- 1Department of Radiation Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin's Clinical Research Center for Cancer, Tianjin, China
- 2Department of Immunology,Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin's Clinical Research Center for Cancer, Tianjin, China
- 3Department of Clinical Laboratory, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin's Clinical Research Center for Cancer, Tianjin, China
Purpose and Objectives: Chemoradiotherapy (CRT) is an important component of treatment for patients with locally advanced esophageal squamous cell carcinoma (ESCC). Recent research findings support the role of CRT in activating an anti-tumor immune response. However, predictors of CRT efficacy are not fully understood. The aim of this study was to measure CRT-induced changes to lymphocyte subpopulations and to evaluate the prognostic value of lymphocyte alterations for patients with ESCC.
Materials and Methods: In total, this pilot study enrolled 64 patients with ESCC who received neo-adjuvant CRT or definitive CRT. Peripheral blood samples were collected before and during treatment and were analyzed by flow cytometry for CD19, CD3, CD4, CD8, CD56, and CD16. Relationships between lymphocyte subset alterations and overall survival (OS) and progression-free survival (PFS) were evaluated using the log-rank test and a Cox regression model.
Results: The median follow-up period was 11.8 months (range, 4.0–20.2 months). Compared to pre-treatment specimens, post-treatment blood samples had decreased proportions of CD19+ B-cells and increased proportions of CD3+ and CD8+ T-cells (all P < 0.05). Univariate and multivariate analysis showed that increased CD4+ T-cell ratios after CRT independently predicted superior PFS (hazard ratio [HR] = 0.383; 95% confidence interval [CI] = 0.173–0.848, P = 0.017) and that increased CD8+ T-cell ratios predicted improved OS (HR = 0.258; 95% CI = 0.083–0.802, P = 0.019). Patients with both increased CD4+ and CD8+ ratios had a superior PFS and OS, compared to patients with an increased CD4+ ratio only or CD8+ ratio only or neither (1-year PFS rate 63 vs. 25%, 1-year OS rate 80 vs. 62%, P = 0.005 and 0.025, respectively).
Conclusions: CRT-induced increases in CD4+ and CD8+ T-cell ratios are reliable biomarker predictors of survival in patients with ESCC.
Introduction
Esophageal carcinoma is a leading cause of cancer and cancer death worldwide. Esophageal squamous cell carcinoma (ESCC) is the major form of esophageal carcinoma in Eastern Europe and Asia, with a 5-year overall survival of 15–25% (1–3). In addition to well-described clinical and therapeutic determinants of survival, the tumor microenvironment and host immune response following treatment may affect prognosis. Lymphocytes play an important role in regulating the host immune and anti-tumor responses (4). Tumor infiltrating lymphocytes (TILs) and peripheral blood lymphocytes (PBLs) have shown promise as predictive biomarkers for their association with improved clinical outcomes for various malignancies (5–8).
Favorable outcomes following neo-adjuvant chemoradiotherapy (neo-CRT) have led to the recognition of neo-CRT alongside definitive chemoradiotherapy (CRT) as a standard treatment option for locally advanced ESCC (9). Although historically viewed as immunosuppressive, CRT has been known to activate immune system through multiple mechanisms including initiating immunogenic cell death (ICD) and production and release of inflammatory factors into tumor microenvironment, leading to improved tumor antigen expression and presentation as well as infiltration by various immune cells (10–12). Peng et al. found that neo-adjuvant chemotherapy (NACT) was associated with increased CD8+ TIL levels in post-treatment tumors (13). Teng and colleagues found increased CD4+ and CD8+ but stable Foxp3+ TIL densities in tumors resected from patients who underwent CRT (6). Similarly, CD8+ TIL densities rise dramatically following CRT in patients with rectal cancer (5). However, alterations to specific PBL subsets during CRT remains unstudied, so little is known about their prognostic value in patients with ESCC.
The present study aimed to evaluate changes in PBL subpopulations among patients with ESCC undergoing CRT. We measured the proportion of each PBL subtype in peripheral blood specimens before treatment and during treatment using flow cytometry. Furthermore, we evaluated the potential value of PBLs for predicting clinical outcomes in patients with ESCC.
Materials and Methods
Study Population
This pilot study enrolled patients with ESCC who received neo-adjuvant chemoradiotherapy (weekly cisplatin and docetaxel chemotherapy for four cycles combined with radiotherapy with a dose of 40 Gy in 20 fractions) followed by radical surgery 4–6 weeks later or definitive chemoradiotherapy (weekly cisplatin and docetaxel chemotherapy for four cycles combined with radiotherapy with a dose of 60 Gy in 30 fractions) between 2015 and 2017. Informed consent was obtained from each patient to undergo blood sample collection before treatment and at the time when the total radiation dose of 40 Gy was reached.
All patients met several inclusion criteria: (i) diagnosis of ESCC based on pathologic evaluation; (ii) age older than 18 years; (iii) Karnofsky performance status of 70 or higher, (iv) receipt of radiation therapy (RT) using a conventional fraction; (v) availability of baseline clinical, laboratory, and follow-up data; (vi) no concomitant receipt of immunosuppressants or antitumor therapy and no prior malignant tumor removal; and (vii) survival time of at least 3 months following CRT. Patients were excluded if they had a history of other malignancy, had not completed CRT, or had evidence of distant metastasis. A total of 64 patients with ESCC were included in the study as of April 2017. All patients were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual: Esophagus and Esophago-gastric Junction. The study was approved by the medical ethics committee of the institute.
Peripheral Blood Lymphocyte Phenotype Detection
Blood samples were obtained by venous puncture before treatment and at the time when the total radiation dose of 40 Gy was reached. Four-color flow cytometric analysis of peripheral lymphocytes was performed. For each patient sample, two tubes were labeled with the sample identification number and A or B. Each tube was aliquoted with 50 μL of mixed, anticoagulated whole blood. Next, a pipette was used to add 20 μL of BD Multitest CD3 FITC/CD8 PE/CD45 PerCP/CD4 APC reagent (catalog no 340503, BD Biosciences, San Jose, CA, USA) into the bottom of each tube labeled A. Then, 20 μL of BD Multitest CD3 FITC/CD16+CD56 PE/CD45 PerCP/CD19 APC reagent (catalog no 340503, BD Biosciences, San Jose, CA, USA) was added into B tubes using a pipette. All samples were mixed gently using a vortex, then incubated for 15 min in the dark at room temperature. Following incubation, 450 μL of lysing solution was added to each tube. After 15 min at room temperature protected from light, cells were washed twice with staining buffer to remove unbound antibodies. Afterward, the samples were immediately analyzed by flow cytometry.
CD45 recognizes human leucocyte antigens in peripheral blood. T cells were defined by CD3 expression and B cells by CD19 expression. T-lymphocyte subsets were identified by the presence of CD4 and CD8. CD56 and CD16 together facilitate identification of the natural killer (NK) lymphocyte population.
Follow-Up and Statistical Analysis
After treatment completion, contrast-enhanced computed tomography (CT) scans of the chest were obtained within 3 months and then every 6 months. Overall survival (OS) was calculated from initiation of CRT to the date of death from any cause or the date of the last follow-up. Patient characteristics were evaluated using descriptive statistics. Weight loss was defined as >5% unintentional weight loss during the 3 months before disease diagnosis. The lymphocyte counts before treatment and during treatment were compared using paired t-tests. CD4+ or CD8+T cell proportion was defined as the percentage of CD4+ or CD8+T cells in the CD45+ lymphocytes. Changes in CD4+ T cell proportion before and after CRT was defined as CD4+ T cell ratio = post-treatment CD4+ T cell proportion/pretreatment CD4+ T cell proportion, and changes in CD8+ T cell proportion before and after CRT was defined as CD8+ T cell ratio = post-treatment CD8+ T cell proportion/pretreatment CD8+ T cell proportion. The log-rank test was used to compare Kaplan-Meier survival curves. Multivariate analyses were performed using Cox proportional hazards regression. All statistical analyses were performed using SPSS software version 20.0 (SPSS Inc., Chicago, IL) and Graph Pad Prism 5 (Graph Pad Software Inc., San Diego, CA, USA). A P < 0.05 was considered statistically significant for two-sided tests.
Results
Baseline Patient Characteristics
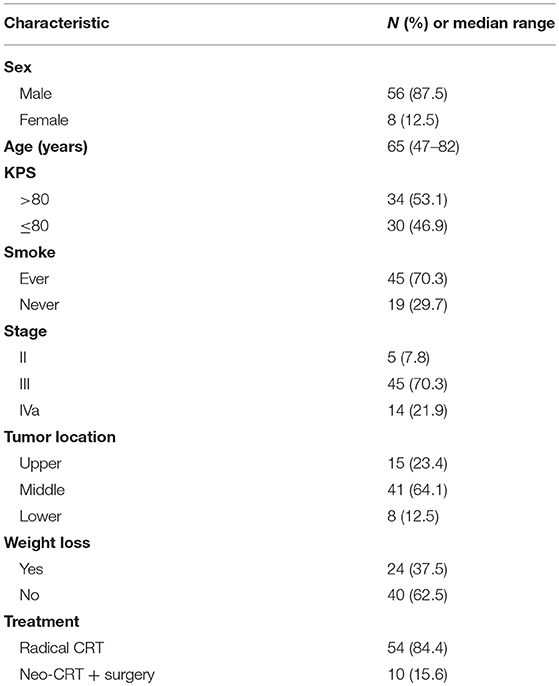
A total of 64 patients met study inclusion criteria and were fully evaluated. Blood specimens were obtained pre-treatment and during treatment for each patient. Of the 64 patients, 56 (87.5%) were men, and 19 (29.7%) had never smoked. The median patient age was 65 years (range, 47–82 years). Baseline patient characteristics are detailed in Table 1.
In present study, 61 patients completed radiotherapy, and 63 completed chemotherapy. Treatment-related toxicities during CRT included nausea (82.8%), leucopenia (62.5 %), and esophagitis (81.2%). The most common (≥5%) grade 3 or 4 toxicity included leucopenia (7.8%), esophagitis (23.4%). No treatment-related Grade 5 toxicity occurred during CRT. Most patients recovered with supportive care. Four patients in this study discontinued treatment as a result of severe hematologic toxicity (n = 1) and esophagitis (n = 3).
Lymphocyte Changes in Peripheral Blood After Chemoradiotherapy
All patients completed treatment. The changes in PBL subset proportions and absolute numbers in patients with ESCC are presented in Figure 1, Tables 2, 3. As shown, the proportion of CD19+ B cells decreased the most markedly following CRT, from 7.5 to 2.9% (P < 0.001). In contrast, proportions rose after treatment for CD8+ cells (26.1–30.6%; P = 0.001) and CD3+ cells (62.4–68.3%; P < 0.001). However, there were no changes in the proportions of CD4+ cells (34.8–35.4%; P = 0.683) or NK cells (26.7–27.7%; P = 0.345).
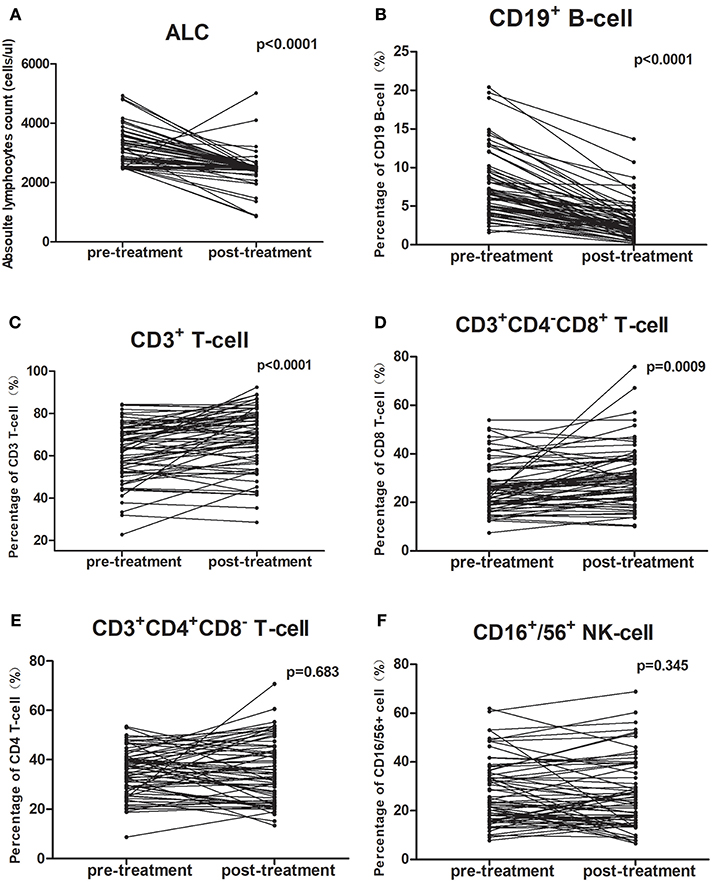
Figure 1. Chemoradiotherapy-induced alterations of circulating lymphocyte subpopulations for all cases. The proportion of each lymphocyte subpopulation before and during treatment were compared using paired t-test. (A,B) The mean absolute lymphocyte counts and proportion of CD19+ B cells were lower after CRT; (C,D) The proportions of CD3+ T-cells and CD3+CD4−CD8+ T-cells were higher after CRT; (E,F) The percentage of CD3+CD4+CD8− T-cells and CD16+/56+ NK cells did not change after CRT.
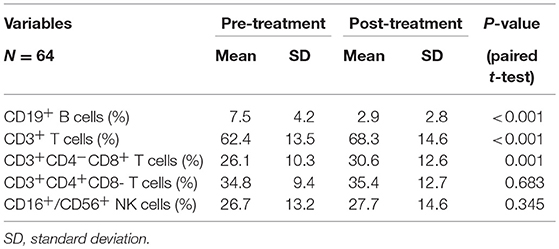
Table 2. Chemoradiotherapy-induced alterations of circulating lymphocyte subpopulation proportion for all cases.
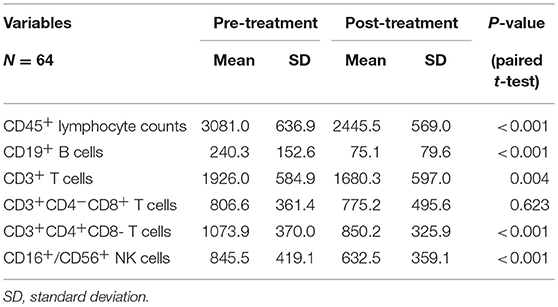
Table 3. Chemoradiotherapy-induced alterations of circulating lymphocyte subpopulation counts for all cases.
The mean absolute lymphocyte counts decreased from 3,081 before CRT to 2,445 after CRT (P < 0.001). Moreover, the absolute counts of CD19+ B cells, CD16+/CD56+ NK cells, CD3+ T cells, and CD4+ T cells also decreased after CRT (P < 0.05, respectively). However, there was no significant changes in the absolute counts of CD8+ T cells (P = 0.623).
Prognostic Significance of Chemotherapy-Induced Changes in CD4+ and CD8+ T-Cell Ratios in Patients With Esophageal Squamous Cell Carcinoma
The median follow-up period was 11.8 months (range, 4.0–20.2 months). At last follow-up, 12 patients (18.7%) had died with disease progression, 14 (21.9%) were alive with disease progression, and 38 (59.4%) were alive without progression.
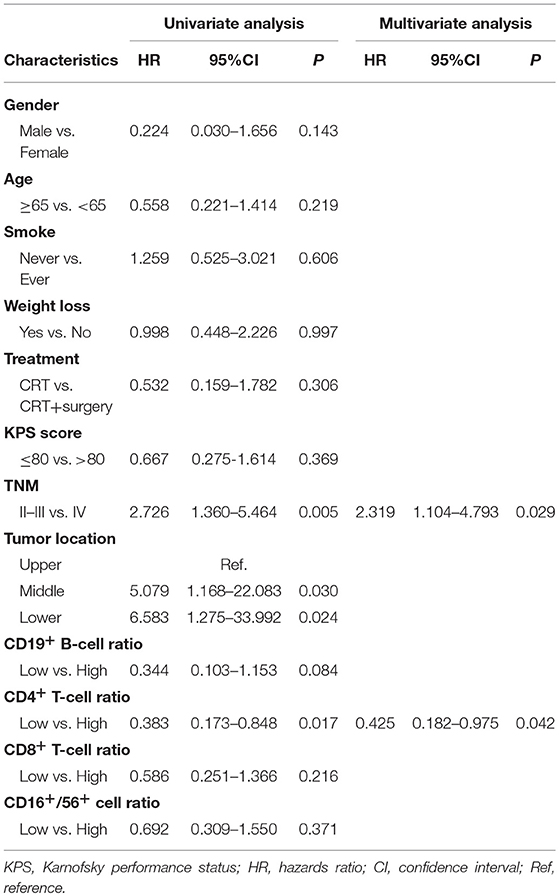
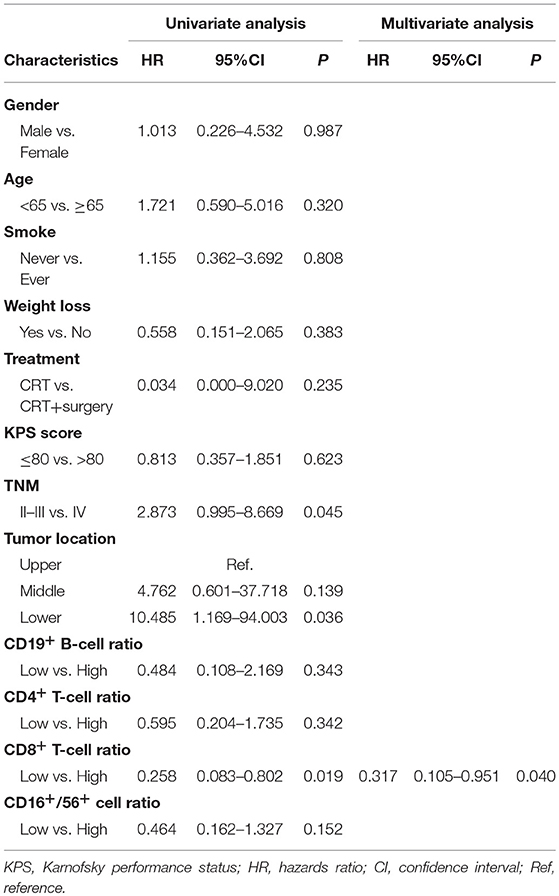
An increased CD4+ T-cell ratio after CRT correlated closely with superior PFS (Figure 2A; hazard ratio [HR] = 0.383; 95% CI = 0.173–0.848, P = 0.017), while CD8+ T-cell ratio was not associated with PFS (Figure 2B, P = 0.216). In univariate analysis, TNM stage, tumor location, and increased CD4+ T-cell ratio were associated with PFS. However, only increased CD4+ T-cell ratio (P = 0.042) and TNM stage (P = 0.029) were independent predictors of PFS on multivariate analysis (Table 4). Similarly, an increased CD8+ T-cell ratio after CRT was associated with improved OS (Figure 2D; HR = 0.258; 95% CI = 0.083–0.802, P = 0.019), while CD4+ T-cell ratio showed no predictive significance (Figure 2C, P = 0.342). More importantly, multivariate analysis showed that increased CD8+ T-cell ratio (P = 0.040) was the only independent predictor of OS (Table 5).
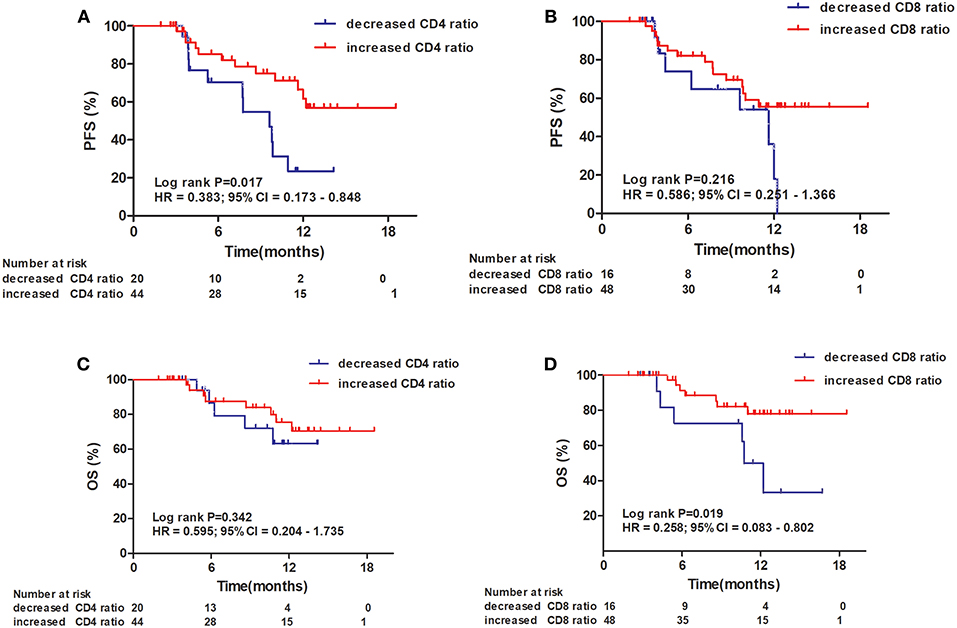
Figure 2. Progression-free survival (PFS) and overall survival (OS) of patients with esophageal squamous cell carcinoma. Progression-free survival curves for patients by CD4+ T-cell ratio (A) and CD8+ T-cell ratio (B). Overall survival curves for patients by CD4+ T-cell ratio (C) and CD8+ T-cell ratio (D).
When the 64 patients with ESCCs were separated into two groups, the 32 patients with both increased CD4+ and CD8+ ratios had a superior PFS and OS, compared to the 32 patients with an increased CD4+ ratio only or CD8+ ratio only or neither (1-year PFS rate 63 vs. 25%, 1-year OS rate 80 vs. 62%, P = 0.005 and 0.025, respectively). Representative Kaplan–Meier survival curves based on these factors are shown in Figures 3A,B.
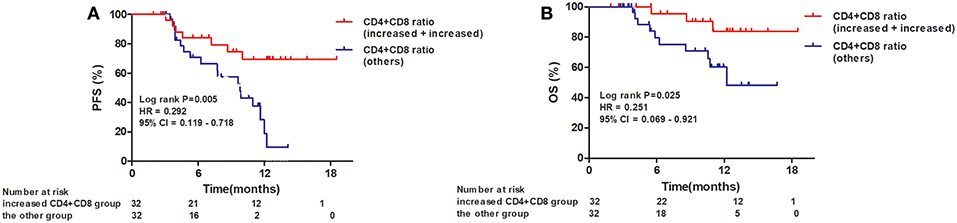
Figure 3. Kaplan–Meier survival curves for overall survival and progression-free survival based on both increased CD4+ T-cell and CD8+ T-cell ratios compared to all others. (A) Progression-free survival curves for patients by combined CD4+ and CD8+ T-cell ratios; (B) Overall survival curves for patients by combined CD4+ and CD8+ T-cell ratios.
Discussion
Neoadjuvant CRT followed by surgery has been considered the standard treatment for locally advanced esophageal cancers, however, the optimal chemotherapy regimen remains controversial (9, 14, 15). Docetaxel is well-known to be a potent radiosensitizer. A phase II trial reported that concurrent CRT with weekly docetaxel and cisplatin was well-tolerated and resulted in favorable activity in terms of both tumor and symptom control (16). Moreover, Xi et al. suggested that, compared to traditional fluorouracil and cisplatin regimen, concurrent chemoradiotherapy with docetaxel and cisplatin in squamous cell esophageal carcinoma was associated with a satisfactory outcome and manageable toxicity (17). Collectively, these findings indicated that the combination of docetaxel and cisplatin for concurrent CRT in esophageal cancer is promising. Growing evidence suggests that CRT plays an important therapeutic role for patients with ESCC, however, factors predicting CRT efficacy, which would facilitate optimal disease management for patients with ESCC, have yet to be fully elucidated. Recent research supports the role of CRT in activating the tumor immune microenvironment to affect tumor response and prognosis (12, 18). Therefore, we hypothesized that circulating lymphocytes, crucial components of human antitumor immunity, may affect response to CRT. This prospective study is the first to measure CRT-induced changes in lymphocyte subpopulations and their prognostic value in ESCC. Our study showed that CRT can activate the immune system and that CD4+ and CD8+ T-cells are positively associated with superior PFS and prolonged survival.
Lymphocytes account for ~30% of the normal human white blood cell population and play an important role in antitumor immunity (4). PBLs are well-known to be extremely sensitive to radiation. Even low-dose total body radiation can decrease circulating lymphocyte counts, which in turn may compromise antitumor immune responses during CRT (19, 20). Previous reports involving non-small cell lung cancer and unresectable hepatocellular cancer, demonstrated a significant correlation between lymphocyte nadirs and radical RT (8, 21). However, the radiosensitivity of different lymphocyte subsets seems to be inconsistent. Stereotactic body radiotherapy for early stage non-small cell lung cancer suppresses all lymphocyte subset counts for 1–4 weeks following treatment completion (22). Our results showed that absolute PBL counts declined after CRT, and that the drop was most pronounced in the B-cell subgroup. This finding was consistent with previous studies that show B-lymphocytes to be the most radiosensitive subpopulation in non-hematologic malignancies. This sensitivity may be caused by the Ku86 protein variant that has a decreased ability to recruit the catalytic component of the DNA-protein kinase complex, which is crucial to DNA double-strand break repair (23). In addition to its immunosuppressive effects, CRT has been known to be immune stimulatory to enhance anti-tumor immune response (10–12). Previous studies that show a positive correlation between CRT and proliferative CD8+ TIL activity (24). Our study also found that tumor-specific CD8+ cytotoxic T lymphocytes (CTLs) levels increased after CRT.
Accumulated clinical studies demonstrated a closely association with high lymphocytes and better patient outcomes in several types of human cancer (8, 21). Previous research on T-cells has long been focused on tumor-specific CD8+ CTLs because of their potent killing activity, while CD4+ T-cells have been studied primarily for their role as helpers to CD8+ CTLs. Recent studies indicated that CD4+ T cells were not a pure cell lineage with a single function, but a diverse cell population with complex functions (25, 26). Moreover, CD4+ T cells may serve not only as helper cells, but also as potent effector cells or partners with macrophages and eosinophils to clear a wide variety of tumors (27, 28). Our results showed that CRT-induced increases in peripheral CD4+ and CD8+ T cell levels are a surrogate marker for immunostimulation and are associated with superior survival in patients with ESCC. Furthermore, our study revealed that the prognosis of patients with both increased CD4+ and CD8+ T-cell ratios was remarkably better than that of patients with an increased CD4+ ratio only or CD8+ ratio only or neither. The finding was consistent with the well-established cooperative role of CD4+ and CD8+ T-cells in tumor eradication (29). Therefore, these results indicated the combination of CD4+ and CD8+ T-cell could be a promising and valuable prognostic marker of anti-tumor immune response after CRT. It may provide the rationale for multipronged approaches to combine CRT with immunotherapy in patients with ESCC, with the goal of enhancing immune response more efficiently to improve survival.
In addition, CRT-induced CD19+ B-cell decreases in our study weakened humoral immunity and presumably resulted in worse outcomes for patients with ESCC (but this difference did not reach statistical significance). In patients with cancer, CD19+ B lymphocytes play an important role in humoral immunity through their specific binding to B-cell activating factor (BAFF) and production of antibodies for tumor-associated antigen. Moreover, B cells can also process and present antigens to induce T cell immune responses and interact with macrophages and the complement system to kill tumor cells (30–32). Notably, natural killer (NK) cells are a small subset of cytotoxic lymphocytes which contribute to innate and adaptive immunity through cytolysis and release of chemokines and large amounts of T-helper cytokines such as IFN-γ (33). Contrary to previous reports involving non-small cell lung cancer, gastric cancer, and colorectal cancer (34, 35), NK cells were not a marker of improved prognosis in this study. Future research is warranted, therefore, to elucidate the relationship between B-cells and NK cells and clinical outcomes.
Some limitations deserve mention. Firstly, our sample size is relatively small, and patients had a short duration of follow-up. Secondly, additional blood collection time points testing of antigen specific CD4+ and CD8+ T cells may allow for more precise characterization of lymphocyte alterations. Further prospective studies are warranted to validate our findings.
Conclusion
In summary, this study was conducted to evaluate the effects of CRT on PBL subpopulations and evaluate possible clinical implications for patients with esophageal cancer. Moreover, multivariate analysis revealed that CRT-induced alterations of CD4+ and CD8+ T-cells are valuable and promising predictors of survival. This finding suggests that CRT might activate the immune response, thereby affecting treatment response and prognosis. Thus, we propose that immune therapy during CRT may promote anti-tumor immune response to confer a survival benefit to patients with esophageal squamous cell carcinoma.
Ethics Statement
This study was carried out in accordance with the recommendations of national ethical guidelines. The protocol was approved by the Medical Ethics Committee of Tianjin Medical University Cancer Institute and Hospital. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
XC and WZ have designed the paper, performed experiments, and wrote the main manuscript. DQ, YG, CY, and XR have been part of every step in this patients' complicated diagnostic and therapeutic course and gave valuable interpretation of data. YL performed flow cytometric analysis of blood samples. YW, HZ, and PE collected clinical data and conducted follow-up. QP and PW designed and directed the overall project. All the coauthors revised paper critically and gave final approval of this version for publishing. They have ensured that all aspects of the work are accurate and have been appropriately investigated and resolved.
Funding
This study was supported by the National Nature Science Foundation of China (NO. 81401948, 81773235, 81773300), subject of Science and Technology Development Fund of Tianjin Education Commission (NO. 20140111).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet (2013) 381:400–12. doi: 10.1016/S0140-6736(12)60643-6
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
4. Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. (2011) 29:4828–36. doi: 10.1200/JCO.2011.38.0899
5. Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. (2014) 21(Suppl. 3):S414–21. doi: 10.1245/s10434-014-3584-y
6. Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. (2015) 166:721–32.e1. doi: 10.1016/j.trsl.2015.06.019
7. Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, et al. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS ONE (2006) 1:e129. doi: 10.1371/journal.pone.0000129
8. Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. (2014) 89:1084–91. doi: 10.1016/j.ijrobp.2014.04.025
9. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6
10. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell (2015) 28:690–714. doi: 10.1016/j.ccell.2015.10.012
11. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer (2012) 12:860–75. doi: 10.1038/nrc3380
12. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. (2015) 41:503–10. doi: 10.1016/j.ctrv.2015.03.011
13. Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. (2015) 75:5034–45. doi: 10.1158/0008-5472.CAN-14-3098
14. van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BPL, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
15. Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. (2007) 8:226–34. doi: 10.1016/S1470-2045(07)70039-6
16. Shim HJ, Kim DE, Hwang JE, Bae WK, Nam TK, Na KJ, et al. A phase II study of concurrent chemoradiotherapy with weekly docetaxel and cisplatin in advanced oesophageal cancer. Cancer Chemother Pharmacol. (2012) 70:683–90. doi: 10.1007/s00280-012-1962-3
17. Xi M, Zhang P, Zhang L, Yang YD, Liu SL, Li Y, et al. Comparing docetaxel plus cisplatin versus fluorouracil plus cisplatin in esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Jpn J Clin Oncol. (2017) 47:683–9. doi: 10.1093/jjco/hyx060
18. Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. (2017) 67:65–85. doi: 10.3322/caac.21358
19. Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. (1987) 139:3199–206.
20. Stratton JA, Byfield PE, Byfield JE, Small RC, Benfield J, Pilch Y. A comparison of the acute effects of radiation therapy, including or excluding the thymus, on the lymphocyte subpopulations of cancer patients. J Clin Invest. (1975) 56:88–97. doi: 10.1172/JCI108084
21. Zhao Q, Xu X, Yue J, Zhu K, Feng R, Jiang S, et al. Minimum absolute lymphocyte counts during radiation are associated with a worse prognosis in patients with unresectable hepatocellular carcinoma. Ther Adv Gastroenterol. (2017) 10:231–41. doi: 10.1177/1756283X16685557
22. Maehata Y, Onishi H, Kuriyama K, Aoki S, Araya M, Saito R, et al. Immune responses following stereotactic body radiotherapy for stage I primary lung cancer. Biomed Res Int. (2013) 2013:731346. doi: 10.1155/2013/731346
23. Louagie H, Van Eijkeren M, Philippe J, Thierens H, de Ridder L. Changes in peripheral blood lymphocyte subsets in patients undergoing radiotherapy. Int J Radiat Biol. (1999) 75:767–71. doi: 10.1080/095530099140113
24. Suwa T, Saio M, Umemura N, Yamashita T, Toida M, Shibata T, et al. Preoperative radiotherapy contributes to induction of proliferative activity of CD8+ tumor-infiltrating T-cells in oral squamous cell carcinoma. Oncol Rep. (2006) 15:757–63. doi: 10.3892/or.15.4.757
25. Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. (1999) 189:753–6. doi: 10.1084/jem.189.5.753
26. Ossendorp F, Toes RE, Offringa R, van der Burg SH, Melief CJ. Importance of CD4(+) T helper cell responses in tumor immunity. Immunol Lett. (2000) 74:75–9. doi: 10.1016/S0165-2478(00)00252-2
27. Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. (1991) 49:281–355. doi: 10.1016/S0065-2776(08)60778-6
28. Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. (1998) 188:2357–68. doi: 10.1084/jem.188.12.2357
29. Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. (2003) 63:1555–9.
30. Schmidt M, Böhm D, von Törne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. (2008) 68:5405–13. doi: 10.1158/0008-5472.CAN-07-5206
31. Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. (2006) 66:5527–36. doi: 10.1158/0008-5472.CAN-05-4128
32. Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. (2006) 16:38–52. doi: 10.1016/j.semcancer.2005.07.006
33. Bellora F, Castriconi R, Dondero A, Carrega P, Mantovani A, Ferlazzo G, et al. Human NK cells and NK receptors. Immunol Lett. (2014) 161:168–73. doi: 10.1016/j.imlet.2013.12.009
34. Gulubova M, Manolova I, Kyurkchiev D, Julianov A, Altunkova I. Decrease in intrahepatic CD56+ lymphocytes in gastric and colorectal cancer patients with liver metastases. APMIS (2009) 117:870–9. doi: 10.1111/j.1600-0463.2009.02547.x
Keywords: esophageal squamous cell carcinoma, clinical outcomes, CD4+ T-cell, CD8+ T-cell, chemoradiotherapy
Citation: Chen X, Zhang W, Qian D, Guan Y, Wang Y, Zhang H, Er P, Yan C, Li Y, Ren X, Pang Q and Wang P (2019) Chemoradiotherapy-Induced CD4+ and CD8+ T-Cell Alterations to Predict Patient Outcomes in Esophageal Squamous Cell Carcinoma. Front. Oncol. 9:73. doi: 10.3389/fonc.2019.00073
Received: 20 June 2018; Accepted: 28 January 2019;
Published: 15 February 2019.
Edited by:
William Small Jr., Stritch School of Medicine, United StatesReviewed by:
Tarita O. Thomas, Loyola University Medical Center, United StatesEric Chi-ching Ko, Cornell University, United States
Copyright © 2019 Chen, Zhang, Qian, Guan, Wang, Zhang, Er, Yan, Li, Ren, Pang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingsong Pang, pangqs_doctor@163.com
Ping Wang, wangping_doctor@163.com
†These authors have contributed equally to this work
 Xi Chen1†
Xi Chen1† Ping Wang
Ping Wang