
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 08 April 2019
Sec. Cancer Epidemiology and Prevention
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00238
 Paramita Dasgupta1
Paramita Dasgupta1 Peter D. Baade1,2,3*
Peter D. Baade1,2,3* Joanne F. Aitken1,4,5
Joanne F. Aitken1,4,5 Nicholas Ralph5,6,7
Nicholas Ralph5,6,7 Suzanne Kathleen Chambers1,2,8,9
Suzanne Kathleen Chambers1,2,8,9 Jeff Dunn1,5,9
Jeff Dunn1,5,9Background: Previous reviews of geographical disparities in the prostate cancer continuum from diagnosis to mortality have identified a consistent pattern of poorer outcomes with increasing residential disadvantage and for rural residents. However, there are no contemporary, systematic reviews summarizing the latest available evidence. Our objective was to systematically review the published international evidence for geographical variations in prostate cancer indicators by residential rurality and disadvantage.
Methods: Systematic searches of peer-reviewed articles in English published from 1/1/1998 to 30/06/2018 using PubMed, EMBASE, CINAHL, and Informit databases. Inclusion criteria were: population was adult prostate cancer patients; outcome measure was PSA testing, prostate cancer incidence, stage at diagnosis, access to and use of services, survival, and prostate cancer mortality with quantitative results by residential rurality and/or disadvantage. Studies were critically appraised using a modified Newcastle-Ottawa Scale.
Results: Overall 169 studies met the inclusion criteria. Around 50% were assessed as high quality and 50% moderate. Men from disadvantaged areas had consistently lower prostate-specific antigen (PSA) testing and prostate cancer incidence, poorer survival, more advanced disease and a trend toward higher mortality. Although less consistent, predominant patterns by rurality were lower PSA testing, prostate cancer incidence and survival, but higher stage disease and mortality among rural men. Both geographical measures were associated with variations in access and use of prostate cancer-related services for low to high risk disease.
Conclusions: This review found substantial evidence that prostate cancer indicators varied by residential location across diverse populations and geographies. While wide variations in study design limited comparisons across studies, our review indicated that internationally, men living in disadvantaged areas, and to a lesser extent more rural areas, face a greater prostate cancer burden. This review highlights the need for a better understanding of the complex social, environmental, and behavioral reasons for these variations, recognizing that, while important, geographical access is not the only issue. Implementing research strategies to help identify these processes and to better understand the central role of disadvantage to variations in health outcome are crucial to inform the development of evidence-based targeted interventions.
Worldwide, prostate cancer is the second most commonly diagnosed invasive cancer and the fifth leading cause of cancer deaths in men (1). Prostate cancer is especially prevalent in developed regions including Australia, United States and Western Europe, with incidence rates varying more than 25-fold between high and low incidence countries (1, 2). In contrast, mortality rates are higher in less developed countries especially among predominantly black Caribbean and sub-Saharan African populations (1, 2). These wide variations in the global burden of prostate cancer reflect the impact of country-specific differences in Prostate Specific Antigen (PSA) testing practices (2, 3), in addition to westernized diet, sedentary lifestyle and obesity (2), genetic differences (2, 3), dissimilar health systems, population life expectancy and competing causes of mortality (4).
Wide and persistent geographical disparities in prostate cancer incidence and mortality (5), treatment (6) and survival have been identified globally (7). Our previously published review (4) of international patterns in disparities along the prostate cancer continuum from detection to incidence, staging, treatment, survival and mortality identified a consistent pattern of poorer outcomes with increasing residential disadvantage and for rural residents. Specifically, men from rural and socioeconomically disadvantaged areas had lower rates of PSA testing, incidence, survival, and access or use of services, but also more aggressive disease at diagnosis and higher mortality rates (4). Although it is likely that the country-specific factors mentioned above also contribute to these within-country differences, a complex interplay of clinical, social, environmental, and behavioral factors are probably also involved (4–7).
Our earlier review (4) was limited in that it was not systematic, considered only a limited number of reference databases, and, given the extent of recent literature on this topic, no longer provides a contemporary summary of international patterns in prostate cancer. Here, we update and extend this work by systematically reviewing current international evidence on the extent of geographical variation in prostate cancer outcomes, with a focus on patterns by rurality and area-level socioeconomic disadvantage. Cancer outcomes along the continuum from PSA testing to diagnosis to mortality and survival are included. This review is intended to identify gaps in knowledge, formulate strategic research priorities and inform the development of evidence-based interventions to address observed inequities.
No patients were directly involved in the development of the research questions, choosing the outcome measures of interest, study design and implementation or interpretation of results.
The studies included in this systematic review used a range of definitions to define rurality and residential disadvantage. For the purposes of this review, “urban” areas were those described as “urban,” “metropolitan,” or “major cities,” with the remainder being categorized as “rural” areas. Advantaged areas were those described as “affluent” or “advantaged,” with the remainder being “disadvantaged.” Where studies reported on geographical variations by intermediate categories, such as suburban groups or quintiles of area-disadvantage, only comparisons between most “extreme” rural and/or most disadvantaged to the least rural and/or disadvantaged categories (such as very remote vs. metropolitan and quintile one vs. quintile five), are presented here.
This review was conducted according to published PRISMA guidelines for conducting systematic reviews (8). Clinical questions to guide the review were clearly defined following a structured framework and agreed upon before commencing the review process. The review addressed six key questions on variations by residential location encompassing the key themes of PSA testing, prostate cancer incidence, tumor characteristics, survival, access to treatment services and prostate cancer mortality (Table 1).
These themes and the relevant questions were repeated when considering differences by residential disadvantage.
The electronic databases: PubMed, EMBASE, CINAHL, and Informit were systematically searched for all indexed articles from 1 January 1998 to 30 June 2018. Final searches were undertaken on 02 July 2018. The Web of Science database was used for cited reference searches.
Search strategies were based on keywords and subject headings to reflect the review aim (Supplemental File 1). Key terms related to prostate cancer, e.g., “prostate neoplasms”; and “prostate cancers” were combined with terms pertaining to geographical aspects and area-based disadvantage, including “geographic inequalities,” “spatial,” “health services accessibility” and “rural health”; and outcome measures of interest, such as “PSA screening,” “incidence,” “stage,” “mortality,” “survival,” “prostatectomy,” “brachytherapy,” and “therapy.” Additional synonyms reflecting each of the key terms were also included.
Studies were eligible if they met the following inclusion criteria:
1) the population included adult male prostate cancer patients or focused on a prostate cancer specific sub-group; and
2) the outcome measure was PSA testing, prostate cancer incidence, stage at diagnosis, survival, access and use of treatment services, or prostate cancer mortality
3) was a quantitative study on geographical differences by:
a) location of residential area (rural vs. urban, non-metropolitan vs. metropolitan comparisons); and/or
b) socioeconomic status of residential location
The scope of the review was limited to English language peer-reviewed original research articles. Reviews, editorials, books, conference abstracts and commentaries were excluded, although when identified through the systematic searches their reference lists were examined for relevant articles.
After removing duplicates, the titles and abstracts of all articles generated by the queries were independently reviewed by two reviewers (first PD, second PB) to assess their relevance to the clinical question(s). Full text versions of all articles of potential relevance were then retrieved for more detailed evaluation by one reviewer (PD). During this process, articles were categorized as “include” or “exclude” with reasons for exclusion being noted.
The quality of all included articles was evaluated using pre-defined criteria (9, 10). Quantitative studies were assessed using a modified (11) version of the Newcastle-Ottawa Scale (NOS) (10), a risk of bias assessment tool for non-randomized studies. The NOS assesses cohort studies on six items over five broad perspectives (a) selection bias; (b) ascertainment of exposure and/or confounders; (c) outcome assessment; (d) follow-up and (e) adjustments for residual confounders (two items). Case-control studies are evaluated on selection of cases and controls, ascertainment of exposure, response rate, adequacy of case-definition and accounting for residual confounders.
The NOS was further modified, by incorporating three additional items evaluating (a) study attrition (missing data), (b) statistical methods, and (c) data presentation, based on published checklists (9). Studies were scored according to the extent that they met each of the nine assessed criteria (Supplemental File 2) using an ordinal scale to rate the risk of bias as 0 (high), 1 (intermediate) and 2 (low) and the individual item scores then summed to give a total quality score. Each article was then assigned a total score (range of 0–18) which was categorized as “high” (12–16), “moderate” (9–13.5), or “low” (< 9) quality. Studies were not excluded based specifically on their quality rating.
Studies were also classified according to the published levels of evidence for quantitative observational studies from the Australian National Health and Medical Research Council (NHMRC) (17) in decreasing order of strength as Level I, Level II, Level III-1, Level III-2, Level III-3 or Level IV.
Information on study features including bibliography (author(s), year, title), setting, time period, design, population (such as sample size, eligibility criteria), outcome measures, assessed geographical unit(s), measure used to define urban/rural residence and/or area disadvantage (if applicable), relevant statistical results and key findings were extracted from all included articles by one reviewer. A selection of these were randomly checked by another reviewer. Disagreements were resolved by discussion until consensus was reached. Any discrepancies were rechecked with the original source.
The process of identifying relevant articles for the review is shown in a PRISMA diagram (Figure 1). A total of 2,568 articles were identified across combined databases with another 54 citations found through other sources (citation searches, reference lists, other reviews) After removing duplicates, 1,418 articles remained of which 1,015 were excluded after initial scanning of the title/abstracts. Following assessment of the remaining 403 full-text articles, 169 met the inclusion criteria for the review. Of the 234 excluded articles, most (more than 95%) did not specifically present quantitative statistics on geographical variations in the outcomes of interest, and/or used only individual-level instead of area-level measures of socioeconomic disadvantage.
Around half (18) of the included studies were from the United States (USA), followed by Australia (19), the United Kingdom (UK, 24), Canada (5), Spain (4), the Netherlands (3), New Zealand (3), Denmark (2), France (2), and Ireland (2). Seven more studies were also from Europe (one each from Belgium, Finland, Germany, Greece, Lithuania, Sweden, and Switzerland), five from Asia (two from Japan, one each from Iran, South Korea and Taiwan), two from other parts of North America (French West Indies, Puerto Rico), one from South America (Colombia), one from Africa (Egypt) and one including Australia and Canada (Supplemental File 3).
Data for 157 (93%) of the included studies were sourced from administrative collections, such as population-based state or national cancer registries, official census and mortality records or non-population based clinical databases. The remaining 12 studies involved medical record reviews and cross-sectional surveys. With respect to study design, the majority (160, 95%) were observational cohort studies, eight were cross-sectional and one was a case-control study.
Of the 169 studies, 46 reported rural/urban differences in at least one of the outcome measures of interest, 82 assessed variations by residential disadvantage while 41 assessed both these measures. Overall, 87 studies looked at differences by residential rurality and 123 by disadvantage, respectively. Studies varied widely in the definition of urban or rural residence. While more than half (62%) of the relevant 87 studies used standardized definitions, such as the USA Department of Agriculture's Rural Urban Continuum Codes (RUCC) and Rural Urban Commuting Area Codes (RUCA) (12, 20) or the Accessibility/Rurality Index of Australia (ARIA+) (13) others defined non-urban and urban areas based on distances to services, degree of urbanization, population size or density. Three studies did not provide detailed information regarding how the geographical classification was derived (Supplemental File 3).
A range of measures were also used to define residential disadvantage (Supplemental File 3). More than three-quarters (76%) of the 63 non-USA-based studies used a standardized definition, such as the Australian Index of Relative Socio-Economic Disadvantage (IRSD) (14), Scottish Index of Multiple Deprivation (SIMD) (15), French Deprivation Index (16), or the UK-based Index of Multiple Deprivation (IMD) (21), Carstairs (22), or Townsend Deprivation Indexes (23). Of the 60 USA-based studies, 36 (60%) used various area-based indicators including median income, education or poverty while the remaining 24 studies created study-specific composite scores derived from these area-based measures.
Around half (24) of all the included studies were graded as high quality and the remaining 85 as moderate quality. There were no studies assessed as low quality. The median quality score was 13.4 (interquartile range of 12–15). Contributing to this higher quality was that most studies (138, 82%) used population-based representative data sources, such as cancer registries and mortality records. The key limiting factors were that 31 (18%) studies did not use a population-based representative sample, while more than a third (61, 36%) did not adjust for confounders or presented only age-adjusted estimates by residential location. Although cancer registries and mortality databases provide representative, reliable and objective data, they present limited information on all plausible prognostic factors. The lack of information on the extent of follow-up also lowered study quality. No study provided Level I evidence, nearly three-quarters (71%) gave Level II evidence, one quarter (24%) Level III evidence and 5% Level-IV evidence (Supplemental File 3).
Studies are summarized below (Figures 2–5; Tables 2–7) according to clinical questions within each of the key themes: (1) PSA testing, (2) incidence, (3) tumor characteristics, (4) survival, (5) access and use of services, and (6) mortality outcomes. Within each clinical question, results are presented separately for variations by rurality and residential disadvantage, further grouped by country within each of the continents (Africa, Americas, Asia, Europe and Oceania). Since several studies reported on multiple outcomes, some studies are repeated either in the same table or across multiple tables.
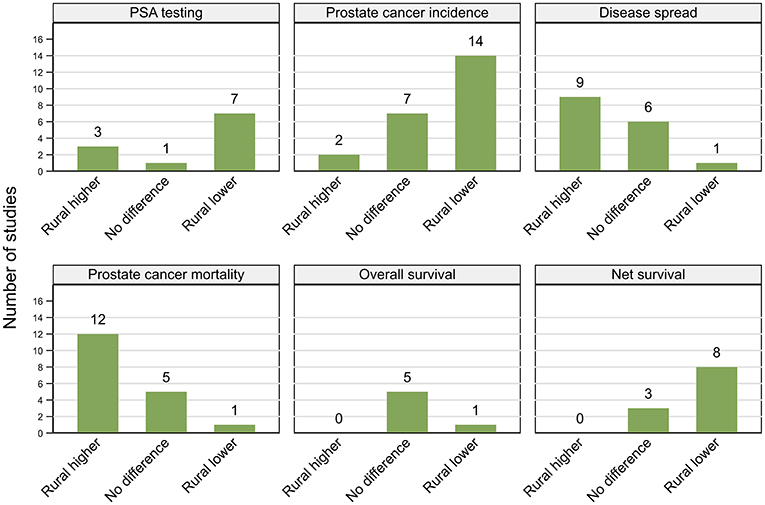
Figure 2. Summary of key patterns by residential rurality for PSA testing, prostate cancer incidence, disease spread, mortality, overall, and net survival.
When multivariate analyses were conducted (136 of 169 studies), only the fully adjusted estimates are reported because many such studies only reported those results. Given the wide heterogeneity among studies in terms of the definitions of geographical measures, time periods, study populations, statistical methods and data presentation, only general trends within and among studies as well as between and within countries have been described. Given these limitations, any summary patterns have been deliberately interpreted with caution. The emphasis is on describing whether there was evidence of geographical variations in the relevant outcome by the reported measure of rurality and/or disadvantage, and if so, the direction and magnitude of the effect.
Results were not consistent across the 11 included studies (Figure 2; Table 2), both within and between countries. All studies included men aged at least 40 years. Based on the 2012 Behavioral Risk Factor Surveillance System (BRFSS) survey, two studies from the USA (one high, one moderate quality), both reported that asymptomatic non-Hispanic white men from rural areas were 11% more likely to undergo PSA testing than those from urban areas, although the geographical differences were not significant among other ethnic groups (26, 27). While urban residence was associated with lower prevalence of PSA testing in a high quality cross-sectional study using the 2010 BFRSS survey (19), the reverse pattern (higher PSA testing in urban areas) was reported by an earlier moderate-quality study, based on the 2001 BRFSS survey (25). Men from urban areas were also more likely to have repeated PSA tests within 3 years in another USA-based moderate quality study (28).
A moderate quality study from the UK found no differences by urban/rural location in PSA testing (23), while three other (moderate quality) studies reported higher rates of PSA testing among men from urban areas in Australia (31, 32) and New Zealand (33). Consistent with this, urban residents were 10% more likely to have ever had PSA testing and 20% more likely to have been tested within the past 2 years from 1992 to 2012 in Switzerland (30), while men living in regions with higher ratio of specialists to general practitioners (GPs) were around eight times more likely to have PSA testing in Canada (29) (both studies moderate quality).
All four (two high, two moderate quality) of the included studies reported that PSA testing was more common in affluent areas (Figure 2; Table 2). Among 212,039 men aged 40–69 years in the UK, men living in most disadvantaged areas were 16% less likely to have a PSA test between 2006 and 2010 than those from affluent areas (23). Similar patterns were reported by one Scottish (35) and two Canadian studies (29, 34).
Of the 23 studies included, 14 (six high, eight moderate quality) reported higher prostate cancer incidence rates in urban areas (25, 32, 36, 38, 40–44, 46, 47, 49, 52, 53), two (moderate) higher rates in rural areas (50, 55) and seven (one high, six moderate) no urban rural differences (31, 37, 39, 45, 48, 51, 54) (Figure 2; Table 3). Six studies used a mixture of data collected both prior to and after the widespread use of PSA testing, which combined with varying time-periods for analysis, could lead to conflicting patterns (31, 32, 39, 49, 51, 55).
While eight of the USA-based studies, reported higher incidence rates among urban residents (or those from areas with higher density of urologists) (25, 38, 40–44, 46), two found no geographical differentials (39, 45). Similarly, two Australian studies reported no significant differences (31, 54), three studies found higher prostate cancer incidence among urban males (32, 52, 53) and one study recorded the reverse pattern (55). In Denmark, urban men had higher incidence rates from 1994 to 2003 (47), but no differential was evident from 2004 to 2008 (48).
Prostate cancer incidence was consistently higher among men from affluent areas across 25 (11 high, 14 moderate quality) of the 30 included studies with only a single (moderate) USA-based case-control study reporting the reverse pattern (66) (Figure 3; Table 3). The remaining four (two moderate, two high quality) studies from the USA (57), UK (51), Japan (70), and French West Indies (67) found no differentials by residential disadvantage.
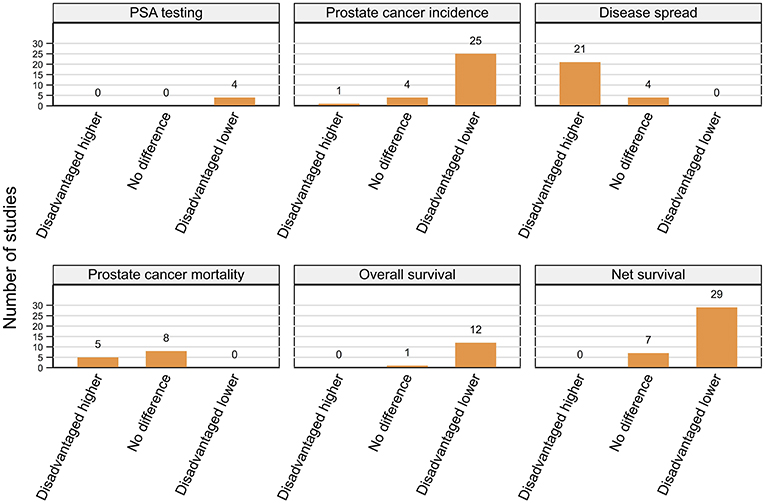
Figure 3. Summary of key patterns by residential disadvantage for PSA testing, prostate cancer incidence, disease spread, mortality, overall, and net survival.
Analysis of the 1988–1992 Surveillance, Epidemiology, and End Results (SEER) data showed that residents of affluent areas had higher prostate cancer incidence than men from deprived areas (60), while rates decreased monotonically with increasing residential disadvantage in the USA from 2005 to 2009 (56). Similar patterns were reported by nine other USA-based (41, 46, 58, 59, 61–65), 11 European, (15, 16, 35, 48, 49, 71–76) and one study each from Australia (53), Puerto-Rico (68), and Iran (69).
There were some variations in the definition of advanced prostate cancer with three studies each basing their classification on tumor size (77, 78, 85) or prostate cancer risk groups (79, 86, 100) and one on pathological Gleason score (90). All remaining studies used a standard cancer staging system (such as the SEER Summary stage or TNM) with Stage I-II cancers consistently referred to as localized disease, Stage III as regional disease and distant/metastatic (Stage IV) cancers as advanced (18, 24, 25, 39, 58, 64, 84, 88, 92, 102), although some collectively categorized both regional and distant cancers as advanced disease (21, 41, 55, 80–83, 87, 89, 91, 93–99, 101).
Findings were not consistent across the 16 included studies (Figure 2; Table 4), with nine (four high, five moderate quality) reporting more advanced tumor characteristics among rural men and six (three high, three moderate) no geographical differentials. However, one high quality study from New Zealand found that men who lived closer to cancer centers were more likely to have advanced disease (87). There were no clear patterns in study findings with characteristics, such as sample size or time period. While three Australian studies (83–85) found no evidence of geographical differentials, three others reported that rural men were more likely to be diagnosed with advanced disease (24, 55, 86). Further discrepancies in findings were evident across the nine USA-based studies, with six reporting more advanced disease among rural residents (25, 77, 81, 82) and those with poorer access to urologists (41, 79), whereas three others found no significant differences in the rates of advanced disease between urban and rural men (39, 78, 80).
A consistent pattern of advanced stage at diagnosis among men from disadvantaged areas was evident across 21 (15 high, six moderate quality) of the 25 included studies (Figure 3; Table 4) despite varying definitions of advanced prostate cancer. Four studies (two high, two moderate), three from the USA (41, 80, 97) and one from New Zealand (87) found no differences by residential disadvantage.
Analysis of 436,251 incident cases from the USA found that males from disadvantaged areas were 1.27 times more likely to be diagnosed with advanced prostate cancer from 2005 to 2009 (88). Similar patterns were reported by 12 other USA-based studies (18, 58, 64, 82, 89–96), although the differential was only evident after 1987 in one instance (64) and among men aged 50–74 years at diagnosis in another study (58). All three studies from Australia (24, 84, 102), four from the UK (21, 99–101), and the single Dutch study (98) also reported positive association between residential disadvantage and being diagnosed with advanced disease.
Any interpretation of patterns and comparability across studies should reflect the type of survival measure used and their respective definitions. Two commonly used measures are overall survival (deaths from all causes) or net survival (includes relative and cancer-specific survival) that is the mortality specifically associated with a cancer diagnosis (186). Patterns are presented separately for overall and net survival.
Of six studies that looked at association between residential rurality and overall survival (Figure 2; Table 5), four (high quality) reported no geographical differentials for men diagnosed with prostate cancer in the USA after controlling for treatment and comorbidities (103–106). Similar patterns were reported by a single (moderate quality) Australian study (108) whereas men living closer to primary care had higher survival in a single (high quality) study from England that did not control for either treatment or comorbidities (107).
However, all four moderate quality studies, one from Denmark (47) and three from Australia (31, 55, 109) that reported poorer prostate cancer relative survival (excess mortality risk of 1.14–2.53, rural vs. urban) for rural residents did not consider comorbidities or treatment, although two did adjust for stage at diagnosis (55, 109). Four (high quality) studies, two Australian (85, 113), and one each from Scotland (112) and Sweden (111) also found consistently lower prostate cancer survival among rural residents. Only one of these studies adjusted for comorbidities (111), two for stage (85, 113) and none for treatment. However, three other studies (one high, two moderate quality), from Australia (114, 115) and the USA (110) found no significant associations with rurality for stage-adjusted estimates. Of the 11 papers in total that reported net survival (relative or prostate-cancer specific survival), only three found no geographical differentials in survival and eight higher survival among urban men (Figure 2; Table 5).
Finally, most of the 17 included papers focused on medium term survival with two reporting survival 10-years after diagnosis (55, 85).
A consistent pattern of poorer overall survival among men diagnosed with prostate cancer while living in disadvantaged areas of the USA was evident across eight (seven high, one moderate quality) (103–106, 116–119) studies (Figure 3; Table 5). Four more studies (three high, one moderate quality), one each from Ireland (120), England (107), Australia (108), and the Netherlands (98) (only among men aged 60–74 years with localized or 60+ years with advanced disease) also reported similar patterns even after adjustment for various combinations of potential explanatory factors, notably stage, treatment and comorbidities (98, 103–106, 108, 116–120). Only one USA-based (high quality) (89) study found no differences by residential disadvantage.
Fifteen studies (seven high, eight moderate quality) reported lower prostate cancer relative survival rates for residents of disadvantaged areas even after adjusting for stage (55, 127, 134) although none controlled for treatment or comorbidities. Six studies were from the UK (22, 126, 127, 129, 131, 132), three from Australia (55, 133, 134) with one each from Colombia (122), France (124), Germany (125), Japan (123), New Zealand (136), and the USA (121). Although the gap in 1-year relative survival for men with prostate cancer from most and least disadvantaged areas in England had narrowed between 1996 and 2013, significant socioeconomic inequalities remained (131, 132). However, two other high quality UK-based studies (128, 130) and one Australian study (moderate quality) (135) found no evidence for differentials in relative survival by residential disadvantage.
Finally, 14 (nine high, five moderate quality) of 18 included studies consistently reported poorer prostate-cancer specific survival among males from disadvantaged areas in the USA (nine studies) (59, 60, 93, 110, 137–141), Australia (two studies) (102, 113), Netherlands (142), Sweden, (111), and Scotland (112) after controlling for diverse explanatory factors. On multivariate analyses, estimated hazard ratios (HR) for increased risk of mortality (poorer survival) ranged from 1.16 to 2.37 (disadvantaged vs. affluent). Three of the four remaining studies (two high, two moderate quality) found no evidence for survival differentials by residential disadvantage in Japan (70), New Zealand (87) and Australia (85) while another Australian study reported that although residential disadvantage was not significantly associated with prostate cancer mortality, male residents of those areas had poorer non prostate-cancer specific mortality (114).
In summary, of 49 included studies, male residents of disadvantaged areas had consistently poorer overall (12 of 13) and net survival (29 of 36) (Figure 3; Table 5) when diagnosed with prostate cancer.
Most studies focused on medium-term survival, with only two (55, 85) following men for longer than 10 years after their cancer diagnosis. Four presented 1-year survival estimates (112, 131, 132, 142).
Treatment of early stage prostate cancer, localized disease or National Comprehensive Cancer Network (NCCN) low to intermediate-risk groups remains controversial with no consensus regarding their optimum management (187, 188). Several treatment types are available depending on clinical features, patient age and preferences. For example, men diagnosed with localized (defined as no identifiable regional lymph nodes or distant metastases) disease have three main options: expectant management (EM) that is monitoring for cancer progression while not having curative therapy, curative surgery typically radical prostatectomy (RP) or radiotherapy (RT), such as brachytherapy (BB) or external beam radiotherapy (EBRT). As such, patterns described below for different treatment types do not necessarily imply adverse outcomes by residential location.
For ease of interpretation we have presented patterns below by different treatment types (Figures 4, 5; Table 6) after an overall summary. No studies reporting geographical variations in use of services for metastatic disease were found.
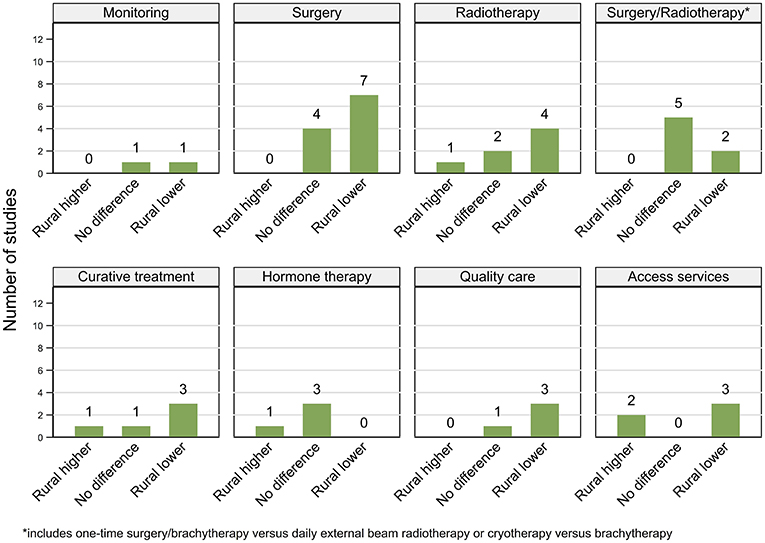
Figure 4. Summary of key patterns by residential rurality for access and use of prostate cancer related services.
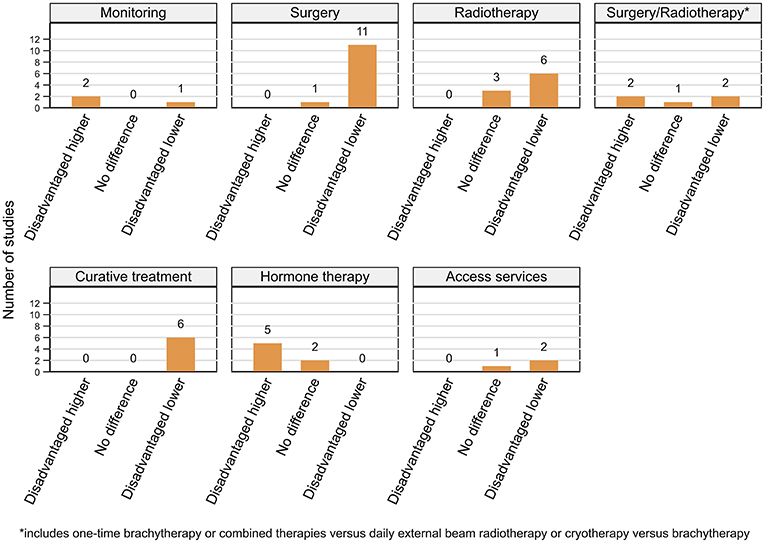
Figure 5. Summary of key patterns by residential disadvantage for access and use of prostate cancer related services.
Twenty-two (11 high, 11 moderate) out of 28 included studies reported geographical variations in access and use of services among men diagnosed with prostate cancer with six (four high, two moderate quality) finding no differences.
Expectant management. While men living furthest away from treating facilities were 8% less likely to have expectant management for very low-risk prostate cancer in the USA from 2010 to 2013 (143), an earlier study reported no differences by residential rurality for localized disease (144).
Radical prostatectomy. Patterns for RP varied with two USA-based (44, 77) and five Australian studies (31, 32, 115, 147, 148) reporting higher rates among urban men or those living closer to major treatment facilities (148). Whereas, two other studies from the USA (144, 145) as well as one each from Australia (108) and England (146) reported no geographical differentials.
Radiotherapy. Included studies gave mixed results for differentials in RT rates for localized or early stage prostate cancer in the USA, with two finding no significant differences (144, 145), one higher rates of BB among rural (77) and one among urban residents (44). Rural men were also 83% less likely to receive intensity-modulated RT (IMRT) for localized prostate cancer (149). Increasing distance from radiation centers in England was associated with lower RT among men diagnosed with prostate cancer (146), while in Australia RT rates were higher among urban than rural males (150).
Type of curative treatment. A study from South Korea (153) and two from the USA (151, 152) found no association between residential rurality and the type of curative treatment received (i.e., RP vs. RT). Moreover, among men with early stage prostate cancer who underwent curative treatment in the USA, urban and rural residents were about equally as likely to receive a one-time treatment (RP or BB) (77), whereas urban residence was associated with greater use of one-time RP or BB rather than daily EBRT for localized disease in another study (44). However, there was no difference by residential location in the use of combined radiotherapies (EBRT and BB vs. EBRT) for intermediate or high-risk disease (116). Urban men were more likely to undergo cryotherapy for localized disease in a single USA-based study (154).
Any curative treatment. Findings from the USA indicated that rural men were 19 to 25% less likely to receive any curative treatment for localized (151) or early-stage (77) disease, whereas a single state-based study found no geographical differences in receipt of curative treatments for localized disease in Wisconsin, USA (152). However, rural residents were more likely to undergo treatment than active surveillance for very low-risk disease (155). Finally, urban men were around two times more likely to undergo curative treatment in one Australian study (86).
Hormone therapy. A consistent pattern of no geographical differentials in hormone therapy (HT) was reported by two USA-based (78, 144) and one South Korean study (153), although in Australia rural men were 36% more likely to undergo orchiectomy (147).
Quality of care. Although one USA-based study found that urban men were more likely to be treated at comprehensive care facilities, no geographical differentials were evident in the timeliness or quality of their care except for receipt of recommended RT dosage (78). Urban residents were more likely to be treated for high-risk disease at academic centers in the USA (156) and by high-volume surgeons or private hospitals in Australia (85).
Access to care. Two USA-based studies reported poorer access to treatment centers among rural men (78, 106), while in Australia, improving access to RT facilities increased its uptake among rural prostate cancer patients (159). Finally, rural residents were more likely than their urban counterparts to undergo prostate cancer-related treatment at larger more comprehensive RT facilities (157) or established centers for robotic surgery rather than nearest facilities (based on travel times) in England (158).
A clear and persistent pattern of variations in the access and use of prostate cancer related services by residential disadvantage was evident across 24 (11 high, 13 moderate quality) of 27 included studies with three (high quality) reporting no difference.
Expectant management. One USA-based study found that residents of affluent areas (vs. disadvantaged) with low-risk disease were two times more likely to be under active surveillance (a strategy of close monitoring, with intent of curative treatment on disease progression) than watchful waiting (monitoring and treating symptoms with palliative intent) (160). By contrast one study from the UK (100) and one from the Netherlands (98) reported that men aged below 60 years from disadvantaged areas were more likely to be under expectant management for low-risk or localized disease, respectively.
Radical prostatectomy. Four studies from the USA reported that residents of affluent areas had higher rates of RP for localized (144, 145), intermediate-risk (104) and non-metastatic prostate cancer (162), while one found no differences (161). Men from disadvantaged areas were consistently less likely (range 17–44% vs. affluent) to undergo RP in the UK (four studies) (99, 100, 146, 163), Australia (two studies) (108, 147) and the Netherlands (98).
Radiotherapy. Higher residential advantage was consistently associated with greater RT usage (OR 0.32–0.85 disadvantaged vs. affluent) in the USA (145, 162) and UK (99, 146, 163) with one USA-based study finding no differences (161). Males from affluent areas in the USA were more likely to receive IMRT for localized disease (149). However, rates of adjuvant RT after surgery did not vary by residential disadvantage in one USA-based (164) and one Canadian study (165).
Type of curative treatment. While one study found that men from disadvantaged areas were less likely to receive RT than surgery for localized prostate cancer in the USA (166), another found no significant differentials by residential disadvantage (167). However, affluent residents had higher usage of combined EBRT and BB (vs. EBRT) for intermediate or high-risk disease (116) and were more likely to undergo BB than cryotherapy for localized disease (154). In the Netherlands, men aged 60–74 from affluent areas were significantly more likely to have one-time BB, whereas higher receipt of EBRT was associated with living in disadvantaged areas, for those aged below 60 years (98).
Any curative treatment. A consistent pattern of men living in disadvantaged areas being 13 to 40% less likely to receive curative treatment when diagnosed with prostate cancer of varying stage or risk group was evident across five studies from the USA (77, 89, 155, 167, 168) and one from England (163).
Hormone therapy. By contrast, residents of disadvantaged areas had higher rates of HT in three studies from the USA (89, 161, 168) and one each from England (163) and Australia (147) with no differentials found in the Netherlands (98). One USA-based study found no association between residential disadvantage and secondary HT after primary curative treatment for non-metastatic prostate cancer (169).
Access to care. Men from affluent areas were more likely to travel beyond their closest treatment centers to larger established centers in England especially those offering robotic surgery (158) or innovative radiation therapies, such as IMRT or proton beam therapy (157). Finally, there were no differences in post-surgery referral rates to radiation oncologists for high-risk disease between affluent and disadvantaged areas in Ontario, Canada (165).
Eleven (five high, six moderate quality) of the 18 included studies consistently reported higher prostate cancer mortality rates among rural residents (25, 31, 32, 45, 172–177, 181), one (moderate) the reverse trend (179) and four (one high, three moderate quality) no differences (171, 178, 180, 182) (Figure 2; Table 7). A (high quality) study by Lagace et al. (170) found higher prostate cancer mortality rates among rural men in Canada and a trend toward higher death rates in rural areas for Australia. Another high quality study also reported higher prostate cancer mortality rates outside urban areas in Australia, although the difference between most extreme remote and urban category was not significant (52).
In the USA, men from rural areas had 3–15% higher prostate cancer mortality rates than those from urban areas across four studies (25, 45, 176, 177) with four more reporting similar patterns (172–175). Although the study by Zhand et al. (178) found no rural urban differences in prostate cancer mortality rates in the Mississippi Delta region, both urban and rural rates were higher compared to corresponding urban and rural areas for other regions in the USA.
Five (two high, three moderate quality) (62, 174, 177, 178, 184) of the seven included studies from the USA reported higher prostate cancer mortality rates in disadvantaged areas with two (high quality) (173, 183) reporting no difference (Figure 3; Table 7). By contrast, all six studies (two high, four moderate) from other countries, one each from Puerto Rico (68), Japan (70), Belgium (180), Finland (72), Spain (185), and Scotland (35) reported no difference. A noteworthy point is that the significantly higher prostate cancer mortality rates among disadvantaged men in Texas between 1996 and 2004 reported by Wan et al. (184) only held for the smaller census block groups and tract-level geographical units of the USA-census, with the effect reversing for larger county-level areas.
Of the 169 articles included, 34 (20%) presented estimates of rurality, adjusted for residential disadvantage, for at least one of the considered outcomes. The proportion of studies including these adjusted estimates varied for each outcome, ranging from 11% (2 of 18) for prostate cancer mortality, 18% for PSA testing (2 of 11) and prostate cancer incidence (4 of 23), 31% for spread of disease (5 of 16), 36% for access and use of services (10 of 28), 65% for net survival (7 of 11) and 100% for overall survival (6 of 6).
The majority of the studies including adjusted estimates were from the USA, followed by Australia and Canada (Supplemental File 4). However, there was wide heterogeneity across studies in the covariates included in the statistical models; this does limit comparisons across them. In addition, only two studies presented both unadjusted and adjusted estimates by rurality, hence we cannot reliably assess the effect of rurality after controlling residential disadvantage. Nevertheless, after adjustment for residential disadvantage, there was a consistent pattern for rurality to remain independently associated with geographical variations in prostate cancer incidence, net survival and access to services (Figure 6), and for no independent association with overall survival. There were no consistent patterns for PSA testing, mortality or advanced spread of disease.
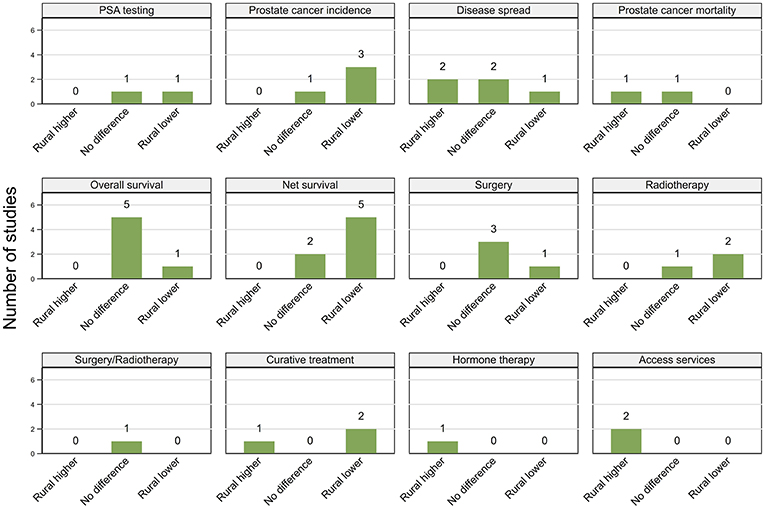
Figure 6. Summary of key patterns by rurality from studies that also adjusted for residential disadvantage.
Overall, for most outcomes except advanced spread of disease and prostate cancer mortality, there appeared to be more consistent evidence of an independent effect for residential disadvantage than for rurality (Figure 7).
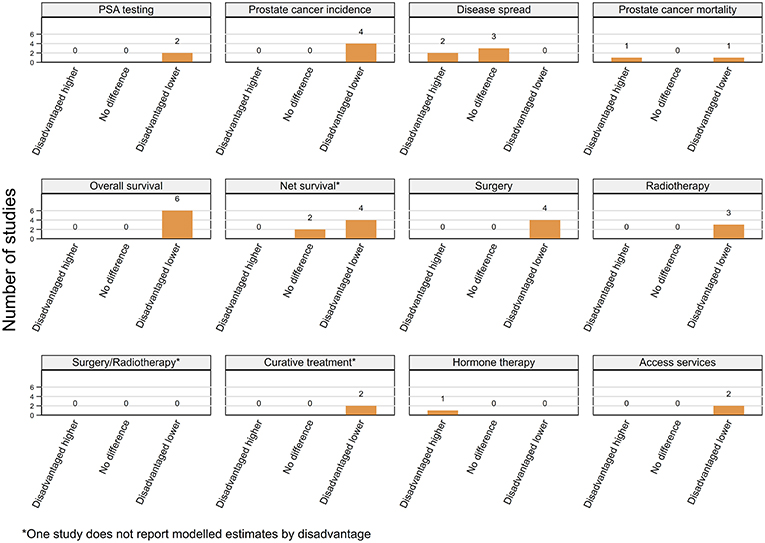
Figure 7. Summary of key patterns by residential disadvantage from studies that also adjusted for rurality.
In summary, included studies did not provide sufficient evidence to conclude that the urban/rural differential in prostate cancer outcomes was completely accounted for by disparities in residential disadvantage. However, there is a suggestion that residential disadvantage may have a stronger effect on prostate cancer disparities than living in an urban or rural area.
This systematic review found a consistent pattern of differences by residential disadvantage across the prostate cancer continuum from PSA testing to incidence, staging, treatment, survival and mortality. Specifically, compared to residents of affluent areas, men living in socioeconomically disadvantaged areas generally had lower PSA testing and prostate cancer incidence, more advanced spread of disease at diagnosis, poorer survival, and higher mortality. Findings by rurality were less consistent. Where a pattern was observed, it was that men from rural areas had lower PSA testing, incidence and survival, more advanced disease and higher prostate cancer mortality than urban residents. There was also evidence that men from more rural or disadvantaged areas had poorer access and use of prostate cancer-related treatment services than those from urban or advantaged areas.
Although the underlying reasons for these variations are not known, there is widespread consensus that they reflect complex and interacting social, genetic, environmental and behavioral processes that can occur at a range of geographical scales (7, 189). The finding that some observed geographical patterns varied by ethnic status, such as with PSA testing for rurality (26, 27), incidence by area disadvantage (41, 46), while others were consistent, such as incidence by rurality (46) highlights the likely complexity of these processes.
Prostate cancer incidence rates increased sharply with the dissemination of PSA-based testing in the early 1990s (5, 190). It is likely that some of the observed geographical patterns in prostate cancer incidence and survival may reflect geographical variations in PSA testing prevalence. Specifically, men from urban (25, 28–33) and affluent (23, 29, 34, 35) areas had higher PSA testing rates than those from rural or disadvantaged areas, respectively. This has been suggested to be due to differential access to screening services and health care (26, 27, 29, 30), GP attitudes (27, 30, 33, 34), health literacy (26–28, 30), socio-cultural norms (26, 27), and help-seeking behaviors (26–28, 30). There was widespread agreement that the higher prostate cancer incidence (15, 16, 31, 39, 41, 42, 47, 48, 53, 56, 62, 68, 73, 76) and lower advanced stage diagnoses (21, 24, 25, 84, 86, 88) among urban and affluent men reflected increased detection of localized and latent cancers through PSA testing. The impact of PSA testing on prostate cancer mortality risks however remains controversial (2, 191). Variations in potential risk factors and health behaviors reflecting physical and social environment (35, 36, 47–49, 56, 60, 62, 125, 177, 180), health care quality, access or utilization (24, 62, 68, 71, 80, 177, 180) and availability of specialists (41, 79, 174) were other commonly cited reasons for the geographical disparities in prostate cancer incidence, stage and mortality.
Non-clinical factors that have been suggested to contribute to geographical patterns of care include differences in access to and availability of treatment modalities (77, 85, 151, 153, 157, 158), clinician practice patterns (86, 108, 116, 145, 151, 153, 162), patient preferences (77, 86, 98, 108, 151, 153, 155, 162), comorbidities (77, 98, 99, 116, 161, 162), and treatment decision-making processes (98, 99, 161, 162). Variations in treatment could also potentially reflect the managing physician's preferences (99, 161, 192) which, in the absence of a definitive treatment guideline for prostate cancer, strongly influences prostate cancer treatment choices (99, 192, 193).
Although stage at diagnosis impacts prostate cancer survival (60, 89, 125, 127), survival differentials by residential location were evident even after adjustment for stage (55, 85, 93, 102, 110, 113, 127, 134, 137, 138, 140), and in some instances also adjusted for treatment (137, 138, 140). Many of the proposed explanations for the geographical variations in survival were multifactorial and included variations in psychosocial factors (93, 98, 105, 108, 111, 113, 117), comorbidities (98, 104, 113, 125, 126, 135, 139, 142), access to high-quality healthcare (55, 60, 93, 98, 103, 104, 106, 107, 110, 113, 117, 125, 127, 135, 139, 141), intensity of clinical follow-up (55, 102, 127, 139), and compliance with recommended treatments (47, 106, 125). Finally, even after adjusting for stage, there is likely to be a residual confounding by PSA testing, in that the observed survival differentials may reflects the diagnosis of latent prostate cancers through PSA testing rather than a true difference in survival (194).
It is likely that inequalities in access to diagnostic and treatment services is a key factor contributing to the geographical disparities in prostate cancer outcomes. These inequalities are influenced by socioeconomic factors, health care policies and proximity to medical services. For rural residents, a diagnosis of prostate cancer can present unique challenges in obtaining appropriate, high-quality care, including limited local services and long travel distances incurring financial, psychosocial and logistical barriers (195–197). Several of the studies in this review found that increasing travel burden impacted treatment (116, 143, 146, 150, 159, 166). Moreover, high-volume specialists and hospitals which have been associated with rapid adoption of innovative treatments and technologies (149, 158, 196), multidisciplinary care (103, 143, 165) and better prostate cancer-related clinical outcomes (103, 106, 198) are typically concentrated in urban areas (196, 199). The close overlap between rurality and residential disadvantage in countries, such as Australia (85, 195) and the USA (77, 200) and the necessity for repeated visits for specific treatments like EBRT are likely to worsen the impact of accessibility-related barriers.
From the studies that reported results adjusted for both area disadvantage and rurality, there was consistent evidence that the strong impact of residential disadvantage remained even after adjusting for rurality. While its effect was diluted, it appears that the urban/rural differential in prostate cancer outcomes was not completely accounted for by disparities in residential disadvantage. Therefore, living in rural areas constitutes an additional disadvantage in terms of prostate cancer outcomes, over and above residential socioeconomic disadvantage itself. As such, rather than considering these two geographical measures separately, both rurality and socioeconomic disadvantage need to be considered together in terms of their impact on inequalities in burden of prostate cancer.
We are not aware of any previous systematic reviews reporting the international evidence for variations along the continuum of prostate cancer outcomes by rurality and residential disadvantage. Our findings were consistent with two earlier systematic reviews on variations in prostate cancer incidence and mortality by rurality (5) and survival by socioeconomic disadvantage (7), respectively. Two earlier reviews also found persistent geographical disparities across a range of prostate cancer outcomes in the USA (201) and worldwide (4). None of these previous reviews critically assessed studies or included the one-third (58 of 169) of articles included in this review published since 2014.
We found only six studies looking at geographical variations in expectant management (EM) for low-risk disease. Differences in use of EM, and the lack of standardized definitions and/or protocols during the time-periods of the included studies impaired the comparability across studies. Only one study (160) specifically distinguished between two main EM strategies of watchful waiting (palliative treatment for symptomatic progression) and active surveillance (curative treatment on evidence of disease progression).
This review highlighted the need for large, high quality studies that include the whole range of prostate cancer indicators within the one cohort. It is likely this will require an innovative combination of population-wide data linkage studies as well as qualitative investigations. Studies based solely on routinely collected population-based data, such as cancer registries and/or hospital separation databases are unable to provide data on the wide range of patient, tumor, clinician and health-care system related factors that are likely to be potential confounders. Collecting information on the characteristics of the geographical areas, in addition to the characteristics of individuals living in those areas, combined with more rigorous analytical approaches, such as multilevel regression (189), will provide greater insights into the key drivers of this geographical variation.
No studies were found describing geographical differences in use of relatively new treatments, such as robotic surgery, management of metastatic prostate cancer, or treatment making decision processes. It was also unclear whether the underlying factors contributing to the key patterns in prostate cancer outcomes are similar for men living in rural and disadvantaged areas.
Strengths of this review include the comprehensive search of current literature over multiple databases for studies describing international patterns in disparities along the prostate cancer continuum by rurality and residential disadvantage, quality checking of all included articles and graphical summary of results. Given it is now well-recognized that rurality and residential disadvantage interactively affect cancer outcomes, rather than acting in isolation (7, 20, 189), we also specifically assessed the impact of adjusting for residential disadvantage on variations in all six of considered outcomes by rurality and vice-versa.
All of the included quantitative studies were observational in nature, although the majority were population-based cohort studies. Around half were graded as high quality, none were graded as low-quality and the majority presented model-based estimates. Large scale population-based studies are able to identify associations between geographical measures and disparities in outcome measures. The challenge is that these associations in themselves provide only limited information in terms of the underlying reasons for observed disparities and hence how they may be reduced.
A key limitation of this review, similar to previous reviews on similar topics (4, 5, 7) was the wide variability in definition of rurality and residential disadvantage both within (especially for the USA) and across countries. For example, area-level disadvantage was variously defined in terms of a single area-level indicator (typically income), a study-specific combination over several area-based measures or a standardized country-specific composite area-based deprivation index. Moreover, the concept of rurality itself may differ between countries, such as Australia (13) and the USA (20) and smaller, more densely populated, countries, such as the UK (202). The choice of geographical measure used (20, 61, 130) and the scale over which it was measured (20, 102, 134, 184) could have impacted study findings, particularly if, for example, the area-level effects are only present in the more extreme values of the remoteness continuum.
Studies also varied widely in their data collection, analytical and reporting methods, the time frames for both diagnostic and survival intervals and covariates included in the statistical analysis. The wide heterogeneity across studies precluded a meta-analysis and limited their comparability.
Finally, despite searching multiple databases with complex queries and evaluating reference lists of identified articles, the possibility that the search term criteria, or choice of literature databases, could have inadvertently caused the exclusion of relevant articles remains.
We found consistent evidence for geographical inequalities across a range of prostate cancer indicators across diverse populations, with men from disadvantaged areas facing a higher prostate cancer burden. Although there was some evidence of an association between rural residence and a higher prostate cancer burden, patterns were less consistent. There needs to be an increased focus on developing more complex research strategies to identify the key underlying drivers that can then be incorporated into evidence-based targeted interventions.
Recognizing the variation in the burden caused by prostate cancer between countries internationally, it is critical to develop strategies to at least ensure equitable access to adequate health care for all men within each country. This would ensure that all male residents of a country have the opportunity to access the same level of care regardless of where they live. Key priorities include diagnosing more aggressive disease early, optimizing informed patient-treatment decision making and providing men the best possible treatment for their disease regardless of their residential location. These tasks pose immense challenges to health providers in each country and will require collaboration over a range of concerned stakeholders.
Current evidence points to the benefit of considering health outcomes underpinned by a multi-level continuum of advantage/disadvantage where resources at an individual, social and community level serve to enable or inhibit certain behaviors and systems over a person's lifetime. Consequently, it is important to examine key variables including socioeconomic, psychosocial, cultural, and geographic characteristics in ways that reflect the complexity of people's lives. Employing such a framework will also limit the misleading reliance on the simplistic rural-urban dichotomy by highlighting the dynamic relationship between geography and disadvantage in understanding inequity in the prostate cancer burden.
All authors, JD, PB, PD, JA, NR, and SC contributed to the design of the study. PB coordinated the study. PD conducted the literature searches. PD and PB acted as reviewers. PD drafted the manuscript. PB contributed to the initial draft of the manuscript and all authors. JD, PB, PD, JA, NR, and SC refined and approved the final version of the paper. Each author has participated sufficiently in the work and takes responsibility for appropriate portions of the content. All authors have read and have given final approval of the version to be published.
This project was supported by the Prostate Cancer Foundation of Australia and University of Southern Queensland Surviving Prostate Cancer in Regional Australia project and a National Health and Medical Research Council Center for Research Excellence in Prostate Cancer Survivorship (APP1098042).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Chloe Henshaw for her assistance in checking the data extract and summary tables.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00238/full#supplementary-material
Supplemental File 1. Systematic review literature search strategies. The file lists data-base specific search queries.
Supplemental File 2. Quality appraisal tools for included quantitative studies. The file lists the criteria and scoring system used for assessing the quality of the included cohort (Table S2.1) and case-control (Table S2.2) studies.
Supplemental File 3. Geographical measures, summary scores, overall grades and levels of evidence for included studies. The file lists the geographical measures, summary scores, overall quality scores and levels of evidence for studies included in the systematic review.
Supplemental File 4. Summary of the included studies that adjusted for both rurality and residential disadvantage.
1. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer (2013). Available online at: http://www-dep.iarc.fr/ (accessed June 10, 2018).
2. Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. (2012) 61:1079–92. doi: 10.1016/j.eururo.2012.02.054
3. Pishgar F, Ebrahimi H, Saeedi Moghaddam S, Fitzmaurice C, Amini E. Global, regional and national burden of prostate cancer, 1990 to 2015: results from the global burden of disease study 2015. J Urol. (2018) 199:1224–32. doi: 10.1016/j.juro.2017.10.044
4. Baade PD, Yu XQ, Smith DP, Dunn J, Chambers SK. Geographic disparities in prostate cancer outcomes–review of international patterns. Asian Pac J Cancer Prev. (2015) 16:1259–75. doi: 10.7314/APJCP.2015.16.3.1259
5. Obertova Z, Brown C, Holmes M, Lawrenson R. Prostate cancer incidence and mortality in rural men–a systematic review of the literature. Rural Remote Health. (2012) 12:2039.
6. Chu DI, Freedland SJ. Prostate cancer. Socioeconomic status and disparities in treatment patterns. Nat Rev Urol. (2010) 7:480–1. doi: 10.1038/nrurol.2010.121
7. Klein J, von dem Knesebeck O. Socioeconomic inequalities in prostate cancer survival: a review of the evidence and explanatory factors. Soc Sci Med. (2015) 142:9–18. doi: 10.1016/j.socscimed.2015.07.006
8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
9. University of York. Systematic Reviews, CRD's Guidance for Undertaking Reviews in Health Care. University of York: Centre for Reviews and Dissemination (2008). Available online at: http://www.york.ac.uk/crd/guidance/ (accessed May 02, 2018).
10. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-analyses. (2016). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed May 02, 2018).
11. Dasgupta P, Baade PD, Youlden DR, Garvey G, Aitken JF, Wallington I, et al. Variations in outcomes by residential location for women with breast cancer: a systematic review. BMJ Open. (2018) 8:e019050. doi: 10.1136/bmjopen-2017-019050
12. United States Department of Agriculture. Rural Classifications. (2013). Available online at: https://www.ers.usda.gov/topics/rural-economy-population/rural-classifications/ (accessed May 10, 2018)
13. Australian Bureau of Statistics. Australian Statistical Geography Standard (ASGS): Volume 5–Remoteness Structure, July 2016. Canberra: ABS (2016). Available online at: http://www.abs.gov.au/ausstats/abs@.nsf/Latestproducts/1270.0.55.005Main%20Features1July%202016?opendocument&tabname=Summary&prodno=1270.0.55.005&issue=July%202016&num=&view= (accessed May 12, 2018).
14. Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2011. Canberra: ABS (2013). Available online at: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2033.0.55.001~2011~Main%20Features~FAQs%20-%20SEIFA%202011~9 (accessed May 12, 2018).
15. Tweed EJ, Allardice GM, McLoone P, Morrison DS. Socio-economic inequalities in the incidence of four common cancers: a population-based registry study. Public Health. (2018) 154:1–10. doi: 10.1016/j.puhe.2017.10.005
16. Bryere J, Dejardin O, Bouvier V, Colonna M, Guizard AV, Troussard X, et al. Socioeconomic environment and cancer incidence: a French population-based study in Normandy. BMC Cancer. (2014) 14:87. doi: 10.1186/1471-2407-14-87
17. NHMRC. NHMRC Levels of Evidence and Grades for Recommendations for Guideline Developers. Canberra: National Health and Medical Research Council (2009). Available online at: https://www.mja.com.au/sites/default/files/NHMRC.levels.of.evidence.2008-09.pdf (accessed May 02, 2018).
18. Greenlee RT, Howe HL. County-level poverty and distant stage cancer in the United States. Cancer Causes Control. (2009) 20:989–1000. doi: 10.1007/s10552-009-9299-x
19. Garg V, Raisch DW, Selig JP, Thompson TA. Health disparities in clinical practice patterns for prostate cancer screening by geographic regions in the United States: a multilevel modeling analysis. Prostate Cancer Prostatic Dis. (2013) 16:193–203. doi: 10.1038/pcan.2013.3
20. Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the United States: issues and challenges. Cancer Epidemiol Biomarkers Prev. (2013) 22:1657–67. doi: 10.1158/1055-9965.epi-13-0404
21. Lyratzopoulos G, Abel GA, Brown CH, Rous BA, Vernon SA, Roland M, et al. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol. (2013) 24:843–50. doi: 10.1093/annonc/mds526
22. Coleman MP, Rachet B, Woods LM, Mitry E, Riga M, Cooper N, et al. Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br J Cancer. (2004) 90:1367–73. doi: 10.1038/sj.bjc.6601696
23. Littlejohns TJ, Travis RC, Key TJ, Allen NE. Lifestyle factors and prostate-specific antigen (PSA) testing in UK Biobank: Implications for epidemiological research. Cancer Epidemiol. (2016) 45:40–6. doi: 10.1016/j.canep.2016.09.010
24. Luo Q, Yu XQ, Smith DP, O'Connell DL. A population-based study of progression to metastatic prostate cancer in Australia. Cancer Epidemiol. (2015) 39:617–22. doi: 10.1016/j.canep.2015.04.013
25. Jemal A, Ward E, Wu X, Martin HJ, McLaughlin CC, Thun MJ. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiol Biomarkers Prev. (2005) 14:590–5. doi: 10.1158/1055-9965.EPI-04-0522
26. Sammon J, Dalela D, Abdollah F, Choueiri T, Han P, Hansen M, et al. Determinants of prostate specific antigen screening among black men in the United States in the contemporary era. J Urol. (2016) 195:e247. doi: 10.1016/j.juro.2015.11.023
27. Trinh QD, Li H, Meyer CP, Hanske J, Choueiri TK, Reznor G, et al. Determinants of cancer screening in Asian-Americans. Cancer Causes Control. (2016) 27:989–98. doi: 10.1007/s10552-016-0776-8
28. Zhu Y, Sorkin JD, Dwyer D, Groves C, Steinberger EK. Predictors of repeated PSA testing among black and white men from the Maryland Cancer Survey, 2006. Prev Chronic Dis. (2011) 8:A114.
29. McAlister FA, Lin M, Bakal J, Dean S. Frequency of low-value care in Alberta, Canada: a retrospective cohort study. BMJ Qual Saf. (2018) 27:340–6. doi: 10.1136/bmjqs-2017-006778
30. Guessous I, Cullati S, Fedewa SA, Burton-Jeangros C, Courvoisier DS, Manor O, et al. Prostate cancer screening in Switzerland: 20-year trends and socioeconomic disparities. Prev Med. (2016) 82:83–91. doi: 10.1016/j.ypmed.2015.11.009
31. Baade PD, Youlden DR, Coory MD, Gardiner RA, Chambers SK. Urban-rural differences in prostate cancer outcomes in Australia: what has changed? Med J Aust. (2011) 194:293–6.
32. Coory MD, Baade PD. Urban-rural differences in prostate cancer mortality, radical prostatectomy and prostate-specific antigen testing in Australia. Med J Aust. (2005) 182:112–5.
33. Obertova Z, Hodgson F, Scott-Jones J, Brown C, Lawrenson R. Rural-urban differences in prostate-specific antigen (PSA) screening and its outcomes in New Zealand. J Rural Health. (2016) 32:56–62. doi: 10.1111/jrh.12127
34. Gorday W, Sadrzadeh H, de Koning L, Naugler C. Association of sociodemographic factors and prostate-specific antigen (PSA) testing. Clin Biochem. (2014) 47:164–9. doi: 10.1016/j.clinbiochem.2014.08.006
35. Morgan RM, Steele RJ, Nabi G, McCowan C. Socioeconomic variation and prostate specific antigen testing in the community: a United Kingdom based population study. J Urol. (2013) 190:1207–12. doi: 10.1016/j.juro.2013.04.044
36. Dey S, Zhang Z, Hablas A, Seifeldein IA, Ramadan M, El-Hamzawy H, et al. Geographic patterns of cancer in the population-based registry of Egypt: possible links to environmental exposures. Cancer Epidemiol. (2011) 35:254–64. doi: 10.1016/j.canep.2010.09.010
37. Holowaty EJ, Norwood TA, Wanigaratne S, Abellan JJ, Beale L. Feasibility and utility of mapping disease risk at the neighbourhood level within a Canadian public health unit: an ecological study. Int J Health Geogr. (2010) 9:21. doi: 10.1186/1476-072x-9-21
38. Altekruse SF, Huang L, Cucinelli JE, McNeel TS, Wells KM, Oliver MN. Spatial patterns of localized-stage prostate cancer incidence among white and black men in the southeastern United States, 1999–2001. Cancer Epidemiol Biomarkers Prev. (2010) 19:1460–7. doi: 10.1158/1055-9965.epi-09-1310
39. Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. (2009) 20:417–35. doi: 10.1007/s10552-008-9256-0
40. Fogleman AJ, Mueller GS, Jenkins WD. Does where you live play an important role in cancer incidence in the U.S.? Am J Cancer Res. (2015) 5:2314–9.
41. Major JM, Norman Oliver M, Doubeni CA, Hollenbeck AR, Graubard BI, Sinha R. Socioeconomic status, healthcare density, and risk of prostate cancer among African American and Caucasian men in a large prospective study. Cancer Causes Control. (2012) 23:1185–91. doi: 10.1007/s10552-012-9988-8
42. Zahnd WE, James AS, Jenkins WD, Izadi SR, Fogleman AJ, Steward DE, et al. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. (2018) 27:1265–74. doi: 10.1158/1055-9965.EPI-17-0430
43. Zahnd WE, Jenkins WD, James AS, Izadi SR, Steward DE, Fogleman AJ, et al. Utility and generalizability of multi-state, population-based cancer registry data for rural cancer surveillance research in the United States. Cancer Epidemiol Biomarkers Prev. (2018) 27:1252–60. doi: 10.1158/1055-9965.EPI-17-1087.
44. Ghali F, Celaya M, Laviolette M, Ingimarsson J, Carlos H, Rees J, et al. Does travel time to a radiation facility impact patient decision-making regarding treatment for prostate cancer? A study of the New Hampshire State Cancer Registry. J Rural Health. (2018) 34:S84–90. doi: 10.1111/jrh.12224
45. Higginbotham JC, Moulder J, Currier M, Rural v. urban aspects of cancer: first-year data from the Mississippi Central Cancer Registry. Fam Community Health. (2001) 24:1–9.
46. Oliver MN, Smith E, Siadaty M, Hauck FR, Pickle LW. Spatial analysis of prostate cancer incidence and race in Virginia, 1990–1999. Am J Prev Med. (2006) 30(2 Suppl.):S67–76. doi: 10.1016/j.amepre.2005.09.008
47. Marsa K, Johnsen NF, Bidstrup PE, Johannesen-Henry CT, Friis S. Social inequality and incidence of and survival from male genital cancer in a population-based study in Denmark, 1994–2003. Eur J Cancer. (2008) 44:2018–29. doi: 10.1016/j.ejca.2008.06.012
48. Meijer M, Bloomfield K, Engholm G. Neighbourhoods matter too: the association between neighbourhood socioeconomic position, population density and breast, prostate and lung cancer incidence in Denmark between 2004 and 2008. J Epidemiol Community Health. (2013) 67:6–13. doi: 10.1136/jech-2011-200192
49. Ocana-Riola R, Sanchez-Cantalejo C, Rosell J, Sanchez-Cantalejo E, Daponte A. Socio-economic level, farming activities and risk of cancer in small areas of Southern Spain. Eur J Epidemiol. (2004) 19:643–50. doi: 10.1023/B:EJEP.0000036808.26094.43
50. Sharp L, Donnelly D, Hegarty A, Carsin AE, Deady S, McCluskey N, et al. Risk of several cancers is higher in urban areas after adjusting for socioeconomic status. Results from a two-country population-based study of 18 common cancers. J Urban Health. (2014) 91:510–25. doi: 10.1007/s11524-013-9846-3
51. Jarup L, Best N, Toledano MB, Wakefield J, Elliott P. Geographical epidemiology of prostate cancer in Great Britain. Int J Cancer. (2002) 97:695–9. doi: 10.1002/ijc.10113
52. AIHW. Cancer in Australia: Actual incidence data from 1991 to 2009 and mortality data from 1991 to 2010 with projections to 2012. Asia Pac J Clin Oncol. (2013) 9:199–213. doi: 10.1111/ajco.12127
53. Cramb SM, Mengersen KL, Baade PD. Identification of area-level influences on regions of high cancer incidence in Queensland, Australia: a classification tree approach. BMC Cancer. (2011) 11:311. doi: 10.1186/1471-2407-11-311
54. Depczynski J, Dobbins T, Armstrong B, Lower T. Comparison of cancer incidence in Australian farm residents 45 years and over, compared to rural non-farm and urban residents–a data linkage study. BMC Cancer. (2018) 18:33. doi: 10.1186/s12885-017-3912-2
55. Yu XQ, Luo Q, Smith DP, O'Connell DL, Baade PD. Geographic variation in prostate cancer survival in New South Wales. Med J Aust. (2014) 200:586–90. doi: 10.5694/mja13.11134
56. Boscoe FP, Johnson CJ, Sherman RL, Stinchcomb DG, Lin G, Henry KA. The relationship between area poverty rate and site-specific cancer incidence in the United States. Cancer. (2014) 120:2191–8. doi: 10.1002/cncr.28632
57. Hastert TA, Beresford SA, Sheppard L, White E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. J Epidemiol Community Health. (2015) 69:168–76. doi: 10.1136/jech-2014-204417
58. Houston KA, King J, Li J, Jemal A. Trends in prostate cancer incidence rates and prevalence of prostate specific antigen screening by socioeconomic status and regions in the United States, 2004 to 2013. J Urol. (2018) 199:676–81. doi: 10.1016/j.juro.2017.09.103
59. Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Natl Cancer Inst Monogr. (2014) 2014:236–43. doi: 10.1093/jncimonographs/lgu020
60. Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. (2017) 2017:2819372. doi: 10.1155/2017/2819372
61. Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. (2014) 25:81–92. doi: 10.1007/s10552-013-0310-1
62. Cheng I, Witte JS, McClure LA, Shema SJ, Cockburn MG, John EM, et al. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control. (2009) 20:1431–40. doi: 10.1007/s10552-009-9369-0
63. Yin D, Morris C, Allen M, Cress R, Bates J, Liu L. Does socioeconomic disparity in cancer incidence vary across racial/ethnic groups? Cancer Causes Control. (2010) 21:1721–30. doi: 10.1007/s10552-010-9601-y
64. Liu L, Cozen W, Bernstein L, Ross RK, Deapen D. Changing relationship between socioeconomic status and prostate cancer incidence. J Natl Cancer Inst. (2001) 93:705–9.
65. Mather FJ, Chen VW, Morgan LH, Correa CN, Shaffer JG, Srivastav SK, et al. Hierarchical modeling and other spatial analyses in prostate cancer incidence data. Am J Prev Med. (2006) 30(2 Suppl.):S88–100. doi: 10.1016/j.amepre.2005.09.012
66. Sanderson M, Coker AL, Perez A, Du XL, Peltz G, Fadden MK. A multilevel analysis of socioeconomic status and prostate cancer risk. Ann Epidemiol. (2006) 16:901–7. doi: 10.1016/j.annepidem.2006.02.006
67. Luce D, Michel S, Dugas J, Bhakkan B, Menvielle G, Joachim C, et al. Disparities in cancer incidence by area-level socioeconomic status in the French West Indies. Cancer Causes Control. (2017) 28:1305–12. doi: 10.1007/s10552-017-0946-3
68. Soto-Salgado M, Suarez E, Torres-Cintron M, Pettaway CA, Colon V, Ortiz AP. Prostate cancer incidence and mortality among Puerto Ricans: an updated analysis comparing men in Puerto Rico with US racial/ethnic groups. P R Health Sci J. (2012) 31:107–13.
69. Haddad-Khoshkar A, Jafari-Koshki T, Mahaki B. Investigating the incidence of prostate cancer in Iran 2005–2008 using Bayesian spatial ecological regression models. Asian Pac J Cancer Prev. (2015) 16:5917–21. doi: 10.7314/APJCP.2015.16.14.5917
70. Miki Y, Inoue M, Ikeda A, Sawada N, Nakaya T, Shimazu T, et al. Neighborhood deprivation and risk of cancer incidence, mortality and survival: results from a population-based cohort study in Japan. PLoS ONE. (2014) 9:e106729. doi: 10.1371/journal.pone.0106729
71. Aarts MJ, van der Aa MA, Coebergh JW, Louwman WJ. Reduction of socioeconomic inequality in cancer incidence in the South of the Netherlands during 1996–2008. Eur J Cancer. (2010) 46:2633–46. doi: 10.1016/j.ejca.2010.07.039
72. Pukkala E, Weiderpass E. Socio-economic differences in incidence rates of cancers of the male genital organs in Finland, 1971–95. Int J Cancer. (2002) 102:643–8. doi: 10.1002/ijc.10749
73. Garcia-Gil M, Elorza JM, Banque M, Comas-Cufi M, Blanch J, Ramos R, et al. Linking of primary care records to census data to study the association between socioeconomic status and cancer incidence in Southern Europe: a nation-wide ecological study. PLoS ONE. (2014) 9:e109706. doi: 10.1371/journal.pone.0109706
74. Vicens GR, Zafra MS, Moreno-Crespi J, Ferrer BC, Marcos-Gragera R. Incidence variation of prostate and cervical cancer according to socioeconomic level in the Girona Health Region. BMC Public Health. (2014) 14:1079. doi: 10.1186/1471-2458-14-1079
75. Maringe C, Mangtani P, Rachet B, Leon DA, Coleman MP, dos Santos Silva I. Cancer incidence in South Asian migrants to England, 1986–2004: unraveling ethnic from socioeconomic differentials. Int J Cancer. (2013) 132:1886–94. doi: 10.1002/ijc.27826
76. Shafique K, Oliphant R, Morrison DS. The impact of socio-economic circumstances on overall and grade-specific prostate cancer incidence: a population-based study. Br J Cancer. (2012) 107:575–82. doi: 10.1038/bjc.2012.289
77. Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. (2013) 119:3067–75. doi: 10.1002/cncr.28037
78. Skolarus TA, Chan S, Shelton JB, Antonio AL, Sales AE, Malin JL, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. (2013) 119:3629–35. doi: 10.1002/cncr.28275
79. Holmes JA, Carpenter WR, Wu Y, Hendrix LH, Peacock S, Massing M, et al. Impact of distance to a urologist on early diagnosis of prostate cancer among black and white patients. J Urol. (2012) 187:883–8. doi: 10.1016/j.juro.2011.10.156
80. McLafferty S, Wang F. Rural reversal? Rural-urban disparities in late-stage cancer risk in Illinois. Cancer. (2009) 115:2755–64. doi: 10.1002/cncr.24306
81. Goovaerts P, Xiao H. Geographical, temporal and racial disparities in late-stage prostate cancer incidence across Florida: a multiscale joinpoint regression analysis. Int J Health Geogr. (2011) 10:63. doi: 10.1186/1476-072X-10-63
82. Xiao H, Tan F, Goovaerts P. Racial and geographic disparities in late-stage prostate cancer diagnosis in Florida. J Health Care Poor Underserved. (2011) 22(4 Suppl.):187–99. doi: 10.1353/hpu.2011.0155
83. Depczynski J, Dobbins T, Armstrong B, Lower T. Stage of diagnosis of prostate, breast and colorectal cancer in farm residents compared with other rural and urban residents in New South Wales. Aust J Rural Health. (2018) 26:56–62. doi: 10.1111/ajr.12392
84. Tervonen HE, Walton R, Roder D, You H, Morrell S, Baker D, et al. Socio-demographic disadvantage and distant summary stage of cancer at diagnosis–a population-based study in New South Wales. Cancer Epidemiol. (2016) 40:87–94. doi: 10.1016/j.canep.2015.10.032
85. Papa N, Lawrentschuk N, Muller D, MacInnis R, Ta A, Severi G, et al. Rural residency and prostate cancer specific mortality: results from the Victorian Radical Prostatectomy Register. Aust N Z J Public Health. (2014) 38:449–54. doi: 10.1111/1753-6405.12210
86. Ruseckaite R, Sampurno F, Millar J, Frydenberg M, Evans S. Diagnostic and treatment factors associated with poor survival from prostate cancer are differentially distributed between regional and metropolitan Victoria, Australia. BMC Urol. (2016) 16:54. doi: 10.1186/s12894-016-0172-4
87. Haynes R, Pearce J, Barnett R. Cancer survival in New Zealand: ethnic, social and geographical inequalities. Soc Sci Med. (2008) 67:928–37. doi: 10.1016/j.socscimed.2008.05.005
88. Boscoe FP, Henry KA, Sherman RL, Johnson CJ. The relationship between cancer incidence, stage and poverty in the United States. Int J Cancer. (2016) 139:607–12. doi: 10.1002/ijc.30087
89. Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. (2008) 113:582–91. doi: 10.1002/cncr.23567
90. Chu DI, Moreira DM, Gerber L, Presti JC Jr., Aronson WJ, Terris MK, et al. Effect of race and socioeconomic status on surgical margins and biochemical outcomes in an equal-access health care setting: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Cancer. (2012) 118:4999–5007. doi: 10.1002/cncr.27456
91. Marlow NM, Halpern MT, Pavluck AL, Ward EM, Chen AY. Disparities associated with advanced prostate cancer stage at diagnosis. J Health Care Poor Underserved. (2010) 21:112–31. doi: 10.1353/hpu.0.0253
92. Weiner AB, Matulewicz RS, Tosoian JJ, Feinglass JM, Schaeffer EM. The effect of socioeconomic status, race, and insurance type on newly diagnosed metastatic prostate cancer in the United States (2004–2013). Urol Oncol. (2018) 36:91.e1–6. doi: 10.1016/j.urolonc.2017.10.023
93. Niu X, Pawlish KS, Roche LM. Cancer survival disparities by race/ethnicity and socioeconomic status in New Jersey. J Health Care Poor Underserved. (2010) 21:144–60. doi: 10.1353/hpu.0.0263
94. Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. (2003) 14:761–6.
95. Goovaerts P, Xiao H, Gwede CK, Tan F, Huang Y, Adunlin G, et al. Impact of age, race and socio-economic status on temporal trends in late-stage prostate cancer diagnosis in Florida. Spat Stat. (2015) 14(Pt 100):321–37. doi: 10.1016/j.spasta.2015.07.002
96. Xiao H, Gwede CK, Kiros G, Milla K. Analysis of prostate cancer incidence using geographic information system and multilevel modeling. J Natl Med Assoc. (2007) 99:218–25.
97. Xiao H, Tan F, Goovaerts P, Adunlin G, Ali AA, Gwede CK, et al. Impact of comorbidities on prostate cancer stage at diagnosis in Florida. Am J Mens Health. (2016) 10:285–95. doi: 10.1177/1557988314564593
98. Aarts MJ, Koldewijn EL, Poortmans PM, Coebergh JW, Louwman M. The impact of socioeconomic status on prostate cancer treatment and survival in the southern Netherlands. Urology. (2013) 81:593–9. doi: 10.1016/j.urology.2012.11.011
99. Lyratzopoulos G, Barbiere JM, Greenberg DC, Wright KA, Neal DE. Population based time trends and socioeconomic variation in use of radiotherapy and radical surgery for prostate cancer in a UK region: continuous survey. BMJ. (2010) 340:c1928. doi: 10.1136/bmj.c1928
100. McVey GP, McPhail S, Fowler S, McIntosh G, Gillatt D, Parker CC. Initial management of low-risk localized prostate cancer in the UK: analysis of the British Association of Urological Surgeons Cancer Registry. BJU Int. (2010) 106:1161–4. doi: 10.1111/j.1464-410X.2010.09288.x
101. Maclean R, Jeffreys M, Ives A, Jones T, Verne J, Ben-Shlomo Y. Primary care characteristics and stage of cancer at diagnosis using data from the national cancer registration service, quality outcomes framework and general practice information. BMC Cancer. (2015) 15:500. doi: 10.1186/s12885-015-1497-1
102. Tervonen HE, Morrell S, Aranda S, Roder D, You H, Niyonsenga T, et al. The impact of geographic unit of analysis on socioeconomic inequalities in cancer survival and distant summary stage–a population-based study. Aust N Z J Public Health. (2017) 41:130–6. doi: 10.1111/1753-6405.12608
103. Chen Y-W, Mahal BA, Muralidhar V, Nezolosky M, Beard CJ, Den RB, et al. Association between treatment at a high-volume facility and improved survival for radiation-treated men with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. (2016) 94:683–90. doi: 10.1016/j.ijrobp.2015.12.008
104. Marsh S, Walters RW, Silberstein PT. Survival outcomes of radical prostatectomy versus radiotherapy in intermediate-risk prostate cancer: a NCDB study. Clin Genitourin Cancer. (2018) 16:E39–46. doi: 10.1016/j.clgc.2017.07.029
105. Prasad SM, Eggener SE, Lipsitz SR, Irwin MR, Ganz PA, Hu JC. Effect of depression on diagnosis, treatment, and mortality of men with clinically localized prostate cancer. J Clin Oncol. (2014) 32:2471–8. doi: 10.1200/jco.2013.51.1048
106. Vetterlein MW, Loppenberg B, Karabon P, Dalela D, Jindal T, Sood A, et al. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer. (2017) 123:3241–52. doi: 10.1002/cncr.30744
107. Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Travel times to health care and survival from cancers in Northern England. Eur J Cancer. (2008) 44:269–74. doi: 10.1016/j.ejca.2007.07.028
108. Hall SE, Holman CD, Wisniewski ZS, Semmens J. Prostate cancer: socio-economic, geographical and private-health insurance effects on care and survival. BJU Int. (2005) 95:51–8. doi: 10.1111/j.1464-410X.2005.05248.x
109. Jong KE, Smith DP, Yu XQ, O'Connell DL, Goldstein D, Armstrong BK. Remoteness of residence and survival from cancer in New South Wales. Med J Aust. (2004) 180:618–22.
110. White A, Coker AL, Du XL, Eggleston KS, Williams M. Racial/ethnic disparities in survival among men diagnosed with prostate cancer in Texas. Cancer. (2011) 117:1080–8. doi: 10.1002/cncr.25671
111. Li X, Sundquist K, Sundquist J. Neighborhood deprivation and prostate cancer mortality: a multilevel analysis from Sweden. Prostate Cancer Prostatic Dis. (2012) 15:128. doi: 10.1038/pcan.2011.46
112. Campbell NC, Elliott AM, Sharp L, Ritchie LD, Cassidy J, Little J. Rural factors and survival from cancer: analysis of Scottish cancer registrations. Br J Cancer. (2000) 82:1863–6. doi: 10.1054/bjoc.1999.1079
113. Tervonen HE, Aranda S, Roder D, You H, Walton R, Morrell S, et al. Cancer survival disparities worsening by socio-economic disadvantage over the last 3 decades in new South Wales, Australia. BMC Public Health. (2017) 17:691. doi: 10.1186/s12889-017-4692-y
114. Thomas AA, Pearce A, Sharp L, Gardiner RA, Chambers S, Aitken J, et al. Socioeconomic disadvantage but not remoteness affects short-term survival in prostate cancer: a population-based study using competing risks. Asia Pac J Clin Oncol. (2017) 13:e31–40. doi: 10.1111/ajco.12570
115. Tan L, Wang LL, Ranasinghe W, Persad R, Bolton D, Lawrentschuk N, et al. Survival outcomes of younger men (< 55 years) undergoing radical prostatectomy. Prostate Int. (2018) 6:31–5. doi: 10.1016/j.prnil.2017.07.002
116. Glaser SM, Dohopolski MJ, Balasubramani GK, Benoit RM, Smith RP, Beriwal S. Brachytherapy boost for prostate cancer: trends in care and survival outcomes. Brachytherapy. (2017) 16:330–41. doi: 10.1016/j.brachy.2016.12.015
117. DeRouen MC, Schupp CW, Koo J, Yang J, Hertz A, Shariff-Marco S, et al. Impact of individual and neighborhood factors on disparities in prostate cancer survival. Cancer Epidemiol. (2018) 53:1–11. doi: 10.1016/j.canep.2018.01.003
118. Hong X, Fei T, Adunlin G, Ali AA, Goovaerts P, Gwede CK, et al. Factors associated with overall survival prostate cancer in Florida: a multilevel analysis. J Health Care Poor Underserved. (2015) 26:266–77. doi: 10.1353/hpu.2015.0007
119. Xiao H, Tan F, Goovaerts P, Ali A, Adunlin G, Gwede CK, et al. Multilevel factors associated with overall mortality for men diagnosed with prostate cancer in Florida. Am J Mens Health. (2014) 8:316–26. doi: 10.1177/1557988313512862
120. Burns RM, Sharp L, Sullivan FJ, Deady SE, Drummond FJ, O'Neill C. Factors driving inequality in prostate cancer survival: a population based study. PLoS ONE. (2014) 9:e0106456. doi: 10.1371/journal.pone.0106456
121. Mariotto A, Capocaccia R, Verdecchia A, Micheli A, Feuer EJ, Pickle L, et al. Projecting SEER cancer survival rates to the US: an ecological regression approach. Cancer Causes Control. (2002) 13:101–11.
122. Bravo LE, García LS, Collazos PA. Cancer survival in Cali, Colombia: a population-based study, 1995–2004. Colomb Med. (2014) 45:110–6.
123. Ito Y, Nakaya T, Nakayama T, Miyashiro I, Ioka A, Tsukuma H, et al. Socioeconomic inequalities in cancer survival: a population-based study of adult patients diagnosed in Osaka, Japan, during the period 1993–2004. Acta Oncol. (2014) 53:1423–33. doi: 10.3109/0284186X.2014.912350
124. Belot A, Remontet L, Rachet B, Dejardin O, Charvat H, Bara S, et al. Describing the association between socioeconomic inequalities and cancer survival: methodological guidelines and illustration with population-based data. Clin Epidemiol. (2018) 10:561–73. doi: 10.2147/CLEP.S150848
125. Jansen L, Eberle A, Emrich K, Gondos A, Holleczek B, Kajüter H, et al. Socioeconomic deprivation and cancer survival in Germany: an ecological analysis in 200 districts in Germany. Int J Cancer. (2014) 134:2951–60. doi: 10.1002/ijc.28624
126. Shack LG, Rachet B, Brewster DH, Coleman MP. Socioeconomic inequalities in cancer survival in Scotland 1986–2000. Br J Cancer. (2007) 97:999–1004. doi: 10.1038/sj.bjc.6603980
127. Shafique K, Morrison DS. Socio-economic inequalities in survival of patients with prostate cancer: role of age and Gleason grade at diagnosis. PLoS ONE. (2013) 8:e56184. doi: 10.1371/journal.pone.0056184
128. Shafique K, Proctor MJ, McMillan DC, Qureshi K, Leung H, Morrison DS. Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow Inflammation Outcome Study. Prostate Cancer Prostatic Dis. (2012) 15:195–201. doi: 10.1038/pcan.2011.60
129. Rowan S, Rachet B, Alexe DM, Cooper N, Coleman MP. Survival from prostate cancer in England and Wales up to 2001. Br J Cancer. (2008) 99:S75–7. doi: 10.1038/sj.bjc.6604595
130. Sloggett A, Young H, Grundy E. The association of cancer survival with four socioeconomic indicators: a longitudinal study of the older population of England and Wales 1981–2000. BMC Cancer. (2007) 7:20. doi: 10.1186/1471-2407-7-20
131. Exarchakou A, Rachet B, Belot A, Maringe C, Coleman MP. Impact of national cancer policies on cancer survival trends and socioeconomic inequalities in England, 1996–2013: population based study. BMJ. (2018) 360:k764. doi: 10.1136/bmj.k764
132. Rachet B, Ellis L, Maringe C, Chu T, Nur U, Quaresma M, et al. Socioeconomic inequalities in cancer survival in England after the NHS cancer plan. Br J Cancer. (2010) 103:446–53. doi: 10.1038/sj.bjc.6605752
133. AIHW. Cancer survival and prevalence in Australia: period estimates from 1982 to 2010. Asia Pac J Clin Oncol. (2013) 9:29–39. doi: 10.1111/ajco.12062
134. Stanbury JF, Baade PD, Yu Y, Yu XQ. Impact of geographic area level on measuring socioeconomic disparities in cancer survival in New South Wales, Australia: a period analysis. Cancer Epidemiol. (2016) 43:56–62. doi: 10.1016/j.canep.2016.06.001
135. Yu XQ, O'Connell DL, Gibberd RW, Armstrong BK. Assessing the impact of socio-economic status on cancer survival in New South Wales, Australia 1996–2001. Cancer Causes Control. (2008) 19:1383–90. doi: 10.1007/s10552-008-9210-1
136. Jeffreys M, Sarfati D, Stevanovic V, Tobias M, Lewis C, Pearce N, et al. Socioeconomic inequalities in cancer survival in New Zealand: the role of extent of disease at diagnosis. Cancer Epidemiol Biomarkers Prev. (2009) 18:915. doi: 10.1158/1055-9965.EPI-08-0685
137. Du XL, Fang S, Coker AL, Sanderson M, Aragaki C, Cormier JN, et al. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. (2006) 106:1276–85. doi: 10.1002/cncr.21732
138. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. (2018) 36:25–33. doi: 10.1200/jco.2017.74.2049
139. Hellenthal NJ, Parikh-Patel A, Bauer K, Ralph W, deVere W, Koppie TM. Men of higher socioeconomic status have improved outcomes after radical prostatectomy for localized prostate cancer. Urology. (2010) 76:1409–13. doi: 10.1016/j.urology.2010.03.024
140. Freeman VL, Ricardo AC, Campbell RT, Barrett RE, Warnecke RB. Association of census tract-level socioeconomic status with disparities in prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev. (2011) 20:2150–9. doi: 10.1158/1055-9965.epi-11-0344
141. Schwartz K, Powell IJ, Underwood W III, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. (2009) 74:1296–302. doi: 10.1016/j.urology.2009.02.058
142. Louwman WJ, Aarts MJ, Houterman S, van Lenthe FJ, Coebergh JW, Janssen-Heijnen ML. A 50% higher prevalence of life-shortening chronic conditions among cancer patients with low socioeconomic status. Br J Cancer. (2010) 103:1742–8. doi: 10.1038/sj.bjc.6605949
143. Parikh RR, Kim S, Stein MN, Haffty BG, Kim IY, Goyal S. Trends in active surveillance for very low-risk prostate cancer: do guidelines influence modern practice? Cancer Med. (2017) 6:2410–8. doi: 10.1002/cam4.1132
144. Schymura MJ, Kahn AR, German RR, Hsieh MC, Cress RD, Finch JL, et al. Factors associated with initial treatment and survival for clinically localized prostate cancer: results from the CDC-NPCR Patterns of Care Study (PoC1). BMC Cancer. (2010) 10:152. doi: 10.1186/1471-2407-10-152
145. des Bordes JKA, Lopez DS, Swartz MD, Volk RJ. Sociodemographic disparities in cure-intended treatment in localized prostate cancer. J Racial Ethn Health Disparities. (2018) 5:104–10. doi: 10.1007/s40615-017-0348-y
146. Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer. (2008) 44:992–9. doi: 10.1016/j.ejca.2008.02.001
147. Hayen A, Smith DP, Patel MI, O'Connell DL. Patterns of surgical care for prostate cancer in NSW, 1993–2002: rural/urban and socio-economic variation. Aust N Z J Public Health. (2008) 32:417–20. doi: 10.1111/j.1753-6405.2008.00272.x
148. Baade PD, Youlden DR, Gardiner RA, Ferguson M, Aitken JF, Yaxley J, et al. Factors associated with treatment received by men diagnosed with prostate cancer in Queensland, Australia. BJU Int. (2012) 110(11 Pt B):E712–9. doi: 10.1111/j.1464-410X.2012.011533.x
149. Cobran EK, Chen RC, Overman R, Meyer AM, Kuo TM, O'Brien J, et al. Racial differences in diffusion of intensity-modulated radiation therapy for localized prostate cancer. Am J Mens Health. (2016) 10:399–407. doi: 10.1177/1557988314568184
150. Henry MJ, Jones P, Morrissy K, Matheson LM, Pitson G, Healy P, et al. Radiotherapy in the Barwon South Western Region: a rural perspective. J Med Imaging Radiat Oncol. (2014) 58:612–7. doi: 10.1111/1754-9485.12208
151. Cary C, Odisho AY, Cooperberg MR. Variation in prostate cancer treatment associated with population density of the county of residence. Prostate Cancer Prostatic Dis. (2016) 19:174. doi: 10.1038/pcan.2015.65
152. Cetnar JP, Hampton JM, Williamson AA, Downs T, Wang D, Owen JB, et al. Place of residence and primary treatment of prostate cancer: examining trends in rural and nonrural areas in Wisconsin. Urology. (2013) 81:540–6. doi: 10.1016/j.urology.2012.09.058
153. Park J, Suh B, Shin DW, Hong JH, Ahn H. Changing patterns of primary treatment in Korean men with prostate cancer over 10 years: a nationwide population based study. Cancer Res Treat. (2016) 48:899–906. doi: 10.4143/crt.2015.212
154. Williams SB, Lei Y, Nguyen PL, Gu X, Lipsitz SR, Yu HY, et al. Comparative effectiveness of cryotherapy vs brachytherapy for localised prostate cancer. BJU Int. (2012) 110(2 Pt 2):E92–8. doi: 10.1111/j.1464-410X.2011.10775.x
155. Mahal BA, Cooperberg MR, Aizer AA, Ziehr DR, Hyatt AS, Choueiri TK, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. (2015) 128:609–16. doi: 10.1016/j.amjmed.2014.12.030
156. Mahal BA, Chen YW, Muralidhar V, Mahal AR, Choueiri TK, Hoffman KE, et al. National sociodemographic disparities in the treatment of high-risk prostate cancer: do academic cancer centers perform better than community cancer centers? Cancer. (2016) 122:3371–7. doi: 10.1002/cncr.30205
157. Aggarwal A, Lewis D, Sujenthiran A, Charman SC, Sullivan R, Payne H, et al. Hospital quality factors influencing the mobility of patients for radical prostate cancer radiation therapy: a national population-based study. Int J Radiat Oncol Biol Phys. (2017) 99:1261–70. doi: 10.1016/j.ijrobp.2017.08.018
158. Aggarwal A, Lewis D, Charman SC, Mason M, Clarke N, Sullivan R, et al. Determinants of patient mobility for prostate cancer surgery: a population-based study of choice and competition. Eur Urol. (2018) 73:822–5. doi: 10.1016/j.eururo.2017.07.013
159. Sharma DK, Vangaveti VN, Larkins S. Geographical access to radiation therapy in North Queensland: a retrospective analysis of patient travel to radiation therapy before and after the opening of an additional radiotherapy facility. Rural Remote Health. (2016) 16:3640.
160. Krishna S, Fan Y, Jarosek S, Adejoro O, Chamie K, Konety B. Racial disparities in active surveillance for prostate cancer. J Urol. (2017) 197:342–9. doi: 10.1016/j.juro.2016.08.104
161. Hoffman RM, Shi Y, Freedland SJ, Keating NL, Walter LC. Treatment patterns for older veterans with localized prostate cancer. Cancer Epidemiol. (2015) 39:769–77. doi: 10.1016/j.canep.2015.07.005
162. Krupski TL, Kwan L, Afifi AA, Litwin MS. Geographic and socioeconomic variation in the treatment of prostate cancer. J Clin Oncol. (2005) 23:7881–8. doi: 10.1200/jco.2005.08.755
163. Fairley L, Baker M, Whiteway J, Cross W, Forman D. Trends in non-metastatic prostate cancer management in the Northern and Yorkshire region of England, 2000–2006. Br J Cancer. (2009) 101:1839–45. doi: 10.1038/sj.bjc.6605424
164. Krupski TL, Kwan L, Litwin MS. Sociodemographic factors associated with postprostatectomy radiotherapy. Prostate Cancer Prostatic Dis. (2005) 8:184–8. doi: 10.1038/sj.pcan.4500791
165. Jin CJ, Hanna TP, Cook EF, Miao Q, Brundage MD. Variation in radiotherapy referral and treatment for high-risk pathological features after radical prostatectomy: results from a population-based study. Clin Oncol. (2018) 30:47–56. doi: 10.1016/j.clon.2017.10.009
166. Muralidhar V, Rose BS, Chen YW, Nezolosky MD, Nguyen PL. Association between travel distance and choice of treatment for prostate cancer: does geography reduce patient choice? Int J Radiat Oncol Biol Phys. (2016) 96:313–7. doi: 10.1016/j.ijrobp.2016.05.022
167. Watson M, Grande D, Radhakrishnan A, Mitra N, Ward KR, Pollack CE. Racial differences in prostate cancer treatment: the role of socioeconomic status. Ethn Dis. (2017) 27:201–8. doi: 10.18865/ed.27.3.201
168. Gilbert SM, Kuo YF, Shahinian VB. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol Oncol. (2011) 29:647–53. doi: 10.1016/j.urolonc.2009.09.004
169. Krupski TL, Saigal CS, Hanley J, Schonlau M, Litwin MS. Patterns of care for men with prostate cancer after failure of primary treatment. Cancer. (2006) 107:258–65. doi: 10.1002/cncr.21981
170. Lagace C, Desmeules M, Pong RW, Heng D. Non-communicable disease and injury-related mortality in rural and urban places of residence: a comparison between Canada and Australia. Can J Public Health. (2007) 98 Suppl. 1:S62–9.
171. Pampalon R, Martinez J, Hamel D. Does living in rural areas make a difference for health in Quebec? Health Place. (2006) 12:421–35. doi: 10.1016/j.healthplace.2005.04.002
172. Colli JL, Amling CL. Prostate cancer mortality rates compared to urologist population densities and prostate-specific antigen screening levels on a state-by-state basis in the United States of America. Prostate Cancer Prostatic Dis. (2008) 11:247–51. doi: 10.1038/pcan.2008.7
173. Jemal A, Kulldorff M, Devesa SS, Hayes RB, Fraumeni JF Jr. A geographic analysis of prostate cancer mortality in the United States, 1970–89. Int J Cancer. (2002) 101:168–74. doi: 10.1002/ijc.10594
174. Odisho AY, Cooperberg MR, Fradet V, Ahmad AE, Carroll PR. Urologist density and county-level urologic cancer mortality. J Clin Oncol. (2010) 28:2499–504. doi: 10.1200/jco.2009.26.9597
175. Rogerson PA, Sinha G, Han D. Recent changes in the spatial pattern of prostate cancer in the U.S. Am J Prev Med. (2006) 30(2 Suppl.):S50–9. doi: 10.1016/j.amepre.2005.09.006
176. Rusiecki JA, Kulldorff M, Nuckols JR, Song C, Ward MH. Geographically based investigation of prostate cancer mortality in four U.S. Northern Plain states. Am J Prev Med. (2006) 30(2 Suppl.):S101–8. doi: 10.1016/j.amepre.2005.09.005
177. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part I-all cancers and lung cancer and part II-colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. (2011) 2011:107497. doi: 10.1155/2011/107497
178. Zahnd WE, Jenkins WD, Mueller-Luckey GS. Cancer mortality in the Mississippi delta region: descriptive epidemiology and needed future research and interventions. J Health Care Poor Underserved. (2017) 28:315–28. doi: 10.1353/hpu.2017.0025
179. Yang CY, Hsieh YL. The relationship between population density and cancer mortality in Taiwan. Jpn J Cancer Res. (1998) 89:355–60.
180. Hagedoorn P, Vandenheede H, Vanthomme K, Gadeyne S. Socioeconomic position, population density and site-specific cancer mortality: a multilevel analysis of Belgian adults, 2001–2011. Int J Cancer. (2018) 142:23–35. doi: 10.1002/ijc.31031
181. Nikolaidis C, Tentes I, Lialiaris T, Constantinidis TC, Kortsaris A. Regional disparities in cancer mortality across the rural-urban axis: a case study from north-eastern Greece. Rural Remote Health. (2015) 15:3013.
182. Smailyte G, Kurtinaitis J. Cancer mortality differences among urban and rural residents in Lithuania. BMC Public Health. (2008) 8:56. doi: 10.1186/1471-2458-8-56
183. Rand AE, Agarwal A, Ahuja D, Ngo T, Qureshi MM, Gupta A, et al. Patient demographic characteristics and disease stage as drivers of disparities in mortality in prostate cancer patients who receive care at a safety net academic medical center. Clin Genitourin Cancer. (2014) 12:455–60. doi: 10.1016/j.clgc.2014.04.005
184. Wan N, Zhan FB, Cai Z. Socioeconomic disparities in prostate cancer mortality and the impact of geographic scale. South Med J. (2011) 104:553–9. doi: 10.1097/SMJ.0b013e31821f99ff
185. Borrell C, Mari-Dell'olmo M, Serral G, Martinez-Beneito M, Gotsens M, Members M. Inequalities in mortality in small areas of eleven Spanish cities (the multicenter MEDEA project). Health Place. (2010) 16:703–11. doi: 10.1016/j.healthplace.2010.03.002
186. Mariotto AB, Noone AM, Howlader N, Cho H, Keel GE, Garshell J, et al. Cancer survival: an overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr. (2014) 2014:145–86. doi: 10.1093/jncimonographs/lgu024
187. Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. (2017) 317:2532–42. doi: 10.1001/jama.2017.7248
188. Parker C, Gillessen S, Heidenreich A, Horwich A, Committee EG. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2015) 26 Suppl. 5:v69–77. doi: 10.1093/annonc/mdv222
189. Zahnd WE, McLafferty SL. Contextual effects and cancer outcomes in the United States: a systematic review of characteristics in multilevel analyses. Ann Epidemiol. (2017) 27:739–48 e3. doi: 10.1016/j.annepidem.2017.10.002
190. Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. (2009) 53:171–84. doi: 10.1002/mnfr.200700511
191. Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen–based screening for prostate cancer: evidence report and systematic review for the us preventive services task force. JAMA. (2018) 319:1914–31. doi: 10.1001/jama.2018.3712
192. Jang TL, Bekelman JE, Liu Y, Bach PB, Basch EM, Elkin EB, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. (2010) 170:440–50. doi: 10.1001/archinternmed.2010.1
193. Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA Cancer J Clin. (2015) 65:265–82. doi: 10.3322/caac.21278
194. Cho H, Mariotto AB, Schwartz LM, Luo J, Woloshin S. When do changes in cancer survival mean progress? The insight from population incidence and mortality. J Natl Cancer Inst Monogr. (2014) 2014:187–97. doi: 10.1093/jncimonographs/lgu014
195. AIHW. Mortality Inequalities in Australia, 2009–2011. Cat no AUS 184. Canberra: Australian Institute of Health & Welfare (2014). Available online at: https://www.aihw.gov.au/getmedia/5683dc4b-796f-49bd-87eb-5a11135956f5/16934.pdf.aspx?inline=true (accessed August 10, 2018).
196. ASCO. The state of cancer care in America, 2017: a report by the American Society of Clinical Oncology. J Oncol Pract. (2017) 13:e353–94. doi: 10.1200/JOP.2016.020743
197. Butow PN, Phillips F, Schweder J, White K, Underhill C, Goldstein D, et al. Psychosocial well-being and supportive care needs of cancer patients living in urban and rural/regional areas: a systematic review. Support Care Cancer. (2012) 20:1–22. doi: 10.1007/s00520-011-1270-1
198. Leow JJ, Leong EK, Serrell EC, Chang SL, Gruen RL, Png KS, et al. Systematic review of the volume–outcome relationship for radical prostatectomy. Eur Urol Focus. (2018) 4:775–89. doi: 10.1016/j.euf.2017.03.008
199. Smith T. A long way from home: access to cancer care for rural Australians. Radiography. (2012) 18:38–42. doi: 10.1016/j.radi.2011.10.041
200. United States Department of Agriculture. Rural Poverty & Well-being. (2018). Available online at: https://www.ers.usda.gov/topics/rural-economy-population/rural-poverty-well-being/#howis (accessed July 12, 2018).
201. Gilbert SM, Pow-Sang JM, Xiao H. Geographical factors associated with health disparities in prostate cancer. Cancer Control. (2016) 23:401–8. doi: 10.1177/107327481602300411
202. Office for National Statistics. Guide to Applying the 2011 Rural Urban Classification to data, July 2016. Department for Environment Food & Rural Affairs, Government, United Kingdom. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/539241/Guide_to_applying_the_rural_urban_classification_to_data.pdf (accessed August 06, 2018).
Keywords: prostate cancer, rural, area-disadvantage, health disparity, systematic review, geographical variations, continuum of care
Citation: Dasgupta P, Baade PD, Aitken JF, Ralph N, Chambers SK and Dunn J (2019) Geographical Variations in Prostate Cancer Outcomes: A Systematic Review of International Evidence. Front. Oncol. 9:238. doi: 10.3389/fonc.2019.00238
Received: 07 September 2018; Accepted: 18 March 2019;
Published: 08 April 2019.
Edited by:
Imtiaz Ahmad Siddiqui, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Francis P. Boscoe, University at Albany, United StatesCopyright © 2019 Dasgupta, Baade, Aitken, Ralph, Chambers and Dunn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter D. Baade, cGV0ZXJiYWFkZUBjYW5jZXJxbGQub3JnLmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.