- 1Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
- 2Department of Animal Sciences, Quaid-i-Azam University, Islamabad, Pakistan
Background: Data on 25-OH VD concentrations and the associated factors in colorectal cancer (CRC) patients are scarce and need to be investigated.
Methods: A total of 200 CRC patients participated in this cross-sectional study conducted in Pakistan. Socio-demographic and other health data were collected in a pretested questionnaire. Serum measurements of Vitamin D (1, 25(OH)2 D3) levels and hormones were performed. Association of age, sex, primary site, effects of hormone therapy and stage of disease and selected reproductive health indicators on vitamin D status were primarily scrutinized by univariate analysis.
Results: Mean age of the population was 55.3 years (±15.6; Range: 20–90 years). Estradiol concentration was considerably elevated in young females compared to young male patients (p < 0.001). The concentrations of FSH, LH testosterone and estradiol were significantly lower in post-menopausal female CRC patients as compared to their male counterparts of old age (p, for all trends < 0.05). Both LH and FSH showed significant gender difference but only in older patients. Level of estrogen was markedly decreased in older post-menopausal CRC patients compared to premenopausal CRC patients, which might be associated with CRC progression. In the group of women, who “ever used hormone therapy” had differences of statistical significance (p, for all trends < 0.05) in their mean serum 25-OH VD concentrations, while in the group of women who “never used hormone therapy” had non-significant differences in their mean serum 25-OH VD concentrations (p, for all trends > 0.05). High 25-OH VD concentrations were observed in women who had their menarche at the age of 15 years or more. Nulliparous women had the highest mean 25-OH VD concentrations as compared to unparious or multiparious women. In addition, women having their menopause at 40–44 years of age had the highest 25-OH VD concentrations, although the difference was not significant (p = 0.08). Women who “never used any oral contraceptive” had higher 25-OH VD concentrations as compared to those “whoever used oral contraceptives.”
Conclusion: Our findings suggest that vitamin D has a positive effect on the development of CRC through the mediation of hormones. Other health and reproductive traits that affect hormone levels may have an indirect effect on the development of CRC. Further potential studies that directly evaluate levels of circulating hormones and hormone therapy in women in association to 25-OH VD concentrations, as well as their possible role in colorectal cancer risk, would be vastly edifying.
Background
The worldwide prevalence of colorectal cancer (CRC) is third among cancer rate of recurrence in men and fourth among women (1, 2). In addition, colonic adenocarcinoma accounts for 37–45% of all metastatic ovarian tumors (3). Even though there is a decline in the death rates for colorectal cancers from 1990 to the present and even with advances in screening and surgical treatment, no cure has been discovered for metastatic cancer and the 5–6-year survival rate is regretfully low (almost 7.8%) (4, 5).
Men are predisposed to have a higher incidence of colorectal cancer than women of the same age (1, 2). In families in the midst of hereditary non-polyposis colorectal cancer (HNPCC), the survival risk of mounting progression of colon cancer to a great extent inferior in females (37%) than in men (78%) (6). The determination of the sex differentiation over decades, in various racial traits and all over the world revealed that sex hormones of both males and females play a vital role in the pathogenesis of CRC and their roles are comparatively defensive (7, 8). Numerous hypotheses regarding the relationship of reproductive factors and use of exogenous sex hormones and progression of colon cancer have been strengthened by results from observational studies (9). Various research analyses have found a reduced risk of colon or CRC in relation to “ever” vs. “never” use of menopausal hormones (7, 8). Murphy et al. found that endogenous revelations to sex hormones didn't reveal significant relation with CRC risk (10). On the other hand, the shielding function of the estrogen pathway in the progression of colon cancer has been reported in various animal models. For example, estrogen therapy has been shown to slow down colon tumors development in rats (11–13). Estrogens role in CRC is under consideration with bottomless attention particularly in context to the patho-physiology of cancers of endometrium, prostate, breast, colon, and rectum (14). The accurate mechanism of function of estrogen in CRC till date remains a controversial topic (15). Nevertheless, endogenous estradiol has been shown to have a vital role in the genetic pathway of CRC and the circulating levels of estradiol are associated with an elevated possibility of cancer (16). Estrogen prejudices the mucosal response to inflammatory injury in colitis, hence encourages inflammation-associated cancer progression (17). A comprehensive and relatively large-scale meta-analysis by Grodstein et al. revealed a positive association between hormone replacement therapy (HRT) and declined risk of colon cancer as much as of approximately 34% (18). The association was further strengthened by the Women's Health Initiative (WHI) Clinical Trial (19, 20), where involvement with estrogen added progestin results a 45% decrease in CRC, whilst only estrogens showed no concerning CRC risk. As epidemiological data support a shielding consequence of HRT on CRC, the links between different amalgamations of HRT and CRC danger remain unrevealed. Very few studies revealed the alliance among sex hormone intensities and colorectal cancer risk in males. Similar to the controversial role of estrogens in females, studies on the association of testosterone concentration and CRC progression are divisive. Studies have hypothesized that lower androgenicity may boost men's risk of mounting CRC probably during the initiation of androgen receptor signaling pathway (21). This advocates that males with lower androgenicity because of diminished AR activity or low circulating androgens were at larger jeopardy of CRC. Whereas, recent studies have revealed, that testosterone increase likelihood of developing CRC in men. Such observations were strengthened by animal studies which showed that orchidectomised mice have a minor risk of mounting CRC than controls. These observations proposed that testosterone may encourage colorectal adenoma formation, resulting in higher male susceptibility to CRC. Besides that, female mice with ovarectomy illustrated the greater risk of mounting CRC when compared with female mice without ovarectomy (22). These observations sustain the impression that sex hormones may confer defense against CRC or encourage the progression of CRC as the case with testosterone. Consequently, quantification of serum testosterone and other sex hormones could be an attractive prospective predictive biomarker of CRC risk. Clinically serum testosterone is used as a biomarker for prostate cancer. Also has been anticipated as a means for quantifying prognosis of breast cancer in postmenopausal women. Nevertheless, data of its use in CRC is limited and promising facts on the role which testosterone plays in CRC warrants auxiliary long-term clinical trials.
The substantial established molecular and genetic data advocate a role of vitamin D against CRC. In addition, many epidemiological studies identified that vitamin D insufficiency is associated with elevated risk of this neoplasia. Worldwide the results accessible on the loss of VDR expression and on the amendment of CYP27B1 and CYP24A1 levels at some stage in CRC development, sustain a role for vitamin D in the preclusion and/or in the therapy of initial stages rather than in the treatment of advanced cases of this neoplasia (23–27). Numerous studies have revealed an association between CRC and 25-OH vitamin D deficiency (28–30). Regardless of the involvement between vitamin D status and jeopardy of colorectal cancer, inadequate data subsists on vitamin D and CRC development across stages I–IV. In addition, veto data exist on vitamin D status and hormones levels in colorectal cancer. A study revealed that 25-OH vitamin D levels in colon cancer cases were higher than the controls. In addition, 25-OH D3 levels showed consistency across the various stages of colorectal cancer, while 1, 25-OH vitamin D diminished with mounting stage (31).
Given the limited data on vitamin D status and association of sex hormones across various stages of CRC and in view of promising clinical data sustaining an impact of vitamin D status on cancer outcome, we examined vitamin D and sex hormones status in 200 CRC patients. Our main objective was to investigate any possible association between sex hormones and vitamin D levels at different stages of the disease in a less studied population of Pakistan.
Methods
The study was carried out at Reproductive Physiology Laboratory, Department of Animal Sciences Quaid-i-Azam University, Islamabad, Pakistan from December 2016 to November 2017. The study was approved by the Institutional Review Board (IRB) of Quaid-i-Azam University, Islamabad, Pakistan. All participants were informed about the study objectives and signed informed consent. The study protocol was carried out in accordance to the principles of the Declaration of Helsinki (32).
Selection of Case Participants and Collection of Blood Samples
Cases of CRC patients were newly diagnosed, histopathologically confirmed and diagnosed with adenocarcinoma of the colon and/or rectum.
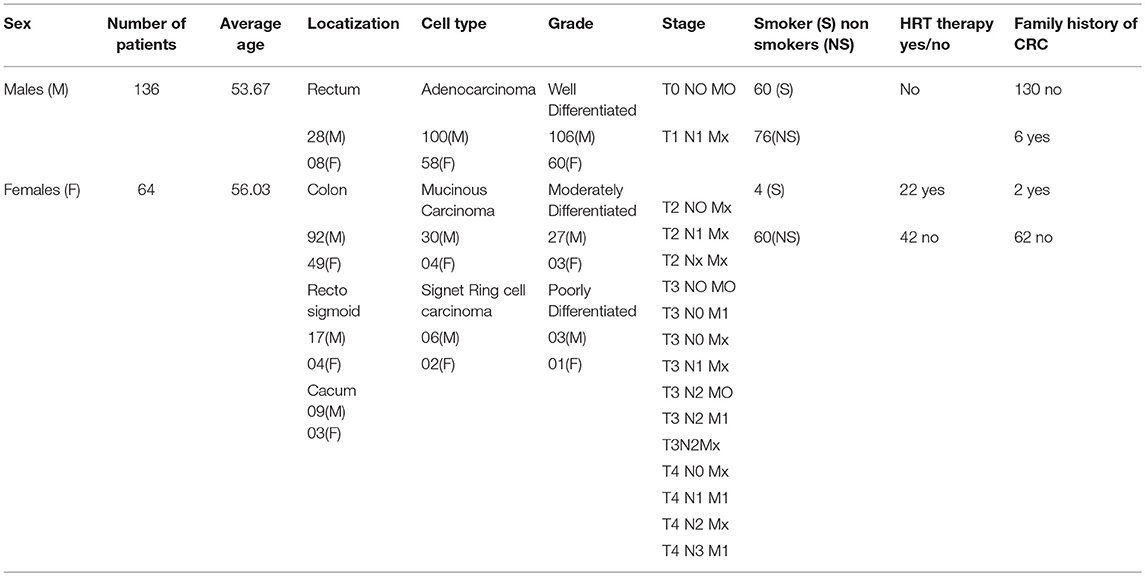
Venous blood samples from all patients were available from the Department of Urology, AFIP, Rawalpindi, Pakistan. At the time of blood sampling, the age of patients ranges from 32 to78 years. In female CRC patients, data on reproductive and menstrual health included data age at menarche, use of hormonal contraceptives, menopausal status, the cause of regularity of the menstrual cycle, menopause, and hormone replacement therapy (HRT). Other details of reproductive history included a number of miscarriages, number/order/sex of the children, gestational age and duration of maternal lactation and information on fertility problems and their treatment was collected. Data on demographic and clinical features were taken and included gender, age at the time of diagnosis, family history, cell type, disease localization, grade, and stage of the tumor (Table 1).
Laboratory Methods
Estimation of Circulating Sex Factors
Serum levels of LH, testosterone, and estradiol representing female and male sex hormones were calculated by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Phoenix Pharmaceuticals, INC, USA), according to manufacturer's instructions.
Measurement of Vitamin D
Serum level of Vitamin D (1, 25(OH)2 D3) was measured by ELISA kit (MyBioSource) following standard manufacturer protocol.
Statistical Analysis
Univariate analysis was used to assess the effect of sex, age, primary site, and stage of disease on vitamin D status. The distribution pattern of sex hormones and 25-OH vitamin D levels in relation to the stage of CRC in young and old CRC patients were evaluated. Pearson's correlation analyses were performed to see the association of 25-OH vitamin D with the stage of the disease in both premenopausal and postmenopausal females with CRC. Odds ratio (OR) was calculated considering ≤16 ng/ml) 25-OH vitamin status as “very low” with respect to the default or reference level (33). To simultaneously consider the impact of age, sex, primary site and stage of disease on 25-OH vitamin status, multiple logistic regression analyses were performed by fitting the log of odds (with “very low” 25-OH vitamin status) on the explanatory variables. We also calculated Hazard Ratio (HR) as described previously. We stratified the data of female patients by hormone therapy use to observe any significant interaction between the mean age of female patients, use of hormone therapy, and serum 25-OH VD status. Statistical significance tests for trend (P-trend) and interaction (P-interaction) were calculated. All data were expressed as mean (STD). An alpha value (p-value) was considered significant at 0.05.
Results
Demographics of Male and Female Patients
Table 1 indicates the demographic characteristics of CRC patients. The percentage number of rectal and colon patients were 20% and 80, respectively. Mean age of the patients 55.3 years (±15.6; Range: 20–90 years). All patients were of normal body weight (mean BMI = 23.5; Range 20–24.8). Mean (SD) 25-OH vitamin D levels was 18.8 (9.11) ng/ml.
Sex Hormones Concentrations in CRC Patients
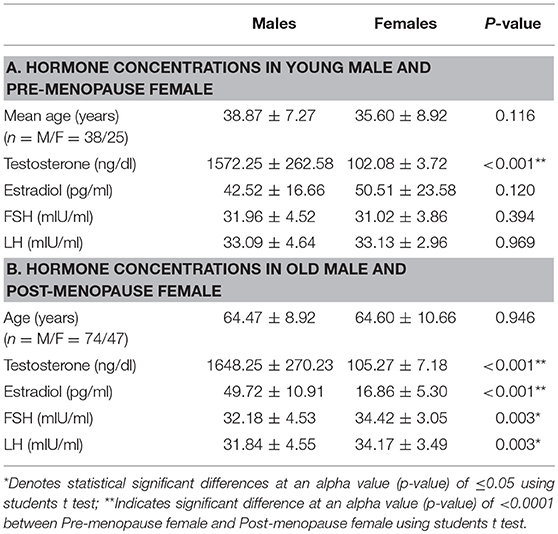
Table 2 shows serum concentrations of testosterone, estradiol, FSH, and LH hormones in young male vs. young female. Only estradiol concentration was considerably elevated in young female than young male patients (p < 0.001). Table 2 also shows serum concentrations of testosterone, estradiol, FSH, and LH hormones in old male vs. post-menopausal female CRC patients. As evident, the concentrations of these hormones were significantly lower in post-menopausal female CRC patients as compared to their male counterparts of old age (p, for all trends < 0.05).
In postmenopausal cases, physiological reduction of estradiol level makes a significant difference (P < 0.001) in estradiol level between males and females with high levels in males of the equivalent ages. Both LH and FSH showed significant gender difference only in older age. Furthermore, the comparison of hormone concentration between premenopausal and post-menopausal females revealed that the level of estrogen is markedly decreased in older post-menopausal CRC patients, which might be associated with CRC progression.
Impact of Patients Demographics on Vitamin D Status
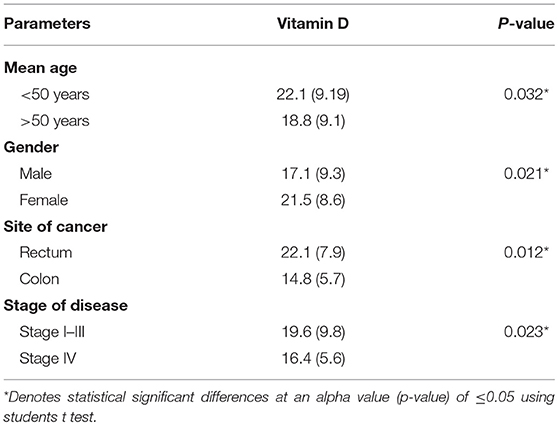
Table 3 shows the distribution of 25-hydroxyvitamin D [25(OH) D] levels in CRC patients. For the current observation, 25-OH VD status was classified into two groups: “very low” and “low to normal.” The “very low” group was defined as ≤16 ng/ml and the “low to normal” group was defined at >16 ng/ml. Levels below 16 ng/ml have been traditionally considered as low. In addition, ≤16 ng/ml corresponds to the lowest quartile of our population. Variables investigated in this study included age, sex, primary site (colon vs. rectum), stage of disease (stages I–III vs. IV). BMI and date of 25-OH vitamin D assay were not considered for regression analysis as all patients had normal BMI (Range 22–24.9) and blood was collected from all patients in the same month of the year thus excluding the possibility of significant seasonal variations in serum vitamin D concentrations (Table 4).
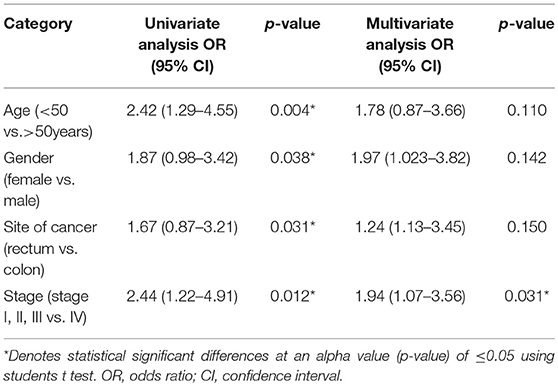
Table 4. Univariate and multivariate logistic regression analysis of low vs. normal 25-OH VD level (≤15 vs. >15 ng/ml).
Reproductive Factors and Distribution of 25-OH VD Concentrations in CRC Patients
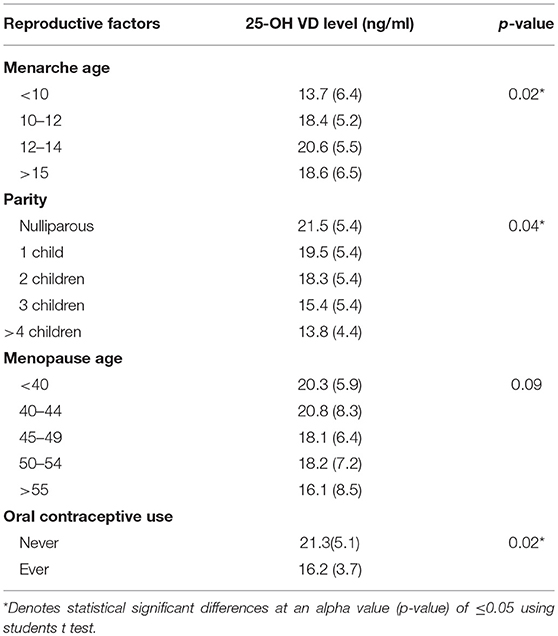
Table 5 shows data on 25-OH VD concentration in female patients according to various reproductive factors. As shown, female patients having their menarche before or after the age of 10–12 years of age had significantly lower 25-OH VD level (p = 0.02). Similarly, the nulliparous female had significantly higher 25-OH VD levels as compared to those having children (p = 0.04).
Women with menopause at age <40 years had higher 25-OH VD level as compared to women who had their menopause after the age of 40. However, this difference didn't reach statistical significance (p = 0.09). Women who ‘never used oral contraceptive’ had higher 25-OH VD levels as compared to those who ‘ever used oral contraceptives’ (p = 0.02).
In the auxiliary investigation, we stratified the data of female patients by hormone therapy use (Table 6) and observed statistically significant differences in serum 25-OH VD concentrations of female patients because of differences in their reproductive health characteristics and use of hormone therapy. In general, the “ever used hormone therapy” female group had differences of statistical significance (p, for all trends < 0.05) in their mean serum 25-OH VD concentrations, while in the group of females who “never used hormone therapy” had non-significant differences in their mean serum 25-OH VD concentrations (p, for all trends > 0.05). Furthermore, taken as single groups (ever use hormone therapy vs. never use of hormone therapy) women with “ever use of hormone therapy” had significantly higher 25-OH VD concentrations as compared to women with “never use of hormone therapy” (p, for all trends < 0.05).

Table 6. Serum 25-OH VD concentrations according to reproductive and menstrual factors by hormone therapy (n = 54).
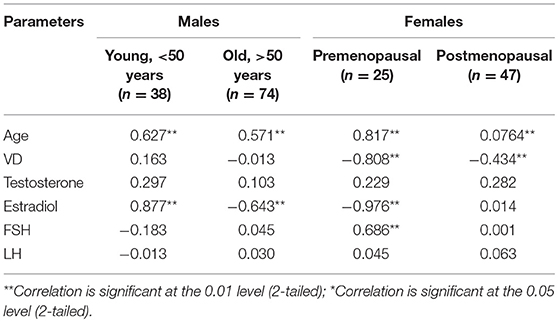
Figures 1, 2 reveals the analyzed distribution pattern of sex hormones and 25-OH vitamin D levels in relation to the stage of CRC in young and old CRC patients. In younger female cases, early stages have relatively higher levels of 25-OH vitamin D and estradiol which get reduced with the progression of the CRC. Furthermore, 25-OH vitamin D showed strong negative correlations with the stage of the disease in both premenopausal and postmenopausal females with CRC (r = −0.808 and −0.434, respectively, P < 0.01, Table 7). This was not the case in males in both age groups. Regarding estradiol level in young males, there was a strong positive correlation with the stage of the disease (r = 0.877, p < 0.01), while in older ages the correlation became negative (r = −0.643, p < 0.01). Females in the reproductive ages and with CRC showed an extremely strong negative correlation with the stage of the disease (r = −0.976, p < 0.01). In both genders and age groups, the severity of the disease stage correlated positively with the age.
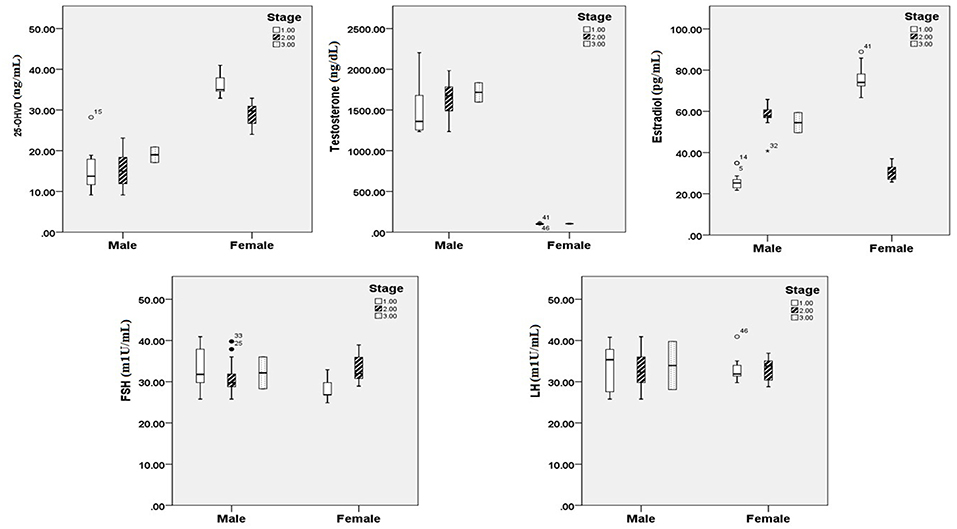
Figure 1. 25 OH vitamin D and sex hormonal patterns in relation to the stage of CRC in young participants (n = 200). Pearson's correlation at alpha = 0.05.
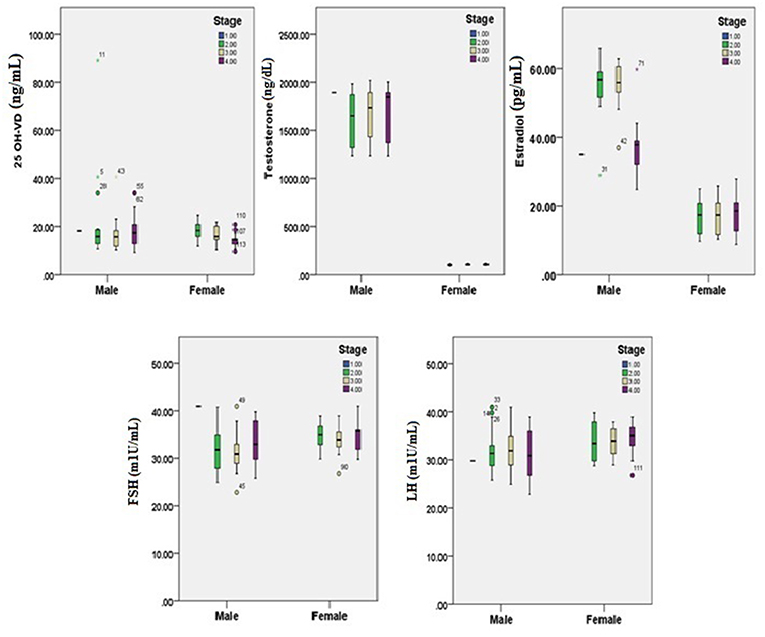
Figure 2. 25-OH vitamin D and sex hormones patterns in relation to the stage of CRC in old participants (n = 200). Pearson's correlation at alpha = 0.05.
Discussion
The present study assessed serum 25-OH Vitamin D concentrations of 200 colorectal cancer (CRC) patients. The sample comprised of 122 male and 78 female with mean age of 55.8 ± 6.9 and 54.5 ± 14.6 years, respectively. The present study report main three findings:
Age and Gender as a Determinant of Hormone Levels
When subjects were divided into young males and female groups, no significant difference was observed in the serum concentrations of hormone levels (p, for all trends > 0.05; Table 2). However, significant differences were observed in the serum concentrations of old male and female (p, for all trends < 0.05; Table 2).
Age, Gender, Site of Cancer, as Determines of Serum 25-OH Vitamin D Concentrations
Findings of the present study show that mean 25-OH vitamin D levels in the patients were 18.8 ± 9.11 ng/ml. 25-OH VD status was classified into two groups “very low” and “low to normal.” The “very low” category was defined as ≤16 ng/ml and the “low to normal” category was defined at >16 ng/ml (34). The other reason for selecting this threshold was that 25-OH VD levels of our subjects were within these ranges. Regression analysis using univariate model show age, gender, site of cancer, and stage of disease were found to be associated with the disease. Age, gender, site of cancer, and stage of disease were found to be 2.42, 1.87, 1.67, and 2.44 times, more likely to be allied with low 25-OH VD (p, for all trends < 0.05; Table 3). However, multivariate logistic regression results show that only stage of disease (OR: 1.94; CI: 1.07–3.56) was significantly associated with low 25-OH VD (p = 0.031; Table 4).
There Is a Close Association Between Serum 25 (OH) D Level and Sex Factors
Early reports have illustrated that amplified levels of testosterone and estrogen, in males and females, are certainly related with serum 25 (OH) D levels (33, 35, 36). We, therefore, analyzed the distribution pattern of sex hormones and 25-OH vitamin D levels in relation to the stage of CRC (Figures 1, 2). Estrogen directly standardizes hepatic hepcidin expression through an efficient estrogen response factor in the promoter region of the hepcidin gene (37). In pre-menopausal women, in particular, 17β-estradiol amplifies iron uptake to reimburse for iron loss during menstruation (38). Nevertheless, postmenopausal women have an accelerated diminution of estrogens caused by menopause. Consequently, in spite of the increase in serum 25 (OH) D concentrations, the ferritin level may be not appreciably dissimilar in postmenopausal women. However, a study proposed that testosterone amplifies ferritin by bracketing hepcidin in men (39).
Effects of Reproductive Characteristics on 25-OH VD Concentrations
We also analyzed some selected reproductive factors as predictors of 25-OH VD concentrations (Table 6). High 25-OH VD concentrations were observed in women who had their menarche at the age of 15 years or more. Nulliparous women had the highest mean 25-OH VD concentrations as compared to unparious or multiparious women. Women having their menopause at 40–44 years of age had the highest 25-OH VD concentrations, although the difference was not significant (p = 0.08). Women who never used any oral contraceptive had higher 25-OH VD concentrations as compared to those who ever used oral contraceptives. Furthermore, it is interesting that these differences were more profound when the women patients were stratified into two groups based on their hormone therapy i.e., ever use hormone therapy and never use hormone therapy (Table 6). So for example, women having menarche at age 15 years or more and who ever used hormone therapy had considerably higher 25-OH VD concentrations as compared to women who had menarche <15 years of age with either ever use hormone therapy or with never use hormone therapy (p, for all trends < 0.05) in the same way being nulliparous and parity (Table 6).
Early menarche is associated to elevated risk of poor health outcomes during adulthood including obesity (40), type 2 diabetes (41), cardiovascular disease (42), and breast (43) and endometrial cancers (44). Women with later onset of menarche demonstrate significantly decreased peak bone mass (45), and bone mass and bone density at skeletal maturity exhibit an inverse relationship with pubertal timing in healthy adolescents (46). Later age of menarche has also been associated with increased fracture risk (47). In this study, we observed mean lower 25-OH VD concentrations in women having their menarche age <10 or >15 years (Table 5). Like age at menopause, age at menarche is a marker of the period of exposure to cyclic ovarian function (48, 49). Studies have confirmed an inverse association between circulating estrogen level and age at menarche. Pregnant women and women breastfeeding for long periods of time are at higher risk of hypovitaminosis D (50–52). Later age at menopause is a recognized risk factor for the elevated number of ovulatory cycles and amplified estrogen exposure allied with later menopause has been theorized to constrain this association (53, 54). The mean age of menopause in Pakistan has been reported that varied greatly i.e., 44.5 years (55) to 47–49 years (56, 57). Similarly, the age of menarche in Pakistani women is also variable (58) and has profound health outcomes. These are important factors and may have significant effects on 25-OH VD concentrations and overall health of women both healthy and those suffering from chronic diseases like CRC.
Limitations of Study
The present study has some limitations. Firstly, relatively small sample size did not allow us to carry out stratified analyses with abundant statistical power, which would have permitted us to estimate females according to the stage of disease and hormone therapy use; two factors that could have a perplexing effect on the alliance between reproductive history and 25-OH VD concentrations in colorectal cancer patients. Secondly, all of the prime variables of concern were based on self-reported reproductive history and therefore we cannot reject the possibility of bias associated to imprecise recall. However, self-reported reproductive history has shown good concurrence with medical records in validation studies (59, 60). Moreover, we could not further stratify the analyses by hormone therapy subtypes, and we lacked more detailed data on other reproductive factors, such as age at first pregnancy or age of mother at the birth of first and last child, breastfeeding history, and history of induced or spontaneous abortion, which have been linked with 25-OH VD concentrations (61–63) and which could help further elucidate the role of sex hormones in colorectal cancer development. Due to its cross-sectional nature, the present study is unable to conclude whether Vitamin D levels and hormonal factors are causative or merely correlative. Further case-control studies on a larger scale are needed to clarify this. Previous studies have shown the presence of 1, 25-(OH)2D3 receptors in some colorectal tumors (64). This needs further investigations into the possible role of 1, 25-(OH)2D3 on colorectal carcinogenesis. We, therefore, would recommend further prospective studies that directly assess levels of circulating hormones and hormone therapy in women in relation to 25-OH VD concentrations as well as their possible role in colorectal cancer risk at clinical as well as molecular and cellular levels.
Conclusion
Our findings suggest that 25-OH VD has a positive effect on the development of CRC through the mediation of hormones. Other health and reproductive traits that affect hormone levels may have an indirect effect on the development of CRC. We suggest that studies in the future may evaluate levels of circulating hormones and hormone therapy in women in association to 25-OH VD concentrations as well as their possible role in colorectal cancer risk would be promising revealing.
Ethics Statement
The study was approved by the Institutional Review Board (IRB) of Quaid-i-Azam University, Islamabad, Pakistan. All participants were informed about the study objectives and signed an informed consent. The study protocol was done in accordance with the principles of the Declaration of Helsinki.
Author Contributions
SR designed the study, conceived the study and analyzed the results. IA, TA, and MA conceived an initial part of the study, performed the experiment, histology and helped in compiling the results. SR and TA performed experiment. AA helped in writing the results. SR and SJ wrote the paper with input from all other authors SJ, SR, TA, and AA made substantial contribution in interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript.
Funding
We are grateful to Department of animal sciences, Quaid-i-Azam University, Islamabad, Pakistan, for funding and assisting us in designing the study and interpretation of results.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the Deanship of Scientific Research at King Saud University for its funding of this research through Research Group Project number 193.
Abbreviations
CRC, Colorectal Cancer; HNPCC, Hereditary non-polyposis colorectal cancer; LH, Luteinizing hormone; FSH, Follicle stimulating hormone; HRT, hormone replacement therapy; EDTA, Ethylenediaminetetraacetic acid; AR, Androgen Receptors; VDR, Vitamin D Receptor; HR, Hazrd Ratio.
References
1. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Prev Biomarkers. (2010) 19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437
2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. (2005) 55:74–108. doi: 10.3322/canjclin.55.2.74
3. Lash RH, Hart WR. Intestinal adenocarcinomas metastatic to the ovaries. A clinicopathologic evaluation of 22 cases. Am J Surg Pathol. (1987) 11:114–21. doi: 10.1097/00000478-198702000-00005
4. Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. (2007) 104:10158–63. doi: 10.1073/pnas.0703478104
5. Mimeault M, Hauke R, Mehta PP, Batra SK. Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med. (2007) 11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x
6. Bonis PA, Trikalinos TA, Chung M, Chew P, Ip S, DeVine DA, et al. Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications. Evid Rep Technol Assess. (2007) 150:1–180.
7. Hendifar A, Yang D, Lenz F, Lurje G, Pohl A, Lenz C, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. (2009) 15:6391–7. doi: 10.1158/1078-0432.CCR-09-0877
8. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. (2011) 61:212–36. doi: 10.3322/caac.20121
9. Lo A-C, Soliman AS, Khaled HM, Aboelyazid A, Greenson JK. Lifestyle, occupational, and reproductive factors and risk of colorectal cancer. Dis Colon Rectum. (2010) 53:830–7. doi: 10.1007/DCR.0b013e3181d320b1
10. Murphy N, Xu L, Zervoudakis A, Xue X, Kabat G, Rohan TE, et al. Reproductive and menstrual factors and colorectal cancer incidence in the Women's Health Initiative Observational Study. Br J Cancer. (2017) 116:117–25. doi: 10.1038/bjc.2016.345
11. -GuoJ J.Y, Li X, Browning JD, Rottinghaus GE, Lubahn DB, Constantinou A, et al. Dietary soy isoflavones and estrone protect ovariectomized ERαKO and wild-type mice from carcinogen-induced colon cancer. J Nutr. (2004) 134:179–82. doi: 10.1093/jn/134.1.179
12. Smirnoff P, Liel Y, Gnainsky J, Shany S, Schwartz B. The protective effect of estrogen against chemically induced murine colon carcinogenesis is associated with decreased CpG island methylation and increased mRNA and protein expression of the colonic vitamin D receptor. Oncol Res. (1999) 11:255–64.
13. Weyant MJ, Carothers AM, Mahmoud NN, Bradlow HL, Remotti H, Bilinski RT, et al. Reciprocal expression of ERα and ERβ is associated with estrogen-mediated modulation of intestinal tumorigenesis. Cancer Res. (2001) 61:2547–51.
14. Arnal J-F, Gourdy P, Lenfant F. In vivo dissection of the estrogen receptor alpha: uncoupling of its physiological effects and medical perspectives. Ann Endocrinol. 2013:82–9. doi: 10.1016/j.ando.2013.03.001
15. Foster PA. Oestrogen and colorectal cancer: mechanisms and controversies. Int J Colorectal Dis. (2013) 28:737–49. doi: 10.1007/s00384-012-1628-y
16. Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. (2008) 68:329–37. doi: 10.1158/0008-5472.CAN-07-2946
17. Heijmans J, Wielenga MC, Rosekrans SL, van Lidth de Jeude JF, Roelofs J, Groothuis P, et al. Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut. (2014) 63:310–6. doi: 10.1136/gutjnl-2012-304216
18. Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. (1999) 106:574–82. doi: 10.1016/S0002-9343(99)00063-7
19. Rossouw JE. Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. (2002) 288:321–33. doi: 10.1001/jama.288.3.321
20. Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. (2004) 350:991–1004. doi: 10.1056/NEJMoa032071
21. Lin JH, Giovannucci E. Sex Hormones and Colorectal Cancer: What Have We Learned so Far? Cary, NC: Oxford University Press (2010).
22. Amos-Landgraf JM, Heijmans J, Wielenga MC, Dunkin E, Krentz KJ, Clipson L, et al. Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc Natl Acad Sci USA. (2014) 111:16514–9. doi: 10.1073/pnas.1323064111
23. Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. (2010) 78:140–5. doi: 10.1038/ki.2010.17
24. Schuster I, Egger H, Herzig G, Reddy GS, Vorisek G. Combination of vitamin D metabolites with selective inhibitors of vitamin D metabolism. In: Vitamin D Analogs in Cancer Prevention and Therapy. Heidelberg: Springer-Verlag (2003). p. 169–88.
25. Gonzalez-Sancho JM, Larriba MJ, Ordonez-Moran P, Palmer HG, Munoz A. Effects of 1α, 25-dihydroxyvitamin D3 in human colon cancer cells. Anticancer Res. (2006) 26:2669–81.
26. Modica S, Gofflot F, Murzilli S, D'Orazio A, Salvatore L, Pellegrini F, et al. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. (2010) 138: 636–48 e12. doi: 10.1053/j.gastro.2009.09.060
27. Sheinin Y, Kaserer K, Wrba F, Wenzl E, Kriwanek S, Peterlik M, et al. In situ mRNA hybridization analysis and immunolocalization of the vitamin D receptor in normal and carcinomatous human colonic mucosa: relation to epidermal growth factor receptor expression. Virchows Archiv. (2000) 437:501–7. doi: 10.1007/s004280000275
28. Garland C, Garland F, Shaw E, Comstock G, Helsing K, Gorham E. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. (1989) 334:1176–8. doi: 10.1016/S0140-6736(89)91789-3
29. Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci EL. A nested case-control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. (2007) 99:1120–9. doi: 10.1093/jnci/djm038
30. Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. (2006) 354:684–96. doi: 10.1056/NEJMoa055222
31. Niv Y, Sperber AD, Figer A, Igael D, Shany S, Fraser G, et al. In colorectal carcinoma patients, serum vitamin D levels vary according to stage of the carcinoma. Cancer. (1999) 86:391–7. doi: 10.1002/(SICI)1097-0142(19990801)86:3<391::AID-CNCR5>3.0.CO;2-A
32. Association WM. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. (2008). Available online at: http://www.wma.net/e/policy/b3.htm (accessed October 23, 2013)
33. Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, et al. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res. (2010) 42:754–7. doi: 10.1055/s-0030-1262837
34. Fakih MG, Trump DL, Johnson CS, Tian L, Muindi J, Sunga AY. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. (2009) 24:219–24. doi: 10.1007/s00384-008-0593-y
35. Wehr E, Pilz S, Boehm BO, März W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol. (2010) 73:243–8. doi: 10.1111/j.1365-2265.2009.03777.x
36. Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. (2011) 43:223–5. doi: 10.1055/s-0030-1269854
37. Hou Y, Zhang S, Wang L, Li J, Qu G, He J, et al. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. (2012) 511:398–403. doi: 10.1016/j.gene.2012.09.060
38. Yang Q, Jian J, Katz S, Abramson SB, Huang X. 17β-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology. (2012) 153:3170–8. doi: 10.1210/en.2011-2045
39. Bachman E, Feng R, Travison T, Li M, Olbina G, Ostland V, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. (2010) 95:4743–7. doi: 10.1210/jc.2010-0864
40. Van Lenthe FJ, Kemper C, van Mechelen W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr. (1996) 64:18–24. doi: 10.1093/ajcn/64.1.18
41. He C, Zhang C, Hunter DJ, Hankinson SE, Buck Louis GM, Hediger ML, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. (2009) 171:334–44. doi: 10.1093/aje/kwp372
42. Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw K-T, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. (2009) 94:4953–60. doi: 10.1210/jc.2009-1789
43. Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer. (1990) 46:796–800. doi: 10.1002/ijc.2910460508
44. Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. (2010) 127:442–51. doi: 10.1002/ijc.25050
45. Armamento-Villareal R, Villareal DT, Avioli LV, Civitelli R. Estrogen status and heredity are major determinants of premenopausal bone mass. J Clin Investig. (1992) 90:2464–71. doi: 10.1172/JCI116138
46. Gilsanz V, Chalfant J, Kalkwarf H, Zemel B, Lappe J, Oberfield S, et al. Age at onset of puberty predicts bone mass in young adulthood. J Pediatrics. (2011) 158: 100–5 e2. doi: 10.1016/j.jpeds.2010.06.054
47. Cooper G, Sandler D. Long-term effects of reproductive-age menstrual cycle patterns on peri-and postmenopausal fracture risk. Am J Epidemiol. (1997) 145:804–9. doi: 10.1093/oxfordjournals.aje.a009173
48. Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. (1989) 44:783–7. doi: 10.1002/ijc.2910440506
49. Macmahon B, Trichopoulos D, Brown J, Andersen A, Cole P, Dewaard F, et al. Age at menarche, urine estrogens and breast cancer risk. Int J Cancer. (1982) 30:427–31. doi: 10.1002/ijc.2910300408
50. Arya V, Bhambri R, Godbole MM, Mithal A. Vitamin D status and its relationship with bone mineral density in healthy Asian Indians. Osteoporosis Int. (2004) 15:56–61. doi: 10.1007/s00198-003-1491-3
51. van der Meer IM, Karamali NS, Boeke AJ, Lips P, Middelkoop BJ, Verhoeven I, et al. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. (2006) 84:350–3. doi: 10.1093/ajcn/84.2.350
52. Eagleton C, Judkins A. Vitamin D deficiency in pregnant New Zealand women. NZ Med J. (2006) 119:U2144.
53. Chavez-MacGregor M, Elias SG, Onland-Moret NC, van der Schouw YT, Van Gils CH, Monninkhof E, et al. Postmenopausal breast cancer risk and cumulative number of menstrual cycles. Cancer Epidemiol Prev Biomarkers. (2005) 14:799–804. doi: 10.1158/1055-9965.EPI-04-0465
54. Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. (1993) 15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115
55. Adhi M, Hasan R, Shoaib S, Tauheed S. Age and symptomatology of menopause in Karachi, Pakistan. Pak J Physiol. (2007) 3:41–4.
56. Yahya S, Rehan N. Age, pattern and symptoms of menopause among rural women of Lahore. Age (Years). (2002) 42:51–60.
57. Wasti S, Robinson S, Akhtar Y, Khan S, Badaruddin N. Characteristics of menopause in three socioeconomic urban groups in Karachi, Pakistan. Maturitas. (1993) 16:61–9. doi: 10.1016/0378-5122(93)90134-4
58. Asif HM, Sultana S, Shafiquee S, Akhtar N. Age at menarche, menstrual pattern and premenstrual symptoms of female students of Islamia University Bahawalpur. RADS J Pharm Pharm Sci. (2017) 5:44–8.
59. Hankinson SE, Colditz GA, Hunter DJ, Manson JE, Willett WC, Stampfer MJ, et al. Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses' Health Study (United States). Cancer Causes Control. (1995) 6:217–24. doi: 10.1007/BF00051793
60. Chubak J, Tworoger SS, Yasui Y, Ulrich CM, Stanczyk FZ, McTiernan A. Associations between reproductive and menstrual factors and postmenopausal sex hormone concentrations. Cancer Epidemiol Prev Biomarkers. (2004) 13:1296–301.
61. Gellert S, Ströhle A, Hahn A Breastfeeding woman are at higher risk of vitamin D deficiency than non-breastfeeding women-insights from the German VitaMinFemin study. International breastfeeding journal. (2016) 12:19. doi: 10.1186/s13006-017-0105-1
62. Li N, Wu H, Hang F, Zhang Y, Li M. Women with recurrent spontaneous abortion have decreased 25 (OH) vitamin D and VDR at the fetal-maternal interface. Braz J Med Biol Res. (2017) 50:e6527. doi: 10.1590/1414-431x20176527
63. Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal Serum 25-Hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women-3. J Nutr. (2010) 140:999–1006. doi: 10.3945/jn.109.119636
Keywords: colorectal cancer, vitamin D (1, 25(OH)2 D3), sex hormones, gender comparison, univariate analysis
Citation: Razak S, Alam I, Afsar T, Abulmeaty MMA, Almajwal A and Jahan S (2019) A Prospective Evaluation of Serum Vitamin D (1, 25(OH)2 D3) and Endogenous Sex Hormone Levels in Colorectal Cancer Patients. Front. Oncol. 9:468. doi: 10.3389/fonc.2019.00468
Received: 21 September 2018; Accepted: 15 May 2019;
Published: 04 June 2019.
Edited by:
Elizabeth J. Ryan, University of Limerick, IrelandReviewed by:
Amit Gupta, All India Institute of Medical Sciences, Rishikesh, IndiaFrancesco Caiazza, University of California, San Francisco, United States
Copyright © 2019 Razak, Alam, Afsar, Abulmeaty, Almajwal and Jahan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suhail Razak, cnVoYWlsMTIzNDVAeWFob28uY29t; c21hcmF6aUBrc3UuZWR1LnNh
 Suhail Razak
Suhail Razak Iftikhar Alam
Iftikhar Alam Tayyaba Afsar
Tayyaba Afsar Mahmoud M. A. Abulmeaty
Mahmoud M. A. Abulmeaty Ali Almajwal1
Ali Almajwal1