
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 18 April 2019
Sec. Cancer Molecular Targets and Therapeutics
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00301
 Takafumi Koyama1
Takafumi Koyama1 Shunsuke Kondo1,2*
Shunsuke Kondo1,2* Toshio Shimizu1
Toshio Shimizu1 Yutaka Fujiwara1
Yutaka Fujiwara1 Chigusa Morizane2
Chigusa Morizane2 Yasunari Sakamoto2
Yasunari Sakamoto2 Takuji Okusaka2
Takuji Okusaka2 Noboru Yamamoto1
Noboru Yamamoto1Chronic viral hepatitis is a risk factor for liver fibrosis and hepatocellular carcinoma (HCC). Patients with advanced HCC have limited effective therapeutic options and are considered potential candidates for early phase clinical trials of anti-cancer agents. The impact of chronic viral hepatitis on the efficacy of anticancer agents for patients with HCC in phase I trials (P-Is) still remains unclear and has not been reported. We retrospectively analyzed the outcomes of consecutive HCC patients in P-Is conducted in a single institute, focusing on chronic viral hepatitis. Of 85 patients enrolled in P-Is, 46 (54%) patients positive and 39 (46%) patients negative for chronic viral hepatitis showed no significant difference in clinical and laboratory variables and on the point of the best response based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria; moreover, the frequency of Grade ≥3 adverse events (AE) was not significantly different. The median time to treatment failure (TTF) and overall survival (OS) from the P-I enrolment were 2.0 and 13.7 months, respectively. No patient experienced reactivation of hepatitis B virus (HBV) or treatment-related death. Chronic viral hepatitis does not independently affect the outcomes of anticancer drugs. Advanced HCC patients with chronic viral hepatitis could be feasible for P-Is.
Hepatocellular carcinoma (HCC) is one of the most commonly found solid tumors and the fourth leading cause of cancer-related death worldwide (1). The incidence of HCC varies widely according to geographic location (2). This difference in distribution of HCC is probably related to regional variation in exposure to hepatitis virus and environmental pathogens. Over 50% of cases are due to underlying chronic viral hepatitis (hepatitis B or C virus infection) (1). The frequency of hepatitis B virus (HBV) carriers is relatively high in sub-Saharan Africa, the People's Republic of China, Hong Kong, and Taiwan (3). The prevalence of hepatitis C virus (HCV) has increased in the United States among individuals born between 1945 and 1965 (~2.5% or >2 million individuals, five times the rate among those born in other years) (4). Japan has one of the highest incidence rates of HCC associated with HBV or HCV infection (5, 6).
Until 2008, no effective therapy existed for patients with advanced-stage HCC or those for which local therapies failed. There has been a resurgence of interest and enthusiasm for systemic treatment of HCC after the emergence of data showing that treatment with the molecular targeting agent sorafenib (7) improved survival compared with that of best supportive care alone. A recent study showed that lenvatinib was non-inferior to sorafenib in patients with no prior systemic treatment for HCC (8). A survival benefit in the second-line treatment with regorafenib (9), ramucirumab (10), and cabozantinib (11) was demonstrated after the failure of sorafenib in HCC patients. Given the lack of established systemic treatment making survival significantly prolonged in patients with advanced HCC, early phase clinical trials are still needed to develop new treatment options for patients with advanced HCC.
Hepatic reserve, as indicated by Child-Pugh (C-P) classification, often dictated the therapeutic options (12). No prospective study has investigated the impact of hepatitis virus on treatment response and adverse events to investigational new drugs in phase I clinical trials (P-I) for advanced HCC patients. Advanced HCC patients with HBV infection might have a poorer prognosis than those with HCV infection (13). The A-P study showed that HCC patients with HBV infection who received sorafenib tended to have longer overall survival (OS) than patients who received a placebo (HR0.68) (14). Increasing data from subgroup analyses in phase III clinical trials suggested that patients with chronic hepatitis C as the etiology of their cirrhosis might show a better response to sorafenib than those with other underlying causes of cirrhosis (8, 15, 16). There were inconclusive data about how HBV or HCV infections affected the safety and efficacy of systemic therapy in patients with HCC during their participation in P-I. The present study was designed to examine the feasibility and efficacy of new anticancer drugs in patients with HCC who enrolled in P-I, from the perspective of HBV or HCV infection.
From July 2009 to August 2017, we retrospectively reviewed electronic medical records of consecutive patients with advanced HCC who enrolled in P-Is at the National Cancer Center Hospital, Tokyo (NCCH). This study was approved by the NCCH institutional review board and performed in compliance with our institutional guidelines.
Patients positive for anti-HCV antibody or HBV antigen (HBsAg) were considered to have HCC due to chronic viral hepatitis, whereas those without anti-HCV antibody nor HBsAg were considered to have HCC by another etiology (non-B/non-C).
All medical records were reviewed to summarize the patients' clinical characteristics, treatments, and outcomes based on the status of chronic viral hepatitis. The baseline clinical characteristics included age, gender, ECOG-performance status (PS), treatment history, spread from the primary site, number of metastatic sites and their locations, laboratory tests, and the C-P classification. The outcomes consisted of the date of P-I enrolment and progression, the date of death or loss to follow-up, the best response to therapy based on Response Evaluation Criteria in Solid Tumors (RECIST), and adverse events based on Common Terminology Criteria for Adverse Events (CTCAE) ver.4.
The primary outcome of interest was the effect of chronic viral hepatitis on the efficacy and feasibility of the drugs on patients with HCC who enrolled in P-Is. We assessed the effectiveness through time to failure (TTF), OS, and the best response, and the feasibility through toxicity.
TTF was defined as the time from the start of P-I enrolment to discontinuation (as judged by the P-I investigators), based on clinical disease progression, treatment intolerance, toxicity, or death. OS was defined as the time from the P-I enrolment to death or the last day at which the patient was known to be alive; patients were censored if they were alive at the last follow-up. Kaplan-Meier product limit estimates were used to generate curves for TTF and OS. Univariate Cox proportional hazards models were fitted to test the effects of chronic viral hepatitis on TTF and OS.
Based on the status of chronic viral hepatitis, differences in the baseline clinical characteristics were evaluated using χ2 tests. The ratio of actual dose (AD) to recommended Phase 2 dose (RP2D) was calculated based on each trial result that was already reported. The distribution of dose ratio for each subgroup of patients was measured based on their hepatitis status and then evaluated using Wilcoxon tests.
The best response was assessed using the RECIST guidelines. Waterfall plots were created to illustrate the responses; subplots were also designed according to the drug classes. Treatment effects were estimated for each subgroup of patients based on their hepatitis status and evaluated using χ2 tests.
Toxicity data were assessed across trials in an aggregate fashion by considering the number of grade 3/4 events recorded. Patients who were taken off therapy because of secondary drug-related toxicity as well as those who died as a result of therapy were also analyzed. The percentage of patients with each type of adverse event was determined by dividing the number of patients with the adverse event by the number of patients based on the status of chronic viral hepatitis or C-P classification. Comparisons between the positive and negative status of chronic viral hepatitis and the correlation between C-P A and B were assessed across P-I studies. The analysis considered the total number of grade 3/4 events across trials with Fisher's exact tests based on the status of chronic viral hepatitis as well as C-P classification.
All tests were two-sided, and a P < 0.05 was considered statistically significant. All statistical analyses were performed using JMP software (version 13; SAS Inc., Cary, North Carolina, USA).
We identified 85 consecutive patients with HCC, who participated in P-Is at the NCCH. Forty-six patients were positive for chronic viral hepatitis, of which 26 were positive for HBsAg, 25 were positive for the anti-HCV antibody, and five were positive for both HBsAg and anti-HCV antibody, whereas 39 patients were negative for chronic viral hepatitis. Based on the status of chronic viral hepatitis, there were no significant differences in the baseline clinical characteristics, between the positive and negative groups (Table 1). There was also no difference in the distribution of dose Ratio of AD to RP2D between the positive and negative groups (P = 0.21).
Fifty-seven patients (67%) had died at the time of the analysis. The TTF and OS from the P-I enrolment were 2.0 months [95% confidence interval (CI): 1.7–2.8] and 13.7 months (95% CI: 8.9–15.9), respectively. The median OS from the P-I enrolment for the populations positive and negative for chronic viral hepatitis was 13.8 months (95% CI: 6.9–16.8 months) and 13.7 months (95% CI: 7.3–18.8 months), respectively (Figure 1A). The 90-day mortality rate was 17.8 and 7.7% for patients positive and negative for chronic viral hepatitis, respectively. The survival rates of the population positive for chronic viral hepatitis at 6, 12, and 18 months were 69.7, 50.4, and 33.4%, respectively. The survival rates of the population negative for chronic viral hepatitis at 6, 12, and 18 months were 87.0, 51.6, and 37.8%, respectively. The TTF for the population positive and negative for chronic viral hepatitis was 1.9 months (95% CI: 1.5–3.5 months) and 2.1 months (95% CI: 1.7–3.5 months), respectively (Figure 1B).
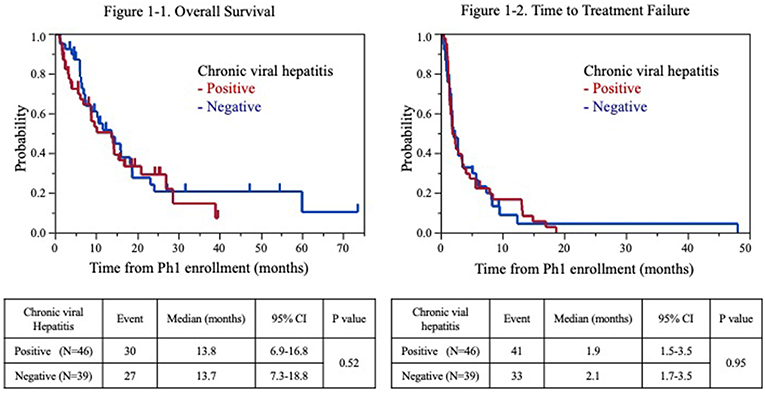
Figure 1. Kaplan-Meier estimates of overall survival (1-1) and time to failure (1-2) for patients with advanced hepatocellular carcinoma.
All patients were evaluated for response based on the RECIST criteria. Based on the status of chronic viral hepatitis, between the positive group and negative group, there was no significant difference in the number of patients who participated in each trial, according to the drug class (Table 2).
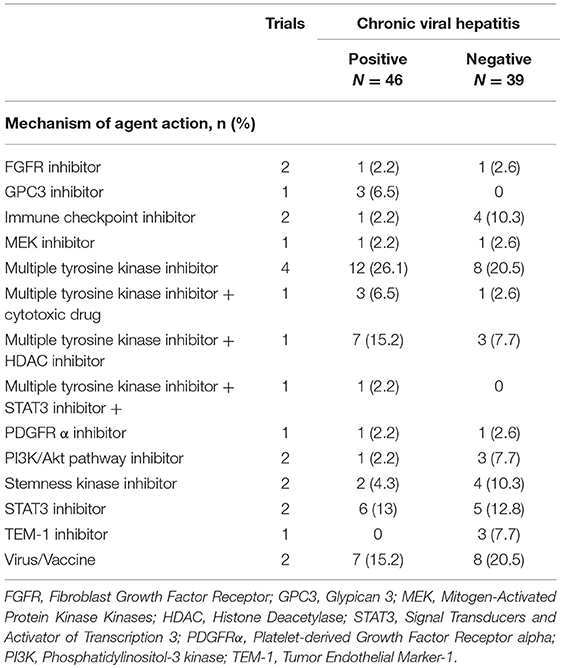
Table 2. Mechanism of agent action in phase I trials enrolling patients in the present study based on the status of chronic viral hepatitis.
There was no significant difference between patients positive (Partial response; 1, stable disease; 19, progressive disease; 25, and not evaluated; 1) and negative for chronic viral hepatitis (Partial response; 2, stable disease; 18, progressive disease; 17, and not evaluated; 2) (P = 0.65) (Figure 2). The best responses of patients according to drug class are shown in Figure 2.
We observed no treatment-related mortality or HBV reactivation. The anti-viral drug Entecavir was prescribed for all patients with chronic HBV infection during the P-Is. Ten patients who received an angiogenesis inhibitor (e.g., Multiple Tyrosine Kinase inhibitor) experienced Grade (Gr) 3 hypertension. An increase in Gr 3 AST or ALT was observed in five patients positive for chronic viral hepatitis during the P-Is (Table 3). Fisher's exact test for the frequency of Gr ≥3 adverse events based on hepatitis virus infection status as well as C-P classification revealed no significant difference (Table 3).
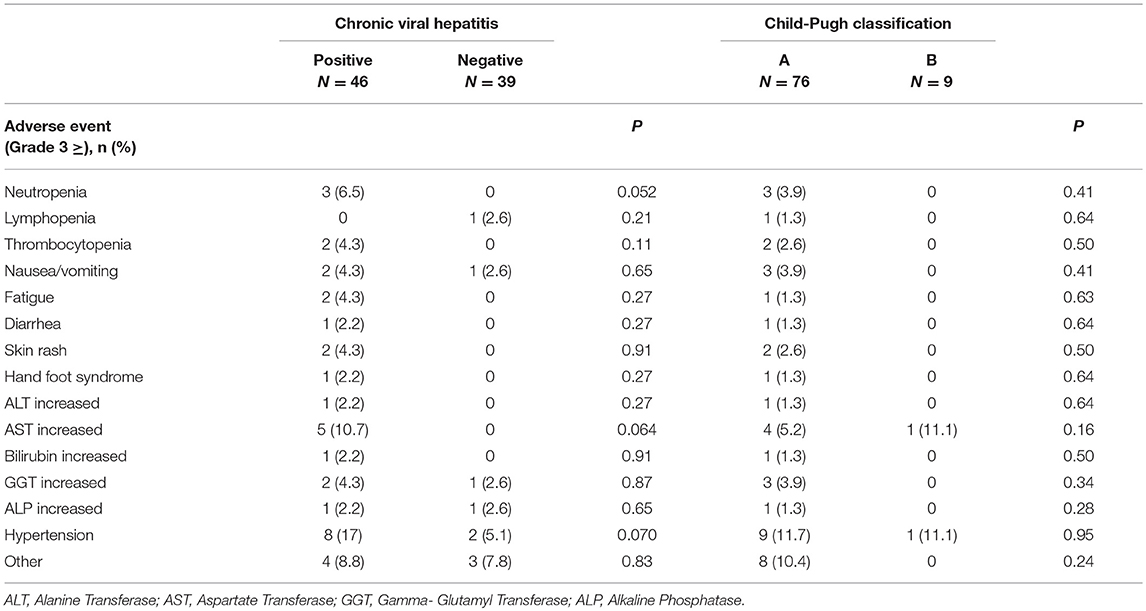
Table 3. Adverse event based on the status of chronic viral hepatitis and Child-Pugh classification.
The reasons for discontinuing the P-I treatment of patients positive for chronic viral hepatitis were disease progression (n = 33, 84.6%), toxicity (n = 4, 10.2%), and patient refusal (n = 2, 5.1%). Toxicity included Gr 3 infection (1), Gr 3 fatigue (1), Gr 3 hand-foot syndrome (1), and Gr 4 neutropenia (1). The reasons for discontinuing the P-I treatment of patients negative for chronic viral hepatitis were disease progression (41 patients; 89.1%), toxicity (4 patients; 8.7%), and patient refusal (1 patient; 2.2%). Here, toxicity included Gr 3 increased gamma-glutamyl transferase (GGT) (1), Gr 3 skin rash (1), Gr 3 hypertension (1), and Gr3 nausea (1).
This is the first report of the impact of chronic viral hepatitis on the efficacy and feasibility of anticancer agents for patients with HCC in P-Is. We conducted a retrospective study on 85 consecutive patients with advanced HCC who enrolled in P-Is independent of the status of chronic viral hepatitis. Patients with chronic viral hepatitis comprised 54.1% of all patients included in the analysis. There was no evidence that positive or negative chronic viral hepatitis affected OS, TTF, and disease control rate. There was no significant difference in the frequency of Gr ≥3 AE, between the positive group and negative group. No patient showed reactivation of hepatitis virus, and no treatment-related mortality was observed.
To date, in most P-I that enrolled patients with all types of solid tumors, patients with chronic viral hepatitis have been excluded. Hence, there are limited clinical data on the effect of chronic viral hepatitis on the efficacy and feasibility of drugs in patients enrolled in P-Is. Recently, the results of a phase I study of nivolumab for patients with advanced HCC (CheckMate 040) showed that efficacy and feasibility were similar in patients with or without chronic viral infection (17). On the other hand, liver function might be related to feasibility in P-Is. In the Phase I study of lenvatinib for patients with advanced HCC, the maximum tolerable dose (MTD) was different between C-P A group (12 mg once daily) and C-P B group (8 mg once daily). The most common Gr 3 toxicities included hypertension in C-P A and hyperbilirubinemia in C-P B (18). Patients with hepatic impairment have higher plasma concentration of lenvatinib and may have increased risk of adverse events compared with patients without hepatic compromise. We have to consider hepatic function when we enroll HCC patients in P- I treatment, which is primarily excreted through the liver (12).
Pre-clinical data from mouse models of cancer with hepatitis virus infection are mostly unavailable because hepatitis viruses do not infect mice. Although mouse models do exist to mimic viral hepatitis, it was difficult to predict whether P- I treatment would cause severe complications such as hepatocyte destruction owing to drug effect on infected hepatocytes.
There is a difference in the mechanism of carcinogenesis between HBV and HCV. Liver inflammation and hepatocyte proliferation driven by host immune responses in hepatocytes with HBV infection are recognized as driving forces of liver cell transformation (19). In the absence of a cytopathic effect in hepatocytes infected with HBV, the oncogenic role of HBV might involve a combination of direct and indirect effects of the virus during the multistep process of liver carcinogenesis (20). The higher level of damage to hepatocytes with chronic HCV infection could be explained by the imbalance in the microenvironments and cytokines of livers infected with the HCV, leading to increased inflammation and cell turnover, which ultimately causes cirrhosis (21). A meta-analysis of three phase III randomized controlled trials demonstrated higher survival benefit to patients positive for HCV than for HBV (15).
This study has some limitations. The first is that it is a retrospective single-center study, which limited the inclusion of some variables because of lack of data. Second, the etiology of an abnormal liver function test is challenging to define in an HCC study population because an abnormal liver function test result may be related to factors such as tumor progression or drug-related toxicity. Third, we did not monitor HBV DNA level and HCV RNA level for each case. Most of the trials we analyzed in this article were conducted before the confirmation by recent studies that effective viral suppression could reduce the risk of HCC (22). Finally, a small number of patients were treated in different trials and at varying doses and achieved different outcomes, which precludes more robust statistical analyses and warrants caution when interpreting our findings. Furthermore, we were unable to conduct an effective multivariate analysis, given the small sample size.
Chronic viral hepatitis did not independently affect the efficacy and feasibility of the P-I treatment, although it was one of the most significant factors that contributed to the pathogenesis of HCC. The poor outcomes and limited treatment options for advanced HCC patients emphasize the need for new approaches. P-Is that are based on an enrichment design such as specific histologic characteristics and particular biomarkers are associated with a higher probability of clinical benefit than those that are not (23). Advanced HCC patients, independent of the status of chronic viral hepatitis, could be feasible for P-Is and might clinically benefit from treatment in the P-Is.
This study was performed under the Declaration of Helsinki with regard to ethical principles for research involving human subjects. The NCCH institutional review board approved this study's design, and the study was performed under its guidelines.
TK and SK contributed to the conceptualization, methodology, investigation, writing the original draft, and revision the manuscript. TS, YF, CM, YS, TO, and NY contributed to the investigation and revision of the manuscript.
TS received consulting fees from Takeda, The Consortium on Harmonization of Institutional Requirements for Clinical Research (CHAIR), Hong Kong and HKSAR; and funds for research expenses from Bristol-Myers Squibb, Daiichi-Sankyo, Takeda-Millennium, FivePrime, PharmaMar, 3D Medicine Symbio Pharmaceuticals, and Chordia Therapeutics. TO received consulting fees from Eli Lilly, Dainippon Sumitomo Pharama, Taiho Pharamaceutical Co and BMS and funds for research expenses from Eli Lilly, Eisai, Dainippon Sumitomo, Taiho Pharamaceutical Co, BMS, Kowa K.K., Kyowa Hakko Kirin Co., Merk Serono Co, Ono Pharamceutical Co., Ono Pharmaceutical Co., Novartis, Pfizer Jana Inc., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co. Ltd., Yakuruto Honsya Co. Ltd., Asrtra Zeneka K.K. and Zeria Pharmaceutical Co. Ltd., and honoria from Eli Lilly, Eisai, Dainippon Sumitomo Pharama, Taiho Pharamaceutical Co, BMS., Ono Pharmaceutical Co., Novartis, Pfizer Jana Inc., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co. Ltd., Yakuruto Honsya Co. Ltd., Asrtra Zeneka K.K., Zeria Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Nippon Chemifa Co. Ltd., EA Pharma Co. Ltd., Fujifilm RI Pharma Co. Ltd., Celgene k.k., Teijin Pharma CO. Ltd., MSD, Shire, Abbvie and Takeda Pharmaceutical Co. Ltd. NY received consulting fees from Eisai, Takeda, Otsuka, Boehringer Ingelheim and Cimic and funds for research expenses from Quintiles, Astellas, Chugai, Esai, Taiho, BMS, Pfizer, Novartis, Daiichi-Sankyo, Bayer, Boehringer Ingelheim, Kyowa-Hakko Kirin, Takeda, ONO and Janssen Pharma and honoraria from BMS, Pfizer, AstraZeneca, Eli Lilly, ONO and Chugai.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Rubi Mukoyama, Keiko Kondo, and Hiroko Hosoi for collecting the clinical data.
1. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and National level: results from the global burden of disease study 2015. JAMA Oncol. (2017) 3:1683–91. doi: 10.1001/jamaoncol.2017.3055
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. (2011) 61:69–90. doi: 10.3322/caac.20107
3. Skolnick AA. Armed with epidemiologic research, China launches programs to prevent liver cancer. JAMA. (1996) 276:1458–9. doi: 10.1001/jama.1996.03540180012005
4. Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. (2016) 122:1312–37. doi: 10.1002/cncr.29936
5. Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology. (2010) 53:39–43. doi: 10.1159/000252782
6. The Research Group for Population-based Cancer Registration in Japan. Cancer incidence and incidence rates in Japan in 1995: estimates based on data from nine population-based cancer registries. Jpn J Clin Oncol. (2000) 30:318–21. doi: 10.1093/jjco/hyd081
7. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
8. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
9. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 389:56–66. doi: 10.1016/S0140-6736(16)32453-9
10. Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2015) 16:859–70. doi: 10.1016/S1470-2045(15)00050-9
11. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. (2018) 379:54–63. doi: 10.1056/NEJMoa1717002
12. Yamamoto S, Kondo S. Oral chemotherapy for the treatment of hepatocellular carcinoma. Expert Opin Pharmacother. (2018) 19:993–1001. doi: 10.1080/14656566.2018.1479398
13. Cantarini MC, Trevisani F, Morselli-Labate AM, Rapaccini G, Farinati F, Del Poggio P, et al. Effect of the etiology of viral cirrhosis on the survival of patients with hepatocellular carcinoma. Am J Gastroenterol. (2006) 101:91–8. doi: 10.1111/j.1572-0241.2006.00364.x
14. Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. (2012) 48:1452–65. doi: 10.1016/j.ejca.2011.12.006
15. Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. (2017) 35:622–8. doi: 10.1200/JCO.2016.69.5197
16. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. (2017) 67:999–1008. doi: 10.1016/j.jhep.2017.06.026
17. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
18. Ikeda M, Okusaka T, Mitsunaga S, Ueno H, Tamai T, Suzuki T, et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. (2016) 22:1385–94. doi: 10.1158/1078-0432.CCR-15-1354
19. Riviere L, Ducroux A, Buendia MA. The oncogenic role of hepatitis B virus. Recent Results Cancer Res. (2014) 193:59–74. doi: 10.1007/978-3-642-38965-8_4
20. Zhao LH, Liu X, Yan HX, Li WY, Zeng X, Yang Y, et al. Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat Commun. (2016) 7:12992. doi: 10.1038/ncomms12992
21. Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. (2006) 80:1197–213. doi: 10.1189/jlb.0506297
22. Zhang W, Wang X, Wang Y, Zhao X, Duan W, Wang Q, et al. Effective viral suppression is necessary to reduce hepatocellular carcinoma development in cirrhotic patients with chronic hepatitis B: Results of a 10-year follow up. Medicine. (2017) 96:e8454. doi: 10.1097/MD.0000000000008454
Keywords: hepatocellular carcinoma (HCC), phase I clinical trials, hepatitis virus, efficacy, feasibility
Citation: Koyama T, Kondo S, Shimizu T, Fujiwara Y, Morizane C, Sakamoto Y, Okusaka T and Yamamoto N (2019) Impact of Hepatitis Virus on the Feasibility and Efficacy of Anticancer Agents in Patients With Hepatocellular Carcinoma in Phase I Clinical Trials. Front. Oncol. 9:301. doi: 10.3389/fonc.2019.00301
Received: 05 February 2019; Accepted: 01 April 2019;
Published: 18 April 2019.
Edited by:
Ruth Plummer, Newcastle University, United KingdomReviewed by:
Min Hee Kang, Texas Tech University Health Sciences Center, United StatesCopyright © 2019 Koyama, Kondo, Shimizu, Fujiwara, Morizane, Sakamoto, Okusaka and Yamamoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunsuke Kondo, c2hrb25kb0BuY2MuZ28uanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.