- 1Department of Thoracic Surgery, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Toxicology, School of Public Health, Women's Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: Despite an increasing understanding about tumor mutation burden (TMB) in cancer immunity and cancer immunotherapy, the comprehensive cognition between TMB and efficiency of immune checkpoint inhibitors (ICIs) is still lacking. A systematic review and meta-analysis was conducted to evaluate the predictive value of TMB on efficacy of ICIs.
Methods: Systematic literature search was conducted on PubMed, EMBASE, Web of Science and Cochrane Library up to June 16, 2019. Pooled odds ratio (OR) of objective response rate (ORR), hazard ratio (HR) of progression-free survival (PFS) and overall survival (OS) were estimated by inverse variance weighted fixed-effects model (I2 ≤ 50%) or DerSimonian-Laird random-effects model (I2 > 50%). In addition, heterogeneity analysis, sensitivity analysis, publication bias and subgroup analysis were conducted. Moreover, fractional polynomial regression was conducted to investigate the dose-response relationship between TMB cutoffs and efficacy of ICIs. Furthermore, we assessed ORR by TMB and programmed cell death ligand 1 (PD-L1) expression after layering each other in studies which the two could be both acquired.
Results: Three thousand six hundred fifty-seven records were retrieved through database searching, and 29 studies with 4,431 patients were finally included in the meta-analysis. TMB high group had significantly improved ORR (pooled OR 3.31, 95% CI 2.61, 4.19, P < 0.001), PFS (pooled HR 0.59, 95% CI 0.49, 0.71, P < 0.001) and OS (pooled HR 0.68, 95% CI 0.53, 0.89, P = 0.004). Sensitivity analyses illustrated the results were stable, and publication bias was identified in ORR. Subgroup analyses showed the predictive value of TMB was significant in non-small-cell lung cancer (except for the OS) and melanoma. In addition, heterogeneity was substantial in targeted next generation sequencing group but tiny in whole exome sequencing group. Furthermore, TMB and PD-L1 expression were capable to predict improved ORR of ICIs after stratification of each other, with tiny heterogeneity.
Conclusions: High tumor mutation burden predicted improved efficacy of immune checkpoint inhibitors in cancers, and targeted next generation sequencing for estimating tumor mutation burden in clinic should be standardized to eliminate heterogeneity in the future. Moreover, tumor mutation burden and programmed cell death ligand 1 expression were independent factors on predicting efficacy of immune checkpoint inhibitors.
Introduction
Immune checkpoint inhibitors (ICIs) have been identified to improve response and survival in diverse solid tumors and hematologic malignancies, including melanoma, non-small-cell lung cancer (NSCLC), urothelial carcinoma, renal-cell carcinoma and Hodgkin's lymphoma (1–6). However, the efficacy seems unsatisfactory in unselected patients (1, 3, 7), suggesting eligible biomarkers are required to identify subgroups appropriate for cancer immunotherapy. At present, scientists have recognized several candidate biomarkers, such as programmed cell death ligand 1 (PD-L1) expression, tumor-infiltrating lymphocytes (TILs), transcriptomic and epigenetic signatures, oncogenic driver mutations and mismatch repair deficiency (dMMR)/microsatellite instability (MSI) (8–10). Among them, tumor mutation burden (TMB), which is defined as the number of mutations (generally non-synonymous somatic mutations) in cancer cells, is likely to be a promising biomarker. It has been reported that patients with high TMB have better response and survival to ICIs than patients with low TMB in melanoma, NSCLC and urothelial carcinoma (11–16). Recently, Samstein et al. have utilized a large cohort of 1,662 patients to validate that high TMB is capable of forecasting preferable overall survival in multiple cancer types (17). Moreover, Singal et al. exploited real-world data from an electronic health records database, further verifying the predictive capability of TMB in NSCLC (18). Furthermore, TMB is widely recognized as a biomarker independent of PD-L1 expression (18–20).
There are quite a lot of evidences supporting the function of TMB. It is associated with neoantigen burden (13, 21), which can activate T lymphocytes to proliferate and kill cancer cells (8). In addition, tumors with dMMR generate a mass of somatic mutations and exhibit MSI which present high TMB (22–24), and dMMR/MSI is connected with response to ICIs (22, 23, 25).
Despite a number of studies uncovering powerful forecasting capability of TMB on efficacy of ICIs, however, negative results are also reported, especially in long-term survival (26–29). Several reasons may explain the heterogeneity of these results. Firstly, since TMB is not significant in all caners (30), it may have predictive value in particular cancer types. Besides, due to diverse cut-off values adopted in different studies (14, 26, 31, 32), the optimum TMB threshold in a wide range of cancers or a typical cancer type is still a mystery. In addition, owing to huge cost and complexity of whole exome sequencing (WES), targeted next generation sequencing (NGS) has been widely adopted to evaluate TMB of cancer cells. However, significant heterogeneity could exist due to quite a lot of variables in different gene panels (33).
Although there are two meta-analyses reporting the predictive value of TMB, the number of studies and patients included is small, and subgroup analyses are insufficient to explain heterogeneity of the results (34, 35). Hence, we did a more comprehensive systematic review and meta-analysis to evaluate the influence of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers, and conduct overall subgroup analyses to identify potential source of heterogeneity.
Methods
Data Sources, Search Strategy, and Selection Criteria
The PRISMA statement was followed in the systematic review and meta-analysis (36). Systematic literature search was conducted on PubMed, EMBASE, Web of Science and Cochrane Library up to June 16, 2019. Two investigators (Wu and Xu) searched the databases independently. The search term was as follows: (PD-1 OR PD-L1 OR CTLA-4 OR Ipilimumab OR Tremelimumab OR Nivolumab OR Pembrolizumab OR Lambrolizumab OR Atezolizumab OR Avelumab OR Durvalumab OR “immune checkpoint inhibitor” OR “immune checkpoint inhibitors” OR “ICI” OR “ICIs” OR “immune checkpoint blocker” OR “immune checkpoint blockers” OR “ICB” OR “ICBs”) AND (mutation burden OR mutational burden OR mutation load OR mutational load OR TMB OR TML). When duplicate reports were identified, the one with larger sample size and more detailed information was selected. We also reviewed references in articles finally included to identify studies potentially missed.
To be eligible, studies had to meet the following inclusion criteria: (1) cohort studies or clinical trials assessed inhibitors of PD-1/PD-L1, CTLA-4, or their combination, in patients with cancer, and the efficiency of therapy was evaluated by TMB which had cut-off value; (2) odds ratio (OR) of objective response rate/overall response rate (ORR), or hazard ratio (HR) of progression-free survival (PFS) or overall survival (OS), and their 95% confidence intervals (95% CI) were given in the article, or sufficient data was available to calculate them; (3) the number of patients accessible for evaluation was no <20; (4) studies were published in English. Reviews, notes, letters, editorials, comments, meeting abstracts, and case reports were excluded on account of insufficient information.
Two investigators (Wu and Xu) independently reviewed the retrieved studies to identify potential applicable articles, and any disagreements about specific articles were discussed and determined with consensus of all investigators.
Data Extraction and Quality Assessment
Two investigators (Wu and Xu) independently extracted data from studies included, and any inconsistencies were conferred and resolved with consensus of all investigators. The following information was extracted from each study: title, first author, year of publication, type of cancer, study design, data source, sample size evaluable for TMB, area of patients, class of immune checkpoint inhibitors, line of therapy, median age, gender, TMB sequencing method, TMB cut-off value, outcomes (ORR, PFS, OS) and their value. When duplicate publications were identified, the most comprehensive one was included.
The Newcastle-Ottawa Scale (NOS) was adopted to assess the quality of studies included (37). The total score ranged from 0 to 9, as 8–9 points indicated high quality of a study, five to seven points indicated medium quality, and studies with points lower than five showed poor quality.
Data Synthesis and Statistical Analysis
The primary endpoint of the meta-analysis was the comparison on efficiency of ICIs between TMB high group and TMB low group, which was measured in terms of OR of ORR, and HR of PFS and OS. Heterogeneity among individual studies was evaluated by the Q test; I2 > 50% and/or P ≤ 0.10 indicated significant heterogeneity (38). Pooled OR or HR with Z test was calculated by DerSimonian-Laird random-effects model when significant heterogeneity was identified, otherwise inverse variance weighted fixed-effects model was adopted. In addition, funnel plots were constructed, and Begg's test and Egger's test were performed to evaluate publication bias (P ≤ 0.10 was considered to be visible publication bias). Besides, sensitivity analysis was used to test the stability of the results in the meta-analysis. To further explore variation of effect of TMB on immunotherapy efficiency, subgroup analyses stratified by cancer type, area of patients, TMB sequencing method, class of immune checkpoint inhibitors, and line of therapy were conducted. Moreover, to investigate the dose-response relationship between TMB cutoffs and efficacy of ICIs, fractional polynomial regression (two degree) was conducted on studies of no <50 patients. To note, total mutation burden detected by WES was converted to mutations per megabase using a linear transformation (39). Furthermore, we evaluated ORR by TMB and PD-L1 expression after layering each other in studies which the two could be both acquired. Stata version 11.0 (Stata Corporation, College Station, TX) was used for analyses mentioned above.
In particular, there were several articles providing original data or graphs without reporting OR or HR. For original response data, STATA 11.0 was used to estimate OR. For original survival data, SPSS 20.0 was used to calculate HR through a Cox proportional hazards regression model. For Kaplan–Meier curves, Engauge Digitizer was used to extract survival data from graphs, then HR was estimated by adopting the method reported by Tierney et al. (40).
Results
Study Characteristics and Data Quality
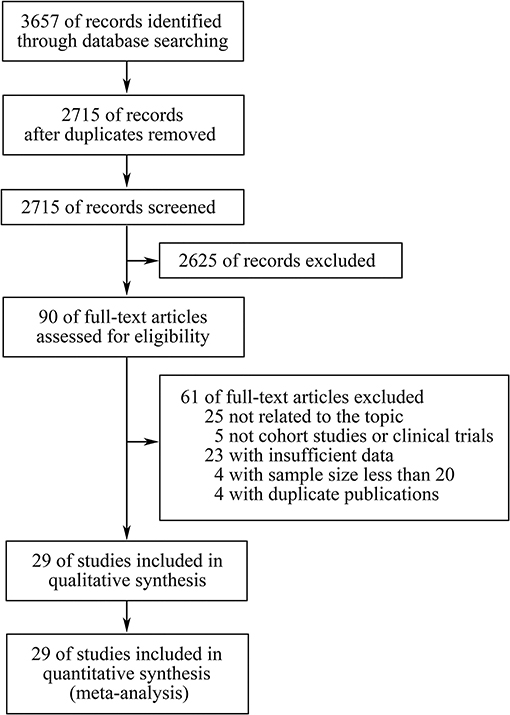
Three thousand six hundred fifty-seven records were retrieved through database searching, from which 90 studies potentially relevant to our topic were identified through screening of titles and abstracts. Subsequently, after full-text screening and qualitative synthesis, 29 studies with 4,431 patients were finally included in the meta-analysis (11–14, 17, 19, 20, 26–29, 31, 32, 41–56), including 26 cohort studies and three clinical trials (Figure 1; Table 1; Supplementary File 1: Table S1). In particular, four duplicate reports (57–60), two studies assessing TMB as a continuous variable (61, 62), and four studies with sample size <20 (63–66) were identified and excluded. There were 11 studies for patients with NSCLC, eight for melanoma, three for gastroesophageal cancer, two for small cell lung cancer (SCLC), two for diverse cancers, one for colorectal cancer, one for melanoma or urologic cancers, and one for three independent cohorts which were pan-tumor, HNSCC and melanoma, respectively. In these studies, 20 articles researched patients in Western countries, six articles investigated patients in Asia, and three articles studied patients in multiple areas. Different classes of ICIs were studied, including 18 studies for anti-PD-(L)1 monotherapy, four for anti-CTLA-4 monotherapy, two for anti-PD-1 in combination with anti-CTLA-4, and four studies comprised anti-PD-(L)1 monotherapy or in combination with anti-CTLA-4. In particular, there was another one study including two independent cohorts with dissimilar classes of ICIs: one was anti-PD-1 monotherapy, the other was anti-PD-1 in combination with anti-CTLA-4. In terms of line of therapy, two studies were done in first-line settings, and 18 studies were done in multiple lines, whereas the rest nine studies didn't mention the line. WES was adopted to detect TMB in 13 studies, and targeted NGS was used in the remaining studies. For the former, TMB was determined by the total number of mutations, and for the latter, TMB was defined as the number of mutations per megabase except for one article which derived the predicted total mutation load (PTML). To note, there were two studies using blood tumor mutation burden (bTMB), one study adopting circulating tumor deoxyribonucleic acid (ctDNA) TMB, and three studies dividing TMB into three segments in which the high TMB group and the low TMB group were included while the medium TMB group was excluded. The results of Newcastle-Ottawa Scale were listed in Supplementary File 1: Table S2. There were seven studies having a high quality, and the remaining studies had a medium quality, which ensured relative high quality of the studies included and enhanced reliability of the meta-analysis. To note, the randomized trial reported by Carbone et al. (53) was also assessed by NOS as patients simply treated with ICIs were included in the meta-analysis.
General Analysis of the Association Between TMB and Efficiency of Immune Checkpoint Inhibitors
Firstly, there were 22 studies (26 cohorts) including 2,013 patients evaluating the correlation between TMB and ORR of ICIs therapy. TMB high group had a significantly better ORR than TMB low group (pooled OR 3.31, 95% CI 2.61, 4.19, P < 0.001; Figure 2). The heterogeneity was insignificant (I2 = 20.0%, P = 0.181; Figure 2), and sensitivity analysis showed that the result was stable (Supplementary File 1: Figure S1a). Begg's test (P = 0.064; Supplementary File 1: Figure S1b) and Egger's test (P = 0.009; Supplementary File 1: Figure S1b) indicated publication bias was present. Since it seemed the publication bias was mostly caused by studies with small sample size, we re-analyzed the association after excluding eight studies with sample size no more than 50. TMB high group still showed an improved ORR (pooled OR 3.09, 95% CI 2.40, 3.97, P < 0.001), and the heterogeneity was tiny (I2 = 0%, P = 0.588). Interestingly, the publication bias disappeared (PBegg = 0.405, PEgger = 0.115). Secondly, the relationship between TMB and PFS of ICIs treatment was assessed in 20 studies (24 cohorts) containing 2,073 patients. PFS was evidently improved in TMB high group (pooled HR 0.59, 95% CI 0.49 0.71, P < 0.001; Figure 3), with a significant heterogeneity existing (I2 = 67.4%, P < 0.001; Figure 3). Sensitivity analysis showed a good stability of the pooled HR (Supplementary File 1: Figure S1c), and no publication bias was recognized (PBegg = 0.673, PEgger = 0.199; Supplementary File 1: Figure S1d). In addition, 15 studies (17 cohorts) including 2,936 patients were evaluated for the relevance between TMB and OS of ICIs. Patients with high TMB had a visibly superior OS than patients with low TMB (pooled HR 0.68, 95% CI 0.53, 0.89, P = 0.004; Figure 4). The result illustrated a significant heterogeneity (I2 = 66.5%, P < 0.001; Figure 4) and a good stability (Supplementary File 1: Figure S1e). Begg's test (P = 0.108; Supplementary File 1: Figure S1f) and Egger's test (P = 0.187; Supplementary File 1: Figure S1f) showed no publication bias.
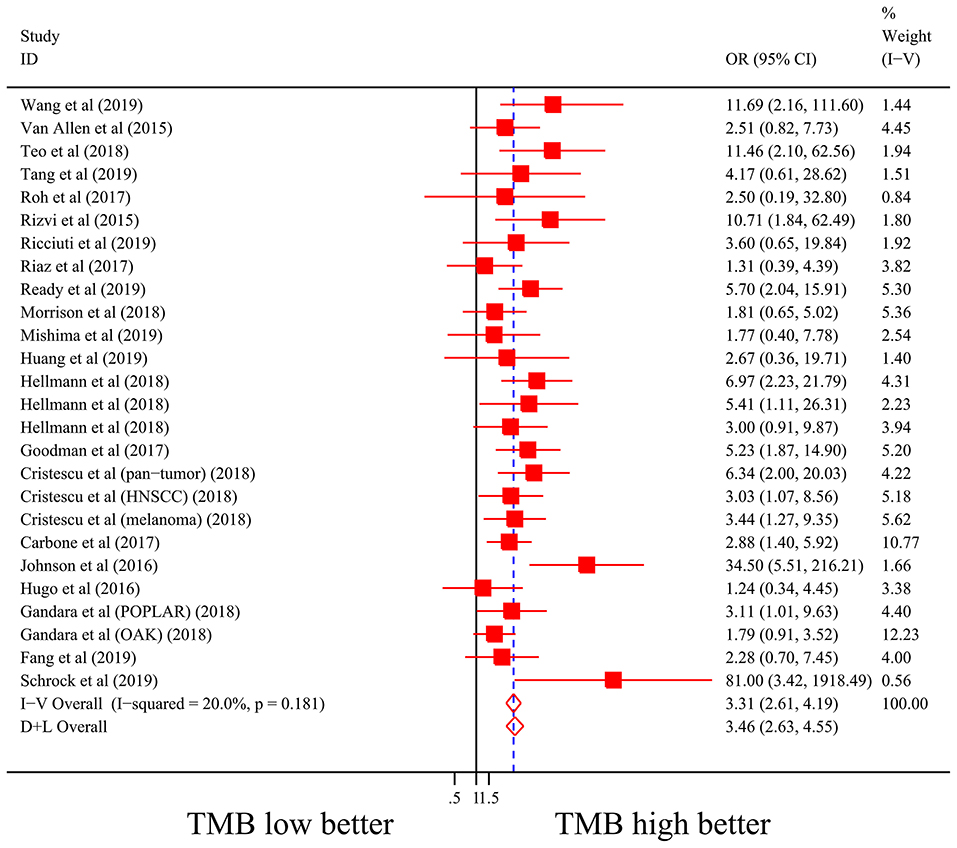
Figure 2. Forest plot of association between TMB and objective response rate of immune checkpoint inhibitors. OR, odds ratio; CI, confidence interval; TMB, tumor mutation burden.
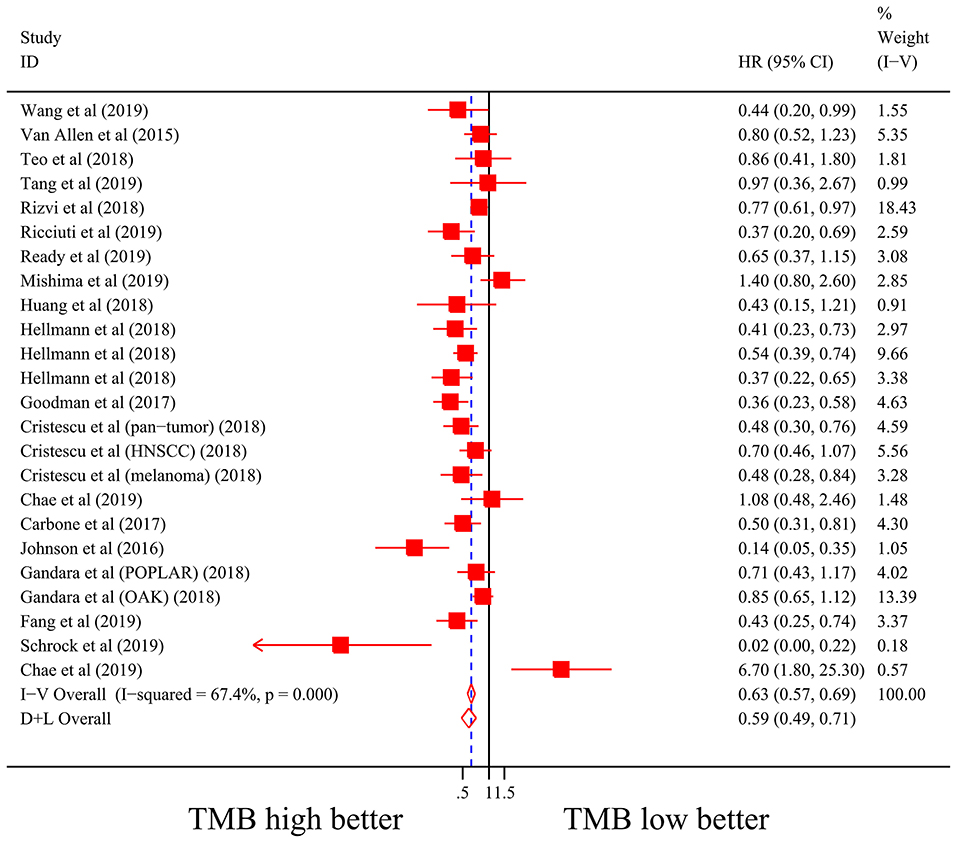
Figure 3. Forest plot of association between TMB and progression-free survival of immune checkpoint inhibitors. HR, hazard ratio; CI, confidence interval; TMB, tumor mutation burden.
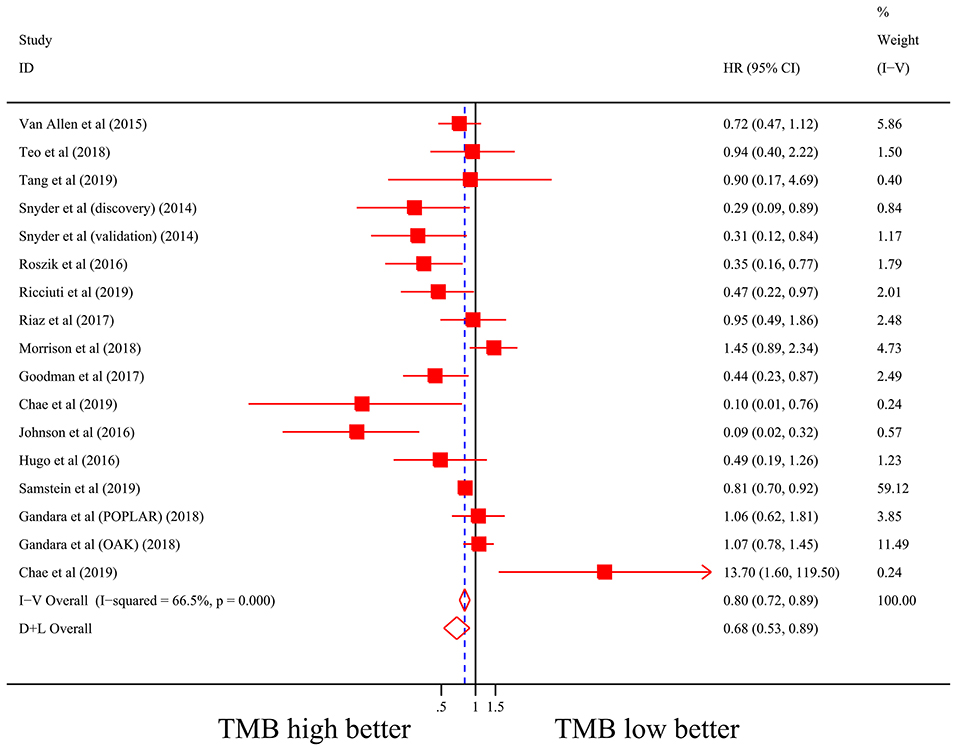
Figure 4. Forest plot of association between TMB and overall survival of immune checkpoint inhibitors. HR, hazard ratio; CI, confidence interval; TMB, tumor mutation burden.
Subgroup Analyses and Fractional Polynomial Regression of the Association Between TMB and Efficiency of Immune Checkpoint Inhibitors
The results of subgroup analyses are shown in Table 2; Supplementary File 1: Table S3. Firstly, in terms of diverse cancer types, it was showed that in NSCLC, TMB high group had significantly better ORR (pooled OR 3.23, 95% CI 2.27, 4.59, P < 0.001) and PFS (pooled HR 0.65, 95% CI 0.50, 0.85, P = 0.001) than TMB low group, while no difference in OS (pooled HR 1.00, 95% CI 0.67, 1.50, P >.99) between the two groups was found. In melanoma, TMB high group had evidently improved ORR (pooled OR 2.55, 95% CI 1.60, 4.05, P < 0.001), PFS (pooled HR 0.46, 95% CI 0.23, 0.94, P = 0.033) and OS (pooled HR 0.55, 95% CI 0.37, 0.82, P = 0.004). In SCLC, superior ORR and PFS were found in TMB high group, while no result with statistical difference was discovered in urothelial carcinoma or gastroesophageal cancer. Secondly, in western countries, patients with high TMB had evidently better ORR, PFS and OS than patients with low TMB; and in Asia, TMB high group showed superior ORR but no better PFS than TMB low group. Besides, no matter whether TMB was measured by WES or targeted NGS, high TMB predicted improved ORR, PFS and OS of ICIs therapy. However, the former showed insignificant heterogeneity while the latter presented substantial heterogeneity. In addition, the efficiency was enhanced in TMB high group with anti-PD-(L)1 monotherapy, anti-CTLA-4 monotherapy or combined therapy, except for ORR and PFS in anti-CTLA-4 monotherapy as well as OS in combined therapy. Moreover, improvement of ORR and PFS was seen in TMB high group with first-line treatment of ICIs.
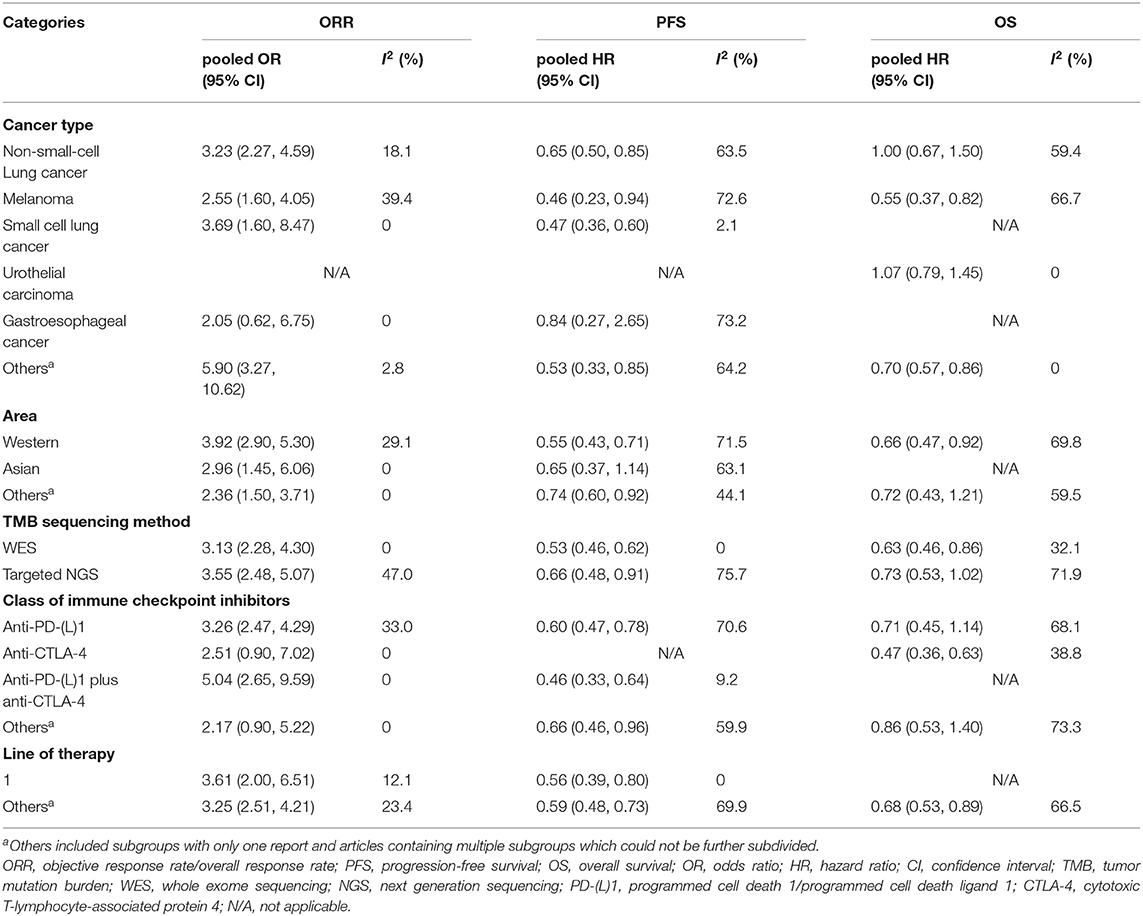
Table 2. Subgroup analyses of the association between TMB and efficiency of immune checkpoint inhibitors.
Moreover, to investigate the dose-response relationship between TMB cutoffs and efficacy of ICIs, fractional polynomial regression was conducted, and the results were shown in Supplementary File 1: Figure S2. Most studies had cutoffs between 5 and 10 muts/Mb. Within this range, the predictive OR of ORR and the predictive HR of PFS and their 95% CIs were meaningful and relatively stable. However, the predictive HR of OS and its 95% CI seemed meaningless within the entire range of cutoffs.
TMB and PD-L1 Expression Were Independent Biomarkers to Predict Objective Response Rate of Immune Checkpoint Inhibitors
To explore whether TMB and PD-L1 expression were separate biomarkers to forecast efficiency of ICIs treatment, we identified 9 studies from articles included in the meta-analysis (13, 20, 26, 27, 31, 48, 51–53), which had sufficient data to calculate ORR in subgroups as follows: group 1, both high expression of TMB and PD-L1; group 2, both low expression of TMB and PD-L1; group 3, low expression of TMB and high expression of PD-L1; group 4, high expression of TMB and low expression of PD-L1. As shown in Table 3; Supplementary File 1: Table S4, patients with high TMB still had superior ORR than patients with low TMB after layering PD-L1 expression. Similarly, ORR could still be enhanced in PD-L1 expression high group after layering TMB level. All results dramatically showed tiny heterogeneity.
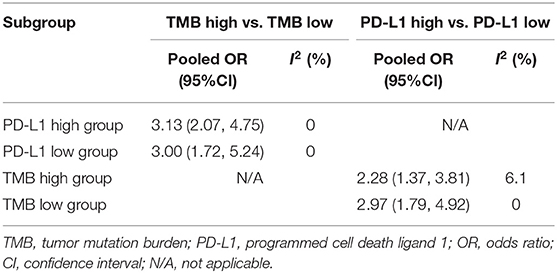
Table 3. TMB and PD-L1 expression were independent biomarkers to predict objective response rate of immune checkpoint inhibitors.
Discussion
The results of this study illustrated that high TMB was responsible for improved efficiency of ICIs therapy. It was significant in melanoma and NSCLC whose TMB level almost topped in diverse cancers (30, 67). However, the predictive value of TMB for long-term survival in NSCLC was still in doubt due to our negative result. Besides, high TMB could predict better ORR and PFS in SCLC, which required further research owing to insufficient number of studies and patients. Most studies were done in Western people, in which the strong association between high TMB and improved immunotherapy efficacy was identified, while more parallel research was required in Asian area. It seemed that high TMB could forecast enhanced efficiency of multiple classes of immune checkpoint inhibitors, especially combined therapy (anti-PD-(L)1 plus anti-CTLA-4). However, the result should be further confirmed due to most of the studies done in PD-(L)1 monotherapy.
Significant heterogeneity was detected in pooled PFS and OS, which could be partially explained by subgroup analyses of cancer type, class of immune checkpoint inhibitors and line of therapy. Interestingly, different TMB sequencing methods might clarify most of the heterogeneity, as it was concentrated in targeted NGS group. Though WES was used to detect TMB in initial studies which discovered that patients with high TMB responded better to ICIs (11, 13, 42), targeted NGS was subsequently widely applicated in research and clinic due to its comparative cheap cost and simplicity. To date, two targeted NGS panels have been approved by Food & Drug Administration (FDA) which are Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) and FoundationOne CDx (F1CDx). However, our results suggested there was significant heterogeneity in dissimilar targeted NGS panels which might affect predictive accuracy and stability. Actually, panel-based TMB evaluation is affected by several experimental factors (e.g., tumor purity or sequencing depth) and the variant calling pipeline, which need to be standardized in different targeted NGS panels (33, 68). In addition, publication bias in pooled ORR should be considered. As the publication bias might be primarily caused by several studies with small sample size due to our results, further research with large sample volume and normative design was demanded.
Moreover, we identified that TMB and PD-L1 expression were capable to predict improved ORR of ICIs after stratification of each other, with dramatically tiny heterogeneity. As it was reported that TMB and PD-L1 expression could independently predict benefit to ICIs (19), our results further supported the view.
One of the most critical issues about TMB is the best threshold on predicting immunotherapy efficacy. Due to our results of fractional polynomial regression, most studies had cutoffs between 5 and 10 muts/Mb, which seemed to present a relative stable predictive value in multiple tumor analysis. However, the number of studies is far from enough to make a convincing conclusion, especially studies with cutoffs above 10 muts/Mb as well as studies reporting the long-term survival data. Actually, as TMB varies greatly in different tumors, there may not be a universal TMB cut-off value for all cancer types, especially cancers with high TMB level such as NSCLC and melanoma (17, 30). Encouragingly, a number of clinical trials are in progress in the context of TMB assessment in diverse cancers (33), which are expected to provide more high quality data to help us identify appropriate TMB cutoffs in certain cancer types.
Interestingly, there is another strategy to divide TMB into three layers, which are TMB high, medium and low groups (12, 49, 53), as the clinical benefit gap between TMB high group and TMB low group seems to be more significant. In addition, it has been reported that the three-tier TMB classification scheme can improve the accuracy of panel-based TMB measurement (69). Actually, there is a great deal of uncertainty on response to ICIs for patients whose TMB level is close to the cutoff. Therefore, the concept of medium TMB could clinically help doctors to comprehensively consider the treatment of such a population, which needs further research.
There are several strengths in our study. First of all, we adopted ORR, PFS and OS as our endpoints to evaluate both short-term and long-term benefits of ICIs therapy, which made it more comprehensive and convincing. Secondly, we did subgroup analyses from diverse aspects, and discovered most of the source of heterogeneity. In addition, sensitivity analyses showed a good stability of our results.
However, the current meta-analysis is restricted by several limitations. Firstly, sample size varied among the included studies, which resulted in large variance in sample volume between different subgroups, and quite a few studies with small sample quantity might be the chief source of publication bias in the meta-analysis. Moreover, a few important clinical characteristics, which has been reported to be responsible for efficiency of ICIs, such as age and sex (70, 71), were not corrected in several studies when calculating effect size.
There are four main conclusions drawn from our study. The first is that high TMB could predict improved efficiency of ICIs. It was significant in NSCLC and melanoma, but the predictive value on long-term survival of NSCLC requires further research. The second is that more studies with large sample size and standardized design are necessitated to further explore the prophetic worth of TMB in certain subgroups, especially in SCLC, Asian area and combined therapy (anti-PD-(L)1 plus anti-CTLA-4). Thirdly, targeted NGS for estimating TMB in clinic should be standardized to eliminate heterogeneity in the future. Moreover, we further validated that TMB and PD-L1 expression were independent factors on predicting response to ICIs. Therefore, the model combining TMB with PD-L1 expression may expand the group benefit from immune checkpoint inhibitors.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
JH contributed to the conception and design of the work. YoW and JX contributed to conception, design, data analysis, and editing the manuscript. CD, YiW, DX, and WL contributed to data acquisition and critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by innovation research grant program for 8-year-system medical students at Zhejiang University (No. 119000-5405A1), National Key R&D Program of China (No. 2017YFC0113500), Major Science and Technology Projects of Zhejiang Province (No. 2014C03032), Key Disciplines of Traditional Chinese Medicine in Zhejiang Province (No. 2017-XK-A33), Chinese Medicine Science and Technology Program Key Research Projects of Zhejiang Province (No. 2015ZZ007), and Natural Science Foundation of Zhejiang Province (No. Q19H160166).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01161/full#supplementary-material
Abbreviations
TMB, tumor mutation burden; ICIs, immune checkpoint inhibitors; OR, odds ratio; ORR, objective response rate/overall response rate; HR, hazard ratio; PFS, progression-free survival; OS, overall survival; CI, confidence interval; PD-L1, programmed cell death ligand 1; NSCLC, non-small-cell lung cancer; TILs, tumor-infiltrating lymphocytes; dMMR, mismatch repair deficiency; MSI, microsatellite instability; WES, whole exome sequencing; NGS, next generation sequencing; NOS, Newcastle-Ottawa Scale; SCLC, small cell lung cancer; PTML, predicted total mutation load; bTMB, blood tumor mutation burden; ctDNA, circulating tumor deoxyribonucleic acid; FDA, Food & Drug Administration; MSK-IMPACT, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets; F1CDx, FoundationOne CDx; HNSCC, head and neck squamous cell carcinoma; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; N/A, not applicable; muts/Mb, mutations per megabase.
References
1. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
2. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
3. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
4. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
5. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. (2015) 373:1803–13. doi: 10.1056/NEJMoa1510665
6. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. (2015) 372:311–9. doi: 10.1056/NEJMoa1411087
7. Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. (2015) 33:1889–94. doi: 10.1200/JCO.2014.56.2736
8. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. (2019) 19:133–50. doi: 10.1038/s41568-019-0116-x
9. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. (2016) 17:e542–51. doi: 10.1016/S1470-2045(16)30406-5
10. Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. (2018) 17:129. doi: 10.1186/s12943-018-0864-3
11. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. (2014) 371:2189–99. doi: 10.1056/NEJMoa1406498
12. Johnson DB, Frampton GM, Rioth MJ, Yusko E, Xu Y, Guo X, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res. (2016) 4:959–67. doi: 10.1158/2326-6066.CIR-16-0143
13. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. (2015) 348:124–8. doi: 10.1126/science.aaa1348
14. Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. (2018) 36:1685–94. doi: 10.1200/JCO.2017.75.7740
15. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
16. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. (2016) 387:1909–20. doi: 10.1016/S0140-6736(16)00561-4
17. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. (2019) 51:202–6. doi: 10.1038/s41588-018-0312-8
18. Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA. (2019) 321:1391–9. doi: 10.1001/jama.2019.3241
19. Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. (2018) 36:633–41. doi: 10.1200/JCO.2017.75.3384
20. Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. (2018) 33:843–52.e4. doi: 10.1016/j.ccell.2018.03.018
21. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. (2015) 160:48–61. doi: 10.1016/j.cell.2014.12.033
22. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
23. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
24. Woerner SM, Tosti E, Yuan YP, Kloor M, Bork P, Edelmann W, et al. Detection of coding microsatellite frameshift mutations in DNA mismatch repair-deficient mouse intestinal tumors. Mol Carcinog. (2015) 54:1376–86. doi: 10.1002/mc.22213
25. Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. (2018) 5:471–8. doi: 10.1001/jamaoncol.2018.5801
26. Tang B, Yan X, Sheng X, Si L, Cui C, Kong Y, et al. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol. (2019) 12:7. doi: 10.1186/s13045-018-0693-2
27. Morrison C, Pabla S, Conroy JM, Nesline MK, Glenn ST, Dressman D, et al. Predicting response to checkpoint inhibitors in melanoma beyond PD-L1 and mutational burden. J Immunother Cancer. (2018) 6:32. doi: 10.1186/s40425-018-0344-8
28. Mishima S, Kawazoe A, Nakamura Y, Sasaki A, Kotani D, Kuboki Y, et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer. (2019) 7:24. doi: 10.1186/s40425-019-0514-3
29. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. (2018) 24:1296–304. doi: 10.1158/1078-0432.CCR-17-2439
30. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. (2017) 9:34. doi: 10.1186/s13073-017-0424-2
31. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. (2019) 37:992–1000. doi: 10.1200/JCO.18.01042
32. Chae YK, Davis AA, Raparia K, Agte S, Pan A, Mohindra N, et al. Association of tumor mutational burden with DNA repair mutations and response to anti–PD-1/PD-L1 therapy in non–small-cell lung cancer. Clin Lung Cancer. (2019) 20:88–96.e6. doi: 10.1016/j.cllc.2018.09.008
33. Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. (2019) 30:44–56. doi: 10.1093/annonc/mdy495
34. Cao D, Xu H, Xu X, Guo T, Ge W. High tumor mutation burden predicts better efficacy of immunotherapy: a pooled analysis of 103078 cancer patients. OncoImmunology. (2019) 8:e1629258. doi: 10.1080/2162402X.2019.1629258
35. Zhu J, Zhang T, Li J, Lin J, Liang W, Huang W, et al. Association between tumor mutation burden (TMB) and outcomes of cancer patients treated with PD-1/PD-L1 inhibitions: a meta-analysis. Front Pharmacol. (2019) 10:673. doi: 10.3389/fphar.2019.00673
36. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
37. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
38. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
39. Szustakowski JD, Green G, Geese WJ, Zerba K, Chang H. Evaluation of tumor mutation burden as a biomarker for immune checkpoint inhibitor efficacy: a calibration study of whole exome sequencing with FoundationOne. Cancer Res. (2018) 78:5528. doi: 10.1158/1538-7445.AM2018-5528
40. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
41. Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. (2019) 5:696–702. doi: 10.1001/jamaoncol.2018.7098
42. Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. (2015) 350:207–11. doi: 10.1126/science.aad0095
43. Roszik J, Haydu LE, Hess KR, Oba J, Joon AY, Siroy AE, et al. Novel algorithmic approach predicts tumor mutation load and correlates with immunotherapy clinical outcomes using a defined gene mutation set. BMC Med. (2016) 14:168. doi: 10.1186/s12916-016-0705-4
44. Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. (2017) 9:eaah3560. doi: 10.1126/scitranslmed.aah3560
45. Ricciuti B, Kravets S, Dahlberg SE, Umeton R, Albayrak A, Subegdjo SJ, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer. (2019) 7:87. doi: 10.1186/s40425-019-0572-6
46. Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. (2017) 171:934–49.e16. doi: 10.1016/j.cell.2017.09.028
47. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. (2016) 165:35–44. doi: 10.1016/j.cell.2016.02.065
48. Huang J. Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer. (2019) 125:742–9. doi: 10.1002/cncr.31855
49. Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer cell. (2018) 33:853–61.e4. doi: 10.1016/j.ccell.2018.04.001
50. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. (2017) 16:2598–608. doi: 10.1158/1535-7163.MCT-17-0386
51. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. (2018) 24:1441–8. doi: 10.1038/s41591-018-0134-3
52. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. (2018) 362:eaar3593. doi: 10.1126/science.aar3593
53. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
54. Fang W, Ma Y, Yin JC, Hong S, Zhou H, Wang A, et al. Comprehensive Genomic profiling identifies novel genetic predictors of response to anti-PD-(L)1 therapies in non-small-cell lung cancer. Clin Cancer Res. (2019) 25:5015–26. doi: 10.1158/1078-0432.CCR-19-0585
55. Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. (2019) 30:1096–103. doi: 10.1093/annonc/mdz134
56. Chae YK, Davis AA, Agte S, Pan A, Simon NI, Iams WT, et al. Clinical implications of circulating tumor DNA tumor mutational burden (ctDNA TMB) in non-small cell lung cancer. Oncologist. (2019) 24:820–8. doi: 10.1634/theoncologist.2018-0433
57. Snyder A, Nathanson T, Funt SA, Ahuja A, Buros Novik J, Hellmann MD, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med. (2017) 4:e1002309. doi: 10.1371/journal.pmed.1002309
58. Nathanson T, Ahuja A, Rubinsteyn A, Aksoy BA, Hellmann MD, Miao D, et al. Somatic mutations and neoepitope homology in melanomas treated with CTLA-4 blockade. Cancer Immunol Res. (2017) 5:84–91. doi: 10.1158/2326-6066.CIR-16-0019
59. Boichard A, Pham TV, Yeerna H, Goodman A, Tamayo P, Lippman S, et al. APOBEC-related mutagenesis and neo-peptide hydrophobicity: implications for response to immunotherapy. OncoImmunology. (2019) 8:1550341. doi: 10.1080/2162402X.2018.1550341
60. Janjigian YY, Sanchez-Vega F, Jonsson P, Chatila WK, Hechtman JF, Ku GY, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. (2018) 8:49–58. doi: 10.1158/2159-8290.CD-17-0787
61. Hu-Lieskovan S, Lisberg A, Zaretsky JM, Grogan TR, Rizvi H, Wells DK, et al. Tumor characteristics associated with benefit from pembrolizumab in advanced non-small cell lung cancer. Clin Cancer Res. (2019) 25:5061–8. doi: 10.1158/1078-0432.CCR-18-4275
62. Yusko E, Vignali M, Wilson RK, Mardis ER, Hodi FS, Horak C, et al. Association of tumor microenvironment T-cell repertoire and mutational load with clinical outcome after sequential checkpoint blockade in melanoma. Cancer Immunol Res. (2019) 7:458–65. doi: 10.1158/2326-6066.CIR-18-0226
63. Sakamuri D, Glitza IC, Betancourt Cuellar SL, Subbiah V, Fu S, Tsimberidou AM, et al. Phase I dose-escalation study of anti-CTLA-4 antibody ipilimumab and lenalidomide in patients with advanced cancers. Mol Cancer Ther. (2018) 17:671–6. doi: 10.1158/1535-7163.MCT-17-0673
64. Rozenblum AB, Ilouze M, Dudnik E, Dvir A, Soussan-Gutman L, Geva S, et al. Clinical impact of hybrid capture–based next-generation sequencing on changes in treatment decisions in lung cancer. J Thorac Oncol. (2017) 12:258–68. doi: 10.1016/j.jtho.2016.10.021
65. Kacew AJ, Harris EJ, Lorch JH, Haddad RI, Chau NG, Rabinowits G, et al. Chromosome 3q arm gain linked to immunotherapy response in advanced cutaneous squamous cell carcinoma. Eur J Cancer. (2019) 113:1–9. doi: 10.1016/j.ejca.2019.03.004
66. Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. (2017) 28:1532–9. doi: 10.1093/annonc/mdx183
67. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. (2017) 23:703–13. doi: 10.1038/nm.4333
68. Fancello L, Gandini S, Pelicci PG, Mazzarella L. Tumor mutational burden quantification from targeted gene panels: major advancements and challenges. J Immunother Cancer. (2019) 7:183. doi: 10.1186/s40425-019-0647-4
69. Budczies J, Allgauer M, Litchfield K, Rempel E, Christopoulos P, Kazdal D, et al. Optimizing panel-based tumor mutational burden (TMB) measurement. Ann Oncol. (2019) 30:1496–506. doi: 10.1093/annonc/mdz205
70. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol. (2018) 19:737–46. doi: 10.1016/S1470-2045(18)30261-4
Keywords: tumor mutation burden, tumor mutational burden, immune checkpoint inhibitors, objective response rate, progression-free survival, overall survival, meta-analysis
Citation: Wu Y, Xu J, Du C, Wu Y, Xia D, Lv W and Hu J (2019) The Predictive Value of Tumor Mutation Burden on Efficacy of Immune Checkpoint Inhibitors in Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 9:1161. doi: 10.3389/fonc.2019.01161
Received: 02 August 2019; Accepted: 17 October 2019;
Published: 05 November 2019.
Edited by:
Brian J. Czerniecki, Moffitt Cancer Center, United StatesReviewed by:
Luis De La Cruz-Merino, Hospital Universitario Virgen Macarena, SpainKawaljit Kaur, University of California, Los Angeles, United States
Copyright © 2019 Wu, Xu, Du, Wu, Xia, Lv and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Hu, ZHJfaHVqaWFuJiN4MDAwNDA7emp1LmVkdS5jbg==
†These authors have contributed equally to this work as co-first authors
 Yongfeng Wu1†
Yongfeng Wu1† Jian Hu
Jian Hu