
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 22 February 2019
Sec. Cancer Molecular Targets and Therapeutics
Volume 8 - 2018 | https://doi.org/10.3389/fonc.2018.00656
This article is part of the Research Topic Targeted Therapies for Glioblastoma: A Critical Appraisal View all 16 articles
 Karishma R. Rajani1†
Karishma R. Rajani1† Lucas P. Carlstrom1†
Lucas P. Carlstrom1† Ian F. Parney1
Ian F. Parney1 Aaron J. Johnson2
Aaron J. Johnson2 Arthur E. Warrington1
Arthur E. Warrington1 Terry C. Burns1*
Terry C. Burns1*Glioblastoma is the most common adult primary brain tumor and carries a dismal prognosis. Radiation is a standard first-line therapy, typically deployed following maximal safe surgical debulking, when possible, in combination with cytotoxic chemotherapy. For other systemic cancers, standard of care is being transformed by immunotherapies, including checkpoint-blocking antibodies targeting CTLA-4 and PD-1/PD-L1, with potential for long-term remission. Ongoing studies are evaluating the role of immunotherapies for GBM. Despite dramatic responses in some cases, randomized trials to date have not met primary outcomes. Challenges have been attributed in part to the immunologically “cold” nature of glioblastoma relative to other malignancies successfully treated with immunotherapy. Radiation may serve as a mechanism to improve tumor immunogenicity. In this review, we critically evaluate current evidence regarding radiation as a synergistic facilitator of immunotherapies through modulation of both the innate and adaptive immune milieu. Although current preclinical data encourage efforts to harness synergistic biology between radiation and immunotherapy, several practical and scientific challenges remain. Moreover, insights from radiation biology may unveil additional novel opportunities to help mobilize immunity against GBM.
Glioblastoma (GBM) is a deadly and highly infiltrative tumor. It is the most common primary brain tumor in adults, causing about 3–4% of all cancer-related deaths (1). Surgery followed by fractionated radiotherapy (RT) and temozolomide (TMZ) has been standard of care for newly diagnosed GBM since 2005 (2). To date, scientific advances in genomics and immunotherapy have failed to translate into effective therapies for GBM, with median survival of just over a year from diagnosis. Once recurrence has occurred, prognosis is extremely guarded with a minority of patients responding meaningfully to second-line therapies or surviving >6 months from time of recurrence (3). Novel approaches to treat GBM are urgently needed and much effort has sought to determine whether immunotherapy may provide a useful adjunct.
Immunotherapies, epitomized by successful trials with checkpoint blockade, have been widely hailed as a breakthrough in cancer therapy over the past decade. Seminal work from the Allison laboratory in 1996 showed that the antibody-blocking cytotoxic T-lymphocyte antigen-4 (CTLA-4) could elicit regression of murine colon carcinoma and fibrosarcoma (4). Since then, several other preclinical models have further validated the effectiveness of blocking CTLA-4 and supported the clinical development of anti-CTLA-4 therapy. The first human phase III study of anti-human CTLA-4 (Ipilimumab) demonstrated improved survival in patients with advanced melanoma (5). Subsequent successes followed with antibodies against programmed cell death-1 (PD-1) and programmed death ligand-1 (PD-L1) (6, 7), confirming the broad utility of blocking inhibitory pathways that interfere with anti-tumor T cell responses.
There is a strong correlation between high somatic mutation burden and the clinical response to immune checkpoint monotherapies (8). Non-synonymous somatic mutations lead to an altered amino acid sequence and give rise to neoepitopes that can serve as neoantigens recognized by the immune system (9, 10), triggering an anticancer immune response. In contrast, GBM has a relatively low burden of neoantigens (11), yielding “cold tumors” for which clinical response immune checkpoint monotherapy is infrequently observed. The “cold” phenotype of GBM is also attributed to recruitment of immunosuppressive immune cell types and secretion of immune suppressive cytokines (12–14). Much work has sought to convert the “cold” GBM phenotype into a “hot” phenotype more responsive to immune checkpoint blockade. To this end, radiation and radiation-induced immune processes have demonstrated particular promise.
Immune infiltration is a doubled sword. Despite the benefit of immune infiltrate for a successful immune therapy response, more aggressive tumors, such as mesenchymal subtype GBM, are typically heavily infiltrated by immune cells (15). In this setting, immune cells are believed to be reprogrammed by the tumor to perform pro-tumorigenic functions. However, whether the presence of robust immune infiltrate is a cause or effect of GBM aggressiveness has been controversial. Mutations in the gene isocitrate dehydrogenase (IDH) are very common in World Health Organization classification of Grade II and III gliomas and in 10% of GBM that have evolved from lower-grade tumors (16, 17). Overproduction of oncometabolite 2-hydroxyglutarate (2HD) in the D-enantiomer is a major hallmark of these glioma subtypes (18). IDH mutation status is an important classifier in stratifying glial tumors. Patients with IDH-mutant gliomas have a substantial survival benefit following chemotherapy and radiation compared to patients with IDH wild type tumors (19). A study by Amankulor et al. used this model to help shed light on the role of immune cells in tumor aggressiveness (20). It is known that IDH-mutant gliomas have fewer tumor-infiltrating immune cells, including T cells, microglia, and macrophages, compared to IDH wild-type tumors; thus IDH-mutant tumors typically exemplify “cold tumors” and may not respond to immunotherapies. The authors generated genetically engineered mice that were identical, except for the presence or absence of IDH mutation, with concomitant increase in 2-HG levels. Decreased leukocyte chemotaxis and prolonged survival was seen in the IDH-mutant tumors supporting the concept of immune infiltration as causatively pathologic in more aggressive gliomas. Whether IDH-mutant gliomas or tumors with inherently lower immune infiltration (e.g., proneural) are inherently less responsive to immunotherapy due to their “cold” phenotype is hypothesized, but remains to be demonstrated clinically. Nevertheless, since radiation is currently standard of care for all subtypes of infiltrative glioma, potential synergy between immunotherapy and radiation is an opportunity to be exploited therapeutically. In such work, the goal will be to promote and maintain an anti-tumorigenic rather than pro-tumorigenic phenotype of recruited leukocytes, even long after completion of radiotherapy.
Preclinical data have provided robust proof of principle that radiation can boost both the local and systemic antitumor immune response to augment tumor control even at sites distant from radiation—eliciting the so-called “abscopal effect.” Although radiation and immunotherapy are both currently employed in early clinical trials of immunotherapy, it is less certain whether their potentially synergistic biology is optimally harnessed using current protocols. Emerging preclinical data suggest that established standards of care for GBM—including radiotherapy fractionation regimens, use of systemically immunosuppressing TMZ, and frequent use of steroids—may need to be revisited before the potential of immunotherapy is fully realized for GBM. This review begins by addressing the current understanding of immune-modulatory effects of radiation and highlights the salient features of the highly immunosuppressive microenvironment of GBM. We then discuss preclinical data supporting the synergistic combination of radiotherapy with immunotherapies targeting both innate and adaptive immune modulators and explore important challenges yet to be overcome in search of a clinically optimal regimen.
The central nervous system (CNS) has long been considered immune privileged due in part to the presence of the blood brain barrier, a unique structural feature that restricts entry of molecules and immune cells into the brain. This view was further supported by relatively low numbers of antigen presenting cells (APCs) and T cells in the brain parenchyma, as well as the historically perceived lack of lymphatic vessels to drain APC and antigen to regional lymph nodes (21). Findings in recent years have challenged long-standing thinking by demonstrating that even the healthy brain is in fact under constant immune surveillance. Brain-derived antigens can entrain peripherally-derived immune cells that in turn penetrate the blood brain barrier (22, 23). Identification of a novel CNS glymphatic system, wherein most APCs could travel from the brain into the cervical lymph nodes and prime T lymphocytes (24, 25), forced reconsideration of the supposedly immune privileged status of the CNS. The revised model is in line with empiric findings of tumor-infiltrating lymphocytes detected in human GBM after vaccination with autologous tumor lysate-pulsed dendritic cells (DCs) (26, 27). It is within this dynamic scientific era that insights are sought from the brain and tumor microenvironments to optimally harness immunotherapy for GBM.
Tumors subvert systemic and local immune mechanisms to establish an immune tolerant microenvironment permissive to infiltration and proliferation. The following sections outline several of the immunosuppressive mechanisms defined to date; the extent to which radiation may help attenuate the immunosuppressive microenvironment of GBM is discussed in section Radiation and GBM.
Like many tumors, GBM express relatively low levels of histocompatibility complex (MHC) class I and II molecules, thereby minimizing display of tumor-associated antigens (28). GBM also secrete immunosuppressive cytokines, such as IL-10 and TGF-β (29). TGF-β is a pleiotropic cytokine that blocks the cytotoxic T cell response and promotes the activity of CD4+ regulatory T cells (Tregs).
Tregs express CD25+ and the transcription factor FoxP3+ (30) and may derive from the periphery (pTregs) from conventional T cells or from the thymus (tTregs) (31). Tregs can be recruited to the tumor or generated via proliferation of pre-existing Tregs in the tumor microenvironment and de novo conversion of tumor-infiltrating CD4+ lymphocytes (TIL) into pTregs (32, 33). Tregs exert their suppressive activity through cell surface molecules such as CTLA-4, perforin, and CD73. These inhibit maturation of APCs and block B7-CD28 co-stimulatory signals. ATP released from dying cells is pro-immunogenic, but is degraded by Tregs. In addition, Tregs can also mediate their suppressive activity via contact-independent mechanisms, secreting inhibitory cytokines that suppress effector T cell function (34).
The enzyme indoleamine 2,3 dioxygenase (IDO) can be produced by both tumor and tumor APCs, including DCs and macrophages (35), to induce immune suppression. IDO contributes to immune tolerance by catabolizing tryptophan to catabolites, such as kynurenine (36). Deprivation of the critical amino acid tryptophan and exposure to metabolites inhibits the proliferation of cytotoxic CD4+ and CD8+ T cells (37), as well as natural killer (NK) cells (38). Preclinical work by Wainwright et al. has demonstrated that GBM tumor-derived IDO increased the recruitment of Tregs and decreased survival of mice with intra-cranial tumors (39). Of note, IDO expression levels tends to positively correlate with glioma grade (40).
Although GBM is confined to the brain, patients with GBM may be profoundly immunosuppressed systemically with decreased numbers (41) and function (42) of circulating lymphocytes. GBM accumulate robust numbers of intra-tumoral activated Tregs that impede the proliferation of, and cytokine secretion by, autologous lymphocytes (43, 44). Furthermore, depletion of Tregs using anti-CD25 antibodies augmented anti-tumor CD4+ and CD8+ T cell responses (45, 46). These studies emphasize the role of GBM-associated Tregs in maintaining a systemic tolerogenic environment that impedes anti-tumor immunity.
Viruses have evolved highly effective strategies for establishing chronic infection and avoiding clearance by the immune response (47, 48). During chronic viral infections, persistent antigen exposure drives CD8+ T cells to increase the expression of inhibitory receptors, dampening their ability to clear the infection (49). This state of decreased proliferation and decreased effector function, including reduced cytokine secretion accompanied by metabolic and transcriptional changes, has been termed “exhaustion” and is also induced by cancers to avoid immune clearance (50, 51). Targeting such T cell exhaustion may be more complex in cancer due to intra-tumoral heterogeneity, resulting from stochastic tumor evolution and spatial gradients within the tumor microenvironment (51). The exhausted T cell phenotype is characterized by upregulation of multiple inhibitory immune checkpoint receptors, such as PD-1 (52), CTLA-4 (4), T cell immunoglobulin 3 (TIM-3) (53), lymphocyte-activation gene 3 (LAG-3), T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT), V-domain Ig Suppressor of T cell Activation (VISTA), and CD39 (54–56). These molecules are prominently expressed on CD8+ TILs from human GBM (57) with stably elevated checkpoint expression restricted TCR repertoire clonality throughout the stages of GBM progression (58). Under normal homeostasis, these molecules play critical immune regulatory roles in mediating tolerance to self-antigens and preventing auto-immunity (59, 60). While it has been known that multiple tumors induce T cell exhaustion to promote survival (61), the degree of T cell exhaustion in patients with GBM was recently determined to be particularly severe (57). To date, the predominant strategy investigated to attenuate T cell exhaustion has included one or more immune checkpoint inhibitors (62). However, modulating metabolic and stromal components in the tumor microenvironment may prove synergistic (51). The potential role of radiation to facilitate such modulation is discussed below.
Numerous preclinical studies have demonstrated efficacy of antibodies targeting CTLA-4 or the PD-1/PD-L1 axis (4, 63, 64). Subsequently, these antibodies have also demonstrated clinical benefit in multiple tumor types, particularly including “hot” tumors with innately high immunogenicity. Monotherapy with ipilimumab, an anti-CTLA-4 antibody, yielded a durable response in ~10% of patients with advanced metastatic melanoma (5). Additionally, lambrolizumab (anti-PD-1) yielded a robust and durable response in about 35% of patients with advanced melanoma (65). Based on numerous such encouraging trials, several immune checkpoint inhibitors have now been FDA approved for multiple cancers. Examples include inhibitors targeting CTLA-4 (ipilimumab), PD-1 (pembrolizumab and nivolumab), and PD-L1 (atezolizumab and avelumab), that have collectively yielded profound impacts on the management of multiple systemic malignancies.
The dysregulation of immune-checkpoint pathways in GBM has provided ample proof of principle suggesting checkpoint inhibitors could also offer a therapeutic avenue for GBM (66). Indeed, in addition to upregulation of inhibitory checkpoint molecules, such as PD-L1 on T-regs and exhausted T-cells, these are also expressed on tumor-associated macrophages and microglia (TAMs) isolated from human GBM (67). Moreover, immunosuppressive cytokines in the GBM microenvironment, including IL-10, promote expression of checkpoint inhibitor expression on GBM itself (67). Despite promising responses in a subset of patients (68), benefits of checkpoint inhibition have yet to be observed in any phase III clinical trial for GBM.
Nevertheless, immune checkpoint dysregulation alone in GBM may be insufficient to portend reliable responses via checkpoint blockade. Increasing data suggest that an elevated tumor mutational burden (69, 70) and a robust lymphocytic infiltrate within the tumor microenvironment (“hot tumors”) correlate with improved clinical response to checkpoint blockade (69, 71, 72). Indeed, consistent with the relatively immunologically “cold” nature of GBM, including modest levels of tumor neoantigens and lymphocytic infiltrate, several late stage clinical trials have failed to demonstrate clinical benefit (see Supplementary Table 1). Nevertheless, promising responses in a subset of patients continue to foster enthusiasm for harnessing checkpoint inhibitors in GBM. The portfolio of checkpoint inhibitors is continuing to expand with preclinical and efficacy data in targeting LAG-3 (73), TIM-3 (74), and TIGIT (75), each showing particular promise in combination with PD-1 inhibition. Moreover, harnessing the use of immunostimulatory strategies, such as radiation, to augment checkpoint responses has generated particularly promising preclinical data (76). The following sections offer additional details regarding the more thoroughly studied checkpoint molecules CTLA-4 and PD1/PDL1 that have provided a foundation for GBM immunotherapy efforts to date.
T cells are typically activated when an MHC-bearing APC presents an antigenic peptide and engages a T cell receptor (TCR). Full activation of T cells requires engagement of the co-stimulatory T cell receptor, CD28, with its ligands, CD80 and CD86, expressed on APC (77). CTLA-4 primarily regulates the early stages of T cell activation. CTLA-4 begins as an intracellular protein, but upon T cell activation translocates to the immunological synapse and co-localizes with TCRs (78, 79). CTLA-4 outcompetes the co-stimulatory TCR CD28 by binding with higher affinity to the ligands CD80 and CD86 expressed on APCs (80). CTLA-4 can also limit conjugation times between T cells and APCs, limit T cell proliferation, and reduce cytokine production (81). CTLA-4 inhibits Akt phosphorylation by activating protein serine/threonine phosphatase PP2A, but does not alter phosphatidylinositol3-kinase (PI3K) activity (62, 82). The intracellular domain of CTLA-4 can recruit the protein phosphatase 2 A to decrease phosphorylation of proteins in the TCR signaling cascade (83). CTLA-4 plays a key role in maintaining immune-regulated homeostasis by enhancing suppressive functions of Tregs (84) and impeding the function of CD4+ helper T cells (85). Anti-CTLA-4 antibodies can mitigate T cell exhaustion by attenuating the inhibitory functions of CTLA-4 and suppressive actions of Tregs. Ipilimumab and tremelimumab were the first anti-CTLA-4 antibodies to enter clinical trials in patients with advanced cancer. Ipilimumab is currently FDA approved for metastatic melanoma and renal cell carcinoma.
In contrast to CTLA-4, which largely regulates T cell activation, PD-1 plays a prominent role in inhibiting proliferation and functions of effector T cell responses. PD-1 is absent on resting naïve and memory T cells, but expressed on tumor infiltrating lymphocytes (TILs) (86). PD-1 is upregulated on activated T cells upon TCR engagement and mediates T cell suppression (87) upon binding PD-L1 (52) or PD-L2 (88). PD-L1, also known as CD274 and B7-H1, is largely undetectable in most normal tissues, but is expressed on macrophages and APCs, particularly in the context of classical (M1) activation (89). PD-L1 is elevated in tumors—not only on APCs, but also tumor cells themselves, promoting tumor cell survival (90, 91). PD-L2 expression is limited to certain immune cell types, mostly DCs, mast cells, and macrophages (87). Both PD-1 and PD-L1 are expressed on Tregs (92). Binding of PD-1 on activated T cells to PD-L1 decreases TCR-mediated signaling by antagonizing PI3K, leading to decreased Akt phosphorylation and thus decreased levels of activation, including decreased IL-2 production and decreased T cell proliferation (62). Engagement of PD-L1 on macrophages to PD-1 promotes IL-10 production, which further promotes immune suppression (93). Currently FDA-approved drugs targeting PD1/PD-L1 for other cancers include the anti-PD1 drug Nivolumab and the anti-PD-L1 drugs pembrolizumab, atezolizumab, and avelumab. No immunotherapeutic drug has been approved to date for glioma.
As exemplified by exhausted T cells, several additional checkpoint molecules exist besides CTLA-4 and PD-1/PD-L1 that regulate T cell activation and are being assessed as targets for immunotherapy (94). Among these, TIM-3 is expressed by IFNγ-secreting T-helper 1 (Th1) cells, DCs, monocytes, CD8+ T cells, and other lymphocyte subsets (95, 96). TIM-3 is expressed on dysfunctional CD8+ T cells in preclinical models of both solid and hematological malignancies (74, 97). Upregulation of TIM-3 is associated with exhaustion of tumor antigen-specific CD8+ T cells in human melanoma and tumor-induced T cell exhaustion is reversed by administration of anti-TIM-3 antibodies (98, 99). TIM-3 is also expressed on Tregs, with TIM-3+ Tregs identified in solid tumors, such as ovarian, colon, and hepatocellular carcinomas (100). As with other checkpoint molecules, including LAG-3 (73) and TIGIT (75), combination therapies blocking TIM-3 in combination with PD-1 exhibited synergistic effects in preclinical tumor models (74, 101). Kim et al. demonstrated that combination therapy of anti-TIM-3 and anti-PD-1 improved survival in mice with GL261 intra-cranial tumors with optimal outcomes observed using both in combination with stereotactic radiosurgery (76). Several of these checkpoint inhibitors are in clinical trials for GBM (see Supplementary Table 1). Available preclinical data suggest a combined strategy of multiple checkpoint inhibitors with pro-immunogenic interventions, such as stereotactic radiosurgery or oncolytic therapy, may yield optimal outcomes. Much work lies ahead to critically and mechanistically evaluate such combinatorial approaches in clinical trials.
The innate immune system, comprising CNS-derived microglia, peripherally-derived neutrophils, macrophages, and lymphoid-derived NK cells, has a central role in both glioma and radiation biology (15). In response to CNS inflammation, activated microglia proliferate, secrete cytokines and chemokines, and upregulate cell surface markers such as CD80, CD86, and MHC-II. Microglia also express pattern recognition receptors and cross-present antigens to activate T cells within the CNS (102, 103). Normally absent from the healthy brain, peripherally-derived macrophages are recruited into the GBM microenvironment where they facilitate antigen presentation, immune induction, and removal of cellular debris. Microglia-derived and infiltrating TAMs can comprise up to half the cells in GBM and play a prominent role in tumor growth and invasion (104). Two distinct polarization states of activated macrophages have been frequently described: classically activated “pro-inflammatory” (M1) and alternatively activated “anti-inflammatory” or “chronic inflammatory” (M2) macrophages (105). M1 macrophages serve an important role in phagocytosis of neoplastic cells (106, 107). However, glioma cells can secrete suppressive immune cytokines, such as IL-10 (108), and TGF-β (109), that promote M2 polarization and suppress the M1 phenotype (110). Characterization of TAMs within human GBM has revealed impaired production of pro-inflammatory cytokines, defective antigen-presentation, and poor induction of T cell proliferation (104). Similarly, the GBM microenvironment can also directly render TAMs tolerogenic. GBM cells can induce downregulation of TNF-alpha production, concomitant with induction of anti-inflammatory cytokine IL-10 from microglia through upregulation of STAT 3 and 5 (108).
Another population of peripherally-derived monocytes within GBM are myeloid-derived suppressor cells (MDSCs) that also act to suppress adaptive immunity (111). MDSCs accumulate in GBM, express PD-L1, and impair CD4+ T cell memory function (112). MDSCs lack macrophage-specific markers, such CD68, CD16, and S100A9 (113), and secrete suppressive cytokines, such as TGF-β (114). Though originally described as pleiotropic cells simultaneously expressing both M1 and M2 polarization markers, more recent work has suggested that MDSC are malleable in their polarization phenotype with M1-polarized MDSCs exhibiting tumoricidal properties (115).
Collectively, these studies illustrate the substantial cross-talk between the multiple constituents of the GBM ecosystem in maintaining a milieu conducive to GBM. The therapeutic potential to reprogram TAMs and MDSCs from pro-tumorigenic to tumoricidal polarization states is an area of intense interest. The following sections provide example mechanisms of innate immune system regulation that could be harnessed to anti-tumor effect. To date, radiotherapy has provided a relatively blunt instrument via which to activate the innate immune system. However, limitations include CNS including CNS toxicity and potential for inadvertent activation of pro-tumorigenic sequelae (15). Improved understanding of innate immune mechanisms may provide opportunities to more effectively attack the tumor, whilst protecting against cognitively deleterious effects of radiation.
Toll-like receptors (TLRs) are pattern recognition receptors (PRRs) expressed by a variety of cell types comprising the innate immune system. The primary function of TLRs is to sense damage and mediate response to pathogens and tumors. TLRs bind to pathogen associated molecular patterns (PAMPs), conserved structures expressed by pathogens, and danger-associated molecular patterns (DAMPs), such as high mobility group box 1 (HMGB1) and fatty acids. TLR 2, 3, 4, and 9 are expressed on human microglia and TAMs (116). DCs also play a prominent role in the development of anti-glioma immunity and anti-tumor response (117). Dead glioma cells release HMGB1 which can activate TLR 2 on DCs, promoting expansion of T cells (118). Preclinical studies with intra-cranial tumors have shown that administration of TLR 3 agonist poly(I:C) attenuated tumor growth in mice (119). Additionally, CpG, in combination with tumor lysate, effectively induced maturation of DCs to control tumor growth (120). Recent work from the Lim laboratory found that that mice treated with poly(I:C) and anti-PD-1 in combination demonstrated increased DC activation, T cell proliferation, and improved tumor control (76). In a phase I clinical study, concomitant administration of DC vaccine, together with adjuvants comprising the TLR7 agonist imiquimod or poly(I:C), appeared safe and increased serum levels of TNF alpha and IL-6 (26). Clinical trials evaluating the safety and efficacy of TLR9 agonist CpG oligodeoxynucleotides demonstrated safety, but no improvement in survival when combined with standard care radiotherapy and TMZ (121–123).
CD47 is a transmembrane immunoglobulin that binds to integrins and serves as a receptor to signal regulatory protein alpha (SIRP1α) and Thrombosponin-1 (TP-1). Expressed on most tumor cells, including GBM (124), CD47 signals “don't eat me” to macrophages. CD47 binding by SIRP1a initiates a signaling cascade that promotes phosphorylation of intracellular ITIMs and activates inhibitory phosphatases SHP-1 and SHP2 (125). These phosphatases dephosphorylate immunoreceptor tyrosine-based activation motifs inhibit pro-phagocytic signals and disrupt cytoskeleton rearrangements necessary for macrophage phagocytosis (125, 126). Antibodies blocking CD47 have been investigated in multiple tumor types to help promote macrophage tumor phagocytosis with efficacy observed in numerous preclinical models, including GBM (124, 127). Clinical trials are underway for both hematologic and solid malignancies (128, 129). Used in combination with radiation, CD47 inhibition has been shown to improve tumor radiosensitivity (130). Anti-CD47 therapy has also been shown to boost antigen presentation (131, 132) and augment cytotoxic CD8+ T cell activity (133). As an adjuvant to radiation therapy, CD47 blockade has the unique advantage of mitigating radiation-induced TSP-1 signaling, which promotes resistance to radiation injury due to decreased inhibition of nitric oxide signaling in normal tissues. As such, whereas most radiation sensitizers increase damage to both tumor and normal tissues alike, the unique biology of CD47 blockade may concurrently enable improved tumor radiosensitivity (via improved phagocytosis) (134), whilst enhancing radioresistance of healthy tissues via increased nitric oxide signaling (130).
Chemokines, such as colony stimulating factor 1 (CSF-1) and its receptor CSF1R, regulate macrophage homeostasis by regulating proliferation, differentiation, migration, and survival. The intra-tumoral presence of CSF1R-expressing macrophages correlates with poor survival of patients with solid tumors (135). Secretion of CSF-1 by GBM impacts tumor progression through CSF1R signaling. Treatment of GBM with the CSF-1R inhibitor, BLZ945, in transgenic mouse and human xenograft models suppressed tumor growth and improved survival. Although the number of TAMs was not affected, the expression of M2 markers was decreased, consistent with a reduced tumor-supportive phenotype (136). TAMs support tumor progression by blocking anti-tumor immunity and secreting factors to promote angiogenesis (137). TAMs secrete cytokines, such as TGF-β and IL-10, which augment Treg populations while inhibiting effector T cell activity (138). TAMs have been shown to reversibly change their functional phenotype upon exposure to the tumor microenvironment (139). Therefore, strategies that alter the microenvironment to facilitate the repolarization of M2-like TAMs to a M1-tumor-suppressive phenotype are a potential clinical strategy (140).
Radiotherapy is a cornerstone of management for GBM with radiation typically delivered to the enhancing tumor and infiltrative margin via 30 fractions of 2.0 Gy, using IMRT or 3D-conformal therapy. Shorter courses have been considered in elderly patients or as a salvage therapy in recurrent disease. Fractionated radiosurgery has been explored on a trial basis without obviously worse outcomes than standard therapies (141), but has not been adopted in standard management protocols. Radiation acts to ablate dividing cells, induce senescence within non-ablated cells (142). Radiation also stimulates local tumor immunity, promoting anti-tumor immune responses via a host of molecular mechanisms (Figure 1).
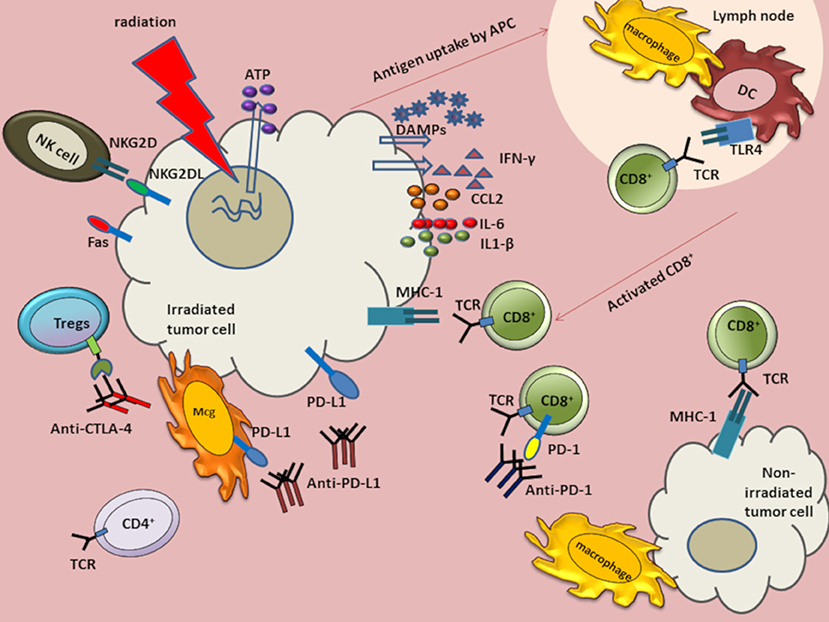
Figure 1. Anti-tumor immune response augmented by the abscopal effect of radiation in combination with immunotherapies. Radiation induces DNA damage and cell death. The dying cells release ATP and DAMPs such as HMGB1 and calreticulin. Although HMGB1 binds TLR4, ATP and calreticulin modulate TLR4 signaling without directly binding to TLR4. Radiation also induces release of tumor antigens to antigen presenting cells (APCs), such as macrophages and dendritic cells (DCs). Antigens are then processed and presented on major histocompatibility complex (MHC) Class I molecules to activate and induce proliferation of CD8+ T cells. The activated cytotoxic CD8+ T cells migrate to tumor sites to induce cell death. Radiation can also induce release of cytokines IL-6 and interferon-gamma (IFN-γ). Radiation also increases tumor cell expression of programmed cell death-1 ligand (PD-L1) and MHC class I molecules. Radiation upregulates immunomodulatory surface proteins, such as Fas and NKG2D ligands on tumor cells. The NKG2D upregulation facilitates NK-mediated tumor cell death. Antibodies, such as α-CTLA-4, α-PD-L1, and α-PD-1 have been used as cancer immunotherapies. When combined with radiation, these antibodies can augment anti-tumor responses in GBM. Anti-CTLA-4 can bind CTLA-4 on Tregs and downregulate suppressive activity. Anti-PDL1 can interact with PD-L1 on tumor cells and on myeloid derived suppressor cells (MDSCs) to curtail suppressive activity induced by MDSCs. Anti-PD-1 antibody can bind to programmed cell death-1 (PD-1) expressed on exhausted T cells.
MHC class I molecules present intracellular peptide fragments to T cells and are expressed on the surface of all nucleated cells, albeit with reduced expression in tumor and stem cells. MHC class 1 molecules are highly expressed on APCs where they may present phagocytosed peptides from tumors. After activation of APCs, such as DCs, antigens are cross-presented to CD8+ T cells. In the healthy brain parenchyma, microglial cells are the main resident antigen-presenting innate immune cell (143). DCs are also present in the choroid plexus (144). After radiation, the extracellular presence of danger-associated molecular patterns (DAMPs) and cytokines, such as MCP1, contribute to rapid microglial activation (145, 146). We have previously shown that radiation induces a unique polarization state in microglia, which is more closely related to M1 than M2, but distinct from both (147). How the transcriptional responses of human microglia and mouse microglia compare following radiation remains to be determined, though persistent microglial activation has been reported in humans even decades following brain radiation (148). Few lymphocytes are typically found in the healthy brain, despite the role of memory CD4+ memory cells in CNS immunosurveillance (21). Murine brain radiation induces a delayed CNS recruitment of T cells, even in the absence of tumor (149).
NK cells are present in relatively low numbers within the GBM microenvironment, when compared to other tumor types (150). Moreover, these NK cells express relatively low levels of the activating receptor natural killer group 2D (NKG2D) (151). Even within the periphery, patients with GBM demonstrate relatively low numbers of circulating NK cells (152), a number that, like T cells (153), falls further after standard radiation and TMZ (152). NKG2D ligands are potent mediators of both the innate and adaptive immune system (154). Radiation upregulates NKG2D ligands in multiple tumor cell lines, which sensitizes them to NK cell mediated cytotoxicity (110). At present, the impact of radiation on NK cell infiltration into GBM is unclear, though may vary as a function of concomitant TMZ and radiation fractionation schemes.
Although GBM display relatively low levels of surface MHC class I (155), radiation increases MHC class I levels, enhancing cross-presentation of tumor associated antigens in the draining lymph nodes and facilitating recognition of antigenic peptides by CD8+ T cells (156–158). Thus, radiation-induced changes can facilitate activation and proliferation of T cell populations to augment anti-tumor immune response.
Interferon (IFN) levels are robustly elevated following radiation and augment systemic anti-tumor immune response. Of the three distinct types of IFN, types I and II play an important role in sculpting anti-viral and anti-microbial defenses. DNA released from irradiated tumor cells is sensed by stimulator of interferon genes (STING) molecules present on DCs to produce type I IFN. Activation of STING pathway and IFN signaling is required for efficient radiation-induced adaptive immune response (116). IFN-γ, a type II interferon, can upregulate MHC class I and NKG2D expression to increase tumor recognition, inhibit development of Tregs, and increase the induction of cytotoxic T cells (159). Radiation-induced production of IFN-γ by CD8+ T cells augments the immunostimulatory anti-tumor effects of radiation (160).
Interestingly, not all of the pro-inflammatory impacts of radiotherapy necessarily serve to enhance anti-tumoral immunity, illustrating the complexity of regulating immune responses. For example, INF-γ and hypoxia—both of which are induced by radiation—upregulate PD-L1 expression on tumor and tumor-associated immune cells (161, 162). Consistent with this finding, anti-PD-L1 therapy has demonstrated synergistic impacts with radiation to promote anti-tumor immunity (161, 163); results that have been found in metastatic melanoma to be further enhanced by deploying radiation in combination with dual checkpoint blockade (164). Recent data in preclinical models indicate the same may likely hold true in GBM (76).
Single tumor radiation has occasionally been clinically reported to decrease growth of tumors at distant sites—a previously poorly-understood phenomenon termed the abscopal (ab: “away from;” scopos: “target”) effect (165). In 2004, Demaria et al. used the growth factor Flt3-Ligand to experimentally enhance numbers of antigen presenting cells providing direct evidence that the abscopal effect is immune mediated and tumor-type specific (166). Numerous studies of metastatic cancers have since demonstrated that radiation in combination with checkpoint inhibitors augment the abscopal effect (167–169). Unlike metastatic cancers for which the abscopal effects may be harnessed to attenuate growth of metastatic lesions elsewhere in the body, GBM is typically restricted to a single (occasionally multifocal) lesion within the CNS. Theoretical limitations of a modest neoantigen repertoire, as well as historically regarded modest CNS immune surveillance, could further confound efforts to elicit an abscopal effect for GBM. Nevertheless, the infiltrative nature of GBM, making it refractory to resection, together with known dose-limiting toxicity of brain radiation, increase motivation to harness abscopal biology against infiltrative tumor cells. Multiple studies have reported that systemic immune status may dictate therapeutic efficacy of radiation (170, 171), providing further impetus to optimize radiation by augmenting immune responsiveness.
Although radiation alone has proven unable to cure glioma, radiation does kill a subset of tumor cells—particularly those that are rapidly dividing. Such cell death facilitates antigen release, as required for adaptive immunity, and stimulates innate immune responses (Figure 1). Radiation induces several types of DNA damage, including simple and complex double stranded breaks (172) with cytotoxic effects (173). Mechanisms of radiation-induced cell death can include necroptosis (174) and p53-dependent apoptosis (175). Radiation-induced mitotic catastrophe may result from radiation, as characterized by aberrant nuclear morphology, multiple nuclei or micronuclei, typically leading to cell death when cells subsequently attempt to divide. However, a small subset of cells may survive with aneuploid or poplyloid karyotypes (176).
Immune activation, as augmented by radiation-induced cell death, facilitates subsequent activation of both the innate and adaptive immune systems against the tumor (177). Immunogenic cell death is mediated by the release of DAMPs directly by tumors or by inflammatory cells present in the microenvironment. Radiation may promote immune activation and immunogenic cell death via at least three mechanisms.
(1) Translocation of Calreticulin (CRT): CRT is a DAMP that is typically restricted to the endoplasmic reticulum. Translocation of CRT to the cell surface of dying cells stimulates DCs to cross-present antigens to cytotoxic T cells (178).
(2) Extracellular release of HMGB1 and ATP: Extracellular HMGB1 induces DC activation through TLR-4. TLRs play an essential role in activation of APCs (179) and microglia (180), as well as release of pro-inflammatory signals, including IFN-γ (156, 160). The physical interaction between HMGB1 and TLR4 further prompts optimal cross-presentation of antigens derived from tumor cells by DCs to T cells (181). ATP release from dying cells can also trigger IL-1- β production and priming of CD8+ T cells by activating P2RX7 and PR2Y2 receptors on DCs and macrophages, respectively (182).
(3) Translocation of heat shock proteins: Cell surface expression of heat shock proteins HSP70 and HSP90 on dying cells induces NK cell activation and promotes cross-presentation of tumor antigens to facilitate DC maturation. Given tumor cell death releases tumor-specific antigens to APCs, including DCs, such cross-presentation of antigens to cytotoxic CD8+ T cells facilitates an anti-tumor T cell response (177, 183).
(4) Upregulated Fas expression: Garnett et al. have demonstrated radiation increases surface expression of Fas on tumor cells, which augments their destruction by antigen-specific immune effector cells via Fas-dependent mechanisms (184). Binding of Fas, a plasma membrane death receptor protein, to its extracellular ligand, Fas-L, activates caspase 3 and triggers apoptosis. The Fas-FasL axis is integral to maintenance of regulation of immune homeostasis (185, 186) and CD8+ T cell-mediated cytotoxicity (187). CD8+ T cell cytotoxicity is a multi-step process in which the effector cells act to induce cell death by forming cell–cell contacts with potential target cells expressing cell death triggering ligands. Following MHC- antigen recognition, CD8+ T cells lyse target cells via secretion of granzyme and perforin and by the engagement of FasL on T cells with Fas expressed on target cells. Both pathways lead to apoptotic cell death (188).
Multiple preclinical studies provide robust proof of principle supporting the combined role of radiation and immunotherapy for GBM (64, 76, 189). In an orthotopic (intracranial) GL261 mouse model, median survival doubled from 27 days with anti-PD1 antibody alone and 28 days in radiation alone, to 53 days when the two modalities were combined. Immunohistochemistry confirmed increased tumor infiltration of cytotoxic CD8+ T cells and decreased regulatory CD4+ T cells in the combination group (64). Similarly, combined radiation and use of an agonist antibody for the co-stimulatory molecule glucocorticoid-induced TNF receptor (GITR) expressed on both regulatory and cytotoxic T cells yielded a cure rate of 24%, compared to 0% for radiation or anti-GITR therapy alone (190).
GL261 is a widely employed mouse GBM line that permits studies in immunocompetent animals (191, 192). As such, many of the seminal studies of immunotherapy with or without radiation have utilized this model. Nevertheless, some have criticized the GL261 model as more highly immunogenic than the immunologically “cold” GBM, thereby potentially over-estimating the clinical potential of immunotherapies for GBM. Numerous genetically engineered models of GBM have been developed, several of which have been well described as “transplantable GEM models” and provide important immunocompetent alternatives to GL261.
As noted in earlier sections, multiple immune checkpoints and other immmunosuppressive strategies are harnessed by GBM to avert immune detection (193). Accordingly, as with preclinical models of metastatic disease, preclinical GBM models have similarly demonstrated improved outcomes with multimodal immune therapy in combination with radiation. Combined use of CTLA-4 blocking antibodies and pro-cytotoxic function CD137 (4-1BB) agonist antibodies with RT yielded 50% survival at 100 days in a GL261 orthotopic model, compared to 20% without RT, and 0% with radiotherapy alone (189). Radiotherapy plus dual checkpoint antibodies against PD-1 and TIM-3 yielded 100% survival of GL261-bearing mice at 100 days, compared to 60% with the best combination of only two of the three treatment modalities (76). Both of these radiation plus dual immunotherapy studies documented elevated CD8+ and CD4+ T cells within the tumor of combined therapy-treated animals (76, 189). Belcaid et al. further performed depletion studies to find that CD4+ but not CD8+ T cells were required for the survival benefit of combined therapy (189).
Most clinical trials of immunotherapy, to date, have enrolled patients with recurrent disease following prior standard therapy. As such, patients would have previously undergone radiation and chemotherapy, though would not typically receive further radiation as part of the trial protocol. As such, it is important to note that Belcaid et al. found a trend toward improved outcomes with concurrent, rather than sequential, administration of radiation and immunotherapy (189). Additionally, prior exposure to TMZ attenuates the immune response to checkpoint inhibitors (194).
Current standard therapy for GBM includes chemotherapy and fractionated radiation, frequently also with administration of corticosteroids, which collectively induce lymphopenia and immune suppression (195–198). Importantly, mathematical modeling indicates that even the fractionated radiation to the tumor itself accounts for lymphotoxic doses of radiation to the entire circulating blood pool, even independent of immunosuppressive chemotherapy and steroids (199). As such, stereotactic radiosurgery has been evaluated as an alternative to standard fractionated radiation with a goal of decreasing immunosuppression and increasing tumor ablation and immune activation. Also of note, traumatic brain injury leads to immune suppression via ill-defined mechanisms (200). Whether additional such mechanisms may further impede immune function following brain radiation, independent of lymphodepletion, remains similarly ill-defined. Most GBMs in humans exceed the size limit (~3 cm) considered acceptable for single fraction radiosurgery, though fractionated radiosurgery has been explored with demonstration of feasibility in a preliminary dose-escalation study (141).
With the increasing clinical prevalence and importance of immune-based strategies, attention has focused on how best to harness immune-activating impacts of radiation. The linear quadratic equation is used to determine which fractionated radiation regimens yield equivalent biologically effective doses (201). Importantly, recent data have revealed that too much radiation in a single fraction may inhibit the very immune mechanism one is attempting to activate through radiation-induced immune activation. In an OVA murine melanoma model, 7.5 Gy/fraction yielded best tumor immunity while minimizing numbers of Tregs (202). Radiation doses above 12 Gy were recently found to activate DNA exonuclease Trex1, which decreases DNA from the cytosol and thereby reduces immunogenicity (203). Current efforts to optimize fractionation schemes to optimize RT-mediated immune activation were recently reviewed elsewhere (204). Importantly, optimal parameters appear tumor-dependent. Few studies to date have addressed this question for GBM, though dedicated clinical trials may be needed to elucidate optimal parameters for human patients. A dose escalation study (25–40 Gy) using 5 Gy/fraction with 5 mm margins revealed a maximum tolerated dose of 40 Gy in 8 fractions and an overall survival of 15 months–similar to standard therapy. Further work would be needed to assess relative efficacy of immunotherapies in such novel paradigms compared to that seen with conventional therapy.
The role of immunotherapy for low grade infiltrative gliomas remains poorly characterized. Preclinical efforts in this domain are hampered by the paucity of available animal models. Low-grade gliomas are ultimately fatal due to transformation into high-grade gliomas. Clinical application of immunotherapies for low-grade gliomas are hampered by the lack of biomarkers for efficacy and prolonged periods of relative clinical stability with existing therapies. Low-grade gliomas demonstrate less immunosuppressive phenotypes compared to high-grade gliomas (196, 205–208). This could portend an improved capacity for inducing an immune response, particularly in the context of a more indolent lesion that affords more time to achieve a therapeutic response before the patient would otherwise succumb to disease (209, 210). Conversely, most low-grade gliomas are IDH-mutant and overproduce 2-hydroxyglutarate, which has been found to be immunosuppressive (211). Nevertheless, the specific IDH1 (R132H) mutation itself could serve as a potential vaccine target (212). Preliminary safety trials of vaccines have been performed in pediatric patients with low-grade glioma. A Poly-IC-containing synthetic peptide-based vaccine against the glioma-associated antigens EphA2, IL-13Rα2, and survivin yielded notable immunologic and radiologic responses in a subset of patients (209, 210, 213). Further work is needed to elucidate prospectively which patients and tumor subtypes could benefit from immunotherapy and how favorable responses can be made more consistent.
Cancer stem cells (CSC) have been identified in numerous tumors and play a role in development, invasion, and metastasis. Glioma stem cells (GSC) (82, 214), represent tumor-initiating cells notable for markers of neural stem cell markers, such as CD133 (214) and Nestin (215). Upregulated markers of pluripotent stem cells, including nanog and Oct4, have also been reported (216). GCS demonstrate therapeutic resistance in part through upregulation of DNA damage checkpoint responses and enhanced DNA repair (217). Radiation can induce de-differentiation of GBM cells into a stem cell-like phenotype with increased self-renewal and tumorigenesis capacity in a survivin-dependent manner (218).
GSCs are primarily enriched in the perivascular niche (219). Both microglia and TAMs are found in the perivascular niche and GSC play a prominent role in immunomodulation by recruiting microglia and TAMs. For example, GSCs secrete periostin to recruit TAMs that largely exhibit an M2 phenotype (220). GSCs have also been shown to activate TLR4 on microglia to induce IL-6 secretion (221). Immune therapies against GSCs have included peptide and DC vaccines. Cantini et al. reported in a GL261that vaccination with GLAST, a CNS protein enriched on radial glial cells, promoted tumor immunity without evidence of autoimmunity (222). DC-based vaccines have been explored using tumor lysate or GSC-associated peptides to stimulate ex vivo DCs. Administration of loaded DCs in human patients induces prolonged anti-tumor immunity against a potentially broad range of antigens (223). In a GL261-murine model, Pellegatta et al. demonstrated that vaccination using CSC antigens yielded improved anti-tumor effects of DC vaccination when compared with vaccination using regular tumor antigens (224). Similarly, in a rat model, Xu et al. showed that rats vaccinated with GSC-enriched lysates from neurospheres survived longer than rats vaccinated with non-GSC-enriched lysates (225). In recent years, such strategies to target GSCs have been extended to clinical trials.
Immune targeting of GSCs ideally seeks to promote immune responses against antigens uniquely expressed on GSCs, but not healthy tissues. However, care may be needed to ensure that rare endogenous tissue stem cells (neural stem cells or oligodendrocyte progenitor cells) are not inadvertently targeted. Currently, this question is complicated in part by controversy surrounding the presence and identity of adult human endogenous neural stem cells (226). Since GSCs likely reactivate more primitive developmental programs than adult CNS or other tissue progenitor populations, targeting these most primitive markers may help minimize depletion of adult progenitor populations. Since the phenotypes of certain human endogenous progenitor populations remains ill-defined, vigilance for cognitive or other toxicities should be maintained in any therapies potentially inducing auto-immunity against non-mutant endogenous peptides.
DCs are one of the most important APCs and have prompted several groups to develop DC-based vaccines for GBM (27, 227–230). DCs have a high capacity to detect maturation signals and process antigens as peptides to generate an efficient and sustained T cell response (231, 232). In an early clinical study of standard chemoradiotherapy followed by GSC-pulsed DC vaccine, 7/11 enrolled patients completed treatment with a median survival of 694 days (233). Currently, it is unclear which factors impact the efficacy of DC vaccination. However, a pre-clinical study by Mitchell et al. showed that DC migration to tumor draining lymphnodes could be enhanced by exogenous administration of the chemokine CCL3 (234). In addition, the authors demonstrated that modulation of CMV-specific DCs with a potent tetanus/diphtheria antigen increased the migratory capacity of DCs and improved the clinical outcomes in patients with GBM (234). A DC vaccine (ICT-107) loaded with six synthetically processed GBM associated peptides (tumor stem cell antigen MAGE-1, her-2, AIM-2, Trp-2, gp100, and IL-13 Rα2) yielded improved progression-free survival and a trend toward improved survival in a randomized, double-blind, placebo-controlled phase II clinical trial for newly diagnosed GBM; however, the study did not meet the primary endpoint of improved overall survival (235). A phase III study was begun, but suspended due to insufficient funding. An initial report demonstrated a median overall survival of 23.1 months in the intention-to-treat population (236). To date, clinical trials have deployed DC therapies following completion of standard chemoradiation therapy. Whether or not modifications to standard therapy could further augment DC-mediated responses remains to be investigated.
Epidermal growth factor receptor (EGFR) variant III (vIII) is expressed in 20–30% of GBM (237). EGFRvIII is absent in normal tissues and selective activation of PI3K/Akt pathway contributes to GBM resistance to radiotherapy (238). Work by Heimberger et al. demonstrated that immunization of DCs mixed with a tumor-specific peptide of EGFRvIII, PEP-3 conjugated to the immune adjuvant keyhole limpet hemocyanin (KLH), resulted in long-term survival of mice with intracranial melanomas (239). The vaccine Rindopepimut, which targets EGFRvIII, has shown efficacy in phase I/II clinical trials, but demonstrated no survival benefit in a phase III trial (see Supplementary Table 1) (240, 241).
Survivin, a regulator of both mitosis and programmed cell death (242), is a tumor associated antigen, making it an attractive candidate for targeted cancer therapy and immunotherapy (242–244). Normal glial cells do not express survivin, whereas survivin is highly expressed in GBM and is associated with poorer prognosis (245). Epitopes of survivin are immunogenic and are presented by MHC Class I complexes. Anti-survivin antibodies have been identified in patients with GBM (246). In an effort to identify a survivin peptide mimic that could elicit a potent T cell response, Ciesielski et al. created SVN53-67/M57, a peptide vaccine derived from survivin. SVN53-67/M57 produced cytotoxic T cell-mediated killing of human glioma cells in vitro and, in combination with GM-CSF, was able to control tumor burden in mice bearing GL-261 glioma tumors (247). A phase II trial of SVN53-67/M57-KLH (SurVaxM) and TMZ is currently recruiting patients with malignant glioma and the therapy has shown to be well tolerated and generates anti-survivin antibody and survivin specific CD8+ T cells (248).
While this review focuses particularly on the facilitating role of radiation in checkpoint blockade, oncolytic viruses may serve a similar role by means of immune activation in GBM (249). Although oncolytic viruses are selected or engineered for their propensity to replicate or selectively kill tumors cells, complete viral-induced lysis of all tumor cells is not observed with the relatively attenuated viral constructs clinically deployed, to date. Instead, the lysis of a subset of tumor cells may serve to promote both anti-viral and anti-tumor immune responses (250). The combination of measles virus-expressing carcinoembryonic antigen with radiation has been shown to improve tumor control (251). Similarly, the combination of radiation with oncolytic DNA viruses, such as herpes-simplex virus-1 and conditionally replicating adenovirus, has demonstrated longer survival and improved outcomes in pre-clinical GBM models (252–254). Despite some case reports of remarkable responses, clinical trials of oncolytic therapies for GBM have proven disappointing, to date, with only marginal therapeutic efficacy reported (249) (ClinicalTrials.gov, Unique Identifier: NCT01280552)1. These findings have prompted ongoing efforts to both better predict which patient populations may respond favorably and how responses may be further augmented.
Translating immunotherapy for GBM has proven challenging. Optimally harnessing radiation to augment the efficacy of immunotherapy is a promising avenue, but is not without its own unique challenges (Figure 2). While many patients seeking clinical trials have recurrent disease, prior radiation may preclude further radiation due to risk of toxicity and may impact immune responses in ways that are difficult to predict. While TMZ may attenuate bone marrow immune responses, TMZ-induced mutations may provide important neoantigens to catalyze immune recognition of the tumor.
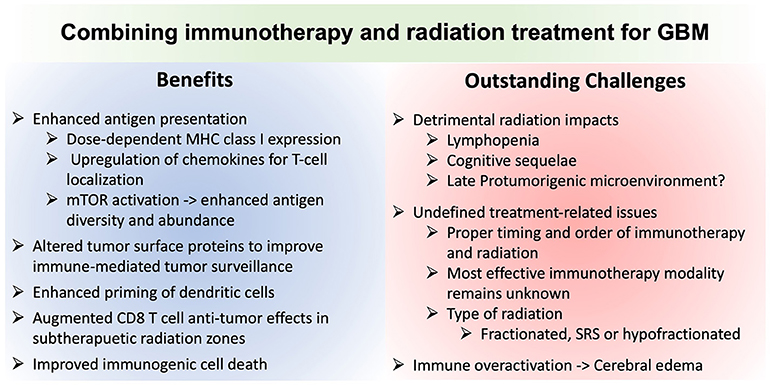
Figure 2. Comparison of the advantages and potential challenges of combining immunotherapy and radiation for glioblastoma treatment. MHCI, Major histocompatibility complex class I molecule; mTOR, mechanistic target of rapamycin; SRS, stereotactic radiosurgery.
Tumor heterogeneity remains a challenge, both within and between patients. Furthermore, human immune responses are complex and will likely require molecular and genetic subtyping to identify potential subclasses and individual “responders” or “partial responders.” For example, the phase II ICT-107 autologous DC vaccine trial suggested clinical responses only in subjects who were HLA-A2 positive (a phase III trial was suspended for financial reasons). Several immunotherapy clinical trials are ongoing for GBM, which is routinely treated with radiation, including DC vaccines, EGFRvIII vaccines, and checkpoint inhibitors, among others. However, few studies, to date, have specifically focused on optimizing synergy between radiation and immunotherapy.
GBM is transcriptionally subclassified into proneural, neural, classical, and mesenchymal based on genomic profiling (255). However, single cell transcriptome data suggest variable representations of each transcriptional cell type within each tumor, challenging selective targeting of the tumor phenotype. Moreover, radiation has been shown to induce a mesenchymal phenotype, notable for its poorest prognosis; likely due in part to radiation-induced upregulation of treatment-resistant stem-like properties (256). Data from other tumor types suggest that cytokines from local tissue in response to immunotherapies may offer an important source of more reliable biomarkers, including biomarkers of therapeutic responsiveness (257). If also true in glioma, this may create impetus to identify technologies for in vivo evaluation of such biomarkers locally within the tumor microenvironment in response to therapy—an avenue our group is currently exploring.
The paucity of prompt biological feedback regarding efficacy remains a challenge. While systemic immune cell populations can be serially accessed to monitor leukocyte numbers and phenotypes, these data are at best an indirect and imperfect indicator of therapeutic efficacy within the tumor. Imaging criteria to interpret immunotherapy responses, despite interpretations challenges of radiation- and immunotherapy-induced pseudoprogression, have been drafted (iRANO). The lack of definitive imaging biomarkers of responsiveness is underscored by the need to follow the trajectory of imaging changes over months to interpret findings (258).
Finally, it is increasingly appreciated that standard management strategies aside from radiation likely inhibit the efficacy of immunotherapy, including immuosuppressive corticosteroids and systemic chemotherapy. Corticosteroids, such as dexamethasone, are used to control vasogenic edema due to infiltrative tumor, surgery, and radiotherapy (259). Pre-clinical models and retrospective data from clinical studies indicate that dexamethasone treatment attenuates the efficacy of radiotherapy, presumably by impeding normal radiation-induced immune responses (260). While TMZ is the cornerstone of the standard STUPP regimen for GBM, experimental data demonstrate that systemic chemotherapy impedes the anti-tumor effects of anti-PD-1, despite the potential for local chemotherapy to augment immunotherapeutic responses (194). These studies highlight practical challenges of optimizing the therapeutic impacts of immunotherapy. Until methods can better predict responses or evaluate therapeutic impact in real time, forgoing the established standard of care (TMZ) to theoretically augment an unproven experimental therapy may prove challenging. Our group recently initiated a clinical trial providing anti-PD-1 in biopsy-proven GBM prior to definitive surgical resection and subsequent chemo/radiation. Insights from early histological analysis of tissue from patients treated with anti-PD-1 may help identify biomarkers and selection criteria for future single and combination immunotherapy trials (ClinicalTrials.gov, Unique Identifier: NCT03197506). As increasing evidence emerges about untoward chronic impacts of radiation on the CNS microenvironment for tumor aggressiveness, could future paradigms replace standard fractionated radiation with combination immunotherapy and hypofractionated SRS applied to just a portion of the tumor? Alternatively, perhaps residual tumor cells after chemo/radiation may be best eliminated with combined immunotherapy and senolytic therapy? Finally, strategies are needed to optimally titrate the immune response to avert potentially severe or fatal toxicities. These may vary in a tumor- and patient-specific manner based on biomarkers of susceptibility and responses that have yet to be identified. We posit that dedicated efforts to understand the human biology of CNS radiation and therapeutic responses may reveal opportunities to optimize safety and efficacy of combined radiation and immunotherapy for glioma.
The dramatic anti-tumor clinical responses observed in certain tumors treated with anti-CTLA-4 and anti-PD-1 antibodies have ushered in a new era for effective cancer therapies. Radiation modulates the tumor microenvironment and offers a potential immune adjuvant to enhance the anti-tumor response in combination with immunotherapies. Preclinical models of GBM illustrate potent opportunities to harness combination immunotherapy with brain radiation. However, several questions remain unanswered regarding the optimal paradigms of combination immunotherapy, timing in relation to radiation, and the potential to mitigate adverse impacts of currently standard treatments, such as fractionated radiotherapy-induced lymphopenia and chemotherapy- and corticosteroid-induced immunosuppression. Preclinical evidence suggests robust opportunities to add optimized strategies of immunotherapy into standard-of-care for GBM. Much work lies ahead to improve translational paradigms that could increase mechanistic insights gleaned from each treated patient and enable iterative improvements in protocols within the life-times of individual patients.
KR, LC, and TB conceived the aim of the review, performed the literature search, and wrote the manuscript with additional intellectual contributions from IP, AJ and AW. KR drafted Figure 1, LC drafted the Supplementary Table 1 and Figure 2.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation, though no other collaboration, with the authors at the time of review.
Funding support TB was provided by NIH K12 NRDCP, Brains Together for a Cure, the Mayo Clinic Grand Forks Career Development Program, the McKelvey family, and Regenerative Medicine Minnesota. We would like to acknowledge the editorial assistance of Kirsten Burns, PsyD, and Superior Medical Experts.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00656/full#supplementary-material
1. ^Pembrolizumab and Standard Therapy in Treating Patients With Glioblastoma. Available online at: https://clinicaltrials.gov/ct2/show/NCT03197506.
1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. (2007) 114:97–109. doi: 10.1007/s00401-007-0243-4
2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. doi: 10.1056/NEJMoa043330
3. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol. (2013) 15:4–27. doi: 10.1093/neuonc/nos273
4. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science (1996) 271:1734–6.
5. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
6. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
7. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
8. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. (2014) 371:2189–99. doi: 10.1056/NEJMoa1406498
9. Castle JC, Kreiter S, Diekmann J, Lower M, van de Roemer N, de Graaf J, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. (2012) 72:1081–91. doi: 10.1158/0008-5472.CAN-11-3722
10. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature (2014) 515:577–81. doi: 10.1038/nature13988
11. Colli LM, Machiela MJ, Myers TA, Jessop L, Yu K, Chanock SJ. Burden of nonsynonymous mutations among TCGA cancers and candidate immune checkpoint inhibitor responses. Cancer Res. (2016) 76:3767–72. doi: 10.1158/0008-5472.CAN-16-0170
12. Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. (1992) 148:1404–10.
13. Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol. (1995) 146:317–22.
14. Tran TT, Uhl M, Ma JY, Janssen L, Sriram V, Aulwurm S, et al. Inhibiting TGF-beta signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. (2007) 9:259–70. doi: 10.1215/15228517-2007-010
15. Gupta K, Burns TC. Radiation-induced alterations in the recurrent glioblastoma microenvironment: therapeutic implications. Front Oncol. (2018) 8:503. doi: 10.3389/fonc.2018.00503
16. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. (2009) 360:765–73. doi: 10.1056/NEJMoa0808710
17. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science (2008) 321:1807–12. doi: 10.1126/science.1164382
18. Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature (2009) 462:739–44. doi: 10.1038/nature08617
19. Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. (2014) 16:81–91. doi: 10.1093/neuonc/not159
20. Amankulor NM, Kim Y, Arora S, Kargl J, Szulzewsky F, Hanke M, et al. Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. (2017) 31:774–86. doi: 10.1101/gad.294991.116
21. Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. (2005) 26:485–95. doi: 10.1016/j.it.2005.07.004
22. Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. (1991) 28:254–60. doi: 10.1002/jnr.490280213
23. Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. (2003) 3:569–81. doi: 10.1038/nri1130
24. Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. (2015) 36:569–77. doi: 10.1016/j.it.2015.08.006
25. Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. (2015) 212:991–9. doi: 10.1084/jem.20142290
26. Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. (2011) 17:1603–15. doi: 10.1158/1078-0432.Ccr-10-2563
27. Prins RM, Wang X, Soto H, Young E, Lisiero DN, Fong B, et al. Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients. J. Immunother. (2013) 36:152–7. doi: 10.1097/CJI.0b013e3182811ae4
28. Zagzag D, Salnikow K, Chiriboga L, Yee H, Lan L, Ali MA, et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Invest. (2005) 85:328–41. doi: 10.1038/labinvest.3700233
29. Zou JP, Morford LA, Chougnet C, Dix AR, Brooks AG, Torres N, et al. Human glioma-induced immunosuppression involves soluble factor(s) that alters monocyte cytokine profile and surface markers. J Immunol. (1999) 162:4882–92.
30. Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity (2009) 30:616–25. doi: 10.1016/j.immuni.2009.04.009
31. Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. (2012) 30:733–58. doi: 10.1146/annurev-immunol-020711-075043
32. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. (2006) 6:295–307. doi: 10.1038/nri1806
33. Burocchi A, Colombo MP, Piconese S. Convergences and divergences of thymus- and peripherally derived regulatory T cells in cancer. Front Immunol. (2013) 4:247. doi: 10.3389/fimmu.2013.00247
34. Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. (2016) 28:401–9. doi: 10.1093/intimm/dxw025
35. Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. (2004) 114:280–90. doi: 10.1172/JCI21583
36. Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. (2003) 9:1269–74. doi: 10.1038/nm934
37. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. (1999) 189:1363–72.
38. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. (2002) 196:459–68.
39. Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. (2012) 18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130
40. Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery (2013) 72:1031–8; discussion 1038–9. doi: 10.1227/NEU.0b013e31828cf945
41. Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. (2006) 66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773
42. McVicar DW, Davis DF, Merchant RE. In vitro analysis of the proliferative potential of T cells from patients with brain tumor: glioma-associated immunosuppression unrelated to intrinsic cellular defect. J Neurosurg. (1992) 76:251–60. doi: 10.3171/jns.1992.76.2.0251
43. Jacobs JF, Idema AJ, Bol KF, Nierkens S, Grauer OM, Wesseling P, et al. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. (2009) 11:394–402. doi: 10.1215/15228517-2008-104
44. Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. (2011) 13:1308–23. doi: 10.1093/neuonc/nor134
45. Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, Wesseling P, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer (2007) 121:95–105. doi: 10.1002/ijc.22607
46. El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. (2006) 105:430–7. doi: 10.3171/jns.2006.105.3.430
47. Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. (1998) 188:2205–13.
48. Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell (2009) 138:30–50. doi: 10.1016/j.cell.2009.06.036
49. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. (2015) 15:486–99. doi: 10.1038/nri3862
50. Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. (2003) 77:4911–27.
51. Kala M, Chrobok J, Dvorak P. A surprising finding by a surgery for lumbar intervertebral disk herniation–Ewing's sarcoma. Acta Univ Palacki Olomuc Fac Med. (1987) 117:185–9.
52. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. (2000) 192:1027–34.
53. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. (2005) 6:1245–52. doi: 10.1038/ni1271
54. Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. (2007) 117:3383–92. doi: 10.1172/JCI31184
55. Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity (2004) 21:503–13. doi: 10.1016/j.immuni.2004.08.010
57. Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-Cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. (2018) 24:4175–86. doi: 10.1158/1078-0432.CCR-17-1846
58. Mohme M, Schliffke S, Maire CL, Runger A, Glau L, Mende KC, et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor- infiltrating lymphocytes. Clin Cancer Res. (2018) 24:clincanres.2617.2017. doi: 10.1158/1078-0432.CCR-17-2617
59. Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity (1995) 3:541–7.
60. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity (1999) 11:141–51.
61. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12:252–64. doi: 10.1038/nrc3239
62. Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. (2005) 25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005
63. van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. (1999) 190:355–66.
64. Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. (2013) 86:343–9. doi: 10.1016/j.ijrobp.2012.12.025
65. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. (2013) 369:134–44. doi: 10.1056/NEJMoa1305133
66. Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. (2016) 18:195–205. doi: 10.1093/neuonc/nov172
67. Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. (2013) 19:3165–75. doi: 10.1158/1078-0432.CCR-12-3314
68. Johanns TM, Miller CA, Dorward IG, Tsien C, Chang E, Perry A, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. (2016) 6:1230–6. doi: 10.1158/2159-8290.CD-16-0575
69. Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. (2012) 61:1019–31. doi: 10.1007/s00262-011-1172-6
70. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (2015) 348:124–8. doi: 10.1126/science.aaa1348
71. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515:568–71. doi: 10.1038/nature13954
72. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature (2014) 515:563–7. doi: 10.1038/nature14011
73. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. (2012) 72:917–27. doi: 10.1158/0008-5472.CAN-11-1620
74. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. (2010) 207:2187–94. doi: 10.1084/jem.20100643
75. Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology (2018) 7:e1466769. doi: 10.1080/2162402X.2018.1466769
76. Kim JE, Patel MA, Mangraviti A, Kim ES, Theodros D, Velarde E, et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res. (2017) 23:124–36. doi: 10.1158/1078-0432.CCR-15-1535
77. Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, et al. The interaction properties of costimulatory molecules revisited. Immunity (2002) 17:201–10.
78. Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity (2002) 16:23–35.
79. Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity (1996) 4:535–43.
80. Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity (1994) 1:793–801.
81. Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci USA. (2002) 99:11790–5. doi: 10.1073/pnas.162359999
82. Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. (2003) 63:5821–8.
83. Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, et al. Molecular basis of T cell inactivation by CTLA-4. Science (1998) 282:2263–6.
84. Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. (2000) 192:303–10.
85. Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. (2009) 206:1717–25. doi: 10.1084/jem.20082492
86. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood (2009) 114:1537–44. doi: 10.1182/blood-2008-12-195792
87. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. (2008) 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331
88. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. (2001) 2:261–8. doi: 10.1038/85330
89. Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. (2003) 100:5336–41. doi: 10.1073/pnas.0931259100
90. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. (2002) 8:793–800. doi: 10.1038/nm730
91. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. (2015) 14:847–56. doi: 10.1158/1535-7163.MCT-14-0983
92. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. (2010) 236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x
93. Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. (2010) 16:452–9. doi: 10.1038/nm.2106
94. Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. (2018) 96:21–33. doi: 10.1111/imcb.1003
95. Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature (2002) 415:536–41. doi: 10.1038/415536a
96. Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science (2007) 318:1141–3. doi: 10.1126/science.1148536
97. Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood (2011) 117:4501–10. doi: 10.1182/blood-2010-10-310425
98. Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. (2011) 121:2350–60. doi: 10.1172/jci46102
99. Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. (2010) 207:2175–86. doi: 10.1084/jem.20100637
100. Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L. Tim-3 expression defines regulatory T cells in human tumors. PLoS ONE (2013) 8:e58006. doi: 10.1371/journal.pone.0058006
101. Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. (2011) 71:3540–51. doi: 10.1158/0008-5472.Can-11-0096
102. Jarry U, Jeannin P, Pineau L, Donnou S, Delneste Y, Couez D. Efficiently stimulated adult microglia cross-prime naive CD8+ T cells injected in the brain. Eur J Immunol. (2013) 43:1173–84. doi: 10.1002/eji.201243040
103. Wei J, Gabrusiewicz K, Heimberger A. The controversial role of microglia in malignant gliomas. Clin Dev Immunol. (2013) 2013:285246. doi: 10.1155/2013/285246
104. Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. (2006) 8:261–79. doi: 10.1215/15228517-2006-008
105. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. (2005) 5:953–64. doi: 10.1038/nri1733
106. Henan X, Toyota N, Yanjiang X, Fujita Y, Zhijun H, Touma M, et al. Enhancement of phagocytosis and cytotoxicity in macrophages by tumor-derived IL-18 stimulation. BMB Rep. (2014) 47:286–91. doi: 10.5483/BMBRep.2014.47.5.152
107. Yuan A, Hsiao YJ, Chen HY, Chen HW, Ho CC, Chen YY, et al. Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci Rep. (2015) 5:14273. doi: 10.1038/srep14273
108. Kostianovsky AM, Maier LM, Anderson RC, Bruce JN, Anderson DE. Astrocytic regulation of human monocytic/microglial activation. J Immunol. (2008) 181:5425–32.
109. Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, et al. IL-10 and TGF-beta2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep. (2011) 38:3585–91. doi: 10.1007/s11033-010-0469-4
110. Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. (2006) 38:474–84. doi: 10.1038/emm.2006.56
111. Sica A, Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J Autoimmun. (2017) 85:117–25. doi: 10.1016/j.jaut.2017.07.010
112. Dubinski D, Wolfer J, Hasselblatt M, Schneider-Hohendorf T, Bogdahn U, Stummer W, et al. CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro Oncol. (2016) 18:807–18. doi: 10.1093/neuonc/nov280
113. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. (2016) 7:12150. doi: 10.1038/ncomms12150
114. Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, et al. Tumor- infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. (2008) 83:1136–44. doi: 10.1189/jlb.0907611
115. Yang WC, Ma G, Chen SH, Pan PY. Polarization and reprogramming of myeloid-derived suppressor cells. J Mol Cell Biol. (2013) 5:207–9. doi: 10.1093/jmcb/mjt009
116. Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity (2014) 41:843–52. doi: 10.1016/j.immuni.2014.10.019
117. Garzon-Muvdi T, Theodros D, Luksik AS, Maxwell R, Kim E, Jackson CM, et al. Dendritic cell activation enhances anti-PD-1 mediated immunotherapy against glioblastoma. Oncotarget (2018) 9:20681–97. doi: 10.18632/oncotarget.25061
118. Curtin JF. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. (2009) 6:e10. doi: 10.1371/journal.pmed.1000010
119. Xiong Z, Ohlfest JR. Topical imiquimod has therapeutic and immunomodulatory effects against intracranial tumors. J Immunother. (2011) 34:264–9. doi: 10.1097/CJI.0b013e318209eed4
120. Wu A, Oh S, Gharagozlou S, Vedi RN, Ericson K, Low WC, et al. In vivo vaccination with tumor cell lysate plus CpG oligodeoxynucleotides eradicates murine glioblastoma. J Immunother. (2007) 30:789–97. doi: 10.1097/CJI.0b013e318155a0f6
121. Carpentier A, Metellus P, Ursu R, Zohar S, Lafitte F, Barrie M, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol. (2010) 12:401–8. doi: 10.1093/neuonc/nop047
122. Ursu R, Carpentier A, Metellus P, Lubrano V, Laigle-Donadey F, Capelle L, et al. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-A phase II multicentric, randomised study. Eur J Cancer (2017) 73:30–7. doi: 10.1016/j.ejca.2016.12.003
123. Carpentier A, Laigle-Donadey F, Zohar S, Capelle L, Behin A, Tibi A, et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol. (2006) 8:60–6. doi: 10.1215/s1522851705000475
124. Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS ONE (2016) 11:e0153550. doi: 10.1371/journal.pone.0153550
125. Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. (2014) 32:25–50. doi: 10.1146/annurev-immunol-032713-120142
126. Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, et al. CD47-signal regulatory protein-alpha (SIRPalpha) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci USA. (2011) 108:18342–7. doi: 10.1073/pnas.1106550108
127. Zhu H, Leiss L, Yang N, Rygh CB, Mitra SS, Cheshier SH, et al. Surgical debulking promotes recruitment of macrophages and triggers glioblastoma phagocytosis in combination with CD47 blocking immunotherapy. Oncotarget (2017) 8:12145–57. doi: 10.18632/oncotarget.14553
128. Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell (2010) 142:699–713. doi: 10.1016/j.cell.2010.07.044
129. Weiskopf K, Weissman IL. Macrophages are critical effectors of antibody therapies for cancer. MAbs (2015) 7:303–10. doi: 10.1080/19420862.2015.1011450
130. Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. (2009) 1:3ra7. doi: 10.1126/scitranslmed.3000139
131. Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. (2013) 110:11103–8. doi: 10.1073/pnas.1305569110
132. Wu L, Yu GT, Deng WW, Mao L, Yang LL, Ma SR, et al. Anti-CD47 treatment enhances anti-tumor T-cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma. Oncoimmunology (2018) 7:e1397248. doi: 10.1080/2162402X.2017.1397248
133. Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. (2014) 74:6771–83. doi: 10.1158/0008-5472.Can-14-0037-t
134. Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J Cancer (2017) 76:100–9. doi: 10.1016/j.ejca.2017.02.013
135. Pedersen MB, Danielsen AV, Hamilton-Dutoit SJ, Bendix K, Norgaard P, Moller MB, et al. High intratumoral macrophage content is an adverse prognostic feature in anaplastic large cell lymphoma. Histopathology (2014) 65:490–500. doi: 10.1111/his.12407
136. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. (2013) 19:1264–72. doi: 10.1038/nm.3337
137. Zheng X, Turkowski K, Mora J, Brune B, Seeger W, Weigert A, et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget (2017) 8:48436–52. doi: 10.18632/oncotarget.17061
138. Di Meglio F, Castaldo C, Nurzynska D, Romano V, Miraglia R, Bancone C, et al. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J Mol Cell Cardiol. (2010) 49:719–27. doi: 10.1016/j.yjmcc.2010.05.013
139. Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. (2005) 175:342–9.
140. Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Ostling J, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. (2016) 15:2000–11. doi: 10.1016/j.celrep.2016.04.084
141. Azoulay M, Ho CK, Fujimoto DK, Modlin LA, Gibbs IC, Hancock SL, et al. A phase I/II trial of 5 fraction stereotactic radiosurgery with 5-mm margins with concurrent and adjuvant temozolomide in newly diagnosed supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. (2016) 96:E131–2. doi: 10.1016/j.ijrobp.2016.06.921
142. Zhang L, Cheng F, Wei Y, Zhang L, Guo D, Wang B, et al. Inhibition of TAZ contributes radiation-induced senescence and growth arrest in glioma cells. Oncogene (2018) doi: 10.1038/s41388-018-0626-0. [Epub ahead of print].
143. Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. (2018) 18:225–42. doi: 10.1038/nri.2017.125
144. Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Compar Neurol. (2008) 508:687–710. doi: 10.1002/cne.21668
145. Chen H, Chong ZZ, De Toledo SM, Azzam EI, Elkabes S, Souayah N. Delayed activation of human microglial cells by high dose ionizing radiation. Brain Res. (2016) 1646:193–8. doi: 10.1016/j.brainres.2016.06.002
146. Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, et al. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. (2006) 21:457–67. doi: 10.1016/j.nbd.2005.08.006
147. Li MD, Burns TC, Kumar S, Morgan AA, Sloan SA, Palmer TD. Aging-like changes in the transcriptome of irradiated microglia. Glia (2015) 63:754–67. doi: 10.1002/glia.22782
148. Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. (2007) 62:515–20. doi: 10.1002/ana.21214
149. Moravan MJ, Olschowka JA, Williams JP, O'Banion MK. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res. (2011) 176:459–73.
150. Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PO, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. (2013) 264:71–83. doi: 10.1016/j.jneuroim.2013.08.013
151. Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. (2010) 12:7–13. doi: 10.1093/neuonc/nop009
152. Fadul CE, Fisher JL, Gui J, Hampton TH, Cote AL, Ernstoff MS. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol. (2011) 13:393–400. doi: 10.1093/neuonc/noq204
153. Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. (2018) 24:1459–68. doi: 10.1038/s41591-018-0135-2
154. Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. (2008) 29:397–403. doi: 10.1016/j.it.2008.04.007
155. Yang I, Kremen TJ, Giovannone AJ, Paik E, Odesa SK, Prins RM, et al. Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex-bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J Neurosurg. (2004) 100:310–9. doi: 10.3171/jns.2004.100.2.0310
156. Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. (2012) 189:558–66. doi: 10.4049/jimmunol.1200563
157. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. (2005) 174:7516–23.
158. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. (2006) 203:1259–71. doi: 10.1084/jem.20052494
159. Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. (2006) 6:836–48. doi: 10.1038/nri1961
160. Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-gamma mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. (2013) 182:2345–54. doi: 10.1016/j.ajpath.2013.02.041
161. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. (2014) 74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258
162. Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett. (2006) 580:755–62. doi: 10.1016/j.febslet.2005.12.093
163. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. (2014) 124:687–95. doi: 10.1172/JCI67313
164. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature (2015) 520:373–7. doi: 10.1038/nature14292
165. Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol. (1973) 46:220–2. doi: 10.1259/0007-1285-46-543-220
166. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. (2004) 58:862–70. doi: 10.1016/j.ijrobp.2003.09.012
167. Bramhall RJ, Mahady K, Peach AH. Spontaneous regression of metastatic melanoma - clinical evidence of the abscopal effect. Eur J Surg Oncol. (2014) 40:34–41. doi: 10.1016/j.ejso.2013.09.026
168. Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. (2005) 11:728–34.
169. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. (2009) 15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265
170. Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. (2010) 70:2697–706. doi: 10.1158/0008-5472.Can-09-2982
171. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. (2011) 71:2488–96. doi: 10.1158/0008-5472.Can-10-2820
172. Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. (1999) 3:77–83.
173. Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. (2010) 31:363–72. doi: 10.1007/s13277-010-0042-8
174. Nehs MA, Lin CI, Kozono DE, Whang EE, Cho NL, Zhu K, et al. Necroptosis is a novel mechanism of radiation-induced cell death in anaplastic thyroid and adrenocortical cancers. Surgery (2011) 150:1032–9. doi: 10.1016/j.surg.2011.09.012
175. Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer (2003) 3:117–29. doi: 10.1038/nrc992
176. Ianzini F, Bertoldo A, Kosmacek EA, Phillips SL, Mackey MA. Lack of p53 function promotes radiation-induced mitotic catastrophe in mouse embryonic fibroblast cells. Cancer Cell Int. (2006) 6:11. doi: 10.1186/1475-2867-6-11
177. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. (2013) 31:51–72. doi: 10.1146/annurev-immunol-032712-100008
178. Perez CA, Fu A, Onishko H, Hallahan DE, Geng L. Radiation induces an antitumour immune response to mouse melanoma. Int J Radiat Biol. (2009) 85:1126–36. doi: 10.3109/09553000903242099
179. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. (2001) 1:135–45. doi: 10.1038/35100529
180. Facci L, Barbierato M, Marinelli C, Argentini C, Skaper SD, Giusti P. Toll-like receptors 2,−3 and−4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1beta release. Sci Rep. (2014) 4:6824. doi: 10.1038/srep06824
181. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. (2007) 13:1050–9. doi: 10.1038/nm1622
182. Ohshima Y, Tsukimoto M, Takenouchi T, Harada H, Suzuki A, Sato M, et al. gamma-Irradiation induces P2X(7) receptor-dependent ATP release from B16 melanoma cells. Biochim Biophys Acta (2010) 1800:40–6. doi: 10.1016/j.bbagen.2009.10.008
183. Medzhitov R, Janeway CAJr. Decoding the patterns of self and nonself by the innate immune system. Science (2002) 296:298–300. doi: 10.1126/science.1068883
184. Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. (2004) 64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525
185. Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. (2000) 1:469–74. doi: 10.1038/82712
186. Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, et al. Mature T lymphocyte apoptosis–immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. (1999) 17:221–53. doi: 10.1146/annurev.immunol.17.1.221
187. Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. (2002) 20:323–70. doi: 10.1146/annurev.immunol.20.100201.131730
188. Henkart PA, Sitkovsky MV. Cytotoxic lymphocytes. Two ways to kill target cells. Curr Biol. (1994) 4:923–5.
189. Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE (2014) 9:e101764. doi: 10.1371/journal.pone.0101764
190. Patel MA, Kim JE, Theodros D, Tam A, Velarde E, Kochel CM, et al. Agonist anti-GITR monoclonal antibody and stereotactic radiation induce immune-mediated survival advantage in murine intracranial glioma. J Immunother Cancer (2016) 4:28. doi: 10.1186/s40425-016-0132-2
191. Szatmari T, Lumniczky K, Desaknai S, Trajcevski S, Hidvegi EJ, Hamada H, et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. (2006) 97:546–53. doi: 10.1111/j.1349-7006.2006.00208.x
192. Trent J, Meltzer P, Rosenblum M, Harsh G, Kinzler K, Mashal R, et al. Evidence for rearrangement, amplification, and expression of c-myc in a human glioblastoma. Proc Natl Acad Sci USA. (1986) 83:470–3.
193. Imai Y, Hasegawa K, Matsushita H, Fujieda N, Sato S, Miyagi E, et al. Expression of multiple immune checkpoint molecules on T cells in malignant ascites from epithelial ovarian carcinoma. Oncol Lett. (2018) 15:6457–68. doi: 10.3892/ol.2018.8101
194. Mathios D, Kim JE, Mangraviti A, Phallen J, Park CK, Jackson CM, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. (2016) 8:370ra180. doi: 10.1126/scitranslmed.aag2942
195. Rosen EM, Fan S, Rockwell S, Goldberg ID. The molecular and cellular basis of radiosensitivity: implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer Invest. (1999) 17:56–72.
196. Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. (2011) 17:5473–80. doi: 10.1158/1078-0432.CCR-11-0774
197. Campian JL, Piotrowski AF, Ye X, Hakim FT, Rose J, Yan XY, et al. Serial changes in lymphocyte subsets in patients with newly diagnosed high grade astrocytomas treated with standard radiation and temozolomide. J Neurooncol. (2017) 135:343–51. doi: 10.1007/s11060-017-2580-z
198. Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. (2012) 1:149–54. doi: 10.2217/cns.12.14
199. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. (2013) 31:140–4. doi: 10.3109/07357907.2012.762780
200. Hazeldine J, Lord JM, Belli A. Traumatic brain injury and peripheral immune suppression: primer and prospectus. Front Neurol. (2015) 6:235. doi: 10.3389/fneur.2015.00235
201. Astrahan M. Some implications of linear-quadratic-linear radiation dose-response with regard to hypofractionation. Med Phys. (2008) 35:4161–72. doi: 10.1118/1.2969065
202. Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. (2012) 83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049
203. Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. (2017) 8:15618. doi: 10.1038/ncomms15618
204. Ko EC, Benjamin KT, Formenti SC. Generating antitumor immunity by targeted radiation therapy: role of dose and fractionation. Adv Radiat Oncol. (2018) 3:486–93. doi: 10.1016/j.adro.2018.08.021
205. Esiri MM, Morris CS. Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease. 2. Non-neoplastic diseases. J Neurol Sci (1991) 101:59–72.
206. Glass R, Synowitz M. CNS macrophages and peripheral myeloid cells in brain tumours. Acta Neuropathol. (2014) 128:347–62. doi: 10.1007/s00401-014-1274-2
207. Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. (1996) 92:288–93.
208. Vega EA, Graner MW, Sampson JH. Combating immunosuppression in glioma. Future Oncol. (2008) 4:433–42. doi: 10.2217/14796694.4.3.433
209. Muller S, Agnihotri S, Shoger KE, Myers MI, Smith N, Chaparala S, et al. Peptide vaccine immunotherapy biomarkers and response patterns in pediatric gliomas. JCI Insight (2018) 3:98791. doi: 10.1172/jci.insight.98791
210. Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Normolle DP, et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro Oncol. (2016) 18:1157–68. doi: 10.1093/neuonc/now026
211. Galluzzi L, Kroemer G. Potent immunosuppressive effects of the oncometabolite R-2-hydroxyglutarate. Oncoimmunology (2018) 7:e1528815. doi: 10.1080/2162402X.2018.1528815
212. Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature (2014) 512:324–7. doi: 10.1038/nature13387
213. Okada H, Butterfield LH, Hamilton RL, Hoji A, Sakaki M, Ahn BJ, et al. Induction of robust type-I CD8+ T-cell responses in WHO grade 2 low-grade glioma patients receiving peptide-based vaccines in combination with poly-ICLC. Clin Cancer Res. (2015) 21:286–94. doi: 10.1158/1078-0432.CCR-14-1790
214. Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. (2004) 64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364
215. Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells (2009) 27:40–8. doi: 10.1634/stemcells.2008-0493
217. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature (2006) 444:756–60. doi: 10.1038/nature05236
218. Dahan P, Martinez Gala J, Delmas C, Monferran S, Malric L, Zentkowski D, et al. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: possible involvement in radioresistance. Cell Death Dis. (2014) 5:e1543. doi: 10.1038/cddis.2014.509
219. Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell (2007) 11:69–82. doi: 10.1016/j.ccr.2006.11.020
220. Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. (2015) 17:170–82. doi: 10.1038/ncb3090
221. Dzaye OD, Hu F, Derkow K, Haage V, Euskirchen P, Harms C, et al. Glioma stem cells but not bulk glioma cells upregulate IL-6 secretion in microglia/brain macrophages via toll-like receptor 4 signaling. J Neuropathol Exp Neurol. (2016) 75:429–40. doi: 10.1093/jnen/nlw016
222. Cantini G, Pisati F, Pessina S, Finocchiaro G, Pellegatta S. Immunotherapy against the radial glia marker GLAST effectively triggers specific antitumor effectors without autoimmunity. Oncoimmunology (2012) 1:884–93. doi: 10.4161/onci.20637
223. Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. (2017) 27:74–95. doi: 10.1038/cr.2016.157
224. Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. (2006) 66:10247–52. doi: 10.1158/0008-5472.CAN-06-2048
225. Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells (2009) 27:1734–40. doi: 10.1002/stem.102
226. Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. (2009) 10:153–63. doi: 10.1038/nrn2571
227. Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. (2001) 61:842–7.
228. Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, Sciot R, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother. (2012) 61:2033–44. doi: 10.1007/s00262-012-1261-1
229. Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. (2005) 11:5515–25. doi: 10.1158/1078-0432.Ccr-05-0464
230. Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. (2004) 64:4973–9. doi: 10.1158/0008-5472.Can-03-3505
231. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer (2012) 12:265–77. doi: 10.1038/nrc3258
232. Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol Rev. (2010) 234:317–34. doi: 10.1111/j.0105-2896.2009.00887.x
233. Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tonnesen P, Suso EM, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. (2013) 62:1499–509. doi: 10.1007/s00262-013-1453-3
234. Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature (2015) 519:366–9. doi: 10.1038/nature14320
235. Wen PY, Reardon DA, Phuphanich S, Aiken R, Landolfi JC, Curry WT, et al. A randomized, double-blind, placebo-controlled phase 2 trial of dendritic cell (DC) vaccination with ICT-107 in newly diagnosed glioblastoma (GBM) patients. J Clin Oncol. (2014) 32:2005. doi: 10.1200/jco.2014.32.15_suppl.2005
236. Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. (2018) 16:142. doi: 10.1186/s12967-018-1507-6
237. Weller M, Kaulich K, Hentschel B, Felsberg J, Gramatzki D, Pietsch T, et al. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int J Cancer (2014) 134:2437–47. doi: 10.1002/ijc.28576
238. Lammering G, Valerie K, Lin PS, Hewit TH, Schmidt-Ullrich RK. Radiation-induced activation of a common variant of EGFR confers enhanced radioresistance. Radiother Oncol. (2004) 72:267–73. doi: 10.1016/j.radonc.2004.07.004
239. Heimberger AB, Archer GE, Crotty LE, McLendon RE, Friedman AH, Friedman HS, et al. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery (2002) 50:158–64; discussion 164–6.
240. Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. (2011) 13:324–33. doi: 10.1093/neuonc/noq157
241. Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. (2010) 28:4722–9. doi: 10.1200/jco.2010.28.6963
242. Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene (2003) 22:8581–9. doi: 10.1038/sj.onc.1207113
243. Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. (2008) 14:5000–5. doi: 10.1158/1078-0432.Ccr-08-0746
244. Garg H, Suri P, Gupta JC, Talwar GP, Dubey S. Survivin: a unique target for tumor therapy. Cancer Cell Int. (2016) 16:49. doi: 10.1186/s12935-016-0326-1
245. Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. (2002) 20:1063–8. doi: 10.1200/jco.2002.20.4.1063
246. Soling A, Plugge EM, Schmitz M, Weigle B, Jacob R, Illert J, et al. Autoantibodies to the inhibitor of apoptosis protein survivin in patients with brain tumors. Int J Oncol. (2007) 30:123–8.
247. Ciesielski MJ, Ahluwalia MS, Munich SA, Orton M, Barone T, Chanan-Khan A, et al. Antitumor cytotoxic T-cell response induced by a survivin peptide mimic. Cancer Immunol Immunother. (2010) 59:1211–21. doi: 10.1007/s00262-010-0845-x
248. Ahluwalia MS, Reardon DA, Abad AP, Curry WT, Wong ET, Belal A, et al. Phase II trial of SurVaxM combined with standard therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. (2018) 36:2041. doi: 10.1200/JCO.2018.36.15_suppl.2041
249. Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer J (2012) 18:69–81. doi: 10.1097/PPO.0b013e31824671c9
250. Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer (2014) 14:559–67. doi: 10.1038/nrc3770
251. Liu C, Sarkaria JN, Petell CA, Paraskevakou G, Zollman PJ, Schroeder M, et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin Cancer Res. (2007) 13:7155–65. doi: 10.1158/1078-0432.CCR-07-1306
252. Geoerger B, Grill J, Opolon P, Morizet J, Aubert G, Lecluse Y, et al. Potentiation of radiation therapy by the oncolytic adenovirus dl1520 (ONYX-015) in human malignant glioma xenografts. Br J Cancer (2003) 89:577–84. doi: 10.1038/sj.bjc.6601102
253. Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. (2002) 62:5736–42.
254. Bradley JD, Kataoka Y, Advani S, Chung SM, Arani RB, Gillespie GY, et al. Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res. (1999) 5:1517–22.
255. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell (2010) 17:98–110. doi: 10.1016/j.ccr.2009.12.020
256. Halliday J, Helmy K, Pattwell SS, Pitter KL, LaPlant Q, Ozawa T, et al. In vivo radiation response of proneural glioma characterized by protective p53 transcriptional program and proneural-mesenchymal shift. Proc Natl Acad Sci USA. (2014) 111:5248–53. doi: 10.1073/pnas.1321014111
257. Lauffenburger D. Systems Analysis of Tumor Microenvironent. In: NCI, Physical Sciences/Oncology Network Symposium. Jacksonville, FL (2018).
258. Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. (2015) 16:e534–42. doi: 10.1016/S1470-2045(15)00088-1
Keywords: radiation, glioblastoma, GBM, PD-1/PD-L1, CTLA-4, immunotherapies, innate and adaptive immune responses
Citation: Rajani KR, Carlstrom LP, Parney IF, Johnson AJ, Warrington AE and Burns TC (2019) Harnessing Radiation Biology to Augment Immunotherapy for Glioblastoma. Front. Oncol. 8:656. doi: 10.3389/fonc.2018.00656
Received: 08 October 2018; Accepted: 12 December 2018;
Published: 22 February 2019.
Edited by:
Shiv K. Gupta, Mayo Clinic, United StatesReviewed by:
Antonio Rozzi, INI, Istituto Neurotraumatologico Italiano, ItalyCopyright © 2019 Rajani, Carlstrom, Parney, Johnson, Warrington and Burns. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Terry C. Burns, YnVybnMudGVycnlAbWF5by5lZHU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.