
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 20 December 2018
Sec. Genitourinary Oncology
Volume 8 - 2018 | https://doi.org/10.3389/fonc.2018.00623
 Marie Christine Hupe1
Marie Christine Hupe1 Christian Philippi2
Christian Philippi2 Doris Roth2
Doris Roth2 Christiane Kümpers2
Christiane Kümpers2 Julika Ribbat-Idel2
Julika Ribbat-Idel2 Finn Becker2
Finn Becker2 Vincent Joerg2
Vincent Joerg2 Stefan Duensing3
Stefan Duensing3 Verena Helena Lubczyk4
Verena Helena Lubczyk4 Jutta Kirfel2
Jutta Kirfel2 Verena Sailer2
Verena Sailer2 Rainer Kuefer5
Rainer Kuefer5 Axel Stuart Merseburger1
Axel Stuart Merseburger1 Sven Perner2*†
Sven Perner2*† Anne Offermann2†
Anne Offermann2†Background: Stratifying prostate cancer (PCa) patients into risk groups at time of initial diagnosis enabling a risk-adapted disease management is still a major clinical challenge. Existing studies evaluating the prognostic potential of PSMA (prostate-specific membrane antigen) for PCa were performed on radical prostatectomy specimens (RPE), i.e., decision making for disease management was already completed at time of sample analysis. Aim of our study was to assess the prognostic value of PSMA expression for PCa patients on biopsies at time of initial diagnosis.
Methods: PSMA expression was assessed by immunohistochemistry on 294 prostate biopsies with corresponding RPE, 621 primary tumor foci from 242 RPE, 43 locally advanced or recurrent tumors, 34 lymph node metastases, 78 distant metastases and 52 benign prostatic samples. PSMA expression was correlated with clinico-pathologic features. Primary endpoint was recurrence free survival. Other clinicopathologic features included WHO/ISUP grade groups, PSA serum level, TNM-stage, and R-status. Chi-square test, ANOVA-analyses, Cox-regression, and log-rank tests were performed for statistical analyses.
Results: High PSMA expression on both biopsy and RPE significantly associates with a higher risk of disease recurrence following curative surgery. The 5-year-recurrence free survival rates were 88.2, 74.2, 67.7 and 26.8% for patients exhibiting no, low, medium, or high PSMA expression on biopsy, respectively. High PSMA expression on biopsy was significant in multivariate analysis predicting a 4-fold increased risk of disease recurrence independently from established prognostic markers. PSMA significantly increases during PCa progression.
Conclusion: PSMA is an independent prognostic marker on biopsies at time of initial diagnosis and can predict disease recurrence following curative therapy for PCa. Our study proposes the application of the routinely used IHC marker PSMA for outcome prediction and decision making in risk-adapted PCa management on biopsies at time of initial diagnosis.
Prostate cancer (PCa) is still the most common cancer type among men with more than 200 new diagnoses per 100,000 men/year in Northern and Western Europe (1). The combination of the serum marker prostate specific antigen (PSA), Gleason score of the prostate biopsy, and clinical stage is currently used to stratify patients into different risk groups for biochemical recurrence (1). Recently, a new Gleason Grading System has been introduced by the International Society of Urological Pathology (ISUP) aiming to increase accuracy and thus the prognostic value (2). However, while the new grading system seems to reduce the rate of upgrading from biopsy to corresponding radical prostatectomy specimens (RPE) it does not seem to significantly improve the prognostic value (3). Thus, further diagnostic tools are urgently needed to optimize risk stratification of PCa patients at time of initial diagnosis.
Commonly used diagnostic markers to confirm PCa include PSA, androgen receptor, or prostate-specific membrane antigen (PSMA) (4). PSMA was first described in 1987 and subsequently characterized as a transmembranous glycoprotein exhibiting hydrolytic activity (5, 6). PSMA is expressed in normal, benign and malignant prostate tissues including intraepithelial neoplasia and metastatic specimens (7, 8). Notably, PSMA expression is significantly higher in primary PCa as compared to benign tissue and also significantly higher in lymph node and distant metastases as compared to primary tumors (4).
PSMA already has a variety of clinical implications in the management of PCa (9). In case of biochemical recurrence following curative therapy, a 68Ga-PSMA PET/CT can be performed to detect local and distant cancer sites. PSMA acts as the target for the radiotracer (10, 11). Similarly, in the course of salvage surgery for recurrent PCa it has been demonstrated that intravenous 99mTc-PSMA can intraoperatively increase detection of metastatic lesions using a gammaprobe known as radio-guided surgery (12). PSMA can also be targeted by PSMA ligands for a radioligand therapy, such as Lutetium-177. This therapy is provided in compassionate use programs or applied as last line treatment option for metastatic castration resistant PCa (13–15).
In RPE specimens, PSMA overexpression has already been linked to an unfavorable biochemical recurrence free survival rate (16–18). In prostate biopsy and RPE specimen, PSMA expression also significantly correlates with Gleason Score (18) implying a potential prognostic value for PSMA in prostate biopsy as well.
Notably, above mentioned studies evaluating the prognostic potential of PSMA for PCa outcome performed analyses at time of therapy by using RPE specimens, i.e., the assessed PSMA level has no impact on treatment decision.
The aim of the present study however was to assess the independent prognostic value of PSMA expression on prostate biopsy specimens enabling a future PSMA based risk stratification of PCa patients with localized disease at time of initial diagnosis.
The cohort used in this study includes 294 preoperative biopsies (Biopsy Cohort), 621 primary tumor foci from 242 patients (RPE Cohort), 43 locally advanced or recurrent tumors obtained from transurethral resection of the prostate, 34 lymph node metastases, and 52 benign prostatic samples from patients who underwent surgery for prostate cancer in the Hospital of Goeppingen, Germany between 2002 and 2014. From 124 patients, corresponding pre-operative needle biopsy and RPE specimen were available. Survival analysis considering biopsies and RPE specimens was performed by including all patients with available follow-up data. Additional 78 distant metastases of patients who were treated at the University Hospital Schleswig-Holstein, Campus Luebeck, Germany, were included in the present study. Patients' characteristics are presented in Table 1.
Disease recurrence was defined as biochemical recurrence (PSA increase above the post-operative nadir following RPE) and used as endpoint for survival analysis.
Ethical approval for the present study was obtained from the Internal Review Board of the University Hospital Schleswig-Holstein, Campus Luebeck.
Immunohistochemical (IHC) analysis of tissues except biopsies was performed on tissue microarrays (TMA). Briefly, formalin-fixed paraffin-embedded tissues were cut in 4-μm thick sections, mounted on slides and relevant tissue regions were circled by a pathologist. Three representative cores of the circled regions measuring 0.6 mm in diameter from each sample were assembled into tissue microarray blocks using a semiautomatic tissue arrayer. For more details see (19).
IHC staining was performed using the Ventana Discovery automated staining system (Ventana Medical System). In brief, slides were incubated at room temperature with primary antibody: anti-PSMA rabbit monoclonal antibody (SP29), SPING Bioscience, and detected with the ultraView Universal DAB Detection Kit (Ventana Medical System).
Membranous PSMA expression was categorized according to its intensity and scored as the following: 0 (no expression), 1 (low expression), 2 (medium expression), and 3 (high expression). Images showing examples for different PSMA scores are shown in Figure 1. IHC analysis was performed by two independent pathologists. In cases of discrepancy, the two pathologists agreed to a consensus.
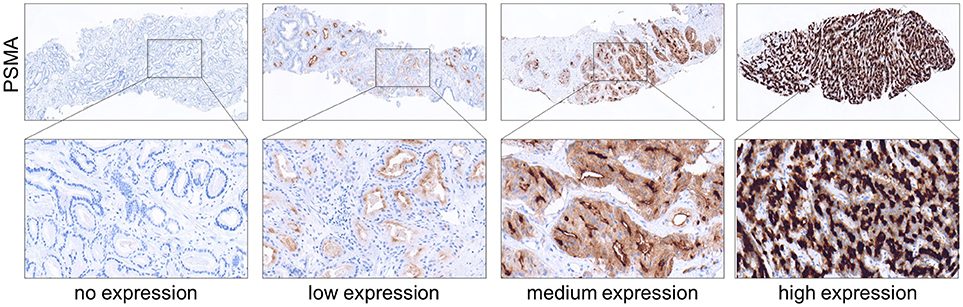
Figure 1. Immunohistochemical images showing PSMA negative, low, medium and high expressing prostate tumors obtained from needle biopsies. (Upper) Magnification 4x. (Lower) Magnification 40x.
For each patient, only the needle biopsy with highest grade group was selected for IHC staining. In cases with consistent grade group between biopsies, the core with highest tumor volume was selected for IHC. In order to take PCa multifocality into account, within RPE specimens, up to 5 largest tumor foci were represented on tissue microarrays. For each patient, the mean PSMA score between all tumor foci, as well as the maximum PSMA score out of the assessed tumor foci were used for further statistical analyses.
Out of 294 biopsies that have been stained, 276 (94%) were suitable for analysis. Following cutting for IHC 29 (10%) biopsies lacked tumor tissue.
Cox proportional-hazards regression models were performed to investigate the association between PSMA expression and iPSA. For multivariate analysis, PSMA expression was adjusted to grade group on biopsy and initial PSA (iPSA) for analysis of biopsies, and to T-stage, iPSA, R-status, N-status, and grade group for analysis of RPE specimens. PSMA was defined as a nominal categorical variable. For all multivariable analyses, we defined “PSMA negative” as the reference group.
Kaplan–Meier curves were used to illustrate biochemical recurrence free survival and statistically proved by log-rank test. Chi-Square test was performed to investigate the association between PSMA expression and grade groups, T-stage, N-status, and iPSA, as well as between PSMA on biopsies and RPE specimens. ANOVA was performed to analyze differences in PSMA expression levels in different tissue types.
All statistical analyses were performed using SPSS 20.0. P levels < 0.05 were considered significant.
PSMA expression is significantly higher expressed in tumor tissue compared to benign prostatic glands. On biopsies, PSMA expression was detectable in tumor tissue and benign glands in 87 and 16% of cases, respectively. Comparing the PSMA expression during PCa progression, Figure 2A shows increasing PSMA expression in lymph node metastases, locally advanced/recurrent tumors and distant metastases compared to primary tumors, and lowest expression in benign prostatic glands (ANOVA p < 0.001, Figure 2A).
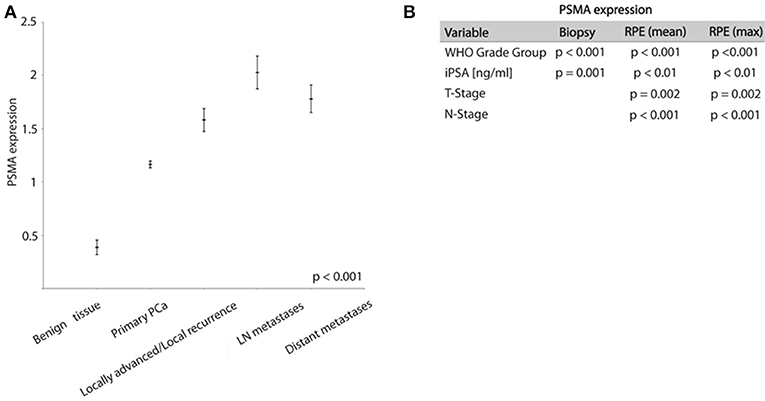
Figure 2. (A) Graph showing PSMA expression in different prostate tissue types (mean ± SEM). (B) Chi-Square test results analyzing association between PSMA expression and shown variables.
High PSMA expression is significantly associated with high grade groups and high iPSA level on both pre-operative biopsy and RPE (Table 2 and Figure 2B). Furthermore, increasing PSMA expression on RPE specimens are associated to higher T-stage and positive nodal status (Figure 2B).
PSMA expression on biopsy is congruent with PSMA expression on the corresponding RPE specimen (considering mean expression on RPE p = 0.006, considering maximum PSMA on RPE p = 0.022).
Increased PSMA expression on tumor tissue obtained from biopsy at time point of initial diagnosis is significantly associated with the likelihood of disease recurrence. During the observation period, 79 out of 235 patients (33.6%) developed disease recurrence following RPE. The frequency of disease recurrence was 16.7, 25.7, 39.2, and 60.7% for patients exhibiting no, low, medium or high PSMA expression on pre-operative biopsy, respectively. Kaplan-Meier curve illustrates reduced recurrence free survival with increasing PSMA expression (log-rank test, p < 0.001). The 5-year-PSA-recurrence free survival rates are 88.2, 74.2, 67.7, and 26.8% for patients exhibiting no, low, medium or high PSMA expression on pre-operative biopsy, respectively (Figure 3A). Compared to PSMA-negative tumors, low, medium and high PSMA expression is associated with 1.940-, 2.893-, and 6.900-fold incidence of developing disease recurrence following RPE, respectively (univariate Cox-regression, p = 0.175, p = 0.028, and p < 0.001, respectively, Figure 3B). Multivariate Cox-regression adjusting PSMA expression to iPSA blood level at time of diagnosis and grade group on biopsy was used to investigate the potential to predict disease recurrence independently from other prognostic marker. High PSMA expression on biopsy remained significant (p = 0.008) in multivariate analysis predicting a 4.024-fold increased risk of disease recurrence in relation to PSMA negative tumors independently from other established prognostic factors (Figure 3C).
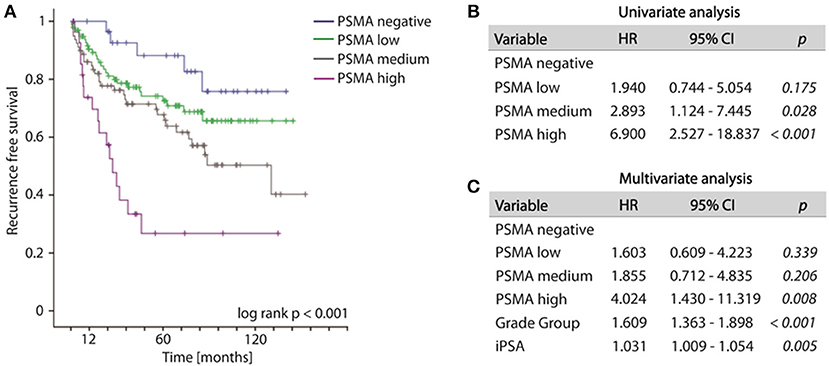
Figure 3. (A) Kaplan-Meier curves showing recurrence free survival of patients following radical prostatectomy stratified by PSMA expression on biopsies, log-rank p < 0.001. (B,C) Cox regression analyses on biopsy cohort for disease recurrence. HR, Hazard ratio; 95%CI,95% confidence interval.
Concordantly to our findings on biopsies, both mean as well as maximum PSMA expression considering multifocal tumors of RPE specimens significantly predict disease recurrence following surgery (log-rank p < 0.01, Figures 4A,B). The 5-year-disease recurrence free survival rates are 87.3, 81.6, 77.0, and 43.8% for patients exhibiting no, low, medium, or high mean PSMA expression, and 87.3, 89.7, 71.0, and 56.3% for patients exhibiting no, low, medium, or high maximum PSMA expression on RPE (Figures 4A,B). Univariate Cox-regression reveals a 1.267-, 1.950-, and 5.623-fold increased risk in low, medium and high PSMA expressing tumors compared to PSMA-negative tumors (95%CI 0.611–2.626, 0.923–4.122, 2.516–12.565; p = 0.525, p = 0.08, p < 0.001) considering PSMA mean expression, and 0.960-, 2.096-, and 4.102-fold increased risk in low, medium and high PSMA expressing tumors compared to PSMA-negative tumors (95%CI 0.439–2.097, 1.021–4.303, 1.863–9.033; p = 0.918, p = 0.04, p < 0.001) considering maximum PSMA expression. Adjusting PSMA expression to other prognostic factors (grade group, T-, N-, R-status, iPSA blood level), multivariate Cox-regression reveals no significant hazard ratio considering both mean and maximum PSMA expression on RPE.
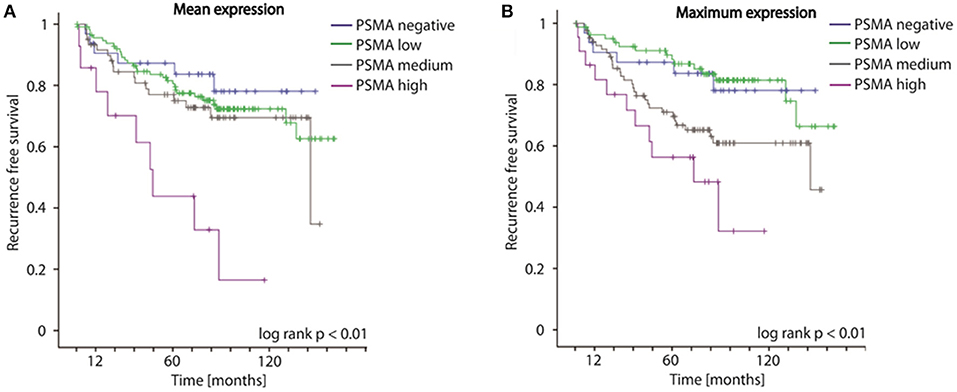
Figure 4. (A,B) Kaplan-Meier curves showing recurrence free survival of patients following radical prostatectomy stratified by mean and maximum PSMA expression on RPE specimens, log-rank p < 0.01.
PCa is characterized by a highly variable disease course, thus stratifying patients into risk groups for individual treatment decisions is still a major clinical challenge. Parameters that are independent from grade group and PSA are urgently needed to improve prognosis at time of initial diagnosis. Considering that PSMA expression on RPE specimens significantly associates with patients' outcome, aim of this study was to assess its prognostic value when assessed on biopsies at time of initial diagnosis, i.e., prior therapy decision. Long term goal of this study is consideration of biopsy PSMA level during decision making for risk-adapted PCa management.
Collectively, our results demonstrate a prognostic value of PSMA expression for disease recurrence following curative surgery at time of initial diagnosis on biopsy. A high PSMA expression on biopsy is associated with a 7-fold increased risk of experiencing disease recurrence following curative therapy compared to negative PSMA expression. In multivariate analyses, high PSMA expression on biopsy predicts disease recurrence independently from established prognostic factors such as Gleason grading or iPSA value. The 5-year-recurrence free survival rates are 88.2, 74.2, 67.7, and 26.8 for patients exhibiting no, low, medium or high PSMA expression on biopsy, respectively. The prognostic potential of PSMA was confirmed on RPE specimens. Furthermore, our study confirms previous study results by other working groups: PSMA expression significantly correlates with the established prognostic parameters grade group and PSA and increases during prostate cancer progression (16–18).
Taken together, PSMA qualifies as an additional prognostic factor determinable on prostate biopsies next to PSA serum levels and grade group. PSMA has the potential to discriminate indolent from aggressive disease and subsequently to stratify patients into risk groups at time of diagnosis. The main advantage is its widespread availability in pathology departments since PSMA is already a well-established and routinely used diagnostic marker for IHC analyses for PCa (4).
There are already a few studies attributing PSMA expression a prognostic value with regard to recurrence free survival. However, PSMA expression in these studies was assessed on RPE specimens, i.e., at time of therapy and thus subsequent to risk stratification of the patient (16, 17). Perner et al. demonstrated that high PSMA expression independently predicts PSA recurrence on RPE samples (HR 1.4, 95%CI 1.1–2.8, p = 0.017) (16). Minner et al. showed that tumors with a high PSMA expression had a higher risk of biochemical recurrence (p = 0.0483) (17).
PSMA also seems to be a marker of cancer progression. Similar to our results, Perner et al. could show that PSMA expression levels significantly differed between benign prostatic tissue, localized PCa and lymph node metastases (16). Also, Queisser et al. and Wright et al. demonstrated an increased PSMA expression in PCa metastases compared to other PCa tissues (4, 8). Notably, PSMA is also overexpressed in prostatic intraepithelial neoplasia (PIN) compared to benign prostatic tissue (20). Jemaa et al. linked the increased PSMA expression in PCa tissues to an increased angiogenesis by performing CD34 staining (21, 22). Nevertheless, this hypothesis needs further validation.
Limitations of the current study are that the analyses are based on a retrospective data set, clinical T-status for multivariate analysis was not available and disease specific survival as endpoint was not assessable due to low frequency of PCa specific deaths in our cohort. We believe that assessing PSMA expression on prostate biopsies has a great potential to be implemented as an additional biomarker in the clinical management of PCa patients.
In conclusion, our data show that PSMA expression assessed by IHC on prostate biopsy has a great prognostic potential at time of initial diagnosis. PSMA is a routinely used marker for PCa in pathology departments and thus ready to use for including in pathological reports. By performing PSMA expression analyses at time of diagnosis, patient stratification into risk groups can be optimized and individual PCa management improved. Patients with high PSMA expression levels in prostate biopsy have a shorter disease free survival following curative therapy.
The datasets generated and/or analyzed during the current study are not publicly available due to data security but are available from the corresponding author on reasonable request.
MH, SP, AM, and AO study concept and design; RK, VL, CP, and AO acquisition of data; MH, SP, AM, AO, CK, JR-I, DR, CP, SD, VJ, and FB analysis and interpretation of data; CP, MH, AO, and SP drafting of the manuscript; All co-authors critical revision of the manuscript; VS, MH, and AO statistical analysis; SP, MH, and AO obtaining funding; RK administrative, technical, or material support; SP, AM, and JK supervision.
The study was supported by a grant from the German Research Foundation (Deutsche Forschungsgemeinschaft (DFG) (PE1179/9-1 and PE1179/11-1) to SP and junior research grants from the University Luebeck to AO and MH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Wenzel Vogel, Eva Dreyer, and Michelle Thiermann for their excellent technical support.
1. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. (2017) 71:618–29. doi: 10.1016/j.eururo.2016.08.003
2. Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. (2016) 69:428–35. doi: 10.1016/j.eururo.2015.06.046
3. Offermann A, Hohensteiner S, Kuempers C, Ribbat-Idel J, Schneider F, Becker F, et al. Prognostic value of the new prostate cancer international society of urological pathology grade groups. Front Med. (2017) 4:157. doi: 10.3389/fmed.2017.00157
4. Queisser A, Hagedorn SA, Braun M, Vogel W, Duensing S, Perner S. Comparison of different prostatic markers in lymph node and distant metastases of prostate cancer. Mod Pathol. (2015) 28:138–45. doi: 10.1038/modpathol.2014.77
5. Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. (1987) 7:927–35.
6. Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. (1996) 2:1445–51.
7. Troyer JK, Beckett ML, Wright GL Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer (1995) 62:552–8.
8. Wright GL Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. (1995) 1:18–28. doi: 10.1016/1078-1439(95)00002-Y
9. Lütje S, Slavik R, Fendler W, Herrmann K, Eiber M. PSMA ligands in prostatecancer - Probe optimization and theranostic applications. Methods (2017) 130:42–50. doi: 10.1016/j.ymeth.2017.06.026
10. Han S, Woo S, Kim YJ, Suh CH. Impact of (68)Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. (2018) 74:179–90. doi: 10.1016/j.eururo.2018.03.030
11. Rauscher I, Duwel C, Haller B, Rischpler C, Heck MM, Gschwend JE, et al. Efficacy, predictive factors, and prediction nomograms for (68)Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. (2018) 73:656–61. doi: 10.1016/j.eururo.2018.01.006
12. Maurer T, Robu S, Schottelius M, Schwamborn K, Rauscher I, van den Berg NS, et al. (99m)Technetium-based prostate-specific membrane antigen-radioguided surgery in recurrent prostate cancer. Eur Urol. (2018). doi: 10.1016/j.eururo.2018.03.013. [Epub ahead of print].
13. Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. (177)Lu-PSMA Radioligand therapy for prostate cancer. J Nucl Med. (2017) 58:1196–200. doi: 10.2967/jnumed.117.191023
14. Heck MM, Retz M, Tauber R, Knorr K, Kratochwil C, Eiber M. PSMA-targeted radioligand therapy in prostate cancer. Urologe A. (2017) 56:32–9. doi: 10.1007/s00120-016-0274-3
15. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche, Krebshilfe, AWMF):, Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose, und Therapie der verschiedenen Stadien des Prostatakarzinoms, Langversion, 5,.0 (2018). AWMF Registernummer: 043/022OL. Available online at: https://www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom/ (Accessed June 07, 2018).
16. Perner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. (2007) 38:696–701. doi: 10.1016/j.humpath.2006.11.012
17. Minner S, Wittmer C, Graefen M, Salomon G, Steuber T, Haese A, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate (2011) 71:281–8. doi: 10.1002/pros.21241
18. Bravaccini S, Puccetti M, Bocchini M, Ravaioli S, Celli M, Scarpi E, et al. PSMA expression: a potential ally for the pathologist in prostate cancer diagnosis. Sci Rep. (2018) 8:4254. doi: 10.1038/s41598-018-22594-1
19. Scheble VJ, Braun M, Beroukhim R, Mermel CH, Ruiz C, Wilbertz T, et al. ERG rearrangement is specific to prostate cancer and does not occur in any other common tumor. Mod Pathol. (2010) 23:1061–67. doi: 10.1038/modpathol.2010.87
20. Marchal C, Redondo M, Padilla M, Caballero J, Rodrigo I, García J, et al. Expression of prostate specific membrane antigen (PSMA) in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. Histol Histopathol. (2004) 19:715–8. doi: 10.14670/HH-19.715
21. Ben Jemaa A, Bouraoui Y, Sallami S, Banasr A, Ben Rais N, Ouertani L, et al. Co-expression and impact of prostate specific membrane antigen and prostate specific antigen in prostatic pathologies. J Exp Clin Cancer Res. (2010) 29:171. doi: 10.1186/1756-9966-29-171
Keywords: disease recurrence, immunohistochemistry, prognostic biomarker, prostate biopsy, prostate cancer, prostate-specific membrane antigen
Citation: Hupe MC, Philippi C, Roth D, Kümpers C, Ribbat-Idel J, Becker F, Joerg V, Duensing S, Lubczyk VH, Kirfel J, Sailer V, Kuefer R, Merseburger AS, Perner S and Offermann A (2018) Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front. Oncol. 8:623. doi: 10.3389/fonc.2018.00623
Received: 08 October 2018; Accepted: 30 November 2018;
Published: 20 December 2018.
Edited by:
George Kulik, Wake Forest University, United StatesReviewed by:
Francesca Sanguedolce, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, ItalyCopyright © 2018 Hupe, Philippi, Roth, Kümpers, Ribbat-Idel, Becker, Joerg, Duensing, Lubczyk, Kirfel, Sailer, Kuefer, Merseburger, Perner and Offermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sven Perner, c3Zlbi5wZXJuZXJAdWtzaC5kZQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.