6:1.0. Non-keratinizing undifferentiated NPC (World Health Organization type III) accounted for the majority (97.5%) of all endemic cases. The proportion of patients with pre-treatment EBV DNA ≥2,000 copies/mL was 57.5%.
In the whole real-world dataset (N = 9,354), all survival curves were significantly disparate except for the comparison of stage II and the overall subgroups of T3N0, T3N1, and T4N0 NPC, which had equivalent OS, FFS, LRRFS, and DMFS (all P ≥ 0.063; Supplementary Figure 3), indicating a good prognosis for T3N0, T3N1, and T4N0 NPC as a whole. As shown in Table 2, T3N1 had equivalent OS (P = 0.116) and LRRFS (P = 0.097) compared with T3N0, and equivalent DMFS (P = 0.511) compared with T4N0, suggesting homogeneity among patients with T3N0, T3N1, and T4N0 NPC.
After adjustment in multivariate analysis, age (P = 0.001), gender (P = 0.007), histological type (P ≤ 0.001), EBV DNA (P ≤ 0.001), albumin (P = 0.011), LDH (P = 0.001), and T category (P ≤ 0.001) were validated to have significant effects on OS (Table 1 and Supplementary Figure 4). All validated predictors were included in RPA to generate risk stratification. After modification of branches based on automatic rpart algorithms, gender and EBV DNA were retained in the final model while inessential factors were discarded.
Figure 1A shows that 2,692 patients with T3N0, T3N1, and T4N0 NPC were categorized into two groups: low-risk group (n = 1,857; EBV DNA titer <2,000 copies/mL, female & EBV DNA titer ≥2,000 copies/mL) and high-risk group (n = 835; male & EBV DNA titer ≥2,000 copies/mL). The low-risk group had significantly higher OS compared with the high-risk group (HR = 2.45, 95% CI = 1.81–3.32; P < 0.001; Figure 1B). Patients with different EBV DNA status in the low-risk group had comparable OS (P = 0.739; Supplementary Figure 5).
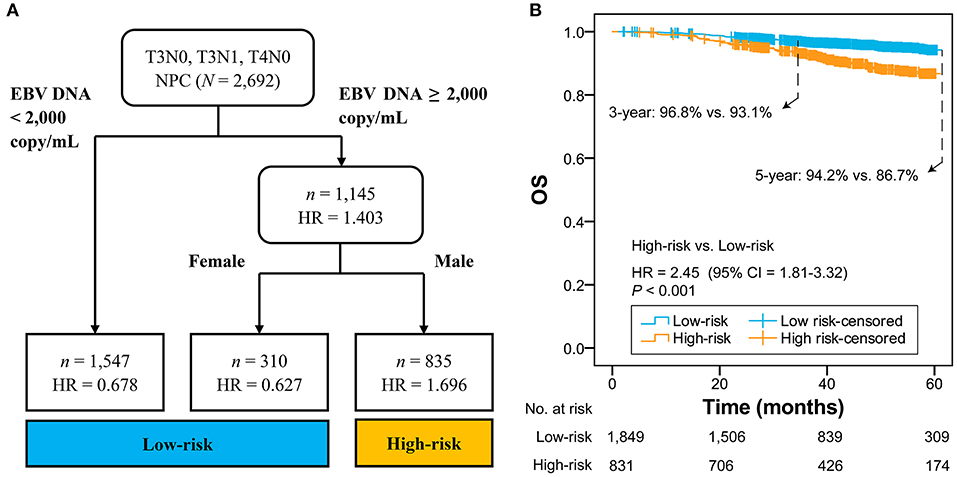
Figure 1. RPA-generated risk stratification (A) and the comparison between high-risk and low-risk groups (B). RPA, recursive partitioning analysis; NPC, nasopharyngeal carcinoma; EBV, Epstein-Barr virus; HR, hazard ratio; CI, confidence interval; OS, overall survival.
In all patients with T3N0, T3N1, T4N0 NPC, OS was not significantly different in the comparison of IC+CCRT and CCRT (HR = 0.77, 95% CI = 0.56–1.04; P = 0.090). In the low-risk group, a non-significant difference in OS was observed between IC+CCRT and CCRT (HR = 0.71, 95% CI = 0.44–1.12; P = 0.138). In the high-risk group, patients receiving IC+CCRT had significantly improved OS compared with their counterparts receiving CCRT alone (HR = 0.64, 95% CI = 0.42–0.97; P = 0.032; Figures 2A–C).
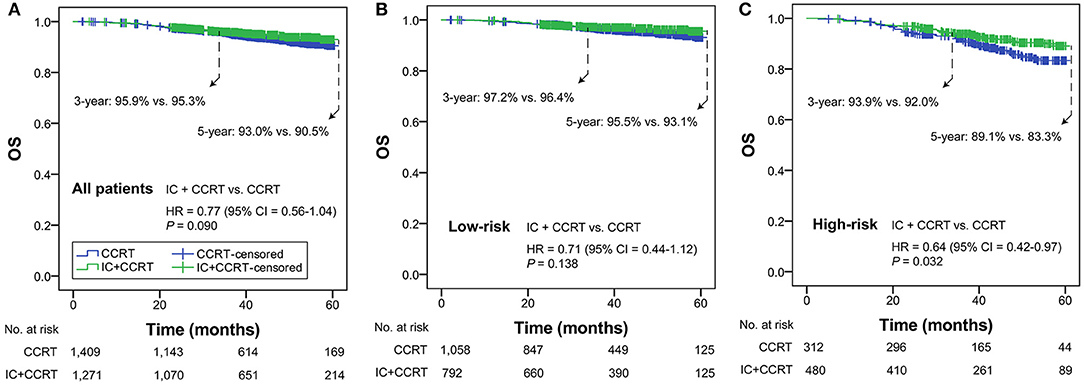
Figure 2. Kaplan-Meier OS curves of IC+CCRT vs. CCRT in the overall T3N0, T3N1, and T4N0 NPC (A), low- (B) and high-risk groups (C). HR, hazard ratio; CI, confidence interval; OS, overall survival; IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy.
Subgroup analysis was performed primarily based on IC regimens and specific LANPC subgroups. The improved, non-adjusted OS of IC+CCRT compared with CCRT was only observed in high-risk patients undergoing TPF (HR = 0.41, 95% CI = 0.22–0.78; P = 0.005) but not PF, TP, or any of the IC regimens in the low-risk group (all P ≥ 0.061; Figure 3). Subgroup analysis was individually performed based on T3N0, T3N1, and T4N0 subgroups. Regardless of the specific clinical stages, low-risk patients treated by IC+CCRT generally had equivalent OS compared with those treated by CCRT alone (all P ≥ 0.065). In high-risk patients, IC+CCRT was found to have significant survival benefit in OS compared with CCRT in the T3N1 subgroup (P = 0.005), but not in the T3N0 or T4N0 subgroups (Figure 4). The sample size of each treatment arm in T3N0 and T4N0 subgroups was very small, ranging from 15 to 44.
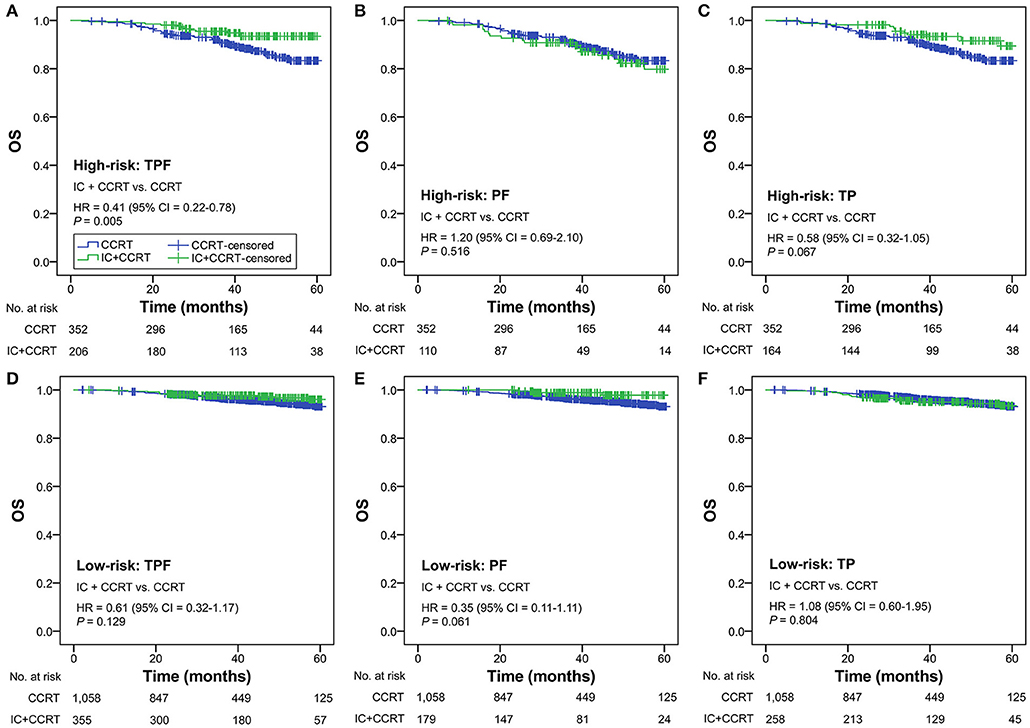
Figure 3. IC+CCRT vs. CCRT in the subgroup analysis based on IC regimen [high-risk: TPF (A), PF (B), TP (C); low-risk: TPF (D), PF (E), TP (F)] without adjustment. IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy; TPF, docetaxel–cisplatin−5-fluorouracil; PF, cisplatin−5-fluorouracil; TP, cisplatin−5-fluorouracil.
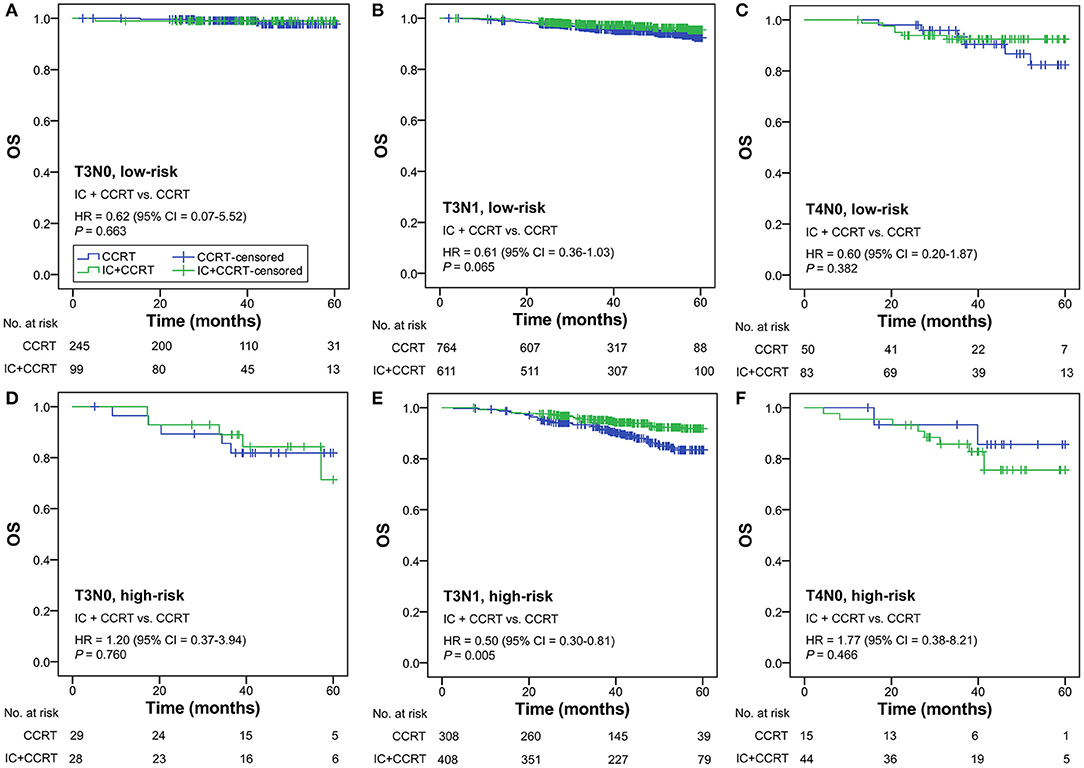
Figure 4. IC+CCRT vs. CCRT in the subgroup analysis based on specific clinical stages [low-risk: T3N0 (A), T3N1 (B), T4N0 (C); high-risk: T3N0 (D), T3N1 (E), T4N0 (F)]. IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy.
The RPA-generated risk stratification showed superb discriminatory ability in all subgroups, except for T4 category (P = 0.065; Table 3). After adjustment of covariates, the superiority of IC+CCRT over CCRT in OS was observed in the high-risk subgroup of age ≥53 years (P = 0.006), LDH ≤ 250 (P = 0.001), T3 category (P = 0.005), N1 category (P = 0.003), and TPF IC regimen (P = 0.003).
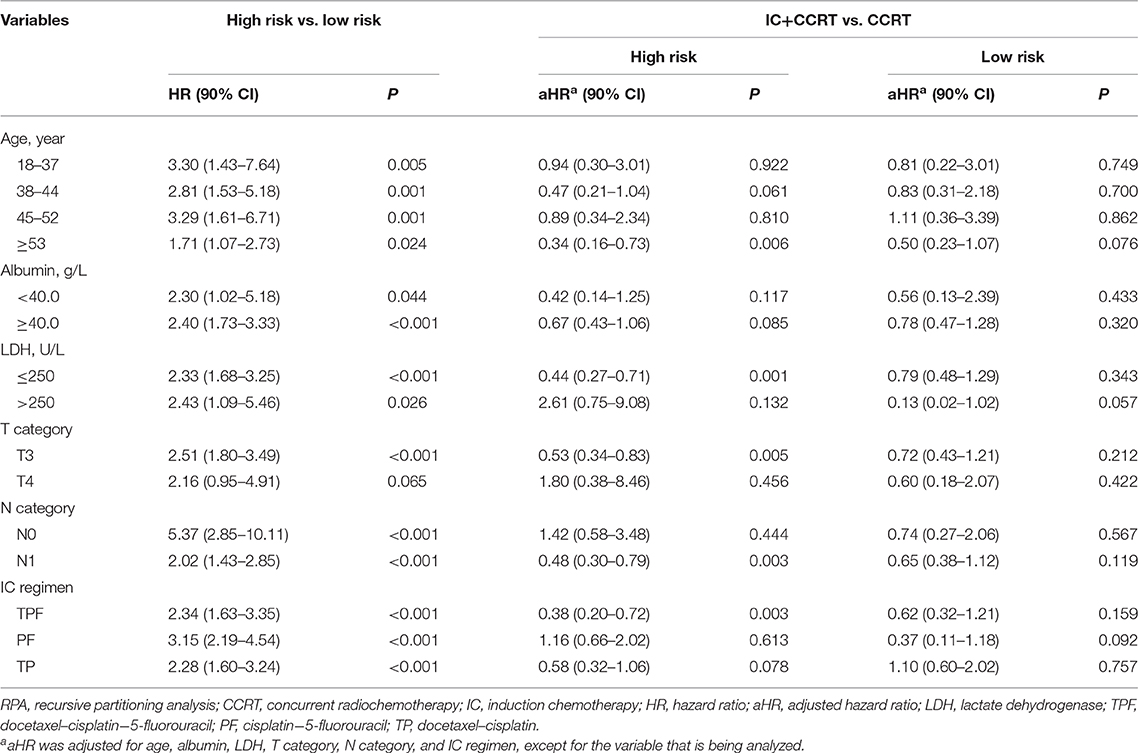
Table 3. Subgroup analysis of RPA-generated risk stratification and IC+CCRT vs. CCRT with adjustment.
A total of 54 patients with T3–4N0 NPC was required to be incorporated into the TPF trial (n = 480) in accordance with the sample size ratio of 1 to 9. Eligible patients were individually selected from all of the T3–4N0 NPC group (n = 594) and high-risk T3–4N0 NPC group (n = 117) to match the trial baselines (e.g., TPF IC regimen, 3-weekly concurrent cisplatin, accumulated concurrent cisplatin ≥200 mg, age, and gender) and produce the validation cohort 1 and cohort 2, respectively. Both cohorts contained 534 patients comparing IC+CCRT with CCRT in LAPNC (267 vs. 267; Figure 5A).
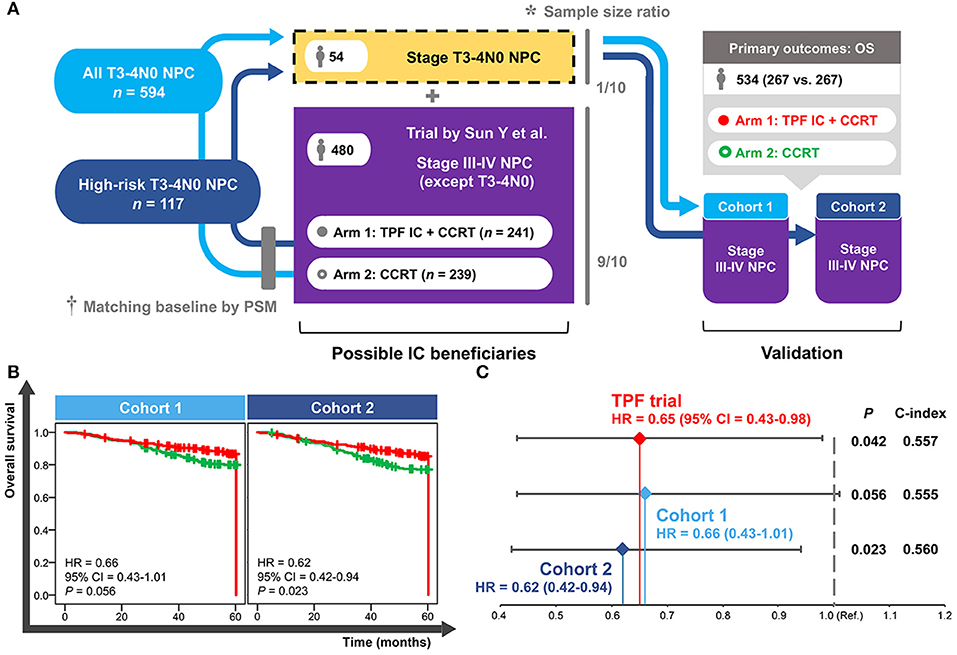
Figure 5. Establishment (A) and validation (B,C) of the cohorts based on real-world and clinical trial data. *Sample size ratio was calculated based on the NPC-specific real-world dataset including 10,126 patients. †Baseline of the selected T3–4N0 NPC patients was matched with the trial data using the PSM method. NPC, nasopharyngeal carcinoma; PSM, propensity score matching; TPF, docetaxel–cisplatin−5-fluorouracil; IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy; OS, overall survival; HR, hazard ratio; CI, confidence interval; Ref., reference.
As shown in Figure 5B, a significantly improved OS of IC+CCRT compared with CCRT was only observed in the validation cohort 2 (HR = 0.62, 95% CI = 0.42–0.94; P = 0.023) but not cohort 1(HR = 0.66, 95% CI = 0.43–1.01; P = 0.056). Cohort 2 showed more obvious superiority of IC+CCRT over CCRT (HR: 0.62 vs. 0.65) and better discrimination performance (c-index: 0.560 vs. 0.557) than the long-term results of the TPF trial (Figure 5C).
With an increasing emphasis on IC in the NCCN guidelines, more robust studies are required to consider evidence on the comparison of IC+CCRT and CCRT in T3N0, T3N1, and T4N0 NPC, given that these patients had been excluded from a majority of IC-related trials and the evidence regarding optimal treatment strategy is limited. In December 2016, the U.S. Congress enacted $$The 21st Century Cures Act, which modified the Food and Drug Administration policies and inspired investigators to provide real-world evidence as supplements to clinical trials, in order to expedite the approval process for innovative research (22). This retrospective, joint analysis based on real-world and clinical trial data is the first attempt to identify IC beneficiaries among patients with T3N0, T3N1, and T4N0 NPC based on EBV DNA status (Supplementary Figure 6). We provide robust real-world evidence that can further complement the contemporary trial results.
The 8th edition AJCC/UICC staging system has successfully incorporated human papillomavirus infection status into the TNM classification of oropharyngeal carcinoma (23), which highlights the possibility of including non-anatomic factors to better differentiate the prognosis of EBV-related NPC. EBV is exclusively detected in tumor cells but not in normal nasopharyngeal epithelium; its cell-free DNA has the same polymorphism as the primary lesion tumor and is considered to be released into the peripheral circulation along with the tumor cells death of Lin et al. (24). Previous studies reported that circulating EBV DNA correlate with the tumor burden, stage classification, and survival of patients with NPC (25–27). The practical application of EBV DNA has expanded from initial diagnosis, detection of metastasis, to population screening and pre-treatment risk stratification (28–30). Only two recent studies have included incorporation of pre-treatment plasma EBV DNA into the 8th edition of TNM staging system (16, 31). These studies indicated that the risk of T3N0, T3N1, and T4N0 NPC could be refined by EBV DNA, since both plans covered the three subgroups. A previous retrospective study reported that patients with T3–4N0–1 NPC receiving CCRT could not benefit from additional IC, which may be influenced by the fact that EBV DNA was not used for the screening of IC beneficiaries (32). Similarly, in this study, we reported a non-significant difference in OS between IC+CCRT and CCRT in the whole patients with T3N0, T3N1, and T4N0 NPC when they had not been stratified (Figure 2A). Therefore, high level EBV DNA may be an indicator for physicians to employ IC in LANPC.
Another validated predictor used for risk stratification in this study is gender. Although both genders had the same improved level of plasma EBV DNA, female patients obtained better OS benefits than males (Supplementary Figure 5). This result was in line with a previous finding, which reported that female is associated with better prognosis in NPC compared with male gender (33). One proposed hypothesis is that female hormones can promote immunological responses and confer higher resistance to oxidative damage (34).
This study demonstrated that the OS benefit for high-risk patients was mainly associated with the TPF regimen but not PF or TP IC regimens. The potent triple agent-based TPF regimen has been shown to be a promising prospect in LANPC, allowing patients to receive stronger intensity treatment, longer hospitalization, improved nursing care, and more supportive therapy than the PF regimen, while intensive management itself can lead to better prognosis (8, 13). In addition, the subgroup analysis based on specific clinical stages only supported the high-risk T3N1 subgroup, but not the T3N0 or T4N0 subgroups, as an IC beneficiary. This result should be regarded with caution, since statistical non-significance may be related to the insufficient sample size of two treatment arms in the high-risk T3N0 (28 vs. 29) and T4N0 (44 vs. 15) subgroups. The PF trial that included all LANPC subgroups except T3N0–1 NPC has recently been updated. It revealed a significant 5-year OS benefit of IC+CCRT compared with CCRT (80.8 vs. 76.8%; P = 0.04) (35), indicating that the T4N0 subgroup should receive IC+CCRT in clinical practice. Hence, the T3N1 subgroup may not be the only IC beneficiary, and all the three subgroups (T3N0, T3N1, T4N0) should be fully investigated. Since individual patient data of the PF trial is not accessible, we only performed the validation analysis using the 5-year data of the TPF trial (20), which successfully verified the effectiveness of RPA-generated risk stratification.
Several limitations to this study should be stated. First, it is important to recognize that different centers adopt different EBV DNA cut-off values, such as 4,000 copies/mL (36), 500 copies/mL (31), and 1,500 copies/mL (24). Moreover, the heterogeneity in PCR-based EBV DNA testing itself is an important problem, with sensitivity ranging from 53 to 96% (37). Assay harmonization of EBV DNA detection is a major hurdle that has to be overcome prior to incorporation of plasma EBV DNA as a clinical decision-making tool. In 2015, a workshop on harmonization of EBV testing for NPC was hosted by the National Cancer Institute. It offered valuable strategies for establishment of harmonized EBV DNA assays and key recommendations guiding future clinical use (37). Second, in this study, results were driven by the T3N1 subgroup (78%), and the OS superiority of IC+CCRT over CCRT in high-risk patients was only observed in the subgroup of T3 and N1 category. Although validation analysis confirmed that patients with high-risk T3–4N0 NPC could effectively benefit from IC, the sample size of T3N0 and T4N0 subgroups was too small to generate a reliable conclusion. Two robust phase 3 RCTs including all LANPC subgroups except T3–4N0 NPC had reported significant OS improvement of 6.0 and 4.3% from the additional TPF (8) and GP (12) IC regimens, respectively. Therefore, the status of the T3N1 subgroup as an IC beneficiary is more explicit than the T3N0 and T4N0 subgroups, while the latter two require more supporting evidences beyond this study. Third, the retrospective nature limits this study to some extent. This study was performed based on the 8th edition of the AJCC/UICC staging system for a better generalizability in real-world clinical practice. Although the clinical trial in validation analysis used the 7th edition of the AJCC/UICC staging system, the difference in staging systems was too subtle to exert obvious influence on results. Re-staging was not performed in this study because the transform of the staging system from the 7th edition into the 8th edition would compromise data integrity. Nonetheless, this real-world study offers essential information to clinical physicians and trialists, helping them make precise clinical decisions and refine future trial design.
RPA-generated risk stratification based on pre-treatment plasma EBV DNA provides good and robust efficacy of OS prediction in T3N0, T3N1, and T4N0 NPC. In comparison with CCRT, IC+CCRT leads to significantly improved OS for patients with high-risk T3N1 NPC, which is mainly due to the TPF IC regimen. Patients with high-risk T3N1 NPC is the definite target population for receiving IC+CCRT in real-world practice. T3N0 and T4N0 subgroups need further investigations in future IC-related studies.
The datasets for this study can be found in the public platform named Research Data Deposit (http://www.researchdata.org.cn) with the identifier RDDA2018000782.
The studies involving human participants were reviewed and approved by Institutional Review Board and the Ethics Committee of Sun Yat-sen University Cancer Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
L-LT and CX: conceptualization. CX, QL, and YG: methodology. CX, LC, and Y-PM: software. CX and W-FL: validation. CX, SZ, and LC: formal analysis. SZ, YG, and Y-PM: investigation. W-FL and JM: resources. SZ and W-FL: data curation. CX and SZ: writing—original draft preparation. W-FL, JM, and L-LT: writing—review and editing. CX: visualization. L-LT: supervision, project administration, and funding acquisition.
This study was supported by grants from the National Natural Science Foundation of China (81930072), Key-Area Research and Development Program of Guangdong Province (2019B020230002), Natural Science Foundation of Guangdong Province (2017A030312003), Health & Medical Collaborative Innovation Project of Guangzhou City, China (201803040003), Innovation Team Development Plan of the Ministry of Education (No. IRT_17R110), Overseas Expertise Introduction Project for Discipline Innovation (111 Project, B14035).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank YiduCloud (Beijing, China) Technology Ltd. for supporting the data extraction and processing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01343/full#supplementary-material
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
2. Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. (2017) 36:90. doi: 10.1186/s40880-017-0257-9
3. Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. (2009) 73:1326–34. doi: 10.1016/j.ijrobp.2008.07.062
4. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. (1998) 16:1310–7. doi: 10.1200/JCO.1998.16.4.1310
5. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. (2012) 13:163–71. doi: 10.1016/S1470-2045(11)70320-5
6. Lee AW, Tung SY, Chua DT, Ngan RK, Chappell R, Tung R, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. (2010) 102:1188–98. doi: 10.1093/jnci/djq258
7. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer. (2017) 75:14–23. doi: 10.1016/j.ejca.2016.12.039
8. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
9. Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, et al. Head and neck cancers, Version 2.2014. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2014) 12:1454–87. doi: 10.6004/jnccn.2014.0142
10. Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol. (2005) 23:1118–24. doi: 10.1200/JCO.2005.12.081.
11. Pfister DG, Spencer S, Adelstein D, Adkins D, Brizel DM, Burtness B, et al. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Head and Neck Cancers. Version 1.2018. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed September 4, 2019).
12. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. New Engl J Med. (2019) 381:1124–35. doi: 10.1056/NEJMoa1905287
13. Frikha M, Auperin A, Tao Y, Elloumi F, Toumi N, Blanchard P, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol. (2018) 29:731–6. doi: 10.1093/annonc/mdx770
14. Xu C, Chen YP, Liu X, Li WF, Chen L, Mao YP, et al. Establishing and applying nomograms based on the 8th edition of the UICC/AJCC staging system to select patients with nasopharyngeal carcinoma who benefit from induction chemotherapy plus concurrent chemoradiotherapy. Oral Oncol. (2017) 69:99–107. doi: 10.1016/j.oraloncology.2017.04.015
15. Lv JW, Chen YP, Huang XD, Zhou GQ, Chen L, Li WF, et al. Hepatitis B virus screening and reactivation and management of patients with nasopharyngeal carcinoma: A large-scale, big-data intelligence platform-based analysis from an endemic area. Cancer. (2017) 123:3540–9. doi: 10.1002/cncr.30775
16. Guo R, Tang LL, Mao YP, Du XJ, Chen L, Zhang ZC, et al. Proposed modifications and incorporation of plasma Epstein-Barr virus DNA improve the TNM staging system for Epstein-Barr virus related nasopharyngeal carcinoma. Cancer. (2019) 125:79–89. doi: 10.1002/cncr.31741
17. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. (1958) 53:457–81. doi: 10.1080/01621459.1958.10501452
18. Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. (1972) 34:187–220. doi: 10.1007/978-1-4612-4380-9_37
19. Atkinson EJ, Therneau TM. An Introduction to Recursive Partitioning Using the RPART Routines. Rochester, NY: Rochester Mayo Foundation (2000).
20. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long-term results of phase 3 randomized controlled trial. Int J Cancer. (2019) 145:295–305. doi: 10.1002/ijc.32099
21. Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. (2006) 59:437–47. doi: 10.1016/j.jclinepi.2005.07.004
22. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med. (2016) 375:2293–7. doi: 10.1056/NEJMsb1609216
23. Amin MB, Edge SB, Greene FL, Schilsky RL, Gaspar LE, Washington MK, et al. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer (2018).
24. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. (2004) 350:2461–70. doi: 10.1056/NEJMoa032260
25. Leung SF, Zee B, Ma BB, Hui EP, Mo F, Lai M, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. (2006) 24: 5414–8. doi: 10.1200/JCO.2006.07.7982
26. Lo YM, Chan LY, Chan AT, Leung SF, Lo KW, Zhang J, et al. Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res. (1999) 59:5452–5.
27. Ma BB, King A, Lo YM, Yau YY, Zee B, Hui EP, et al. Relationship between pretreatment level of plasma Epstein-Barr virus DNA, tumor burden, and metabolic activity in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. (2006) 66:714–20. doi: 10.1016/j.ijrobp.2006.05.064
28. Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, et al. Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer. N Engl J Med. (2017) 377:513–22. doi: 10.1056/NEJMoa1701717
29. Takes RP, Rinaldo A, Silver CE, Piccirillo JF, Haigentz M Jr, Suarez C, et al. Future of the TNM classification and staging system in head and neck cancer. Head Neck. (2010) 32:1693–711. doi: 10.1002/hed.21361
30. Wang WY, Twu CW, Chen HH, Jan JS, Jiang RS, Chao JY, et al. Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res. (2010) 16:1016–24. doi: 10.1158/1078-0432.CCR-09-2796
31. Lee VH, Kwong DL, Leung TW, Choi CW, O'Sullivan B, Lam KO, et al. The addition of pretreatment plasma Epstein-Barr virus DNA into the eighth edition of nasopharyngeal cancer TNM stage classification. Int J Cancer. (2019) 144:1713–22. doi: 10.1002/ijc.31856
32. Wu LR, Yu HL, Jiang N, Jiang XS, Zong D, Wen J, et al. Prognostic value of chemotherapy in addition to concurrent chemoradiotherapy in T3-4N0-1 nasopharyngeal carcinoma: a propensity score matching study. Oncotarget. (2017) 8:76807–15. doi: 10.18632/oncotarget.20014
33. Xu C, Liu X, Chen YP, Mao YP, Guo R, Zhou GQ, et al. Impact of marital status at diagnosis on survival and its change over time between 1973 and 2012 in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Cancer Med. (2017) 6:3040–51. doi: 10.1002/cam4.1232
34. Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. (2019) 119:87–96. doi: 10.1016/j.ejca.2019.07.007.
35. Austad SN, Fischer KE. Sex Differences in Lifespan. Cell Metab. (2016) 23:1022–33. doi: 10.1016/j.cmet.2016.05.019
36. Chan AT, Lo YM, Zee B, Chan LY, Ma BB, Leung SF, et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. (2002) 94:1614–9. doi: 10.1093/jnci/94.21.1614
Keywords: nasopharyngeal carcinoma, Epstein-Barr virus, induction chemotherapy, concurrent chemoradiotherapy, recursive partitioning analysis
Citation: Xu C, Zhang S, Li W-F, Chen L, Mao Y-P, Guo Y, Liu Q, Ma J and Tang L-L (2019) Selection and Validation of Induction Chemotherapy Beneficiaries Among Patients With T3N0, T3N1, T4N0 Nasopharyngeal Carcinoma Using Epstein-Barr Virus DNA: A Joint Analysis of Real-World and Clinical Trial Data. Front. Oncol. 9:1343. doi: 10.3389/fonc.2019.01343
Received: 14 September 2019; Accepted: 15 November 2019;
Published: 29 November 2019.
Edited by:
David I. Rosenthal, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Sarbani Ghosh Laskar, Tata Memorial Hospital, IndiaCopyright © 2019 Xu, Zhang, Li, Chen, Mao, Guo, Liu, Ma and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-Long Tang, dGFuZ2xsQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.