
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 21 May 2019
Sec. Gastrointestinal Cancers
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00347
Prediction of prognosis of hepatocellular carcinoma (HCC) has shown an important role in improving treatment outcomes and preventing disease progression, however, the prognostic indicator of HCC is still lacking. The purpose of this study is to investigate the predictive value of GLR (gamma-glutamyl transpeptidase to lymphocyte count ratio) in single HCC with a tumor size (TS) ≤ 5 cm. A retrospective analysis was performed on 272 patients with TS ≤ 5 cm who underwent radical resection. The Pearson χ2 test was applied to discuss the relationship between HCC and GLR, alpha-fetoprotein (AFP). Then univariate and multivariate analysis was utilized to predict the risk factors for survival prognosis in patients. In this study, GLR showed a positive relation with tumor size, tumor-node-metastasis (TNM) stage, microvascular invasion, early recurrence, and serum aspartate aminotransferase (AST) level, while the AFP value only correlated with drinking. Elevated GLR value had poor overall survival (OS) and progression-free survival (PFS) of TS ≤ 5 cm HCC patients, GLR level and tumor size were closely related to the prognosis of small HCC patients compared with AFP. GLR may serve as a prognostic marker for dynamic monitoring of HCC patients with single TS ≤ 5 cm after radical resection.
Hepatocellular carcinoma (HCC) is one of the most common cancers and the major cause of cancer-related death in the world. It accounts for about 50% of the total incidence and mortality of liver cancer in China (1, 2). Guangxi is one of the regions with the highest incidence of HCC in China, accounting for 40% of the total cancer mortality, ranking first in cancer mortality spectrum. Compare to HCC patients with large tumor size (TS), there are more options for treatments of small HCC tumor patients. Resection is still the modality of first choice for the treatment of patients with TS ≤ 5 cm HCC, which has an excellent prognosis after surgery compare to the large HCC (3, 4). Although the diagnosis and treatment of HCC and postoperative management are improving, the long-term prognosis after hepatectomy is still not optimistic due to the high recurrence and metastasis rate of HCC (5). Serum alpha-fetoprotein (AFP) screening has become a routine clinical practice to diagnosis early stage liver cancer and monitoring recurrence in many parts of the developed world (6). However, serum AFP level is a less sensitive and specific indicator in predicting the prognosis of HCC, and has no diagnostic and prognostic value for small HCC (7). Thus, it is urgent to find an effective indicator to predict and surveil HCC.
As we know, the occurrence and development of hepatocellular carcinoma are closely related to inflammatory factors and immune factors (8). In view of the presence of neutrophils, lymphocytes, monocytes, liver enzymes, etc. in peripheral blood, it is easy to obtain the indicators of inflammation, immunity and liver function by using the economic and non-invasive blood biochemical routine. Liver function indicators can directly reflect the state of the liver, and associate with the prognosis of HCC patients. Gamma-glutamyl transferase (GGT) is an enzyme which acts as a marker for variety of cancers such as renal cell carcinoma, breast cancer, lung cancer and ovarian cancer (9–11), and elevated levels of GGT can be a convincing prognostic indicator for the recurrence of HCC after hepatectomy (12, 13). Recently, some researchers have proposed GGT-to-platelet ratio as an independent prognostic predictor for HBV-related HCC overall survival (14, 15). Lymphocytes have been shown to play a key role in anti-tumor in the human immune system, and the infiltration of lymphocytes is usually associated with a better prognosis in cancer, total lymphocyte infiltration has prognostic significance in HCC (16–18). More studies have shown that the neutrophil-lymphocyte ratio could predict prognosis for HCC after hepatectomy (19, 20). Furthermore, some studies have revealed that the ratio of certain indicators is related to the prognosis of HCC, such as AFP to tumor volume ratio, alkaline phosphatase to lymphocyte ratio, aspartic acid to lymphocyte ratio and neutrophil times γ-glutamyl transpeptidase to lymphocyte ratio, etc. (21–24).
In this article, we put forward a model, GLR (gamma-glutamyltransferase to lymphocyte count ratio), which has been shown to be a prognostic marker for nonfunctional pancreatic neuroendocrine tumor patients underwent curative resection (25). The purpose of this study is to demonstrate whether GLR can be used as a prognostic marker for hepatocellular carcinoma. To achieve this goal, we explored the association of GLR with clinical character, overall survival (OS), and progression free survival (PFS) in HCC patients with TS ≤ 5 cm after surgery, to provide guidance for postoperative monitoring and treatment of HCC patients after hepatectomy.
In this study, the specimens and clinical data of 272 HCC patients who underwent hepatic resection were obtained at the Affiliated Hospital of Guilin Medical University, from 1993 to 2011. Patients eligible for the following criteria were enrolled to the retrospective study: (1) a confirmed diagnosis of HCC with single nodule and a diameter < 5 cm; (2) after treatment of radical resection; (3) complete laboratory biochemical data and follow-up records; (4) no lymphatic system disease and blood system disease. Routine examination of these 272 HCC patients, including ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) and blood biochemical examination were performed before the surgery. Data were collected from patients including age, gender, family history, drinking history, smoking history, HBsAg, serum AFP, liver cirrhosis, tumor-node-metastasis (TNM) stage, NEUT, LYMPH, aspartate aminotransferase (AST), alanine aminotransferase (ALT), recurrence, GGT and so on.
Long term follow-up was performed in 272 cases of HCC patients after radical resection. In the first 2 years, abdominal ultrasonography, blood routine, liver function index, and serum AFP were performed every 2 months after surgery, and same examinations were conducted every 6 months after 2 years. Overall survival was calculated as the interval between the date of operation and the date of death or the last follow-up date. Progression free survival was calculated as the interval between the date of operation and the date of disease progression or death. Recurrence was defined as the presence of clinical symptoms associated with hepatic ultrasound or serum AFP abnormalities, and the recurrence of HCC within 2 years was defined as early recurrence.
The data conforming to Gaussian distribution was expressed as mean ± standard deviation (SD) and assessed by independent t-test, while categorical data were compared using Chi square (χ2) test or the Fisher exact test. Kaplan-Meier survival curve analysis was utilized to compare the survival difference between different HCC groups, and survival distributions were compared with log-rank test. Univariate analysis and multivariate Cox proportional hazards regression model were performed to determine independent prognostic factors. All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL) and P < 0.05 were found to be statistically significant.
A total of 272 patients (226 males and 46 females) diagnosed with HCC were enrolled in this study. The average age of these patients was 51.90 ± 11.37 years. The mean values of NEUT ( × 109/L), LYMPH ( × 109/L), Platelets (109/L), Albumin (g/L), Globuline (g/L), ALT (U/L), AST (U/L) and ALP (U/L) in these patients were 3.38 ± 1.50, 1.80 ± 0.62, 162.24 ± 72.42, 39.22 ± 4.50, 30.61 ± 5.02, 46.17 ± 44.06, 44.45 ± 41.83, and 87.32 ± 61.22, respectively. And the median of AFP, GGT and GLR were 43.3 ng/ml (range 0.20–9012.00), 44.46 U/L (range 10.84–517.35) and 28.97 (range 4.86–361.75), respectively. Other parameters and more details are shown in Table 1.
On the base of receiver operating characteristic (ROC) curve analysis, the optimal cut-off value of GLR was 23.3, with a sensitivity of 69.3% and a specificity of 76.6%. The AUC of the GLR was 0.796 [95% confidence interval (CI), 0.756–0.840] for predicting the prognosis in patients with HCC. The relationships between preoperative GLR, AFP level and clinicopathologic parameters of HCC patients with a diameter ≤ 5 cm were analyzed and showed in Table 2. All cases were divided into two groups based on GLR level, 160 patients (58.82%) were identified as high-GLR group with an elevated GLR (>23.3), and 112 patients (41.18%) were identified as low-GLR ( ≤ 23.3) group. High AFP level group (AFP > 200 ng/ml) and low AFP level group (AFP ≤ 200 ng/ml) were also sorted in this study. The results showed that positive relationships were existed between GLR level and tumor size (P = 0.038), TNM stage (P = 0.006), microvascular invasion (P = 0.048), early recurrence (P = 0.015), and serum AST level (P < 0.001). However, there was no significant relationship between GLR level and gender, age, family history, drinking, HBsAg, cirrhosis, and serum AFP level (all P > 0.05). Relatively speaking, the levels of AFP were not significantly correlated with other clinicopathologic parameters (all P > 0.05), except drinking (P = 0.002).
The results of univariate analysis indicated that tumor size (>3 cm), TNM stage (III–IV), microvascular invasion, serum AST (>40 U/L) and (GLR > 23.3) were important factors affecting OS and PFS of HCC. In addition, early recurrence was an important factor affecting OS of HCC (Table 3). After adjusting other positive predictors, the factors mentioned above were assessed using the multivariate Cox regression analysis showed that microvascular invasion (HR = 2.58, 95% CI = 1.70–4.16, P < 0.001), early recurrence (HR = 1.76, 95% CI = 1.25–2.55, P = 0.001), serum AST > 40 U/L (HR = 1.58, 95% CI = 1.07–2.38, P = 0.021) and GLR > 23.3 (HR = 2.13, 95% CI = 1.51–3.05, P < 0.001) can play as independent predictors of OS for HCC with a diameter ≤ 5 cm. Moreover, the findings also revealed that those factors include tumor size > 3 cm (HR = 1.55, 95% CI = 1.08–2.23, P = 0.019), microvascular invasion (HR = 2.31, 95% CI = 1.60–3.82, P < 0.001), and GLR > 23.3 (HR = 2.01, 95% CI = 1.40–2.92, P < 0.001) were independent predictors of PFS for HCC with TS ≤ 5 cm (Table 3).
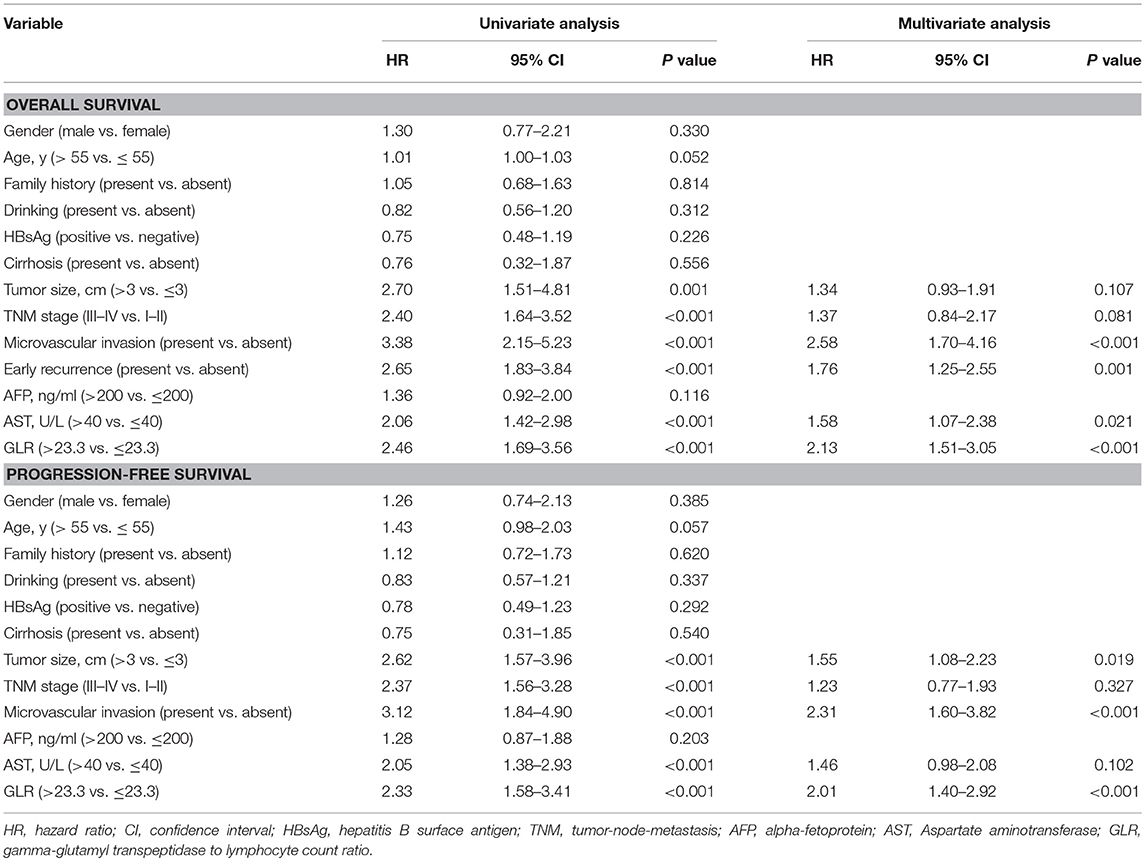
Table 3. Analysis Predictors of Overall Survival and Progression-free Survival in Patients with HCC.
Kaplan-Meier survival analysis showed that the 1-, 3-, and 5-year OS and PFS rates of 272 patients were 93.2, 77.3, and 55.6%; 80.7, 59.3, and 48.5%, respectively. The GLR > 23.3 was significantly associated with poorer OS (Figure 1A) and PFS (Figure 1B). Mean OS was 61.3 months (95% CI, 54.08–68.56) in GLR > 23.3 group and 89.7 months (95% CI, 84.20–95.06) in GLR ≤ 23.3 group (P < 0.0001), while mean PFS was 47.12 months (95% CI, 39.21–55.37) and 82.96 months (95% CI, 74.66–90.23) (P < 0.0001), respectively. Compared with AFP ≤ 200 ng/ml group, AFP > 200 ng/ml was also associated with poorer OS (P = 0.011, Figure 1C) and PFS (P = 0.033, Figure 1D).
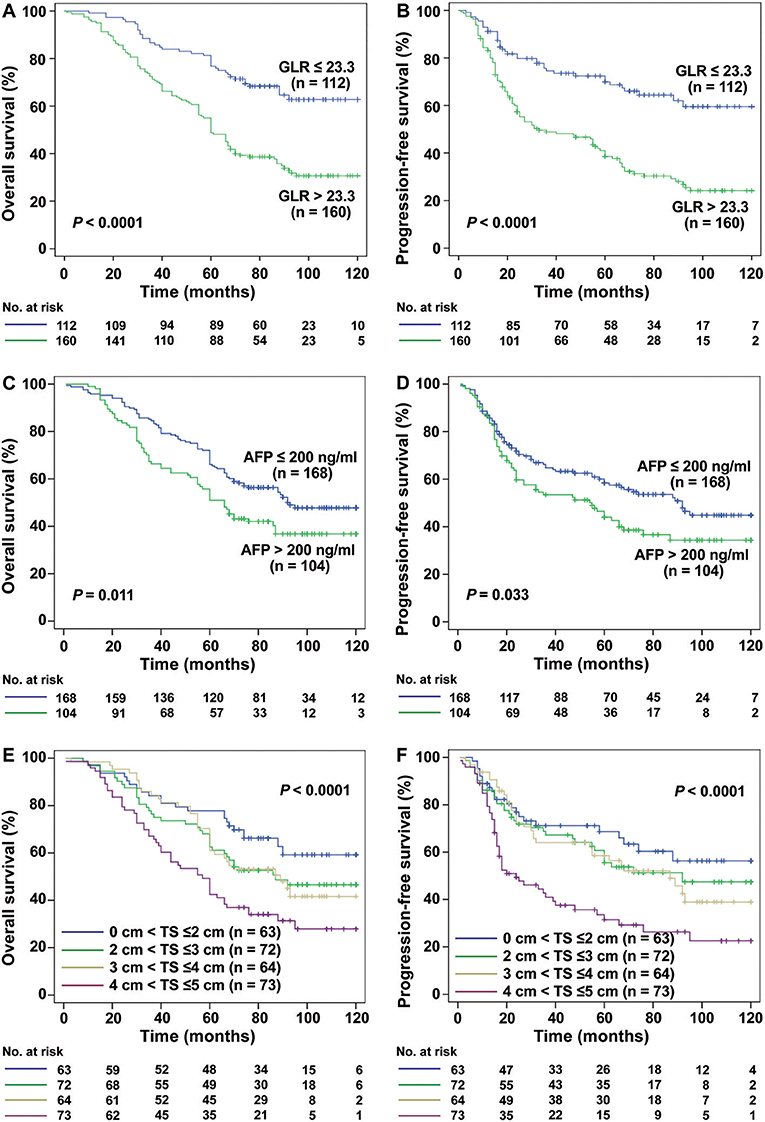
Figure 1. Kaplan-Meier estimated survival curves of HCC (TS ≤ 5 cm) patients after radical resection. (A,B) Kaplan-Meier analysis showed that the GLR ≤ 23.3 group (n = 112) had a higher rate of OS and PFS than those with GLR > 23.3 (n = 160) (both P < 0.0001). (C,D) The OS and PFS rate of AFP ≤ 200 ng/ml group (n = 168) were also higher than AFP > 200 ng/ml group (n = 104) (both P < 0.05). (E,F) In the different tumor size groups, the smaller the tumor size is, the higher the rate of OS and PFS in HCC ( ≤ 5 cm) patients (P < 0.0001).
Furthermore, different TS was also significantly associated with the mean OS (0 cm < TS ≤ 2 cm: 92.38 months, 95% CI, 82.68–102.09; 2 cm < TS ≤ 3 cm: 83.89 months, 95% CI, 74.37–93.42; 3 cm < TS ≤ 4 cm: 81.76 months, 95% CI, 72.48–90.97; 4 cm < TS ≤ 5 cm: 64.95 months, 95% CI, 55.50–74.37; P < 0.0001, Figure 1E) and mean PFS (0 cm < TS ≤ 2 cm: 83.02 months, 95% CI, 70.75–95.29; 2 cm < TS ≤ 3 cm: 75.11 months, 95% CI, 63.79–86.40; 3 cm < TS ≤ 4 cm: 71.83 months, 95% CI, 60.52–82.27; 4 cm < TS ≤ 5 cm: 48.31 months, 95% CI, 37.36–59.21; P < 0.0001, Figure 1F).
Interestingly, for HCC patients with TS ≤ 3 cm (n = 131), the mean OS was 64.51 months in GLR > 23.3 group and 95.47 months in GLR ≤ 23.3 group (P < 0.0001, Figure 2A), while mean PFS was 51.50 months and 89.51 months (P < 0.0001, Figure 2B), respectively. Similarly, the mean OS in GLR > 23.3 group and GLR ≤ 23.3 group were 62.88 months and 99.89 months (P = 0.002, Figure 3A), while the mean PFS in GLR > 23.3 group and GLR ≤ 23.3 group were 44.28 months and 91.63 months (P = 0.003, Figure 3B) in TS ≤ 2 cm HCC patients (n = 62). Conversely, AFP level has no prognostic value in TS ≤ 3 or ≤ 2 cm HCC patients (all P > 0.05, Figures 2C,D and 3C,D). These results suggest that GLR level and tumor size were closely related to the prognosis of small HCC patients.
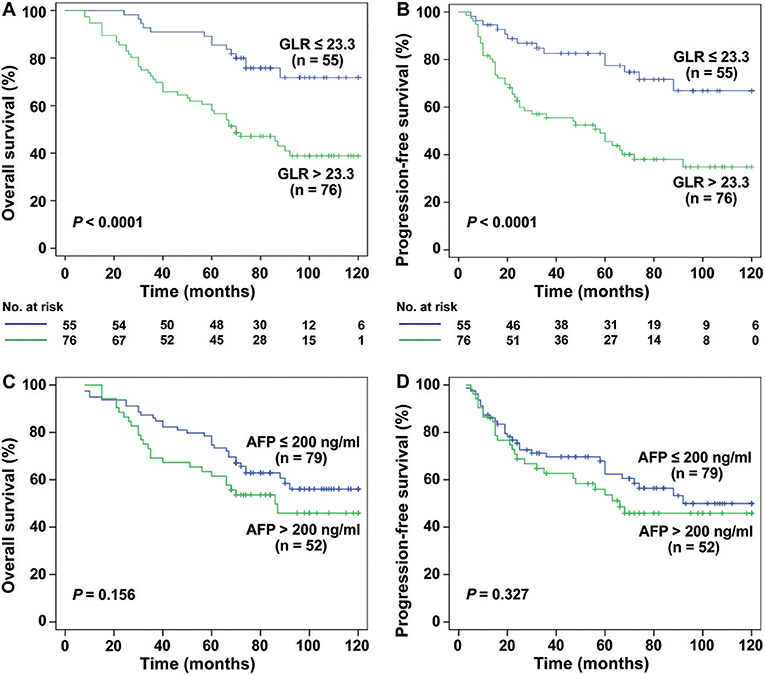
Figure 2. The relation between the GLR, AFP level and the prognosis of TS ≤ 3 cm HCC patients. (A,B) Kaplan-Meier analysis showed that the GLR ≤ 23.3 group (n = 55) had a higher rate of OS and PFS than the GLR > 23.3 group (n = 76) in TS ≤ 3 cm HCC patients (both P < 0.0001). (C,D) There was no significant difference between the AFP ≤ 200 ng/ml group (n = 79) and AFP > 200 ng/ml group (n = 52) in the rate of OS and PFS (all P > 0.05).
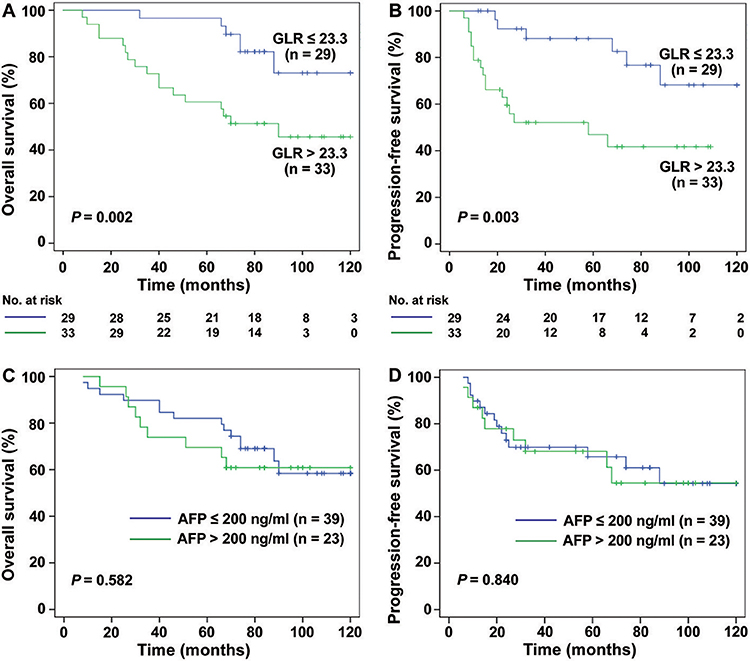
Figure 3. The survival curves of HCC (TS ≤ 2 cm) patients with different levels of GLR and AFP. (A,B) The results showed that the rate of OS and PFS in GLR ≤ 23.3 group (n = 29) was higher than the GLR > 23.3 group (n = 33) in TS ≤ 2 cm HCC patients (n = 42) (P = 0.002, P = 0.001). (C,D) The rate of OS and PFS in AFP ≤ 200 ng/ml group (n = 39) had no significant difference with AFP > 200 ng/ml group (n = 23) (all P > 0.05).
In this study, we compared the relationship between AFP or GLR and clinical characters, OS, and PFS in HCC patients with TS ≤ 5 cm, aimed to validate GLR can serve as an independent prognostic factor of HCC patients after radical resection.
Based on previous studies, the size of tumor has always been one of the most important factors that affect the prognosis of small hepatocellular carcinoma (SHCC) (26). Chen et al. reported that SHCC is defined as a single HCC nodule, which is < 5 cm, and different cut-off values of tumor size may lead to a variety of prognostic significance (27). Nevertheless, the definition of SHCC is based solely on the size of tumor, and it's different from early stage liver cancer which is a concept based on biological behavior (4). And Some SHCC cases may have advanced liver cancer characteristics, which were usually associated with poor prognosis of liver cancer (28), including high TNM stage, vascular invasion, distant metastasis, etc. Of these 272 HCC patients we investigated, there was a certain proportion of TNM III-IV (23%), microvascular invasion (8.5%), and these data confirmed the above point of view (Table 2).
Liver cancer is a typical inflammation-associated cancers, the infiltration of inflammatory cells causes the liver damage by releasing inflammatory factors, and the accumulation of chronic liver injury gives rise to the mutation of liver cells, leading to the occurrence of cancer (29). Globally, 78% of the HCC was caused by HBV (53%) or HCV (25%) chronic infection, which usually accounted for 57% of cirrhosis (30). And our data also supported this conclusion. Of these 272 cases of HCC patients, 227 cases were Hepatitis B surface antigen positive, and 262 cases were liver cirrhosis (Table 1).
Over the years, serum AFP was used as a marker for clinical diagnosis and screening of HCC because of the higher overall survival of patients with preoperative low AFP, and it is believed that AFP is an independent risk factor for postoperative survival (31, 32). However, there was a controversy about the role of AFP in the prognosis of HCC (33). In a survey of 205 patients with SHCC (TS ≤ 3 cm), it was concluded that AFP has no value in predicting the prognosis of SHCC after surgery (7). More studies have also confirmed this phenomenon (34). Similarly, in our results (Figures 1–3), AFP was associated with OS and PFS in these 272 patients with HCC (TS ≤ 5 cm), but there was no prognostic meaning for AFP in the HCC patients with tumor size ≤ 3 cm or ≤ 2 cm. On the other hand, in addition to serum AFP for postoperative monitoring, there are many models or index that can reflect the prognosis of patients with HCC after curative hepatectomy, such as SII (35), GPR (14), NLR (19), etc., and there are some gene expression signatures succeeded in prognosis prediction and treatment responses for HCC (36, 37). However, Gene related prediction models are relatively costly and not conducive to dynamic monitoring dynamic levels in vivo, considering the poor specificity and accuracy with single factor to predict the prognosis of SHCC, perhaps we can combine other parameters instead of the single influencing factor to as a prognostic factor for SHCC.
Some studies have reported that vascular invasion was identified as a poor independent prognostic factor for OS and PFS in patients with SHCC, and tumor tissue differentiation was closely related to vascular invasion (38). Our research also proved this point. Other studies have revealed that the disease-free survival rate of the hepatocellular carcinoma ≤ 5 cm without vascular invasion was significantly improved (39). Hence, it is very important for SHCC patients with vascular invasion to closely monitor the situation of early recurrence and take treatment measures to improve the long-term survival rate of patients.
Obviously, there are several limitations in our study. First, the population in our study came from the same third-grade class-A hospital, which will create a selection bias. Secondly, the sample size of the study was too small and had a poor representation and potential bias. Finally, due to the retrospective nature of this study, further prospective with large sample studies are needed to confirm our results.
In conclusion, our findings revealed that GLR was associated with tumor size, TNM stage, microvascular invasion, early recurrence, serum AST level, OS and PFS in the HCC patients with tumor size ≤ 5 cm. GLR can be served as a prognostic marker for < 5 cm HCC patients after radical resection. Therefore, using GLR index to evaluate HCC patients with TS ≤ 5 cm and taking individualized treatment is helpful to improve the long-term prognosis of HCC patients after hepatectomy.
This study was approved by the research ethics committee of the Affiliated Hospital of Guilin Medical University, and the informed consent were obtained from all patients.
ML and WQ performed the statistical analysis and wrote the first draft of the manuscript. YL and RY collected cases. JY and WL contributed to the conception and design of the study. All authors listed have made a substantial, direct and intellectual contribution to manuscript revision, read and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported in part by the National Natural Science Foundation of China (No. 81773148), the National Key Sci-Tech Special Project of China (No. 2018ZX10302207) and the Natural Science Foundation of Guangxi (No. 2018GXNSFAA139111).
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortettieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2011) 61:69–90. doi: 10.3322/caac.20107
2. Chen JG, Zhang SW. Liver cancer epidemic in China: past, present and future. Semin Cancer Biol. (2011) 21:59–69. doi: 10.1016/j.semcancer.2010.11.002
3. Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC, Ye SL, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. (2015) 91:1479–86. doi: 10.1002/1097-0142(20010415)91:8<1479::AID-CNCR1155>3.0.CO;2-0
4. Cong WM, Wu MC. Small hepatocellular carcinoma: current and future approaches. Hepatol Int. (2013) 7:805–12. doi: 10.1007/s12072-013-9454-z
5. Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. (2007) 141:330–9. doi: 10.1016/j.surg.2006.06.028
6. Danta M, Barnes E, Dusheiko G. The surveillance and diagnosis of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. (2005) 17:491. doi: 10.1097/00042737-200505000-00004
7. Giannini EG, Marenco S, Borgonovo G, Savarino V, Farinati F, Poggio PD, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. (2012) 56:1371–9. doi: 10.1002/hep.25814
8. Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, et al. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol. (2012) 40:1733. doi: 10.3892/ijo.2012.1408
9. Hofbauer SL, Stangl KI, De MM, Lucca I, Haitel A, Shariat SF, et al. Pretherapeutic gamma-glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma. Br J Cancer. (2014) 111:1526–31. doi: 10.1038/bjc.2014.450
10. Staudigl C, Concin N, Grimm C, Pfeiler G, Nehoda R, Singer CF, et al. Prognostic relevance of pretherapeutic gamma-glutamyltransferase in patients with primary metastatic breast cancer. PLoS ONE. (2015) 10:e0125317. doi: 10.1371/journal.pone.0125317
11. Xia J, Song P, Sun Z, Sawakami T, Jia M, Wang Z. Advances of diagnostic and mechanistic studies of γ-glutamyl transpeptidase in hepatocellular carcinoma. Drug Discov Ther. (2016) 10:181. doi: 10.5582/ddt.2016.01052
12. Fu S, Guo Z, Li S, Kuang M, Hu W, Hua Y, et al. Prognostic value of preoperative serum gamma-glutamyltranspeptidase in patients with hepatocellular carcinoma after hepatectomy. Tumor Biol. (2016) 37:3433–40. doi: 10.1007/s13277-015-4136-1
13. Fu SJ, Zhao Q, Ji F, Chen MG, Wu LW, Ren QQ, et al. Elevated preoperative serum gamma-glutamyltranspeptidase predicts poor prognosis for hepatocellular carcinoma after liver transplantation. Sci Rep. (2016) 6:28835. doi: 10.1038/srep28835
14. Park YE, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, et al. Gamma-glutamyl transpeptidase-to-platelet ratio is an independent predictor of hepatitis B virus-related liver cancer. J Gastroenterol Hepatol. (2016) 64:S343. doi: 10.1016/S0168-8278(16)00490-6
15. Wang WL, Zheng XL, Zhang ZY, Zhou Y, Hao J, Tang G, et al. Preoperative γ-glutamyl transpeptidase to platelet ratio (GPR) is an independent prognostic factor for HBV-related hepatocellular carcinoma after curative hepatic resection. Medicine. (2016) 95:e4087. doi: 10.1097/MD.0000000000004087
16. Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. (2007) 19:203–8. doi: 10.1016/j.coi.2007.02.001
17. Rezvani G, Davarmanesh M, Azar MR, Salehipour M, Sedaghat R, Karimi F, et al. Tumor lymphocyte infiltration and prognosis in patients with hepatocellular carcinoma treated by liver transplantation. Transplant Proc. (2013) 27:1333–4. doi: 10.1016/j.jhep.2005.12.027
18. Nakagawa S, Umezaki N, Yamao T, Kaida T, Okabe H, Mima K, et al. Survival impact of lymphocyte infiltration into the tumor of hepatocellular carcinoma in hepatitis B virus-positive or non-B non-C patients who underwent curative resection. Hepatol Res. (2017) 48:E126–32. doi: 10.1111/hepr.12936
19. Liao R, Tang ZW, Li DW, Luo SQ, Huang P, Du CY. Preoperative neutrophil-to-lymphocyte ratio predicts recurrence of patients with single-nodule small hepatocellular carcinoma following curative resection: a retrospective report. World J Surg Oncol. (2015) 13:265. doi: 10.1186/s12957-015-0670-y
20. Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. (2013) 58:58–64. doi: 10.1016/j.jhep.2012.08.017
21. Yu YQ, Li J, Liao Y, Chen Q, Liao WJ, Huang J. The preoperative alkaline phosphatase-to-platelet ratio index is an independent prognostic factor for hepatocellular carcinoma after hepatic resection. Medicine. (2016) 95:e5734. doi: 10.1097/MD.0000000000005734
22. Jin J, Zhu P, Yan L, Li J, Liao W, He S. Elevated preoperative aspartate aminotransferase to lymphocyte ratio index as an independent prognostic factor for patients with hepatocellular carcinoma after hepatic resection. Oncotarget. (2015) 6:19217–27. doi: 10.18632/oncotarget.4265
23. Furihata T, Sawada T, Kita J, Iso Y, Kato M, Rokkaku K, et al. Serum alpha-fetoprotein level per tumor volume reflects prognosis in patients with hepatocellular carcinoma after curative hepatectomy. Hepatogastroenterology. (2008) 55:1705–9. doi: 10.1136/gut.2007.129296corr1
24. Li J, Liao Y, Suo L, Zhu P, Chen X, Dang W, et al. A novel prognostic index-neutrophil times γ-glutamyl transpeptidase to lymphocyte ratio (NγLR) predicts outcome for patients with hepatocellular carcinoma. Sci Rep. (2017) 7:9229. doi: 10.1038/s41598-017-09696-y
25. Zhou B, Zhan C, Wu J, Liu J, Zhou J, Zheng S. Prognostic significance of preoperative gamma-glutamyltransferase to lymphocyte ratio index in nonfunctional pancreatic neuroendocrine tumors after curative resection. Sci Rep. (2017) 7:13372. doi: 10.1038/s41598-017-13847-6
26. Zhang W, Wang X, Jiang R, Hou J, Mu X, Li G, et al. Effect of tumor size on cancer-specific survival in small hepatocellular carcinoma. Mayo Clin Proc. (2015) 90:1187–95. doi: 10.1016/j.mayocp.2015.06.018
27. Chen YL, Ko CJ, Chien SY, Chen LS, Chen ML, Chi CW, et al. Tumor size as a prognostic factor in resected small hepatocellular carcinoma: a controversy revisited. J Gastroenterol Hepatol. (2011) 26:851–7. doi: 10.1111/j.1440-1746.2010.06595.x
28. Choi KK, Kim SH, Choi SB, Lim JH, Choi GH, Choi JS, et al. Portal venous invasion: the single most independent risk factor for immediate postoperative recurrence of hepatocellular carcinoma. J Gastroenterol Hepatol. (2011) 26:1646–51. doi: 10.1111/j.1440-1746.2011.06780.x
29. Rehermann B. Intrahepatic T cells in hepatitis b: viral control versus liver cell injury. J Exp Med. (2000) 191:1263. doi: 10.1084/jem.191.8.1263
30. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. (2006) 45:529–38. doi: 10.1016/j.jhep.2006.05.013
31. Aghoram R, Cai P, Dickinson JA. Alpha-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. Cochrane Database Syst Rev. (2012) 12:CD002799. doi: 10.1002/14651858.CD002799.pub2
32. Yao M, Zhao J, Lu F. Alpha-fetoprotein still is a valuable diagnostic and prognosis predicting biomarker in hepatitis B virus infection-related hepatocellular carcinoma. Oncotarget. (2016) 7:3702–8. doi: 10.18632/oncotarget.6913
33. Asrih M, Lenglet S, Mach FO, Montecucco F. Alpha-fetoprotein: a controversial prognostic biomarker for small hepatocellular carcinoma. World J Gastroenterol. (2013) 19:328–30. doi: 10.3748/wjg.v19.i3.328
34. Wang GY, Zhang J, Yang Y, Zhang Q, Chen GH. 186 pretransplant serum alpha-fetoprotein has no prognostic role in patients with HBV-associated hepatocellular carcinoma without vascular invasion. J Hepatol. (2013) 58:S82–S82. doi: 10.1016/S0168-8278(13)60188-9
35. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
36. van't Veer LJ, Bernards R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature. (2008) 452:564. doi: 10.1038/nature06915
37. Roessler S, Jia HL, Budhu A, Forgues M, Ye QH, Lee JS, et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. (2010) 70:10202–12. doi: 10.1158/0008-5472.CAN-10-2607
38. Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. (2014) 14:38. doi: 10.1186/1471-2407-14-38
Keywords: hepatocellular carcinoma, GLR, AFP, survival, prognosis
Citation: Liao M, Qin W, Liao Y, Yao R, Yu J and Liao W (2019) Prognostic Value of Gamma-Glutamyl Transpeptidase to Lymphocyte Count Ratio in Patients With Single Tumor Size ≤ 5 cm Hepatocellular Carcinoma After Radical Resection. Front. Oncol. 9:347. doi: 10.3389/fonc.2019.00347
Received: 27 August 2018; Accepted: 15 April 2019;
Published: 21 May 2019.
Edited by:
Cornelis F. M. Sier, Leiden University, NetherlandsReviewed by:
Engin Altintas, Mersin University, TurkeyCopyright © 2019 Liao, Qin, Liao, Yao, Yu and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junxiong Yu, eXVqdW54aW9uZzI4OTczMjBAMTYzLmNvbQ==
Weijia Liao, bGlhb3dlaWppYTI4OEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.