- 1Department of Epidemiology, Second Military Medical University, Shanghai, China
- 2Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China
Background and aims: The contribution of hepatitis B virus (HBV) infection to the aggressiveness of primary liver cancer (PLC) remains controversial. We aimed to characterize this in eastern China.
Methods: We enrolled 8,515 PLC patients whose specimens were reserved at the BioBank of the hepatobiliary hospital (Shanghai, China) during 2007–2016. Of those, 3,124 who received primary radical resection were involved in survival analysis. A nomogram was constructed to predict the survivals using preoperative parameters.
Results: Hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined hepatocellular cholangiocarcinoma (CHC) accounted for 94.6, 3.7, and 1.7%, respectively. The rates of HBV infection were 87.5, 49.2, and 80.6%, respectively. HBV infection was significantly associated with 10-year earlier onset, more cirrhosis, higher α-fetoprotein, higher carbohydrate antigen 19-9 (CA19-9), more microvascular invasion (MVI), lower neutrophil-to-lymphocyte ratio (NLR), and lower platelet-to-lymphocyte ratio (PLR) in HCC. HBV infection was also associated with 7-year earlier onset, more cirrhosis, higher α-fetoprotein, more MVI, and lower PLR in ICC. In the multivariate Cox analysis, high circulating HBV DNA, α-fetoprotein, CA19-9, NLR, tumor size, number, encapsulation, Barcelona Clinic Liver Cancer (BCLC) stage, and MVI predicted an unfavorable prognosis in HCC; only CA19-9 and BCLC stage, rather than HBV-related parameters, had prognostic values in ICC. A nomogram constructed with preoperative HBV-related parameters including HBV load, ultrasonic cirrhosis, and α-fetoprotein perform better than the current staging systems in predicting postoperative survival in HCC.
Conclusion: HBV promotes the aggressiveness of HCC in Chinese population. The contributions of HBV to ICC and other etiological factors to HCC might be indirect via arousing non-resolving inflammation.
Introduction
Primary liver cancer (PLC), comprising hepatocellular carcinoma (HCC, 70–90%), intrahepatic cholangiocarcinoma (ICC, 10–20%), and rare histotypes including combined hepatocellular cholangiocarcinoma (CHC), is the second leading cause of cancer death in men and the sixth leading cause of cancer death in women worldwide (1, 2). The incidence rates of PLC remain highest in Asia. China alone accounts for half of global PLC (1). Over decades, the mortalities increased in Europe and America and decreased in East Asia (1).
Of global PLC, 56% were attributable to hepatitis B virus (HBV) and 20% to hepatitis C virus (HCV) (3). Chronic HBV infection is the major cause of HCC in Asian and African countries (4). Although HCV infection is the leading cause of HCC in most European and American countries, the contribution of HBV is increasing possibly because of immigration. Aflatoxin B1 exposure, alcohol consumption, sugar consumption, and diabetes also contribute to the development of HCC (1–5). Aflatoxin B1 exposure or smoking increases the occurrence of HCC caused by other factors (6, 7). Infection with Opisthorchis viverrini and Clonorchis sinensis, hepatolithiasis, and primary sclerosing cholangitis are associated with cholangiocarcinoma (8). Chronic infection with HBV or HCV also increase the risk of ICC (9, 10). However, it remains to be identified whether the risk factors promote the development of HCC or ICC directly or indirectly via inducing inflammation.
The association of etiological factors and PLC prognosis remains controversial. Data from Australia and the United States indicate that HBV-related HCC has a better prognosis than HCV-related HCC, which is hardly repeated in other populations (11–15). Thus, we performed this large epidemiological study to clarify the contribution of HBV infection to the aggressiveness of major PLC histotypes.
Materials and Methods
Patient Enrollment
From January 1st 2007 to March 31st 2016, 8,515 consecutive PLC patients who received hepatectomy at the Eastern Hepatobiliary Surgery Hospital (Shanghai, China) and had their removed tissues reserved in the BioBank were enrolled in this study. Their diagnoses were pathologically confirmed. Radical resection was defined as follows: (i) complete resection of all tumor nodules, with the cut surface free of cancer cells by pathologic examination; (ii) no macroscopic tumor thrombosis in the portal vein (main trunk or two major branches), hepatic veins, or bile duct; (iii) number of tumor nodules not exceeding three; (iv) serum α-fetoprotein (AFP), if positive, declined to undetectable level 2 months after surgery; and (v) no extrahepatic metastasis. Six months after surgical treatment, patients were regularly followed up through telephone by the same team of professional staff, followed by another five sequential follow-ups at the time point of 1, 2, 3, 4, and 5 years after surgery. In the telephone interview, we collected data including survival situation, treatment(s) received after surgery, as well as the exact date of death in case of death. The final date of follow-up was August 31st, 2017. Patients who survived were censored at their last follow-up. Patients with microvascular invasion (MVI) were recommended to receive postoperative transcatheter arterial chemoembolization (TACE) as previously described (16). Patients with imaging evidence of tumor recurrence were recommended to receive second resection or radiofrequency ablation (RFA) (17).
Data Collection
Demographical information, pathological examinations (including nodule number, capsule integrity of tumor, and MVI), and results of the latest pre-operative laboratory examinations [including serum AFP, carbohydrate antigen 19-9 (CA19-9), HBV parameters, routine blood test, and liver function tests] were extracted from electronic medical records. Child–Pugh score and Barcelona Clinic Liver Cancer (BCLC) stage were determined as previously described (18, 19).
Statistical Analysis
HBV infection was defined if a patient was seropositive for HBsAg and/or HBV DNA (20). Occult HBV infection (OBI) was defined as the presence of HBV DNA in a patient seronegative for HBsAg (21). Neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) were calculated as neutrophil count and platelet count divided by lymphocyte count, respectively. Their cutoff values were determined using X-tile software (http://www.tissuearray.org/rimmlab/xtile.html, RRID: SCR_005602). Cutoff values of AFP, CA19-9, total bilirubin, direct bilirubin, and albumin were the same as those in previous studies (22, 23). Categorical variables including the positive rates of hepatitis B surface antigen (HBsAg) were compared by χ2 test or Fisher's exact test when appropriate. Continuous variables with skewed distribution were compared by Kruskal–Wallis ANOVA for multiple group comparison and Mann–Whitney U-test for double group comparison. The Bonferroni correction was applied for multiple comparisons. The Kaplan–Meier method was applied to estimate overall survival (OS), and the log-rank test was performed to compare the difference between survival curves. A Cox proportional hazard model was applied to calculate the hazard ratio (HR) and its 95% confidence interval (CI) for each variable. Significant variables in the univariate Cox analysis were introduced into the multivariate Cox model to determine the factors that independently contributed to postoperative survival. Our cohort was randomly dichotomized into a training cohort and a validation cohort. A Cox model utilizing pre-operatively available variables was fitted in training cohort. A final model was selected by a backward stepwise selection procedure following the Akaike information criterion (24). A nomogram was formulated by applying the rms package in R (25). The performance of nomogram was measured by the concordance index (C-index) and calibration plots with 1,000 bootstraps. Comparisons of the prediction power between the nomogram and independent prognostic factors or clinical staging systems were performed using the rcorrp.cens package in Hmisc in R and were evaluated by the C-index (26). The accuracy of the nomogram was validated in the validation cohort. All statistical analyses were two-sided and performed using SPSS V21.0 for Windows (http://www-01.ibm.com/software/uk/analytics/spss/, RRID: SCR_002865) and RStudio V3.9.2 (http://www.rstudio.com/RRID:SCR_000432). P < 0.05 was considered as statistically significant.
Results
Patients were from almost all provinces of mainland China except Tibet. Patients from eastern China accounted for 93.9% (Supplementary Figure 1). They are self-reported Chinese with a median age of 53 years [interquartile range (IQR), 46–61 years]. Patients were predominantly male, with a male-to-female ratio of 6.15. Of the 8,515 patients, 8,056 (94.6%) had HCC, 314 (3.7%) had ICC, and 145 (1.7%) had CHC. The proportions of patients seropositive for HBsAg, HBV DNA, and anti-HCV antibody were 87.3, 51.7and 1.7% in HCC, 49.2, 33.8, and 1.8% in ICC, and 80.6, 41.5, and 1.4% in CHC, respectively. OBI accounted for 0.2% in HCC. Compared with ICC patients, HCC patients had a higher male-to-female ratio, higher proportions of AFP positivity, HBsAg positivity, and HBV DNA positivity, and a lower proportion of CA19-9 positivity. Compared with HCC patients, CHC patients had higher proportions of CA19-9 seropositivity, NLR (>3.3), and PLR (>117). These data are shown in Supplementary Table 1.
Demographical and Clinical Characteristics Between Primary Liver Cancer Patients With Hepatitis B Virus Infection and Those Without Hepatitis B Virus Infection
Compared with HCC patients without HBV infection, HCC patients with HBV infection were 10 years younger and had higher proportions of positive AFP (≥20 ng/ml), positive CA19-9 (≥37 U/ml), the presence of liver cirrhosis, high direct bilirubin (>7 μmol/L), advanced BCLC stage, and the presence of MVI and lower proportions of NLR (>3.3) and PLR (>117; Table 1). Compared with ICC patients without HBV infection, those with HBV infection were 7 years younger and had a higher male-to-female ratio, higher proportions of positive AFP, cirrhosis, and MVI and lower proportions of PLR (>117) and advanced Child–Pugh score (B vs. A; Table 2). Similarly, CHC patients with HBV infection were 9 years younger and had higher proportions of AFP positivity and cirrhosis, and lower proportions of NLR (>3.3) and PLR (>117) than those without HBV infection (Supplementary Table 2).
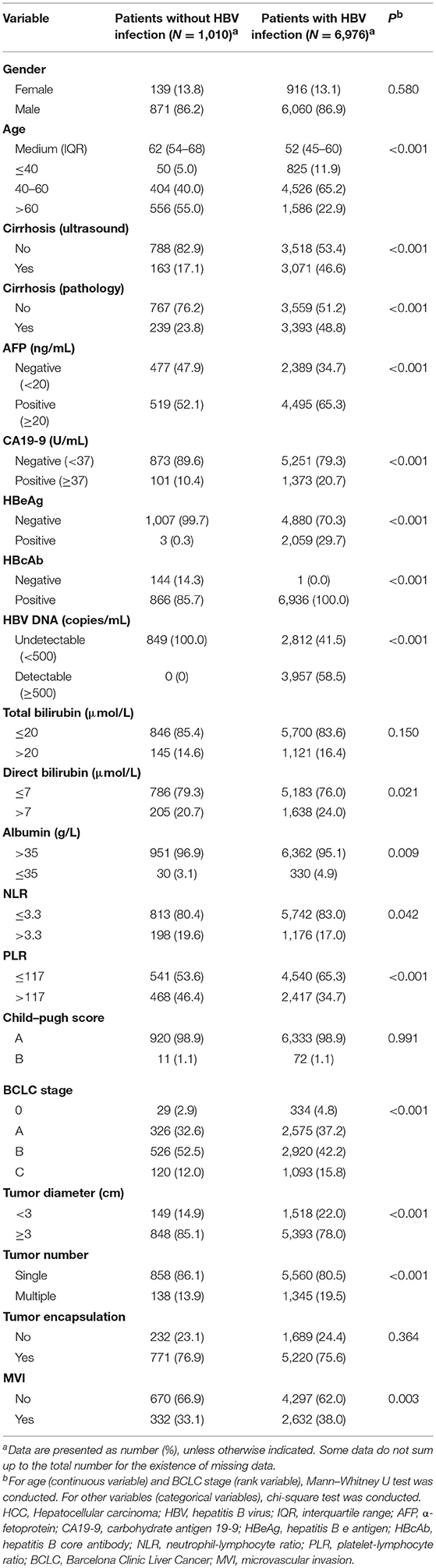
Table 1. Comparison of demographical and clinical characteristics between HCC patients with HBV infection and those without HBV infection.
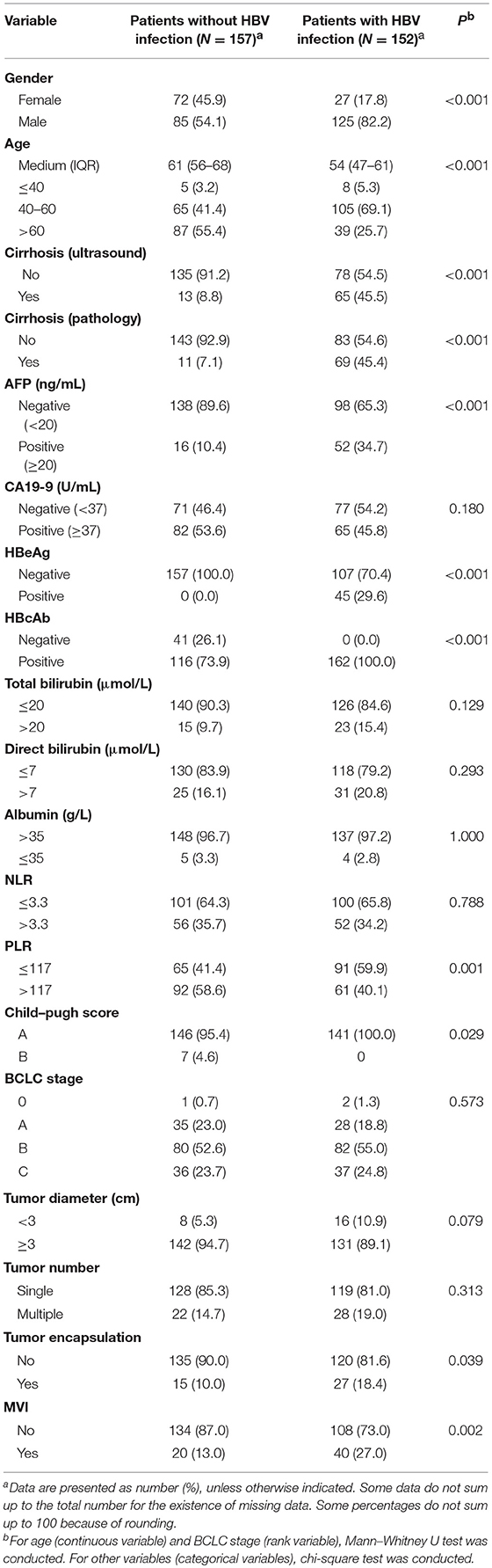
Table 2. Comparison of demographical and clinical characteristics between ICC patients with HBV infection and those without HBV infection.
Postoperative Survival
Patients who received first radical resection at the study hospital (n = 5,602) were invited to join in the survival analysis. Of those, 2,478 (1,932 refused to be followed-up and 546 were lost in the follow-up) were excluded from survival analysis. The remaining 3,124 patients were included in survival analysis (Supplementary Figure 2). The median follow-up time was 1.18 years, with an IQR of 0.78–2.35 years. Supplementary Table 3 shows the baseline characteristics of patients involved in survival analysis and those not involved. Of the 3,124 patients, 1,443 died of this malignancy during follow-up, with the 1-, 3-, and 5-year survival rates of 79.7, 47.5, and 28.6%, respectively. Postoperative 1-, 3-, and 5-year survival rates of patients with each histotype are shown in Supplementary Table 4. Multivariate Cox regression analysis indicated that serum HBV DNA (≥500 copies/ml), AFP (>20 ng/ml), CA19-9 (>37 U/ml), NLR (>3.3), tumor size (≥3 cm in diameter), multiple tumor nodules, incomplete tumor capsule, later more advanced BCLC stage, and MVI independently predicted shorter OS in HCC. Second resection and RFA independently improved OS (Table 3). CA19-9 (>37U/ml), multiple tumor nodules, and BCLC were significantly associated with shorter OS in univariate Cox analysis, while CA19-9 and more advanced BCLC stage were independently associated with OS in ICC (Supplementary Table 5). To further clarify the effect of HBV parameters on the aggressiveness of HCC, multivariate Cox regression analysis was conducted in HBV-positive HCC patients. It was found that HBV DNA (≥500 copies/ml) was significantly associated with shorter OS (Supplementary Table 6), indicating that HCC patients with active HBV replication had shorter OS than those with inactive HBV replication.
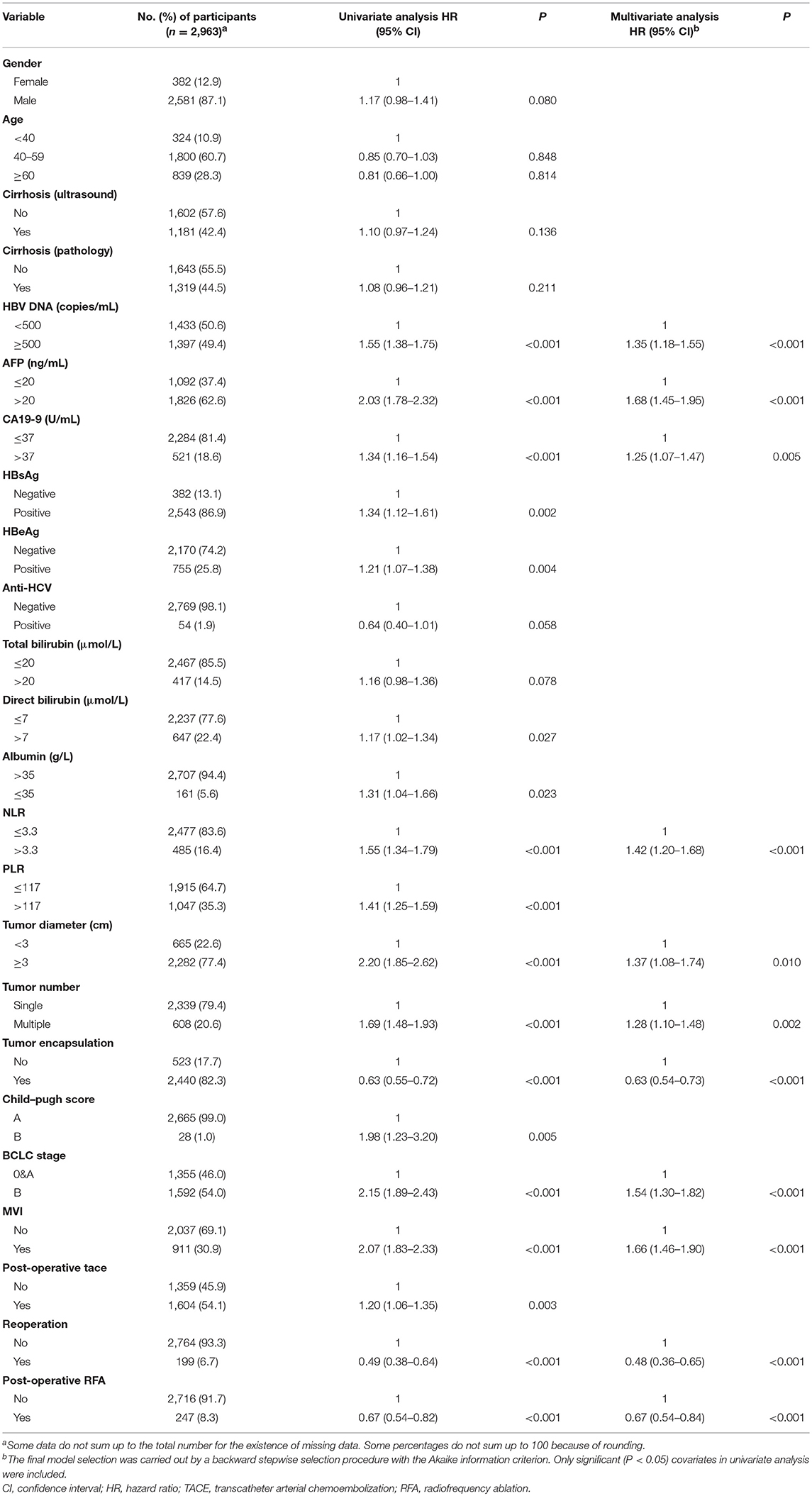
Table 3. Univariate and multivariate Cox regression analysis of prognostic factors for postoperative survival in HCC patients.
Predication for Postoperative Prognosis Using Preoperative Parameters
To evaluate if HBV-related clinical parameters harvested preoperatively could predict postoperative prognosis, we developed a nomogram using the independent prognostic factors. HCC patients with radical resection (n = 2,963) were randomly dichotomized into the training cohort (n = 1,482) and validation cohort (n = 1,481). All demographical and clinical characteristics were balanced between the training cohort and validation cohort except CA19-9 (Supplementary Table 7). Multivariate Cox analysis in the training cohort showed that preoperative ultrasound cirrhosis, AFP, BCLC stage, HBV DNA, and tumor size were independently associated with OS (Supplementary Table 8). A nomogram that integrated all independent prognostic factors in the training cohort is shown in Figure 1A. The C-index for survival prediction of the nomogram in the training cohort was 0.699 (95% CI, 0.669–0.729). The calibration plot for the probability of postoperative OS showed good agreement between the prediction by nomogram and actual observation (Figures 1B,C). The results were faithfully replicated in the validation cohort. The C-index in the validation cohort was 0.700 (95% CI, 0.670–0.730), and a calibration curve showed a good agreement between prediction and actual observation in the probability of 3- and 5-year survivals (Figures 1D,E). The C-index was 0.644, 0.638, 0.597, and 0.546 by tumor size, AFP, MVI, and incomplete tumor capsule, respectively, which were significantly lower than that by the nomogram (P < 0.001 for each comparison). We then compared the accuracy between the nomogram and each of the clinical staging systems including American Joint Committee on Cancer (AJCC) Staging Manual, 7th ed., Okuda, Chinese University Prognostic Index (CUPI), Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire Prognostic classification (GETCH), and BCLC (16, 27–31). The AJCC 7th, Okuda, CUPI, GETCH, and BCLC systems showed good stratification for the postoperative prognosis of HCC patients in both the training cohort and the validation cohort (Supplementary Figure 3). In the training cohort, the C-index of the nomogram was significantly higher than the AJCC 7th (0.644, P < 0.001), Okuda (0.564, P < 0.001), CUPI (0.514, P < 0.001), GETCH (0.611, P < 0.001), and BCLC (0.608, P < 0.001). Thus, the nomogram resulted in more accurate prediction for postoperative prognosis of HCC than the prevailing prognostic factors and well-established clinical staging systems.
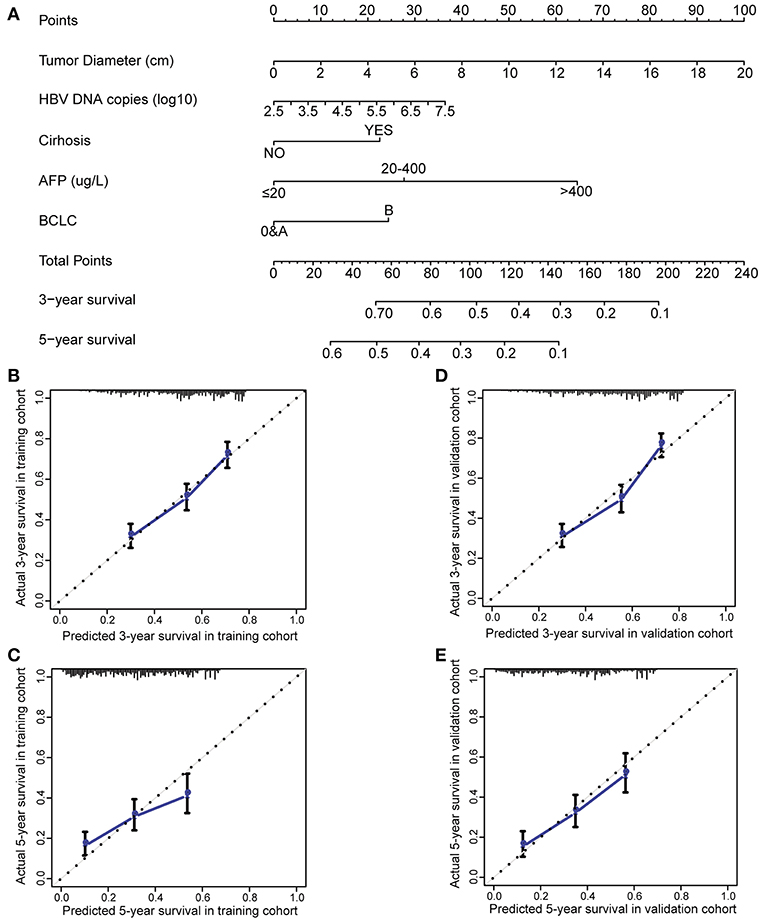
Figure 1. Preoperative nomogram and the calibration curve for predicting postoperative survival of patients with HCC. (A) The nomogram. To use this nomogram, a patient's value is located on each variable axis, and a line represents the number of points received for each variable value. The sum of these numbers is located on “Total Points” axis, and a line is drawn downward to the survival axes to determine the likelihood of postoperative 3- or 5-year survival. (B) The calibration curve for predicting postoperative 3-year survival in the training cohort. (C) The calibration curve for predicting postoperative 5-year survival in the training cohort. (D) The calibration curve for predicting postoperative 3-year survival in the validation cohort. (E) The calibration curve for predicting postoperative 5-year survival in the validation cohort. AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma.
Discussion
In this study, we found that HBV infection was associated with 10-year earlier onset and higher proportions of positive AFP, positive CA19-9, the presence of liver cirrhosis, high direct bilirubin, advanced BCLC stage, and the presence of MVI in HCC. AFP, whose expression can be driven by HBV X protein, plays a critical role in promoting the stemness of HCC cells (32). Liver cirrhosis represents anti-inflammatory immune responses to hepatitis B flares or hepatic injuries caused by other chronic inflammation (33). HBV, especially its evolved forms generated during chronic infection and its integrated forms, directly promotes the development of HCC (34–36). This may explain why HBV-related HCC occurs 10 years earlier and is more aggressive than HCC caused by other etiological factors. HBV was inversely associated with NLR and PLR, the well-established inflammatory factors (37, 38), indicating that the non-HBV etiological factors may cause HCC via inducing non-resolving inflammation. Interestingly, the HBV-related parameters including HBV DNA, AFP, CA19-9, BCLC stage, and MVI predicted an unfavorable postoperative prognosis independently. Furthermore, the nomogram constructed with HBV-related parameters harvested preoperatively including HBV DNA, liver cirrhosis, AFP, and BCLC stage accurately predicted an unfavorable postoperative prognosis. The prediction power is better than the current clinical staging systems. These evidences indicate that HBV infection promotes the aggressiveness of HCC, at least in the HBV endemic areas. The non-HBV etiological factors promote the development of HCC possibly via inducing non-resolving inflammation.
Surprisingly, HBV infection was also associated with 7-year earlier onset, more cirrhosis, higher AFP, more MVI, and lower PLR and Child–Pugh score in ICC. We believe that the changes in these clinical parameters in ICC reflect the role of HBV in generating inflammatory background from which ICC develops, rather than direct etiological role of HBV in ICC. Compared to HCC patients, ICC patients had significantly lower proportions of positive HBsAg, positive HBV DNA, the presence of liver cirrhosis, positive AFP, and the presence of MVI and lower male-to-female ratio, the HBV-related parameters. By contrast, ICC patients had higher proportions of NLR (>3.3) and PLR (>117). These data indicate that HBV-caused inflammation, rather than HBV itself, play a major role in inducing ICC in HBV-infected subjects. CA19-9 and BCLC stage were not associated with HBV infection in ICC, but they predicted an unfavorable prognosis in ICC independently. These data indicate that HBV infection is not related to the aggressiveness of ICC. HBV promotes the development of ICC indirectly via inducing non-resolving inflammation. Antiviral treatment reduces the risk of ICC (9, 10), possibly via reducing the non-resolving inflammation caused by active HBV infection.
HBV infection accounted for 87.5% of HCC, while HCV infection only accounted for 1.7%. This difference might be fundamentally related to the genetic predispositions. The genotypes and/or allele of HLA-DQ, HLA-DP, and NFKBIA single-nucleotide polymorphisms (SNPs) that significantly increased the risk of chronic HBV infection are more frequent in Chinese population than in European population (http://www.hapmap.org/) (39–42). These genetic predispositions in Chinese population facilitate chronic transformation of HBV infection, possibly via weakening the corresponding antiviral immune function. HBV that evolved in chronic inflammatory microenvironment promotes the occurrence and aggressiveness of HCC. Instead, the C/C genotype of a SNP (rs12979860) of the IL28B gene, which is strongly associated with spontaneous clearance of HCV, is more frequent in Chinese population than in African or European population (http://www.hapmap.org/) (43). Thus, HCV might be more apt to cause HCC-inducing non-resolving inflammation in Western populations than in Chinese population. Non-resolving inflammation caused by chronic HCV infection might promote the aggressiveness of HCC, especially in Western populations.
Our study has several limitations. First, selection bias cannot be avoided in a single center. Second, severity of cirrhosis, family history, exposure to aflatoxin, metabolic syndrome, dietary changes, alcohol consumption, and cigarette smoking were not included because these data were not intact in their medical records. Third, compared with patients lost to follow-up, patients who were successfully followed-up had higher AFP level, higher Child–Pugh score, higher BCLC stage, larger tumor diameter, lower albumin level, larger tumor diameter, higher proportion of multiple tumor nodules, tumor encapsulation, and MVI, most of which were associated with shorter OS in HCC, so the survival rates of HCC should be underestimated. Fourth, the effects of postoperative radiotherapy, chemotherapy, stereotactic radiation, percutaneous ethanol injection, antiviral treatment, and targeted therapy as well as their combinations were not evaluated because of very small sample size. Fifth, prognosis prediction analysis was not carried out in ICC or CHC because of small sample sizes. Sixth, HBV replication was not found to be significantly associated with the progression of ICC, which might also be related to insufficient power due to small sample size.
Conclusively, this large epidemiological study demonstrates that HBV infection contributes to the aggressiveness of HCC in China and possibly in other HBV-endemic areas. The contribution of HBV to the aggressiveness of ICC and other risk factors to the aggressiveness of HCC might be indirect via arousing non-resolving inflammation.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Ethics Statement
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the ethics committee of Eastern Hepatobiliary Surgery Hospital.
Author Contributions
FY, LM, and WL contributed to the data organization, data analyses, and data interpretation. YY, JZ, MW, SC, and FS contributed to the patient enrolment, surgical treatment, and follow-up. XC, HZ, and HW conducted data collection and analyses. WZ performed surgical treatment as well as construction and maintenance of the database and BioBank. GC contributed to the study design, supervision, and writing of the manuscript.
Funding
This study was supported by the National Key Basic Research Program of China (973 Program) (grant numbers 2015CB554000 to GC and 2014CB542102 to WZ), the National Natural Science Foundation of China (grant numbers 81520108021, 91529305, and 81673250 to GC, 81502882 to XC, and 81521091 to HW), the State Key Infection Disease Project of China (grant numbers 2017ZX10203208 to WZ and 2017ZX10201201-006-001 to HZ), and the National Human Genetic Resources Sharing Service Platform (grant number 2005DKA21300 to WZ).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the medical staff including Fengqiu Chen, Yuanping Tao, Jutang Li, and Huifen Li for their excellent work in conducting follow-up study and managing the clinical information.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00370/full#supplementary-material
Abbreviations
AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CA19-9, carbohydrate antigen 19-9; CHC, combined hepatocellular cholangiocarcinoma; CI, confidence interval; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HR, hazard ratio; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; IQR, interquartile range; MVI, microvascular invasion; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; PLC, primary liver cancer; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:187–08. doi: 10.3322/caac.21262
2. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. (2017) 152:745–61. doi: 10.1053/j.gastro.2016.11.048
3. Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. (2018) 142:2471–7. doi: 10.1002/ijc.31280
4. de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. (2015) 62:1190–200. doi: 10.1002/hep.27969
5. Aleksandrova K, Boffetta P, Tjønneland A, Franceschi S. Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in Western Europeans. Ann Oncol. (2013) 24:543–53. doi: 10.1093/annonc/mds434
6. Chu YJ, Yang HI, Wu HC, Liu J, Wang LY, Lu SN, et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int J Cancer. (2017) 141:711–20. doi: 10.1002/ijc.30782
7. Liu X, Baecker A, Wu M, Zhou JY, Yang J, Han RQ, et al. Interaction between tobacco smoking and hepatitis B virus infection on the risk of liver cancer in a Chinese population. Int J Cancer. (2018) 142:1560–7. doi: 10.1002/ijc.31181
8. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. (2013) 145:1215–29. doi: 10.1053/j.gastro.2013.10.013
9. Zhou Y, Zhao Y, Li B, Huang J, Wu L, Xu D, et al. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. (2012) 12:289. doi: 10.1186/1471-2407-12-289
10. Lee TY, Hsu YC, Yu SH, Lin JT, Wu MS, Wu CY. Effect of nucleos(t)ide analogue therapy on risk of intrahepatic cholangiocarcinoma in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. (2018) 16:947–54.e4. doi: 10.1016/j.cgh.2017.09.031
11. Mgaieth S, Kemp W, Gow P, Fink M, Lubel J, Nicoll A, et al. Impact of viral hepatitis aetiology on survival outcomes in hepatocellular carcinoma: a large multicentre cohort study. J Viral Hepat. (2017) 24:982–9. doi: 10.1111/jvh.12717
12. Younossi ZM, Stepanova M, Saab S, Ahmed A, Lam B, Srishord M, et al. The impact of viral hepatitis-related hepatocellular carcinoma to post-transplant outcomes. J Viral Hepat. (2016) 23:53–61. doi: 10.1111/jvh.12449
13. Munaf A, Memon MS, Kumar P, Ahmed S, Kumar MB. Comparison of viral hepatitis-associated hepatocellular carcinoma due to HBV and HCV—cohort from liver clinics in Pakistan. Asian Pac J Cancer Prev. (2014) 15:7563–7. doi: 10.7314/APJCP.2014.15.18.7563
14. Li Q, Li H, Qin Y, Wang PP, Hao X. Comparison of surgical outcomes for small hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a Chinese experience. J Gastroenterol Hepatol. (2007) 22:1936–41. doi: 10.1111/j.1440-1746.2006.04619.x
15. Makarova M, Krettek A, Valkov MY, Grjibovski AM. Hepatitis B and C viruses and survival from hepatocellular carcinoma in the Arkhangelsk region: a Russian registry-based study. Int J Circumpolar Health. (2013) 72:20282. doi: 10.3402/ijch.v72i0.20282
16. Ueno M, Hayami S, Shigekawa Y, Kawai M, Hirono S, Okada K, et al. Prognostic impact of surgery and radiofrequency ablation on single nodular HCC?5 cm: cohort study based on serum HCC markers. J Hepatol. (2015) 63:1352–9. doi: 10.1016/j.jhep.2015.07.013
17. Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. (2014) 61:82–8. doi: 10.1016/j.jhep.2014.03.012
18. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. (1973) 60:646–9.
19. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. (1999) 19:329–38.
21. Chen L, Zhao H, Yang X, Gao JY, Cheng J. HBsAg-negative hepatitis B virus infection and hepatocellular carcinoma. Discov Med. (2014) 18:189–93.
22. Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. (2013) 31:3647–55. doi: 10.1200/JCO.2012.48.5896
23. Yin J, Wang J, Pu R, Xin H, Li Z, Han X, et al. Hepatitis B virus combo mutations improve the prediction and active prophylaxis of hepatocellular carcinoma: a clinic-based cohort study. Cancer Prev Res. (2015) 8:978–88. doi: 10.1158/1940-6207.CAPR-15-0160
24. Harrell FE Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15:361–87.
25. Frank E, Harrell Jr. Rms: Regression Modeling Strategies. R Package version 3.4-0. Available online at: https://cran.r-project.org/web/packages/rms/index.html (accessed May 8, 2019)
26. Harrell FE. Hmisc: Harrell Miscellaneous. R Package version 3.9-2. Available online at: https://cran.r-project.org/web/packages/Hmisc/index.html (accessed May 8, 2019)
27. Huitzil-Melendez FD, Capanu M, O'Reilly EM, Duffy A, Gansukh B, Saltz LL, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. (2010) 28:2889–95. doi: 10.1200/JCO.2009.25.9895
28. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
29. Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. (1985) 56:918–28.
30. Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. (2002) 94:1760–9. doi: 10.1002/cncr.10384
31. Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma: Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. (1999) 31:133–41.
32. Zhu M, Li W, Lu Y, Dong X, Lin B, Chen Y, et al. HBx drives alpha fetoprotein expression to promote initiation of liver cancer stem cells through activating PI3K/AKT signal pathway. Int J Cancer. (2017) 140:1346–55. doi: 10.1002/ijc.30553
33. Lin J, Wu JF, Zhang Q, Zhang HW, Cao GW. Virus-related liver cirrhosis: molecular basis and therapeutic options. World J Gastroenterol. (2014) 20:6457–69. doi: 10.3748/wjg.v20.i21.6457
34. Huang Y, Tong S, Tai AW, Hussain M, Lok AS. Hepatitis B virus core promoter mutations contribute to hepatocarcinogenesis by deregulating SKP2 and its target, p21. Gastroenterology. (2011) 141:1412–21. doi: 10.1053/j.gastro.2011.06.048
35. Yin J, Xie J, Liu S, Zhang H, Han L, Lu W, et al. Association between the various mutations in viral core promoter region to different stages of hepatitis B, ranging of asymptomatic carrier state to hepatocellular carcinoma. Am J Gastroenterol. (2011) 106:81–92. doi: 10.1038/ajg.2010.399
36. Yang X, Wu L, Lin J, Wang A, Wan X, Wu Y, et al. Distinct hepatitis B virus integration patterns in hepatocellular carcinoma and adjacent normal liver tissue. Int J Cancer. (2017) 140:1324–30. doi: 10.1002/ijc.30547
37. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. (2017) 67:999–1008. doi: 10.1016/j.jhep.2017.06.026
38. Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. (2019) 125:127–34. doi: 10.1002/cncr.31778
39. Ji X, Zhang Q, Li B, Du Y, Yin J, Liu W, et al. Impacts of human leukocyte antigen DQ genetic polymorphisms and their interactions with hepatitis B virus mutations on the risks of viral persistence, liver cirrhosis, and hepatocellular carcinoma. Infect Genet Evol. (2014) 28:201–9. doi: 10.1016/j.meegid.2014.09.032
40. Zhang Q, Yin J, Zhang Y, Deng Y, Ji X, Du Y, et al. HLA-DP polymorphisms affect the outcomes of chronic hepatitis B virus infections, possibly through interacting with viral mutations. J Virol. (2013) 87:12176–86. doi: 10.1128/JVI.02073-13
41. Zhang Q, Ji XW, Hou XM, Lu FM, Du Y, Yin JH, et al. Effect of functional nuclear factor-kappaB genetic polymorphisms on hepatitis B virus persistence and their interactions with viral mutations on the risk of hepatocellular carcinoma. Ann Oncol. (2014) 25:2413–9. doi: 10.1093/annonc/mdu451
42. Li Z, Hou X, Cao G. Is mother-to-infant transmission the most important factor for persistent HBV infection? Emerg Microbes Infect. (2015) 4:e30. doi: 10.1038/emi.2015.30
Keywords: primary liver cancer, hepatitis virus, radical resection, prognosis, aggressiveness
Citation: Yang F, Ma L, Yang Y, Liu W, Zhao J, Chen X, Wang M, Zhang H, Cheng S, Shen F, Wang H, Zhou W and Cao G (2019) Contribution of Hepatitis B Virus Infection to the Aggressiveness of Primary Liver Cancer: A Clinical Epidemiological Study in Eastern China. Front. Oncol. 9:370. doi: 10.3389/fonc.2019.00370
Received: 22 March 2019; Accepted: 23 April 2019;
Published: 21 May 2019.
Edited by:
Tianhui Chen, Zhejiang Academy of Medical Sciences, ChinaReviewed by:
Wei-Hua Jia, Sun Yat-sen University Cancer Center (SYSUCC), ChinaHongping Yu, Tumor Hospital of Guangxi Medical University, China
Copyright © 2019 Yang, Ma, Yang, Liu, Zhao, Chen, Wang, Zhang, Cheng, Shen, Wang, Zhou and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiping Zhou, RUhQSFdQQDEyNi5jb20=
Guangwen Cao, Z2Nhb0BzbW11LmVkdS5jbg==
†These authors have contributed equally to this work
 Fan Yang
Fan Yang Longteng Ma
Longteng Ma Yuan Yang2†
Yuan Yang2† Wenbin Liu
Wenbin Liu Xi Chen
Xi Chen Hongwei Zhang
Hongwei Zhang Guangwen Cao
Guangwen Cao