- 1Henan Office for Cancer Control and Research, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 2Office of Cancer Screening, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Nursing Department, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 4Department of Head and Neck Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Department of Head Neck and Thyroid Surgery, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
Background: The burden of head and neck cancer in China is heavier, and studies have shown that it may be associated with HPV infection, especially high-risk HPV.
Objectives: We aimed to conduct a meta-analysis to estimate the high-risk HPV-16 prevalence of head and neck cancer in the Chinese population.
Methods: The reports on HPV and head and neck cancer in a Chinese population published between Jan 1, 2006 and Oct 23, 2018 were retrieved via WANFANG/CNKI/MEDLINE/EMBASE databases. The pooled prevalence and corresponding 95% confidence intervals was calculated by a random-effect model.
Results: The meta-analysis included a total of 2,896 head and neck cancer cases from 28 studies. Overall, the pooled HPV-16 prevalence among head and neck cancer cases was 24.7% (20.2–29.3%) in China, 31.6% (21.7–41.5%) in oropharyngeal cancer, 28.5% (18.2–38.7%) in laryngeal cancer and 14.9% (10.1–19.7%) in oral cancer, 25.3% (14.8–35.8%) in fresh or frozen biopsies and 25.0% (19.5–30.5%) in paraffin-embedded fixed biopsies, 36.5% (17.9–55.1%) by E6/E7 region and 14.3% (6.4–22.1%) by L1 region of HPV gene. The highest HPV-16 prevalence was found in Central China.
Conclusions: High prevalence of HPV-16 was found in the samples of Chinese head and neck cancers. Preventive HPV-vaccination may reduce the burden of HPV-related head and neck cancer in China.
Introduction
As a member of the papillomavirus family of viruses, human papillomavirus (HPV) can infect humans by attacking the squamous cell of skin and mucous membranes, including those of the cervix, anogenital region and head and neck (1–3). As we all know, nasopharyngeal carcinoma is related to EB virus infection. Except that, more than 11% of the remaining squamous cell carcinomas of head and neck are caused by high-risk human papillomavirus infection (4), and the incidence of such subtypes is increasing year by year (5). However, the frequency of HPV infection in head and neck cancers varies between 3 and 84% in different studies (6). Based on the different nucleotide sequences, HPV can be divided into more than 200 genotypes by DNA sequencing, of which HPV16 and HPV18 are more closely related to malignant tumors as the main high-risk types (7, 8). However, unlike cervical and oral carcinogenesis, the role of HPV-16/18 in the development of other head and neck cancers has not been clearly defined.
Even in the same country, the HPV prevalence in head and neck cancers range widely (9). Demographic and racial factors, sample condition, cancer location and the viral detection method have been proposed to identify possible causes of differences in the results. However, as one of the most common subtype of HR-HPV, the HPV-16 prevalence in China has not been estimated so far.
The aim of the meta-analysis is to estimate the prevalence of HPV-16 detected in head and neck cancer cases and the different cancer sites, influence of regions, specimen types, and detection methods in China from all published studies of English and Chinese language literature.
Materials and Methods
Literature Search Strategies
This meta-analysis was reported following the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (10). The key words “human papillomavirus,” “papillomavirus infections,” “HPV,” “head and neck cancer,” “laryngeal cancer,” “oropharyngeal cancer,” “oral cancer,” “neck cancer,” “head cancer,” and corresponding carcinoma in English language or in Chinese language were used in combination to search. The retrieved databases included MEDLINE (via PubMed), Excerpta Medica database (EMBASE), Wanfang Data Knowledge Service Platform and Chinese National Knowledge Infrastructure (CNKI). Date of the literature was assigned between Jan 1, 2006 and Oct 23, 2018. Firstly, we excluded the duplicates. Secondly, we screened each title and abstract to evaluate its possible relevance. Thirdly, we downloaded full text for detailed evaluation if titles and abstracts weren't enough to make decision. All papers were independently reviewed by two authors (YFN and LSZ). Uncertainties and discrepancies were resolved by consensus after discussing with a senior researcher (SXB). If the data we needed were not explicitly reported or could not be derived from the papers, we also emailed authors to obtain related data. Additional studies were also identified using cross-referencing.
Inclusion and Exclusion Criteria
According to the PRISMA statement, the literatures contained in this study must meets the following criteria: (a) to inform at least 10 cases of head and neck cancer confirmed by biopsy or histopathology, (b) to use polymerase chain reaction (PCR)-based methods (including type-specific PCR primers, broad-spectrum PCR primers, or a combination of both kinds of primers) or in situ hybridization (ISH) to amplify HPV DNA, (c) to report the prevalence of HPV-16 in head and neck cancer tissue samples with clear anatomical subsite.
The literatures excluded in this study were mainly due to the following reasons: were cellular or animal studies; were not conducted in Chinese; unable to extract or calculate the necessary data directly from the original article; without clear definition of anatomical subsite; reviews.
Data Extraction
All studies included in the final meta-analysis extracted the following data: first author's name, publication year, geographical areas, cancer site, clinical stage, numbers of cases and HPV positive cases, HPV test method, specimen types (formalin-fixed paraffin-embedded biopsies [FFPE], fresh, or frozen biopsies [FF]).
Statistical Analysis
Overall pooled point estimate and 95% confidence interval (95% CI) for HPV-16 prevalence were calculated through the method of DerSimonian and Laird (11) using the assumptions of a random-effects model. For a variety of HPV infections (including HPV-16), the multiple HPV types were classified into different types and the HPV-16 type-specific prevalence represents types for cases with either single HPV-16 infection and multiple HPV-16 infection.
Cochrane Q test (P < 0.10 indicated a high level of statistical heterogeneity) and I2 (values of 25, 50, and 75% corresponding to low, moderate and high degrees of heterogeneity, respectively) was used to assess the heterogeneity between eligible studies (12). Subgroup analyses for HPV-16 prevalence were subsequently carried out according to the geographical areas of the study origin, cancer site, year of publication, number of patients, HPV detection method and types of specimen. In the eligible studies, three studies, which contained different cancer sites, were treated as the separate studies. Meta-regression analyses were used to examine the association of the geographical areas of the study origin, cancer site, year of publication, number of patients, HPV detection method and types of specimen with the prevalence of HPV-16. Sensitivity analysis was also performed to assess the impact of each individual study on the strength and stability of the meta-analytic results. Each time, one study in the meta-analysis was excluded to show that study's impact on the overall impact size. Funnel plot and Begg adjusted rank correlation test for funnel plot asymmetry were performed to test any existing publication bias.
Statistical analysis was performed using STATA SE version 15.1 (StataCorp LP, College Station, TX, USA) for Windows. P < 0.05 with two-tailed was considered statistically significant.
Results
Systematic Review and Study Characteristics
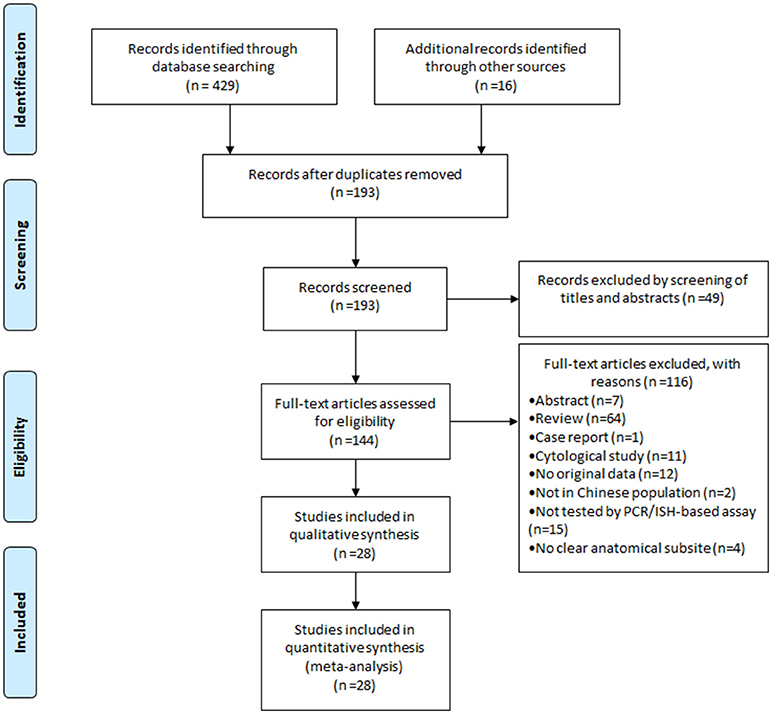
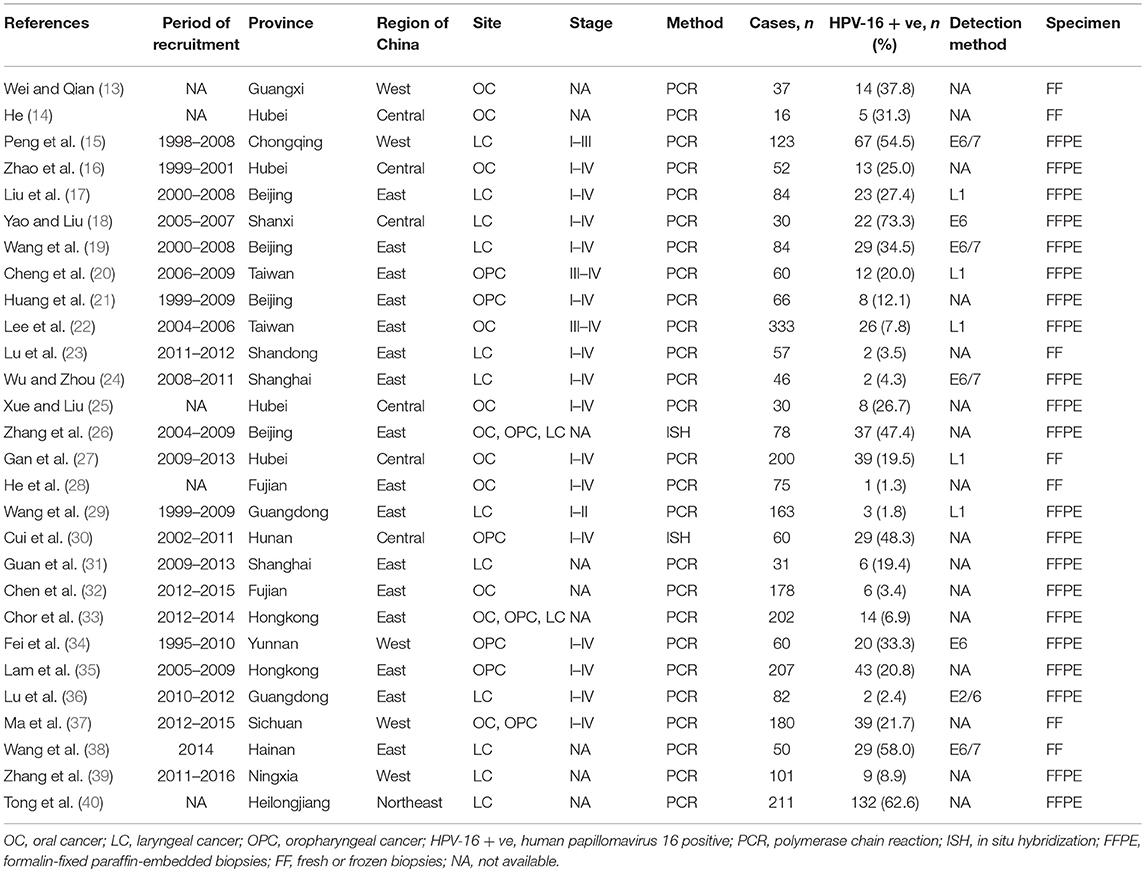
Figure 1 showed the flow diagram of systematic literature search. Generally speaking, the search strategy generated 445 citations, of which 193 were considered of potential value after screening of titles and abstracts and the full text was retrieved for detailed evaluation. 116 articles were subsequently excluded from the meta-analysis for various reasons, including 64 were reviews, 15 were not tested by PCR/ISH-based assay, 12 that did not provide HRs or CIs, 11 were cytological study, 7 were abstract, 4 had not clear anatomical subsite, 2 were not in Chinese population and 1 was case report. So, 28 studies (11 in English and 17 in Chinese) were eligible and included in this meta-analysis (13–40). Individual characteristics of the included 28 studies were summarized in Table 1. The size of the study samples ranged from 10 to 333 cases of head and neck cancer (median = 60). Summing up the studies, a total of 2,896 cases of head and neck cancer were identified. As shown in Table 1, more than half of the studies were conducted in Eastern China (n = 16, 57.14%), and the remaining studies distributed in three other regions of China as follows: 6 (21.43%) studies in Central China, 5 (17.86%) studies in Western China and 1 (3.57%) study in Northeastern China. 14 (50.00%) studies conducted in laryngeal cancer cases, 11 (39.29) conducted in oral cancer cases, and 8 (28.57%) conducted in oropharyngeal cancer cases. For HPV detection methods, 26 (92.86%) studies used PCR, and 2 (3.57%) studies used ISH. 21 (75.00%) studies used formalin-fixed paraffin-embedded biopsies (FFPE), and 7 (25.00%) studies used fresh or frozen biopsies. Besides, 7 (25.00%) studies used the gene detected from HPV E6/E7 region, and 5 (17.86%) used the gene detected from HPV L1 region.
Meta-Analysis of HPV-16 Prevalence in Head and Neck Cancer Cases
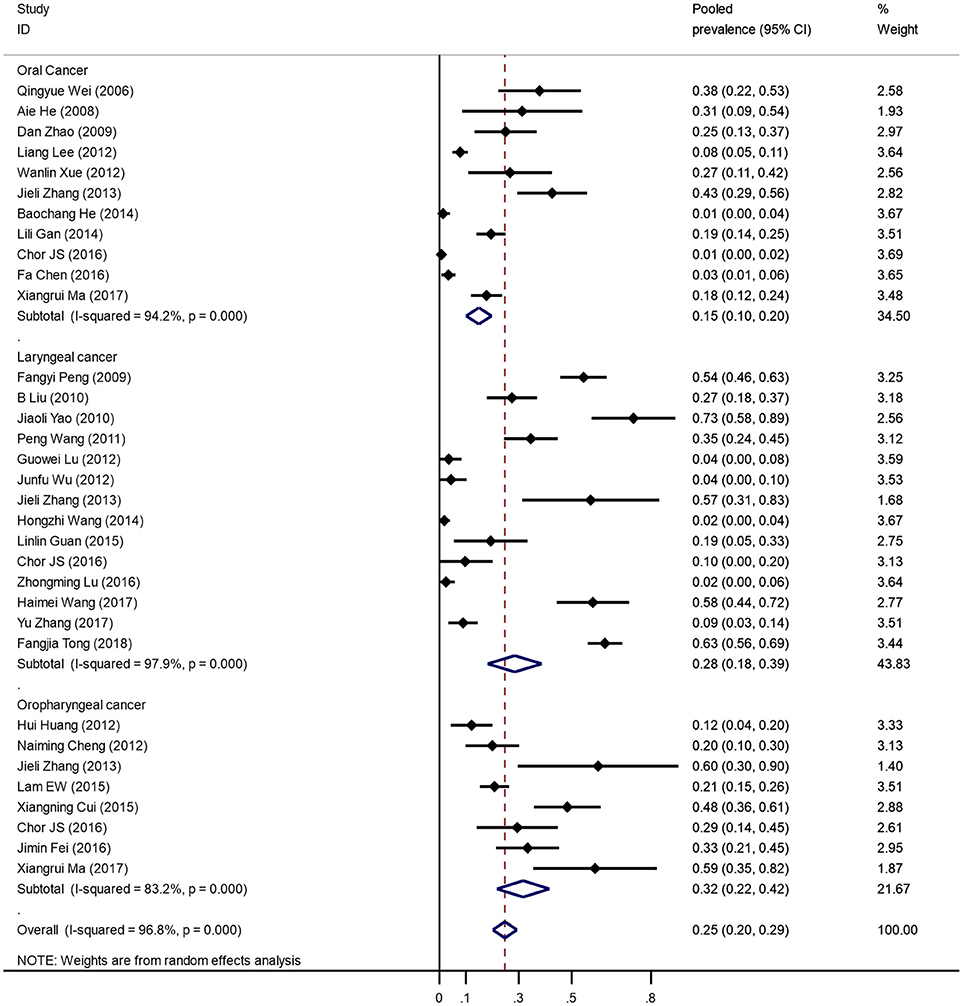
Figure 2 was the forest plot illustrated the individual and pooled prevalence estimates derived from a random effect model analysis. In this study, the prevalence of HPV-16 ranged from 1.3 to 58.0%. The pooled prevalence for HPV-16 was 24.7% (95% CI, 20.2–29.3%) in head and neck cancer cases in the Chinese population. Overall, high heterogeneity was observed in the studies included (Q-test Pheterogeneity < 0.001, I2 = 96.8%).
Subgroup Analysis and Meta-Regression
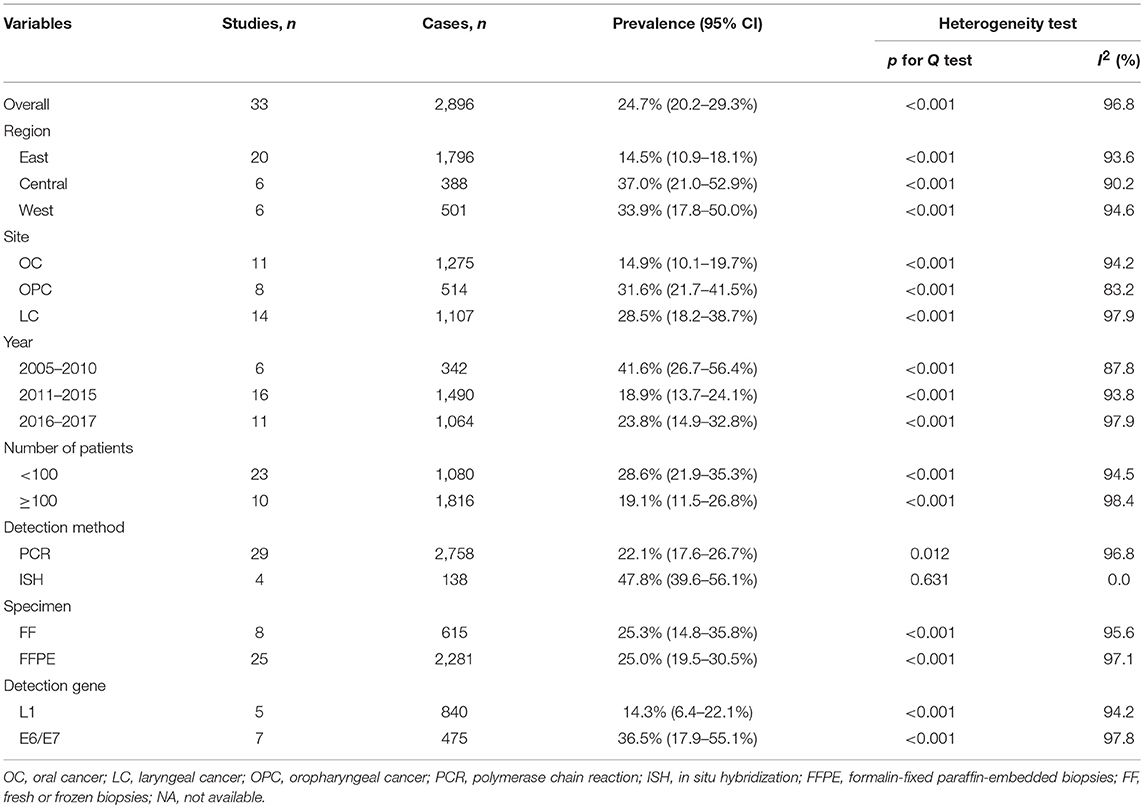
Table 2 presents detailed results of subgroup analyses. As shown in Table 2, the highest pooled HPV-16 prevalence was observed in Central China (37.0%; 95% CI, 21.0–52.9%), followed by that in Western China (33.9%; 95% CI, 17.8–50.0%), and in Eastern China (14.5%; 95% CI, 10.9–18.1%). Oropharyngeal cancer had the highest infection rate of HPV-16 (31.6%; 95% CI, 21.7–41.5%), followed by laryngeal cancer (28.5%; 95% CI, 18.2–38.7%) and oral cancer (14.9%; 95% CI, 10.1–19.7%). Stratified analysis by published year showed that head and neck cancer before the year 2011 had the highest HPV-16 prevalence (41.6%; 95% CI, 26.7–56.4%) as compared with the period from 2011 to 2015 (18.9%; 95% CI, 13.7–24.1%) and the period from 2016 to 2017 (23.8%; 95% CI, 14.9–32.8%). Stratified analysis by number of patients showed that head and neck cancer patients < 100 has the higher HPV-16 prevalence (28.6%; 95% CI, 21.9–35.3%) as compared with those more than 100 (19.1%; 95% CI, 11.5–26.8%). HPV-16 prevalence with PCR method (22.1%; 95% CI, 17.6–26.7%) was lower than with ISH method (47.8%; 95% CI, 39.6–56.1%). Hierarchical analysis by specimen types showed that head and neck cancer in FF tissue (25.3%; 95% CI, 14.8–35.8%) had almost the same prevalence of HPV-16 compared with the FFPE tissue (25.0%; 95% CI, 19.5–30.5%). Regarding the HPV-16 test methods, the prevalence of head and neck cancer in the E6/E7 region of HPV gene (36.5%; 95% CI, 17.9–55.1%) was significantly higher than that in the L1 region (14.3%; 95% CI, 6.4–22.1%). In short, the estimated heterogeneity for studies included decreased to some extent but not disappeared. Meta-regression analyses found significant association of HPV-16 prevalence with HPV detection method (slope = 2.78, P = 0.027).
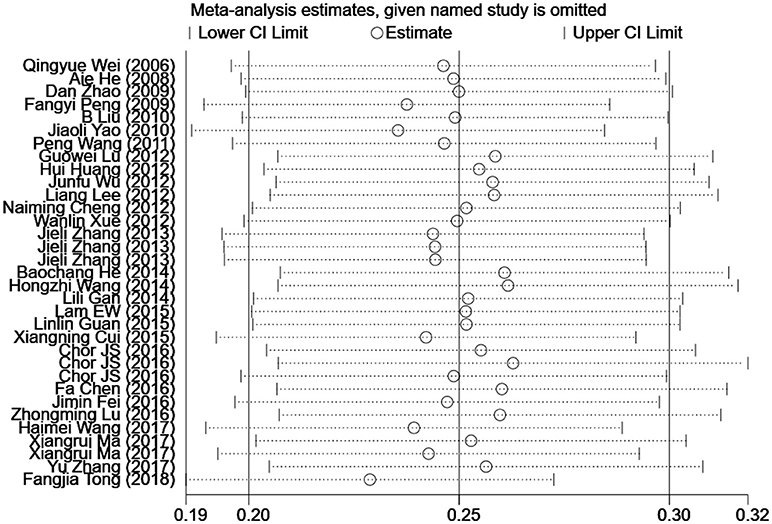
Influence Analysis of Individual Studies
To address the potential bias due to the quality of the included studies, we performed the sensitivity analysis by calculating pooled HPV-16 prevalence again when omitting one study at a time. Figure 3 showed the results of sensitivity analysis. The HPV-16 prevalence ranged from 23.0% (95% CI, 18.7–27.3%) to 26.4% (95% CI, 20.9–31.9%). Point estimates for all results of influence analysis were within 95% CIs of the pooled prevalence. The meta-analysis of pooled HPV-16 prevalence in head and neck cancer cases was not significantly affected by any of the 33 individual studies that was not analyzed, indicating that each study did not affect the stability of overall HPV-16 prevalence estimate.
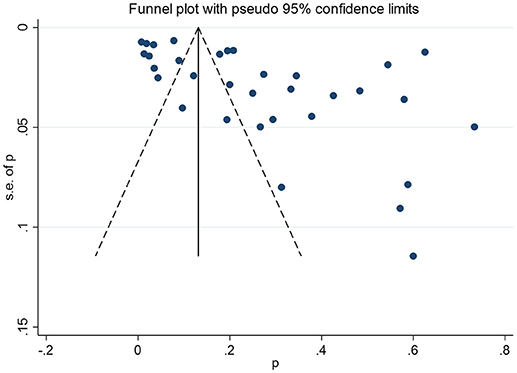
Publication Bias
The non-significant P-values of Begg's test (0.09), Eegg's test (0.07), and the near-symmetric funnel plot (Figure 4) demonstrated that there was no evidence of publication bias.
Discussion
China has the highest burden of head and neck cancer around the world, which was rational for studying the prevalence of certain types of HPV (type-16) in China for the highly lethal cancer. As we know, this is the first meta-analysis to exploring the prevalence of HPV-16 in Chinese head and neck cancer tissues. The results of the meta-analysis showed that about 25% cases of head and neck cancer harbored HPV-16, indicating a high level of HPV 16 infection in head and neck cancer cases of China. Our results were little higher than the global prevalence (41).
Characterizing the HPV-16 prevalence in head and neck cancer is an important preliminary step in assessing the relationship between HPV-16 and head and neck cancer. Estimates of the HPV prevalence in various cancer sites of head and neck cancer vary considerably. Our meta-analysis showed that oropharyngeal cancer had the highest HPV-16 prevalence (31.6%), followed by that in laryngeal cancer (28.5%), and in oral cancer (14.9%). Interesting, the prevalence of HPV-16 in oropharyngeal cancer and oral cancer were much lower than Asian average, but much higher in laryngeal cancer (41). More cases involved and areas covered could be helpful in estimating the prevalence of HPV-16 in different head and neck cancer sites in the future.
The detection rate of HPV-16 DNA in FF tissue was found somewhat higher than that in FFPE tissue, given significant DNA degradation could be observed in FFPE tissue (42). The HPV infection status was determined on FFPE tissue in the majority of included studies. It is known that the low detection rate of HPV DNA occur with the fabric of FFPE, especially when a long DNA fragment was amplified.
Some possible reasons for variation in HPV prevalence among studies include small study sizes, different HPV testing, inter-laboratory variability, and manipulation of specimens leading to contamination (43–45). In our meta-analysis, PCR and ISH were just used as HPV detection methods, which focused on the HPV DNA detection method. Besides, for better PCR and ISH sensitivity and specificity, the literature time was limited from January 1, 2006 to Oct 23, 2018. HPV, a double-stranded circular DNA virus, encodes early proteins (E1, E2, E5, E6, and E7) and late proteins (L1, L2, and E4) with about 8,000 bp genome size (46, 47). When stratifying the L1 and E6/E7 gene fragments, we found that, in E6/E7 gene fragment (35.8%), the detection rate of HPV-16 DNA was much higher than in L1 gene fragment (14.3%). This is mainly due to the disruption of L1 region when HPV is integrated into the host genome (48), which may be an important event in promoting and triggering head and neck cancer.
The highlights of this meta-analysis includes a large sample size, both English and Chinese published studies are included with a strict inclusion criteria. By including English and Chinese studies, the selection bias caused by the publication language was avoided. Finally, by astricting studied published after 2006, limiting PCR and ISH detection methods and excluding studies without specific subsites, we tried to minimize the HPV prevalence variation as much as possible.
However, the meta-analysis has several limitations. First, the studies included in the meta-analysis are heterogeneous, which could be explained by changes in the population, the cancer site, the year of publication, the number of patients, the HPV detection method, the sample collection method, and the sensitivity of HPV primer PCR different protocols. To solve this issue, the random-effects model was used in the meta-analysis to combine data if significant heterogeneity was found. We directly tested heterogeneity by describing the HPV-16 prevalence in head and neck cancer cases by study area, cancer site, year of publication, number of patients, method of HPV detection and sample types. Of course, we have not been fully able to explain the heterogeneity. Even in stratified outcomes (for example, studies in different parts of China), the prevalence estimates are still uneven. Second, the study estimates may be biased because the accuracy of these estimates depends on the test method used and the type of HPV evaluated. That is, some studies use multiple probes or wide primers to detect multiple types of HPV, while other studies only detect HPV-16 type. Finally, the possibility of confounder cannot be ruled out. Limited studies have implicated the effect of age and smoking on the prevalence of HPV. We could not determine whether the variation of HPV prevalence was due to differences in environmental factors: age, sexual habits, smoking, alcohol consumption and other ethnic and cultural differences, as little or no information on these potential confounders in the studies involved. Therefore, studies with good designs to explore HPV infection by major confounding factors are likely to be required in future studies.
In short, the current meta-analysis provides a quantitative estimate of HPV-16 prevalence in head and neck cancer lesions in China. Although this review is a preliminary step in assessing the relationship between HPV-16 and head and neck cancer in China, it may be useful to evaluate the effect of HPV-16/18 prophylactic vaccines against carcinogenesis in the future. Considering that HPV infection plays an important role in the tumorigenesis of head and neck, other scientific studies deserve to be done in the future.
Author Contributions
All authors have made substantial contributions to the conception and design of the study. LG and FY led protocol design, search, data extraction, statistical analysis, and manuscript drafting. YY and SL contributed to search, data extraction, and manuscript modifications. PL, XZ, DC, YL, JW, KW, YZ, QL, and XW contributed to quality assessment and revision of the manuscript. XS contributed to data interpretation and revision of the manuscript. All authors have reviewed and approved the final version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81703298).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. (2005) 14:467–75. doi: 10.1158/1055-9965.epi-04-0551
2. Stelzer MK, Pitot HC, Liem A, Schweizer J, Mahoney C, Lambert PF. A mouse model for human anal cancer. Cancer Prev Res (Phila). (2010) 3:1534–41. doi: 10.1158/1940-6207.capr-10-0086
3. Ciapponi A, Bardach A, Glujovsky D, Gibbons L, Picconi MA. Type-specific HPV prevalence in cervical cancer and high-grade lesions in Latin America and the Caribbean: systematic review and meta-analysis. PLoS ONE (2011) 6:e25493. doi: 10.1371/journal.pone.0025493
4. Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. (2016) 108:djv403. doi: 10.1093/jnci/djv403
5. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. (2008) 26:612–9. doi: 10.1200/jco.2007.14.1713
6. Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. (2010) 2:15. doi: 10.1186/1758-3284-2-15
7. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology (2004) 324:17–27. doi: 10.1016/j.virol.2004.03.033
8. Guo LW, Zhang SK, Liu SZ, Chen Q, Zhang M, Quan PL, et al. Human papillomavirus type-18 prevalence in oesophageal cancer in the Chinese population: a meta-analysis. Epidemiol Infect. (2016) 144:469–77. doi: 10.1017/s0950268815001703
9. Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta-analysis of trends by time and region. Head Neck (2013) 35:747–55. doi: 10.1002/hed.22015
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
12. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
13. Wei Q, Qian C. Relationship between oral squamous cell carcinoma and human papillomavirus subtypes. J Guangxi Med Univ. (2006) 23:767–8. doi: 10.3969/j.issn.1005-930X.2006.05.032
14. He A. Human papillomavirus and human cytomegalovirus in oral carcinoma. J Clin Stomatol. (2008) 24:528–9. doi: 10.3969/j.issn.1003-1634.2008.09.006
15. Peng F, Jiang H, Liu F, Du Z, Lin Z, Peng F, et al. Detection and sequencing of different subtypes of HPV in laryngeal carcinoma. J Chongqing Med Univ. (2009) 34:1689–92. doi: 10.13406/j.cnki.cyxb.2009.12.037
16. Zhao D, Xu QG, Chen XM, Fan MW. Human papillomavirus as an independent predictor in oral squamous cell cancer. Int J Oral Sci. (2009) 1:119–25. doi: 10.4248/ijos.09015
17. Liu B, Lu Z, Wang P, Basang Z, Rao X. Prevalence of high-risk human papillomavirus types (HPV-16, HPV-18) and their physical status in primary laryngeal squamous cell carcinoma. Neoplasma (2010) 57:594–600. doi: 10.4149/neo_2010_06_594
18. Yao J, Liu T. Expression of human herpesvirus 6 and human papillomavirus 16/18 in the laryngeal squamous cell carcinoma and their relations. J Shanxi Med Univ. (2010) 41:316–22. doi: 10.3969/j.issn.1007-6611.2010.04.009
19. Wang P, Rao X, Li Y, Ning T, Liu B. Detection of high-risk human papillomavirus types-16 and-18 in laryngeal squamous cell carcinoma. Cancer Res Clin. (2011) 23:14–7. doi: 10.3760/cma.j.issn.1006-9801.2011.01.005
20. Cheng NM, Chang JT, Huang CG, Tsan DL, Ng SH, Wang HM, et al. Prognostic value of pretreatment (1)(8)F-FDG PET/CT and human papillomavirus type 16 testing in locally advanced oropharyngeal squamous cell carcinoma. Eur J Nucl Med Mol Imaging (2012) 39:1673–84. doi: 10.1007/s00259-012-2186-9
21. Huang H, Zhang B, Chen W, Zhou SM, Zhang YX, Gao L, et al. Human papillomavirus infection in oropharyngeal squamous cell carcinoma and prognosis: a preliminary analysis of 66 cases. Chin J Otorhinolaryngol Head Neck Surg. (2012) 47:207–11. doi: 10.3760/cma.j.issn.1673-0860.2012.03.007
22. Lee LA, Huang CG, Liao CT, Lee LY, Hsueh C, Chen TC, et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS ONE (2012) 7:e40767. doi: 10.1371/journal.pone.0040767
23. Lu G, Wang L, Sun Y, Li W, Hua H, Ge R. Detection of different subtypes of HPV DNA in 57 cases of laryngeal carcinoma in easter of the shandong province. J Otolaryngol Ophthalmol Shandong Univ. (2012) 26:16–9. doi: 10.6040/j.issn.1673-3770.2012.06.006
24. Wu J, Zhou J. Detection of different type of human papillomavirus in benign, premalignant and malignant lesions of the larynx. J Mod Oncol. (2012) 20:1813–6. doi: 10.3969/j.issn.1672-4992.2012.09.16
25. Xue W, Liu C. Human papillomavirus type 16 infection in patients with oral squamous cell carcinomas and leukoplakia. J Clin Stomatol. (2012) 28:293–4. doi: 10.3969/j.issn.1003-1634.2012.05.015
26. Zhang J, Sun Z, Huo Z, Wang D, Cui Q, Bai C. Comparison of RT-PCR and in situ hybridization in detecting HPV16/18 DNA infection in tissues of head and neck squamous cell cancer. Basic Clin Med. (2013) 33:593–6. doi: 10.16352/j.issn.1001-6325.2013.05.027
27. Gan LL, Zhang H, Guo JH, Fan MW. Prevalence of human papillomavirus infection in oral squamous cell carcinoma: a case-control study in Wuhan, China. Asian Pac J Cancer Prev. (2014) 15:5861–5. doi: 10.7314/APJCP.2014.15.14.5861
28. He B, Chen F, Cao Y, Zheng X, Lin L, Cai L. A case control study on the association between HPV infection and oral cancer in Fujian area. J Fujian Med Univ. (2014) 48:100–5.
29. Wang H, Sun R, Hu W. Human papillomavirus infection and HRAS, PIK3CA mutations analysis in patients with early laryngeal carcinoma. Cancer Res Clin. (2014) 26:361–5. doi: 10.3760/cma.j.issn.1006-9801.2014.06.001
30. Cui X, Zhang X, Xu J, Huang D, Tang Y, Tian Y, et al. HPV16 infection and p16 protein expression in oropharyngeal squamous cell carcinoma and their clinical significance. J Mod Oncol. (2015) 23:1813–8. doi: 10.3969/j.issn.1672-4992.2015.13.09
31. Guan L, Sun N, Sun G, Fang Q, Meng Y, Zhao X, et al. Analysis of subtypes of HPV infection in laryngeal squamous cell carcinoma and precancerous lesions and its clinical significance. J Clin Otorhinolaryngol Head Neck Surg. (2015) 19:1549–52. doi: 10.13201/j.issn.1001-1781.2015.17.015
32. Chen F, Yan L, Liu F, Huang J, Liu F, Wu J, et al. Oral human papillomavirus infection, sexual behaviors and risk of oral squamous cell carcinoma in southeast of China: a case-control study. J Clin Virol. (2016) 85:7–12. doi: 10.1016/j.jcv.2016.10.011
33. Chor JS, Vlantis AC, Chow TL, Fung SC, Ng FY, Lau CH, et al. The role of human papillomavirus in head and neck squamous cell carcinoma: a case control study on a southern Chinese population. J Med Virol. (2016) 88:877–87. doi: 10.1002/jmv.24405
34. Fei J, Wu Y, Zeng W, Zhang R, Xi Y, Li M. The correlation of HPV infection with angiogenesis in oropharyngeal squamous cell carcinomas. J Mod Oncol. (2016) 24:541–4. doi: 10.3969/j.issn.1672-4992.2016.04.010
35. Lam EW, Chan JY, Chan AB, Ng CS, Lo ST, Lam VS, et al. Prevalence, clinicopathological characteristics, and outcome of human papillomavirus-associated oropharyngeal cancer in southern Chinese patients. Cancer Epidemiol Biomarkers Prev. (2016) 25:165–73. doi: 10.1158/1055-9965.epi-15-0869
36. Lu Z, Li Y, Xu M, Chen L, Luo X, Huang Y, et al. The infection and integration status of high-risk human papillomavirus 16/18 in laryngeal cancer. J Pract Med. (2016) 32:1422–4. doi: 10.3969/j.issn.1006-5725.2016.09.014
37. Ma X, Sheng S, Wu J, Jiang Y, Gao X, Cen X, et al. LncRNAs as an intermediate in HPV16 promoting myeloid-derived suppressor cell recruitment of head and neck squamous cell carcinoma. Oncotarget (2017) 8:42061–75. doi: 10.18632/oncotarget.14939
38. Wang H, Mu Z, Zhou X, Zhaou X. Relationship between high-risk human papillomavirus E7 mRNA and laryngeal lesions. Chin J Gerontol. (2017) 37:2875–7. doi: 10.3969/j.issn.1005-9202.2017.12.006
39. Zhang Y, Chen X, Li X, Li C, Lu D, Ma R, et al. Correlation of positive expressions of HPV and EBV with laryngeal carcinoma. J Pract Med. (2017) 33:2117–22. doi: 10.3969/j.issn.1006-5725.2017.13.012
40. Tong F, Geng J, Yan B, Lou H, Chen X, Duan C, et al. Prevalence and prognostic significance of HPV in laryngeal squamous cell carcinoma in northeast China. Cell Physiol Biochem. (2018) 49:206–16. doi: 10.1159/000492858
41. Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsague X, Laporte L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. (2014) 15:1319–31. doi: 10.1016/s1470-2045(14)70471-1
42. Srinivasan M, Taioli E, Ragin CC. Human papillomavirus type 16 and 18 in primary lung cancers–a meta-analysis. Carcinogenesis (2009) 30:1722–8. doi: 10.1093/carcin/bgp177
43. Poljak M, Cerar A, Seme K. Human papillomavirus infection in esophageal carcinomas: a study of 121 lesions using multiple broad-spectrum polymerase chain reactions and literature review. Hum Pathol. (1998) 29:266–71. doi: 10.1016/S0046-8177(98)90046-6
44. Hubbard RA. Human papillomavirus testing methods. Arch Pathol Lab Med. (2003) 127:940–5. doi: 10.1043/1543-2165(2003)127<940:hptm>2.0.co;2
45. Kamangar F, Qiao YL, Schiller JT, Dawsey SM, Fears T, Sun XD, et al. Human papillomavirus serology and the risk of esophageal and gastric cancers: results from a cohort in a high-risk region in China. Int J Cancer (2006) 119:579–84. doi: 10.1002/ijc.21871
46. Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. (2002) 89:213–28. doi: 10.1016/S0168-1702(02)00190-9
47. Garcia-Vallve S, Alonso A, Bravo IG. Papillomaviruses: different genes have different histories. Trends Microbiol. (2005) 13:514–21. doi: 10.1016/j.tim.2005.09.003
Keywords: head and neck cancer, human papillomavirus, prevalence, meta-analysis, China
Citation: Guo L, Yang F, Yin Y, Liu S, Li P, Zhang X, Chen D, Liu Y, Wang J, Wang K, Zhu Y, Lv Q, Wang X and Sun X (2018) Prevalence of Human Papillomavirus Type-16 in Head and Neck Cancer Among the Chinese Population: A Meta-Analysis. Front. Oncol. 8:619. doi: 10.3389/fonc.2018.00619
Received: 12 September 2018; Accepted: 29 November 2018;
Published: 12 December 2018.
Edited by:
Tianhui Chen, Zhejiang Academy of Medical Sciences, ChinaReviewed by:
Lu Yin, National Center for Cardiovascular Diseases (CAMS), ChinaJerry Polesel, Centro di Riferimento Oncologico di Aviano (IRCCS), Italy
Copyright © 2018 Guo, Yang, Yin, Liu, Li, Zhang, Chen, Liu, Wang, Wang, Zhu, Lv, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xibin Sun, eGJzdW4yMUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Lanwei Guo
Lanwei Guo Funa Yang3†
Funa Yang3† Xibin Sun
Xibin Sun