- 1Department of Radiotherapy-Oncology, Ghent University Hospital, Ghent, Belgium
- 2Department of Medical Oncology, Ghent University Hospital, Ghent, Belgium
- 3Department of Thoracic Oncology, Ghent University Hospital, Ghent, Belgium
Background: The majority of lung cancer patients are diagnosed with advanced non-small cell lung cancer (NSCLC), the bulk of which receive palliative systemic treatment with the goal to provide effective symptom palliation and safeguard health-related quality of life (HRQoL). Advanced NSCLC trials with HRQoL endpoints face methodological constraints limiting interpretability.
Objectives: We provide a comprehensive overview of recent clinical trials evaluating the impact of systemic therapies on HRQoL in advanced NSCLC, focusing on the methodological quality, with the ultimate goal to improve interpretation, comparison and reporting of HRQoL data.
Methods: A systematic literature review was performed. Prospective studies published over the last decade evaluating the impact of systemic treatments on HRQoL in advanced NSCLC were included. Methodological quality of HRQoL reporting was assessed with the CONSORT-PRO extension.
Results: Hundred-twelve manuscripts describing 85 trials met all criteria. No formal conclusion can be drawn regarding the impact on HRQoL of different treatments. We report an important variety in methodological quality in terms of definitions of HRQoL, missing data points, lack of standardization of analyzing and presenting HRQoL and no standard follow-up time. The quality of HRQoL data reporting varies substantially between studies but improves over time.
Conclusion: This review shows that in the heterogeneous landscape of trials addressing HRQoL in advanced stage NSCLC. Methodology reporting remains generally poor. Adequate reporting of HRQoL outcome data is equally important to support clinical decision-making as to correctly inform health policy regarding direct approval and reimbursement of the new drugs and combinations that will come online.
Introduction
Lung cancer contributes to 1.6 million deaths a year, making it the deadliest cancer worldwide (1). Approximately 80% of primary lung cancers are non-small cell lung cancer (NSCLC) (2). Locally advanced (stage IIIB) or metastatic (stage IV) NSCLC is diagnosed in most patients (65%), leading, despite the development of novel systemic therapies, to poor 5-years survival rates of 5 and 1%, respectively. Palliative therapy aims to prolong survival and to offer acceptable quality of life (QoL). The latter is especially important because besides poor survival, patients with advanced NSCLC also frequently suffer from high symptom burden and toxic therapeutic side effects (3).
Health-related quality of life (HRQoL) has become an integral endpoint in clinical trials for advanced cancer (4, 5). HRQoL is a multi-dimensional concept that addresses the functional effect of a health status and/or a patient's treatment. It embodies physical, role, emotional, social, cognitive, sexual and spiritual functioning on individual levels (6–8). HRQoL data enables treatment comparisons, supports daily clinical treatment decision-making, improves communication between patients and treating clinicians and facilitates clinical and economic evaluations to define the most efficient allocation of healthcare resources (3). Patient-reported HRQoL data particularly aids clinicians to better understand toxicity and symptoms experienced by patients, as subjective symptoms, such as fatigue and pain are frequently under-reported (9). Finally, baseline HRQoL is an independent predictive value for therapy response and survival (5, 10–13), performing better than certain classic endpoints, such as performance status (5). In order to guide treatment decision making and health policy, qualitative reporting and analysis of HRQoL outcomes are essential. Methodological flaws might lead to inaccurate interpretation of outcomes and hinder implementation in clinical practice. Correspondingly, poor data quality and methodological concerns have limited the influence of HRQoL data on the Food and Drug Administrations' regulatory decision making process (3). Methodological constraints in HRQoL data reporting and analysis have been previously highlighted (5).
A number of systematic literature reviews have already been conducted on clinical trials using HRQoL endpoints in patients with advanced NSCLC, all studying a specific question (Table 1). This systematic literature review aims to add to the available evidence by providing a comprehensive overview of all prospective studies published over the last decade, evaluating the impact of various systemic treatments on HRQoL in advanced NSCLC. In addition, the methodological quality of this set of papers is analyzed with the ultimate goal to discuss challenges in and recommendations for the interpretation and comparison of HRQoL evidence obtained from randomized-controlled trials (RCTs).
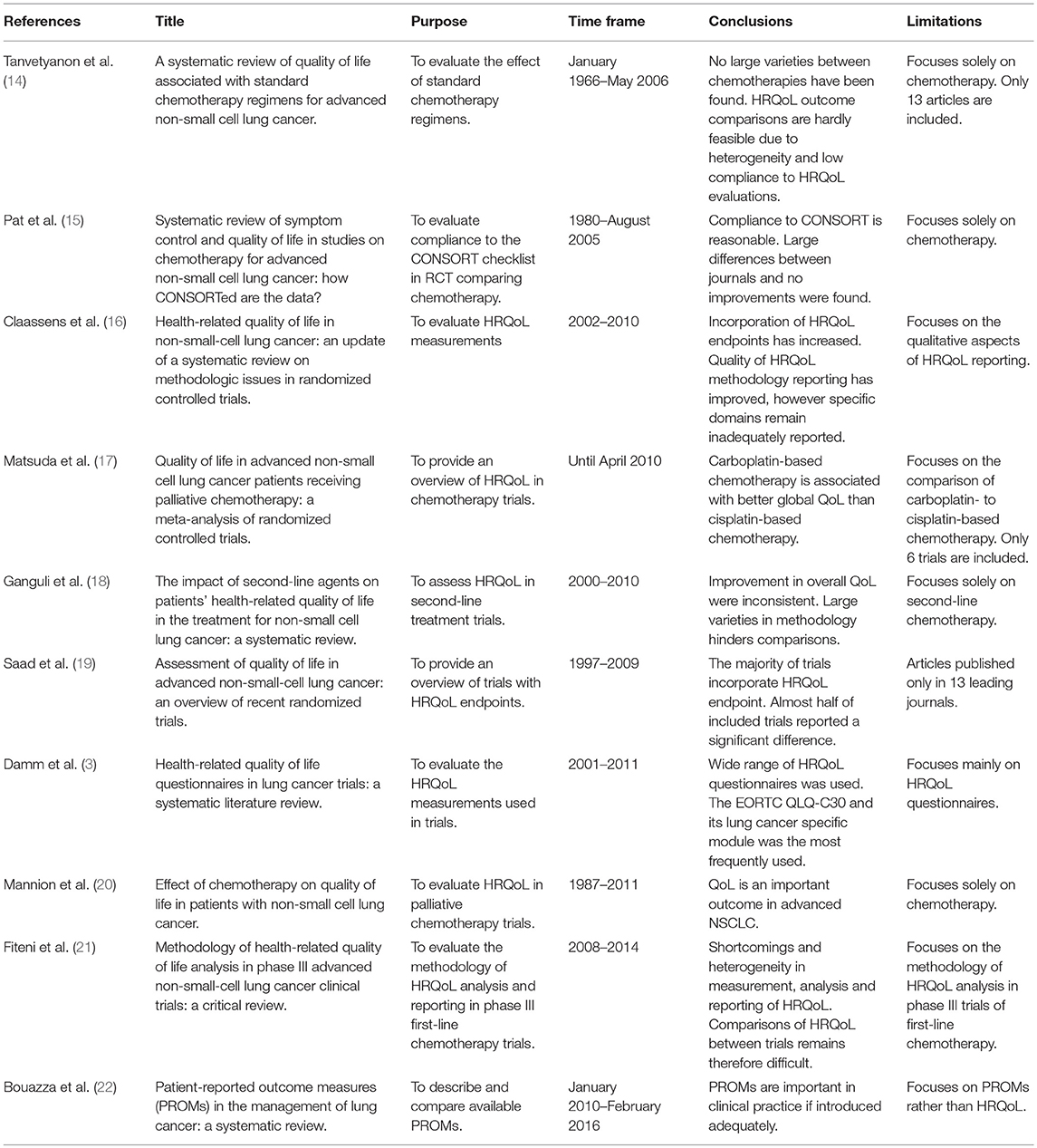
Table 1. Overview of systematic literature reviews of HRQoL in advanced NSCLC trials published since 2007 in chronological order.
Materials and Methods
Literature Search
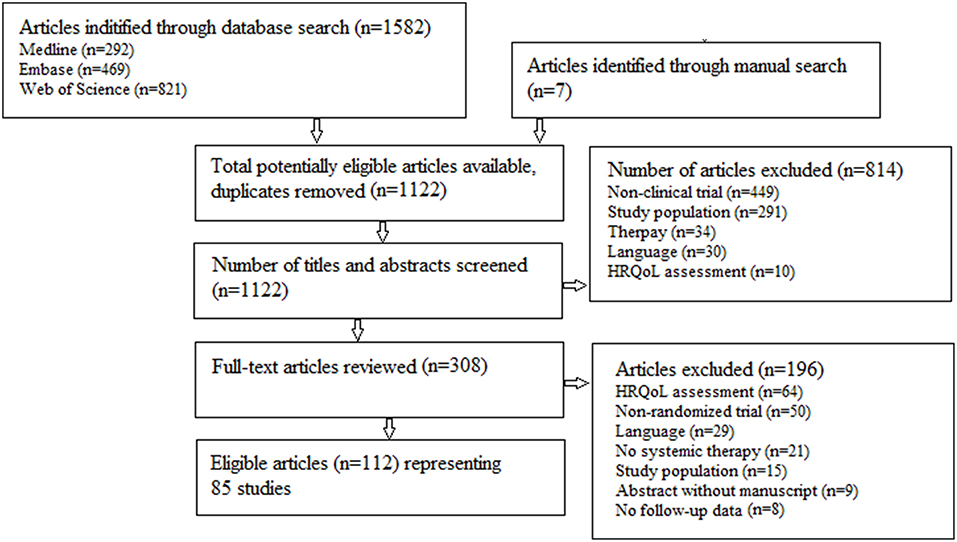
A literature search, following the PRISMA principles (23), was performed in Medline, Web of Science and Embase with both systematic and free text terms concerning HRQoL, advanced and NSCLC (Figure 1). The last search was performed May 29, 2018.
Study Selection
The search focuses on articles published between January 2007 and December 2017, including those electronically available within this period. Non-randomized trials, pilot and retrospective studies, abstracts without manuscript and non-English articles were excluded. Articles were eligible if the study population included patients with stage IIIB and/or IV NSCLC receiving palliative systemic therapy and measuring HRQoL for more than one time points. Secondary articles, covering the same study population as the primary article, were included and combined into the data synthesis, provided they added information concerning HRQoL.
Data Extraction
Study selection was two-staged. Firstly, title and abstract screening against selection criteria was undertaken by a single reviewer (LVDW). The other authors were consulted for disagreement resolution. Secondly, full-text articles were extracted based on the eligibility criteria to select the final sample of studies.
Methodological Quality
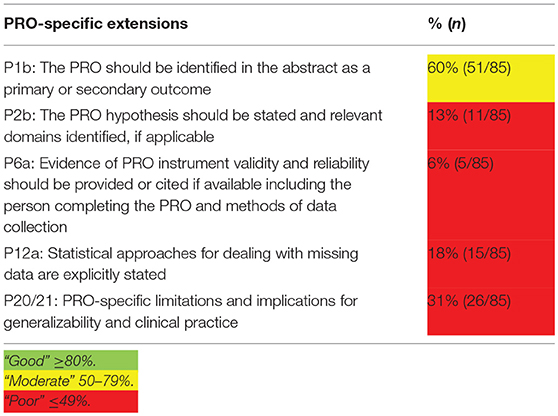
The quality of HRQoL data reporting of the included studies was assessed with the CON-solidated Standards of Reporting Trials (CONSORT)—Patient-Reported Outcomes (PRO) checklist (24). The five PRO-specific minimum recommendations for reporting randomized controlled trials were used to assess quality of reporting.
Cut-off scores for reporting quality are: “good” ≥80%; “moderate” 50–79%; and “poor” ≤ 49% (24). In case items were not applicable, such as allocation concealment mechanism, they were excluded in the total outcome. In case secondary manuscripts were published on HRQoL outcomes, both the primary and secondary manuscript were included in the quality assessment.
Results
Eleven hundred and twenty-two abstracts were identified for initial review. A total of 308 articles underwent in-depth analysis. Of these, 112 articles representing 85 trials were included in this review. The review process is summarized in Figure 1.
Study Characteristics
In brief, 34,897 patients were enrolled in 85 RCTs, ranging from 37 to 1,433 patients per study. Most of the identified literature presented phase III (n = 59; 69%) RCTs. Fifty-nine studies (69%) analyzed first-line therapy. The most frequently used primary (co-) endpoint was survival (n = 34; 40%), followed by progression free survival (PFS) (n = 32; 38%), response rate (n = 9; 11%) and QoL (n = 6; 7%).
In total, 41 studies compared different chemotherapies, 4 studies compared different targeted therapies. Twenty studies compared chemotherapy with targeted therapy; 1 with immunotherapy. Respectively, 14 and 1 studies compared targeted and chemotherapy to placebo. Four studies compared different sequential therapies consisting of both targeted therapy and chemotherapy. A brief synopsis of the main study characteristics is presented in the Supplementary Material.
Figure 2A provides an overview of studies incorporating different therapies per year, illustrating that the interest for the HRQoL impact of systemic treatments progressively shifted from chemotherapy to chemotherapy vs. targeted therapy and targeted therapy vs. placebo.
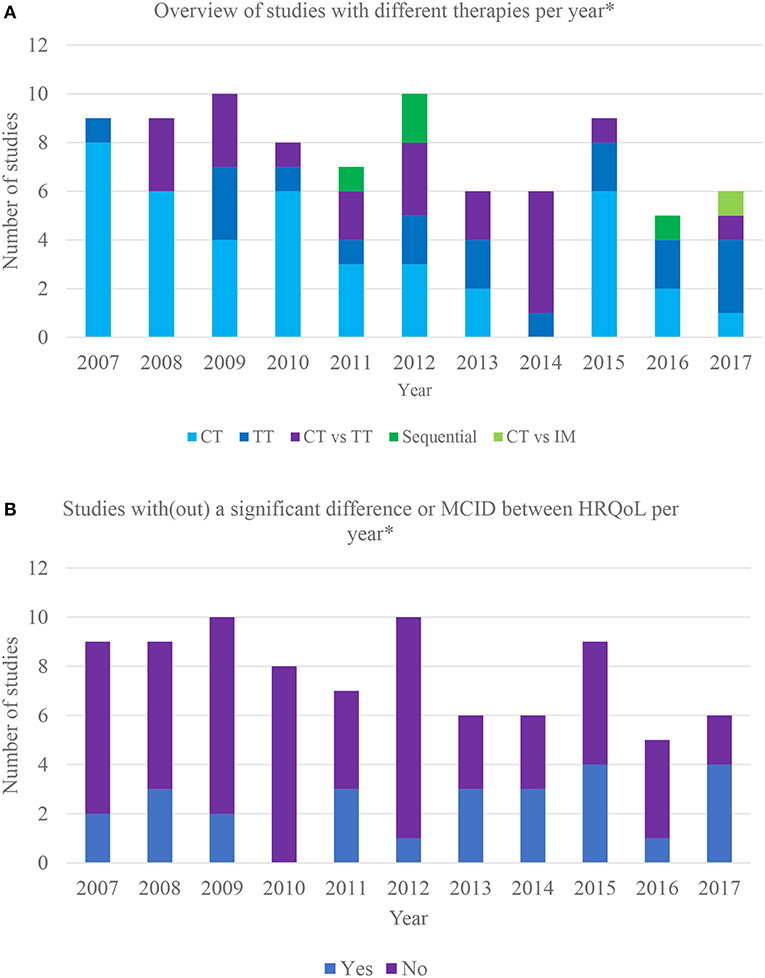
Figure 2. (A) Overview of different therapies incorporated in clinical trials over the last 10 years. *Based on the year of HRQoL publication; CT, chemotherapy vs. chemotherapy or placebo; TT, targeted therapy vs. targeted therapy or placebo; CT vs. TT, chemotherapy vs. targeted therapy; sequential: sequential therapy consisting of both targeted and chemotherapy; CT vs. IM, chemotherapy vs. immunotherapy. (B) Overview of clinical trials with/without significant differences in HRQoL between therapy arms over the last 10 years. *Based on the year of HRQoL publication; MCID, meaningful clinically important difference.
Impact of Systemic Treatments on HRQoL
Twenty-six (31%) studies found a statistically or minimal clinically important difference (MCID) in HRQoL between therapy arms. Furthermore, some reported a difference in certain domains or symptoms. MCID refers to smallest change in an outcome that is important to the patient (25). Respectively, 22% (n = 9) (26–36) and 50% (n = 2) (37–39) of the trials assessing the impact on HRQoL of various chemotherapeutic combinations and different targeted therapies reported a difference. The only trial (40, 41) comparing chemotherapy to placebo reported no difference, only 2 out of 14 studies showed that targeted therapy causes a positive impact on HRQoL compared to placebo (42–44). Eleven studies (55%) comparing chemo- with targeted therapy reported a difference (45–60), with targeted therapy favored in nine. One study reported that gefitinib is favored in patients with EGFR mutations and carboplatin/paclitaxel in those without mutations (61, 62). The only study comparing chemotherapy to immunotherapy favored the latter (63, 64). One out of 4 sequential therapy RCTs reported a difference between arms (65, 66). The results are summarized in Figure 2B.
Quality of HRQoL Data
Eighteen different HRQoL measurement tools were used, including generic, cancer-, lung cancer-, and symptom specific tools. The cancer-specific European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) alone or together with its lung cancer-specific EORTC QLQ-LC13 supplementary module was the dominantly used HRQoL instrument (n = 38; 45%). The Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire was employed 27 (32%) times and the lung cancer symptom scale (LCSS) tool was used in 11 (13%) trials.
HRQoL data analysis is based on a large variety of statistical techniques, ranging from Mann–Whitney U test (n = 11; 13%), logistic regression model (n = 8; 9%) to t-test (n = 6; 7%). Twenty-three studies lacked clarification on statistical methods applied.
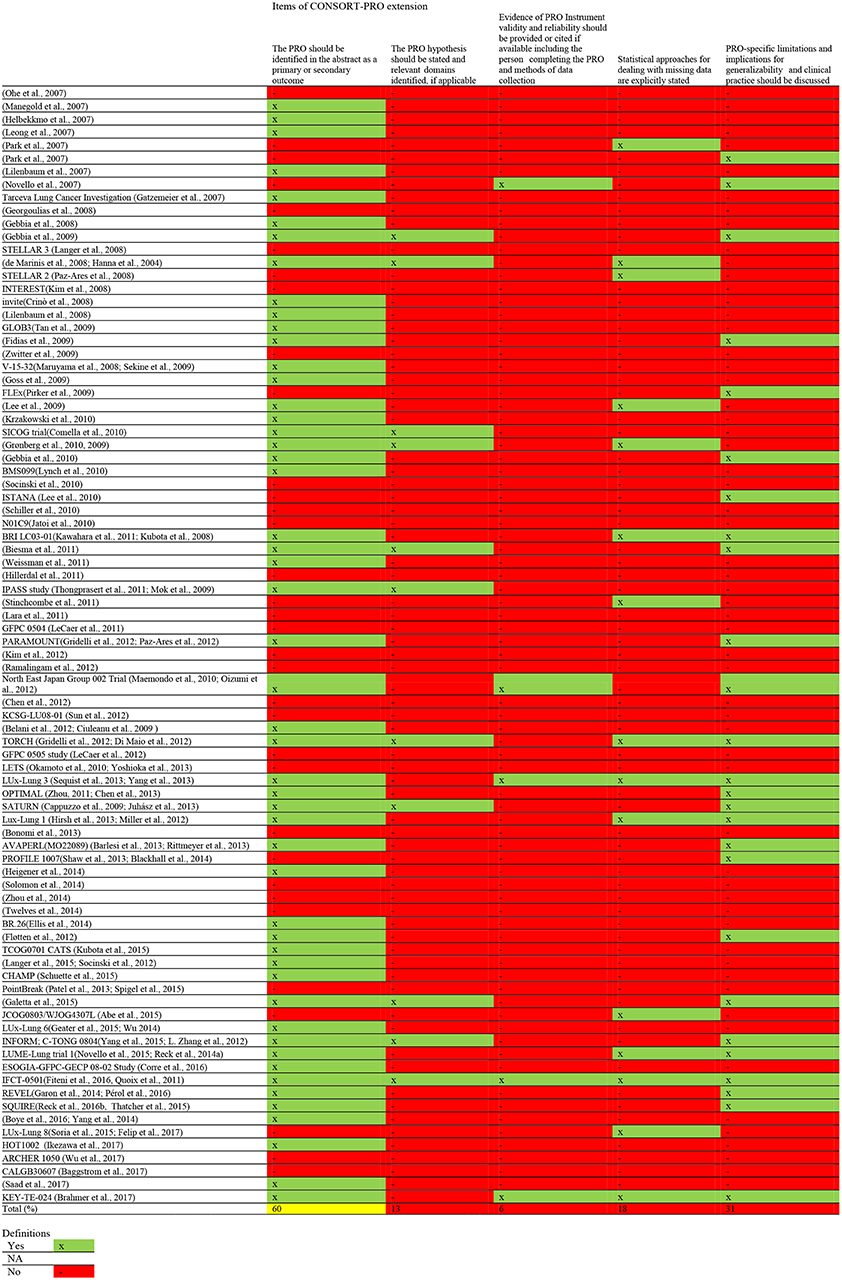
The quality of HRQoL reporting is summarized in Table 2. Figure 3 provides a comprehensive overview of the quality aspects concerning PROs of each individual study. All PRO items were scored poorly, except for identification PRO as a primary or secondary outcome in the abstract which was scored moderate. Only one study fulfilled all criteria (67, 68).
Discussion
A number of systematic literature reviews on HRQoL in advanced NSCLC trials have already been conducted (Table 1), all focusing on specific treatments or aspects. This review addresses the entire spectrum of nowadays' systemic treatment strategies, newer drugs as well as chemotherapy, in trials published over the last 10 years. In addition, in contrast to the other reviews, it provides both a synopsis of study characteristics and methodological quality of HRQoL reporting.
Maintenance and improvement of HRQoL in advanced NSCLC as a result of treatment is important due to the limited impact which therapies have on prolonging life. A qualitative study on chemotherapy preferences reported that advanced NSCLC patients favor chemotherapy over best supportive care in case HRQoL improves with chemotherapy, even without improving survival (69). Accordingly, the Food and Drug Administration started approving new drugs, such as erlotinib, based on the combination of longer PFS with positive impact on HRQoL or other PROs, without significantly improving overall survival. To-date however, the patient perspective is not yet fully embedded in drug approval, and when PROs are used, they are typically limited to improvement in symptoms rather than HRQoL (70). It has however been shown that PROs are more strongly associated with measures of daily health status than clinician assessment (71). High quality patient-reported HRQoL data may therefore provide meaningful information for risk-benefit evaluation and should be used to support drug approval and reimbursement policy. In this review, 85 studies on advanced NSCLC with HRQoL end-points were identified, enrolling almost 35,000 patients. Despite these large numbers, the vast heterogeneity of agents used does not allow one to draw overall conclusions, nor on the actual HRQoL gains, nor on the most effective drugs to improve HRQoL. Based on the study results, targeted therapies seem to be favored over chemotherapy in terms of HRQoL, but interpretation on a case by case basis remains necessary. One review concluded that carboplatin-based chemotherapy is associated with better global QoL compared to cisplatin-based regimes; no other conclusions were drawn in the reviews (17).
Although, only one immunotherapy RCT has been included in this review, immunotherapy is an emerging and continuously evolving field within thoracic oncology. The first data on HRQoL from immunotherapy trials is now appearing. These initial results show that HRQoL is maintained and improved more significantly with immunotherapy compared to chemotherapy (72–75). More data on HRQoL in immunotherapy is expected in the near future.
In addition to the actual outcome data supporting clinical decision-making, consistency in collecting, analyzing and reporting HRQoL data is important to guide health policy. Especially in an era where new, expensive and potentially toxic drug combinations are frequent. HRQoL data may have an impact on reimbursement policies. Previous studies have highlighted the multidimensional nature of HRQoL, diversity in research questions, lack of a priori hypothesis, repeated measures and high likelihood of missing data which hampers drawing meaningful conclusions (76). Standardization—in HRQoL end-points included in trials, in analysis and reporting of HRQoL data—is, therefore, needed. Paucity of standardization limits the interpretability and comparability of HRQoL data between therapies and has implications on decision-making (3). The Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data (SISAQOL) consortium has been established to develop recommendations on analysis and interpretation of PROs in oncology trials (76). When designing an oncology trial with HRQoL endpoints and interpreting HRQoL data, in the future it will be important to consult the recommendations of the SISAQOL. Furthermore, HRQoL results should be presented according to the CONSORT-PRO checklist, aiming to facilitate optimal reporting of PRO data in RCTs (24). Several HRQoL data capturing tools are used, including generic, condition- and symptom-specific questionnaires. Outcome data derived from these questionnaires differ substantially in terms of HRQoL parameters and lung cancer-associated symptoms (3). Montazeri et al. recommends using the EORTC QLQ-C30 questionnaire and its lung cancer-specific module (QLQ-LC13) in lung cancer trials (77, 78). However, since the introduction of the QLQ-LC13 in 1994, major developments have occurred in the diagnosis and treatment of lung cancer. The EORTC has recognized this challenge and has therefore updated this questionnaire (QLQ-LC29), encompassing the impact of new and innovative treatment strategies on HRQoL (79).
The EORTC QLQ C30 is used in almost half of the analyzed studies. Yet inter-comparison among studies remains difficult as some selectively report certain domains or symptoms of HRQoL, for example an improvement in cough, pain, dyspnea and physical functioning using erlotinib, without mentioning the remaining HRQoL parameters (80). Other studies solely report significant differences between treatment arms, but lack reporting on whether HRQoL is maintained, improved or whether it deteriorates (81–83). Other studies report percentile symptom improvements or the mean HRQoL deterioration time (55), moreover, the majority of RCTs stop HRQoL measurement some months after end of therapy or solely rely on HRQoL evaluations during and at the end of treatment, not capturing long-term effects. Although toxicities associated with targeted and chemotherapy regimens, influencing HRQoL, may be either reversible or continue to prevail during most of the patient's life, recommendations on duration of the follow-up period are still inexistent (84).
From a statistical point of view, an even larger variation exists. If longitudinal HRQoL data is available, standardized approaches to analyze and define MCID scores are necessary. MCID should be applied, as statistically significant improvement or worsening of HRQoL scores may be too small to be clinically relevant to the patient (85). As mentioned, the lack of standardization hinders drawing conclusions on the clinical meaningfulness of HRQoL data, hence, assisting treatment decisions (3). As a result of these issues, the SISAQOL consortium was established in 2016 (76). Missing multiple data points also remains problematic and seems inevitable in longitudinal HRQoL datasets in the advanced NSCLC population (5). Patients with poor health at baseline typically have poor baseline HRQoL, may have worse disease progression and have a HRQoL that deteriorates faster. These patients generally drop out earlier than patients with better baseline HRQoL. This potentially leads to bias, affects trial results and conclusions, and eventually clinical practice. (68, 86). Accordingly, missing data in repeated measurements over time combined with the multidimensionality of HRQoL data requires statistical analysis techniques capable of dealing with these issues (69).
Qualitative and complete reporting of HRQoL results in scientific articles is crucial to allow applying HRQoL evidence from clinical trials into daily clinical decision-making and treatment policies. Our data demonstrates persisting inadequate presentation of specific domains and overall poor reporting quality, not dissimilar from the observations made in previous reviews (16). The limited methodological quality and lack of certain crucial aspects hinders comparing and interpreting HRQoL data, necessary to support optimal decision-making, problematic.
Apart from the quality issues described, the very nature of clinical trials per se may hamper translating evidence into practice. Strict inclusion criteria concerning age and performance status limit the overall generalization of HRQoL data. Of the 85 analyzed studies, only 12 focused particularly on elderly patients, whereas they represent the majority of the lung cancer patient population (35, 46, 47, 49, 67, 68, 87–93). Hence, collecting HRQoL real-life data has added value to determine the best standard of care and to define the most efficient healthcare resource allocation involving patients with advanced NSCLC (20).
Finally, although this review aimed to provide a comprehensive review of the recent peer-reviewed literature, it may have failed to capture potentially important evidence, as only full-text publications were included and studies not published in English excluded. The exclusion of gray literature has nonetheless been applied to guarantee the validity of the included articles in terms of methodological quality (94). Another factor to be considered is that the publication period was restricted to 10 years, in order to capture evidence on the drugs most relevant to date.
Conclusion
Our systematic review provides a comprehensive overview of recent RCTs evaluating the impact of systemic therapies on HRQoL in advanced NSCLC. It focuses on the methodological quality of these papers.
A vast variety in HRQoL measurements, data collection time points and reporting and analyzing of HRQoL data makes comparisons of outcomes hardly feasible. Quality of reporting HRQoL outcomes remains poor with certain aspects being systematically underreported.
Nevertheless, adequate and complete reporting is critical to inform health policy and clinical decision-making to sustain and improve HRQoL in this critical patient population. This is particularly important since new, expensive and potentially toxic therapies, often in combination, are being introduced.
Future clinical trials exploring novel therapies for advanced NSCLC should focus on reporting HRQoL data in a clinically meaningful and methodologically qualitative way. Additionally, further research should focus on developing standards to optimize and on defining MCID scores.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
Funding Agency for Innovation by Science and Technology (IWT), Brussels, Belgium. Contract number: IWT TBM 130262.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00715/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: Recent developments. Lancet. (2013) 382:709–19. doi: 10.1016/S0140-6736(13)61502-0
3. Damm K, Roeske N, Jacob C. Health-related quality of life questionnaires in lung cancer trials: a systematic literature review. Health Econ Rev. (2013) 3:15. doi: 10.1186/2191-1991-3-15
4. Goodwin PJ, Black JT, Bordeleau LJ, Ganz PA. Health-related quality-of-life measurement in randomized clinical trials in breast cancer–taking stock. J Natl Cancer Inst. (2003) 95:263–81. doi: 10.1093/jnci/95.4.263
5. Osoba D. Health-related quality of life and cancer clinical trials. Ther Adv Med Oncol. (2011) 3:57–71. doi: 10.1177/1758834010395342
6. McKenna SP. Measuring patient-reported outcomes: moving beyond misplaced common sense to hard science. BMC Med. (2011) 9:86. doi: 10.1186/1741-7015-9-86
7. Siddiqui F, Kohl R, Swann S, Watkins-Bruner D, Movsas B. Gender differences in pretreatment quality of life in a prospective lung cancer trial. J Support Oncol. (2008) 6:33–9.
8. van der Weijst L, Surmont V, Schrauwen W, Lievens Y. Systematic literature review of health-related quality of life in locally-advanced non-small cell lung cancer: has it yet become state-of-the-art? Crit Rev Oncol Hematol. (2017) 119:40–9. doi: 10.1016/j.critrevonc.2017.10.006
9. Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, et al. Patient versus clinician symptom reporting using the National Cancer Institute common terminology criteria for adverse events: results of a questionnaire-based study. Lancet Oncol. (2006) 7:903–9. doi: 10.1016/S1470-2045(06)70910-X
10. Efficace F, Osoba D, Gotay C, Sprangers M, Coens C, Bottomley A. Has the quality of health-related quality of life reporting in cancer clinical trials improved over time? Towards bridging the gap with clinical decision making. Ann Oncol. (2007) 18:775–81. doi: 10.1093/annonc/mdl494
11. Lemonnier I, Guillemin F, Arveux P, Clément-Duchêne C, Velten M, Woronoff-Lemsi MC, et al. Quality of life after the initial treatments of non-small cell lung cancer: a persistent predictor for patients' survival. Health Qual Life Outcomes. (2014) 12:73. doi: 10.1186/1477-7525-12-73
12. Movsas B, Moughan J, Sarna L, Langer C, Werner-Wasik M, Nicolaou N, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol. (2009) 27:5816–22. doi: 10.1200/JCO.2009.23.7420
13. Langendijk H, Aaronson NK, de Jong JMA, ten Velde GPM, Muller MJ, Wouters M. The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol. (2000) 55:19–25. doi: 10.1016/S0167-8140(00)00158-4
14. Fiteni F, Anota A, Westeel V, Bonnetain F. Methodology of health-related quality of life analysis in phase III advanced non-small-cell lung cancer clinical trials: a critical review. BMC Cancer. (2016) 16:122. doi: 10.1186/s12885-016-2152-1
15. Bouazza Y Ben, Chiairi I, El Kharbouchi O, De Backer L, Vanhoutte G, Janssens A, et al. Patient-reported outcome measures (PROMs) in the management of lung cancer: a systematic review. Lung Cancer. (2017) 113:140–51. doi: 10.1016/j.lungcan.2017.09.011
16. Corre R, Greillier L, Le Caër H, Audigier-Valette C, Baize N, Bérard H, et al. Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non–small-cell lung cancer: the phase III randomized ESOGIA-GFPC-GECP 08-02 Study. J Clin Oncol. (2016) 34:1476–83. doi: 10.1200/JCO.2015.63.5839
17. Matsuda A, Yamaoka K, Tango T. Quality of life in advanced non-small cell lung cancer patients receiving palliative chemotherapy: a meta-analysis of randomized controlled trials. Exp Ther Med. (2012) 3:134–40. doi: 10.3892/etm.2011.368
18. Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. (2007) 18:317–23. doi: 10.1093/annonc/mdl377
19. Park SH, Choi SJ, Kyung SY, An CH, Lee SP, Park JW, et al. Randomized phase II trial of two different schedules of docetaxel plus cisplatin as first-line therapy in advanced nonsmall cell lung cancer. Cancer. (2007) 109:732–40. doi: 10.1002/cncr.22446
20. Ganguli A, Wiegand P, Gao X, Carter JA, Botteman MF, Ray S. The impact of second-line agents on patients' health-related quality of life in the treatment for non-small cell lung cancer: a systematic review. Qual Life Res. (2012) 22:1015–26. doi: 10.1007/s11136-012-0229-0
21. Novello S, Bruzzi P, Barone C, Buosi R, Masotti A, Michetti G, et al. Phase III study in stage IV non-small-cell lung cancer patients treated with two courses of cisplatin/gemcitabine followed by a randomization to three additional courses of the same combination or gemcitabine alone. Ann Oncol. (2007) 18:903–8. doi: 10.1093/annonc/mdm061
22. Gebbia V, Galetta D, Lorusso V, Caruso M, Verderame F, Pezzella G, et al. Cisplatin plus weekly vinorelbine versus cisplatin plus vinorelbine on days 1 and 8 in advanced non-small cell lung cancer: a prospective randomized phase III trial of the G.O.I.M. (Gruppo Oncologico Italia Meridionale). Lung Cancer. (2008) 61:369–77. doi: 10.1016/j.lungcan.2008.01.010
23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 89:873–80. doi: 10.1371/journal.pmed.1000097
24. Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials. J Am Med Assoc. (2013) 309:814–22. doi: 10.1001/jama.2013.879
25. Mercieca-Bebber R, Rouette J, Calvert M, King MT, McLeod L, Holch P, et al. Preliminary evidence on the uptake, use and benefits of the CONSORT-PRO extension. Qual Life Res. (2017) 26:1427–37. doi: 10.1007/s11136-017-1508-6
26. McGlothilin AE, Lewis RJ. Minimally clinically important difference. defining what really matters to patients. JAMA Guid Stat Methods. (2014) 312:1342–3. doi: 10.1001/jama.2014.13128
27. Manegold C, Koschel G, Hruska D, Schott-von-Römer K, Mezger J, Pilz LR. Open, randomized, phase II study of single-agent gemcitabine and docetaxel as first- and second-line treatment in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. (2007) 8:245–51. doi: 10.3816/CLC.2007.n.001
28. Park JO, Kim SW, Ahn JS, Suh C, Lee JS, Jang JS, et al. Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non-small-cell lung cancer. J Clin Oncol. (2007) 25:5233–9. doi: 10.1200/JCO.2007.10.8134
29. Patel JD, Socinski MA, Garon EB, Reynolds CH, Spigel DR, Olsen MR, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. (2013) 31:4349–57. doi: 10.1200/JCO.2012.47.9626
30. Georgoulias V, Androulakis N, Kotsakis A, Hatzidaki D, Syrigos K, Polyzos A, et al. Docetaxel versus docetaxel plus gemcitabine as front-line treatment of patients with advanced non-small cell lung cancer: a randomized, multicenter phase III trial. Lung Cancer. (2008) 59:57–63. doi: 10.1016/j.lungcan.2007.07.021
31. Zwitter M, Kovac V, Smrdel U, Vrankar M, Zadnik V. Gemcitabine in brief versus prolonged low-dose infusion, both combined with cisplatin, for advanced non-small cell lung cancer: a randomized phase II clinical trial. J Thorac Oncol. (2009) 4:1148–55. doi: 10.1097/JTO.0b013e3181ae280f
32. Weissman CH, Reynolds CH, Neubauer MA, Pritchard S, Kobina S, Asmar L. A Phase III randomized trial of gemcitabine–oxaliplatin versus carboplatin–paclitaxel as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. (2011) 6:358–64. doi: 10.1097/JTO.0b013e3181ffe8ef
33. Socinski MA, Langer CJ, Okamoto I, Hon K, Hirsh V, Dakhil SR, et al. Safety and efficacy of weekly nab®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol. (2013) 24:314–21. doi: 10.1093/annonc/mds461
34. Langer CJ, Hirsh V, Okamoto I, Lin F-J, Wan Y, Whiting S, et al. Survival, quality-adjusted survival, and other clinical end points in older advanced non-small-cell lung cancer patients treated with albumin-bound paclitaxel. Br J Cancer. (2015) 113:20–9. doi: 10.1038/bjc.2015.181
35. Kubota K, Sakai H, Katakami N, Nishio M, Inoue A, Okamoto H, et al. A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol. (2015) 26:1401–8. doi: 10.1093/annonc/mdv190
36. Abe T, Takeda K, Ohe Y, Kudoh S, Ichinose Y, Okamoto H, et al. Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: the intergroup trial JCOG0803/WJOG4307L. J Clin Oncol. (2015) 33:575–81. doi: 10.1200/JCO.2014.55.8627
37. Spigel DR, Patel JD, Reynolds CH, Garon EB, Hermann RC, Govindan R, et al. Quality of life analyses from the randomized, open-label, phase III PointBreak study of pemetrexed-carboplatin-bevacizumab followed by maintenance pemetrexed-bevacizumab versus paclitaxel-carboplatin-bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Thorac Oncol. (2015) 10:353–9. doi: 10.1097/JTO.0000000000000277
38. Soria J-C, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. (2015) 16:897–907. doi: 10.1016/S1470-2045(15)00006-6
39. Felip E, Hirsh V, Popat S, Cobo M, Fülöp A, Dayen C, et al. Symptom and quality of life improvement in LUX-Lung 8, an open-label phase III study of second-line afatinib versus erlotinib in patients with advanced squamous cell carcinoma of the lung after first-line platinum-based chemotherapy. Clin Lung Cancer. (2017) 19:74–83.e11. doi: 10.1016/j.cllc.2017.06.002
40. Wu Y-L, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. (2017) 18:1454–66. doi: 10.1016/S1470-2045(17)30608-3
41. Belani CP, Brodowicz T, Ciuleanu TE, Krzakowski M, Yang SH, Franke F, et al. Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): results from a randomised, double-blind, phase 3 study. Lancet Oncol. (2012) 13:292–9. doi: 10.1016/S1470-2045(11)70339-4
42. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. (2009) 374:1432–40. doi: 10.1016/S0140-6736(09)61497-5
43. Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. (2012) 13:528–38. doi: 10.1016/S1470-2045(12)70087-6
44. Hirsh V, Cadranel J, Cong XJ, Fairclough D, Finnern HW, Lorence RM, et al. Symptom and quality of life benefit of afatinib in advanced non-small-cell lung cancer patients previously treated with erlotinib or gefitinib. J Thorac Oncol. (2013) 8:229–37. doi: 10.1097/JTO.0b013e3182773fce
45. Baggstrom MQ, Socinski MA, Wang XF, Gu L, Stinchcombe TE, Edelman MJ, et al. Maintenance sunitinib following initial platinum-based combination chemotherapy in advanced-stage IIIB/IV non-small cell lung cancer: a randomized, double-blind, placebo-controlled phase III study-CALGB 30607 (Alliance). J Thorac Oncol. (2017) 12:843–9. doi: 10.1016/j.jtho.2017.01.022
46. Kim ES, Hirsh V, Mok TS, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. (2008) 372:1809–18. doi: 10.1016/S0140-6736(08)61758-4
47. Crinò L, Cappuzzo F, Zatloukal P, Reck M, Pesek M, Thompson JC, et al. Gefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized, phase II study. J Cinical Oncol. (2008) 26:4253–60. doi: 10.1200/JCO.2007.15.0672
48. Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. (2013) 368:2385–94. doi: 10.1056/NEJMoa1214886
49. Blackhall F, Kim D-W, Besse B, Nokihara H, Han J-Y, Wilner KD, et al. Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J Thorac Oncol. (2014) 9:1625–33. doi: 10.1097/JTO.0000000000000318
50. Heigener DF, Deppermann KM, Pawel J V, Fischer JR, Kortsik C, Bohnet S, et al. Open, randomized, multi-center phase II study comparing efficacy and tolerability of Erlotinib vs. Carboplatin/Vinorelbin in elderly patients (>70 years of age) with untreated non-small cell lung cancer. Lung Cancer. (2014) 84:62–6. doi: 10.1016/j.lungcan.2014.01.024
51. Solomon BJ, Mok TSK, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
52. Geater SL, Xu CR, Zhou C, Hu CP, Feng J, Lu S, et al. Symptom and quality of life improvement in LUX-lung 6. J Thorac Oncol. (2015) 10:883–9. doi: 10.1097/JTO.0000000000000517
53. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. (2014) 15:213–22. doi: 10.1016/S1470-2045(13)70604-1
54. Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. (2008) 26:4244–52. doi: 10.1200/JCO.2007.15.0185
55. Sekine I, Ichinose Y, Nishiwaki Y, Yamamoto N, Tsuboi M, Nakagawa K, et al. Quality of life and disease-related symptoms in previously treated Japanese patients with non-small-cell lung cancer: Results of a randomized phase III study (V-15-32) of gefitinib versus docetaxel. Ann Oncol. (2009) 20:1483–8. doi: 10.1093/annonc/mdp031
56. Oizumi S, Kobayashi K, Inoue A, Maemondo M, Sugawara S, Yoghizawa H, et al. Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of north east Japan study group 002 trial. Oncologist. (2012) 17:863–70. doi: 10.1634/theoncologist.2011-0426
57. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. (2010) 362:2380–8. doi: 10.1056/NEJMoa0909530
58. Chen G, Feng J, Zhou C, Wu YL, Liu XQ, Wang C, et al. Quality of life (QoL) analyses from optimal (CTONG-0802), a phase III, randomised, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC). Ann Oncol. (2013) 24:1615–22. doi: 10.1093/annonc/mdt012
59. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X
60. Yang JCH, Hirsh V, Schuler M, Yamamoto N, O'Byrne KJ, Mok TSK, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. (2013) 31:3342–50. doi: 10.1200/JCO.2012.46.1764
61. Sequist LV, Yang JCH, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. (2013) 31:3327–34. doi: 10.1200/JCO.2012.44.2806
62. Mok TS, Wu YL, Thongprasert S, Yang JCH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
63. Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JCH, Chu DT, et al. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J Thorac Oncol. (2011) 6:1872–80. doi: 10.1097/JTO.0b013e31822adaf7
64. Brahmer JR, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. (2017) 18:1600–9. doi: 10.1016/S1470-2045(17)30690-3
65. Addeo R. A new frontier for targeted therapy in NSCLC: clinical efficacy of pembrolizumab in the inhibition of programmed cell death 1 (PD-1). Expert Rev Anticancer Ther. (2017) 17:199–201. doi: 10.1080/14737140.2017.1286986
66. Boye M, Wang X, Srimuninnimit V, Kang JH, Tsai CM, Orlando M, et al. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian never-smoker patients with locally advanced or metastatic nonsquamous non-small cell lung cancer: final overall survival results from. Clin Lung Cancer. (2016) 17:150–60. doi: 10.1016/j.cllc.2015.12.004
67. Yang JCH, Kang JH, Mok T, Ahn MJ, Srimuninnimit V, Lin CC, et al. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non-squamous non-small cell lung cancer: a randomised, phase 3 trial. Eur J Cancer. (2014) 50:2219–30. doi: 10.1016/j.ejca.2014.05.011
68. Fiteni F, Anota A, Bonnetain F, Oster J-P, Pichon E, Wislez M, et al. Health-related quality of life in elderly patients with advanced non-small cell lung cancer comparing carboplatin and weekly paclitaxel doublet chemotherapy with monotherapy. Eur Respir J. (2016) 48:861–72. doi: 10.1183/13993003.01695-2015
69. Quoix E, Zalcman G, Oster J-P, Westeel V, Pichon E, Lavole A, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. (2011) 378:1079–88. doi: 10.1016/S0140-6736(11)60780-0
70. Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. BMJ. (1998) 317:771–5. doi: 10.1136/bmj.317.7161.771
71. Demuro C, Clark M, Doward L, Evans E, Mordin M, Gnanasakthy A. Assessment of PRO label claims granted by the FDA as compared to the EMA (2006-2010). Value Heal. (2013) 16:1150–5. doi: 10.1016/j.jval.2013.08.2293
72. Bottomley A, Pe M, Sloan J, Basch E, Bonnetain F, Calvert M, et al. Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Lancet Oncol. (2016) 17:e510–4. doi: 10.1016/S1470-2045(16)30510-1
73. Bezjak A, Tu D, Seymour L, Clark G, Trajkovic A, Zukin M, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Cinical Oncol. (2006) 24:3831–7. doi: 10.1200/JCO.2006.05.8073
74. Helbekkmo N, Sundstrøm SH, Aasebø U, Brunsvig PF, von Plessen C, Hjelde HH, et al. Vinorelbine/carboplatin vs gemcitabine/carboplatin in advanced NSCLC shows similar efficacy, but different impact of toxicity. Br J Cancer. (2007) 97:283–9. doi: 10.1038/sj.bjc.6603869
75. Langer CJ, O'Byrne KJ, Socinski MA, Mikhailov SM, Lesniewski-Kmak K, Smakal M, et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J Thorac Oncol. (2008) 3:623–30. doi: 10.1097/JTO.0b013e3181753b4b
76. Gebbia V, Gridelli C, Verusio C, Frontini L, Aitini E, Daniele B, et al. Weekly docetaxel vs. docetaxel-based combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer patients. The DISTAL-2 randomized trial. Lung Cancer. (2009) 63:251–8. doi: 10.1016/j.lungcan.2008.05.027
77. Wintner LM, Sztankay M, Giesinger JM, Aaronson N, Bottomley A, Velikova G, et al. The use of EORTC measures in daily clinical practice—A synopsis of a newly developed manual. Eur J Cancer. (2016) 68:73–81. doi: 10.1016/j.ejca.2016.08.024
78. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. (2003) 41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C
79. Fielding S, Maclennan G, Cook JA, Ramsay CR. A review of RCTs in four medical journals to assess the use of imputation to overcome missing data in quality of life outcomes. Trials. (2008) 9:51. doi: 10.1186/1745-6215-9-51
80. Donaldson GW, Moinpour CM. Learning to live with missing quality-of-life data in advanced-stage disease trials. J Clin Oncol. (2005) 23:7380–4. doi: 10.1200/JCO.2005.07.022
81. Hamel J-F, Saulnier P, Pe M, Zikos E, Musoro J, Coens C, et al. A systematic review of the quality of statistical methods employed for analysing quality of life data in cancer randomised controlled trials. Eur J Cancer. (2017) 83:166–76. doi: 10.1016/j.ejca.2017.06.025
82. Claassens L, van Meerbeeck JP, Coens C, Quinten C, Ghislain I, Sloan E, et al. Health-related quality of life in non-small-cell lung cancer: an update of a systematic review on methodologic issues in randomized controlled trials. J Clin Oncol. (2011) 29:2104–20. doi: 10.1200/JCO.2010.32.3683
83. LeCaer H, Barlesi F, Corre R, Jullian H, Bota S, Falchero L, et al. A multicentre phase II randomised trial of weekly docetaxel/gemcitabine followed by erlotinib on progression, vs the reverse sequence, in elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0504 study). Br J Cancer. (2011) 105:1123–30. doi: 10.1038/bjc.2011.331
84. Chen Y-M, Tsai C-M, Fan W-C, Shih J-F, Liu S-H, Wu C-H, et al. Phase II randomized trial of erlotinib or vinorelbine in chemonaive, advanced, non-small cell lung cancer patients aged 70 years or older. J Thorac Oncol. (2012) 7:412–8. doi: 10.1097/JTO.0b013e31823a39e8
85. Leong SS, Toh CK, Lim WT, Lin X, Tan SB, Poon D, et al. A randomized phase II trial of single-agent gemcitabine, vinorelbine, or docetaxel in patients with advanced non-small cell lung cancer who have poor performance status and/or are elderly. J Thorac Oncol. (2007) 2:230–6. doi: 10.1097/JTO.0b013e318031d06f
86. Biesma B, Wymenga ANM, Vincent A, Dalesio O, Smit HJM, Stigt JA, et al. Quality of life, geriatric assessment and survival in elderly patients with non-small-cell lung cancer treated with carboplatin-gemcitabine or carboplatin-paclitaxel: NVALT-3 a phase III study. Ann Oncol. (2011) 22:1520–7. doi: 10.1093/annonc/mdq637
87. Stinchcombe TE, Peterman AH, Lee CB, Moore DT, Beaumont JL, Bradford DS, et al. A randomized phase II trial of first-line treatment with gemcitabine, erlotinib, or gemcitabine and erlotinib in elderly patients (age ≥70 years) with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol. (2011) 6:1569–77. doi: 10.1097/JTO.0b013e3182210430
88. Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer. (2010) 68:234–9. doi: 10.1016/j.lungcan.2009.06.020
89. Lilenbaum R, Rubin M, Samuel J, Boros L, Chidiac T, Seigel L, et al. A randomized phase II trial of two schedules of docetaxel in elderly or poor performance status patients with advanced non-small cell lung cancer. J Thorac Oncol. (2007) 2:306–11. doi: 10.1097/01.JTO.0000263713.38826.8e
90. Mannion E, Gilmartin JJ, Donnellan P, Keane M, Waldron D. Effect of chemotherapy on quality of life in patients with non-small cell lung cancer. Support Care Cancer. (2014) 22:1417–28. doi: 10.1007/s00520-014-2148-9
91. Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. (2007):MR000010. doi: 10.1002/14651858.MR000010.pub3
92. Tanvetyanon T, Soares HP, Djulbegovic B, Jacobsen PB, Bepler G. A systematic review of quality of life associated with standard chemotherapy regimens for advanced non-small cell lung cancer. J Thorac Oncol. (2007) 2:1091–7. doi: 10.1097/JTO.0b013e31815cff64
93. Pat K, Dooms C, Vansteenkiste J. Systematic review of symptom control and quality of life in studies on chemotherapy for advanced non-small cell lung cancer: how CONSORTed are the data? Lung Cancer. (2008) 62:126–38. doi: 10.1016/j.lungcan.2008.02.018
94. Saad ED, Adamowicz K, Katz A, Jassem J. Assessment of quality of life in advanced non-small-cell lung cancer: an overview of recent randomized trials. Cancer Treat Rev. (2012) 38:807–14. doi: 10.1016/j.ctrv.2012.02.012
95. Gridelli C, de Marinis F, Pujol J-L, Reck M, Ramlau R, Parente B, et al. Safety, resource use, and quality of life in paramount: a phase III study of maintenance pemetrexed versus placebo after induction pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. (2012) 7:1713–21. doi: 10.1097/JTO.0b013e318267cf84
96. Schuette W, Behringer D, Stoehlmacher J, Kollmeier J, Schmager S, Fischer Von Weikersthal L, et al. CHAMP: A phase II study of panitumumab with pemetrexed and cisplatin versus pemetrexed and cisplatin in the treatment of patients with advanced-stage primary nonsquamous non-small-cell lung cancer with particular regard to the KRAS status. Clin Lung Cancer. (2015) 16:447–56. doi: 10.1016/j.cllc.2015.05.009
97. Krzakowski M, Ramlau R, Jassem J, Szczesna A, Zatloukal P, Von Pawel J, et al. Phase III trial comparing vinflunine with docetaxel in second-line advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy. J Clin Oncol. (2010) 28:2167–73. doi: 10.1200/JCO.2009.23.4146
98. Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. (2010) 11:521–9. doi: 10.1016/S1470-2045(10)70112-1
99. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
100. Garon EB, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. (2014) 384:665–73. doi: 10.1016/S0140-6736(14)60845-X
101. Fløtten Ø, Grønberg BH, Bremnes RM, Amundsen T, Sundstrøm SH, Rolke H, et al. Vinorelbine and gemcitabine vs vinorelbine and carboplatin as first-line treatment of advanced NSCLC. A phase III randomised controlled trial by the Norwegian lung cancer study group. Br J Cancer. (2012) 107:442–7. doi: 10.1038/bjc.2012.284
102. Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. (2010) 16:1307–14. doi: 10.1158/1078-0432.CCR-09-1903
103. Yoshioka H, Okamoto I, Morita S, Ando M, Takeda K, Seto T, et al. Efficacy and safety analysis according to histology for s-1 in combination with carboplatin as first-line chemotherapy in patients with advanced non-small-cell lung cancer: updated results of the west japan oncology group lets study. Ann Oncol. (2013) 24:1326–31. doi: 10.1093/annonc/mds629
104. Thatcher N, Hirsch FR, Luft A, Szczesna A, Ciuleanu TE, Dediu M, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. (2015) 16:763–74. doi: 10.1016/S1470-2045(15)00021-2
105. Socinski MA, Raju RN, Stinchcombe T, Kocs DM, Couch LS, Barrera D, et al. Randomized, phase II trial of pemetrexed and carboplatin with or without enzastaurin versus docetaxel and carboplatin as first-line treatment of patients with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol. (2010) 5:1963–9. doi: 10.1097/JTO.0b013e3181fd42eb
106. Kawahara M, Tada H, Tokoro A, Teramukai S, Origasa H, Kubota K, et al. Quality-of-life evaluation for advanced non-small-cell lung cancer: a comparison between vinorelbine plus gemcitabine followed by docetaxel versus paclitaxel plus carboplatin regimens in a randomized trial: Japan Multinational Trial Organization LC00-03 (BRI LC03-01). BMC Cancer. (2011) 11:356. doi: 10.1186/1471-2407-11-356
107. Saad AS, Ghali RR, Shawki MA. A prospective randomized controlled study of cisplatin versus carboplatin-based regimen in advanced squamous nonsmall cell lung cancer. J Cancer Res Ther. (2017) 13:198–203. doi: 10.4103/0973-1482.187287
108. Ikezawa Y, Asahina H, Oizumi S, Watanabe M, Takamura K, Kawai Y, et al. A randomized phase II trial of erlotinib vs. S-1 as a third- or fourth-line therapy for patients with wild-type EGFR non-small cell lung cancer (HOT1002). Cancer Chemother Pharmacol. (2017) 80:955–63. doi: 10.1007/s00280-017-3432-4
109. Tan EH, Rolski J, Grodzki T, Schneider CP, Gatzemeier U, Zatloukal P, et al. Global lung oncology branch trial 3 (GLOB3): final results of a randomised multinational phase III study alternating oral and i.v. vinorelbine plus cisplatin versus docetaxel plus cisplatin as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol. (2009) 20:1249–56. doi: 10.1093/annonc/mdn774
110. Di Maio M, Leighl NB, Gallo C, Feld R, Ciardiello F, Butts C, et al. Quality of life analysis of TORCH, a randomized trial testing first-line erlotinib followed by second-line cisplatin/gemcitabine chemotherapy in advanced non-small-cell lung cancer. J Thorac Oncol. (2012) 7:1830–44. doi: 10.1200/JCO.2011.41.2056
111. Reck M, Socinski MA, Luft A, Szczesna A, Dediu M, Ramlau R, et al. The effect of necitumumab in combination with gemcitabine plus cisplatin on tolerability and on quality of life: results from the phase 3 SQUIRE trial. J Thorac Oncol. (2016) 11:808–18. doi: 10.1016/j.jtho.2016.03.002
112. Goss G, Ferry D, Wierzbicki R, Laurie SA, Thompson J, Biesma B, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol. (2009) 27:2253–60. doi: 10.1200/JCO.2008.18.4408
113. Grønberg BH, Bremnes RM, Fløtten Ø, Amundsen T, Brunsvig PF, Hjelde HH, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. (2009) 27:3217–24. doi: 10.1200/JCO.2008.20.9114
114. Grønberg BH, Sundstrøm S, Kaasa S, Bremnes RM, Fløtten Ø, Amundsen T, et al. Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer. (2010) 46:2225–34. doi: 10.1016/j.ejca.2010.04.009
115. Rittmeyer A, Gorbunova V, Vikström A, Scherpereel A, Kim JH, Ahn MJ, et al. Health-related quality of life in patients with advanced nonsquamous non-small-cell lung cancer receiving bevacizumab or bevacizumab-plus-pemetrexed maintenance therapy in AVAPERL (MO22089). J Thorac Oncol. (2013) 8:1409–16. doi: 10.1097/JTO.0b013e3182a46bcf
116. Novello S, Kaiser R, Mellemgaard A, Douillard J-Y, Orlov S, Krzakowski M, et al. Analysis of patient-reported outcomes from the LUME-Lung 1 trial: a randomised, double-blind, placebo-controlled, phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer. Eur J Cancer. (2015) 51:317–26. doi: 10.1016/j.ejca.2014.11.015
117. Juhász E, Kim JH, Klingelschmitt G, Walzer S. Effects of erlotinib first-line maintenance therapy versus placebo on the health-related quality of life of patients with metastatic non-small-cell lung cancer. Eur J Cancer. (2013) 49:1205–15. doi: 10.1016/j.ejca.2012.11.006
118. Gebbia V, Lorusso V, Galetta D, Caruso MM, Palomba G, Riccardi F, et al. First-line cisplatin with docetaxel or vinorelbine in patients with advanced non-small-cell lung cancer: a quality of life directed phase II randomized trial of Gruppo Oncologico Italia Meridionale. Lung Cancer. (2010) 69:218–24. doi: 10.1016/j.lungcan.2009.10.008
119. Lee SM, Rudd R, Woll PJ, Ottensmeier C, Gilligan D, Price A, et al. Randomized double-blind placebo-controlled trial of thalidomide in combination with gemcitabine and carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. (2009) 27:5248–54. doi: 10.1200/JCO.2009.21.9733
120. Gridelli C, Ciardiello F, Gallo C, Feld R, Butts C, Gebbia V, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol. (2012) 30:3002–11. doi: 10.1097/JTO.0b013e318275b327
121. Ramalingam SS, Blackhall F, Krzakowski M, Barrios CH, Park K, Bover I, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. (2012) 30:3337–44. doi: 10.1200/JCO.2011.40.9433
122. Kubota K, Kawahara M, Ogawara M, Nishiwaki Y, Komuta K, Minato K, et al. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: a randomised, open-label, phase III study. Lancet Oncol. (2008) 9:1135–42. doi: 10.1016/S1470-2045(08)70261-4
123. Schiller JH, von Pawel J, Schutt P, Ansari RH, Thomas M, Saleh M, et al. Pemetrexed with or without matuzumab as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol. (2010) 5:1977–85. doi: 10.1097/JTO.0b013e3181f4a5c9
124. Hanna N, Shepherd FA, Fossella FV, Pereira JR, Demarinis F, Von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. (2004) 22:1589–97. doi: 10.1200/JCO.2004.08.163
125. Zhou Q, Cheng Y, Yang JJ, Zhao MF, Zhang L, Zhang XC, et al. Pemetrexed versus gefitinib as a second-line treatment in advanced nonsquamous nonsmall-cell lung cancer patients harboring wild-type EGFR (CTONG0806): a multicenter randomized trial. Ann Oncol. (2014) 25:2385–91. doi: 10.1093/annonc/mdu463
126. Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, random. Lancet Oncol. (2012) 13:247–55. doi: 10.1016/S1470-2045(12)70063-3
127. Kim ST, Uhm JE, Lee J, Sun J-M, Sohn I, Kim SW, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer. (2012) 75:82–8. doi: 10.1016/j.lungcan.2011.05.022
128. Galetta D, Cinieri S, Pisconti S, Gebbia V, Morabito A, Borsellino N, et al. Cisplatin/pemetrexed followed by maintenance pemetrexed versus carboplatin/paclitaxel/bevacizumab followed by maintenance bevacizumab in advanced nonsquamous lung cancer: the GOIM (Gruppo Oncologico Italia Meridionale) ERACLE phase III randomized trial. Clin Lung Cancer. (2015) 16:262–73. doi: 10.1016/j.cllc.2014.12.002
129. Comella P, Chiuri VE, De Cataldis G, Filippelli G, Maiorino L, Vessia G, et al. Gemcitabine combined with either pemetrexed or paclitaxel in the treatment of advanced non-small cell lung cancer: a randomized phase II SICOG trial. Lung Cancer. (2010) 68:94–8. doi: 10.1016/j.lungcan.2009.05.008
130. Bonomi PD, Mace J, Mandanas RA, Min M, Olsen M, Youssoufian H, et al. Randomized phase II study of cetuximab and bevacizumab in combination with two regimens of paclitaxel and carboplatin in chemonaive patients with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol. (2013) 8:338–45. doi: 10.1097/JTO.0b013e318282ded5
131. Ellis PM, Shepherd FA, Millward M, Perrone F, Seymour L, Liu G, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol. (2014) 15:1379–88. doi: 10.1016/S1470-2045(14)70472-3
132. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the tarceva lung cancer investigation trial. J Clin Oncol. (2007) 25:1545–52. doi: 10.1200/JCO.2005.05.1474
133. Lynch TJ, Patel T, Dreisbach L, McCleod M, Heim WJ, Hermann RC, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol. (2010) 28:911–7. doi: 10.1200/JCO.2009.21.9618
134. Hillerdal G, Sederholm C, Andersson K. Randomized phase II study of gemcitabine and carboplatin +/– sequential docetaxel in non-small cell lung cancer. Lung Cancer. (2011) 71:178–81. doi: 10.1016/j.lungcan.2010.05.007
135. Lara PN, Douillard JY, Nakagawa K, Von Pawel J, McKeage MJ, Albert I, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol. (2011) 29:2965–71. doi: 10.1200/JCO.2011.35.0660
136. Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, Bromund JL, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. (2009) 27:591–8. doi: 10.1200/JCO.2008.17.1405
137. Yang Y-P, Ma Y-X, Huang Y, Zhao Y-Y, Fang W-F, Hong S-D, et al. QoL analyses from INFORM study, a phase III study of gefitinib versus placebo as maintenance therapy in advanced NSCLC. Sci Rep. (2015) 5:11934. doi: 10.1038/srep11934
138. Lilenbaum R, Axelrod R, Thomas S, Dowlati A, Seigel L, Albert D, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol. (2008) 26:863–9. doi: 10.1200/JCO.2007.13.2720
139. Twelves C, Chmielowska E, Havel L, Popat S, Swieboda-Sadlej A, Sawrycki P, et al. Randomised phase II study of axitinib or bevacizumab combined with paclitaxel/carboplatin as first-line therapy for patients with advanced non-small-cell lung cancer. Ann Oncol. (2014) 25:132–8. doi: 10.1093/annonc/mdt489
140. Zhang L, Ma S, Song X, Han B, Cheng Y, Huang C, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol. (2012) 13:466–75. doi: 10.1016/S1470-2045(12)70117-1
141. de Marinis F, Pereira JR, Fossella F, Perry MC, Reck M, Salzberg M, et al. Lung cancer symptom scale outcomes in relation to standard efficacy measures–an analysis of the phase III study of pemetrexed versus docetaxel in advanced non-small cell lung cancer. J Thorac Oncol. (2008) 3:30–6. doi: 10.1097/JTO.0b013e31815e8b48
142. Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol. (2013) 31:3004–11. doi: 10.1200/JCO.2012.42.3749
143. Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. (2009) 373:1525–31. doi: 10.1016/S0140-6736(09)60569-9
144. LeCaer H, Greillier L, Corre R, Jullian H, Crequit J, Falchero L, et al. A multicenter phase II randomized trial of gemcitabine followed by erlotinib at progression, versus the reverse sequence, in vulnerable elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0505 study). Lung Cancer. (2012) 77:97–103. doi: 10.1016/j.lungcan.2012.02.004
145. Pérol M, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Quality of life results from the phase 3 REVEL randomized clinical trial of ramucirumab-plus-docetaxel versus placebo-plus-docetaxel in advanced/metastatic non-small cell lung cancer patients with progression after platinum-based chemotherapy. Lung Cancer. (2016) 93:95–103. doi: 10.1016/j.lungcan.2016.01.007
146. Sun JM, Lee KH, Kim SW, Lee DH, Min YJ, Yun HJ, et al. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. (2012) 118:6234–42. doi: 10.1002/cncr.27630
147. Okamoto I, Yoshioka H, Morita S, Ando M, Takeda K, Seto T, et al. Phase III trial comparing oral S-1 plus carboplatin with paclitaxel plus carboplatin in chemotherapy-naïve patients with advanced non-small-cell lung cancer: results of a west Japan oncology group study. J Clin Oncol. (2010) 28:5240–6. doi: 10.1200/JCO.2010.31.0326
148. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. (2014) 15:143–55. doi: 10.1016/S1470-2045(13)70586-2
Keywords: quality of life, review–systematic, lung cancer, methodological quality, NSCLC
Citation: Van Der Weijst L, Lievens Y, Schrauwen W and Surmont V (2019) Health-Related Quality of Life in Advanced Non-small Cell Lung Cancer: A Methodological Appraisal Based on a Systematic Literature Review. Front. Oncol. 9:715. doi: 10.3389/fonc.2019.00715
Received: 19 April 2019; Accepted: 18 July 2019;
Published: 12 August 2019.
Edited by:
Sacha I. Rothschild, University of Basel, SwitzerlandReviewed by:
Francesca Mazzoni, Careggi University Hospital, ItalyIacopo Petrini, University of Pisa, Italy
Copyright © 2019 Van Der Weijst, Lievens, Schrauwen and Surmont. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lotte Van Der Weijst, bG90dGUudmFuZGVyd2VpanN0JiN4MDAwNDA7dWdlbnQuYmU=
 Lotte Van Der Weijst
Lotte Van Der Weijst Yolande Lievens1
Yolande Lievens1 Wim Schrauwen
Wim Schrauwen