
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 28 November 2019
Sec. Women's Cancer
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00937
Placental site trophoblastic tumor (PSTT) is a rare type of gestational trophoblastic disease originating from the intermediate trophoblast. Compared with hydatidiform mole, invasive hydatidiform mole and choriocarcinoma, the diagnosis of PSTT is more complicated and lacks specific and sensitive tumor markers. Most PSTT patients demonstrate malignant potential, and the primary treatment of PSTT is hysterectomy. However, metastasis occasionally occurs and even causes death in a small number of PSTT patients. Most PSTT patients are young women hence fertility preservation is an important consideration. The major obstacle for PSTT patient prognosis is chemotherapy resistance. However, the current understanding of the pathogenesis of PSTT and clinical treatment remains elusive. In this review, we summarized the research progress of PSTT in recent years from three aspects: mechanism, clinical presentation, and treatment and prognosis. Well-conducted multi-center studies with sufficient sample sizes are of great importance to better examine the pathological progress and evaluate the prognosis of PSTT patients, so as to develop prevention and early detection programs, as well as novel treatment strategies, and finally improve prognosis for PSTT patients.
Placental site trophoblastic tumor (PSTT) is a rare type of gestational trophoblastic disease (GTD) originating from the placental implantation site. It was first named as “trophoblastic pseudotumor” by Kurman and Scully in 1976 (1). With more understanding of this disease, the terminology of “PSTT” has gradually been acknowledged to feature both the benign and malignant potentials of this specific type of tumor. The World Health Organization (WHO) listed PSTT as the fourth trophoblastic disease in 1944, along with hydatidiform mole, invasive hydatidiform mole, and choriocarcinoma. Among these tumors, partial or complete hydatidiform mole is considered to be benign hyperplasia of the villi, while choriocarcinoma and PSTT are considered as tumor tissues (2). A global survey showed that the disease-specific mortality of PSTT is higher than other GTD subtypes (16.1% for PSTT, 6.5% for hydatidimolars, and 13.4% for choriocarcinoma) (3). The characteristics of PSTT lies in its unpredictable biological behavior, poor prognosis compared with other subtypes of GTD, and less responsiveness to chemotherapy. PSTT accounts for 0.2% of GTD, with a morbidity of around 1/100,000 per delivery. According to studies published in recent years, there are subtle differences in incidence among different regions around the world (4–13). The clinical features of PSTT are usually benign, but a few of them can relapse and metastasize, demonstrating the malignant biological behavior. The early diagnosis of PSTT remains uncertain, and it is difficult to distinguish the benign and malignant forms of PSTT in the early stage (14). The present obstacles in managing PSTT include malignancy prediction, fertility preservation, recurrence and chemotherapy resistance, and identification of possible treatment targets. We discussed the updates concerning these problems and summarized them to three main aspects: mechanism, clinical presentation, and treatment and prognosis.
Gestational trophoblastic neoplasia (GTN) includes hydatidiform mole, choriocarcinoma, PSTT and epithelial trophoblastic tumor (ETT). PSTT originates from placental site intermediate trophoblasts, which refer to the cells at the distal villus that attach to the endometrium and become dispersed and independent cell lines, and then acquire the abilities of proliferation and migration (Figure 1A) (15). In normal pregnancy, those cells migrate away from placenta and invade the decidual artery and uterine spiral artery to remodel the blood vessels, which in turn provide nutrition for the embryo (15, 16). These features of extravillous trophoblast are similar to those of tumor cells, and facilitate successful placental implantation. The above process is subject to strict temporal and spatial regulation in normal placentation (17). It is considered that PSTT is caused by hyperplasia of intermediate trophoblast, while hydatidiform mole and choriocarcinoma are results of abnormal or malignant proliferation of syncytiotrophoblast and cytotrophoblast (18, 19). Others propose that PSTT forms during the process of placenta detaching from the uterus, and the small nodules of the placental tissue remaining in the myometrium, and being reabsorbed over time. During this complex process, cell mitosis increases and becomes either atypical placental nodules or PSTT (Figure 1B) (18). The occurrence of PSTT is closely related to the change of the invasive ability of trophoblasts, a process involving many cytokines, extracellular matrix components, and enzymes.
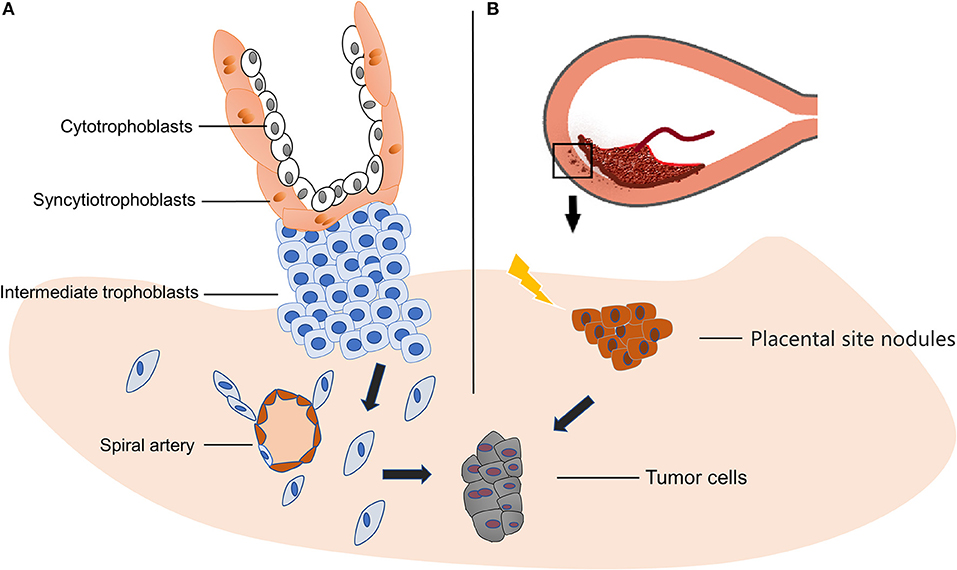
Figure 1. Origin of PSTT. (A) PSTT originates from extravillous trophoblasts, and then acquires the abilities of proliferation and migration. Afterwards, those cells migrate away from placenta and invade the decidual artery and uterine spiral artery to remodel the blood vessels which in turn provide nutrition for the embryo. The disruption of this well-regulated invasion process may lead to PSTT. (B) During delivery, placenta detaches from decidual, leaving small nodules and form placental site nodules in the myometrium. In the process of reabsorbing, some adverse trigger or stimuli may cause atypical mitosis and result in neoplasm.
The alterations of intracellular signaling pathways, intercellular information transmission, and extracellular matrix contribute to the occurrence of tumor. Studies have indicated that ERK, MAPK, mTOR signaling pathway, transcription factor NF-κB, Kiss-1, and GATA3 may play key roles in the invasion and metastasis of PSTT (Figure 2) (20–22). Adhesion molecules mediating cell-to-cell and cell-to-matrix communication can cause metastasis and local inflammation. For example, integrin-α5β1, which expresses on the cell surface and belongs to the adhesion molecule family, can interact with extracellular matrix components and is capable of fixing the cytoskeleton. Studies have found that P-selectin and integrin-α5β1 are related to the occurrence, development, invasion and metastasis of PSTT (23). Other molecules, such as P65, CD44v6, CD146, P21, FBI-1, and E-cadherin, are reported to play a similar role (21, 24, 25). In addition, the destruction of extracellular matrix components facilitates cancer cell invasion and metastasis. Heparanase (HPA) can promote tumor metastasis by degrading heparinase sulfate proteoglycan (HSPG) in extracellular matrix (26). Normally, the expression of HPA is limited to placenta and immune organs (23). It is reported that the expression of HPA is elevated in invasive malignant tumors, such as breast cancer, esophageal cancer, rectal cancer, and bladder cancer (20), and is found to be expressed in extravillous trophblasts (27). Researchers have speculated that HPA is related to the invasion of PSTT. Furthermore, it is implied that matrix metalloproteinase (MMP), tissue inhibitor of metalloproteinase (TIMP), and other molecules in the matrix may promote PSTT metastasis (28).
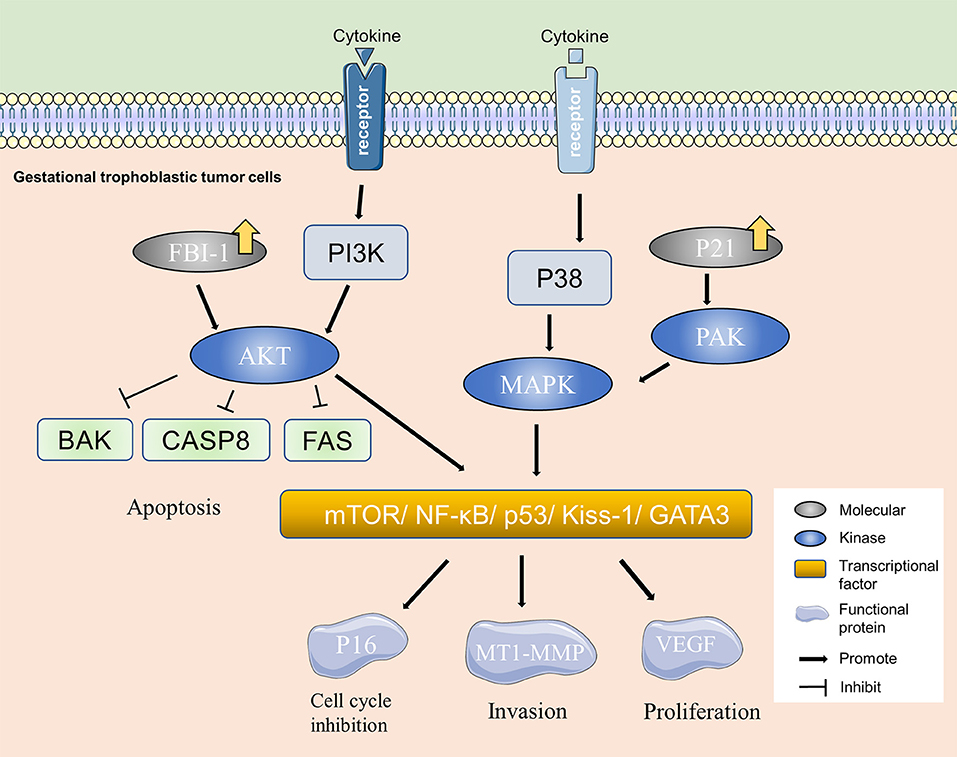
Figure 2. Molecular mechanism of PSTT. PI3K/AKT and MAPK are important molecular pathways in PSTT. Over-expression of molecules, such as FBI-1 and P21 can activate kinases, such as PAK and AKT in gestational trophoblastic tumor cells and consequently cause changes in the adhesion-associated and cell cycle regulation proteins, resulting in alterations of biological behaviors including invasion and proliferation of PSTT.
Several types of GTD are characterized by distinct genetic modes (29). There are various hypotheses about the underlying genetic mechanism of PSTT. A study analyzed the status of X chromosome inactivation of PSTT cases, and found that PSTT present a unique genetic mode requiring the presence of a paternal X (Xp) chromosome and PSTT are derived from the extraembryonic tissue of an antecedent female conceptus (30). Duplication of Xp chromosome is considered to cause abnormal genetic overdosing and plays a role in trophoblastic proliferation (30). Evidences have shown that X chromosome-linked genetically imprinted genes regulate extraembryonic tissues (31, 32). X chromosome inactivation is crucial to the development of normal extraembryonic tissues (33). It is believed that uniparental gene expression can lead to cancer predisposition. The hypothesis is that Xp harbors a dominant oncogene or tumorigenesis results from abnormal dosage of functional X chromosomes. Possible oncogenes include Esx1, Pem, MYCL2, and IAP (32–35). Using short sequence analysis of X chromosome, Zhao et al. confirmed the importance of maternal X chromosome in the occurrence of PSTT (36).
PHLDA2 (platelet-leukocyte C kinase substrate analog family A-2) is a paternally imprinted gene while expressed maternally, and is located at 11p15.5 which belongs to a known tumor suppressor gene region (37). The PHLDA2 protein is exclusively expressed in extravillous trophoblasts. Studies have found that PHLDA2 increased apoptosis-associated proteins and decreased synthesis of cyclin and cyclin-dependent kinase, thus inducing apoptosis of trophoblasts and reducing the proliferation ability of trophoblasts (37). Genomic hybridization studies have observed regional chromosomal gains involving 21q in PSTT cases, suggesting that chromosomal gains involving 21q may be associated with PSTT pathogenesis (38). We summarized major findings from current genetic studies in Table 1. Future studies are needed to further elucidate the involvement of these genes in PSTT.
Tumor cells can survive immunological surveillance, escape immunological elimination, and sometimes cause immunological tolerance. The role of immune system in the occurrence of gestational trophoblastic diseases has attracted many attentions in recent years. Immune response disorder on maternal-fetal interface may be related to abnormal reproductive disease. HLA-G is a non-classical class I histocompatibility complex expressed in EVT, which can protect cells from being killed by natural killer (NK) cells and CD40T cells, and maintaining maternal and fetal immunological tolerance. Trophoblasts can up-regulate the expression of HLA-G, which makes it conducive to abnormal proliferation, infiltration, and metastasis of trophoblasts (45). Th1/Th2 and Th17/Treg balance are also closely related to placenta formation and pregnancy maintenance. The changes of Th1/Th2 and Th17/Treg balance in PSTT have not been reported, and should be examined comprehensively (45, 46).
PSTT often occurs in women of childbearing age, with an average age of 32 years. The occurrence of PSTT can follow term pregnancy, premature delivery, hydatidiform mole, and choriocarcinoma, with an interval between the occurrence and previous pregnancy ranging from months to several years. Most PSTT develop within 1 year after the antecedent pregnancy. Tumors of early stage are confined to the uterus and grow slowly. The main symptoms of PSTT include colporrhagia and amenorrhea (2, 13, 47–49). Compared to other trophoblastic tumors, the human chorionic gonadotropin (hCG) serum level is slightly elevated in most PSTT patients. In rare cases, the serum level of hCG can reach even 100,000 IU/L (50), which may be a result of tumors mixing with choriocarcinoma tissues. Although the metastasis tendency of PSTT is lower than that of choriocarcinoma, it occurs occasionally. The main sites of metastasis include lung and the central nervous system (13, 51, 52). Other symptoms, such as hemoptysis, nephrotic symptoms and abdominal mass will appear while the tumor progresses or metastasizes. Very few cases are associated with nephropathy, such as proteinuria and thrombotic microangiopathy (53, 54). One study reported a PSTT case coexisted with paraneoplastic nodular regenerative hyperplasia of the liver (55). Some scholars believe that these symptoms may be caused by paracancerous syndrome which might be induced by the immune response to cancer cells or humoral factors. These paraneoplastic disorders usually resolve after hysterectomy and/or chemotherapy. Table 2 summarizes atypical presentations of PSTT.
Different trophoblastic diseases have distinct origins and pathological manifestations. In PSTT, the lesion is generally located at the placenta implantation site, and is massive or polypoid, protruding uterine cavity or infiltrating the uterus. Hemorrhagic focus can be found in PSTT. There is hardly normal villus structure under microscopic observation (58, 59). In addition, there are abundant intermediate trophoblasts, but no cytotrophoblasts or syncytiotrophoblasts (58). PSTT tumor cells infiltrate into the uterine myometrium in a strip-like manner, revealing the invasive ability of the intermediate trophoblasts at the implantation site. PSTT invades deep myometrium of the uterus and extends to serosa in some cases (58). The mitotic figures of PSTT vary. It can be observed that the nuclei are at different stages of mitosis. Tumor cells present monomorphic population of large polyhedral cells with irregular hyperchromatic nuclei. Besides, there is eosinophilic or transparent substance in the cytoplasm that could be large amount of fibrin (52, 60). Despite evident infiltration, the artery walls are usually intact, with a handful of bleeding foci and mild inflammation or necrosis (Figure 3). Immunohistochemistry analyses have shown that the expression of prolactin (hPL), cytokeratin (CK), melanoma adhesion molecule (Mel-CAM), Cyclin E and CD146 is diffuse positive in the cytoplasm of PSTT tumor cells, while the expression of hCG is locally positive and Vimentin is negative (60–63). It has been shown that inhibiting, epithelial membrane antigen (EMA) and placental alkaline phosphatase (PLAP) are focally positive (64). The expression of Ki67, a type of nuclear antigen, is positively correlated to the proliferation ability of tumor tissue. In PSTT, Ki67-positive cells could reach 15%, while in choriocarcinoma it may surge to 60–70% (61).
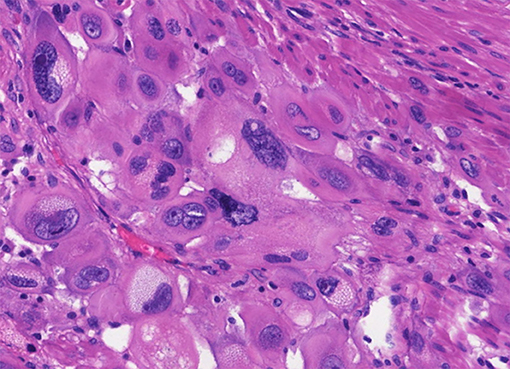
Figure 3. Histopathological features of PSTT. Tumor cells present monomorphic population of large polyhedral cells with irregular hyperchromatic nuclei, which are at different stages of mitosis. Besides, there is eosinophilic or transparent substance in the cytoplasm that could be large amount of fibrin. Tumor cells grow like nest or bands into myometrium, with a handful of bleeding foci and mild inflammation or necrosis.
Comparatively, ETT arises from chorionic-type intermediate trophoblast cells. For ETT, the lesions locate at decidua outside the implantation site, and can be found in the uterine body, fundus and cervical canal. ETT lesions are generally solid or cystic, with certain degrees of hemorrhage and necrosis. Tumor cells display a nested or bulk pattern of growth, similar to the biological behavior of smooth chorion under microscopic observation. There are characteristic geographic changes, referring to necrosis and eosinophilic vitreous matrix around the tumor cells (2). CK18 and P63 present diffuse positive immunoreactivity while hPL is negative or focally positive in the cytoplasm (65). CD146 is negative or locally positive but PLAP, E-cadherin and EGFR are positive on the membrane (66). ETT can be differentiated from PSTT by the expression of hPL and P63 (61). Previous studies have compared the expression of P40 and P63 in PSTT and ETT, and suggested that P40 is an equivalent immunohistochemical marker of ETT (62).
Choriocarcinoma originates from choriotrophoblasts, and the lesions appear in the myometrium of the uterus. Choriocarcinoma can also protrude into the uterine cavity or perforate the serosa. The choriocarcinoma lesions feature significant necrosis and abundant blood supply (67). Microscopically, there are typical cytotrophoblasts and syncytiotrophoblasts with mitotic figure >10/10 HP. Choriocarcinoma has significant vascular infiltration, accompanied by massive hemorrhage and necrosis. In choriocarcinoma, hCG shows strong positive immunoreactivity with the presence of rich cytotrophoblasts alternate with syncytiotrophoblasts, and hPL is focally positive or negative (67). There are a couple of points to help differentiate choriocarcinoma diagnosis from PSTT: Ki67-positive cells exceeding 50% and elevated serum beta-hCG level (67). A small number of studies also looked at other possible markers, such as pregnancy-associated major basic protein (pMBP), which has been shown to stain positively in large exaggerated placenta sites and PSTT cells (63). Table 3 summarizes the different expression levels of above discussed makers. Because of the chemoresistance of PSTT, it is important to establish biomarkers to aid diagnosis and stratification. It is found that glypican-3(GPC3), carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1), GATA binding protein 3 (GATA3), and HLA-G were positively expressed in PSTT (68–71), while bcl-2 and spalt-like transcription factor 4 (SALL4) were negatively expressed in PSTT (69, 70). Among these markers, SALL4 and bcl-2 are modestly positive in choriocarcinoma (69, 70), and HLA-G is exclusively positive in intermediate trophoblasts (71). Table 4 summarized studies evaluating differential diagnoses and potential diagnostic biomarkers of PSTT.
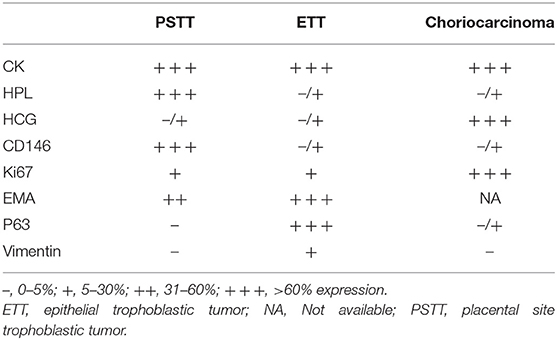
Table 3. Summary of the expression patterns of different markers in three types of gestational trophoblastic neoplasia (GTN) tissues.
Taken together, different trophoblastic diseases have distinct origins and pathological manifestations. Although the immunochemistry markers as well as serum beta-hCG level contribute to the differential diagnosis of GTN, it remains ambiguous for some patients. It is helpful to develop more non-invasive diagnostic markers to aid accurate diagnosis.
In recent years, liquid biopsy, which contains circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), circulating tumor RNA (ctRNA) and extracellular vesicles (EVs), has become a useful tool in cancer diagnosis. Studies have shown that solid tumors release enough ctDNA into the bloodstream to be detected. The analysis of ctDNA can help with molecular genotyping, mutational analysis, diagnosis of cancer, and surveillance after treatment. Previous study has shown that it is feasible to extract cell free DNA (cfDNA) to utilize as “liquid biopsy” in patients without histopathological diagnosis (72). There is huge potential of liquid biopsy in PSTT. First of all, liquid biopsy can be used for diagnosis and differential diagnosis. In combination with molecular technology, such as short tandem repeat (STR), single nucletide polymorphism (SNP) and amplification refractory mutation system-PCR (ARMS-PCR), liquid biopsy can detect paternal or maternal alleles to aid the diagnosis of GTN. Given the presence of non-maternal DNA in GTN tumors, the characteristics of these tumor cells in circulation may be easily detected. Then, the amount of ctDNA may reflect tumor burden and has been found to be associated with HCG in invasive molar disease (72–74). Besides, liquid biopsy can be used for post-operative monitoring of recurrence and metastasis. When histology is unavailable or minimal metastasis occurs which cannot be detected by imaging, ctDNA may be used to aid early diagnosis with advantages of painlessness, low risk and low cost (75). In addition, ctDNA may provide unique genetic information that can help developing personalized treatment. Furthermore, the level of ctDNA may be affected by chemotherapy, therefore ctDNA may be used to detect drug resistance mutations (75).
Imaging manifestation including ultrasound, computer tomography (CT) and PET-CT can assist the diagnosis of PSTT. Detailed gynecologic ultrasonography is used for ultrasound-guided dilation and curettag (D&C) or hysteroscopically guided biopsy to help with the diagnosis and preoperative staging (76). In combination with clinical history and hCG levels, ultrasonography is a first-line imaging method for GTN diagnosis because of its convenience and economy (76, 77). Both transabdominal and transvaginal ultrasound are performed. The sonographic findings can show changes in size, morphology, echo, surrounding lymph nodes and the relationship between the uterus, and the surrounding organs. It is important to assess the extent of the lesion and the choice of treatment. PSTT can be classified according to the imaging findings of the lesion location. There are three types of PSTT based on transvaginal ultrasound: most of the tumors protruding into the uterine cavity (Type I), the lesion is located in the uterine cavity and part of the myometrium (Type II), and the whole lesion is in the myometrium (Type III). Most lesions are located at the uterine corpus, and very few cases reported lesions in the uterus cervix or pelvic wall (78–80). According to the literature, the size of lesions ranges from 7 to 62 mm (81, 82). The lesions are solid or cystic or mixed with solid capsules. About one-half of the lesions appear to be hypoechoic or echogenic (77). There is no obvious boundary between the lesion and the surrounding tissue. Those cystic and mixed cystic lesions can be misdiagnosed as choriocarcinoma or hydatidiform mole. The blood flow signal of the PSTT lesion is slow, and the blood flow resistance index is low (76, 77, 83). The hemodynamic parameters of PSTT tumor vessels show that the Doppler signal is distributed in the lesion and the blood flow signal is rich, which may be caused by vasodilation or formation of arteriovenous fistula (Figures 4A,B) (84). PSTT can be divided into vessel abundant and relatively low blood vessel types detected by the Doppler signal. D&C should be avoided in the vessel abundant type. Compared to PSTT, the ultrasound of ETT is characterized by an echogenic mass located in the myometrium of the uterus, with abundant blood flow signals (83). Geographic necrosis is usually presented in ETT.
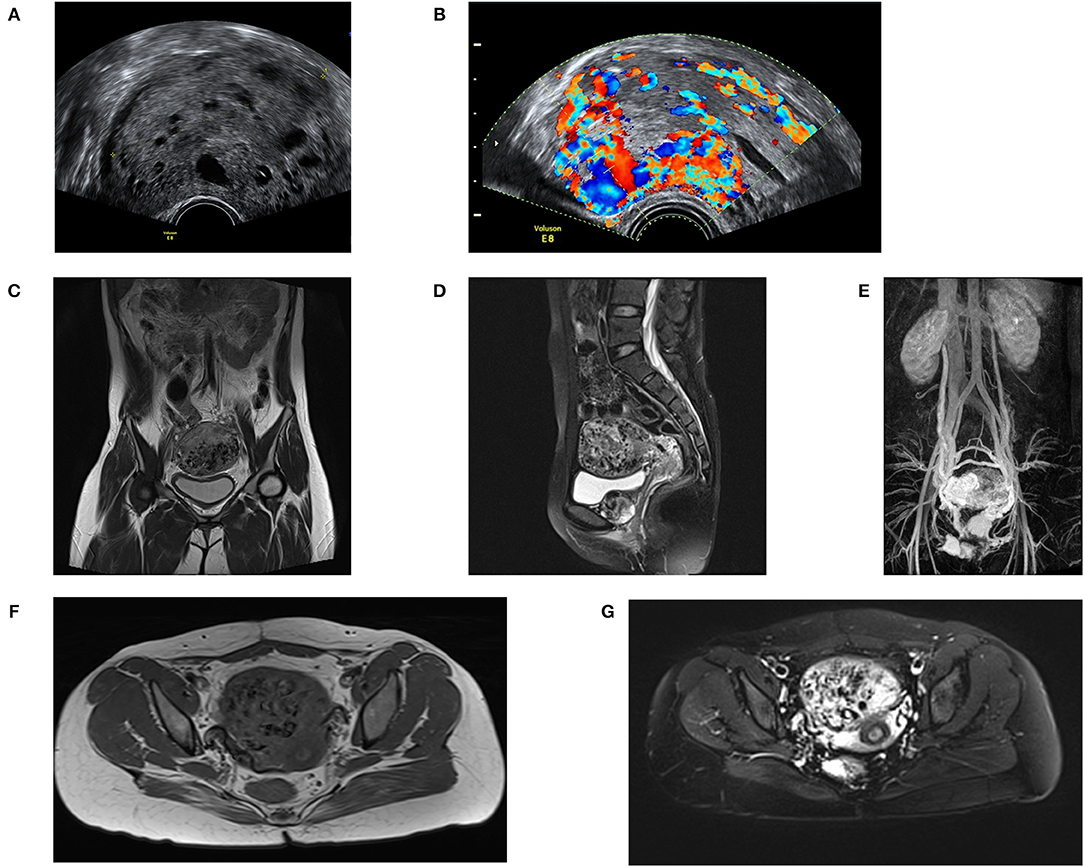
Figure 4. Imaging features of PSTT. (A) Ultrasound feature of PSTT: the mass shows as a mixed mass with intact capsule and presents as echogenic bulk. (B) Ultrasound feature of PSTT: formation of arteriovenous fistula can be observed. (C,D) MRI imaging of sagittal position: PSTT presents as uterine dilatation with short T1 and long T2 signals. (E,F) MRI imaging of axial position. (G) Enhancement imaging: the parauterine artery can be observed.
Although ultrasound performance is not considered as a diagnostic criterion for PSTT according to the International Federation of Gynecology and Obstetrics (FIGO), it is believed that solid lesions combined with low blood flow signals and low levels of serum βHCG constitute the diagnostic conditions of PSTT (85). In addition to the diagnostic value, ultrasound is a powerful imaging method in subsequent treatment and monitoring processes. Some researchers have suggested that the uterine artery pulsation index (UAPI) <1 can be used as a predictor of chemoresistance risk. Moreover, color Doppler ultrasound is a highly sensitive method for observing residual lesions (86).
MRI is also an imaging method for assessing the condition of PSTT. An abnormal heterogeneous signal changes can be observed in the wall of uterus in the T2-weighted image. In reported cases, MRI usually presents as uterine dilatation with short T1 and long T2 signals (Figures 4C,D,F,G) (77), and enhancement of vascular empty effect in myometrium and bilateral para-uterine tissue. Enhanced heterogeneous tumor and parauterine tissue can be observed after administration of contrast material (Figure 4E). For patients whose tumors cannot be detected by ultrasound, MRI can be used for localization (77, 87).
PET is not considered as regular imaging tools. It has not been widely used in assessing PSTT and other types of GTD cases (88). However, it is reported that PET is highly consistent with ultrasound, chest X-ray, and CT in the staging of GTN, which is not superior to traditional imaging methods, but nevertheless plays an important role in the diagnostic evaluation of high-risk patients. Therefore, it is believed that there is great potential of PET in assessing the range of lesions as well as metastases (88–90). In addition, there is high sensitivity of PET in detecting metastatic lesions (91). F-FDG PET can observe active tumor lesions and hence can be used to monitor PSTT recurrence and chemoresistance.
A study from the United States suggested implementing MRI to the head and pelvis, and CT to the chest and abdominal, and Doppler ultrasonography to the pelvis (77). The location and size of the lesion will be more comprehensive and precise after integrating the results from CT, ultrasound and MRI. The value of FDG-PET in the initial staging is still unclear, and clinicians tend to reserve it for selected cases with unsure lesions or to determine the resection site (91, 92). Considering the unreliability of hCG monitoring, there are great expectations for imaging tools in prognosis surveillance.
Generally, there are two treatment strategies based on patient evaluation: simple hysterectomy and systemic therapy (93–95). The consensuses are that patients of FIGO stage I can be cured by simple hysterectomy with or without pelvic lymph node biopsy (48). In a 17-year clinical trial of PSTT, of all the patients underwent surgery, 5 out of 13 patients relapsed and the recurrence rate was up to 50% (96). This high rate of recurrence reveals that the management of metastatic lesions is crucial in surgery. It is now generally accepted that lymphatic metastasis is more likely to occur in PSTT compared with choriocarcinoma (97). The retroperitoneal lymph nodes, especially the paraaortic lymph nodes, are the most frequent sites of lymphatic metastasis. Some researchers believed that paraaortic lymph nodes were an important part of lymphatic communication and metastasis between PSTT tumor and pelvic lymphoid tissue. They observed that lymph node metastasis existed in 39.1% of the 24 patients in the study (97). For patients with stage II to IV PSTT, lymph node biopsy should be carried out to evaluate the status of lymphatic metastasis, and provide guidance of whether to perform lymphadenectomy. Multidisciplinary management is important in treating PSTT. It remains controversial in terms of whether to perform oophrectomy. Individual study reported metastasis of PSTT to the ovary (98), which illustrates the possibility of ovarian metastasis and the necessity of oophrectomy in the operation. However, it requires comprehensive evaluation of patient conditions including age, staging, metastasis, basic condition, and fertility requirements before deciding whether to perform oophrectomy. Besides, researchers suggest ovarian retention during hysterectomy should take consideration of family history of ovarian diseases. For PSTT patients with a strong family history of ovarian diseases, bilateral oophrectomy should be seriously considered to reduce the risk of ovarian metastasis.
Systemic therapy is considered according to risk factors after the surgery. The current treatment guidelines of PSTT vary (Table 5) (46, 99–101). Noticeably, WHO prognostic index score for GTN does not apply to PSTT. Various researches have reported several criteria related to poor prognosis of PSTT, including interval between antecedent pregnancy >2 years, deep infiltration, necrosis, mitotic index >5/10 under microscope. Patients with high risk are recommended to use multi-drug combined chemotherapy. For patients of FIGO stage II–IV, hysterectomy followed by adjuvant chemotherapy is an acceptable choice (52). For patients with metastasis, it is believed that targeted surgery and high-dose platinum-containing chemotherapy containing platinum or etoposide are advisable treatment options. Chemotherapy regimens with reported better response include EMA/EP (etoposide, methotrexate, actinomycin-D/etoposide, cisplatin), EMA/CO (etoposide, methotrexate, actinomycin-D/vincristine, cyclophosphamide), and TE/TP (taxol, etoposide/taxol, cisplatin) (102). Studies have shown that EMA/EP lead to a higher remission rate; however, it has high toxicity and possible subsequent hematological side effects, such as neutropenia (103). TP and TE regimens are also used in the clinic. For relapsed patients, EMA/EP and TP show more chemosensitivity than other regimens. Therefore, it is the first line treatment choice especially for refractory or relapsed PSTT patients. In addition, salvage chemotherapy regimens including BEP (bleomycin/etoposide/platinum) and ICE (ifosfamide/cisplatin/etoposide) are used in chemotherapy-resistance patients (104).
It is reported that high-dose chemotherapy regimen containing platinum significantly increased overall survival in patients with high-risk GTD, and 5 of 11 high-risk PSTT patients receiving the treatment were in remission (105). In UK, researchers adopted an 8–12 weeks EP-EMA regime based on patient tolerance, after which stem cell mobilization is initiated, then several courses of high dose chemotherapy are applied. Following the above processes, autologous stem cell transplant support sequential therapy is implemented (106). The authors proposed that early initiation of high dose chemotherapy and peripheral blood stem cell support therapy in high-risk PSTT patients with an interval between antecedent pregnancy >2 years, and in younger patients with low HCG levels are more effective (106, 107). The disadvantages of high-dose chemotherapy are toxic side effects and induced premature ovarian failure (POF) which will affect the patient's future fertility. Chemotherapy may lead to decreased follicular cells, thus decreased ovarian reserve, and premature ovarian failure. Anti-Müllerian hormone (AMH) is a valuable marker that reflects the function of particles and sertoli cells. It can be used for assessing female ovarian reserve and monitoring menopausal process. Researchers have observed that serum AMH levels are significantly decreased after using etoposide (VP16) in patients with GTN, and proposed that monitoring AMH levels during chemotherapy is beneficial for ovarian protection (108).
In general, PSTT patients are prone to chemotherapy resistance and should be treated with appropriate surgical treatment in time. In addition, chemotherapy response for each patient should be determined timely so as to avoid low reactivity. For patients with metastasis, comprehensive treatment strategies, such as high-dose multidrug combination chemotherapy, surgery, imaging evaluation, and the use of colony stimulating factor (G-CSF) are implemented. To the best of our knowledge, there is currently no literature on how to evaluate chemotherapeutic reactivity of trophoblastic tumors. Treatment experiences of other types of tumor have shown that measurement of chemotherapy efficacy can base on clinical benefit response (CBR), which is tumor-related symptoms after chemotherapy improvement; drug susceptibility test; imaging evaluation including CT, MRI, radionuclide scanning, and angiography; tumor size; tumor blood supply; and tumor density changes (99). Besides, currently there is no monitoring of drug resistance genes in PSTT patients, which could also be a direction for future research to explore.
Since most patients with PSTT are young women, the preservation of fertility during chemotherapy is a critical issue. At present, the means of conservation is mainly based on surgery and chemotherapy. The choice of surgical methods includes abdominal resection, laparoscopic resection, and hysteroscopic resection (6, 109, 110). There is also report of using a modified Strausmann procedure (MSP) on suitable candidates (111). Some researchers have observed significant effects of arterial infusion of chemotherapy drugs, which significantly increased uterine preservation therapy (112).
The success rate of fertility preservation treatment is relatively high in young women. However, a small number of patients who went through fertility preservation therapy eventually underwent hysterectomy, probably due to incomplete removal of residual lesions. A study conducted in a single hospital reported that about 21% of patients who preserved uterus in surgery successfully preserved fertility (6). Another study reported that when trying to perform local resection of tumors, five out of six PSTT patients finally underwent total hysterectomy because of failure to completely remove hidden lesions (111). The obstacle of fertility-preserving treatment lies in the establishment of criteria to distinguish patients that are suitable to preserve fertility. Scholars have proposed appropriate conditions for fertility preservation, those factors include stage I, >35 years old, a strong fertility requirement, acceptable chemotherapy responsiveness, no malignant prognostic factors, such as deep myometrium infiltration (110, 113). More data are needed to confirm those findings.
Developing new strategy for treatment of PSTT is of great significance. Recently, certain treatment strategies, such as targeted therapy for vascular growth factors and immunological checkpoints, as well as the application of mifepristone have drawn attention (114). Targeted therapy can increase the efficacy while reducing side effects because they selectively target specific pathways. Standard chemotherapy affects most proliferating cells, while target-based treatment is designed to inactivate molecular pathways, such as PI3K and MAPK signaling pathways that are essential for tumor-cell growth and survival (115). Studies have found increased expression of vascular endothelial growth factor (VEGF) and TGFβ3 in PSTT tissues (116, 117). VEGF and TGFβ3 are both growth factors that play an important role in angiogenesis, embryo implantation and placenta formation. TGFβ3 has many regulatory effects on trophoblasts, such as inhibiting proliferation and invasiveness. Importantly, the time sequence of TGFβ3 expression is consistent with the regulation of trophoblast invasion throughout pregnancy. It is believed that overexpression of VEGF and TGFβ3 may enhance the invasiveness of trophoblastic tumors and associated with poor prognosis (118). Another vascular growth factor, endocrine gland-derived vascular endothelial growth factor (EG-VEGF), is exclusively expressed in endocrine tissues (ovary, testis, adrenal cortex, and placenta), but has no effect on endothelial cells of other tissues. EG-VEGF can induce phosphorylation of p42/p44 MAP kinases and Akt pathway through receptors PKR1 and PKR2. It is reported that there were differences in the expression of EG-VEGF and PKR1/PKR2 between normal villous tissues and choriocarcinoma cell lines, and that PKR1 was mainly expressed in cytotrophoblasts, while PKR2 was mainly expressed in extravillous trophoblasts and syncytiotrophoblasts (119). Inhibition of PKR2 expression by small interfering RNA demonstrated that EG-VEGF can inhibit EVT invasion by regulating MMP-2 and MMP-9, suggesting that EG-VEGF may be an important factor in the development of trophoblastic tumors (119). Further studies are needed to investigate whether targeted therapy of VEGF, TGFβ3, and EG-VEGF can assist PSTT diagnosis or treatment.
Immunotherapy has been proven to be successful in treating several types of cancer patients after first-line chemotherapy (120–123). It has been proposed that there is potential of immunotherapy in treating PSTT patients (124). The immunological checkpoints have gained wide attention in recent years. Programmed death 1 (PD1) is a transmembrane receptor expressed on the surface of T cells, B cells, NK cells, as well as antigen presenting cells. After binding to its ligand (PD-L1), PD-1/PD-L1 can inhibit the production of activated T cells, which is involved in the regulation of immunosuppressive function and immune tolerance. Inhibitors against this membrane surface protein can block the binding of PD-1/PD-L1, thereby relieving the immunosuppressive effect and promoting the killing effect of T cells on tumor cells (125, 126). It has been shown that PD-L1 is widely expressed in all trophoblasts except cytotrophoblasts, while PD-L1, B7-H3, and VISTA were positively expressed in PSTT tissue, indicating that PD-L1 blocker may be a potential treatment for PSTT (125, 126). PD-1 blockers (Nivolumab, Pembrolizumab) and PD-L1 blockers (Atezolizumab, Avelumab, Durvalumab) have been used in treatment of cancer (120–123). A recent study has shown that Pembrolizumab is a remarkably effective drug in patients with chemotherapy-resistant gestational tumors (124). Besides, it has been shown that intravenous infusion of Pembrolizumab is well-tolerated with an accepted toxicity profile, which makes it a promising treatment choice (124).
Since most PSTT patients are young women preferring fertility-preserving treatment, which usually is simple local resection, there is elevated risk of recurrence and metastasis. Most PSTT patients develop chemotherapy resistance to platinum-based regime, and PD-1 inhibitors, such as Nivolumab and Pembrolizumab have been used to treat patients with platinum-resistant ovarian cancer patients (121). Therefore, the use of PD-1 antibody might be a life-saving therapy for chemotherapy-resistant patients and improve the prognosis of PSTT. In addition, PD-1/PD-L1 can be used in combination with a CTLA4 inhibitor or an anti-angiogenic targeted drug to enhance the therapeutic effect (127, 128). Because of the memory function of the immune system, once the PD-1 inhibitor works, patients will have a chance to achieve long-term cure, which has already been observed in malignant tumors, such as malignant melanoma (129–131). The prediction of responsiveness to immunotherapy could be based on human lymphocyte antigen class I (HLA-G), microsatellite instability-high (MSI-H), deficient mismatch repair (dMMR), microsatellite detection (MSI), tumor mutation burden (TMB), and tumor infiltrating lymphocytes (122, 124). However, the application of immunotherapy in treating PSTT patients requires further clinical investigation.
Immune checkpoint inhibitors are expected to increase the treatment success rate, while currently there is no research about pregnancy outcomes after using immune checkpoint inhibitors in patients with trophoblastic diseases. Existing literatures have reported several immune-related side effects caused by immune checkpoint inhibitors, such as endocrine system disorders, sexual dysfunction, and damage to the reproductive system (132). Another theory is that inhibition of the PD-1 pathway can reduce immune tolerance of the maternal-fetal interface during pregnancy (133, 134). Whether and/or how these side effects will affect the long-term reproductive function remains to be elucidated in the future.
The prognosis of most PSTT cases is desirable; however, metastasis or recurrence does occur. Detailed information regarding prognosis has been summarized in a previous review (48). FIGO staging is the main factor affecting PSTT prognosis (6, 10). A retrospective study with 62 PSTT cases reported a 10-years survival rate of 90% for stage I patients, 52% for the stage II patients, 49% for stage III and IV patients (87).
Patients with stage I PSTT usually experience favorable prognosis after total hysterectomy, and the 10-years survival rate can be as high as 100% (112). However, those PSTT patients became infertile due to hysterectomy and may suffer from subsequent psychological and social stress (113). The prognosis of patients with stage II-IV PSTT is relatively poor. Although the progression of PSTT placental trophoblastic tumor is generally slow, it has certain malignant potential. According to the statistics from Britain, the 10-years survival rate of patients with stage II-IV PSTT was around 49% during 1976–2006 (112). The prognosis of patients with recurrent or refractory diseases is worse, with a 5-years survival rate of about 22% (112). A study reviewed and analyzed the prognosis of 88 PSTT cases and reported that the survival rate of patients with stage III–IV PSTT was only 30% (135).
Chemotherapy resistance is a major obstacle of PSTT prognosis. A study from Beijing showed that among all 108 PSTT cases only 7 deaths were observed, which were due to poor chemotherapy response and recurrence (6), indicating early diagnosis and accurate chemotherapy are essential to improved outcome. There is an urgent need for prognostic and predictive biomarkers for patients' stratification. It is suggested that immunoactivity of VEGF and EGFR indicates the efficacy of targeted therapy (97). A study has shown that p53 successfully discriminated confined and metastatic PSTT cases (69). There are also markers, such as MMP and TIMP that could predict biological behaviors including invasive and metastatic abilities of PSTT (28). At present, there are few studies investigating the mechanism of PSTT chemotherapy resistance. A better understanding of the molecular mechanism of PSTT chemotherapy resistance is crucial to develop novel and more effective treatment strategies of PSTT.
To summarize, PSTT is a type of trophoblastic neoplasm with very low incidence and benign characteristics. Similar to many other types of tumors, PSTT may be a result of comprehensive interaction between genetic, immunological, and environmental factors. The prognosis of most PSTT cases is desirable; however, metastasis or recurrence occurs in a few cases. Most PSTT patients are young women hence fertility preservation is an important consideration. Chemotherapy resistance is a main obstacle for PSTT patient prognosis, but the underlying mechanism is still unclear. Table 6 discussed related treatments and survival outcomes of PSTT reported in the recent literature, and Figure 5 summarized the diagnosis, treatment, and prognosis of PSTT patients. Since the disease is very rare, it is important to have a centralized registration system and well-kept data, so as to facilitate PSTT research. Well-conducted multi-center studies with sufficient sample sizes are of great importance to better examine the pathological progress and evaluate the prognosis of PSTT patients. More studies are needed to explore the exact mechanism of PSTT, so as to develop prevention and early detection programs, as well as novel treatment strategies, and finally improve prognosis for PSTT patients.
The study protocol was approved by the Institutional Review Board (IRB) of the Obstetrics and Gynecology Hospital of Fudan University (Reference number: 2016-26; Date of approval: 18 April 2016). The patients gave written informed consent.
XF: extensive literature search and drafting. ZW: figures. SZ: extensive literature search. YD: literature search and critical revision. HZ: conception of the work and final version approval. All authors read and approved the final manuscript.
This work was funded by the National Natural Science Foundation of China (Grant number: 81571457, 81971394), Shanghai Talent Development Fund (Grant Number 2017090), and Shanghai Pujiang Program (Grant number: 15PJ1400900).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Kurman RJ, Scully RE, Norris HJ. Trophoblastic pseudotumor of the uterus: an exaggerated form of “syncytial endometritis” simulating a malignant tumor. Cancer. (1976) 38:1214–26. doi: 10.1002/1097-0142(197609)38:3<1214::AID-CNCR2820380323>3.0.CO;2-J
2. Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. (2011) 204:11–8. doi: 10.1016/j.ajog.2010.06.072
3. Kohorn EL. Worldwide survey of the results of treating gestational trophoblastic disease. J Reprod Med. (2014) 59:145–53.
4. Zhang Y, Zhang S, Huang W, Chen T, Yuan H, Zhang Y. Intermediate trophoblastic tumor: the clinical analysis of 62 cases and prognostic factors. Arch Gynecol Obstet. (2019) 299:1353–64. doi: 10.1007/s00404-018-05037-0
5. Hyman DM, Bakios L, Gualtiere G, Carr C, Grisham RN, Makker V, et al. Placental site trophoblastic tumor: analysis of presentation, treatment, and outcome. Gynecol Oncol. (2013) 129:58–62. doi: 10.1016/j.ygyno.2012.12.029
6. Zhao J, Lv WG, Feng FZ, Wan XR, Liu JH, Yi XF, et al. Placental site trophoblastic tumor: a review of 108 cases and their implications for prognosis and treatment. Gynecol Oncol. (2016) 142:102–8. doi: 10.1016/j.ygyno.2016.05.006
7. Moutte A, Doret M, Hajri T, Peyron N, Chateau F, Massardier J, et al. Placental site and epithelioid trophoblastic tumours: diagnostic pitfalls. Gynecol Oncol. (2013) 128:568–72. doi: 10.1016/j.ygyno.2012.11.010
8. Behtash N, Karimi Zarchi M. Placental site trophoblastic tumor. J Cancer Res Clin Oncol. (2008) 134:1–6. doi: 10.1007/s00432-007-0208-y
9. Choi MC, Jung SG, Park H, Joo WD, Lee C, Lee JH, et al. Placental site trophoblastic tumors: analysis of the clinicopathologic characteristics of 20 cases in Korea. Int J Gynecol Cancer. (2016) 26:1515–20. doi: 10.1097/IGC.0000000000000799
10. Baergen RN, Rutgers JL, Young RH, Osann K, Scully RE. Placental site trophoblastic tumor: a study of 55 cases and review of the literature emphasizing factors of prognostic significance. Gynecol Oncol. (2006) 100:511–20. doi: 10.1016/j.ygyno.2005.08.058
11. Braga A, Uberti EM, Fajardo Mdo C, Viggiano M, Sun SY, Grillo BM, et al. Epidemiological report on the treatment of patients with gestational trophoblastic disease in 10 Brazilian referral centers: results after 12 years since International FIGO 2000 Consensus. J Reprod Med. (2014) 59: 241–7.
12. van Trommel NE, Lok CA, Bulten H, Thomas CM, Massuger LF. Long-term outcome of placental site trophoblastic tumor in The Netherlands. J Reprod Med. (2013) 58: 224–8.
13. Karimi-Zarchi M, Mortazavizadeh MR, Soltani-Gerdefaramrzi M, Rouhi M, Yazdian-Anari P, Ahmadiyeh MH. Investigation of risk factors, stage and outcome in patients with gestational trophoblastic disease since 2001 to 2011 in Iran-Yazd. Int J Biomed Sci. (2015) 11:166–72.
14. Braga A, Mora P, de Melo AC, Nogueira-Rodrigues A, Amim-Junior J, Rezende-Filho J, et al. Challenges in the diagnosis and treatment of gestational trophoblastic neoplasia worldwide. World J Clin Oncol. (2019) 10:28–37. doi: 10.5306/wjco.v10.i2.28
15. Cheung AN. Pathology of gestational trophoblastic diseases. Best Pract Res Clin Obstet Gynaecol. (2003) 17:849–68. doi: 10.1016/S1521-6934(03)00094-4
16. Shih IM, Seidman JD, Kurman RJ. Placental site nodule and characterization of distinctive types of intermediate trophoblast. Hum Pathol. (1999) 30:687–94. doi: 10.1016/S0046-8177(99)90095-3
17. Fujiwara H, Higuchi T, Sato Y, Nishioka Y, Zeng BX, Yoshioka S, et al. Regulation of human extravillous trophoblast function by membrane-bound peptidases. Biochim Biophys Acta. (2005) 1751:26–32. doi: 10.1016/j.bbapap.2005.04.007
18. Shih IM, Kurman RJ. The pathology of intermediate trophoblastic tumors and tumor-like lesions. Int J Gynecol Pathol. (2001) 20:31–47. doi: 10.1097/00004347-200101000-00004
19. Kurman RJ, Shih Ie M. Discovery of a cell: reflections on the checkered history of intermediate trophoblast and update on its nature and pathologic manifestations. Int J Gynecol Pathol. (2014) 33:339–47. doi: 10.1097/PGP.0000000000000144
20. Köbel M, Pohl G, Schmitt WD, Hauptmann S, Wang TL, Shih IeM. Activation of mitogen-activated protein kinase is required for migration and invasion of placental site trophoblastic tumor. Am J Pathol. (2005) 167:879–85. doi: 10.1016/S0002-9440(10)62059-7
21. Huang BX, Guo XB, Pan HX, Nie X. Expression of transcriptional factor GATA3 in trophoblastic tissues and its implications. J Clin Exp Pathol. (2016) 32:652–5. doi: 10.13315/j.cnki.cjcep.2016.06.012
22. Banet N, Gown AM, Shih IeM, Kay Li Q, Roden RB, Nucci MR, et al. GATA-3 expression in trophoblastic tissues: an immunohistochemical study of 445 cases, including diagnostic utility. Am J Surg Pathol. (2015) 39:101–8. doi: 10.1097/PAS.0000000000000315
23. Yang FG, Ma WJ, Wang C, Bai XW, Qi JM. Advances in the study of P-selectin, integrin-α5β1 and HPA in PSTT. J Chengde Med Coll. (2017) 34:516–518. doi: 10.15921/j.cnki.cyxb.2017.06.031
24. Siu MK, Yeung MC, Zhang H, Kong DS, Ho JW, Ngan HY, et al. p21-Activated kinase-1 promotes aggressive phenotype, cell proliferation, and invasion in gestational trophoblastic disease. Am J Pathol. (2010) 176:3015–22. doi: 10.2353/ajpath.2010.091263
25. Mak VC, Wong OG, Siu MK, Wong ES, Ng WY, Wong RW, et al. FBI-1 is overexpressed in gestational trophoblastic disease and promotes tumor growth and cell aggressiveness of choriocarcinoma via PI3K/Akt signaling. Am J Pathol. (2015) 185:2038–48. doi: 10.1016/j.ajpath.2015.03.011
26. Vlodavsky I, Goldshmidt O, Zcharia E, Atzmon R, Rangini-Guatta Z, Elkin M, et al. Mammalian heparanase: involvement in cancer metastasis, angiogenesis and normal development. Semin Cancer Biol. (2002) 12:121–9. doi: 10.1006/scbi.2001.0420
27. Haimov-Kochman R, Friedmann Y, Prus D, Goldman-Wohl DS, Greenfield C, Anteby EY, et al. Localization of heparanase in normal and pathological human placenta. Mol Hum Reprod. (2002) 8:566–73. doi: 10.1093/molehr/8.6.566
28. Singh M, Kindelberger D, Nagymanyoki Z, Ng SW, Quick CM, Elias KM, et al. Matrix metalloproteinases and their inhibitors and inducer in gestational trophoblastic diseases and normal placenta. Gynecol Oncol. (2011) 122:178–82. doi: 10.1016/j.ygyno.2011.03.025
29. Alifrangis C, Seckl MJ. Genetics of gestational trophoblastic neoplasia: an update for the clinician. Future Oncol. (2010) 6:1915–23. doi: 10.2217/fon.10.153
30. Hui P, Parkash V, Perkins AS, Carcangiu ML. Pathogenesis of placental site trophoblastic tumor may require the presence of a paternally derived X chromosome. Lab Invest. (2000) 80:965–72. doi: 10.1038/labinvest.3780099
31. Goto T, Wright E, Monk M. Paternal X-chromosome inactivation in human trophoblastic cells. Mol Hum Reprod. (1997) 3:77–80. doi: 10.1093/molehr/3.1.77
32. Li Y, Lemaire P, Behringer RR. Esx1, a novel X chromosome-linked homeobox gene expressed in mouse extraembryonic tissues and male germ cells. Dev Biol. (1997) 188:85–95. doi: 10.1006/dbio.1997.8640
33. Lin TP, Labosky PA, Grabel LB, Kozak CA, Pitman JL, Kleeman J, et al. The Pem homeobox gene is X-linked and exclusively expressed in extraembryonic tissues during early murine development. Dev Biol. (1994) 166:170–9. doi: 10.1006/dbio.1994.1305
34. Redolfi E, Pizzuti A, Di Bacco A, Susani L, Labella T, Affer M, et al. Mapping of the MYCL2 processed gene to Xq22-23 and identification of an additional L MYC-related sequence in Xq27.2. FEBS Lett. (1999) 446:273–7. doi: 10.1016/S0014-5793(99)00243-4
35. Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. (1997) 388:300–4. doi: 10.1038/40901
36. Zhao S, Sebire NJ, Kaur B, Seckl MJ, Fisher RA. Molecular genotyping of placental site and epithelioid trophoblastic tumours; female predominance. Gynecol Oncol. (2016) 142:501–7. doi: 10.1016/j.ygyno.2016.05.033
37. Tunster SJ, Creeth HDJ, John RM. The imprinted Phlda2 gene modulates a major endocrine compartment of the placenta to regulate placental demands for maternal resources. Dev Biol. (2016) 409: 251–60. doi: 10.1016/j.ydbio.2015.10.015
38. Hui P, Riba A, Pejovic T, Johnson T, Baergen RN, Ward D. Comparative genomic hybridization study of placental site trophoblastic tumour: a report of four cases. Mod Pathol. (2004) 17:248–51. doi: 10.1038/modpathol.3800025
39. Fisher RA, Paradinas FJ, Newlands ES, Boxer GM. Genetic evidence that placental site trophoblastic tumours can originate from a hydatidiform mole or a normal conceptus. Br J Cancer. (1992) 65:355–8. doi: 10.1038/bjc.1992.72
40. Fukunaga M, Ushigome S. Metastasizing placental site trophoblastic tumor. An immunohistochemical and flow cytometric study of two cases. Am J Surg Pathol. (1993) 17:1003–10. doi: 10.1097/00000478-199310000-00005
41. Oldt RJ III, Kurman RJ, Shih IeM. Molecular genetic analysis of placental site trophoblastic tumors and epithelioid trophoblastic tumors confirms their trophoblastic origin. Am J Pathol. (2002) 161:1033–7. doi: 10.1016/S0002-9440(10)64264-2
42. Xue WC, Guan XY, Ngan HY, Shen DH, Khoo US, Cheung AN. Malignant placental site trophoblastic tumor: a cytogenetic study using comparative genomic hybridization and chromosome in situ hybridization. Cancer. (2002) 94:2288–94. doi: 10.1002/cncr.10424
43. Hui P, Wang HL, Chu P, Yang B, Huang J, Baergen RN, et al. Absence of Y chromosome in human placental site trophoblastic tumor. Mod Pathol. (2007) 20:1055–60. doi: 10.1038/modpathol.3800941
44. Dotto J, Hui P. Lack of genetic association between exaggerated placental site reaction and placental site trophoblastic tumor. Int J Gynecol Pathol. (2008) 27:562–7. doi: 10.1097/PGP.0b013e31816d1d00
45. Helige C, Ahammer H, Moser G, Hammer A, Dohr G, Huppertz B, et al. Distribution of decidual natural killer cells and macrophages in the neighbourhood of the trophoblast invasion front: a quantitative evaluation. Hum Reprod. (2014) 29:8–17. doi: 10.1093/humrep/det353
46. Xiang Y, Zhou Q, Wu XH, Liu JH, Li L, Zhu XQ, et al. Guidelines for the diagnosis and treatment of gestational trophoblastic diseases (Fourth Edition). Chin J Prac Gynecol Obstetr. (2018) 34:994–1001. doi: 10.19538/j.fk2018090113
47. Ngan HY, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. (2015) 131(Suppl 2):S123–6. doi: 10.1016/j.ijgo.2015.06.008
48. Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Placental site trophoblastic tumor and epithelioid trophoblastic tumor: clinical and pathological features, prognostic variables and treatment strategy. Gynecol Oncol. (2019) 153:684–693. doi: 10.1016/j.ygyno.2019.03.011
49. Hassadia A, Gillespie A, Tidy J, Everard RGNJ, Wells M, Coleman R, et al. Placental site trophoblastic tumour: clinical features and management. Gynecol Oncol. (2005) 99:603–7. doi: 10.1016/j.ygyno.2005.06.054
50. De Nola R, Schonauer LM, Fiore MG, Loverro M, Carriero C, Di Naro E. Management of placental site trophoblastic tumor: Two case reports. Medicine (Baltimore). (2018) 97:e13439. doi: 10.1097/MD.0000000000013439
51. Bower M, Paradinas FJ, Fisher RA, Nicholson SK, Rustin GJ, Begent RH, et al. Placental site trophoblastic tumor: molecular analysis and clinical experience. Clin Cancer Res. (1996) 2:897–902.
52. Horowitz NS, Goldstein DP, Berkowitz RS. Placental site trophoblastic tumors and epithelioid trophoblastic tumors: biology, natural history, and treatment modalities. Gynecol Oncol. (2017) 144:208–14. doi: 10.1016/j.ygyno.2016.10.024
53. Xiao C, Zhao J, Li M, Zhao D, Xiang Y. Lupus nephritis associated with placental site trophoblastic tumor: a case report and review of the literature. Gynecol Oncol Case Rep. (2014) 9:26–8. doi: 10.1016/j.gynor.2014.05.003
54. Sawamura M, Komatsuda A, Nara M, Togashi M, Wakui H, Takahashi N. Disappearance of a thrombotic microangiopathy-like glomerular lesion in a patient with a placental site trophoblastic tumor after hysterectomy. Clin Nephrol Case Stud. (2018) 6:27–30. doi: 10.5414/CNCS109440
55. Dumas K, Wilbur M, Dillon J, Cliby W, Langstraat C, Fader AN, et al. Paraneoplastic nodular regenerative hyperplasia of the liver associated with placental site trophoblastic tumor. Gynecol Oncol Rep. (2019) 29:16–9. doi: 10.1016/j.gore.2019.05.003
56. Batra V, Kalra OP, Mathur P, Kavita Dev G. Membranous glomerulopathy associated with placental site trophoblastic tumour: a case report. Nephrol Dial Transplant. (2007) 22:1766–8. doi: 10.1093/ndt/gfl786
57. Brewer CA, Adelson MD, Elder RC. Erythrocytosis associated with a placental-site trophoblastic tumor. Obstet Gynecol. (1992) 79:846–9.
58. Hui P. Gestational trophoblastic tumors: a timely review of diagnostic pathology. Arch Pathol Lab Med. (2019) 143:65–74. doi: 10.5858/arpa.2018-0234-RA
59. Zhao J, Xiang Y. Diagnosis and treatment of placental trophoblastic tumor. J Pract Oncol. (2008) 23:5–7. doi: 10.13267/j.cnki.syzlzz.2008.01.003
60. Luiza JW, Taylor SE, Gao FF, Edwards RP. Placental site trophoblastic tumor: immunohistochemistry algorithm key to diagnosis and review of literature. Gynecol Oncol Case Rep. (2014) 7:13–5. doi: 10.1016/j.gynor.2013.11.001
61. Kar A, Mishra C, Biswal P, Kar T, Panda S, Naik S. Differential expression of cyclin E, p63, and Ki-67 in gestational trophoblastic disease and its role in diagnosis and management: A prospective case-control study. Indian J Pathol Microbiol. (2019) 62: 54–60. doi: 10.4103/IJPM.IJPM_82_18
62. McCarthy WA, Paquette C, Gundogan F, Lawrence WD. Comparison of p63 and p40 immunohistochemical stains to distinguish epithelioid trophoblastic tumor from other trophoblastic lesions. Int J Gynecol Pathol. (2018) 37:401–4. doi: 10.1097/PGP.0000000000000420
63. Rhoton-Vlasak A, Wagner JM, Rutgers JL, Baergen RN, Young RH, Roche PC, et al. Placental site trophoblastic tumor: human placental lactogen and pregnancy-associated major basic protein as immunohistologic markers. Hum Pathol. (1998) 29:280–8. doi: 10.1016/S0046-8177(98)90048-X
64. He YM, Jiang W, Li L. Clinical and pathological analysis of 13 cases of placental trophoblastic tumor. J Sichuan Univ (Med Sci Edi). (2017) 48:647–649. doi: 10.13464/j.scuxbyxb.2017.04.033
65. Jiang N, Wang HY, Sui YX, Deng Y, Hou HL. The clinicopathologic analysis of placental site trophoblastic tumor. Mod Oncol. (2016) 24:2778–81. doi: 10.3969/j.issn.1672-4992.2016.17.031
66. Müller-Höcker J, Obernitz N, Johannes A, Löhrs U. P53 gene product and EGF-receptor are highly expressed in placental site trophoblastic tumor. Hum Pathol. (1997) 28:1302–6. doi: 10.1016/S0046-8177(97)90206-9
67. Xin LH, Hui J. Expression and significance of ykl-40, HCG and HPL in choriocarcinoma and placental trophoblastic tumors. J Clin Exp Pathol. (2019) 35:320–2. doi: 10.13315/j.cnki.cjcep.2019.03.017
68. Ou-Yang RJ, Hui P, Yang XJ, Zynger DL. Expression of glypican 3 in placental site trophoblastic tumor. Diagn Pathol. (2010) 5:64. doi: 10.1186/1746-1596-5-64
69. Nagai Y, Kamoi S, Matsuoka T, Hata A, Jobo T, Ogasawara T, et al. Impact of p53 immunostaining in predicting advanced or recurrent placental site trophoblastic tumors: a study of 12 cases. Gynecol Oncol. (2007) 106:446–52. doi: 10.1016/j.ygyno.2007.04.025
70. Stichelbout M1, Devisme L, Franquet-Ansart H, Massardier J, Vinatier D, Renaud F, et al. SALL4 expression in gestational trophoblastic tumors: a useful tool to distinguish choriocarcinoma from placental site trophoblastic tumor and epithelioid trophoblastic tumor. Hum Pathol. (2016) 54:121–6. doi: 10.1016/j.humpath.2016.03.012
71. Singer G, Kurman RJ, McMaster MT, Shih IeM. HLA-G immunoreactivity is specific for intermediate trophoblast in gestational trophoblastic disease and can serve as a useful marker in differential diagnosis. Am J Surg Pathol. (2002) 26:914–20. doi: 10.1097/00000478-200207000-00010
72. Openshaw MR, Harvey RA, Sebire NJ, Kaur B, Sarwar N, Seckl MJ, et al. Circulating cell free DNA in the diagnosis of trophoblastic tumors. EBioMed. (2015) 4:146–52. doi: 10.1016/j.ebiom.2015.12.022
73. Lavoie JM, Alcaide M, Fisher RA, Seckl MJ, Morin R, Tinker AV. Targeted error-suppressed detection of circulating paternal dna to establish a diagnosis of gestational trophoblastic neoplasm. JCO Precision Oncol. (2017) 1–6. doi: 10.1200/PO.17.00154
74. Fisher RA, Savage PM, MacDermott C, Hook J, Sebire NJ, Lindsay I, et al. The impact of molecular genetic diagnosis on the management of women with hCG-producing malignancies. Gynecol Oncol. (2007) 107:413–9. doi: 10.1016/j.ygyno.2007.07.081
75. Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease–latest advances and implications for cure. Nat Rev Clin Oncol. (2019) 16:409–24. doi: 10.1038/s41571-019-0187-3
76. Zhou Y, Lu H, Yu C, Tian Q, Lu W. Sonographic characteristics of placental site trophoblastic tumor. Ultrasound Obstet Gynecol. (2013) 41:679–84. doi: 10.1002/uog.12269
77. Shaaban AM, Rezvani M, Haroun RR, Kennedy AM, Elsayes KM, Olpin JD, et al. Gestational trophoblastic disease: clinical and imaging features. Radiographics. (2017) 37:681–700. doi: 10.1148/rg.2017160140
78. Zwischenberger BA, Boren T. Placental site trophoblastic tumor presenting as a friable cervical mass. Eur J Gynaecol Oncol. (2010) 31:570–2.
79. Tang X, Yang F, Jia L, Yao XY, Yang KX. Placental site trophoblastic tumor in the pelvic wall: a case report and review of the literature. Indian J Pathol Microbiol. (2013) 56:300–2. doi: 10.4103/0377-4929.120405
80. Gupta M, Gnanasekaran KK, Manojkumar R, Thomas A, Sebastian A. Extrauterine placental site trophoblastic tumor involving the vagina. Int J Gynecol Pathol. (2017) 36:294–9. doi: 10.1097/PGP.0000000000000318
81. Niknejadi M, Ahmadi F, Akhbari F. Imaging and clinical data of placental site trophoblastic tumor: a case report. Iran J Radiol. (2016) 13:e18480. doi: 10.5812/iranjradiol.18480
82. Zeng X, Liu X, Tian Q, Xue Y, An R. Placental site trophoblastic tumor: a case report and literature review. Intractable Rare Dis Res. (2015) 4:147–51. doi: 10.5582/irdr.2015.01013
83. Qin J, Ying W, Cheng X, Wu X, Lu B, Liang Y, et al. A well-circumscribed border with peripheral Doppler signal in sonographic image distinguishes epithelioid trophoblastic tumor from other gestational trophoblastic neoplasms. PLoS ONE. (2014) 9:e112618. doi: 10.1371/journal.pone.0112618
84. Ichikawa Y, Nakauchi T, Sato T, Oki A, Tsunoda H, Yoshikawa H. Ultrasound diagnosis of uterine arteriovenous fistula associated with placental site trophoblastic tumor. Ultrasound Obstet Gynecol. (2003) 21:606–8. doi: 10.1002/uog.145
85. Sita-Lumsden A, Medani H, Fisher R, Harvey R, Short D, Sebire N, et al. Uterine artery pulsatility index improves prediction of methotrexate resistance in women with gestational trophoblastic neoplasia with FIGO score 5-6. BJOG. (2013) 120:1012–5. doi: 10.1111/1471-0528.12196
86. Xu Y, Zhao FG, Zhang H, Sun L, Gong FB, Ren YY. The clinical value of color Doppler ultrasonography in diagnosis of placental site trophoblastic tumor. Fudan Univ J Med Sci. (2016) 43:65–9. doi: 10.3969/j.issn.1672-8467.2016.01.01
87. Brandt KR, Coakley KJ. MR appearance of placental site trophoblastic tumor: a report of three cases. AJR Am J Roentgenol. (1998) 170:485–7. doi: 10.2214/ajr.170.2.9456970
88. Cerci SS, Erdemoglu E, Bozkurt KK, Yalcn Y, Erdemoglu E. Placental-site trophoblastic tumor and fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography. Hell J Nucl Med. (2015) 18: 264–7. doi: 10.1967/s002449910310
89. Mangili G, Bergamini A, Giorgione V, Picchio M, Petrone M, Mapelli P, et al. [181F]fluorodeoxyglucose positron emission tomography/computed tomography and trophoblastic disease: the gynecologist perspective. Q J Nucl Med Mol Imaging. (2016) 60:103–16.
90. Shaw SW, Wang CW, Ma SY, Ng KK, Chang TC. Exclusion of lung metastases inplacental site trophoblastic tumor using [18F]fluorodeoxyglucose positron emission tomography: a case report. Gynecol Oncol. (2005) 99:239–42. doi: 10.1016/j.ygyno.2005.06.037
91. Chang TC, Yen TC, Li YT, Wu YC, Chang YC, Ng KK, et al. The role of 18F-fluorodeoxyglucose positron emission tomography in gestational trophoblastic tumours: a pilot study. Eur J Nucl Med Mol Imaging. (2006) 33:156–63. doi: 10.1007/s00259-005-1873-1
92. Nieves L, Hoffman J, Allen G, Currie J, Sorosky JI. Placental-site trophoblastic tumor with PET scan-detected surgically treated lung metastasis. Int J Clin Oncol. (2008) 13:263–5. doi: 10.1007/s10147-007-0721-7
93. Papadopoulos AJ, Foskett M, Seckl MJ, McNeish I, Paradinas FJ, Rees H, et al. Twenty-five years' clinical experience with placental site trophoblastic tumors. J Reprod Med. (2002) 47:460–4.
94. Pisal N, North C, Tidy J, Hancock B. Role of hysterectomy in management of gestational trophoblastic disease. Gynecol Oncol. (2002) 87:190–2. doi: 10.1016/S0090-8258(02)96814-9
95. Hanna RK, Soper JT. The role of surgery and radiation therapy in the management of gestational trophoblastic disease. Oncologist. (2010) 15:593–600. doi: 10.1634/theoncologist.2010-0065
96. Feltmate CM, Genest DR, Wise L, Bernstein MR, Goldstein DP, Berkowitz RS. Placental site trophoblastic tumor: a 17-year experience at the New England Trophoblastic Disease Center. Gynecol Oncol. (2001) 82: 415–9. doi: 10.1006/gyno.2001.6265
97. Lan C, Li Y, He J, Liu J. Placental site trophoblastic tumor: lymphatic spread and possible target markers. Gynecol Oncol. (2010) 116: 430–7. doi: 10.1016/j.ygyno.2009.10.056
98. Milingos D, Doumplis D, Savage P, Seckl M, Lindsay I, Smith JR. Placental site trophoblastic tumor with an ovarian metastasis. Int J Gynecol Cancer. (2007) 17: 925–7. doi: 10.1111/j.1525-1438.2007.00881.x
99. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Gestatioanl Trophoblastic Neoplasia. Available online at: https://www.nccn.org/professionals/physician_gls/default.aspx#site (accessed July 9, 2019).
100. Hancock B, Froeling F, Ramaswami R, Horsman J, Ellis L, Seckl M, on behalf of the ISSTD PSTT database collaborators. The ISSTD global placental site and epithelioid trophoblastic tumor (PSTT/ETT) database–an analysis of 326 patients. Abstract # 12. In: ISSTD XVIII World Congress on Gestational Trophoblastic Disease. Bali (2015).
101. Tempfer C, Horn LC, Ackermann S, Beckmann MW, Dittrich R, Einenkel J, et al. Gestational and Non-gestational Trophoblastic Disease. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry No. 032/049, December 2015). Geburtshilfe Frauenheilkd. (2016) 76:134–44. doi: 10.1055/s-0041-111788
102. Deng L, Zhang J, Wu T, Lawrie TA. Combination chemotherapy for primary treatment of high-risk gestational trophoblastic tumour. Cochrane Database Syst Rev. (2013) CD005196. doi: 10.1002/14651858.CD005196.pub4
103. Essel KG, Bruegl A, Gershenson DM, Ramondetta LM, Naumann RW, Brown J. Salvage chemotherapy for gestational trophoblastic neoplasia: utility or futility? Gynecol Oncol. (2017) 146:74–80. doi: 10.1016/j.ygyno.2017.04.017
104. Ajithkumar TV, Abraham EK, Rejnishkumar R, Minimole AL. Placental site trophoblastic tumor. Obstet Gynecol Surv. (2003) 58:484–8. doi: 10.1097/01.OGX.0000077466.40895.32
105. El-Helw LM, Seckl MJ, Haynes R, Evans LS, Lorigan PC, Long J, et al. High-dose chemotherapy and peripheral blood stem cell support in refractory gestational trophoblastic neoplasia. Br J Cancer. (2005) 93:620–1. doi: 10.1038/sj.bjc.6602771
106. Froeling FEM, Ramaswami R, Papanastasopoulos P, Kaur B, Sebire NJ, Short D, et al. Intensified therapies improve survival and identification of novel prognostic factors for placental-site and epithelioid trophoblastic tumours. Br J Cancer. (2019) 120:587–94. doi: 10.1038/s41416-019-0402-0
107. Frijstein MM, Lok CAR, Short D, Singh K, Fisher RA, Hancock BW, et al. The results of treatment with high-dose chemotherapy and peripheral blood stem cell support for gestational trophoblastic neoplasia. Euro J Cancer. (2019) 109:162–71. doi: 10.1016/j.ejca.2018.12.033
108. Iwase A, Sugita A, Hirokawa W, Goto M, Yamamoto E, Takikawa S, et al. Anti-mullerian hormone as a marker of ovarian reserve following chemotherapy in patients with gestational trophoblastic neoplasia. Eur J Obstet Gynecol Reprod Biol. (2013) 167:194–8. doi: 10.1016/j.ejogrb.2012.11.021
109. Tsuji Y, Tsubamoto H, Hori M, Ogasawara T, Koyama K. Case of PSTT treated with chemotherapy followed by open uterine tumor resection to preserve fertility. Gynecol Oncol. (2002) 87:303–7. doi: 10.1006/gyno.2002.6827
110. Chiofalo B, Palmara V, Lagana AS, Triolo O, Vitale SG, Conway F, et al. Fertility sparing strategies in patients affected by placental site trophoblastic tumor. Curr Treat Options Oncol. (2017) 18:58. doi: 10.1007/s11864-017-0502-0
111. Saso S, Haddad J, Ellis P, Lindsay I, Sebire NJ, McIndoe A, et al. Placental site trophoblastic tumours and the concept of fertility preservation. BJOG. (2012) 119:369–74; discussion 74. doi: 10.1111/j.1471-0528.2011.03230.x
112. Schmid P, Nagai Y, Agarwal R, Hancock B, Savage PM, Sebire NJ, et al. Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. Lancet. (2009) 374:48–55. doi: 10.1016/S0140-6736(09)60618-8
113. Shen X, Xiang Y, Guo L, Feng F, Wan X, Xiao Y, et al. Fertility-preserving treatment in young patients with placental site trophoblastic tumors. Int J Gynecol Cancer. (2012) 22:869–74. doi: 10.1097/IGC.0b013e31824a1bd6
114. Brown J, Naumann RW, Seckl MJ, Schink J. 15years of progress in gestational trophoblastic disease: scoring, standardization, and salvage. Gynecol Oncol. (2017) 144:200–7. doi: 10.1016/j.ygyno.2016.08.330
115. Shih IeM. Gestational trophoblastic neoplasia–pathogenesis and potential therapeutic targets. Lancet Oncol. (2007) 8:642–50. doi: 10.1016/S1470-2045(07)70204-8
116. Bolat F, Haberal N, Tunali N, Aslan E, Bal N, Tuncer I. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor 1 alpha (HIF-1alpha), and transforming growth factors beta1 (TGFbeta1) and beta3 (TGFbeta3) in gestational trophoblastic disease. Pathol Res Pract. (2010) 206:19–23. doi: 10.1016/j.prp.2009.07.017
117. Singh M, Kindelberger D, Nagymanyoki Z, Ng SW, Quick CM, Yamamoto H, et al. Vascular endothelial growth factors and their receptors and regulators in gestational trophoblastic diseases and normal placenta. J Reprod Med. (2012) 57:197–203.
118. Boufettal H, Feige JJ, Benharouga M, Aboussaouira T, Nadifi S, Mahdaoui S, et al. Potential role of the angiogenic factor “EG-VEGF” in gestational trophoblastic diseases. Pathol Biol (Paris). (2013) 61:178–83. doi: 10.1016/j.patbio.2013.02.001
119. Hoffmann P, Saoudi Y, Benharouga M, Graham CH, Schaal JP, Mazouni C, et al. Role of EG-VEGF in human placentation: physiological and pathological implications. J Cell Mol Med. (2009)13:2224–35. doi: 10.1111/j.1582-4934.2008.00554.x
120. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. (2017) 18:312–22. doi: 10.1016/S1470-2045(17)30065-7
121. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-pd-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. (2015) 33:4015–22. doi: 10.1200/JCO.2015.62.3397
122. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
123. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
124. Ghorani E, Kaur B, Fisher RA, Short D, Joneborg U, Carlson JW, et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. (2017) 390:2343–5. doi: 10.1016/S0140-6736(17)32894-5
125. Lu B, Teng X, Fu G, Bao L, Tang J, Shi H, et al. Analysis of PD-L1 expression in trophoblastic tissues and tumors. Hum Pathol. (2019) 84: 202–12. doi: 10.1016/j.humpath.2018.10.001
126. Veras E, Kurman RJ, Wang TL, Shih IM. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol. (2017) 36:146–53. doi: 10.1097/PGP.0000000000000305
127. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. (2018) 15:325–40. doi: 10.1038/nrclinonc.2018.29
128. Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. (2013) 19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214
129. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. (2019). doi: 10.1001/jamaoncol.2019.2187. [Epub ahead of print].
130. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19:1480–92. doi: 10.1016/S1470-2045(18)30700-9
131. Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 Trial. JAMA Oncol. (2018) 5:187–94. doi: 10.1001/jamaoncol.2018.4514
132. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
133. Meggyes M, Miko E, Szigeti B, Farkas N, Szereday L. The importance of the PD-1/PD-L1 pathway at the maternal-fetal interface. BMC Pregnancy Childbirth. (2019) 19:74. doi: 10.1186/s12884-019-2218-6
134. Poulet FM, Wolf JJ, Herzyk DJ, DeGeorge JJ. An evaluation of the impact of PD-1 pathway blockade on reproductive safety of therapeutic PD-1 inhibitors. Birth Defects Res B Dev Reprod Toxicol. (2016) 107:108–19. doi: 10.1002/bdrb.21176
135. Chang YL, Chang TC, Hsueh S, Huang KG, Wang PN, Liu HP, et al. Prognostic factors and treatment for placental site trophoblastic tumor-report of 3 cases and analysis of 88 cases. Gynecol Oncol. (1999) 73:216–22. doi: 10.1006/gyno.1999.5344
136. Lee HJ, Shin W, Jang YJ, Choi CH, Lee JW, Bae DS, et al. Clinical characteristics and outcomes of placental site trophoblastic tumor: experience of single institution in Korea. Obstet Gynecol Sci. (2018) 61:319–27. doi: 10.5468/ogs.2018.61.3.319
137. Nie JC, Chen GH, Yan AQ, Liu XS. Postoperative chemotherapy on placental site trophoblastic tumor in early stage: analysis of 60 cases. Eur J Gynaecol Oncol. (2017) 38:431–440.
138. Zheng Y, Bao L, Ning Y, Lu X, Hua K, Yi X. Retrospective analysis of the clinicopathologic and prognostic characteristics of stage I placental site trophoblastic tumor in China. Int J Gynaecol Obstet. (2015) 129:67–70. doi: 10.1016/j.ijgo.2014.10.027
Keywords: placenta site trophoblastic tumor, clinical presentation, mechanism, treatment, prognosis
Citation: Feng X, Wei Z, Zhang S, Du Y and Zhao H (2019) A Review on the Pathogenesis and Clinical Management of Placental Site Trophoblastic Tumors. Front. Oncol. 9:937. doi: 10.3389/fonc.2019.00937
Received: 23 May 2019; Accepted: 06 September 2019;
Published: 28 November 2019.
Edited by:
Rebecca Stone, Johns Hopkins Medicine, United StatesReviewed by:
Marilyn Huang, University of Miami Health System, United StatesCopyright © 2019 Feng, Wei, Zhang, Du and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Du, c29waGllZHVfNjFAMTYzLmNvbQ==; Hongbo Zhao, emhhb2hvbmdibzAxQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.