- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
Background: In this study, publicly datasets with organs at risk (OAR) structures were used as reference data to compare the differences of several observers. Convolutional neural network (CNN)-based auto-contouring was also used in the analysis. We evaluated the variations among observers and the effect of CNN-based auto-contouring in clinical applications.
Materials and methods: A total of 60 publicly available lung cancer CT with structures were used; 48 cases were used for training, and the other 12 cases were used for testing. The structures of the datasets were used as reference data. Three observers and a CNN-based program performed contouring for 12 testing cases, and the 3D dice similarity coefficient (DSC) and mean surface distance (MSD) were used to evaluate differences from the reference data. The three observers edited the CNN-based contours, and the results were compared to those of manual contouring. A value of P<0.05 was considered statistically significant.
Results: Compared to the reference data, no statistically significant differences were observed for the DSCs and MSDs among the manual contouring performed by the three observers at the same institution for the heart, esophagus, spinal cord, and left and right lungs. The 95% confidence interval (CI) and P-values of the CNN-based auto-contouring results comparing to the manual results for the heart, esophagus, spinal cord, and left and right lungs were as follows: the DSCs were CNN vs. A: 0.914~0.939(P = 0.004), 0.746~0.808(P = 0.002), 0.866~0.887(P = 0.136), 0.952~0.966(P = 0.158) and 0.960~0.972 (P = 0.136); CNN vs. B: 0.913~0.936 (P = 0.002), 0.745~0.807 (P = 0.005), 0.864~0.894 (P = 0.239), 0.952~0.964 (P = 0.308), and 0.959~0.971 (P = 0.272); and CNN vs. C: 0.912~0.933 (P = 0.004), 0.748~0.804(P = 0.002), 0.867~0.890 (P = 0.530), 0.952~0.964 (P = 0.308), and 0.958~0.970 (P = 0.480), respectively. The P-values of MSDs are similar to DSCs. The P-values of heart and esophagus is smaller than 0.05. No significant differences were found between the edited CNN-based auto-contouring results and the manual results.
Conclusion: For the spinal cord, both lungs, no statistically significant differences were found between CNN-based auto-contouring and manual contouring. Further modifications to contouring of the heart and esophagus are necessary. Overall, editing based on CNN-based auto-contouring can effectively shorten the contouring time without affecting the results. CNNs have considerable potential for automatic contouring applications.
Introduction
The correct contouring of organs at risk (OARs) and target volumes is important for ensuring radiation quality during radiation treatment planning (RTP). Studies have shown that the dosimetric impact of the variation in the contouring of targets and OARs can be significant depending on the degree of variation and the plan dose gradient (1, 2). Differences in structure delineation impact DVH calculation, tumor control probability (TCP), and normal tissue complication probability (NTCP). The accuracy of primary gross tumor contouring could have a positive impact on tumor control and patient survival (3–5). Interobserver variation in the delineation of OARs primarily originates from various subjective interpretations of organ boundaries and objective contouring variation (6, 7). Reproducibility in the delineation of tumor and normal tissues is crucial for optimal treatment quality and outcomes (8). Variations in contouring have a direct impact on the quality and evaluation of RTP, especially for dose distribution of OARs (2). Intensity-modulated radiotherapy (IMRT) is a key treatment for lung cancer, particularly for patients with advanced stages (III and IV) (9). Cui Y et al. reported that the planned target volume (PTV) showed large variation among institutions. The PTV coverage of institutions dramatically decreased when re-evaluated using the consensus PTV contour (10). E.M. Gore et al. evaluated five thoracic radiation oncologists who collectively contoured cardiac structures for each available case, guided by a common atlas. The defined anatomic structures were the pericardium (P), ventricles (V), atria (A) and coronary spaces (CS). Large variation was found among observers, creating uncertainty regarding the dose delivered to OARs (11).
Standardized guidelines and anatomic atlases have been used to reduce interobserver variation and subjective diversity in clinical practice. The use of knowledge-based auto-contouring software, including atlas-based methods, has gained popularity because it is clinically acceptable, saves time and improves the consistency of contours created by various observers (1, 12–14). Rapid development has recently occurred for deep-learning methods, especially high-accuracy deep convolutional neural networks (CNNs), which can be used for computer vision, image recognition, and feature extraction (15–17). Neural networks are starting to be used for auxiliary diagnosis of medical images and contouring based on CT images (18, 19). Nevertheless, few studies have focused on the examination and comparison of the clinical use of neural networks regarding multiple OARs in CT images of lung cancer in RTP, particularly with respect to the following three questions: 1) Is there any difference between the results of CNN-based contouring and observer contouring? If so, which organs are different? 2) In clinical use, can interobserver variation and contouring time be reduced by editing the CNN-based auto-contouring results? 3) Based on these data, can CNN-based auto-contouring for OARs achieve an acceptable level for clinical use?
Datasets provided by the American Association of Physicists in Medicine (AAPM) in the thoracic auto-segmentation challenge were used as reference data. Variations among observers and the CNN and the clinical impact of editing based on CNN auto-contouring were evaluated.
Materials and Methods
Datasets
Publicly available lung cancer datasets were provided by AAPM for the thoracic auto-segmentation challenge in 2017 (20–22). The datasets were provided by three institutions: MD Anderson Cancer Center (MDACC), Memorial Sloan-Kettering Cancer Center (MSKCC) and the MAASTRO clinic. Each case had a CT volume and a reference contour. The contours were checked for quality and edited to adhere to the RTOG 1106 contouring guidelines (20). The OARs included heart, esophagus, spinal cord, left lung and right lung. Each image had 512 × 512 pixels and a layer thickness of 1.25–3 mm. There were 115–214 slices per case. The contours provided by the public datasets were used as the standards in the following analysis and the labeled data. A total of 60 cases were divided randomly into two groups, including a group of 48 cases for CNN training and a group of 12 cases for testing and evaluation.
CNN-Based Auto-Contouring
A CNN is a specific type of multilevel perceptron architecture that can make predictions regarding an image. The largest difference between image contouring and image classification is that, in image contouring, the category of an object present in the image has to be identified, and the boundary of the object has to be depicted pixel by pixel (23, 24). The U–net architecture was first designed for biomedical image segmentation (25). The encoder gradually reduces the number of spatial dimensions and identifies the features of the image, while the decoder gradually modifies the details and spatial dimensions of the object and determines its boundary on a pixel-by-pixel basis. By considering that the volumes of the OARs are different in the thoracic region, DeepLabv3+ architecture combines the advantages of spatial pyramid pooling modules and encode-decoder structures. It uses atrous convolutions and atrous spatial pyramid pooling (ASPP) as the encoder for the segmentation of objects at multiple scales, and it uses a bilinear upsampling decoder module to refine the segmentation results, especially along the object boundaries (26). In the last layer, a 1 × 1 convolution with a softmax activation function reduces the number of feature maps to the number of labels.
Image Preprocessing and CNN Training
To effectively increase the number of training samples, the training data were shuffled, and the following random processing tasks were performed during training: 1) each CT image was randomly cropped to regions of interest (ROIs) of 256 × 256 (columns × rows) pixels; the randomly cropped ROIs could overlap, but there was at least one labeled pixel in each ROI; and 2) the HU value was randomly shifted by ± 40 HU for each pixel.
To highlight soft tissue, bone, and spinal cord tissue, a window-level transformation was applied in which each original slice was transformed using a soft-tissue window (window width: 350; window level: 40), a bone window (window width: 1000; window level: 400), and a brain window (window width: 100; window level: 50) to generate three new images, and then, these images were integrated with the original image as an additional channel. The input size of the CNN was 256 × 256 × 4 (columns × rows × channels).
The training process requires automatic segmentation to be performed simultaneously for multiple organs that vary in size. Therefore, during the training process, the convergence rates vary. The class rebalancing properties of the generalized dice overlap, which is a recognized metric for segmentation assessment and a robust and accurate deep-learning loss function for unbalanced tasks (27). Adam optimizers were used to train the CNN, and the learning rate was 0.001. The following default values provided in the original paper were used for the other parameters: beta_1 = 0.9, beta_2 = 0.999, and epsilon = 1e−8 (28). The training batch size was 2, and the models were trained for 16 epochs.
Interobserver Comparison of Contouring
To compare the differences among observers, 12 test cases were manually contoured by three observers. Observer A and observer B were experienced senior radiation oncologists specializing in the thoracic region with more than 10 years of work experience. Observer C was a dosimetrist with 6 years of work experience. The original structures of the test cases were deleted, and the three observers independently contoured the CT images using RTOG 1106 OAR contouring guidelines. Manual contouring was performed using Monaco (Elekta AB, Stockholm, Sweden). The observers were not shown the contours produced by the other observers.
Additionally, to evaluate whether the errors of the auto-contouring based on CNN lie into the variability of the experts, CNN-based auto-contouring was used as observer D and compared with the results of the three other observers.
Using the original structure of the test case as the reference data, the four contouring results (three manual and one automatic) were compared and analyzed in terms of the significant differences among the observers.
Edited CNN-Based Contouring
The original structures of the test cases were deleted, and auto-contouring was performed on the test cases by the CNN. To minimize recall bias, the three observers independently reviewed and edited the final multisubject auto-contouring results of the OARs using consensus guidelines at a minimum of 1 month after manual contouring. The edited results were compared with the reference data for analysis.
Quantitative and Statistical Analyses
Two indicators were used as evaluation criteria in the 3D region: the dice similarity coefficient (DSC) and the mean surface distance (MSD).
The DSC is commonly used to assess the degree of overlap between two structures in medical images (29). A higher level of overlap between two structures is reflected by a greater DSC. The DSC (0 ≤ DSC ≤ 1) is defined as follows:
where V1 is the volume of the reference structure and V2 is the volume of the comparison structure.
The formula for the MSD is:
where , , and x and y are points belonging to different structures. d(x,y) is the distance between x and y.
Statistical analysis was performed on the contouring results of the observers using the ranked Wilcoxon test. All analyses were performed using SPSS version 24.0 (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Comparison of Contouring Between Observers
Table 1 lists the 95% confidence interval (CI) and P-values for the statistical analysis of the DSCs for the OARs using pairwise comparisons among the observers. Except for the heart and esophagus, which were significantly different between observer D and observers A, B, and C (P < 0.05), no significant differences were found among observers for the other OARs. Table 2 lists the 95% CI and P-values for statistical analysis of the MSDs for OARs for pairwise comparisons among observers. Similar to the DSC results, significant differences were observed between observer D and observers A, B, and C only for the heart and esophagus (P < 0.05). The mean DSCs of the observers for the heart, esophagus, spinal cord, and left and right lungs met the commonly accepted threshold value for the DSC (DSC ≥ 0.7) (13, 14). The mean ± deviation of the DSC and MSD values for all observers compared to the reference data for the 12 test cases is listed in the Supplementary Material.
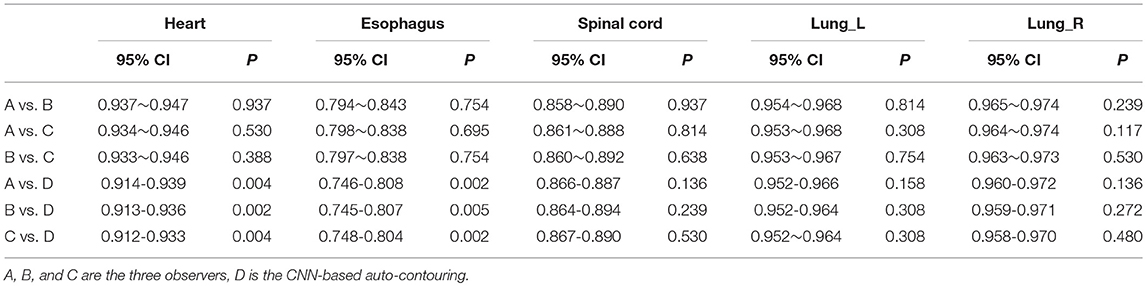
Table 1. 95% CI and P-value of Wilcoxon signed rank test comparing the DSC results generated by the individual observers.
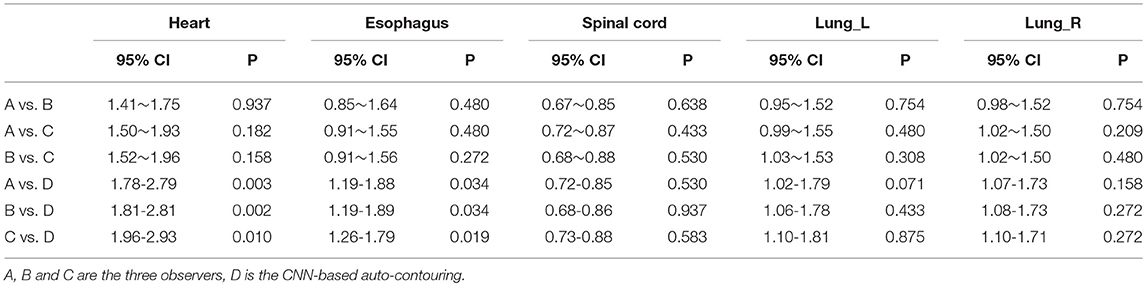
Table 2. 95% CI and P-value of Wilcoxon signed rank test comparing the MSD results generated by individual observers.
Observer Editing of CNN-Based Contouring
Tables 3, 4 provide the 95% CI and P-values for the statistical analysis of the differences among manual contouring by the three observers and the edited contouring based on contours generated by the CNN. No statistically significant differences were found between independent manual contouring and the edited contouring for each OAR. However, the time required to edit the contours was reduced from 40–50 min to 15–20 min, effectively shortening the contouring time. The mean ± deviation of DSCs and MSDs for the CNN-based structures edited by the three observers with the reference data is listed in the Supplementary Material.
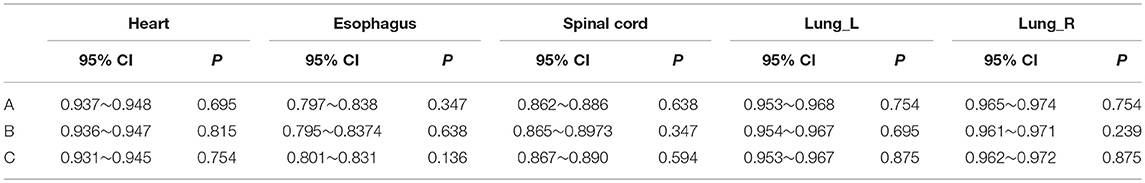
Table 3. 95% CI and P-value of Wilcoxon signed rank test comparing the DSCs generated by the manual method to those of the edited method for the individual observers.
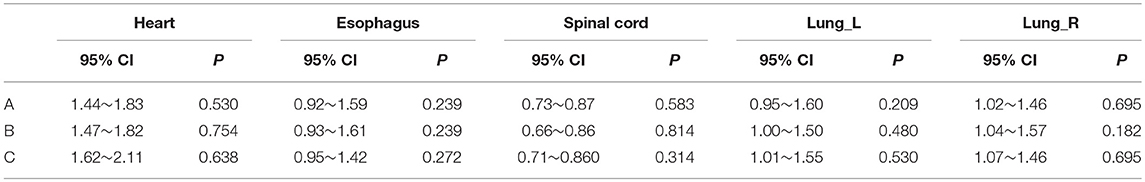
Table 4. 95% CI and P-value of Wilcoxon signed rank test comparing the MSDs generated by the manual method to those of the edited method for the individual observers.
Discussion
In this study, based on publicly available lung cancer datasets provided by AAPM, CNN-based auto-contouring was used as an observer (observer D) and compared to manual contouring performed by three separate observers. The differences among observers were analyzed for structures in publicly available datasets, which were used as the reference data. We found that, if the clinically acceptable level (DSC ≥ 0.7) was used as the standard (13, 14), the average DSCs of the heart, esophagus, spinal cord, and left and right lungs for the observers (including CNN auto-contouring) met the standard. However, for RTP, attention is focused on the difference in dosimetry parameters for various structures. Yunfeng Cui et al. (10) reported that, for non-small-cell lung cancer (NSCLC), the dosimetric impact of the variation of contouring OARs is dependent on the proximity of the OAR to the target and the dose gradient in the OAR region. OAR dosimetry was not highly affected by contouring in the observed variation range in their report.
For the spinal cord and left and right lungs, in the comparison with the reference data, the DSCs and MSDs were not significantly different between the results of CNN auto-contouring and the manual contouring of other observers because these three OARs have high contrast differences on CT, and their boundaries can be clearly identified. For the heart, most of the regional boundaries were clear on CT, and the average DSCs obtained by the four observers were >0.9. However, the boundaries of the starting and ending positions of the heart are not clear. The superior aspect begins at the level of the inferior aspect of the pulmonary artery. The HU value of the end position of the heart is close to those of the mediastinum and liver. Therefore, a significant difference was observed between CNN-based auto-contouring and manual contouring. Compared to those of manual contouring, the average DSC was reduced by 0.04 and the MSD was increased by 2.0 mm in CNN-based auto-contouring. Due to the poor soft-tissue contrast on CT images, the indistinct boundary of the esophagus due to surrounding soft tissues, and its irregular shape, both the DSCs and MSDs of CNN-based auto-contouring were significantly different from those of manual contouring. The average DSC was reduced by 0.08, and the MSD was increased by 0.59 mm. It is possible that the 48 cases used for training did not include patients with various esophageal shape changes and density variations. Therefore, the use of more uniform standard structures as training data may improve the results.
For the heart, esophagus, spinal cord, and left and right lungs, using the same standardized guidelines, no statistically significant differences were observed between the reference data and the three observers for the DSCs and MSDs. Dawn C. (30) reported that the magnitude of the discrepancies did not appear to be correlated with the experience of the dosimetrist for the heart, esophagus and spinal cord.
In the study, we found that using CNN-based contouring as a first pass for manual segmentation can increase the work efficiency. For RTP, precise delineation of OARs is a time-consuming process, especially because some OARs are difficult to differentiate from the other structures. On some CT slices, even experts have difficulties reliably defining boundaries (such as the esophagus), which leads to a tedious interpretation of CT findings and makes the process time-consuming and highly prone to interobserver variability. Some studies have shown that user editing of contours autogenerated by software is a viable strategy for reducing the contouring time of OARs while conforming to local clinical standards (18, 31). In this study, when editing CNN-based contours, the time could be reduced to 15–20 min on average. More importantly, no significant differences were found in the results of manual contouring and edited contouring. Therefore, adjustment of the results generated by a CNN can save the time required for OAR contouring while maintaining the accuracy and consistency of the contours. Nevertheless, the results presented in this study did not show that interobserver variation was reduced by editing CNN-based auto-contouring results. Unlike multi-institutional comparisons, the results presented in this study were generated by observers at the same institution who follow the same clinical contouring practices and have similar subjective interpretations of organ boundaries. Yunfeng Cui et al. (10) reported that a segmentation atlas improved the contour agreement for the esophagus and heart in a multi-institutional preclinical trial planning study. In a future study, multi-institutional observers should be included to determine the areas of agreement. Further investigation is needed to determine whether auto-contouring methods as described in this study could potentially reduce the interinstitutional observer variability for OARs.
This study is a preliminary clinical study on the examination and comparison of the clinical use of neural networks regarding multiple OARs in CT images of lung cancer in RTP. The total size of the data was limited to 60 cases, which were split for training and testing. The training data size would limit the CNN performance. However, assembling a large well-labeled dataset with consistent standards is very difficult. We hope to have higher quality data in the future. To effectively increase the number of training samples, the training data were shuffled, and random processing tasks were performed during training. These image generator preprocessing tasks can reduce the training difficulty caused by having too few samples, reduce model overfitting and increase the stability of the model. The results are statistically significant.
Conclusions
In this study, publicly available lung cancer datasets were used as reference data. We compared and analyzed the differences between manual contouring by several observers and CNN-based auto-contouring for OARs. For the spinal cord and left and right lungs, no statistically significant differences were found between CNN-based auto-contouring and manual contouring. Further modifications to the heart and esophagus were necessary. Overall, editing CNN-based auto-contouring results can effectively shorten the contouring time while ensuring contouring quality.
Author Contributions
All authors contributed to the research. JiZ completed the CNN program, performed the data analysis, and drafted the manuscript. LC developed the study concept, including the study design and coordination. YL, JuZ, and YW performed the manual contouring. All authors read and approved the final manuscript.
Funding
This study was supported by the Guangdong Esophageal Cancer Institute Science and Technology Program (No. M201813).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00627/full#supplementary-material
References
1. Tao CJ, Yi JL, Chen NY, Ren W, Cheng J, Tung S, et al. Multi-subject atlas-based auto-segmentation reduces interobserver variation and improves dosimetric parameter consistency for organs at risk in nasopharyngeal carcinoma: A multi-institution clinical study. Radiother Oncol. (2015) 115:407–11. doi: 10.1016/j.radonc.2015.05.012
2. Nelms BE, Tomé WA, Robinson G, Wheeler J. Variations in the contouring of organs at risk: test case from a patient with oropharyngeal cancer. Int J Radiation Oncol Biol Phys. (2012) 82:368–78. doi: 10.1016/j.ijrobp.2010.10.019
3. Lin L, Dou Q, Jin YM, Zhou GQ, Tang YQ, Chen WL, et al. Deep learning for automated contouring of primary tumor volumes by MRI for nasopharyngeal carcinoma. Radiology. (2019) 291:677–86. doi: 10.1148/radiol.2019182012
4. Chen AM, Chin R, Beron P, Yoshizaki T, Mikaeilian AG, Cao M. Inadequate target volume delineation and local-regional recurrence after intensity-modulated radiotherapy for human papillomavirus-positive oropharynx cancer. Radiother Oncol. (2017) 123:412–8. doi: 10.1016/j.radonc.2017.04.015
5. Rosewall T, Bayley AJ, Chung P, Le LW, Xie J, Baxi S, et al. The effect of delineation method and observer variability on bladder dose-volume histograms for prostate intensity modulated radiotherapy. Radiother Oncol. (2011) 101:479–85. doi: 10.1016/j.radonc.2011.06.039
6. Jameson MG, Holloway LC, Vial PJ, Vinod SK, Metcalfe PE. A review of methods of analysis in contouring studies for radiation oncology. J Med Imaging Radiat Oncol. (2010) 54:401–10. doi: 10.1111/j.1754-9485.2010.02192.x
7. Brouwer CL, Steenbakkers RJ, van den Heuvel E, Duppen JC, Navran A, Bijl HP, et al. 3D Variation in delineation of head and neck organs at risk. Radiat Oncol. (2012) 7:32. doi: 10.1186/1748-717X-7-32
8. Peters LJ, O'Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. (2010) 28:2996–3001. doi: 10.1200/JCO.2009.27.4498
9. Jett JR, Schild SE, Keith RL, Kesler KA, American College of Chest P. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. (2007) 132(3 Suppl.), 266S−76S. doi: 10.1378/chest.07-1380
10. Cui Y, Chen W, Kong FM, Olsen LA, Beatty RE, Maxim PG, et al. Contouring variations and the role of atlas in non-small cell lung cancer radiation therapy: Analysis of a multi-institutional preclinical trial planning study. Pract Radiat Oncol. (2015) 5:e67–75. doi: 10.1016/j.prro.2014.05.005
11. Gore EM, Hu C, Ad VB, Robinson CG, Wheatley MD, Bogart JA, et al. Impact of incidental cardiac radiation on cardiopulmonary toxicity and survival for locally advanced non-small cell lung cancer: reanalysis of NRG oncology/RTOG 0617 with centrally contoured cardiac structures. Int J Radiation Oncol Biol Phys. (2016) 96:S129. doi: 10.1016/j.ijrobp.2016.06.316
12. Ciardo D, Gerardi MA, Vigorito S, Morra A, Dell'acqua V, Diaz FJ, et al. Atlas-based segmentation in breast cancer radiotherapy: Evaluation of specific and generic-purpose atlases. Breast. (2017) 32:44–52. doi: 10.1016/j.breast.2016.12.010
13. Conson M, Cella L, Pacelli R, Comerci M, Liuzzi R, Salvatore M, et al. Automated delineation of brain structures in patients undergoing radiotherapy for primary brain tumors: from atlas to dose-volume histograms. Radiother Oncol. (2014) 112:326–31. doi: 10.1016/j.radonc.2014.06.006
14. Dolz J, Kirisli HA, Fechter T, Karnitzki S, Oehlke O, Nestle U, et al. Interactive contour delineation of organs at risk in radiotherapy: clinical evaluation on NSCLC patients. Med Phys. (2016) 43:2569. doi: 10.1118/1.4947484
16. Shin HC, Roth HR, Gao M, Lu L, Xu Z, Nogues I, et al. Deep convolutional neural networks for computer-aided detection: CNN Architectures, Dataset Characteristics and Transfer Learning. IEEE Trans Med Imaging. (2016) 35:1285–98. doi: 10.1109/TMI.2016.2528162
17. Ali I, Hart GR, Gunabushanam G, Liang Y, Muhammad W, Nartowt B, et al. Lung nodule detection via deep reinforcement learning. Front Oncol. (2018) 8:108. doi: 10.3389/fonc.2018.00108
18. Lustberg T, van Soest J, Gooding M, Peressutti D, Aljabar P, van der Stoep J, et al. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiother Oncol. (2018) 126:312–7. doi: 10.1016/j.radonc.2017.11.012
19. Men K, Chen X, Zhang Y, Zhang T, Dai J, Yi J, et al. Deep deconvolutional neural network for target segmentation of nasopharyngeal cancer in planning computed tomography images. Front Oncol. (2017) 7:315. doi: 10.3389/fonc.2017.00315
20. Yang JS, Veeraraghavan G., van Elmpt H, Dekker W, Lustberg A, Gooding T, et al. Data from Lung CT Segmentation Challenge. Cancer Imaging Archive. (2017) doi: 10.7937/K9/TCIA.2017.3r3fvz08
21. Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, et al. The cancer imaging archive (TCIA): maintaining and operating a public information repository. J Digital Imaging. (2013) 26:1045–57. doi: 10.1007/s10278-013-9622-7
22. Yang J, Veeraraghavan H, Armato SG 3rd, Farahani K, Kirby JS, Kalpathy-Kramer J, et al. Autosegmentation for thoracic radiation treatment planning: A grand challenge at AAPM 2017. Med Phys. (2018) 45:4568–81. doi: 10.1002/mp.13141
23. Dolz J, Desrosiers C, Ben Ayed I. 3D fully convolutional networks for subcortical segmentation in MRI: a large-scale study. Neuroimage. (2017) 170:456–70. doi: 10.1016/j.neuroimage.2017.04.039
24. Shelhamer E, Long J, Darrell T. Fully Convolutional Networks for Semantic Segmentation. IEEE Trans Pattern Anal Mach Intell. (2017) 39:640–51. doi: 10.1109/TPAMI.2016.2572683
25. Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. MICCAI. (2015) 9351:234–41. doi: 10.1007/978-3-319-24574-4_28
26. Chen L, Zhu Y, Papandreou G, Schroff F, Adam H. Encoder-Decoder with Atrous Separable Convolution for Semantic Image Segmentation. arXiv: Computer Vision and Pattern Recognition, (2018).
27. Sudre CH, Li W, Vercauteren T, Ourselin S, Jorge Cardoso M. Generalised Dice Overlap as a Deep Learning Loss Function for Highly Unbalanced Segmentations. Cham: Springer International Publishing, (2017).
28. Kingma DP, Ba J. Adam: A Method for Stochastic Optimization. CoRR. (2014) abs/1412.6980.[Preprint] Available online at: https://arxiv.org/abs/1412.6980
29. Dice LR. Measures of the amount of ecologic association between species. Ecology. (1945) 26:297–302. doi: 10.2307/1932409
30. Collier DC, Burnett SS, Amin M, Bilton S, Brooks C, Ryan A, et al. Assessment of consistency in contouring of normal-tissue anatomic structures. J Appl Clin Med Phys. (2003) 4:17–24. doi: 10.1120/1.1521271
Keywords: contour variation, deep convolutional neural network, organs at risk, auto-contouring, lung cancer
Citation: Zhu J, Liu Y, Zhang J, Wang Y and Chen L (2019) Preliminary Clinical Study of the Differences Between Interobserver Evaluation and Deep Convolutional Neural Network-Based Segmentation of Multiple Organs at Risk in CT Images of Lung Cancer. Front. Oncol. 9:627. doi: 10.3389/fonc.2019.00627
Received: 13 October 2018; Accepted: 25 June 2019;
Published: 05 July 2019.
Edited by:
Issam El Naqa, University of Michigan, United StatesReviewed by:
Bilgin Kadri Aribas, Bülent Ecevit University, TurkeyYoganand Balagurunathan, Moffitt Cancer Center, United States
Copyright © 2019 Zhu, Liu, Zhang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Chen, Y2hlbmx4QHN5c3VjYy5vcmcuY24=
 Jinhan Zhu
Jinhan Zhu Yimei Liu
Yimei Liu