- 1Orthopaedic Pathophysiology and Regenerative Medicine Unit, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy
- 2Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
Mesenchymal stromal cells (MSC) have essential functions in building and supporting the tumour microenvironment, providing metastatic niches, and maintaining cancer hallmarks, and it is increasingly evident that the study of the role of MSC in cancer is crucial for paving the way to clinical opportunities for novel anti-cancer therapies. To date, the vast majority of preclinical models that have been used for studying the effect of reactive MSC on cancer growth, metastasis, and response to therapy has been mainly based on in vitro flat biology, including the co-culturing with cell compartmentalization or with cell-to-cell contact, and on in vivo cancer models with different routes of MSC inoculation. More complex in vitro 3D models based on spheroid structures that are formed by intermingled MSC and tumour cells are also capturing the interest in cancer research. These are innovative culture systems tailored on the specific tumour type and that can be combined with a synthetic extracellular matrix, or included in in silico technologies, to more properly mimic the in vivo biological, spatial, biochemical, and biophysical features of tumour tissues. In this review, we summarized the most popular and currently available preclinical models for evaluating the role of MSC in cancer and their specific suitability, for example, in assaying the MSC-driven induction of epithelial-to-mesenchymal transition or of stem-like traits in cancer cells. Finally, we enlightened the need to carefully consider those parameters that might unintentionally strongly affect the secretome in MSC-cancer interplay and introduce confounding variables for the interpretation of results.
Introduction
Cancer is a complex disease that thrives in a heterogeneous and adaptive tumour microenvironment (TME) admixed with reactive elements surrounding or infiltrating the tumour cells. Among these, endothelial, immune, and mesenchymal stromal cells (MSC) or cancer-associated fibroblasts (CAF) are frequently observed, playing an important role during carcinogenesis and cancer progression (1, 2). As a part of the tumour-supporting mesenchymal stroma, CAF have been suggested to originate from MSC, thereby sharing several features (3–5). For the distinguishing between MSC and CAF we recommend other previous review (5), since in this review, we will focus on MSC. Regardless the tissue origins, MSC are inherently tumour-homing and are a considerable component of the general host response to tissue damage caused by cancer cells. Cancer has been associated with MSC at all stages of disease progression with contradicting conclusions. Indeed, MSC have been also shown to have anti-cancer activities (6). More often, MSC are considered a foe in cancer for the immunosuppressive ability that creates a protective milieu for tumour cells by recruiting immunosuppressive tumour-associated macrophage TAM (7), for the promotion of tumour angiogenesis, proliferation, and metastasis, but also tumour dormancy and drug resistance (5).
CSC and MSC
Under physiological conditions, MSC have a major role in the maintenance of stem cell niches, like for the hematopoietic niche (8). Similarly, in cancer, MSC are relevant for the formation and maintenance of cancer stem cells (CSC). CSC are considered quiescent cells that have been isolated from a number of tumours (e.g., hematopoietic malignancies, breast carcinoma, glioblastoma, and sarcomas) by using different techniques (9–12).
Research on CSC has defined them as the driving force in tumour formation as they are characterized by self-renewal ability and can give rise to heterogeneous lineages that recapitulate the main tumour features (13). Yet, it has gradually become clear that CSC, like normal stem cells, do not necessarily have to be rare and/or quiescent; multiple examples now show that they can be abundant and can proliferate vigorously. Furthermore, it is emerging that stem cell hierarchies may be much more plastic than previously appreciated, a phenomenon that complicates the identification and eradication of CSC (14).
Despite recent data are therefore questioning the validity of the CSC model, the CSC-MSC interaction is well documented and is crucial for 3D growth and stemness of tumour cells (15), or for the generation of an hybrid MSC-CSC cell populations, occurring via entosis, i.e., a form of cell-to-cell internalization, or via fusion, as demonstrated in different tumours (16–20). Indeed, the selection processes of hybrid cells after MSC–tumour cell fusion contribute to CSC development (20, 21). CSC-MSC interaction occurs via soluble factors (22), exosomes, or direct interaction (23). Another form of contact has been described for glioblastoma, where the CSC state is regulated by a transient interaction between cancer cells and platelets. This contact induces an epithelial-to-mesenchymal (EMT) transition program leading to the expression of mesenchymal features which may coincide with a CSC-like state. In turn, once CSC have seeded a distant organ, they can orchestrate stromal cells to their needs (23) as CSC may promote, as an example, the release of TGF-β by MSC that further fosters EMT and increases the CSC stem-like state (24).
Given their mesenchymal origin, EMT cannot be proposed in sarcomas. However, after we demonstrated the existence of CSC (25), we showed that, by using a 2D co-culture system that also included CSC spheroids, MSC increased the CSC migratory capacity via TGF-β1 that, in turn, stimulated the secretion of the pro-inflammatory cytokine IL-6 that fostered osteosarcoma stemness and aggressiveness (26). Blocking the TGF- β1 signalling pathway in the same MSC-CSC co-cultures inhibited the CSC dedifferentiation, clonogenicity, and self-renewal capacity (27).
It follows that the study of the interaction between MSC and cancer cells, with or without stem-like properties, is crucial to bring out clinical opportunities for new cancer therapies. However, the set up of the appropriate models according to the specific study aim is crucial. Here, we summarized the currently available and most popular preclinical models and their specific features for modelling MSC-cancer cells interplays.
In vitro Models
The set up of pre-clinical models with MSC requires in vitro expansion thereby possibly causing meaningful changes in MSC behaviour, and affecting the interpretation of results. However, this is a bias that, to date, cannot be overcome to meet the demand for MSC-cancer cells preclinical modelling. The current in vitro preclinical models are mainly based on two-dimensional (2D) surfaces and include co-culture systems. These type of models have the enormous advantage to easily allow the control of the experimental conditions, and the analysis of expression of specific molecular signalling that can be distinguished between the two different cell populations. The first and simplest example is the treatment of cancer cells with conditioned medium of MSC cultures and is useful to study the effect of MSC-secreted soluble factors on cancer cell behaviour (28–30). A more complex system is based on the use of transwell with the two cell types seeded onto separate compartments and is suitable for keeping the reciprocal paracrine interactions for studying the effect of MSC secretome on tumour migratory, invasive, and stemness potentials (26, 31, 32). Finally, the co-seeding of the two cell populations on the same compartment of the culture support is also possible and has been used, for example, to evaluate the transfer of mitochondria from MSC to breast carcinoma cells (33), or to evaluate the metabolic symbiosis (34). Notably, the co-seeding is the most appropriate 2D approach to resemble the in vivo phenotype: it allows the cell-to-cell direct contact interactions. For this model, immunostaining is the easiest way of analysis, combined with the observation of either fixed or live cells through confocal or optical microscopes. However, for more complex molecular analysis, expensive techniques to sort single cells are needed, like immunomagnetic separation or, after the cell transfection with a fluorescent reporter, the cell retrieval by flow cytometry (34, 35). In conclusion, 2D in vitro culture systems are easily handled but they are falling short in predicting biological responses since they cannot thoroughly recapitulate both the complexity and the specificity of living tissues. Indeed, tumours are not merely clusters of proliferating cancer cells that grow on plastic 2D surfaces, but rather highly complex 3D structures with a dynamic extracellular matrix (ECM) and reactive stromal cells with a precise spatial relationship. Thus, 2D models should be not used for studying complex processes that cannot be reproduced in this type of culture, like drug perfusion in the tissue, intravasion, or extravasion of tumour cells, invasion of tumour cells through an ECM with a 3D structure, cytotoxicity of anticancer-drugs that might be affected by solute or gas gradient or by different transcriptome or proteome signature that is affected by the tumour-stroma interaction in 3D structures. Based on that, significant effort has been put forward to develop more sophisticated 3D structures, like cells aggregates alone or combined with bioprinting or microfluidics techniques.
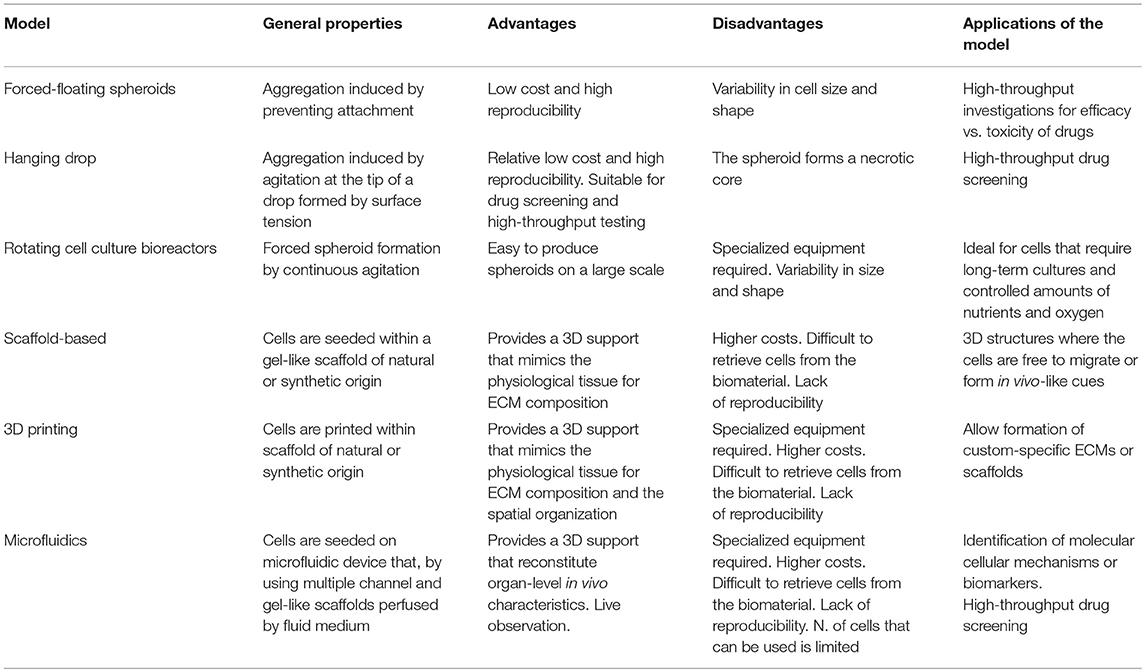
The key point to improve 3D co-culturing model is that functional unit of the tissue must be considered rather than single cells, including cell-cell contact and, depending on the cell types, a polarized morphology, a basement membrane and an ECM. For a list and brief description of the most commonly used 3D culturing systems see Table 1. The simplest model of 3D cultures is based on multicellular tumour spheroids without the addition of external ECM component. Despite cells can per se secrete ECM proteins, 3D spheroids are commonly considered ECM-free models. Spheroid cultures have been established from several cancers, also to study tumour-MSC interaction, including glioma, breast, colon, ovary, and prostate carcinoma (36). These multicellular structures mimic in vivo growth via the formation of a central necrotic core, a solute/ion gradient from the periphery to the centre, and a 3D cellular spatial organization. Forced-floating, hanging drop spheroids, spheroids obtained by using bioreactors are examples of this type of cell aggregates mixed with MSC and tumour cells, at different ratio (e.g., 3:1, respectively) (36, 37). Forced-floating cell aggregates are obtained by avoiding cell attachment to the well bottom. The hanging-drop method is the most widely used and is obtained by seeding a small aliquot of single-cell suspension in a volume that exceeds the well volume. By inverting the plate, the volume generates a drop in which cells are kept in place by surface tension and are then densely packed in spheroid-like structures with high reproducibility. Both forced-floating and hanging-drop spheroids are extensively used for drug screening (38, 39), thanks to the high number of spheroids/plate that can be obtained and the low cost. On the contrary, rotating cell culture bioreactor and spinner flasks force spheroid formation by continuous agitation (37, 40). However, the different size and the fact that spheroids formed in bioreactors must be related to be tested for drug screening, makes them unsuitable for this application. Nevertheless, bioreactors are the best options when long term culture and carefully monitoring of the environmental conditions (such as oxygen and nutrients) are required.
To recreate the interstitial space, it is essential to add the ECM component to the multicellular spheroids (41). For this aim, tumour cell and MSC co-cultures can be admixed to high biocompatible scaffolds of natural origin (i.g. collagen, hyaluronan, matrigel, elastin), or synthetic origin (polyethylenglycol, polyvinvyl alcohol, ceramics, or biomaterials), or also ECM isolated from tumour biopsies to mimic microenvironmental conditions (42). Within the scaffold, cells can interact one with the other (essential in the case of MSC-tumour studies), migrate through the pores and re-create in vivo-like communication strategies that mimic physiology. More the used matrix resembles the real tumour matrix and more predictive is assumed to be the model. For the addition of ECM in 3D cancer models, 3D bioprinting has stolen the spotlight since it allows the formation of high-resolution 3D structures by dispensing cell-laden biomaterials in a precisely and spatially defined way (43). In this technique, a hydrogel-like pre-polymer solution with encapsulated cells is stored into the ink cartridge that is connected to a printer head. The printer heads are deformed by a thermal or piezoelectric actuators and squeezed to generate bioink droplets of controllable size. However, to date, with very few exceptions (44), the bioprinting has been barely explored to study the MSC and cancer cells interactions.
Finally, tailored innovative platforms that combine spheroid technologies with disease-specific in silico models by using microfluidics, the so called “organ-on-a-chip” technologies that reconstitute organ-level in vivo characteristics (45, 46), have emerged also in cancer research. Although more expensive and less practical, this cutting-edge approach facilitates the identification of molecular mechanisms behind the disease or the identification of novel biomarkers, and is also particularly useful for drug screening. Microfluidics allows the study of complex phenomena under the combination of multiple biochemical and biophysical parameters, coupled with high-resolution real-time imaging. This type of approach has been mainly developed to study the interaction of tumour cells with blood vessels that can be recreated in the microfluidic chips. Few examples have also been reported for co-culturing MSC with tumour cells, like for lung cancer (47), or to recapitulate the bone metastatic niche that also includes MSC, like for acute lymphoid myeloma, acute lymphoblastic leukaemia, or breast carcinoma (48–50). By using this approach, it is possible to evaluate on real-time the induction of tumour apoptosis, proliferation, migration and invasion, the activation of the reactive stroma, the secretion of cytokines by tumour cells, the activation of specific oncogenes, and stroma-mediated extravasion and intravasion.
In vivo Models
To study the role of MSC on cancer development and progression, several animal models has been developed, mainly xenograft and syngenic small rodents, with MSC co-injected with tumour cells (51–53). In these models, MSC participate to tumour pathophysiology, ultimately facilitating the metastatic spread of weakly metastatic cancer (52, 54–56). MSC/tumour cells ratio seems to be particularly relevant like for tumour dormancy/growth, as demonstrated in melanoma or osteosarcoma models (57, 58). However, the isolation of MSC with different methods and from different tissues (e.g., bone marrow and adipose tissues) have made it difficult to reach consistent conclusions.
Heterotopic injections are the most used and include the subcutaneous injections (52), the easiest and most reproducible model that rarely gives origin to metastases, and that is quite far from the human disease since the host tissue surrounding the tumor might be very different from the tumor-associated stroma of the normal tissue where the tumor develops.
Systemically infused MSC localize within injured, inflamed, and cancerous tissues. Thus, to study the tropism of MSC to the tumour, MSC have been injected into circulation through the tail vein (59, 60). MSC have been also systemically administered via alternative routes, like via intratracheal (61), internal carotid artery (62), intraperitoneal (63), like for gliomas, breast, colon, ovarian, and lung carcinomas (52, 53, 61–66). Systemic injection of MSC may be useful also to enhance their viability along the experiment. Indeed, as it appeared from studies on MSC-based cell therapy, MSC survival is very low (67–70). Thus, periodic injections of MSC after the first injection might enhance their engraftment in the tumour and ensure the continuous secretion of MSC-derived protein factors.
Finally, the use of orthotropic injection site mimics the fate of MSC that have been already chemoattracted by tumour cells and have migrated at the primary tumour site, and it better recapitulates the human disease. However, this model has a great variability and requires a higher number of animals to obtain results that may be correctly interpreted.
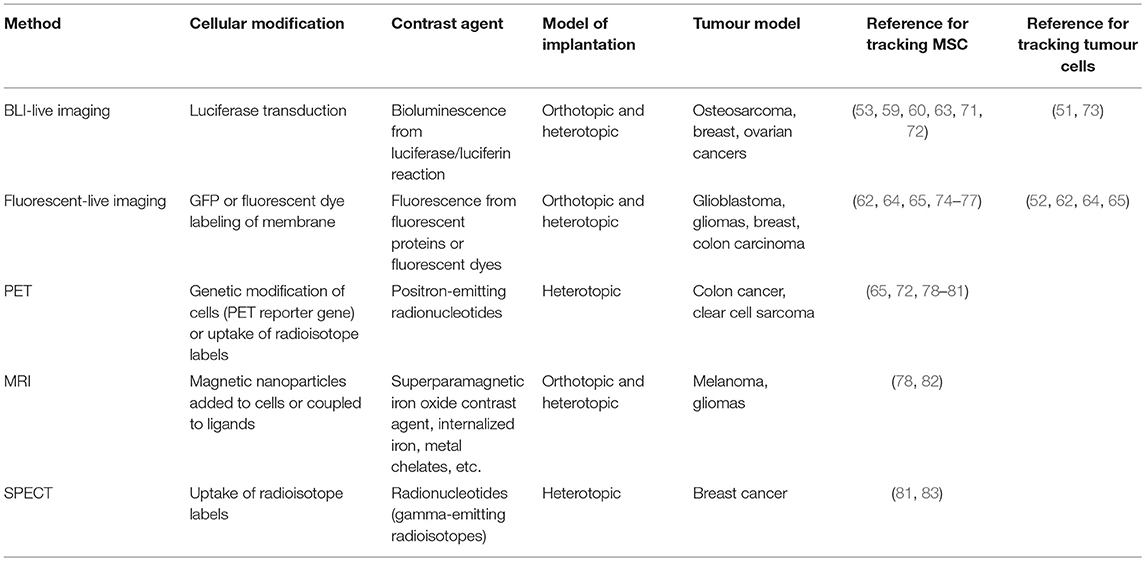
It is clear that, due to the short-term survival of injected MSC, monitoring their fate in vivo is crucial to support the conclusions about their pro- or anti-cancer activities. As for tumour cells, tracking MSC fate has been obtained by different approaches (see Table 2). In principle, the most powerful technique should allow non-invasive live imaging by optical or not-optical methods to gain real-time information. After animal sacrifice, also histology, immunofluorescence, immunohistochemistry, or flow cytometry techniques on isolated live or fixed cells can be used. Among the live-imaging techniques, the most used are the pre-labelling of MSC with a lipophilic fluorescent dyes (e.g., DiL or Cell TrackerTM) (54), or the pre-tagging of MSC by specific gene transfection, like luciferase or green fluorescent protein for the detection of bioluminescence (60, 71, 84) or fluorescence (55), respectively. Notably, bioluminescent imaging of luciferase-expressing cells is also a quantitative technique for the direct assessment of tumour growth (51, 53), whereas non-optical methods, such as magnetic resonance imaging (MRI) (82), positron emission tomography (PET) (65) and single photon emission computed tomography (SPECT) (83) provide a high spatial resolution and three-dimensional whole-body imaging.
In conclusion, to develop a more clinical relevant in vivo model that accurately reflects the human tumour biology is an urgent need to better predict the response of the tumour to the treatments and for identifying those steps that are crucial for tumour progression to be targeted or prevented for an improved clinical outcome. The addition of MSC in the model is a step forward in this direction, although for the development of in vivo pre-clinical models of MSC-tumour cells interaction several parameters need to be carefully considered according to the specific aim, like MSC:tumour cells ratio, the via of co-injection, and the tracking of MSC to check their fate. Last but not least, variables affecting the secretome should be very carefully analysed.
The Secretome
Studies on MSC-cancer interplay and analyses of cell conditioned media in the mentioned preclinical models have allowed the identification of soluble mediators of the indirect communication between MSC and tumour cells. To summarise, cancer cells frequently secrete IL-1 and TGF-β which switch MSC to a pro-inflammatory phenotype (85, 86), and the monocyte chemotactic protein-1 (MCP-1) which stimulates MSC migration (87). Conversely, MSC produce a plethora of cytokines which, in turn, modulates cancer cell behaviour: IL-6 and IL-8 that promote tumour cell proliferation, survival, migration, and invasion of different tumour cells (26, 88–90), CCL5 that support metastasis in several cancers (52, 91–93), the pro-angiogenic cytokine VEGF that enhances tumour growth and metastasis (94, 95), and TGF-β that fosters tumour invasion and metastasis via EMT (96). Cell communication within the tumour microenvironment is mediated also by exosomes, extracellular nanovesicles that deliver a functional cargo of proteins, lipids, and nucleic acids (97). Tumour-derived exosomes are able to co-opt and reprogram MSC by enhancing their pro-tumourigenic functions, including the pro-angiogenic activity and the production of the pro-inflammatory cytokines IL6 (51, 98). On the other side, exosomes derived from MSC are able to influence tumour development (99), and to increase the tumour stemness (100). Besides, several metabolites, like glutamine, lactate, and ketone bodies, that are released both by tumours cells and by MSC in the extracellular space might fuel the energetic metabolism of cells of the TME (101) or may act as signalling molecules, ultimately stimulating cancer motility, survival, or self-renewal (2, 102–106). Also in osteosarcoma, we recently demonstrated that tumour cells cause an oxidative stress in MSC that, in response, acquire a Warburg phenotype and produce a large amount of lactate.
In this context, it is worth to highlight that the in vitro conditions, both 2D and 3D, might induce secretory modifications per se (107), thereby affecting the interpretation of results, like by using experimental conditions that can unintentionally exert a stressing stimulus for the cells. Thus, we suggest to evaluate results by considering cells, secretome, and three-dimension as an integrated whole. This add complexity to the system, and careful attention has to be paid when setting up the experiment according to the specific aim and during the interpretation of data.
Conclusions
Several model systems are now available to characterize the MSC-tumour interplay in the TME. These offer early promise in establishing robust preclinical platforms for the identification of crucial molecular pathways and for the assessment of clinical efficacy of novel drugs to inhibit cancer development and progression. However, selection of the right model for a given study should be shaped on the purpose, and should also consider fixed biological, biochemical, and biophysical parameters according to the specific tumour type. Finally, in order to get reliable and useful results to be translated to the clinic, it should be always kept in mind the careful comparisons in the prediction of human outcomes by the developed model.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This study was funded by the Italian Association for Cancer Research (AIRC IG# 21403 to NB) and by the Ministry of Health 5x1000 fundings (to NB at Rizzoli Orthopaedic Institute).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. (1996) 76:69–125. doi: 10.1152/physrev.1996.76.1.69
2. Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. (2015) 368:7–13. doi: 10.1016/j.canlet.2015.07.039
3. Barclay M, Skipski VP, Terebus-Kekish O, Merker PL, Cappuccino JG. Serum lipoproteins in rats with tumors induced by 9,10-dimethyl-1,2-benzanthracene and with transplanted Walker carcinosarcoma 256. Cancer Res. (1967) 27:1158–67.
4. Borriello L, Nakata R, Sheard MA, Fernandez GE, Sposto R, Malvar J, et al. Cancer-associated fibroblasts share characteristics and protumorigenic activity with mesenchymal stromal cells. Cancer Res. (2017) 77:5142–57. doi: 10.1158/0008-5472.CAN-16-2586
5. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. (2016) 16:582–98. doi: 10.1038/nrc.2016.73
6. Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. (2008) 10:657–67. doi: 10.1080/14653240802486517
7. Gok Yavuz B, Gunaydin G, Gedik ME, Kosemehmetoglu K, Karakoc D, Ozgur F, et al. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1(+) TAMs. Sci Rep. (2019) 9:3172. doi: 10.1038/s41598-019-39553-z
8. Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. (2015) 16:239–53. doi: 10.1016/j.stem.2015.02.019
9. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. (1994) 367:645–8. doi: 10.1038/367645a0
10. Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. (2005) 7:967–76. doi: 10.1593/neo.05394
11. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. (2003) 100:3983–8. doi: 10.1073/pnas.0530291100
12. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. (2007) 445:111–5. doi: 10.1038/nature05384
13. Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. (2006) 66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126
14. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. (2017) 23:1124–34. doi: 10.1038/nm.4409
15. Shamai Y, Alperovich DC, Yakhini Z, Skorecki K, Tzukerman M. Reciprocal reprogramming of cancer cells and associated mesenchymal stem cells in gastric cancer. Stem Cells. (2019) 37:176–89. doi: 10.1002/stem.2942
16. Yang Y, Otte A, Hass R. Human mesenchymal stroma/stem cells exchange membrane proteins and alter functionality during interaction with different tumor cell lines. Stem Cells Dev. (2015) 24:1205–22. doi: 10.1089/scd.2014.0413
17. Wei HJ, Nickoloff JA, Chen WH, Liu HY, Lo WC, Chang YT, et al. FOXF1 mediates mesenchymal stem cell fusion-induced reprogramming of lung cancer cells. Oncotarget. (2014) 5:9514–29. doi: 10.18632/oncotarget.2413
18. Xue J, Zhu Y, Sun Z, Ji R, Zhang X, Xu W, et al. Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness. BMC Cancer. (2015) 15:793. doi: 10.1186/s12885-015-1780-1
19. Melzer C, Yang Y, Hass R. Interaction of MSC with tumor cells. Cell Commun Signal. (2016) 14:20. doi: 10.1186/s12964-016-0143-0
20. Melzer C, von der Ohe J, Lehnert H, Ungefroren H, Hass R. Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Mol Cancer. (2017) 16:28. doi: 10.1186/s12943-017-0595-x
21. Sottile F, Aulicino F, Theka I, Cosma MP. Mesenchymal stem cells generate distinct functional hybrids in vitro via cell fusion or entosis. Sci Rep. (2016) 6:36863. doi: 10.1038/srep36863
22. Cortini M, Avnet S, Baldini N. Mesenchymal stroma: role in osteosarcoma progression. Cancer Lett. (2017) 405:90–9. doi: 10.1016/j.canlet.2017.07.024
23. Fessler E, Dijkgraaf FE, De Sousa EMF, Medema JP. Cancer stem cell dynamics in tumor progression and metastasis: is the microenvironment to blame? Cancer Lett. (2013) 341:97–104. doi: 10.1016/j.canlet.2012.10.015
24. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. (2008) 133:704–15. doi: 10.1016/j.cell.2008.03.027
25. Salerno M, Avnet S, Bonuccelli G, Eramo A, De Maria R, Gambarotti M, et al. Sphere-forming cell subsets with cancer stem cell properties in human musculoskeletal sarcomas. Int J Oncol. (2013) 43:95–102. doi: 10.3892/ijo.2013.1927
26. Cortini M, Massa A, Avnet S, Bonuccelli G, Baldini N. Tumor-activated mesenchymal stromal cells promote osteosarcoma stemness and migratory potential via IL-6 secretion. PLoS ONE. (2016) 11:e0166500. doi: 10.1371/journal.pone.0166500
27. Zhang H, Wu H, Zheng J, Yu P, Xu L, Jiang P, et al. Transforming growth factor beta1 signal is crucial for dedifferentiation of cancer cells to cancer stem cells in osteosarcoma. Stem Cells. (2013) 31:433–46. doi: 10.1002/stem.1298
28. Iser IC, Ceschini SM, Onzi GR, Bertoni AP, Lenz G, Wink MR. Conditioned medium from adipose-derived stem cells (ADSCs) promotes epithelial-to-mesenchymal-like transition (EMT-Like) in glioma cells >in vitro. Mol Neurobiol. (2016) 53:7184–99. doi: 10.1007/s12035-015-9585-4
29. Hernanda PY, Pedroza-Gonzalez A, van der Laan LJ, Broker ME, Hoogduijn MJ, Ijzermans JN, et al. Tumor promotion through the mesenchymal stem cell compartment in human hepatocellular carcinoma. Carcinogenesis. (2013) 34:2330–40. doi: 10.1093/carcin/bgt210
30. Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. (2007) 21:3763–70. doi: 10.1096/fj.07-8832com
31. Avnet S, Di Pompo G, Chano T, Errani C, Ibrahim-Hashim A, Gillies RJ, et al. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-kappaB activation. Int J Cancer. (2017) 140:1331–45. doi: 10.1002/ijc.30540
32. Chiovaro F, Martina E, Bottos A, Scherberich A, Hynes NE, Chiquet-Ehrismann R. Transcriptional regulation of tenascin-W by TGF-beta signaling in the bone metastatic niche of breast cancer cells. Int J Cancer. (2015) 137:1842–54. doi: 10.1002/ijc.29565
33. Caicedo A, Fritz V, Brondello JM, Ayala M, Dennemont I, Abdellaoui N, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. (2015) 5:9073. doi: 10.1038/srep09073
34. Bonuccelli G, Avnet S, Grisendi G, Salerno M, Granchi D, Dominici M, et al. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget. (2014) 5:7575–88. doi: 10.18632/oncotarget.2243
35. Molloy AP, Martin FT, Dwyer RM, Griffin TP, Murphy M, Barry FP, et al. Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer. (2009) 124:326–32. doi: 10.1002/ijc.23939
36. Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. (2017) 108:283–9. doi: 10.1111/cas.13155
37. Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. (2008) 3:1172–84. doi: 10.1002/biot.200700228
38. Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. (2007) 130:601–10. doi: 10.1016/j.cell.2007.08.006
39. Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. (2010) 148:3–15. doi: 10.1016/j.jbiotec.2010.01.012
40. Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. (2005) 15:365–77. doi: 10.1016/j.semcancer.2005.05.002
41. Breslin S, O'Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. (2013) 18:240–9. doi: 10.1016/j.drudis.2012.10.003
42. Saforo D, Omer L, Smolenkov A, Barve A, Casson L, Boyd N, et al. Primary lung cancer samples cultured under microenvironment-mimetic conditions enrich for mesenchymal stem-like cells that promote metastasis. Sci Rep. (2019) 9:4177. doi: 10.1038/s41598-019-40519-4
43. Zhang YS, Duchamp M, Oklu R, Ellisen LW, Langer R, Khademhosseini A. Bioprinting the Cancer Microenvironment. ACS Biomater Sci Eng. (2016) 2:1710–21. doi: 10.1021/acsbiomaterials.6b00246
44. Braham MVJ, Ahlfeld T, Akkineni AR, Minnema MC, Dhert WJA, Oner FC, et al. Endosteal and perivascular subniches in a 3D bone marrow model for multiple myeloma. Tissue Eng Part C Methods. (2018) 24:300–12. doi: 10.1089/ten.tec.2017.0467
45. Portillo-Lara R, Annabi N. Microengineered cancer-on-a-chip platforms to study the metastatic microenvironment. Lab Chip. (2016) 16:4063–81. doi: 10.1039/C6LC00718J
46. Chung M, Ahn J, Son K, Kim S, Jeon NL. Biomimetic model of tumor microenvironment on microfluidic platform. Adv Healthc Mater. (2017) 6:196. doi: 10.1002/adhm.201700196
47. Hao Y, Zhang L, He J, Guo Z, Ying L, Xu Z, et al. Functional investigation of NCI-H460-inducible myofibroblasts on the chemoresistance to VP-16 with a microfluidic 3D co-culture device. PLoS ONE. (2013) 8:e61754. doi: 10.1371/journal.pone.0061754
48. Narkhede AA, Shevde LA, Rao SS. Biomimetic strategies to recapitulate organ specific microenvironments for studying breast cancer metastasis. Int J Cancer. (2017) 141:1091–109. doi: 10.1002/ijc.30748
49. Houshmand M, Soleimani M, Atashi A, Saglio G, Abdollahi M, Nikougoftar Zarif M. Mimicking the acute myeloid leukemia niche for molecular study and drug screening. Tissue Eng Part C Methods. (2017) 23:72–85. doi: 10.1089/ten.tec.2016.0404
50. Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. (2014) 35:2454–61. doi: 10.1016/j.biomaterials.2013.11.050
51. Baglio SR, Lagerweij T, Perez-Lanzon M, Ho XD, Leveille N, Melo SA, et al. Blocking tumor-educated MSC paracrine activity halts osteosarcoma progression. Clin Cancer Res. (2017) 23:3721–33. doi: 10.1158/1078-0432.CCR-16-2726
52. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. (2007) 449:557–63. doi: 10.1038/nature06188
53. Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. (2011) 71:614–24. doi: 10.1158/0008-5472.CAN-10-0538
54. Zhang P, Dong L, Long H, Yang TT, Zhou Y, Fan QY, et al. Homologous mesenchymal stem cells promote the emergence and growth of pulmonary metastases of the rat osteosarcoma cell line UMR-106. Oncol Lett. (2014) 8:127–32. doi: 10.3892/ol.2014.2127
55. Ke CC, Liu RS, Suetsugu A, Kimura H, Ho JH, Lee OK, et al. In vivo fluorescence imaging reveals the promotion of mammary tumorigenesis by mesenchymal stromal cells. PLoS ONE. (2013) 8:e69658. doi: 10.1371/journal.pone.0069658
56. Fregni G, Quinodoz M, Moller E, Vuille J, Galland S, Fusco C, et al. Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. EBioMedicine. (2018) 29:128–45. doi: 10.1016/j.ebiom.2018.02.017
57. Kucerova L, Matuskova M, Hlubinova K, Altanerova V, Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. (2010) 9:129. doi: 10.1186/1476-4598-9-129
58. Avril P, Le Nail LR, Brennan MA, Rosset P, De Pinieux G, Layrolle P, et al. Mesenchymal stem cells increase proliferation but do not change quiescent state of osteosarcoma cells: potential implications according to the tumor resection status. J Bone Oncol. (2016) 5:5–14. doi: 10.1016/j.jbo.2015.11.002
59. Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, et al. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. (2009) 27:1548–58. doi: 10.1002/stem.81
60. Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. (2007) 67:11687–95. doi: 10.1158/0008-5472.CAN-07-1406
61. Xin H, Sun R, Kanehira M, Takahata T, Itoh J, Mizuguchi H, et al. Intratracheal delivery of CX3CL1-expressing mesenchymal stem cells to multiple lung tumors. Mol Med. (2009) 15:321–7. doi: 10.2119/molmed.2009.00059
62. Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. (2005) 65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874
63. Komarova S, Roth J, Alvarez R, Curiel DT, Pereboeva L. Targeting of mesenchymal stem cells to ovarian tumors via an artificial receptor. J Ovarian Res. (2010) 3:12. doi: 10.1186/1757-2215-3-12
64. Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. (2010) 70:10044–50. doi: 10.1158/0008-5472.CAN-10-1254
65. Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. (2005) 11:7749–56. doi: 10.1158/1078-0432.CCR-05-0876
66. Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, et al. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. (2009) 69:8862–7. doi: 10.1158/0008-5472.CAN-09-1912
67. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. (2009) 4:206–16. doi: 10.1016/j.stem.2009.02.001
68. Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. (2013) 2013:732742. doi: 10.1155/2013/732742
69. Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. (2012) 3:297. doi: 10.3389/fimmu.2012.00297
70. Braid LR, Wood CA, Wiese DM, Ford BN. Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy. (2018) 20:232–44. doi: 10.1016/j.jcyt.2017.09.013
71. Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. (2009) 27:2614–23. doi: 10.1002/stem.187
72. Love Z, Wang F, Dennis J, Awadallah A, Salem N, Lin Y, et al. Imaging of mesenchymal stem cell transplant by bioluminescence and PET. J Nucl Med. (2007) 48:2011–20. doi: 10.2967/jnumed.107.043166
73. Kim JB, Urban K, Cochran E, Lee S, Ang A, Rice B, et al. Non-invasive detection of a small number of bioluminescent cancer cells in vivo. PLoS ONE. (2010) 5:e9364. doi: 10.1371/journal.pone.0009364
74. Pacioni S, D'Alessandris QG, Giannetti S, Morgante L, Cocce V, Bonomi A, et al. Human mesenchymal stromal cells inhibit tumor growth in orthotopic glioblastoma xenografts. Stem Cell Res Ther. (2017) 8:53. doi: 10.1186/s13287-017-0516-3
75. Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. (2009) 17:183-90. doi: 10.1038/mt.2008.229
76. Wang N, Fallavollita L, Nguyen L, Burnier J, Rafei M, Galipeau J, et al. Autologous bone marrow stromal cells genetically engineered to secrete an igf-I receptor decoy prevent the growth of liver metastases. Mol Ther. (2009) 17:1241–9. doi: 10.1038/mt.2009.82
77. Pavon LF, Sibov TT, de Souza AV, da Cruz EF, Malheiros SMF, Cabral FR, et al. Tropism of mesenchymal stem cell toward CD133(+) stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res Ther. (2018) 9:310. doi: 10.1186/s13287-018-1049-0
78. Patel D, Kell A, Simard B, Xiang B, Lin HY, Tian G. The cell labeling efficacy, cytotoxicity and relaxivity of copper-activated MRI/PET imaging contrast agents. Biomaterials. (2011) 32:1167–76. doi: 10.1016/j.biomaterials.2010.10.013
79. Yaghoubi SS, Gambhir SS. PET imaging of herpes simplex virus type 1 thymidine kinase (HSV1-tk) or mutant HSV1-sr39tk reporter gene expression in mice and humans using [18F]FHBG. Nat Protoc. (2006) 1:3069–75. doi: 10.1038/nprot.2006.459
80. Schonitzer V, Haasters F, Kasbauer S, Ulrich V, Mille E, Gildehaus FJ, et al. In vivo mesenchymal stem cell tracking with PET using the dopamine type 2 receptor and 18F-fallypride. J Nucl Med. (2014) 55:1342–7. doi: 10.2967/jnumed.113.134775
81. Lim M, Wang W, Liang L, Han ZB, Li Z, Geng J, et al. Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res Ther. (2018) 9:129. doi: 10.1186/s13287-018-0888-z
82. Wu X, Hu J, Zhou L, Mao Y, Yang B, Gao L, et al. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. (2008) 108:320–9. doi: 10.3171/JNS/2008/108/2/0320
83. Dwyer RM, Ryan J, Havelin RJ, Morris JC, Miller BW, Liu Z, et al. Mesenchymal Stem Cell-mediated delivery of the sodium iodide symporter supports radionuclide imaging and treatment of breast cancer. Stem Cells. (2011) 29:1149–57. doi: 10.1002/stem.665
84. Compte M, Cuesta AM, Sanchez-Martin D, Alonso-Camino V, Vicario JL, Sanz L, et al. Tumor immunotherapy using gene-modified human mesenchymal stem cells loaded into synthetic extracellular matrix scaffolds. Stem Cells. (2009) 27:753–60. doi: 10.1634/stemcells.2008-0831
85. Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. (2012) 2:840–55. doi: 10.1158/2159-8290.CD-12-0101
86. Tu B, Peng ZX, Fan QM, Du L, Yan W, Tang TT. Osteosarcoma cells promote the production of pro-tumor cytokines in mesenchymal stem cells by inhibiting their osteogenic differentiation through the TGF-beta/Smad2/3 pathway. Exp Cell Res. (2014) 320:164–73. doi: 10.1016/j.yexcr.2013.10.013
87. Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. (2007) 13:5020–7. doi: 10.1158/1078-0432.CCR-07-0731
88. Touboul C, Lis R, Al Farsi H, Raynaud CM, Warfa M, Althawadi H, et al. Mesenchymal stem cells enhance ovarian cancer cell infiltration through IL6 secretion in an amniochorionic membrane based 3D model. J Transl Med. (2013) 11:28. doi: 10.1186/1479-5876-11-28
89. Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. (2009) 28:2745–55. doi: 10.1038/onc.2009.130
90. Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. (2007) 117:3988–4002. doi: 10.1172/JCI32533
91. Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. (2011) 32:477–87. doi: 10.1093/carcin/bgr009
92. Luo J, Ok Lee S, Liang L, Huang CK, Li L, Wen S, et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. (2014) 33:2768–78. doi: 10.1038/onc.2013.233
93. Xu WT, Bian ZY, Fan QM, Li G, Tang TT. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. (2009) 281:32–41. doi: 10.1016/j.canlet.2009.02.022
94. Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. (2008) 99:622–31. doi: 10.1038/sj.bjc.6604508
95. Zhang K, Shi B, Chen J, Zhang D, Zhu Y, Zhou C, et al. Bone marrow mesenchymal stem cells induce angiogenesis and promote bladder cancer growth in a rabbit model. Urol Int. (2010) 84:94–9. doi: 10.1159/000273474
96. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. (2009) 19:156–72. doi: 10.1038/cr.2009.5
97. Ruivo CF, Adem B, Silva M, Melo SA. The biology of cancer exosomes: insights and new perspectives. Cancer Res. (2017) 77:6480–8. doi: 10.1158/0008-5472.CAN-17-0994
98. Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol. (2018) 35:69–79. doi: 10.1016/j.smim.2017.12.003
99. Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem. (2017) 42:2242–54. doi: 10.1159/000479998
100. Li H, Li F. Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. Br J Cancer. (2018) 119:744–55. doi: 10.1038/s41416-018-0254-z
101. Wilde L, Roche M, Domingo-Vidal M, Tanson K, Philp N, Curry J, et al. Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin Oncol. (2017) 44:198–203. doi: 10.1053/j.seminoncol.2017.10.004
102. Corbet C, Feron O. Cancer cell metabolism and mitochondria: nutrient plasticity for TCA cycle fueling. Biochim Biophys Acta Rev Cancer. (2017) 1868:7–15. doi: 10.1016/j.bbcan.2017.01.002
103. Danhier P, Banski P, Payen VL, Grasso D, Ippolito L, Sonveaux P, et al. Cancer metabolism in space and time: beyond the Warburg effect. Biochim Biophys Acta Bioenerg. (2017) 1858:556–72. doi: 10.1016/j.bbabio.2017.02.001
104. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. (2016) 380:272–80. doi: 10.1016/j.canlet.2015.10.027
105. Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. (2014) 25:47–60. doi: 10.1016/j.semcancer.2014.01.005
106. Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. (2012) 72:5130–40. doi: 10.1158/0008-5472.CAN-12-1949
Keywords: mesenchymal stromal cells, tumour microenvironment, stemness, secretome, 3D models, metastasis
Citation: Avnet S, Lemma S, Cortini M, Di Pompo G, Perut F and Baldini N (2019) Pre-clinical Models for Studying the Interaction Between Mesenchymal Stromal Cells and Cancer Cells and the Induction of Stemness. Front. Oncol. 9:305. doi: 10.3389/fonc.2019.00305
Received: 31 December 2018; Accepted: 02 April 2019;
Published: 30 April 2019.
Edited by:
Cyril Corbet, Catholic University of Louvain, BelgiumReviewed by:
Peiman Hematti, University of Wisconsin-Madison, United StatesMarco A. Velasco-Velazquez, National Autonomous University of Mexico, Mexico
Copyright © 2019 Avnet, Lemma, Cortini, Di Pompo, Perut and Baldini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Baldini, fisiopatologia@ior.it
 Sofia Avnet
Sofia Avnet Silvia Lemma
Silvia Lemma Margherita Cortini
Margherita Cortini Gemma Di Pompo1
Gemma Di Pompo1 Francesca Perut
Francesca Perut