
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 19 June 2019
Sec. Molecular and Cellular Oncology
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00544
Tumor cells require high rates of lipid synthesis to support membrane biogenesis for their exacerbated growth. The only two proteins known that activate phospholipid synthesis are Fra-1 and c-Fos, two members of the AP-1 family of transcription factors. These proteins that are overexpressed in human breast malignant tumors increase the rate of phospholipid synthesis at the endoplasmic reticulum through a mechanism independent of their nuclear function. The aim of this study was to inhibit breast tumor cell proliferation by modulating c-Fos and Fra-1 and regulate membrane biogenesis by controlling lipid synthesis rates. The molecular mechanism by which Fra-1 and c-Fos activate phospholipid synthesis was examined. Both proteins physically associate with the rate limiting enzyme CDP-DAG synthase through their N-terminus domain and activate it through their basic domain; neither protein associates to or activates the enzyme phosphatidylinositol synthase as determined through in vitro enzymatic reactions and FRET experiments. The N-terminus domain of both proteins act as negative dominant peptides that physically associate with CDP-DAG synthase but do not activate it. Proliferation of MDA-MB231 and 4T1 cells was impaired in vitro after inducing them to proliferate in the presence of the negative dominant peptides derived from Fra-1 and c-Fos. When tumors generated in Balb/c mice with the breast tumor cell line 4T1 were treated with these negative dominant peptides, a significant reduction in tumor growth was observed. Consequently, these Fra-1 and c-Fos negative dominant peptides can be exploited as a new therapeutic strategy to impair breast tumor cell proliferation.
Breast cancer is the most frequent cancer among women (25% of all cancers) and the main cause of cancer deaths in less developed countries (1). According to the World Health Organization, its incidence is increasing worldwide. Although prevention and early detection significantly reduce death risk, they do not eliminate most breast cancers that develop in low- and middle-income countries where these are diagnosed at late stages and generally result in patient death. Breast cancer is the fifth cause of cancer deaths after lung (1.6 million deaths), liver (745,000 deaths), stomach (723,000 deaths), and colorectal (694,000 deaths) cancers (2). So, development of new therapeutic strategies targeted to inhibit breast tumor cell proliferation in established tumors is essential.
Fra-1 and c-Fos are two well-known oncogenes, members of the AP-1 family of transcription factors (3, 4). Their nuclear activity and overexpression in diverse tumors has been widely reported (5). Fra-1 is overexpressed in breast, lung, colon, esophagus, bladder, thyroid and hepatocellular tumors, among others (5–13). Moreover, Fra-1 has been implicated in cervical cancer stem cell radio-resistance (14), in the malignant progression of esophageal squamous cell carcinoma through MAPK/MEK/ERK/FRA-1 pathway activation (15), in epithelial-mesenchymal transition regulation of colorectal cancer cells (16, 17), in cancer stem cell formation from non-stem cells in a PKCα-dependent activation of Fra-1 (18), and in the inhibition of p53-dependent apoptosis in lung cancer (19). However, despite having been studied for almost 30 years, the cellular consequences of c-Fos and Fra-1 expression upon mitogen stimulation have not been completely elucidated.
In breast tumors, the transcription factors Fra-1 and c-Fos are also expressed in the cytoplasm where they activate phospholipid synthesis (20). Phospholipids are the quantitatively most important components of cell membranes thus making their production essential to sustain cell proliferation, differentiation and growth. Tumor cells are characterized by their exacerbated growth and so, demand high rates of phospholipid synthesis to support increased membrane biogenesis. Fra-1 and c-Fos show a similar capacity to activate phospholipid synthesis in proliferating breast tumor cells in culture (MDA-MB231 and MCF-7 cells) and in human breast tumor tissues. In more than 200 malignant human breast tumors examined (Invasive ductal carcinoma, Medullary carcinoma, Phyllodes sarcoma, Mucinous carcinoma, lobular carcinoma in situ and squamous cell carcinoma) 95% of both proteins were significantly overexpressed and 100% had either Fra-1 or c-Fos overexpressed contrasting with their undetectable levels in normal tissue. Fra-1 was found mainly in the cytoplasm: 69% of tumor samples showed only cytoplasmic Fra-1, while the remaining 31% contained both nuclear and cytoplasmic Fra-1. c-Fos was also preferentially in the cytoplasm of the tumor samples: 100% showed cytoplasmic c-Fos and 63% also contained nuclear c-Fos. In all cases, Fra-1 and c-Fos localized with the Endoplasmic Reticulum (ER) marker calnexin where they both participate in the bulk phospholipid synthesis. Silencing Fra-1 and c-Fos simultaneously and, more importantly, blocking the cytoplasmic activity of these proteins with specific antibodies significantly inhibits lipid synthesis activation and cell proliferation in MDA-MB231 cells (20). Based on these results, cytoplasmic c-Fos and Fra-1 deserve to be considered as potential targets to control proliferation of breast cancer cells. To seek a therapeutic application of these results, we determined the molecular mechanism by which these proteins activate phospholipid synthesis. For c-Fos to promote activation, it must associate with enzymes of the lipid synthesis pathway in the ER, through its N-terminal domain (aa 1–138) and increases their catalytic activity through its basic domain (aa 139–159) (21). c-Fos association to the ER is regulated by the phosphorylation status of its tyrosine-residues #10 and #30 (22).
c-Fos activates several and the same enzymes in different cell types. Specifically, c-Fos activates 1-acylglycerol-3-phosphate acyltransferase, CDP-diacylglycerol synthase (CDS) the rate-limiting enzyme of the phosphoinositide synthesis pathway, phosphatidylinositol 4-kinase II α (PI4KIIα) and Lipin1β which drives phosphatidic acid into the Kennedy pathway. However, c-Fos does not modify the activity of phosphatidylserine synthases 1 and 2, phosphatidylinositol synthase (PIS) or PI4KIIβ (21, 23, 24). A similar effect is observed for glycosphingolipid synthesis, in which c-Fos activates glucosylceramide synthase but does not affect glucosylceramide galactosyltransferase 1 or lactosylceramide sialyltransferase 1 (25). Fra-1 also activates the overall synthesis of phospholipids and has been shown to activate Lipin 1β (20, 26). However, the mechanism by which Fra-1 activates phospholipid synthesis still remains unexplored.
Herein, we examine the mechanism by which Fra-1 activates phospholipid synthesis in a breast tumor cell model. Two enzymes were examined; one that was previously shown to be activated by c-Fos (CDS) and one whose activity is not modified by c-Fos (PIS). We observed that Fra-1 associates to CDS1 and activates total CDS, whereas it neither associates to nor activates PIS.
Results shown herein indicate that Fra-1 and c-Fos can be the foundation for a novel therapeutic strategy to inhibit breast tumor growth by impairing membrane biogenesis.
MDA-MB231 and 4T1 cells from ATCC were cultured as indicated by the supplier. Mycoplasma control was regularly performed. Cultured cell quiescence was achieved after culturing 48 h using DMEM without FBS and phenol red. Cells were induced to re-enter growth by adding 20% FBS.
Transfections were performed following the manufacturer's instructions in 24 multi-well plates using Lipofectamine 2000 (Invitrogen) plus 400 ng of pTQ2-Fra-1-NA or pTQ2-c-Fos-NA, or a mixture of 200 ng of each. Control cells received 400 ng of the pTQ2 vector. Twenty-four hours after transfection, cells were cultured in serum-free media for 48 h to achieve quiescence (0 h), then 20% FBS was added to the culture medium (mitotic stimulus) and cells cultured for an additional 30 h. Turquoise fluorescence was used to determine the number of transfected cells; total cell number by propidium iodide staining and double blind cell counting in each condition was performed in at least 20 fields per experiment. By subtracting the number of transfected cells from total cells, the number of non-transfected cells was determined at 0 h and at 30 h. Cell proliferation was calculated as the ratio between the number of cells at 30 h/0 h.
Recombinant proteins were expressed as His-tagged recombinant proteins in the BL21 strain of Escherichia coli and purified as described previously (27, 28) by nickel affinity chromatography. Cells were re-suspended in lysis buffer containing 6 M urea for wild type proteins or without 6 M urea for deletion mutants and lysed using the Avestin Emulsiflex C3. Cell homogenates were run through a HisTrap HF column (GE Healthcare Life Sciences), washed thoroughly and proteins purified using an Äkta Purifier 10 (GE Healthcare Life Sciences). Elution buffer (0.5 M NaCl, 20 mM Tris-HCl pH 8, 500 mM imidazole) with Urea (6M) was used for c-Fos and Fra-1, and without urea for Fra-1 deletion mutants and c-Fos-NA. Final imidazole concentration in the eluted proteins was 171 mM.
Reactions performed in a final volume of 80 μL contained, 100 μg of quiescent MDA-MB231 cell homogenate protein in 50 mM Tris-HCl, pH 8 (enzyme source). For enzyme activity assays in animal excised tumors, homogenates were prepared in water containing protease inhibitors using a Potter-Elvehjem Tissue Grinder, prior to sonication. Total CDS activity was assayed as described by Lykidis et al. (29) with or without the recombinant c-Fos, Fra-1 or its deletion mutants Fra-1-NA, Fra-1-NB, Fra-1-BC, as stated in each case, suspended in 3 μL of elution buffer or an equal volume of elution buffer for control reactions.
Total PIS activity reactions were as described by Lykidis et al. (29) in the presence of c-Fos or Fra-1 suspended in 3 μL of elution buffer or with elution buffer for control reactions.
MDA-MB231 cells were co-transfected with pEGFP-Fra-1 or pEGFP-c-Fos and both NA deletion mutants fused to the HA tag or the control with the empty vector. Immunofluorescence was performed using anti-HA (mouse Sigma #H9658 1:100) and anti-calreticulin (rabbit Thermo Scientific #PA3-900 1:800), secondary antibodies: anti-mouse 546 and anti-rabbit 643 (1:600). A spectral Olympus (Center Valley, PA) FV 1200 confocal laser scanning microscope with a plan apochromatic 60x (SC), numerical aperture 1.4, oil immersion objective lens zoomed digitally 5x was used. Mander's colocalization coefficient M1 (proportion of Fra-1 or c-Fos present in the ER) with threshold was used to quantify colocalization using the Fiji software and the Jacop plugin.
FRET experiments were performed as described previously (21). Cells transfected with GFP derivatives-coupled enzymes and proteins/peptides as indicated were arrested for 48 h. CDS1 and PIS (pSYFP2-N1) and pTQ2-c-Fos were previously used (21). Wild type Fra-1 and truncated mutants of Fra-1 were in pTurquoise2-N1. Synthesis of endogenous c-Fos and Fra-1 was impaired using 50 μg/mL of cycloheximide 1 h prior to inducing cells to re-enter growth with FBS. Fifteen minutes later, cells were fixed with 4% paraformaldehyde for 10 min at room temperature. Mounted Coverslips were visualized using an Olympus FV300 confocal laser scanning microscope with a plan apochromatic 63x, numerical aperture 1.4, oil immersion objective lens zoomed digitally 2x. FRET determinations were by measuring sensitized emission (30, 31). Images analyzed with Fiji (NIH, USA) were pseudo-colored to illustrate the calculated efficiencies distribution in cells.
This study was carried out in accordance with the recommendations of the ethics committee of experimental protocols for the use of animals (Facultad de Ciencias Químicas, Universidad Nacional de Córdoba). The protocol was approved by the ethics committee of experimental protocols for the use of animals (Facultad de Ciencias Químicas, Universidad Nacional de Córdoba. Resolution N° 2102). The mouse-4T1 breast tumor model was used as described in Pulaski et al. (32). Ten days after inoculation, animals received an intra-tumor injection at 3-days intervals of either Fra-1-NA + c-Fos-NA-containing liposomes (treated) or empty liposomes (controls). Liposomes were prepared by the lipid film hydration method (33, 34). Briefly, diphosphatidylcholine, diphosphatidylserine, and cholesterol (Avanti Polar Lipids 850355P, 840037P, 700000P) (70:10:20 ratio) were dissolved in chloroform (5 mg/mL) and evaporated under nitrogen. Hydration was performed by addition of the peptide solution or vehicle (peptide elution buffer) followed by five cycles of incubation at 55 °C and vortexing. Liposome size was determined by light scattering. A total amount of 22.5 nmol of peptides/mouse in 5 injections delivered 3 times a week was used in low dose experiments. Each injection contained 2.5 nmol of Fra-1-NA (30 μg) plus 2 nmol of c-Fos-NA (30 μg) contained in liposomes re-suspended in 50 μL of peptide elution buffer for the first 2 injections and in 100 μL for the last 3 injections. Empty liposomes (controls) were re-suspended in elution buffer as for the treated animals. For high dose experiments, total peptide administered was 45 nmol/mouse in 5 injections delivered 3 times a week, each containing 5 nmol of Fra-1-NA (60 μg) plus 4 nmol of c-Fos-NA (60 μg) re-suspended in 50 μL of elution buffer for the first 2 injections and in 100 μL for the last 3 injections. Injection volume was varied due to the variation of the tumor volume.
When each mouse harbored 2 tumors, animals received a 2 × 105 4T1 cell inoculation into each fat pad and upon tumor generation, one tumor was subjected to the high dose treatment protocol described above whereas the other tumor of the same animal was treated as described for the control group. In all cases, tumors (length, width) were measured using a caliper. For western blot experiments, tumors were excised and frozen (−80°C).
Using sonication, tumor tissues were processed in ice-cold RIPA buffer (150 mM NaCl, 1% Triton, 50 mM Tris-HCl pH 6.8, 0.5 mM EDTA, SDS 0.01%) plus protease inhibitor (Roche) and centrifuged for 10 min at 12,000 g. Protein extracts were separated on a 12% SDS-PAGE and transferred to nitrocellulose membrane (Pall Corporation). After blocking with 5% non-fat milk in 1X PBS—0.05% Tween-20, membranes were incubated with primary antibodies (mouse monoclonal anti-α-tubulin, Abcam ab7291; 1:5,000 and anti-PCNA, Santa Cruz PC10; 1:250) overnight at 4°C. After washing, blots were incubated with donkey anti-mouse IRDye IgG secondary antibody (LI-COR Bioscience, Lincoln, NE, USA; 1:25,000) at room temperature for 1 h. Immuno-detection was performed using ODYSSEY Infrared Imaging System (LI-COR Bioscience).
Previous results from human breast tumors show that 100% of these overexpress either Fra-1 or c-Fos or both oncoproteins; furthermore, non-fixed samples showed activated phospholipid synthesis with respect to control human breast tissue (20). To evaluate the potential therapeutic use of peptides derived from these proteins, we determined if these two proteins share a common mechanism to activate the synthesis of phospholipids, by evaluating their capacity to regulate two enzymes of the pathway of phospholipid synthesis.
To assess the capacity of Fra-1 to activate the enzymes CDS and PIS in vitro, total homogenate (TH) from quiescent MDA-MB231 cells was used as enzyme source. Total CDS activity is linear up to 60 min of incubation (Figure 1A) whereas PIS activity is linear up to 15 min of incubation (Figure 1B). At linearity, CDS activity increased in the presence of 0.5 ng of recombinant Fra-1/μg of TH in comparison with the control. By contrast, PIS activity was not significantly modified by the addition of Fra-1 to the assay as compared to the control (Figure 1B). For PIS, increasing concentrations of recombinant Fra-1 were also tested with no changes under any of the experimental conditions evaluated [0–3 ng of Fra-1/μg TH (Supplementary Figure 1)]. Essentially the same results were found when recombinant c-Fos (0.5 ng/μg of TH) was assayed (Figures 1A,B).
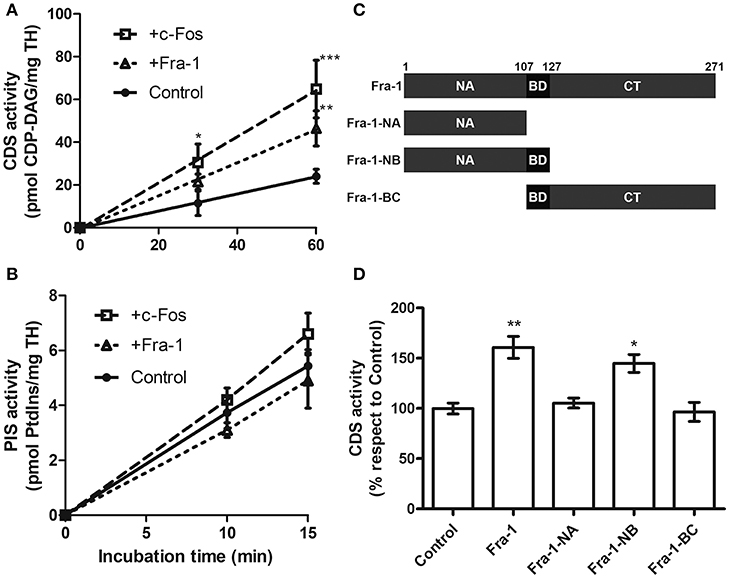
Figure 1. Fra-1 activates CDS through its NB domain. (A,B) Total homogenate (TH) from quiescent MDA-MB231 cells was used as enzyme source to evaluate total CDS and PIS activity in the presence of Fra-1 (0.5 ng of recombinant Fra-1 per ug of TH; short dashed line) or c-Fos (0.5 ng of recombinant c-Fos per ug of TH; long dashed line) in comparison with the controls that contained only the vehicle used to re-suspend Fra-1 or c-Fos (continuous line). Both c-Fos and Fra-1 significantly activate CDS (A) at the incubation times examined whereas neither of them significantly modified PIS activity (B). (C) Schematic representation of the deletion mutants of Fra-1. The numbers on the top of the scheme indicate the amino acid position. Fra-1-NA: N-terminus (aa 1–107), Fra-1-NB: N-terminus plus basic domain (aa 1–127), Fra-1-BC: basic domain plus the C-terminus (aa 108–271). (D) CDS activity was evaluated in the presence of recombinant Fra-1 or of its deletion mutants. Only Fra-1-NB activates the enzyme to similar levels as those obtained with the full-length version, whereas Fra-1-NA that lacks the BD or Fra-1-BC that lacks the NA do not retain this activating capacity. *p < 0.05; **p < 0.01; ***p < 0.001 (Two-way ANOVA in A,B and 1 way ANOVA in D). Results are the mean CDS activity ± SEM of three independent experiments performed in triplicate.
To determine which domains of Fra-1 are involved in CDS activation, CDS was assayed in the presence of the deletion mutants of Fra-1 schematized in Figure 1C: Fra-1-NA (aa 1–107), Fra-1-NB (aa 1–127) and Fra-1-BC (aa 108–271). Fra-1-NB activates CDS to comparable levels as full-length Fra-1 whereas no activation was observed with Fra-1-NA or Fra-1-BC (Figure 1D). Note that Fra-1-NB is the only mutant assayed that contains both the NA and the BD domains of Fra-1.
FRET experiments were performed to evaluate if Fra-1 physically interacts with CDS1 or PIS. MDA-MB231 cells were co-transfected with constructs encoding full length Fra-1 fused to a donor FRET-pair (pTQ2) and each enzyme assayed, CDS1 or PIS, fused to an acceptor FRET-pair (pSYFP). Results show that Fra-1 associates with CDS1 (Figure 2A, row 2) but not with PIS (Figure 2A, row 3). Cells co-transfected with the pTQ2 vector and CDS1 fused to pSYFP show negative FRET values (negative control) (Figure 2A, row 1). Positive FRET values were also found with tagged c-Fos and CDS1 but not with tagged c-Fos and PIS (Figure 2A, rows 4 and 5). Quantification shows statistically significant FRET efficiencies with CDS1 when compared to negative controls whereas neither Fra-1 nor c-Fos shows positive FRET efficiencies when assayed with PIS (Figure 2B). These results evidence that Fra-1 and c-Fos physically associate with the enzyme (s) they activate, like CDS1, but not with those they do not activate, such as PIS.
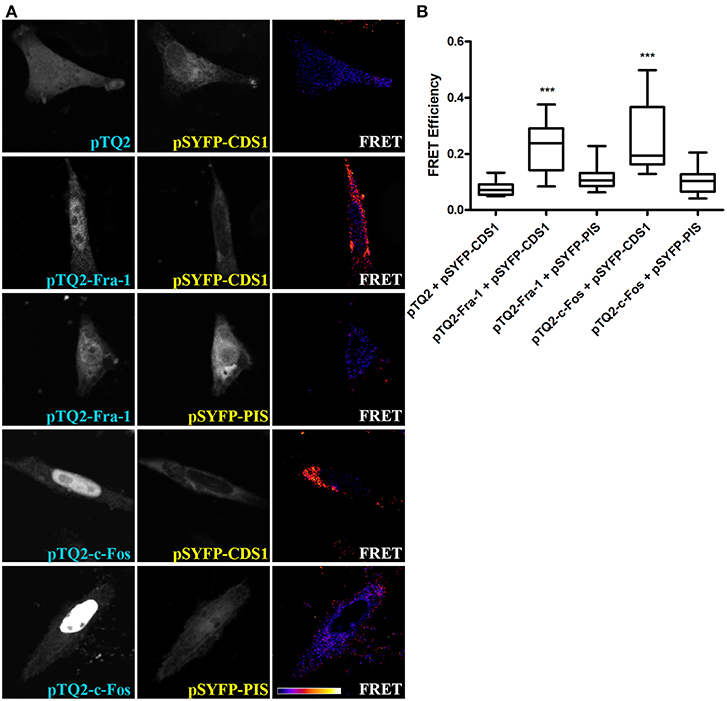
Figure 2. Fra-1 and c-Fos physically associate with CDS1 but not with PIS. (A) MDA-MB231 cells were co-transfected to express pTQ2 and pSYFP-CDS1 (first row) as a negative control, pTQ2-Fra-1 and pSYFP-CDS1 (second row), pTQ2-Fra-1 and pSYFP-PIS (third row), pTQ2-c-Fos and pSYFP-CDS1 (fourth row) or pTQ2-c-Fos and pSYFP-PIS (last row), and examined by confocal microscopy; for FRET efficiency quantification, the sensitized emission method was applied. The first column shows tagged Fra-1 or c-Fos expression, the second, the expression of the tagged enzyme and the third column shows FRET efficiency; the scale bar on the bottom row shows an increasing FRET efficiency scale from violet (no FRET) to white. (B) FRET efficiency quantification is depicted as boxplot graphs representing the medians (central horizontal bars), the 25–75th percentile interquartile range (box limits), and the lowest and highest values (whiskers). ***p < 0.001 (Kruskal-Wallis test, Dunn's post-test). One representative experiment of three performed that gave essentially the same results, is shown.
To determine which domain (s) of Fra-1 is/are involved in physical association with CDS1, we performed FRET experiments with the three Fra-1 deletion mutants Fra-1-NA, Fra-1-NB and Fra-1-BC (Figure 1C). MDA-MB231 cells were co-transfected with constructs codifying for Fra-1 or its deletion mutants fused to pTQ2 and CDS1 fused to pSYFP. The mutants Fra-1-NA and Fra-1-NB show positive FRET efficiencies with CDS1, similar to that observed with full-length Fra-1 and statistically different from the negative control (pTQ2 and CDS1 fused to SYFP) (p < 0.001 and p < 0.01, respectively) (Figures 3A,B). By contrast, Fra-1-BC that lacks the N-terminus domain, does not associate with CDS1 showing similar FRET efficiency to that of the negative control (p > 0.05) (Figure 3A, row 5 and Figure 3B). These experiments evidence that Fra-1 associates to CDS1 through its N-terminus domain and that the BD domain is not essential for this association.
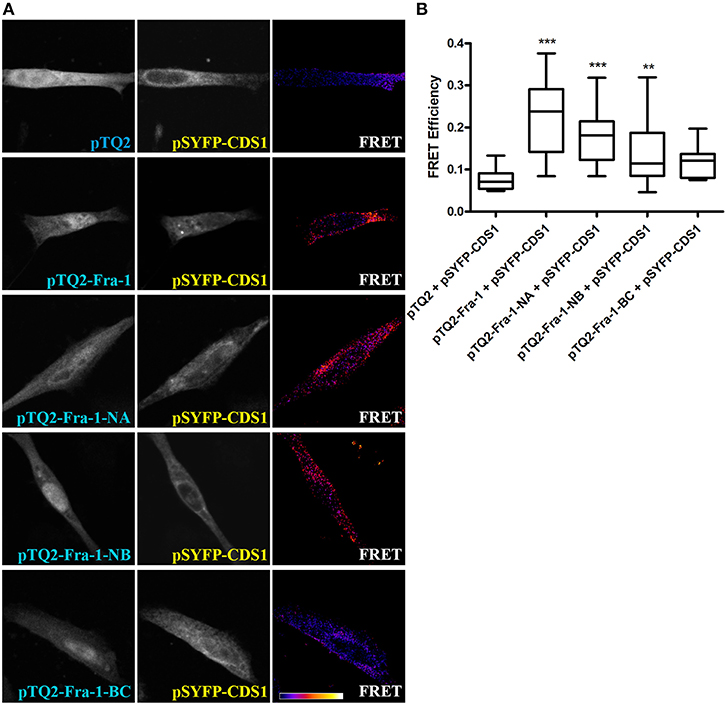
Figure 3. The N-terminus domain of Fra-1 physically associates with CDS1. (A) MDA-MB231 cells were co-transfected to express the negative control pTQ2 and pSYFP-CDS1 (first row), pTQ2-Fra-1 and pSYFP-CDS1 (second row), pTQ2-Fra-1-NA and pSYFP-CDS1 (third row), pTQ2-Fra-1-NB and pSYFP-CDS1 (fourth row) or pTQ2-Fra-1-BC and pSYFP-CDS1 (last row), and were examined by confocal microscopy. FRET quantification was performed by the sensitized emission method. The first column shows the expression of tagged Fra-1 or of its deletion mutants, the second of the tagged enzyme and the third column shows FRET efficiency. The scale bar on the bottom row of the right column shows an increasing FRET efficiency scale from violet (negative) to white (maximum). (B) FRET efficiency quantification depicted as boxplot graphs representing the medians (central horizontal bars), the 25–75th percentile interquartile range (box limits), and the lowest and highest values (whiskers). **p < 0.01; ***p < 0.001 (Kruskal-Wallis test, Dunn's post-test). One representative experiment of three performed, which gave essentially the same results, is shown.
Previous reports indicate that Fra-1 and c-Fos colocalize with the ER marker calnexin in human breast tumor samples, and in MDA-MB231 and MCF7 breast tumor cell lines (20). Herein, we quantified the colocalization between Fra-1 or c-Fos and the ER marker calreticulin in the absence or in the presence of Fra-1-NA plus c-Fos-NA deletion mutants by determining Mander's colocalization coefficient M1. For this, MDA-MB231 cells were co-transfected with pEGFP-Fra-1 or pEGFP-c-Fos and the N-terminal deletion mutants fused to the HA tag, that is Fra-1-NA-HA and c-Fos-NA-HA or the control with the HA plasmid. GFP signal was directly detected whereas HA and calreticulin were detected by indirect immunofluorescence using specific antibodies. The results in Supplementary Figure 2 show that Mander's colocalization coefficient M1 that is the proportion of Fra-1 or c-Fos localized in the ER compartment, decreases in the presence of Fra-1-NA and c-Fos-NA.
Since Fra-1 and c-Fos physically associate with CDS1 through their N-terminus domain and activate it through their BD domain, and both are overexpressed in breast tumor tissues in comparison with their undetectable levels in normal tissue (20), we hypothesized that the NA domains of both proteins, could function as negative dominants of the cytoplasmic activity of c-Fos and Fra-1 by associating with the enzymes that the full-length proteins activate, but without activating them. So, it is expected that the presence of these deletion mutants, will promote a decrease in phospholipid biosynthesis rates and therefore will decrease or inhibit membrane biogenesis-dependent cell proliferation. To test this hypothesis, we transiently transfected MDA-MB231 cells to overexpress either Fra-1-NA, c-Fos-NA, or both, tagged with a TQ2 fluorescent protein. At 48 h after transfection, quiescent cells were induced to re-enter growth and proliferation assessed as described under materials and methods. Overexpression of either of the NA deletion mutants alone or, both mutants together, significantly decrease the number of cells at 30 h after the mitotic stimulus as compared to controls (Figure 4A). Similar experiments were performed using the 4T1 mouse mammary carcinoma cell line showing similar results (Figures 4B,C). The presence of the pTQ2 vector did not significantly alter 4T1 (Figures 4B,C) or MDA-MB231 cell proliferation as compared to non-transfected cells.
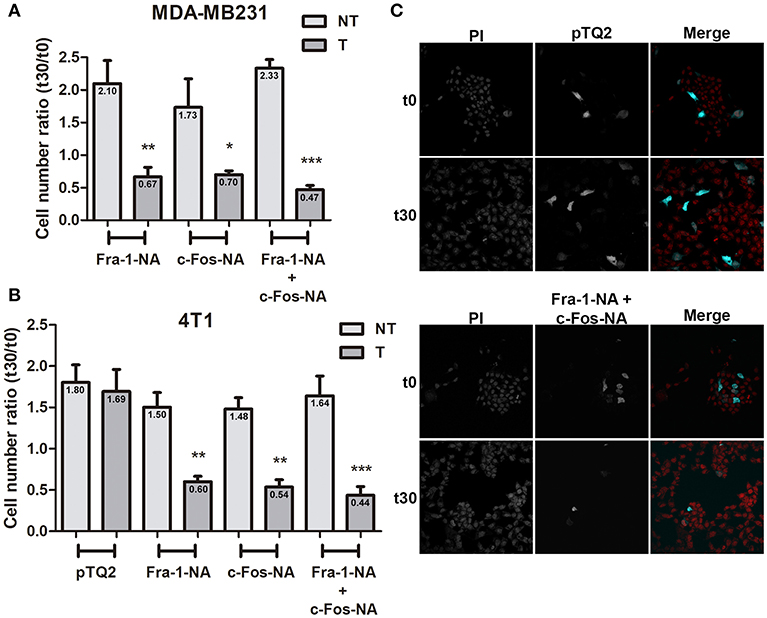
Figure 4. Fra-1-NA and c-Fos-NA inhibit MDA-MB231 and 4T1 cell proliferation. MDA-MB231 (A) or 4T1 cells (B) were transfected to express pTQ2-Fra-1-NA, or pTQ2-c-Fos-NA, or pTQ2-c-Fos-NA + pTQ2-Fra-1-NA. The number of cells expressing the turquoise fluorescent protein (T: dark gray bars) and the number of non-transfected (NT: light gray bars) cells (propidium iodide stained cells—turquoise cells) present in the same well were counted at 0 h (arrested cells: t0) and 30 h after a mitotic stimulus (20% fetal bovine serum: t30). The ratio between the number of T or NT cells at t30 and t0 is shown and the mean indicated in each bar. Results are expressed as mean ± SEM (n = 3). *p < 0.05; **p < 0.01 ***p < 0.001 (ANOVA, Bonferroni post-test). (C) Representative images of 4T1 cells at t0 (arrested cells) and t30 (after 30 h of a mitotic stimulus) of the control condition transfected with the pTQ2 vector (upper panel) and the condition transfected with pTQ2-c-Fos-NA + pTQ2-Fra-1-NA (lower panel). Total cells stained with PI (left column), and transfected cells (middle column) are shown in black and white and the colored merge in right column. PI: propidium iodide (red), TQ2 (cyan). Original Magnification 400×.
As both Fra-1-NA and c-Fos-NA interfere with the proliferation of breast tumor cells in culture, we verified if these deletion mutants were capable of interfering with breast tumor growth in vivo. For this, the 4T1 breast tumor model was used (32). As indicated under Materials and Methods, two protocols were used for treatment, one with a 60 μg dose of 30 μg each of Fra-1-NA plus c-Fos-NA per injection (4.5 nmol per injection) and the other with a 120 μg dose of 60 μg of each peptide per injection (9 nmol per injection). After 15 days of treatment with both doses of Fra-1-NA- plus c-Fos-NA-containing liposomes, a significant reduction in tumor volume and tumor growth rate was found as compared to control tumors (Figures 5A,C: low dose; Figures 5B,D: high dose). However, by day 15 of treatment (day 25 post inoculation), control tumors reached a tumor volume ~50% larger than those treated with the lowest dose of both N-terminus deletion mutants whereas they almost doubled the size of those treated with the higher dose. No significant differences were observed in the body weight between groups (Figures 5E,F).
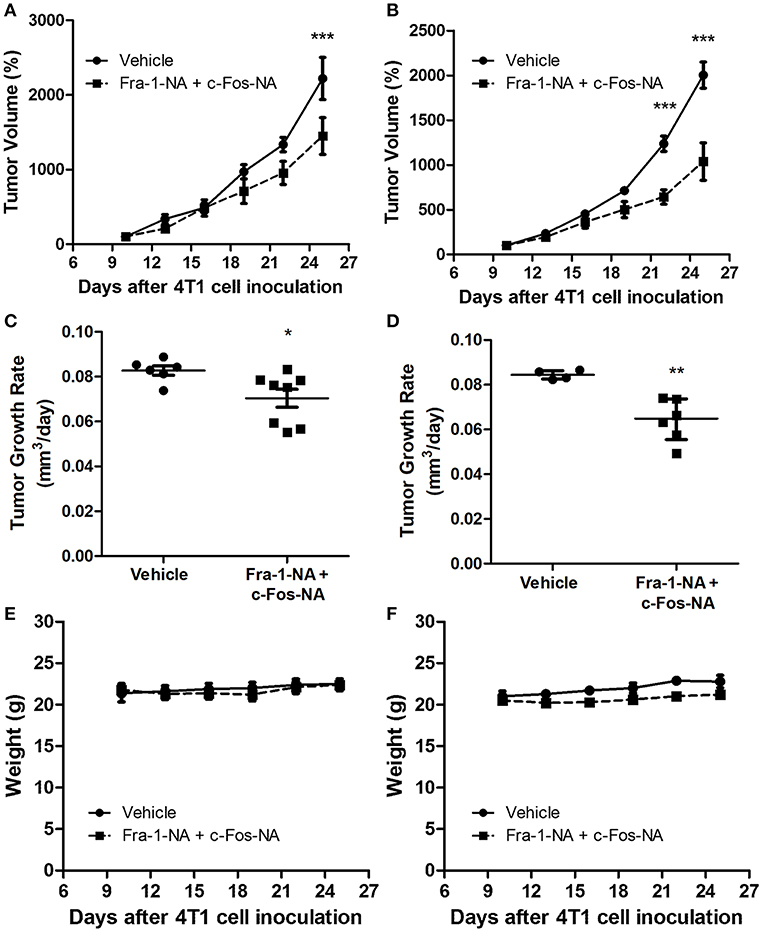
Figure 5. Fra-1-NA plus c-Fos-NA inhibit breast tumor growth. Mice bearing one tumor received an intratumoral injection of liposomes containing Fra1-NA plus c-Fos-NA. Peptide administered: 5 injections containing 4.5 nmol per injection (dashed line) or vehicle (continuous line) (A,C,E) or 9 nmol per injection (B,D,F) delivered three times a week for 15 days. In (A,C,E), control group: n = 6; treated group: n = 8; in (B,D,F), control group: n = 4; treated group: n = 6. (A,B) Tumor volumes were measured at 3-day intervals during the treatment period. Results are expressed as the percent volume taking the volume of the tumor at 10 days of inoculation as 100%. ***p < 0.001 (Two-way ANOVA, Bonferroni post-test). (C,D) Tumor growth rates were calculated for each group at the end of the treatment as the slope of the curve obtained by plotting the log10 of the tumor volume in function of the days of treatment. Horizontal bars represent mean (C) or median (D) values according to the distribution of the data (parametric and non-parametric ANOVA, respectively) *p < 0.05; **p < 0.01. (E,F) Body weight of animals during the treatment period.
To evaluate the proliferating status of the treated and control tumors, once tumors were excised from the animals of the high dose experiment, protein extracts were prepared from the tumors and proliferating cell nuclear antigen (PCNA) protein levels determined by western blot. Fra-1-NA plus c-Fos-NA-treated tumors showed a significant decrease in PCNA protein levels in comparison to the controls (Figure 6). The lowest PCNA levels in individual samples were found in the tumors with the lowest growth rate (data not shown), indicating a good correlation between the amount of PCNA found and the tumor growth rate calculated for each tumor.
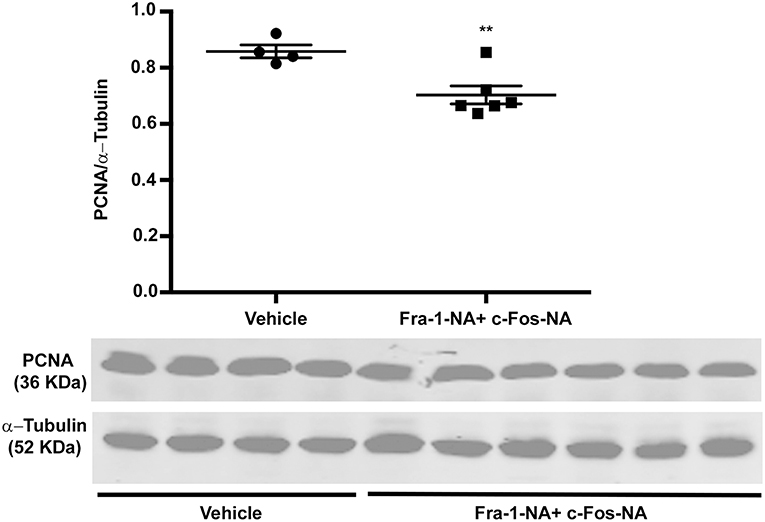
Figure 6. Fra-1-NA plus c-Fos-NA treatment decreases PCNA protein levels. Protein extracts prepared from the tumors excised from tumor-bearing Balb/c mice treated or not with Fra-1-NA plus c-Fos-NA were subjected to western blot analysis to examine PCNA and α-tubulin (as loading control) levels as described under Materials and Methods. PCNA and α-tubulin levels of all the tumors of the high dose treatment experiment with their controls are shown. Scatter dot plot represents the densitometric quantification and horizontal bars the mean ± SEM (Student t-test). **p < 0.01. Control group: n = 4; treated group: n = 6.
To evaluate if the Fra-1-NA plus c-Fos-NA-treatment was triggering a systemic effect on tumor growth, assays were performed inoculating 4T1 cells into both flanks of each mouse and 10 days later, one of the two tumors generated was treated with the high dose protocol whereas the other was treated with vehicle. Consistently, tumors treated with the deletion mutants grew to a significantly smaller tumor volume and evidenced a decreased tumor growth rate when compared with the contralateral control tumor (Figures 7A,B). Once excised from the animals, paired control and treated tumors from two mice were used to evaluate total CDS activity. In both cases, CDS activity measured in TH of the vehicle-treated tumor was significantly higher than that obtained in TH of the paired Fra-1-NA plus c-Fos-NA-treated tumor from the same mouse (light gray bars in Figures 7C,D). Furthermore, when these tumor samples were stripped of associated proteins with 1M KCl and subjected to subcellular fractionation by ultracentrifugation, CDS activity in the microsomal fraction (MF) was no longer different in the control as compared to the treated tumors (white bars in Figures 7C,D). As expected, the addition of recombinant Fra-1 (dark gray bars in Figure 7C) or c-Fos (dark gray bars in Figure 7D) activated CDS but if in addition to the recombinant Fra-1 or c-Fos proteins, the N-terminus deletion mutants were included in the reaction, CDS activity decreased to similar levels of those obtained in the MF treated with KCl 1M of each condition (black bars in Figures 7C,D).
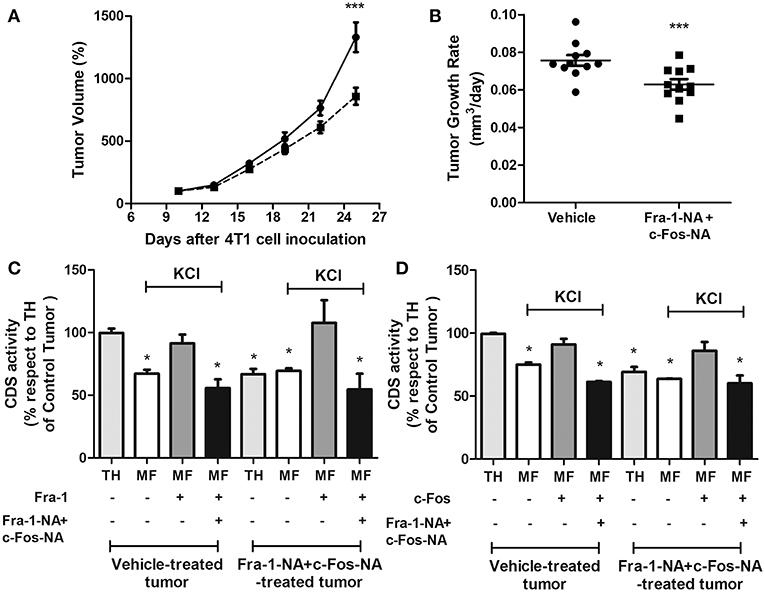
Figure 7. When grown in the same animal, Fra-1-NA+c-Fos-NA treatment impairs tumor growth and CDS activity. Mice bearing two tumors received intratumoral injections of liposomes containing equal concentrations of Fra1-NA plus c-Fos-NA into one tumor and empty liposomes into the other. Peptide administered: 5 injections containing a total of 9 nmol of Fra-1-NA plus c-Fos-NA peptides per injection (dashed line) or vehicle (continuous line) delivered three times a week for 15 days. (A) Tumor volumes were measured at 3-day intervals during the treatment period. Results are expressed as the percent volume taking the volume of the tumor at 10 days of inoculation as 100%. ***p < 0.001 (Two-way ANOVA, Bonferroni post-test) (B) Tumor growth rates were calculated for each group at the end of the treatment as the slope of the curve obtained by plotting the log10 of the tumor volume in function of the days of treatment. Horizontal bars represent mean values. ***p < 0.001 (Paired t-test). Mice examined: n = 11. (C,D) CDS activity was measured in TH (light gray bars) and the microsomal fraction (MF) stripped with KCl 1M (white, dark gray and black bars) from vehicle-treated tumors and Fra-1-NA plus c-Fos-NA-treated tumors of two mice. Recombinant wild type Fra-1 (C), recombinant wild type c-Fos (D) and/or Fra-1-NA + c-Fos-NA (C,D) were added to the reaction as indicated in the figure. Experiments were performed in triplicate; bar graphs represent the mean ± SEM. All experimental situations were compared to TH from the non-treated samples. *p < 0.05 (One-way ANOVA, Dunnett post-test).
Most studies regarding c-Fos and Fra-1 are related to their role as transcription factors. However, both proteins also activate phospholipid synthesis by a non-genomic mechanism (35). In breast tumor models, c-Fos and Fra-1 activate phospholipid synthesis and both do so through a mechanism that is independent of their genomic activity (20). We previously showed for c-Fos that its NA is implicated in the physical association with the enzymes it activates whereas its BD is essential for activation to occur (21, 26). Herein, we demonstrate that in order to activate phospholipid synthesis in an invasive triple negative breast tumor cell model, the MDA-MB231 cell line, Fra-1, and c-Fos physically associate with CDS1 and enhance total CDS activity. By contrast, neither protein associates with or activates PIS. Both proteins associate with the enzyme they activate through their NA domain and activate it through their BD domain. Furthermore, in the presence of both NA deletion mutants, colocalization of full-length Fra-1 and c-Fos with the ER marker calreticulin decreases.
Of all the Fos family members, Fra-1 and c-Fos share the highest homology, especially on their basic domains: key for lipid synthesis activation (20). Although homology between their N-terminus domains reaches only a 34%, the in silico predicted disorder shows high order correlation in the central region of these peptides (Supplementary Figure 3), suggesting that these ordered regions are relevant for the oncoprotein-enzyme interaction.
We hypothesized that the NA domains of Fra-1 and c-Fos act as negative dominants that physically associate with enzymes involved in the phospholipid synthesis pathway but without activating them. Consequently, no proliferation can be expected if activation of membrane biogenesis is impaired. Indeed, the overexpression of Fra-1-NA, c-Fos-NA or both together, significantly impairs MDA-MB231 and 4T1 cell proliferation in comparison with control cells or cells transfected with the empty vector pTQ2. Other authors and our previous reports have shown that the silencing of only Fra-1 or c-Fos does not significantly affect MDA-MB231 cell proliferation (18, 20). However, silencing both proteins with siRNA or blocking their cytoplasmic activities using specific antibodies, inhibits MDA-MB231 cell proliferation (20). We inhibit tumor cell proliferation by decreasing or blocking the cytoplasmic function of Fra-1 and c-Fos without affecting their nuclear function as AP-1 transcription factors because both deletion mutants examined, lack the AP-1 dimer formation domain (LZ) of the C-terminus of these proteins and the recognition domain for their nuclear translocator system (BD) (36, 37).
Fra-1 and c-Fos are overexpressed in breast tumor tissues as compared with their undetectable levels found in normal cells (20) with a direct relationship reported for the cytoplasmic expression of Fra-1 and malignancy in breast tumor (38). Furthermore, Fra-1 mRNA is overexpressed in triple-negative breast tumors, compared to breast tumors positive for estrogen or progesterone receptors (39). Fra-1 and c-Fos overexpression together with PCNA immunoreaction are clearly observed in tumor samples, whereas PCNA immunoreactivity and c-Fos and Fra-1 expression levels are almost undetectable in the non-pathological counterparts (20). Herein, the inhibitory effect on proliferation of both NA deletion mutants were evident both in vitro and in vivo in the 4T1 breast tumor model. After the intratumoral delivery of liposomes containing Fra-1-NA plus c-Fos-NA, a significant decrease in tumor size and tumor growth rate was observed. This response seems to be due to a direct effect of the peptides rather than to a stress or systemic effect on the animal since when two tumors were induced in a single mouse, the one treated with Fra-1-NA plus c-Fos-NA showed a decrease in tumor volume, tumor growth rate and CDS activity as compared to its own control treated with empty liposomes in the same mouse. In addition, PCNA protein levels in the excised tumors from the treated group showed lower expression levels than the control, evidencing that treated tumors are less proliferative. Two different doses were tested in these experiments, and, although in both cases a decrease in tumor growth rate was observed, the highest concentration was more effective. It should be mentioned that the doses assayed were empirically determined since, although previous data on cancer treatment with proteins is available, mostly antibodies [reviewed in Scott et al. (40)], no previous reports were found using the peptides evaluated herein.
These results highlight the potential use of Fra-1-NA and c-Fos-NA as a new therapeutic strategy. Other authors demonstrated that Fra-1 plays a key role in mediating the transition to and in maintaining the population of breast cancer stem cells and also observed from a compendium of clinical data sets, that high Fra-1 mRNA expression correlates with a poor, metastasis-free survival (18). In this context, further studies are necessary to evaluate if the N-terminal deletion mutants are also capable of inhibiting the proliferation of breast cancer stem cells.
The therapy proposed in this manuscript using c-Fos plus Fra-1 deletion mutants has some advantages. First, Fra-1 and c-Fos overexpression in breast tumor tissues in comparison with undetectable levels in normal tissue confers per se high tumorigenic tissue specificity because the deletion mutants will interfere only with the activity of these proteins in cells overexpressing their full-length versions. Moreover, these peptides should not interfere with the canonical function of the AP-1 transcription factors in normal cells since they do not contain the domain required for AP-1 dimerization of full-length c-Fos and Fra-1. The therapeutic use of peptides and proteins is recognized as being highly selective for their in vivo targets resulting in exceptionally high potencies of action and, at the same time, relatively safe and well-tolerated with few off-target effects (41, 42).
The application of these mutants as an adjuvant therapeutic strategy to treat malignant breast tumors is promising and, since other tumors such as brain, lung, liver, cervical, bladder also show over expression of one or both of these proteins, it is expected that this future therapeutic strategy will be readapted or slightly modified to inhibit the growth and progression of all tumors overexpressing Fra-1 and/or c-Fos.
The datasets generated for this study are available on request to the corresponding author.
This study was carried out in accordance with the recommendations of the ethics committee of experimental protocols for the use of animals (Facultad de Ciencias Químicas, Universidad Nacional de Córdoba). The protocol was approved by the ethics committee of experimental protocols for the use of animals (Facultad de Ciencias Químicas, Universidad Nacional de Córdoba. Resolution N° 2102).
AR: conception and design of the study, experimental work and acquisition of data, analysis and interpretation of data, manuscript writing, and final approval of the manuscript. CP: conception and design of the study, experimental work and acquisition of data, analysis and interpretation of data, revising draft critically, and final approval of the manuscript. BC: conception and design of the study, analysis and interpretation of data, manuscript writing, and final approval of the manuscript.
This work was supported by Ministerio de Ciencia, Tecnología e Innovación Productiva, Argentina (PICT 2012 #2792, PICT 2013 #2996, and PICT 2015 #3702 to BC) and Instituto Nacional del Cancer, Argentina (to BC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Dr. Hugo Maccioni for critical reading of the manuscript. Excellent technical assistance with animal handling from Laura Argañaras and Gimena Farias, and the technical and imaging assistance from Dr. Carlos Mas and Dr. Cecilia Sampedro from the Centro de Micro y Nanoscopía de Córdoba, -CEMINCO- CONICET, is also acknowledged. AR acknowledges the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET Argentina) for a postdoctoral fellowship; BC, CP, and AR are Career Investigators of CONICET.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00544/full#supplementary-material
1. American Cancer Society. Global Cancer Facts & Figures 4th Edition. Atlanta: American Cancer Society (2018).
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
3. Cohen DR, Curran T. Fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. (1988) 8:2063–9.
5. Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. (2005) 41:2449–2461. doi: 10.1016/j.ejca.2005.08.008
6. Belguise K, Kersual N, Galtier F, Chalbos D. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene. (2005) 24:1434–44. doi: 10.1038/sj.onc.1208312
7. Battista S, de Nigris F, Fedele M, Chiappetta G, Scala S, Vallone D, et al. Increase in AP-1 activity is a general event in thyroid cell transformation in vitro and in vivo. Oncogene. (1998) 17:377–85. doi: 10.1038/sj.onc.1201953
8. Debinski W, Gibo DM. Fos-related antigen 1 modulates malignant features of glioma cells. Mol Cancer Res. (2005) 3:237–49. doi: 10.1158/1541-7786.MCR-05-0004
9. Gao X-Q, Ge Y-S, Shu Q-H, Ma H-X. Expression of Fra-1 in human hepatocellular carcinoma and its prognostic significance. Tumor Biol. (2017) 39:101042831770963. doi: 10.1177/1010428317709635
10. Kustikova O, Kramerov D, Grigorian M, Berezin V, Bock E, Lukanidin E, et al. Fra-1 induces morphological transformation and increases in vitro invasiveness and motility of epithelioid adenocarcinoma cells. Mol Cell Biol. (1998) 18:7095–105.
11. Usui A, Hoshino I, Akutsu Y, Sakata H, Nishimori T, Murakami K, et al. The molecular role of Fra-1 and its prognostic significance in human esophageal squamous cell carcinoma. Cancer. (2012) 118:3387–96. doi: 10.1002/cncr.26652
12. Young MR, Colburn NH. Fra-1 a target for cancer prevention or intervention. Gene. (2006) 379:1–11. doi: 10.1016/j.gene.2006.05.001
13. Zajchowski DA, Bartholdi MF, Gong Y, Webster L, Liu HL, Munishkin A, et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. (2001) 61:5168–78.
14. Tyagi A, Vishnoi K, Kaur H, Srivastava Y, Roy BG, Das BC, et al. Cervical cancer stem cells manifest radioresistance: association with upregulated AP-1 activity. Sci Rep. (2017) 7:4781. doi: 10.1038/s41598-017-05162-x
15. Wang C, Li Z, Shao F, Yang X, Feng X, Shi S, et al. High expression of Collagen Triple Helix Repeat Containing 1 (CTHRC1) facilitates progression of oesophageal squamous cell carcinoma through MAPK/MEK/ERK/FRA-1 activation. J Exp Clin Cancer Res. (2017) 36:84. doi: 10.1186/s13046-017-0555-8
16. Iskit S, Schlicker A, Wessels L, Peeper DS. Fra-1 is a key driver of colon cancer metastasis and a Fra-1 classifier predicts disease-free survival. Oncotarget. (2015) 6:43146–61. doi: 10.18632/oncotarget.6454
17. Zhang N, Shen Q, Zhang P. miR-497 suppresses epithelial-mesenchymal transition and metastasis in colorectal cancer cells by targeting fos-related antigen-1. Onco Targets Ther. (2016) 9:6597–604. doi: 10.2147/OTT.S114609
18. Tam WL, Lu H, Buikhuisen J, Soh BS, Lim E, Reinhardt F, et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. (2013) 24:347–64. doi: 10.1016/j.ccr.2013.08.005
19. Zhong G, Chen X, Fang X, Wang D, Xie M, Chen Q. Fra-1 is upregulated in lung cancer tissues and inhibits the apoptosis of lung cancer cells by the P53 signaling pathway. Oncol Rep. (2016) 35:447–53. doi: 10.3892/or.2015.4395
20. Motrich RD, Castro GM, Caputto BL. Old players with a newly defined function: Fra-1 and c-Fos support growth of human malignant breast tumors by activating membrane biogenesis at the cytoplasm. PLoS ONE. (2013) 8:e53211. doi: 10.1371/journal.pone.0053211PONE-D-12-26591
21. Alfonso Pecchio AR, Cardozo Gizzi AM, Renner ML, Molina-Calavita M, Caputto BL. c-Fos activates and physically interacts with specific enzymes of the pathway of synthesis of polyphosphoinositides. Mol Biol Cell. (2011) 22:4716–4725. doi: 10.1091/mbc.E11-03-0259
22. Ferrero GO, Velazquez FN, Caputto BL. The kinase c-Src and the phosphatase TC45 coordinately regulate c-Fos tyrosine phosphorylation and c-Fos phospholipid synthesis activation capacity. Oncogene. (2012) 31:3381–3391. doi: 10.1038/onc.2011.510
23. Caputto BL, Cardozo Gizzi AM, Gil GA. c-Fos: an AP-1 transcription factor with an additional cytoplasmic, non-genomic lipid synthesis activation capacity. Biochim Biophys Acta. (2014) 1841:1241–1246. doi: 10.1016/j.bbalip.2014.05.007
24. de Arriba Zerpa GA, Guido ME, Bussolino DF, Pasquare SJ, Castagnet PI, Giusto NM, et al. Light exposure activates retina ganglion cell lysophosphatidic acid acyl transferase and phosphatidic acid phosphatase by a c-fos-dependent mechanism. J Neurochem. (1999) 73:1228–35.
25. Crespo PM, Silvestre DC, Gil GA, Maccioni HJF, Daniotti JL, Caputto BL. c-Fos activates glucosylceramide synthase and glycolipid synthesis in PC12 cells. J Biol Chem. (2008) 283:31163–31171. doi: 10.1074/jbc.M709257200
26. Cardozo Gizzi AM, Prucca CG, Gaveglio VL, Renner ML, Pasquaré SJ, Caputto BL, et al. The catalytic efficiency of Lipin 1beta increases by physically interacting with the protooncoprotein c-Fos. J Biol Chem. (2015) 290:29578–29592. doi: 10.1074/jbc.M115.678821
27. Borioli GA, Caputto BL, Maggio B. c-Fos is surface active and interacts differentially with phospholipid monolayers. Biochem Biophys Res Commun. (2001) 280:9–13. doi: 10.1006/bbrc.2000.4081
28. Gaggiotti MC, Del Boca M, Castro G, Caputto BL, Borioli GA. The immediate-early oncoproteins Fra-1, c-Fos, and c-Jun have distinguishable surface behavior and interactions with phospholipids. Biopolymers. (2009) 91:710–8. doi: 10.1002/bip.21212
29. Lykidis A, Jackson PD, Rock CO, Jackowski S. The role of CDP-diacylglycerol synthetase and phosphatidylinositol synthase activity levels in the regulation of cellular phosphatidylinositol content. J Biol Chem. (1997) 272:33402–9.
30. Esposito A, Schlachter S, Schierle GSK, Elder AD, Diaspro A, Wouters FS, et al. Quantitative fluorescence microscopy techniques. Methods Mol Biol. (2009) 586:117–42. doi: 10.1007/978-1-60761-376-3_6
31. Gordon GW, Berry G, Liang XH, Levine B, Herman B. Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy. Biophys J. (1998) 74:2702–2713. doi: 10.1016/S0006-3495(98)77976-7
32. Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. (2001) Chapter 20:Unit 20.2. doi: 10.1002/0471142735.im2002s39
33. Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA. (1996) 93:14164–9.
34. Moeller EH, Holst B, Nielsen LH, Pedersen PS, Østergaard J. Stability, liposome interaction, and in vivo pharmacology of ghrelin in liposomal suspensions. Int J Pharm. (2010) 390:13–8. doi: 10.1016/j.ijpharm.2009.05.067
35. Cardozo Gizzi AM, Caputto BL. Mechanistic insights into the nongenomic regulation of phospholipid synthesizing enzymes. IUBMB Life. (2013) 65:584–592. doi: 10.1002/iub.1173
36. Arnold M, Nath A, Wohlwend D, Kehlenbach RH. Transportin is a major nuclear import receptor for c-Fos: a novel mode of cargo interaction. J Biol Chem. (2006) 281:5492–9. doi: 10.1074/jbc.M513281200
37. Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. (1988) 55:917–924.
38. Song Y, Song S, Zhang D, Zhang Y, Chen L, Qian L, et al. An association of a simultaneous nuclear and cytoplasmic localization of Fra-1 with breast malignancy. BMC Cancer. (2006) 6:298. doi: 10.1186/1471-2407-6-298
39. Kharman-Biz A, Gao H, Ghiasvand R, Zhao C, Zendehdel K, Dahlman-Wright K. Expression of activator protein-1 (AP-1) family members in breast cancer. BMC Cancer. (2013) 13:441. doi: 10.1186/1471-2407-13-441
40. Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. (2012) 12:278–87. doi: 10.1038/nrc3236
41. Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. (2013) 81:136–47. doi: 10.1111/cbdd.12055
Keywords: c-Fos, Fra-1, phospholipids, CDP-diacylglycerol synthase, phosphatidylinositol synthase
Citation: Racca AC, Prucca CG and Caputto BL (2019) Fra-1 and c-Fos N-Terminal Deletion Mutants Impair Breast Tumor Cell Proliferation by Blocking Lipid Synthesis Activation. Front. Oncol. 9:544. doi: 10.3389/fonc.2019.00544
Received: 05 April 2019; Accepted: 04 June 2019;
Published: 19 June 2019.
Edited by:
Daniel Christian Hoessli, University of Karachi, PakistanReviewed by:
Michael P. Lisanti, University of Salford, United KingdomCopyright © 2019 Racca, Prucca and Caputto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beatriz Leonor Caputto, YmNhcHV0dG9AZmNxLnVuYy5lZHUuYXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.