- 1Department of Radiation Oncology, Shandong University Affiliated Shandong Cancer Hospital and Institute, Jinan, China
- 2Key Laboratory of Radiation Oncology of Shandong Province, Jinan, China
- 3Department of Pathology, Shandong University Affiliated Shandong Cancer Hospital and Institute, Jinan, China
- 4Department of General Surgery, Shandong University Affiliated Shandong Cancer Hospital and Institute, Jinan, China
- 5Department of Oncology, Shandong University Affiliated Shandong Cancer Hospital and Institute, Jinan, China
- 6Department of Oncology, Yucheng City People's Hospital, Dezhou, China
- 7Department of Thorax Surgery, Shandong University Affiliated Shandong Cancer Hospital and Institute, Jinan, China
- 8Department of Nuclear Medicine, Shandong University Affiliated Shandong Cancer Hospital and Institute, Jinan, China
Background and Objectives: An accurate delineation of the primary clinical target volume (CTVp) in esophageal squamous cell carcinoma (ESCC) significantly affects the outcomes of radiotherapy. However, when basing the CTVp on the primary gross tumor volume, there are no consistent guidelines for the size of the margin. We compared preoperative 18F-fluorodeoxyglucose (FDG) PET/CT images and large slices of resected pathological ESCC specimens for evidence and prediction of subclinical lesions. We also investigated associations between the maximum standardized uptake value (SUVmax), metabolic tumor volumes (MTVs), and lesions to improve estimates of the CTVp.
Methods: 55 patients underwent FDG PET/CT before surgery, and the SUVmax and MTVs were determined. To ensure that the in situ distances between the primary and secondary tumors were preserved, the esophageal specimens collected during radical surgery were processed to minimize shrinkage, and subclinical lesions were characterized by pathological examination. A 2-dimensional logistic regression model was used to assess the associations between clinicopathological features and microscopic spread of the lesions.
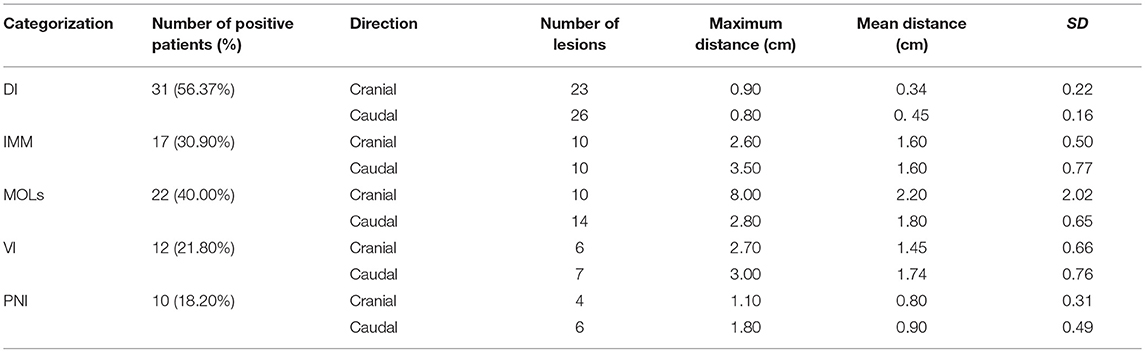
Results: Subclinical lesions in pathological specimens were characterized as direct invasion, multicentric occurrence lesions, intra-mural metastasis, vascular invasion, and perineural invasion in 56.4, 40.0, 30.9, 21.8, and 18.2% of patients, respectively. The mean distances of the subclinical lesions from the primary tumor were 0.79 ± 1.28 cm and 0.87 ± 1.00 cm in the cranial and caudal directions, respectively. Together the SUVmax and MTV values could predict the presence of subclinical lesions that were not detectable in PET/CT images.
Conclusions: To cover 94.5% of ESCC subclinical lesions in the CTVp, a 3-cm margin along the cranial-caudal axis should be added to the primary gross tumor volume as defined by FDG-PET/CT, as well as a cutoff SUVmax value of 2.5. Although preoperative FDG PET/CT images may not reveal lesions directly, the SUVmax and MTV measurements together could predict their presence.
Background
Esophageal carcinoma often occurs as squamous cell carcinoma (ESCC), a highly aggressive malignancy with a poor prognosis worldwide. The incidence rates of ESCC are particularly high in China (1). Most patients with ESCC have locally advanced disease (2, 3). Important treatment strategies for locally advanced ESCC include neoadjuvant chemoradiation and definitive chemoradiation or radiation therapy. However, the overall survival and local control rates remain unsatisfactory—the 2-year survival rate is merely 30–40%, and the local relapse rate may reach up to 50% (4–6).
In ESCC radiotherapy, an accurate delineation of the primary clinical target volume (CTVp) importantly influences the success of the outcome, with higher local regional control, and less toxicity. Wu et al. suggested that CTVp is generally a 3–4 cm superior and inferior expansion of the gross tumor volume (GTV) and a 1 cm radial expansion, according to an expert consensus (7). However, when basing the CTVp on the size of the primary gross tumor, there are no established or standard guidelines for how large the margin should be based on precision data. Countries, institutions and even physicians have different viewpoints on this issue. We reviewed the original literature in ESCC research pathology (8) and found that the CTVp commonly consists of the primary tumor and surrounding secondary lesions, which frequently include direct, vascular and perineural invasion, intra-mural metastasis, and multicentric occurrence lesions.
Currently, there is no technology capable of accurately detecting subclinical tumor lesions, and the optimal CTVp in esophageal cancer remains controversial. To provide a theoretical basis for the clinical determination of the CTVp in esophageal cancer, this study examined the pathology of the longitudinal distribution of subclinical lesions in long resected specimens. For the closest estimate of the length of the primary gross tumor volume, we previously reported a standardized 18F-fluorodeoxyglucose (FDG) uptake cutoff value of 2.5 in positron emission tomography (PET)/computed tomography (CT) (9, 10). In this study, we investigated the correspondence between the results of ESCC pathology and FDG PET/CT, as well as pathological results that help determine the most appropriate CTVp in these patients.
Methods
The study was designed and conducted at Shandong University Affiliated Shandong Cancer Hospital as a prospective study with clinical samples handled in vitro (without registration). The Ethics Committee of Shandong University Affiliated Shandong Cancer Hospital and Institute approved the study protocol on 5 March 2011 (Ethics Approval No. SDTHEC201103009). All patients gave their written informed consent for experiments, in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Patients
In general, to meet the basic requirements of model estimation, the sample size n should be >30, or n ≥ 3(k + 1), where k is the number of independent variables. However, while a sample size of 30 is considered the minimum for a quantitative study, subclinical lesions are usually found in <10% of ESCC patients (7). Thus, we sought an enrolled sample size of 55, and the final study population was comprised of 55 patients treated between January 2012 and September 2015 at Shandong University Affiliated Shandong Cancer Hospital.
All of the analyzed patients conformed to the following inclusion criteria: histologically proven ESCC, Karnofsky performance status (KPS) score >70, routine pretreatment evaluation, including barium esophagography, gastroscopy, ultrasound evaluation of the neck and abdomen, and pulmonary function testing; radical surgery 3–5 d after a FDG PET/CT scan, no previous chemotherapy or radiotherapy, and no history of a malignant tumor. Patients were excluded from this study if any of the above inclusion criteria were not met.
All eligible patients without distant metastasis, or definite direct invasion of adjacent organs detected on imaging, were scheduled as routine for esophageal resection and extensive regional lymph node dissection. They underwent a standard esophagectomy based on the McKeown method. A 3-field lymph node dissection was also performed, if necessary.
Imaging Protocols and the Parameters of FDG PET/CT in Patients
The patients were required to fast for 6 h and to rest for 15 min before i.v. injection of 370 MBq (10 mCi) FDG. Patients were also asked to drink 500 mL of water before imaging to stimulate FDG excretion from the renal calyces and subsequent voiding.
The images were acquired 60 min p.i. using a standard clinical PET/CT scanner (Discovery LS; GE Healthcare) on the basis of the clinical situation and with the patient's consent. Emission scans were performed in whole-body mode for 5 min per field of view from head to thigh. Each field covered 14.5 cm at an axial sampling thickness of 4.25 mm/slice. The PET/CT system was used for 4-slice helical CT acquisition, followed by a full-ring dedicated PET scan of the same axial range. The CT component was operated with an X-ray tube peak voltage of 120 keV at 90 mA, 6:1 pitch, 4.25 mm slice thickness and a rotational speed of 0.8 s/ rotation. Both the PET and the CT scans were acquired during normal tidal breathing.
PET images were reconstructed with CT-derived attenuation correction by iterative reconstruction using ordered-subset expectation maximization (OSEM). The images (attenuation-corrected PET, CT, and fused PET/CT) were reviewed in the axial, coronal and sagittal planes on a dedicated workstation (Xeleris; GE Healthcare) computer monitors, as was a cine display of the maximum intensity projections of the PET data.
The PET images were visually inspected, and the maximum standardized uptake value (SUVmax) was determined from circular regions of interest drawn around the lesion in all consecutive image slices covering the entire lesion. The metabolic tumor volume (MTV) was delineated in FDG PET/CT images using a fixed SUV threshold value of 2.5 (8, 9). The imaging area covered the metabolically active area. The boundary was outlined layer by layer, and to calculate the volume the area of each layer was multiplied by the layer thickness. Then, the volumes of each layer were combined to calculate the MTV. If the SUVmax was less than the standard value, the MTV was calculated as a single voxel with volume of 0.1 mL.
Due to PET partial volume effects, the determination of the MTV were excluded in the cavity regions. All PET images were further reviewed by two physicians who were experienced in nuclear medicine.
Preparation of Large Pathological Slices
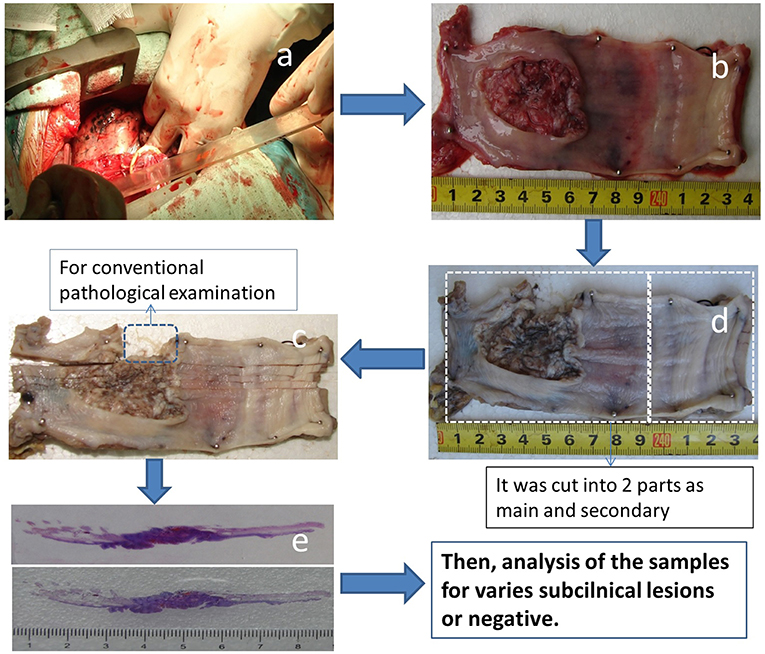
The process for large slice preparation is shown in Figure 1. The longitudinal length of the esophagus to be resected was measured in situ during surgery prior to resection (Figure 1a). To correct for shrinkage of the surgical specimen, each fresh esophageal specimen was opened along the longitudinal axis after resection (with care taken not to cut through the tumor). The specimen was then carefully stretched to the same length as in vivo, pinned to a flat expanded polystyrene board (Figure 1b) and photographed for record keeping.
The specimen, along with the flat board, was fixed in 10% formaldehyde (Figure 1c), cut vertically into 0.5-cm-wide tissue strips that included the upper and lower cut edges (Figure 1d) and used as a large pathologic slice for examination (Figure 1e). During this step, shrinkage of the specimen was recorded after obtaining a large pathologic slice and the specimen was re-formed to its in vivo length.
If the length of the surgical specimen was >9 cm and ≤ 18 cm, it was cut into two parts (the main part and the secondary part), which were perpendicular to the longitudinal axis. If the specimen was >18 cm, it was cut into three parts (one main part and two secondary parts), and each part was labeled in sequence from the most cranial to the most caudal. The percent shrinkage was also calculated from the time the specimen was cut into 0.5-cm strips (Figure 1d) until the pathological examination (Figure 1e). The samples were then analyzed.
The resected lymph nodes were separately dissected and the sites were described using the nomenclature of the Japanese Society for Esophageal Diseases (11).
Diagnostic Criteria of the Subclinical Lesions
The subclinical ESCC lesions were classified as: direct invasion (involving the intramucosal, submucosal, and muscular layers), intra-mural metastasis, multicentric occurrent lesions (differentiated from a second carcinoma of non-esophageal origin and other lesions, e.g., intra-mural metastasis), vascular invasion, or perineural invasion (Figure 2) (12–14).
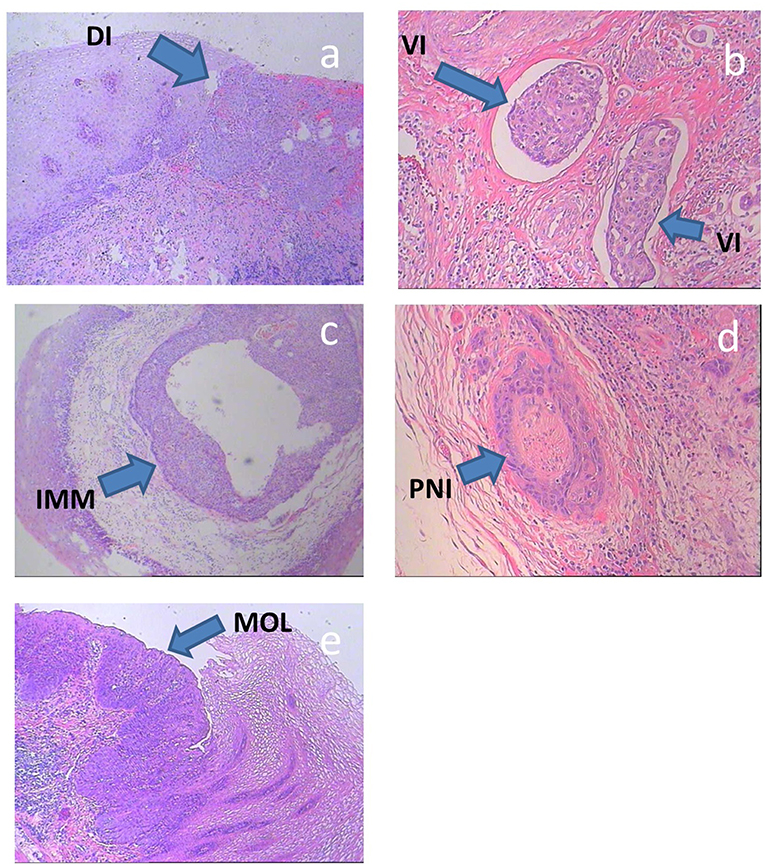
Figure 2. (a–e) Histologic specimens of typical subclinical lesions of ESCC. DI, direct invasion; IMM, intra-mural metastasis; MOL, multicentric occurrent lesions; VI, vascular invasion; PNI, perineural invasion.
Direct invasion involving the intramucosal, submucosal and muscular layers includes secondary lesions that infiltrate in all directions from the main tumor. In this study, direct invasion was only measured in the cranial-caudal direction, because only subclinical lesions in this direction affect the length of the CTVp.
Intra-mural metastasis, also known as salutatory metastasis, was defined in accordance with the following standard macroscopic and histologic criteria: clearly separated from the primary tumor, located in the esophageal wall, the gross appearance of a submucosal tumor without intra-epithelial extension of the tumor, of the same histological type as the primary tumor, and without evidence of intravascular growth.
Multicentric occurrent lesions must be differentiated from a second carcinoma of non-esophageal origin and other lesions. The main tumor is located in the esophagus or the gastroesophageal junction. Multicentric occurrent lesions have definite malignant features, as determined by pathological analysis, and individual lesions are discontinuous. The main tumor and secondary tumor coexist, and the main tumor is larger and more deeply invasive than the secondary tumor. The secondary tumor lesions include intraepithelial carcinomas, such as atypical hyperplasia and carcinoma in situ, metastatic lesions were excluded. The secondary tumors that occur after the primary esophageal carcinoma (~1 year later) are usually known as heterochronic multi-esophageal carcinoma and should not be included in the multicentric lesion category.
Vascular invasion is defined as the infiltration of tumor cells into lymph and blood vessels, including tumor embolus formation.
Perineural invasion involves cancer cell infiltration into the perineurium or fasciculus and can be detected at the boundary of the deepest tumor invasion, as well as by metastasis outside of the primary tumor site (15).
Statistical Analysis
SPSS software (version 18.0; SPSS, Chicago, IL, USA) was used for statistical analyses. Sensitivities were compared using the chi-squared or Fisher's exact tests. A 2-dimensional logistic regression model was used to estimate the associations between the clinicopathological features and the microscopic spread of lesions.
Results
Subclinical Lesions and Clinicopathological Parameters
There were 55 patients with ESCC enrolled in this study. The following clinicopathological parameters were associated with the presence of subclinical lesions (according to the chi-squared and Fisher's exact tests): tumor length, differentiation, pathologic tumor status, pathologic lymph node status and stage (Table 1). The age and gender of the patient and the tumor location were not statistically related to subclinical lesions.
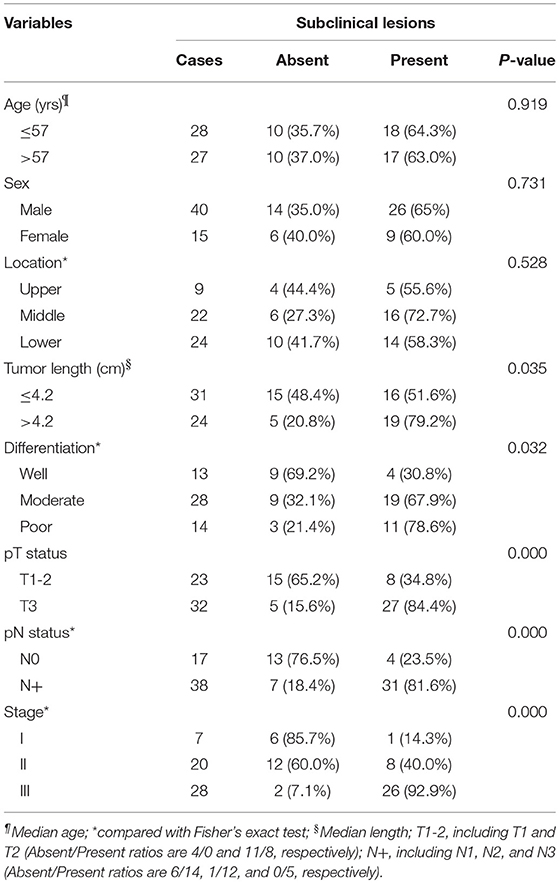
Table 1. Correlation of subclinical lesions with patients' clinicopathological parameters in esophageal SCC.
Shrinkage of the Large Pathological Slice Specimens
The length of the esophagus to be resected was measured in situ, and again after fixation in 10% formaldehyde (Figure 1c). The mean percent shrinkage was 0.081 ± 0.041% for the 113 specimens. Fifty-two of the specimens were separated into two parts (51 specimens between 9 and 18 cm, one equal to 18 cm). Three specimens were separated into three parts (those >18 cm).
Presence and Distance of Subclinical Lesions Beyond the Gross Tumor
Subclinical lesions were found in 63.64% (35/55) of patients (including direct invasion, intra-mural metastasis, multicentric occurrent lesions, vascular invasion, and perineural invasion; Table 2). Of the 35 ESCC patients with subclinical lesions, 3.29 ± 1.25 lesions were observed.
Considering all the histological specimens of all patients, the greatest distances of the subclinical lesions beyond the gross tumor were 0.79 ± 1.28 cm (cranial) and 0.87 ± 1.00 cm (caudal; Figure 3).
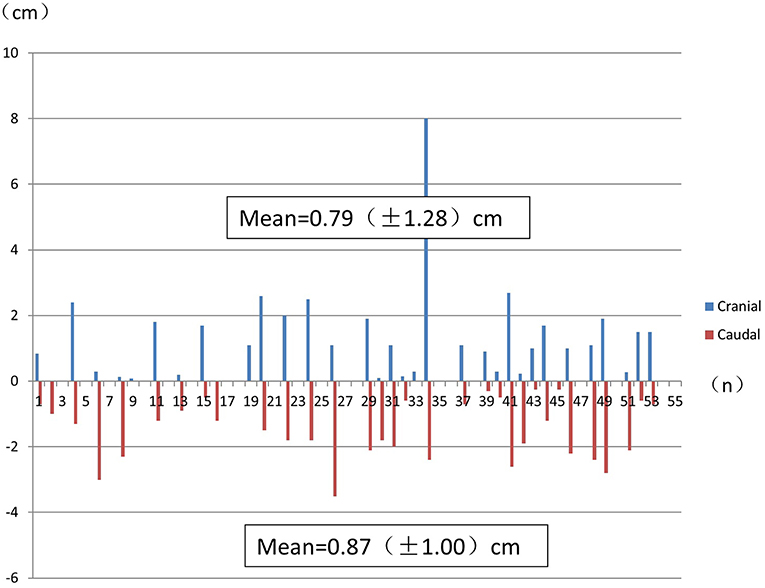
Figure 3. The greatest distance of subclinical lesions beyond the gross tumor for each histological specimen from each patient. Numbers indicate the mean ± SD.
The furthest distance that a subclinical lesion was observed from the main tumor was 8.0 cm, which involved a multicentric lesion located cranial to the main tumor site in a 53-year-old male patient with middle thoracic ESCC. This lesion was not detected in situ by FDG PET/CT (Figure 4).
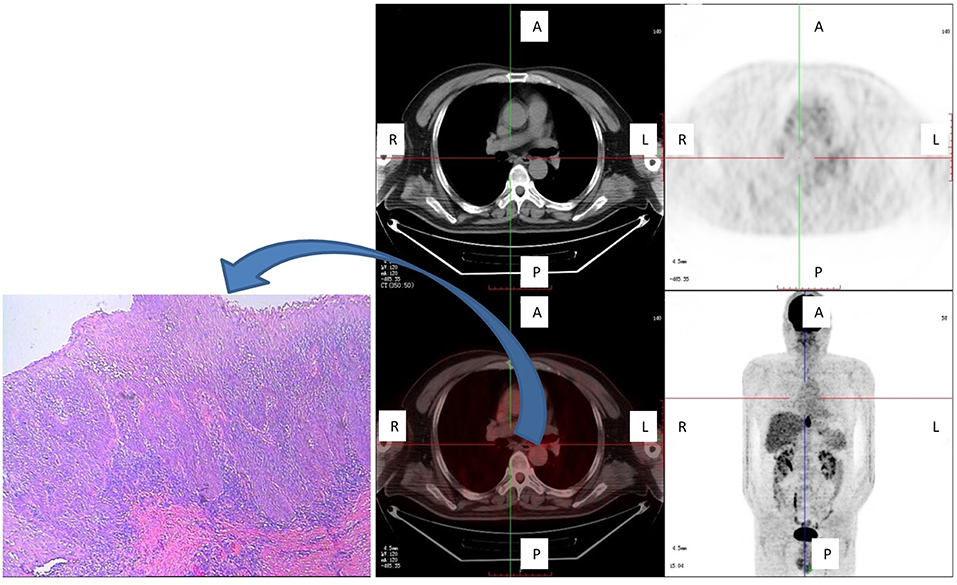
Figure 4. One patient with a subclinical (multicentric) lesion, which was the greatest cranial distance from the main tumor. This lesion was not detected in situ by FDG PET/CT, but was detected by pathological examination (carcinoma in situ) and was located cranial to the main tumor. Shown are axial PET, axial fused PET/CT, wholebody maximum intensity projections, and pathological specimen of secondary tumor manifestation at the plane marked by the cross-sign.
Association Between Subclinical Lesions and Clinical Pathological Parameters
The number of subclinical lesions correlated with the gross length of the tumor (R = 0.356, 95% CI = 0.028–0.667, P = 0.035) and with the FDG PET/CT SUVmax (R = 0.487, 95% CI = 0.119–0.689, P = 0.003; Table 3). Additionally, there was a trend in association between the SUVmax values of the primary tumor and the number of subclinical lesions. In addition, the number of subclinical lesions correlated with the MTV (R = 0.342, 95% CI = −0.099–0.661, P = 0.044).
Discussion
An objective of this study was to clarify the most appropriate CTVp margins in patients with ESCC. To determine this distance and its correspondence with FDG PET/CT results, we employed large pathological slices that included the entire tumor and the resected proximal tissue. This long and complex process provided an intact specimen in which the anatomical relationship between the tumor and the surrounding tissue can be observed, which was essential for assessing the presence of lesions.
Using various pathological techniques, previous studies have reported different rates of subclinical lesion occurrence. However, there has not been a comprehensive report that evaluated all types of subclinical lesions that make up the CTV in esophageal cancer, and the CTVp range is still controversial. Although there have been several studies addressing this, they seldom used large pathological slices (12, 16–18).
Kuwano et al. (19) reported that the mean distance of direct invasion from the main tumor was 4.11 mm (range: 1.2–9.5 mm). However, according to Tsutsui et al. (20), direct invasion was usually <30 mm. In our study, direct invasion was observed in 56.37% of the patients (cranial and caudal) and the greatest distance was 9.5 mm. Other studies have reported finding intra-mural metastasis in 4.19–26.0% of patients, and the distance of intra-mural metastasis from the primary tumor ranged from 1–130 mm, with maximum cranial and caudal distances of 130 mm and 95 mm, respectively (21–24). Our research results indicated that 30.90% of patients had intra-mural metastasis, with a maximum distance of 35.0 mm.
Several studies have reported that the incidence of multicentric occurrent lesions ranges from 20.20 to 31.00% in patients who did not receive preoperative irradiation, with a cranial distance from the primary tumor of 0.88–7.14 cm and a caudal distance of 0.57–6.26 cm (10, 12). In our study, the incidence of multicentric occurrent lesions was 40.00%, and the greatest distance that a lesion was observed was 8.0 cm in the cranial direction.
Several clinical trials have shown that preoperative radiation can reduce the incidence of multicentric occurrent lesions. For example, Kuwano et al. (12) reported that multicentric occurrent lesions were found in 11.70% (19/162) of patients who received preoperative irradiation and in 25.60% (11/143) of those who did not. In addition, Tsutsui et al. (20) reported that only 5.61% (17/303) of patients had multicentric occurrent lesions, most of whom were administered preoperative irradiation.
The Japanese researchers Lam et al. (17) observed vascular invasion in 16.67% (16/96) of ESCC patients, mostly at the base of the tumor, and occasionally distal to the primary tumor, at a maximum distance of 5 cm. Vascular invasion has been putatively associated with advanced tumor stages, with rates of 13.89% (15/108) in the early stage (25) to 39.10% (143/366) in the advanced stage (26). In our study, vascular invasion was found in 21.80% of the study population, and the greatest distance was 3.00 cm in the caudal direction.
Sarbia et al. (27) first reported perineural invasion in 26.10% (42/161) of patients with ESCC in 1995. Through univariate and multivariate survival analyses, the authors concluded that perineural invasion was not a prognostic factor. In our study, perineural invasion was present in 18.20% of patients, and the greatest distance was 1.80 cm in the caudal direction. Conversely, Tanaka et al. (28) reported perineural invasion in 46.20% (48/104) of resected ESCCs and concluded that perineural invasion is an important prognostic factor for local relapse. Recently, Chen et al. (29) also reported that perineural invasion is a prognostic factor for ESCC and suggested that perineural invasion status should be considered when planning therapy strategies.
Kato et al. (30) reported that it is difficult to detect lymph node metastases of 0.6 to 0.8-cm size with FDG PET/CT. In our study, lesions with a diameter < 0.5 cm could not be detected using FDG PET/CT. Furthermore, subclinical lesions of several microns could not be detected using FDG PET/CT (Figure 3).
Even with today's advanced imaging technology, some subclinical lesions cannot be detected directly. In this study, there was a trend toward higher FDG PET/CT SUVmax and MTV values associating with the presence of subclinical lesions. SUVmax correlated with the number of lesions (R = 0.487, 95% CI = 0.119–0.689, P = 0.003), as did the MTV (R = 0.342, 95% CI = −0.099–0.661, P = 0.044). As imaging technology advances, the ability to detect smaller cancer foci may improve.
Regardless of the controversy concerning CTVp data, clinical experience has shown that enlarged radiation fields do not improve local control rates or overall survival, despite the extremely toxic nature of radiation (31). The extent of subclinical lesions exceeds the conventional extent of the margins, as the results of the current research indicate. In many authors' views, it is also a good choice to employ involved-field irradiation; therefore, radiation-related toxicity would decline in cases where the target volume was diminished.
In theory, the treatment of subclinical lesions may benefit from lower doses of radiation. A total dosage of 50 Gy administered in 2-Gy fractions is effective for achieving an overall 90% reduction in subclinical metastases, and the cancer cell burden in some patients can be wiped out by these low doses. Thus, significant rates of disease control can still be achieved when patient tolerance suggests lower-than-optimal doses, as shown by the linear association and absence of a significant threshold in the dose-response curve. Small lesions can be treated by low doses of radiation (autoimmune extermination), but the optimal dose for this treatment still needs further study.
In our study, we concluded that the SUVmax and MTV obtained via FDG PET/CT may be able to indicate the existence of subclinical lesions. Imaging guided by field involvement will benefit more patients with ESCC in terms of dose-limitation for the organs at risk, such as the lungs, heart and spinal cord (32).
Because our sample size was limited, our analysis did not include all variables associated with subclinical lesions. The safe surgical margin for primary ESCC tumors classified as Tis (high-grade dysplasia) or T1 (invasion of lamina propria, muscularis mucosae, or submucosa) is usually 1 cm in most endoscopic mucosal resections and dissections (33). However, for T2 (invasion of the muscularis propria) and more aggressive diseases, the safe margin is uncertain, and studies with a larger sample size are needed.
Secondary lesions with diameters from several microns to 2 mm, as well as multicentric occurrent lesions, are more likely to occur in patients with certain risk factors. These include male gender, heavy drinking or smoking, and a family history of carcinoma of the upper digestive tract (11). The CTVp margin should be enlarged appropriately if these risk factors are present.
To correct for the shrinkage of the resected esophagus, we stretched the surgical specimen to the same length as in situ by pinning it to a board prior to fixing it with formaldehyde. The amount of shrinkage was low; however, other authors have reported greater degrees of shrinkage. For example, Siu et al. (34) reported that shrinkage was usually 50% of the in situ length.
Conclusions
Our results suggest that to cover 94.5% of subclinical lesions in the CTVp of ESCC, a 3-cm margin in the cranial-caudal direction should be added to the primary gross tumor volume. Both the SUVmax and MTV obtained through FDG PET/CT may predict subclinical lesions, although the imaging did not detect subclinical lesions directly. By our findings, it may be safe to use involved-field radiation for ESCC, although validation of this should be sought through future prospective and randomized studies.
Ethics Statement
All patients gave their written informed consent in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments.
Shandong University Affiliated Shandong Cancer Hospital Ethics Committee allows the use of patient data for research, provided that any person's related data are kept anonymous. The Ethics Approval authorization is SDTHEC201103009, approved on 5 March 2011.
Author Contributions
JY and DH designed the experiments. YY, JC, AR, PS, and ZF carried out experiments and data collection. GZ, LW, and DH analyzed experimental results and developed analysis tools. DH wrote and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported partly by grants from Natural Science Foundation of China, No. 81101699, National Key R&D Program 2018YFC1313200, Shandong Province Natural Science Foundation, ZR2011HL028, ZR2017MH129, and ZR2017MH115, Shandong Province Key R&D Program, No. 2015GSF118017 and 2018GSF118031, and the Shandong Chinese Medicine Science and Technology Development Project, No. 2013-212. These foundations had no involvement in study design, data collection, or analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CT, computed tomography; CTVp, primary clinical target volume; ESCC, esophageal squamous cell carcinoma; FDG, 18F-fluorodeoxyglucose; MTV, metabolic tumor volume; PET, positron emission tomography; SUV, standardized uptake value.
References
1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. (2013) 381:400–12. doi: 10.1016/S0140-6736(12)60643-6
2. Zanoni A, Verlato G, Giacopuzzi S, Weindelmayer J, Casella F, Pasini F, et al. Neoadjuvant concurrent chemoradiotherapy for locally advanced esophageal cancer in a single high-volume center. Ann Surg Oncol. (2013) 20:1993–9. doi: 10.1245/s10434-012-2822-4
3. Roeder F, Nicolay NH, Nguyen T, Saleh-Ebrahimi L, Askoxylakis V, Bostel T, et al. Intensity modulated radiotherapy (IMRT) with concurrent chemotherapy as definitive treatment of locally advanced esophageal cancer. Radiat Oncol. (2014) 9:191. doi: 10.1186/1748-717X-9-191
4. Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Rad Therapy Oncol Group JAMA. (1999) 281:1623–7. doi: 10.1001/jama.281.17.1623
5. Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. (2002)20:1167–74. doi: 10.1200/JCO.2002.20.5.1167
6. Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6
7. Wu AJ, Bosch WR, Chang DT, Hong TS, Jabbour SK, Kleinberg LR, et al. Expert consensus contouring guidelines for intensity modulated radiation therapy in esophageal and gastroesophageal junction cancer. Int J Radiat Oncol Biol Phys. (2015) 92:911–20. doi: 10.1016/j.ijrobp.2015.03.030
8. Han D, Yuan Y, Song X, Yu Y, Yu J. What is the appropriate clinical target volume for esophageal squamous cell carcinoma? Debate and consensus based on pathological and clinical outcomes. J Cancer. (2016) 7:200–6. doi: 10.7150/jca.13873
9. Zhong X, Yu J, Zhang B, Mu D, Zhang W, Li D, et al. Using 18F- fluorodeoxyglucose positron emission tomography to estimate the length of gross tumor in patients with squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. (2009) 73:136–41. doi: 10.1016/j.ijrobp.2008.04.015
10. Han D, Yu J, Yu Y, Zhang G, Zhong X, Lu J, et al. Comparison of (18)F-fluorothymidine and (18)F-fluorodeoxyglucose PET/CT in delineating gross tumor volume by optimal threshold in patients with squamous cell carcinoma of thoracic esophagus. Int J Radiat Oncol Biol Phys. (2010) 76:1235–41. doi: 10.1016/j.ijrobp.2009.07.1681
11. Japanese Society for Esophageal Diseases. Guidelines for clinical and pathologic studies on carcinoma of the esophagus, ninth edition: preface, general principles, part I. Esophagus. (2004) 161–88.
12. Kuwano H, Ohno S, Matsuda H, Mori M, Sugimachi K. Serial histologic evaluation of multiple primary squamous cell carcinomas of the esophagus. Cancer. (1988) 61:1635–8.
13. Morita M, Kuwano H, Yasuda M, Watanabe M, Ohno S, Saito T, et al. The multicentric occurrence of squamous epithelial dysplasia and squamous cell carcinoma in the esophagus. Cancer. (1994) 74:2889–95. doi: 10.1002/1097-0142(19941201)74:11<2889::AID-CNCR2820741102>3.0.CO;2-K
14. Morita M, Araki K, Saeki H, Sakaguchi Y, Baba H, Sugimachi K, et al. Risk factors for multicentric occurrence of carcinoma in the upper aerodigestive tract-analysis with a serial histologic evaluation of the whole resected-esophagus including carcinoma. J Surg Oncol. (2003) 83:216–21. doi: 10.1002/jso.10276
15. Liebl F, Demir IE, Mayer K, Schuster T, D'Haese JG, Becker K, et al. The impact of neural invasion severity in gastrointestinal malignancies: a clinicopathological study. Ann Surg. (2014) 260:900–7; discussion 907–8. doi: 10.1097/SLA.0000000000000968
16. Gao XS, Qiao X, Wu F, Cao L, Meng X, Dong Z, et al. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol Biol Phys. (2007)67:389–96. doi: 10.1016/j.ijrobp.2006.09.015
17. Lam KY, Ma LT, Wong J. Measurement of extent of spread of oesophageal squamous carcinoma by serial sectioning. J Clin Pathol. (1996) 49:124–9. doi: 10.1136/jcp.49.2.124
18. Koenig AM, Prenzel KL, Bogoevski D, Yekebas EF, Bubenheim M, Faithova L, et al. Strong impact of micrometastatic tumor cell load in patients with esophageal carcinoma. Ann Surg Oncol. (2009) 16:454–62. doi: 10.1245/s10434-008-0169-7
19. Kuwano H, Masuda N, Kato H, Sugimachi K. The subepithelial extension of esophageal carcinoma for determining the resection margin during esophagectomy: a serial histopathologic investigation. Surgery. (2002) 131:S14–21. doi: 10.1067/msy.2002.119289
20. Tsutsui S, Kuwano H, Watanabe M, Kitamura M, Sugimachi K. Resection margin for squamous cell carcinoma of the esophagus. Ann Surg. (1995) 222:193–202. doi: 10.1097/00000658-199508000-00012
21. Nishimaki T, Suzuki T, Tanaka Y, Aizawa K, Hatakeyama K, Muto T. Intramural metastases from thoracic esophageal cancer: local indicators of advanced disease. World J Surg. (1996) 20:32–7. doi: 10.1007/s002689900006
22. Takubo K, Sasajima K, Yamashita K, Tanaka Y, Fujita K. Prognostic significance of intramural metastasis in patients with esophageal carcinoma. Cancer. (1990) 65:1816–9. doi: 10.1002/1097-0142(19900415)65:8<1816::AID-CNCR2820650825>3.0.CO;2-L
23. Kuwano H, Watanabe M, Sadanaga N, Kamakura T, Nozoe T, Yasuda M, et al. Univariate and multivariate analyses of the prognostic significance of discontinuous intramural metastasis in patients with esophageal cancer. J Surg Oncol. (1994) 57:17–21. doi: 10.1002/jso.2930570106
24. Yuasa N, Miyake H, Yamada T, Oda K, Nimura Y, Nagasaka T, et al. Prognostic significance of the location of intramural metastasis in patients with esophageal squamous cell carcinoma. Langenbecks Arch Surg. (2004) 389:122–7. doi: 10.1007/s00423-003-0453-8
25. Amano T, Matsumoto T, Hayashi T, Arakawa A, Sonoue H, Kajiyama Y, et al. Subepithelial extension of squamous cell carcinoma in the esophagus: histopathological study using D2-40 immunostaining for 108 superficial carcinomas. Pathol Int. (2007) 57:759–64. doi: 10.1111/j.1440-1827.2007.02171.x
26. Brücher BL, Stein HJ, Werner M, Siewert JR. Lymphatic vessel invasion is an independent prognostic factor in patients with a primary resected tumor with esophageal squamous cell carcinoma. Cancer. (2001) 92:2228–33. doi: 10.1002/1097-0142(20011015)92:8<2228::AID-CNCR1567>3.0.CO;2-4
27. Sarbia M, Porschen R, Borchard F, Horstmann O, Willers R, Gabbert HE. Incidence and prognostic significance of vascular and neural invasion in squamous cell carcinomas of the esophagus. Int J Cancer. (1995) 61:333–6. doi: 10.1002/ijc.2910610310
28. Tanaka A, Matsumura E, Yosikawa H, Uchida T, Machidera N, Kubo R, et al. An evaluation of neural invasion in esophageal cancer. Surg Today. (1998) 28:873–8. doi: 10.1007/s005950050245
29. Chen JW, Xie JD, Ling YH, Li P, Yan SM, Xi SY, et al. The prognostic effect of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. (2014) 14:313. doi: 10.1186/1471-2407-14-313
30. Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Ojima H, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer. (2002) 94:921–8. doi: 10.1002/cncr.10330
31. Minsky BD. Primary combined-modality therapy for esophageal cancer. Oncology. (2006) 20:497–505; discussion 505–6, 511–3.
32. Nkhali L, Thureau S, Edet-Sanson A, Doyeux K, Benyoucef A, Gardin I, et al. FDG-PET/CT during concomitant chemo radiotherapy for esophageal cancer: reducing target volumes to deliver higher radiotherapy doses. Acta Oncol. (2015) 54:909–15. doi: 10.3109/0284186X.2014.973062
33. Kurokawa Y, Muto M, Minashi K, Boku N, Fukuda H. A phase II trial of combined treatment of endoscopic mucosal resection and chemoradiotherapy for clinical stage I esophageal carcinoma: Japan Clinical Oncology Group Study JCOG0508. Jpn J Clin Oncol. (2009) 39:686–9. doi: 10.1093/jjco/hyp078
Keywords: pathology, subclinical lesion, clinical target volume, esophageal squamous cell carcinoma, 18F-fluorodeoxyglucose
Citation: Han D, Yuan Y, Chai J, Zhang G, Wang L, Ren A, Song P, Fu Z and Yu J (2019) Subclinical Lesions of the Primary Clinical Target Volume Margin in Esophageal Squamous Cell Carcinoma and Association With FDG PET/CT. Front. Oncol. 9:336. doi: 10.3389/fonc.2019.00336
Received: 25 November 2018; Accepted: 11 April 2019;
Published: 30 April 2019.
Edited by:
Freimut Dankwart Juengling, Universität Bern, SwitzerlandReviewed by:
Orazio Schillaci, University of Rome Tor Vergata, ItalyWilliam Ian Duncombe Rae, University of Sydney, Australia
Paul Romesser, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2019 Han, Yuan, Chai, Zhang, Wang, Ren, Song, Fu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Fu, YWJiYWFiNzdAYWxpeXVuLmNvbQ==
Jinming Yu, eXVqaW5taW5nc2RAMTYzLmNvbQ==
 Dali Han
Dali Han Yinping Yuan3
Yinping Yuan3 Jinming Yu
Jinming Yu