- Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Beijing, China
This study is to compare the survival outcomes of laparoscopic radical hysterectomy (LRH) to those of abdominal radical hysterectomy (ARH) for patients with locally advanced cervical cancer (LACC). Patients with the International Federation of Gynecology and Obstetrics (FIGO) 2009 stage IB2 to IIB LACC who underwent radical hysterectomy between 2001 and 2015 were identified. The disease-free survival (DFS) and overall survival (OS) were compared according to the surgical approach and were adjusted based on clinicopathologic characteristics. A total of 396 patients were included in the study, with 179 (45.2%) and 217 (54.8%) patients in the ARH and LRH groups, respectively. The LRH group showed a significantly lower amount of estimated blood loss, lower blood transfusion rate and shorter length of hospital stay. Overall, there were no significant differences in the 5-year DFS and 5-year OS between the LRH and ARH groups with the Kaplan-Meier method. However, multivariate analyses identified LRH as an independent prognostic factor for a poor DFS (hazard ratio [HR] 2.5; 95% confidence interval [95% CI] 0.19 to 0.87; p = 0.02). The analysis of stage IB2 disease and the squamous subtype (61.9% and 87.9% of all participants, respectively) reached the same conclusion. When stratifying by FIGO stage, the patients with IB2 (n = 348) in the ARH group had a significantly better DFS (HR 0.14, 95% CI 0.05–0.42, p < 0.01) and OS (HR 0.17, 95% CI 0.04–0.67, p = 0.11) than those in the LRH group in the Cox regression model. However, no differences were found in patient with stage IIA1, IIA2, or IIB in Cox regression model. When stratifying by histological types, for the patients with squamous carcinomas (n = 375), in Cox model, ARH had a significantly superior DFS compared with those who underwent LRH (HR 0.45, 95% CI 0.25–0.82, p = 0.01), but the OS was not statistically significant (HR 0.57, 95% CI 0.27–1.20, p = 0.14). However, no differences were found in patient with adenocarcinoma and adenosquamous carcinomas in the Cox model. Therefore, ARH was associated with a higher DFS than LRH in patients with LACC, especially in patients with stage IB2 disease or the squamous subtype.
Introduction
Cervical cancer (CC) is one of most common cancer-related deaths among women worldwide (1). China also has a high burden of disease and a relatively high prevalence of advanced-stage disease (2). Locally advanced cervical cancer (LACC) refers to stage IB2, IIA, and IIB carcinomas, classified by the International Federation of Gynecology and Obstetrics (FIGO) staging system (3). The treatment for women with LACC remains challenging. The standard treatment for LACC is cisplatin-based concurrent chemoradiotherapy (CCRT) (4, 5). However, surgical treatment, including radical hysterectomy (RH) followed by adjuvant treatment is also a preferred treatment option for LACC in some circumstances (6, 7) and could offer potential benefits, including reducing the burden of tumor, preserving ovarian function and precisely determining the postoperative stage on the basis of histopathologic findings, thereby allowing quality of life improvements in young patients and individualizing postoperative treatment (4).
Recently, a phase III trial (8) and an epidemiological study (9) revealed that women undergoing minimally invasive surgery (MIS) through RH for early CC had a higher recurrence and worse survival than those who underwent abdominal radical hysterectomy (ARH). Nevertheless, only a few studies have compared the surgical and oncologic outcomes of MIS through RH to those of ARH for LACC. In the report from Zanagnolo et al. (10), ARH and robotic RH after neoadjuvant chemotherapy (NAC) in women with LACC were associated with similar perioperative and oncologic outcomes. Type B RH after NAC in well-selected patients is a safe procedure that could potentially reduce the operative time and late postoperative morbidity, without detrimental effects to survival (11). However, these trials lack sufficient sample sizes, long-term follow-ups and good comparators, which limit the generalizability of their conclusions.
In this study, we aimed to compare the survival outcomes of LRH with those of ARH for patients with FIGO stage IB2 to IIB CC at a tertiary institutional hospital in China from 2001 to 2015. All major procedures, consisting of parametrium resection and systematic lymphadenectomy, were performed by the corresponding authors.
Methods
Ethical Approval
The Institutional Review Board from the study center approved the study (No. ZS-1427). All patients provided consent before treatment. The registration number is NCT03291236 (clinicaltrials.gov). All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Study Design and Patient Enrollment
A total of 428 patients with CC classified as stage IB2 to IIB, according to the FIGO staging system of 2009, who received class III or type C ARH or LRH based on the Q-M classification from February 2, 2001 to November 11, 2015 at the study center were enrolled in this retrospective study. The inclusion criteria consisted of the following: FIGO stage IB2 to IIB cancer diagnosed by pelvic examinations conducted by two experienced physicians of gynecologic oncology; histopathologically proven primary cervical squamous carcinoma, adenocarcinoma or adenosquamous carcinoma; aged 18 years or older; and Eastern Cooperative Oncology Group performance status scores of 0 or 1. Patients were excluded if they had distant metastasis based on presurgical imaging.
The patients were divided into ARH and LRH groups according to their definitive surgical route. The primary objective was to compare the disease-free survival (DFS) and overall survival (OS) between the two groups. The secondary endpoints included the surgical outcomes of the two groups.
Treatments and Follow-up
Surgical treatment consisted of LRH and ARH, with bilateral salpingo-oophorectomy, and lymphadenectomy of the pelvic lymph nodes (PLNs) and para-aortic lymph nodes (PALNs). Salpingectomy was performed in young patients, along with translocation of the ovaries to the peritoneum above the level of the anterior superior spine. All the major surgical procedures (resection of parametrium and systematic lymphadenectomy) were primarily performed by the corresponding authors, and the choice of LRH and ARH was due to the experiences and learning curve of the surgeons. The surgical extent followed the specifications of class III of the Piver classification (before 2011) (12) or type C of the Q-M classification (after 2011) (13, 14). The nerve-sparing RH procedures have been described in another study (15). The complications related to ARH and LRH that occurred within 3 months were reviewed and collected from the medical records as adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.03 (16). As currently there were no criteria or standard for utilizing NAC, NAC was given for fractional patients with bulky tumors after a discussion with the patients. The NAC protocols consisted of TC (paclitaxel 175 mg/m2, carboplatin AUC 5 on day 1 in a 21-day cycle administered via intravenous infusion), TP (paclitaxel 175 mg/m2, cisplatin 70 mg/m2 on day 1 of a 21-day cycle via intravenous infusion) or PF (fluorouracil 1,000 mg/m2 on days 1 to 4, cisplatin 70 mg/m2 on day 1 of a 28-day cycle via intravenous or transuterine arterial infusion). Postoperative adjuvant therapies were provided for the eligible patients and included systematic chemotherapy, radiotherapy, radiotherapy and concurrent chemoradiotherapy (CCRT) or a combination of these regimens. The administration of all adjuvant therapies followed the relevant contemporary guidelines (17).
All tumor specimens were investigated with detailed pathological examinations used to determine the characteristics of the pathological subtypes, lymphovascular space invasion (LVSI), invasion depth of the stroma, lymphatic metastasis, involvement of the uterus or parametrium, and status of the incision margin. For RH performed before 2009, the staging was reviewed and redefined according to FIGO 2009 criteria (18).
All patients were followed until March 1, 2017. The patients were closely followed up according to the customized protocol: all patients were asked to visit an outpatient clinic every 3 months for the first year, every 4 months for the second year, every 6 months for the third year, and every year for the remainder of the follow-up time. The patients underwent physical examinations, cytology tests, and imaging evaluations. Recurrence was validated by imaging examination and/or biopsy. The recurrent sites were divided into categories including within the pelvic cavity and at distant sites. Mortality was confirmed by reviewing the medical records and interviews by telephone and/or email. DFS was defined as the length of time after receiving primary treatment for a cancer that a patient lived without any signs or symptoms of that cancer, and OS was defined as the length of time that the patient was alive after receiving primary treatment for that cancer. Specifically, the survival analysis was performed again in patients of stage IB2 to IIA2.
Statistical Analysis
SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The characteristics of different patients were compared between the ARH and LRH groups using Student's t-tests, Wilcoxon rank sum tests and chi-square tests. The Kaplan-Meier method with log-rank tests was used to compare the survival outcomes between the two groups as univariate methods. In multivariate analyses, the hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression models. The following factors were used to adjust the hazard ratios of survivals between ARH and LRH groups in a Cox regression model: operative period (before and after 2010), menstrual status, nerve-sparing radical hysterectomy (NSRH), FIGO stage, pathological subtype, differentiation status, lymph node metastasis, involvement of the parametrium and vaginal margin, invasion depth of stroma, LVSI, residual lesions, postoperative complications and adjuvant therapies. All reported statistical significances were two-tailed at a level of 0.05.
Results
Baseline Clinical and Pathological Characteristics
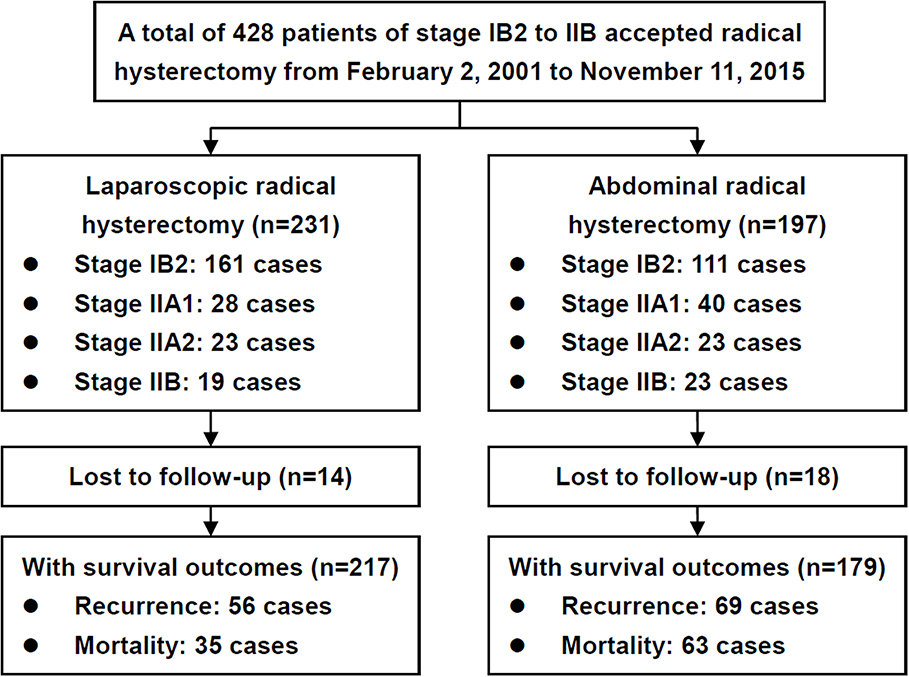
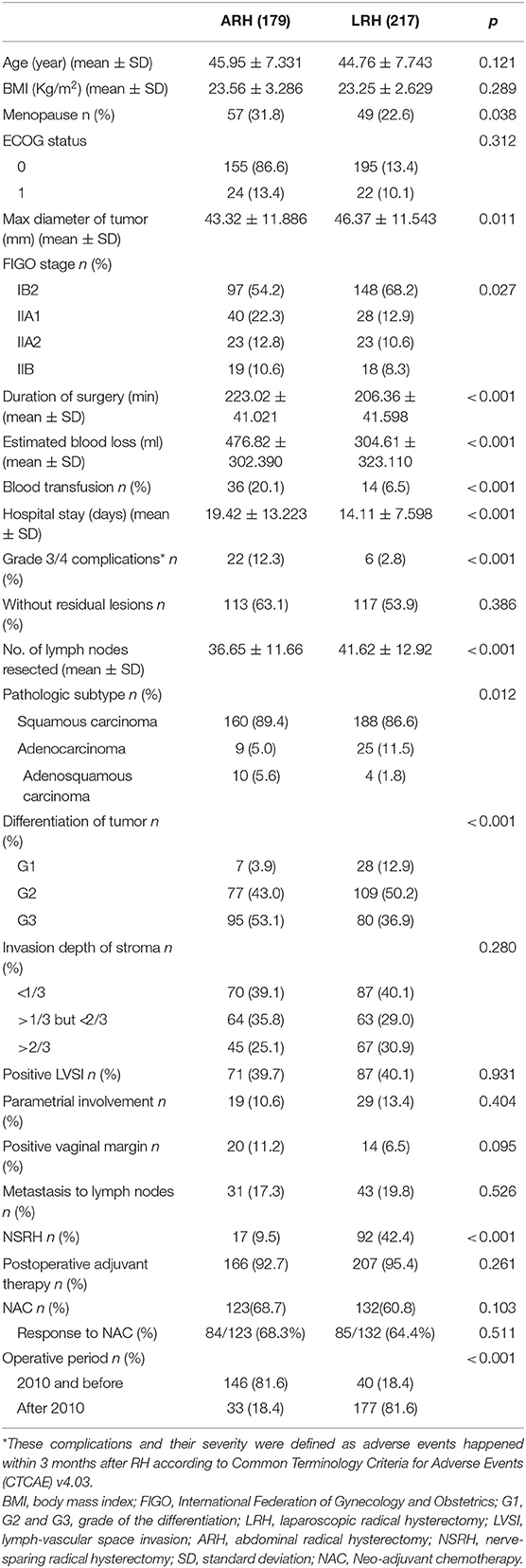
During the study period, 428 patients were included, and 396 patients had definitive survival outcomes (Figure 1; Supplement File 1). The baseline clinical and pathological characteristics are presented in Table 1. In total, 179 patients (45.2%) underwent ARH, and 217 patients (54.8%) underwent LRH; the patients had mean ages of 45.95 years (SD = 7.33) and 44.76 years (SD = 7.74), respectively. No conversion from LRH to ARH occurred. Most of the baseline and pathological characteristics between these two groups were well-balanced, including preoperative NAC and postoperative adjuvant therapy. However, the patients in the ARH group presented with a more advanced FIGO stage (p = 0.027), earlier surgical period (p < 0.001), smaller tumor size (p = 0.011) and poorer differentiation (p < 0.001) than those in the LRH group.
Specifically, 123 (68.7%) and 132 (60.8%) patients in the ARH and LRH groups received NAC (p = 0.103), and 84 (68.3%) and 85 (64.4%) patients achieved partial or complete response (p = 0.511), respectively. There were no significant differences in the NAC protocols and administration route between the two groups (all p-values > 0.05).
Survival Outcomes
The median follow-up was 41.3 months (range 6–193.5), the 5 and 10-years DFS of the whole population were 70 and 60%, and the 5 and 10-years OS rates were 75% and 68%, respectively. In the ARH and LRH groups, there were 69 (38.5%) and 56 recurrences (25.8%, p = 0.007), and 63 (35.2%) and 35 deaths (16.1%, p < 0.001), respectively. Among the recurrent cases, 46 of 69 (66.7%) cases in the ARH group and 35 of 56 (62.5%) cases in the LRH group occurred within the pelvic cavity (p = 0.628). However, vaginal vault recurrence occurred in 14 of 69 (20.3%) patients of the ARH group and 4 of 56 (7.1%) of the LRH group (p = 0.037), respectively. In Kaplan-Meier analysis, the DFS and OS were related with several clinicopathological risk factors, including operative period (before and after 2010), menstrual status, nerve-sparing radical hysterectomy (NSRH), FIGO stage, pathological subtype, differentiation status, lymph node metastasis, involvement of the parametrium and vaginal margin, invasion depth of stroma, LVSI, residual lesions, postoperative complications and adjuvant therapies (all p <0.05).
The 5-year OS rates in the ARH and LRH groups were 71% and 77% (p = 0.10, Figure 2A), and the 5-year DFS rates were 69 and 69% (p = 0.47 Figure 2B), respectively. However, multivariate analyses of the Cox regression model identified ARH as an independent protective prognostic factor for DFS (HR 0.4; 95% CI 0.19–0.87; p = 0.02, Figure 2C). There were no significant differences in OS (HR 0.59; 95% CI 0.24–1.45; p = 0.25, Figure 2D) between the two groups.
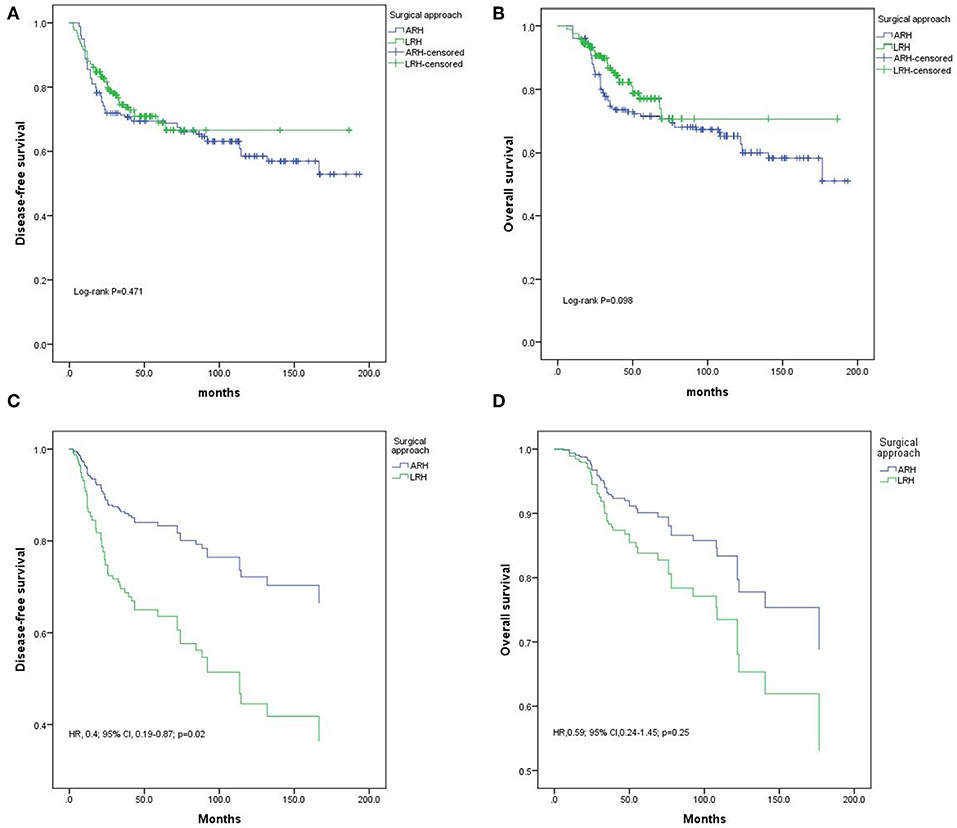
Figure 2. Disease-free survival (DFS) and overall survival (OS) of the whole population. (A) DFS results by the Kaplan-Meier method; (B) OS results by the Kaplan-Meier method; (C) DFS results by Cox regression analysis; (D) OS results by Cox regression analysis.
Specifically, in our study, NAC did not provide a more superior DFS or OS in the whole population, in the LRH and ARH groups, or in patients with various stages of disease in the multivariate analyses compared with non-NAC patients in Cox regression model (Supplement Table 1).
In particular, ARH still had more superior DFS but not OS than LRH in 386 patients of stage IB2 to IIA2 (i.e., excluding stage IIB patients) in Cox regression model: DFS, HR 0.2, 95% CI 0.1–0.5, p < 0.001; OS, HR 0.5, 95% CI 0.2–1.3, p = 0.169.
Survival Analysis of Different FIGO Stages
For patients with stage IB2 (n = 272), Kaplan-Meier analysis revealed no differences in DFS (p = 0.817, Figure 3A) or OS (p = 0.128, Figure 3B). In the Cox regression model, the patients in the ARH group had a significantly better DFS (HR 0.14, 95% CI 0.05–0.42, p < 0.01, Figure 3C) and OS (HR 0.17, 95% CI 0.04–0.67, p < 0.01, Figure 3D) than those in the LRH group.
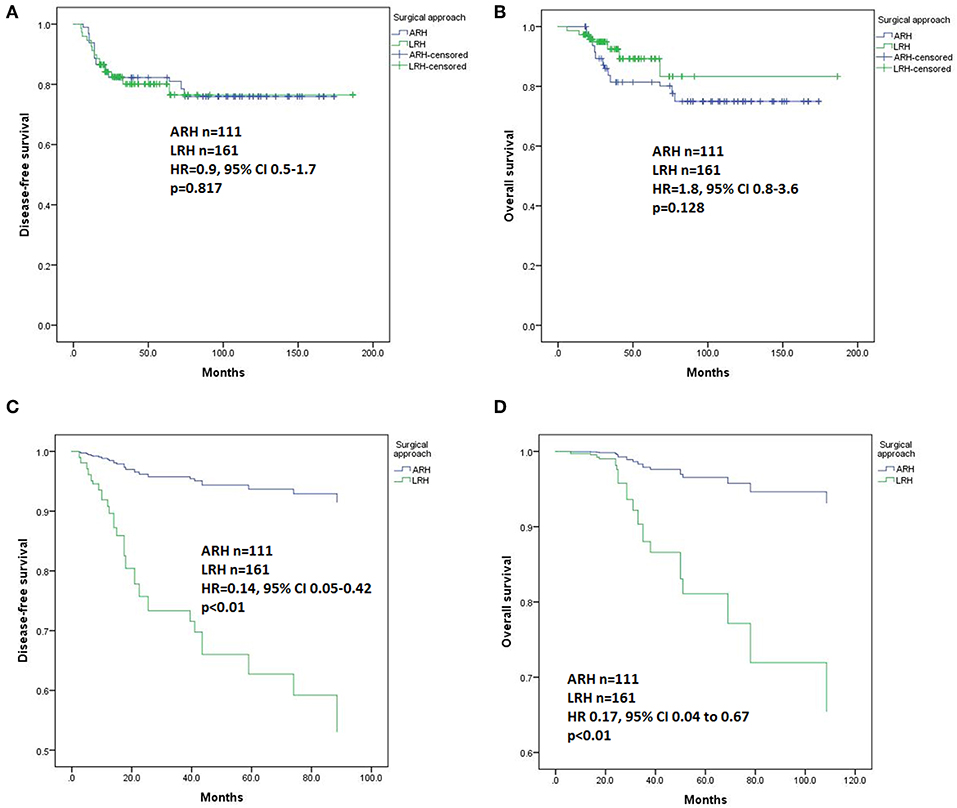
Figure 3. Disease-free survival (DFS) and overall survival (OS) of the patients with stage IB2. (A) DFS results by the Kaplan-Meier method; (B) OS results by the Kaplan-Meier method; (C) DFS results by Cox regression analysis; (D) OS results by Cox regression analysis.
For patients with IIA1 (n = 68) and IIA2 (n = 46) disease, Kaplan-Meier analysis revealed no differences in DFS (p = 0.09 and 0.86, respectively, Figures 4A,B) or OS (p = 0.40 and 0.42, respectively, Figures 4D,E) between the LRH and ARH groups. However, for patients with stage IIB disease (n = 42), the ARH group had a significantly worse DFS and OS than the LRH group (p < 0.01, Figures 4C,F). However, they all had no significant differences in the Cox regression model.
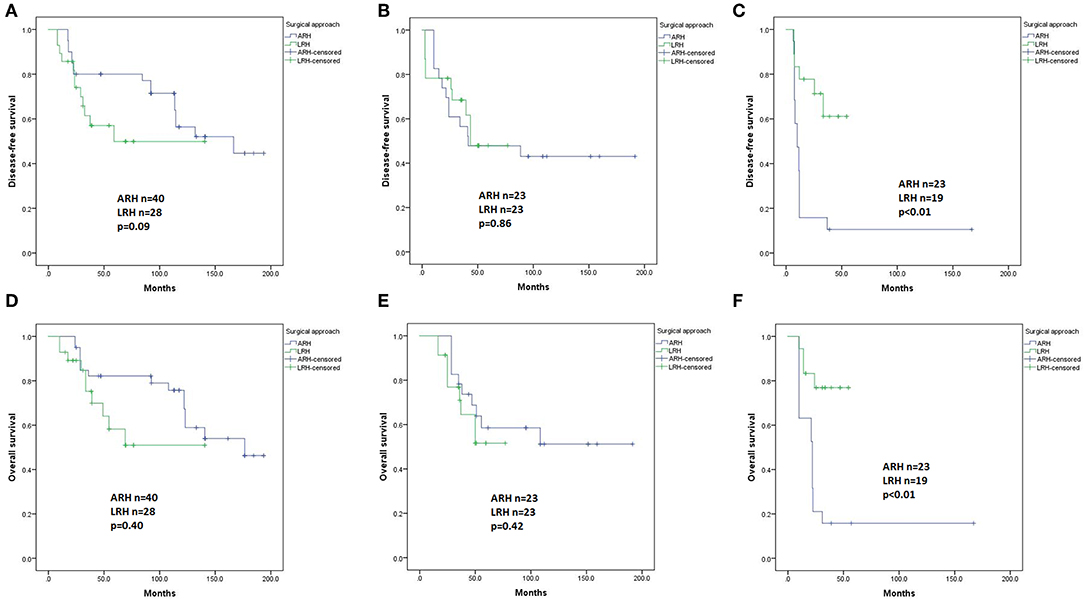
Figure 4. Disease-free survival (DFS) and overall survival (OS) of the patients with stage IIA1, IIA2, and IIB disease by the Kaplan-Meier method. (A) DFS of stage IIA1 patients; (B) DFS of stage IIA2 patients; (C) DFS of stage IIB patients; (D) OS of stage IIA1 patients; (E) OS of stage IIA2 patients; (F) OS of stage IIB patients.
Survival Analysis of Different Histological Subtypes
In univariate analysis, in patients with squamous carcinomas (n = 375), there were no significant differences in DFS (p = 0.96, Figure 5A) or OS (p = 0.33, Figure 5B) based on the type of surgery. In Cox regression model, in patients with squamous carcinomas, those who underwent ARH had a significantly superior DFS compared with those who underwent LRH (HR 0.45, 95% CI 0.25–0.82, p = 0.01), but the OS was not statistically significant (HR 0.57, 95% CI 0.27–1.20, p = 0.14).
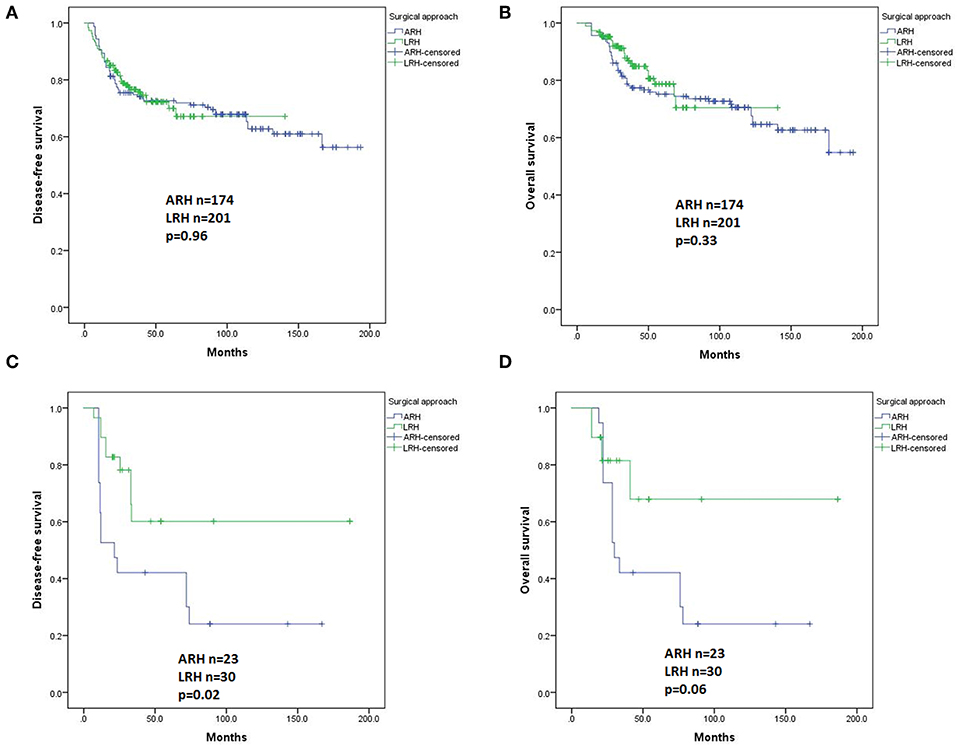
Figure 5. Disease-free survival (DFS) and overall survival (OS) of the patients with different histological subtypes by the Kaplan-Meier method. DFS (A) and OS (B) of patients with squamous carcinomas; DFS (C) and OS (D) of patients with adenocarcinomas plus adenosquamous carcinomas.
In patients with adenocarcinoma and adenosquamous carcinomas (n = 53), the ARH group showed a worse DFS and worse OS than the LRH group (p = 0.02 and p = 0.06, Figures 5C,D respectively). However, the difference disappeared in the Cox regression model.
Surgical Outcomes
Compared with the ARH group, the LRH group had better surgical outcomes, including a significantly lower amount of estimated blood loss, lower blood transfusion rate, shorter operation time and hospital stay, more LNs resected, higher NSRH rate and lower grade 3/4 complications (p < 0.001).
Discussion
In this study, we reported a large cohort of LACC patients who underwent RH with or without NAC. The surgical and survival outcomes between ARH and LRH could become the basis of decision making for physicians and patients, which could probably improve the quality of life of these relatively young patients. In the United States, CCRT is typically preferred over radical surgery for patients with LACC (17). However, to reduce the size of the primary tumor (19), improve the chances of operative curability and safety, and reduce long-term morbidity due to radiotherapy (20), NAC followed by RH had been attempted for patients with LACC. As previously reported, NAC followed by RH was suggested as a useful strategy (21), even for patients with nonsquamous cell carcinoma of the uterine cervix (22). Currently, no conclusive evidence is available regarding the relative benefits and drawbacks of primary RH vs. primary chemoradiotherapy for LACC (23). In a phase 3 randomized controlled study of the squamous subtype of LACC, NAC followed by chemoradiotherapy could improve DFS but not the OS compared with NAC followed by RH, and NAC had more toxicity (24). Judged by these findings, the impact of RH on the surgical or oncologic benefits in patients with LACC still requires more substantial evidence.
In our study, compared to LRH, ARH had a significantly superior survival outcome, especially in terms of DFS. The non-significant differences in OS between the two groups may be due to the insufficient follow-up periods in the LRH group. These findings are in accordance with previously reported results of early stage cervical cancer (8, 9). In a subgroup analysis, ARH for stage IB2 and the squamous carcinoma subtype led to a better DFS than LRH did, mainly because the ARH patients comprised the majority of the study population. However, despite several assumptions, the reasons for the inferior survival outcomes of MIS are unknown.
The learning curves of RH were most likely the most important reason, since almost all the LRH procedures were performed after 2011, and all the ARH procedures were performed before 2011. Due to the complex and complicated characteristics of radical surgeries, a lengthy learning process is needed to achieve comparable survival outcomes, which is equally essential for laparotomy or laparoscopy (25). Surgeons in the United States began to adopt minimally invasive RH for the treatment of cervical cancer in 2006 (26). In recent published study, the adoption of MIS was associated with a significant change in the survival trends and coincided with the beginning of a decline of survival rate between 2006 and 2010 (9). No definite reasons could explain this phenomenon except for the surgical route, and very few studies have considered factors that involve surgeons and their learning curves (25). Mastery of LRH requires experience with at least 20 cases and up to 50 cases (27–29), which shows the gradual slope of the learning curve (29). The learning curve for LRH and LN dissection would reach a turning point at 40 cases (30). These studies support the essential evaluation of the learning curves of a demanding surgical modality, both in early stage cervical cancer and LACC.
Other perspectives in our study need further consideration. A better description of the surgical procedure for the purpose of quality control is essential for future studies, since no conversions occurred in our study, which differs from other reports with rates of 1.5–3.5% (31). The significantly higher recurrence at the vaginal vault in the ARH group was comparable to the previously reported results (8). It is supposed that total laparoscopic/robotic intracorporeal colpotomy under CO2 pneumoperitoneum might pose a risk for implantation in vaginal cuff margin and intraperitoneal spread (32). ARH may limit the chance of intra-abdominal seeding during cancer cell removal by placing two sets of clamps across the vagina below the cervix (33). However, all of these considerations lack the support of validated evidence and cannot explain the poor survival outcomes of LRH.
Studies suggest that histopathology is an independent prognostic factor that plays an essential role in the outcomes of LACC patients (34). Most reports suggest that patients with adenocarcinoma and adenosquamous carcinoma have a worse prognosis than patients with squamous carcinoma (34). In our study, there was a meaningful survival difference between the ARH and LRH groups in patients with squamous carcinoma, which is the primary subtype. The results indicate that further exploration of other less common histological subtypes is needed. At present, based on our findings, ARH should be the procedure of choice for LACC, at least for the squamous subtype.
In our study, as expected, LRH resulted in better surgical outcomes than ARH, including a shorter mean operating time, less blood loss, and shorter hospital stay, all of which are in accordance with previously reported findings (23). However, these surgical benefits still could not compensate for the low survival. A long, comprehensive evaluation of the surgical protocols is essential to benefit patients.
In our study, NAC did not provide survival benefits in the whole cohort or in the subgroups of various surgical routes or stages. Despite of numerous cohort studies guaranteeing the safety and effectiveness of NAC, randomized controlled trials (35) and meta-analyses (36) have proved that NAC had no positive impact on the survival outcomes of patients with LACC. Therefore, the applicability of NAC in LACC is controversial (17). However, current randomized studies of NAC did not rigorously follow uniform chemotherapy protocols (35). Currently, some expert opinions provided the appropriate selection of patients for the application of NAC (37).
The strengths of our study were the relatively large cohort and uniform RH procedures performed by experienced physicians. However, there are several major limitations. Similar to previous studies about LACC, our conclusion was also limited by inadequate power and probable residual confounding. As patients were all from a single center and a single team, selection bias would probably greatly interfere with the interpretation of the oncologic outcomes. The lack of uniform NAC protocols also limits the conclusions on the influence of NAC. Data on long-term survival assessed in randomized trials or in large cohort studies are needed for patients with LACC. The insufficient number of disease cases from stages other than IB2 and subtypes other than squamous carcinoma also limits the generalizability of our conclusions. Last, the inclusion of patients with stage IIB did not accord with current guideline (17). We have performed separate analysis for stage IB2 to IIA2, and have achieved similar conclusions. However, we wish our data, failure or possible experiences would provide knowledge and insight on the topic of treatment for LACC, the troublesome and confusing type.
Conclusions
Despite its favorable surgical outcomes, LRH was associated with a shorter DFS than ARH for patients with LACC (IB2 to IIB) as a whole population, and in patients with stage IB2 disease or the squamous subtype. However, in patients with stage IIA and IIB, and in patients of non-squamous subtypes, LRH hand no significant differences with ARH in the survival prognosis, probably due to the limited sample size in these subgroups. NAC had no significant impact on the survival outcomes.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The Institutional Review Board of Peking Union Medical College Hospital approved this study (No. ZS-1427). The registration No. is NCT03291236 (clinicaltrials.gov).
Author Contributions
LL and MW conceived of the original idea for the study, interpreted the results, carried out the statistical analysis, edited the paper, and were the overall guarantors. MW completed all the major procedures of the radical hysterectomy, WW obtained ethical approval, contributed to the preparation of the data set, interpreted the results and contributed to drafts of the paper. SZ, SM, and XT contributed to the study design, interpretation of the results, and commented on drafts of the paper.
Funding
This study was supported by the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-2017-I2M-1-002). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01331/full#supplementary-material
Supplement Table 1. Survival outcomes of patients with and without NAC followed by RH and systematic lymphadenectomy and the stratified analysis according to stages in Cox regression model.
Supplement File 1. Raw data of the study.
Abbreviations
95% CI, 95% confidence interval; ARH, abdominal radical hysterectomy; CC, cervical cancer; DFS, disease-free survival; HR, hazard ratio; LACC, locally advanced cervical cancer; LRH, laparoscopic radical hysterectomy; MIS, minimally invasive surgery; NAC, neoadjuvant chemotherapy; OS, overall survival.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Cai J, Yang L, Dong W, Wang H, Xiong Z, Wang Z. Retrospective comparison of laparoscopic versus open radical hysterectomy after neoadjuvant chemotherapy for locally advanced cervical cancer. Int J Gynaecol Obstet. (2016) 132:29–33. doi: 10.1016/j.ijgo.2015.06.042
4. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. (2019) 393:169–82. doi: 10.1016/s0140-6736(18)32470-x
5. Wang N, Guan QL, Wang K, Zhou X, Gao C, Yang HT, et al. Radiochemotherapy versus radiotherapy in locally advanced cervical cancer: a meta-analysis. Arch Gynecol Obstet. (2011) 283:103–8. doi: 10.1007/s00404–010-1385–5
6. Kim SI, Cho JH, Seol A, Kim YI, Lee M, Kim HS, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol Oncol. (2019) 153:3–12. doi: 10.1016/j.ygyno.2019.01.008
7. Chantalat E, Vidal F, Leguevaque P, Lepage B, Lambaudie E, Hebert T, et al. Para-aortic workup in locally advanced cervical cancer: heterogeneity is still the rule. Results from a retrospective multicenter study. Arch Gynecol Obstet. (2016) 293:1081–6. doi: 10.1007/s00404-015-3885-9
8. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. (2018) 379:1895–904. doi: 10.1056/NEJMoa1806395
9. Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. (2018) 379:1905–14. doi: 10.1056/NEJMoa1804923
10. Zanagnolo V, Minig L, Cardenas-Rebollo JM, Achilarre MT, Garbi A, Patrono MG, et al. Robotic versus open radical hysterectomy in women with locally advanced cervical cancer after neoadjuvant chemotherapy: a single-institution experience of surgical and oncologic outcomes. J Minim Invasive Gynecol. (2016) 23:909–16. doi: 10.1016/j.jmig.2016.04.014
11. Panici PB, Di Donato V, Palaia I, Visentin VS, Marchetti C, Perniola G, et al. Type B versus type C radical hysterectomy after neoadjuvant chemotherapy in locally advanced cervical carcinoma: a propensity-matched analysis. Ann Surg Oncol. (2016) 23:2176–82. doi: 10.1245/s10434-015-4996-z
12. Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. (1974) 44:265–72.
13. Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol. (2008) 9:297–303. doi: 10.1016/s1470–2045(08)70074-3
14. Cibula D, Abu-Rustum NR, Benedetti-Panici P, Kohler C, Raspagliesi F, Querleu D, et al. New classification system of radical hysterectomy: emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol Oncol. (2011) 122:264–8. doi: 10.1016/j.ygyno.2011.04.029
15. Li L, Ma S, Tan X, Zhong S, Wu M. The urodynamics and survival outcomes of different methods of dissecting the inferior hypogastric plexus in laparoscopic nerve-sparing radical hysterectomy of type C: a randomized controlled study. Ann Surg Oncol. (2019) 26:1560–8. doi: 10.1245/s10434-019-07228-8
16. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. (2009). Available online at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50
17. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Cervical Cancer. Version 2.2019 - October 12, 2018. (2018). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
18. Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. (2009) 105:107–8. doi: 10.1016/j.ijgo.2009.02.009
19. deSouza NM, Soutter WP, Rustin G, Mahon MM, Jones B, Dina R, et al. Use of neoadjuvant chemotherapy prior to radical hysterectomy in cervical cancer: monitoring tumour shrinkage and molecular profile on magnetic resonance and assessment of 3-year outcome. Br J Cancer. (2004) 90:2326–31. doi: 10.1038/sj.bjc.6601870
20. Benedetti Panici P, Bellati F, Plotti F, Di Donato V, Antonilli M, Perniola G, et al. Neoadjuvant chemotherapy followed by radical surgery in patients affected by vaginal carcinoma. Gynecol Oncol. (2008) 111:307–11. doi: 10.1016/j.ygyno.2008.07.005
21. Li L, Wu M, Ma S, Tan X, Zhong S. Neoadjuvant chemotherapy followed by radical hysterectomy for stage IB2-to-IIB cervical cancer: a retrospective cohort study. Int J Clin Oncol. 24:1440–8. (2019). doi: 10.1007/s10147–019-01510–1
22. Shimada M, Nagao S, Fujiwara K, Takeshima N, Takizawa K, Shoji T, et al. Neoadjuvant chemotherapy with docetaxel and carboplatin followed by radical hysterectomy for stage IB2, IIA2, and IIB patients with non-squamous cell carcinoma of the uterine cervix. Int J Clin Oncol. (2016) 21:1128–35. doi: 10.1007/s10147-016-1010-0
23. Nama V, Angelopoulos G, Twigg J, Murdoch JB, Bailey J, Lawrie TA. Type II or type III radical hysterectomy compared to chemoradiotherapy as a primary intervention for stage IB2 cervical cancer. Cochrane Database Syst Rev. (2018) 10:CD011478. doi: 10.1002/14651858.CD011478.pub2
24. Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. (2018) 36:1548–55. doi: 10.1200/JCO.2017.75.9985
25. Liu Y, Li L, Wu M, Ma S, Tan X, Zhong S, et al. The impact of the surgical routes and learning curve of radical hysterectomy on the survival outcomes in stage IB cervical cancer: a retrospective cohort study. Int J Surg. (2019) 68:72–7. doi: 10.1016/j.ijsu.2019.06.009
26. Wright JD, Burke WM, Tergas AI, Hou JY, Huang Y, Hu JC, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol. (2016) 34:1087–96. doi: 10.1200/JCO.2015.65.3212
27. Chong GO, Park NY, Hong DG, Cho YL, Park IS, Lee YS. Learning curve of laparoscopic radical hysterectomy with pelvic and/or para-aortic lymphadenectomy in the early and locally advanced cervical cancer: comparison of the first 50 and second 50 cases. Int J Gynecol Cancer. (2009) 19:1459–64. doi: 10.1111/IGC.0b013e3181b76640
28. Reade C, Hauspy J, Schmuck ML, Moens F. Characterizing the learning curve for laparoscopic radical hysterectomy: buddy operating as a technique for accelerating skill acquisition. Int J Gynecol Cancer. (2011) 21:930–5. doi: 10.1097/IGC.0b013e3182157a44
29. Kong TW, Chang SJ, Paek J, Park H, Kang SW, Ryu HS. Learning curve analysis of laparoscopic radical hysterectomy for gynecologic oncologists without open counterpart experience. Obstet Gynecol Sci. (2015) 58:377–84. doi: 10.5468/ogs.2015.58.5.377
30. Hwang JH, Yoo HJ, Joo J, Kim S, Lim MC, Song YJ, et al. Learning curve analysis of laparoscopic radical hysterectomy and lymph node dissection in early cervical cancer. Eur J Obstet Gynecol Reprod Biol. (2012) 163:219–23. doi: 10.1016/j.ejogrb.2012.05.005
31. Park JY, Nam JH. Laparotomy conversion rate of laparoscopic radical hysterectomy for early-stage cervical cancer in a consecutive series without case selection. Ann Surg Oncol. (2014) 21:3030–5. doi: 10.1245/s10434–014-3707–5
32. Pennington KP, Urban RR, Gray HJ. Revisiting minimally invasive surgery in the management of early-stage cervical cancer. J Natl Compr Canc Netw. (2019) 17:86–90. doi: 10.6004/jnccn.2018.7263
33. Doo DW, Kirkland CT, Griswold LH, McGwin G, Huh WK, Leath CA III, et al. Comparative outcomes between robotic and abdominal radical hysterectomy for IB1 cervical cancer: results from a single high volume institution. Gynecol Oncol. (2019) 153:242–7. doi: 10.1016/j.ygyno.2019.03.001
34. Seamon LG, Java JJ, Monk BJ, Penson RT, Brown J, Mannel RS, et al. Impact of tumour histology on survival in advanced cervical carcinoma: an NRG Oncology/Gynaecologic Oncology Group Study. Br J Cancer. (2018) 118:162–70. doi: 10.1038/bjc.2017.400
35. Katsumata N, Yoshikawa H, Kobayashi H, Saito T, Kuzuya K, Nakanishi T, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102). Br J Cancer. (2013) 108:1957–63. doi: 10.1038/bjc.2013.179
36. Kim HS, Sardi JE, Katsumata N, Ryu HS, Nam JH, Chung HH, et al. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur J Surg Oncol. (2013) 39:115–24. doi: 10.1016/j.ejso.2012.09.003
Keywords: locally advanced cervical cancer, radical hysterectomy, survival, neoadjuvant chemotherapy, mini-invasive surgery, open surgery
Citation: Wang W, Li L, Wu M, Ma S, Tan X and Zhong S (2019) Laparoscopic vs. Abdominal Radical Hysterectomy for Locally Advanced Cervical Cancer. Front. Oncol. 9:1331. doi: 10.3389/fonc.2019.01331
Received: 09 September 2019; Accepted: 14 November 2019;
Published: 27 November 2019.
Edited by:
Zongbing You, Tulane University, United StatesReviewed by:
Qingli Li, Sichuan University, ChinaJiangyan Lou, Sichuan University, China
Li Li, Guangxi Medical University, China
Copyright © 2019 Wang, Li, Wu, Ma, Tan and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Li, bGlsZWlnaEAxNjMuY29t
 Wenhui Wang
Wenhui Wang Lei Li
Lei Li