- 1Division of Oncology, Radio-oncology Department, Vaudois University Hospital Centre (CHUV), Lausanne, Switzerland
- 2Radio-Oncology Research Laboratory, Vaudois University Hospital Centre (CHUV), Epalinges, Switzerland
- 3Radiation Oncology Department, Hôpital Neuchâtelois, La Chaux-de-Fonds, Switzerland
- 4Department of Oncology, Breast Center, Vaudois University Hospital Centre (CHUV), Lausanne, Switzerland
- 5Department of Oncology, Center of Experimental Therapeutics, Ludwig Center for Cancer Research, University of Lausanne, Lausanne, Switzerland
The association of radiotherapy and immunotherapy has recently emerged as an exciting combination that might improve outcomes in many solid tumor settings. In the context of breast cancer, this opportunity is promising and under investigation. Given the heterogeneity of breast cancer, it might be meaningful to study the association of radiotherapy and immunotherapy distinctly among the various breast cancer subtypes. The use of biomarkers, such as tumor infiltrating lymphocytes, which are also associated to breast cancer heterogeneity, might provide an opportunity for tailored studies. This review highlights current knowledge of the association of radiotherapy and immunotherapy in the setting of breast cancer and attempts to highlight the therapeutic opportunities among breast cancer heterogeneity.
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer and leading cause of cancer death among females worldwide. BC survival is closely related to cancer biology and disease stage, in a disease setting that presents a tremendous heterogeneity in terms of natural history (1). Historically, Halsted proposed that BC represents a local disease that progressively spreads to adjacent tissues through lymphatics (2); Fisher then underlined the systemic component of the disease (3, 4) and finally Hellman suggested that BC is heterogeneous, varying from a solely local disease throughout its whole course vs. systemic disease at presentation (5, 6). Local and systemic treatments address therapeutically these two BC elements. The current challenge becomes the introduction of emerging therapies, such as immunotherapy, in association to established modalities, such as radiotherapy (RT), chemotherapy or targeted treatments in order to improve oncological outcomes. This review will examine existing data highlighting potential combinations of immunotherapy and RT in the setting of BC. The possible implications of BC heterogeneity on RT-immunotherapy combinations will also be discussed.
Biological Considerations of BC
The molecular biological basis of BC heterogeneity was poorly understood until 2000 when Perou and colleagues brought into light at least four distinct BC subtypes (7). Tumors were classified based on their gene expression and the fact that variations of their transcriptional program were implicated in their diversity. A classification system based upon the gene expression profile of a tumor was thus developed, with selection of particular gene groups (intrinsic gene subset) constantly present in the same tumor and distinct among different tumors. Others have subsequently further dissected BC heterogeneity (8) providing tailored therapeutic targets to distinct subsets of BC.
Nowadays, BC is no longer considered a unique disease, but the clinical manifestation of several. The classification of 4 subtypes, associated to distinct clinical behaviors and natural histories of BC, has permitted refining systemic therapy in order to improve patient's outcome and minimize toxicities (9–11). Despite this, a complete image of the biological heterogeneity of BC in regards to molecular alterations, sensitivity to treatment and cellular composition, is still lacking. In clinical practice, most BC tumors are classified as Luminal A or B, HER-2 positive, or triple negative (TN) based on pathological parameters in immunohistochemistry, such as hormone receptor status, HER-2 status, grade, and proliferation index (Ki-67), that have been well defined (12), although they do not perfectly correspond to the molecular subtypes defined by Perou (13). Luminal A is the most favorable subtype in terms of prognosis and endocrine sensitivity (14), while triple negative remains a subtype with highly aggressive biology, associated with an increased risk of locoregional recurrence (15) and systemic failure (16).
Established Treatment Options in BC
Surgery is an important treatment modality for non-metastatic disease of every stage. Neo/adjuvant systemic treatments, namely chemotherapy, endocrine therapy, and targeted treatments, have been been combined with surgery in the non-metastatic setting given their ability to reduce the risk of systemic and local recurrence (17). Systemic treatments have been the cornerstone in the metastatic setting and have been providing overall survival gains within the last decades (18). Radiotherapy is a local/locoregional treatment with the potential to sterilize residual microscopic disease in BC. It is often indicated after mastectomy, when prognostic factors imply an increased risk of locoregional recurrence (LR), and systematically after breast conserving surgery, permitting equal locoregional control rates to mastectomy and conferring an overall survival benefit (19).
The indication of a systemic treatment is now refined by the use of surrogates, which permit the selection of patients at highest risk for relapse, and thus in need for an additional “preventive” treatment (prognostic factors), as well as the patients with enhanced probabilities of response to a given treatment (predictive factors, a concept known as treatment personalization. BC is perhaps the paradigm of personalized therapy, since data accumulation, due to its high incidence, has permitted unprecedented insight into disease heterogeneity.
Cancer Immunotherapy
Immunotherapy is an emerging modality in cancer treatment. The basic principle for introducing immunotherapy in cancer treatment is that although tumors are finally poorly immunogenic entities, which, according to the “immunosurveillance hypothesis,” escape immune detection (20), they present as initially immunogenic and are eliminated by the immune system. Natural selection results in the persistence of the less immunogenic clones, through the expression of immunosuppressive cytokines and growth factors (21), though a procedure known as “immunoediting,” which progressively enriches tumor microenvironment by immunosuppressive cell populations, such as Treg (CD4+CD25+regulatory T cells) and hijacked plasmacytoid dendritic cells (pDCs) (21, 22).
The central idea of cancer immunotherapy is to identify tumor specific antigens, not present in essential normal tissues (so that autoimmune phenomena are avoided), which could induce a tumor-specific immune response and promote adaptive immunity to fight back the tumor within a given cancer patient. Immunotherapies comprise active, passive or immunomodulatory strategies, although some of them overlap (23). Vaccines or adoptive-cell therapies with autologous T-cells are active strategies aiming to increase the ability of the patients' own immune system to mount an immune response against the patient's tumor (24).
Truly tumor-specific antigens, “tumor-associated antigens” (TAAs) are rare (25), but still can be detected within the tumor and in the peripheral blood of patients with specific tumors (26). In order to identify and clone TAAs, tumor infiltrating lymphocytes (TILs) have been isolated, and emerge as a therapeutic tool (27). Both antigen presentation and lymphocyte activation depend on the tumor microenvironment (28), while the interaction of lymphocytes with antigen-presenting cells occurs in regional lymph nodes, where dendritic cells (DCs, which are professional Ag-presenting cells) migrate during their maturation process (Figure 1). DCs present antigens to lymphocytes, activating them to identify, target and destroy tumor cells (29). The microenvironment can induce tumor-suppressing and promoting pathways, including the secretion of cytokines and growth factors by stromal cells and promoting macrophage polarization, angiogenic switch and immune suppression or evasion by affecting in situ immune cells of myeloid and lymphoid lineage (30).
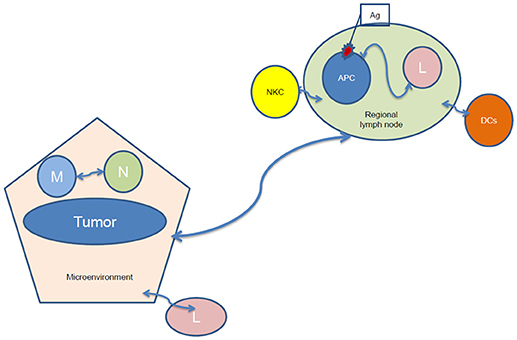
Figure 1. Tumor-regional lymph node communication after irradiation. NKC, natural killer cells; Ag, antigen; APC, antigen-presenting cells; L, lymphocytes; DCs, dendritic cells; M, macrophages; N, Neutrophil.
Tumor-infiltrating lymphocytes (TILs): A biomarker of BC immunogenicity
TILs as a Biomarker
TILs infiltrating BC are tumor specific T cells chronically exposed to tumor associated antigens (TAAs) (31). Whereas normal breast tissue does not contain large quantities of immune cells (32), TILs infiltration can be observed in specific subtypes of BC (Table 1). Notably, TILs are mainly present in TNBC and HER-2 positive BC, in which subtypes their increased number has a positive prognostic impact. Increased TILs infiltration has also been correlated to better overall prognosis and response to neo-/adjuvant chemotherapy (34) and recently, TILs have been proven an independent prognostic factor for disease-free survival (DFS) and overall survival (OS) in TNBC (34). The first studies of TILs have been published in a pivotal study published in 1992, in which the predictive value of TILs for axillary lymph-node status, tumor diameter and histological and morphometric variables has been reported in 489 BC patients after 10-year follow-up (35). More importantly, they correlated to recurrence-free survival and BC-specific survival in rapidly proliferating, axillary lymph-node negative disease (36).

Table 1. The median percentage of stromal tissue TILs within the various BC subtypes (33).
In the last five years, TILs have been evaluated in about 16,000 patients, thus highlighting the growing interest in this biomarker (35–39). Loi et al. have shown in 2,009 patients participating in the Breast International Group (BIG) 02-98 trial (a phase III trial in the adjuvant setting of early BC), that TILs are an important prognostic biomarker, but in TN patients only (33). In that study, after calculation of tumor lymphocyte infiltration (defined as the percentage of mononuclear cells within the epithelium of the invasive tumor nests) and stromal lymphocyte infiltration (defined as the percentage of infiltrating lymphocytes into the stroma), a lymphocyte predominant phenotype (LPBC) was defined as >50% infiltration of either tumoral or stromal TILs (sTILs).
As expected, TILs were higher in TN and HER-2 positive subgroups given they are highly proliferative tumors. TILs were independent predictors of DFS and OS in the TN subgroup of patients only. Importantly, for every 10% increase in TILs there was a 15–17% (stromal vs. intratumoral TILs) decrease in the risk of recurrence and a 17–27% decrease in the risk of death (stromal vs intratumoral TILs). The 5-year DFS was 92 vs. 62%, and the 5-year OS was 92 vs. 71% for TN patients with a LPBC phenotype vs a non LPBC phenotype, respectively. However, it should be noted that only 27 patients had a LPBC in the TN group, while 229 patients had not. In that study, TILs were also predictive of response to taxanes within the HER-2 positive subtype only (33).
The OS and DFS benefit has recently been confirmed in a meta-analysis in TNBC, where a 15–20% gain in any recurrence or mortality was shown for every 10% TILs' increase (40). In TNBC patients, intratumoral or stromal presence of TILs has been consistently associated with a survival benefit. In a meta-analysis that was conducted to identify the prognostic value of TILs and/or TILs subsets in BC patients stratified by infiltration sites, the presence of TILs was associated to improved disease-free survival (DFS) (HR = 0.82; 95% CI, 0.76–0.88 8) and overall survival (OS) in TNBC patients; (HR = 0.79; 95% CI, 0.71–0.87). Both intratumoral and stromal TILs were associated with good prognosis, while LPBC was a surrogate of a particularly significant survival benefit (41).
It was then shown in 12,439 BC patients that the presence of CD8+ TILs is associated with good prognosis in HER-2 positive patients (regardless of ER positivity) also (37). In that study, a 21–28% reduction (stromal vs intratumoral TILs, respectively) in the hazard of BC-specific mortality was shown in all ER negative tumors (HER-2 positive and TN); for HER-2- and ER-positive tumors, a 27% reduction in the hazard of BC-specific mortality was shown with intratumoral CD8+ TILs (37). Finally, in multivariate analyses of combined data coming from two large phase III randomized adjuvant BC trials, the prognostic value of TILs in TNBC has once more been confirmed (35, 42). Moreover, it was shown that the likelihood of absence of TILs increased as the number of positive nodes increased (36).
Molecular and Physiological Features of TILs
TILs can be detected with heamatoxylin and eosin (H&E) staining on histological slides as well as with light microcopy. Additional immunohistochemistry (IHC) helps to characterize specific lymphocyte markers (31). In BC, TILs consist mainly of heterogeneous lymphocyte populations phenotyped as CD8+ (cytotoxic) and CD4+ (T helper) T cells, as well as CD19 B cells and natural killer (NK) cells (31, 43). These cell types have different functions with a variable functional significance and impact in the context of BC. They possess cytolytic and cytokine secretion properties, as well as the property to recognize unique tumor antigens (31).
TILs are functionally important, since immunomodulatory gene activation, as well as high expression of immunological gene signatures has been detected in patients with enriched TILs (44) and associated to intrinsic tumor qualities (45). These analyses were undertaken in full-face tissue sections, consisting of the entire tumor, whereas Mahmud et al. showed a prognostic significance for CD8+ lymphocytes at distance of the tumor (>1cm diameter from the tumor) (44). Functionally, these studies suggested that TILs in BC have a Th1 polarization and express immune checkpoint molecules, such as programmed cell death-1 (PD-1) (46). TILs express mRNA related to genes involved in T-cell activation and T-cell checkpoint receptors, such as indoleamine 2,3-dioxygenase 1 (IDO1) and markers of T-regs (34, 47). Notably, the expression of immunosuppressive markers also increases with TILs' infiltration but should not be considered as an ineffective immunity signal (31, 34, 48). It seems indeed that the stimulus of immune recognition of breast tumors is the repertoire of tumor mutant peptides, while a variable correlation between tumor mutation burden and T-cell effector function has been recognized (31, 49). TNBC is generally known to possess an increased mutation rate while TNBC tumors accumulate mutations 13.3 times faster than luminal tumors (50). It was shown that the relapse rate after chemotherapy and radiotherapy is higher in patients with BC when they carry a TLR4 loss-of-function allele, which induces an impaired innate immune response to tumor-cell death (51).
In the meta-analysis of TNBC, a clear benefit in OS was maintained for CD8+ and FOXP3+ (regulatory) TILs (40), however, given that data on these TILs' phenotype are limited, their specific prognostic value should be considered with caution. Others have shown that CD8+, CD3+, and CD20+ TILs are associated to better response to neo-adjuvant chemotherapy (52). Moreover, the prognostic value of FOXP3+ TILs seems to be related to ER positivity: FOXP3+ TILs are related to improved prognosis in ER-negative tumors while they are significantly associated with poor survival in ER-positive BC (53). The expression of PDL-1 in BC tumor cells is associated with elevated TILs and longer recurrence-free survival suggesting a functional link between TILs and tumor PD-L1 upregulation (48, 54). High intratumoral and stromal CD3+ , CD4+, and CD8+ TILs have been also shown to be prognosticators of OS in 150 patients with BC (all subtypes represented) (54). In a meta-analysis, CD3+, CD8+ and the ratio of CD8+/FOXP3+ TILs presented the most significant positive effect on survival (hazard ratio (HR): 0.59 (confidence interval (CI) 0.43–0.78; HR:0.71 (CI:0.62–0.82 and HR:0.48, CI:0.34–0.68, respectively) (55). The relevance of local lymphoid structures to support immune activation in response to RT has also been recently suggested by our group in medullary BC (56), a type of BC infiltrated with tertiary lymphoid structures (TLS). We showed acute and transient TLS depletion after hypo-fractionated RT, followed by a restoration phase and identified possible cellular targets (i.e., Tregs) that could be selectively modulated in subsequent studies to optimize anti-tumor immune response (56).
Hormone-receptor (HR)-positive BC is less proliferative and is expected to be less immunogenic. In fact, TILs have not been shown to maintain their prognostic value in the context of HR-positive BC and might be associated with worse survival, as shown in a pooled analysis of 3,771 patients treated with neo-adjuvant therapy (57). The role of TILs seems to be unclear in this setting (58), while the surprising finding that TILS are associated with poorer prognosis has also been observed in metastatic HR-positive BC patients treated with metronomic chemotherapy (59). In fact, CD4+ TILs display a positive prognostic value in patients with HR-negative tumors, while FOXP3+/CD8+ TILs display a negative prognostic value in those with HR-positive tumors (60). However, it has been shown that HR-positive tumors possess lower CD8+ TILs, while a minority possesses high FOXP3+ cells (61).
TILs, a Biomarker Beyond Prognosis
Despite the emerging importance of TILs as prognosticators and predictive factors, several points need to be addressed before their introduction into clinical practice. This has also been the case in other tumor sites, such as ovarian cancer (62). In this disease, a genetic background has been implicated in the mechanism associated with TILs infiltration (63).
Our group believes that TILs represent a biomarker beyond prognosis with major therapeutic implications in BC (64). It remains an open question if this intrinsic quality can be manipulated and a host can be “pushed” to a more favorable immunologic response within the different contexts of disease heterogeneity. This is particularly important mainly in the early BC setting, where a favorable immune response could eradicate microscopic, dormant disease that would eventually manifest as metastasis; moreover, this could also optimize local control, therefore providing an essential component of disease eradication both at the local, as well as at the regional and distant setting. According to the immunosurveillance hypothesis, poorly differentiated tumors were shown to be more antigenic and therefore stimulate a stronger immunogenic response (65). As discussed by Loi, this immunogenic response might not be sufficient to eradicate existing tumor, but might be important in preventing recurrence after surgery, as also shown in BC in the setting of preventing metastasis (42).
Immunotherapy in BC
Vaccines have reached a relatively advanced stage of clinical evaluation in BC. Vaccines attempt to enhance tumor killing by reinforcing tumor-dependent cellular cytotoxicity, which relies mainly upon NK and CD8+ T-cells. In a study that has evaluated immunological effects of conventional treatment in preoperative and postoperative BC patients, as well as in healthy controls, NK cells' quantity and functional cytotoxicity, as well as T-cell functions were decreased in post-operative, post-chemo/radiotherapy patients, while cytokine counts were increased in pre-operative patients (66). This study suggests that early introduction of immunotherapy interventions might be more efficient (66). Immunotherapy merits testing in early disease stages, where tumor burden is less important and immunotherapy might be more efficient in eradicating microscopic disease. Therefore, the design of early phase window studies needs to be emphasized in order to fully unveil the potential of this modality.
Existing BC vaccines have been reviewed by Soliman (67). In these studies, patients with minimal tumor burden and those not heavily pretreated seemed to benefit most from vaccines. A hypothesis would be that antigen-specific, cytotoxic T-cells activated after vaccination are not numerically sufficient to fight against an increased tumor burden, while they might be capable of eradicating microscopic, indolent disease.
A detailed review of the role of immunotherapy in BC has suggested various strategies to introduce this modality into clinical trials (68). The concept of BC heterogeneity is important, since it has been shown that not all BC subtypes are immunogenic. The notion of immunogenic tumors, as detected by increased TILs counts, is relevant for highly proliferating tumors and notably for the HER-2 positive and TN subtypes.
Immunotherapy in TNBC
In the paradigm of TNBC, checkpoint inhibitors have been tested and shown interesting activity profiles (69). It has been shown that 20% of TNBC express PD-L1 (70). The overall response rate (ORR) in a phase IB study of 28 metastatic TNBC patients with pembrolizumab monotherapy was 18.5% (71). Atelizolizumab has shown an ORR of 24% in 21 metastatic TNBC bearing PD-L1-positive tumors (72). Two ongoing studies of monotherapy with checkpoint inhibitors are underway (KEYNOTE-086 (NCT02447003) (phase II) and KEYNOTE-119 (NCT02555657) (randomized phase III) (69). Studies of combination of checkpoint inhibitors with chemotherapy have also been promising. A phase Ib trial of atezolizumab and nab-paclitaxel in the same setting has shown ORR of 42% (73) and two studies are currently ongoing: IMpassion130 (NCT02425891), and KEYNOTE-355 (NCT02819518) (both phase III) (69).
The TONIC trial recently showed that nivolumab in TNBC induced an ORR of 22% with a median response duration of 9 months in responders (74). Interestingly, nivolumab treatment was initiated after priming the tumor microenvironment with either irradiation or chemotherapy, resulting in a promising response rate that appeared higher than expected based on previous PD-1/PD-L1 blockade monotherapy studies in unselected TNBC. Here, the median time to response was 2.1 months, and the median response duration was 9.0 months. Median progression-free survival was 3.4 months (95% CI 2.5–3.7 months). Among patients with a complete or partial response, the 1-year overall survival rate was 83%, compared with 13% in the one patient who had stable disease.
Immunotherapy in HER-2 Positive BC
In HER-2 positive disease, trastuzumab downregulates HER-2 signaling by blocking heterodimers; it has also been shown to activate killing of HER-2 overexpressing cells by antibody-dependent means, through activation of NK cells (75, 76). Patients with immunoglobulin fragment (IgG Fc) polymorphisms have better trastuzumab responses, through the enhancement of an immune response (77). Trastuzumab has already been combined to HER-2-specific vaccines resulting in enhanced responses (78). However, patients in the metastatic setting eventually progress and acquire resistance to these treatments. Despite trastuzumab resistance, HER-2-based vaccines, that induce polyclonal antibody responses against HER-2 have shown enhanced anti-tumor activity when administered with lapatinib in murine models. In a phase I study of a HER-2-based cancer vaccine combined with lapatinib in 12 patients with metastatic, trastuzumab-refractory, HER-2-overexpressing BC, the regimen was well tolerated and anti-HER-2-specific Ab was induced in all patients, while very satisfactory overall survival rates (1y-OS: 92%) have been observed (79).
For a comprehensive and recent overview of undergoing immunotherapy studies in BC, the reader is referred to the works of Puzstai et al. (80), Vonderheide et al. (58), and Kroemer et al. (81). Within all this evidence, it becomes apparent that immunotherapy might be an interesting strategy for maintenance or prevention of micrometastasis, while in the setting of very advanced stages with an important disease burden, it might be insufficient by itself to eradicate the disease.
Immunotherapy in HR-Positive BC
Given the poor immunogeneicity and the ambivalent role of TILs in HR-positive BC, immunotherapy is not expected to have a major therapeutic role in this setting (69). Immunotherapy is mostly effective when sufficient neo-antigens exist, so that T-cells can be activated and BC has been shown to possess a medium mutational load (82). In fact, estrogen-receptor (ER)-positive tumors with high mutational load are associated with poorer survival (83) and immunotherapy might be an appropriate strategy in this setting, but clinical data is lacking and very few studies focused on immunotherapy in hormone-receptor positive BC are ongoing.
RT and Immunological Effects
Theoretical Advantages of Associating RT to Immunotherapy
The association of RT and immunotherapy has gained extensive attention in the last few years, as RT is able to modulate each parameter of the immune cycle: 1-antigen release; 2-Antigen presentation; 3-Priming and activation of T-cells; 4-T- cells trafficking to tumors; 5-T cells tumor infiltration; 6-Recognition of tumors by T cells; 7-Killing of tumor cells by T cells (25, 84–91). First, irradiation has a known tumoricidal action by provoking DNA damage through single- or double-strand DNA breaks, whose insufficient repair leads to cell death and antigen release. Therefore, intrinsic tumor radiosensitivity depends also on the immunocompetence of the host (92), and cell death caused by irradiation is also “immunogenic” (51). It has been shown that RT enhances tumor immunogenicity and increases the presence of effector immune cells to the tumor site (25, 93–95). Recently RT has been characterized as “immunomodulatory” (96) and considered as signaling “danger,” through the induction of pro-inflammatory cytokines, such as TNF-α and IL-1β (97), capable of generating an in vivo vaccination effect. The group of Formenti and Demaria has extensively summarized the concept of the interaction of RT with the immune system (25, 93–95).
RT-induced increased availability of tumor antigens and its immunomodulatory consequences, such as antigen capture, cell migration to the lymph nodes, polarization toward a tolerogenic or immunogenic phenotype or migration of lymphocytes into the tumor might promote tumor killing (83, 98). Combining RT with immunotherapy would therefore induce tumor cell death resulting in antigens release and promotion of DCs' maturation, enhancing the cytotoxic capacity of T cells. Therefore, a synergistic model can be conceived, where systemic effects of irradiation and immunotherapy are more effective for cancer treatment than any of both treatments administered alone (83, 98). A synergistic model of the effects of combined immunotherapy and RT is visualized in
Figure 2.Various combinations of RT and immunotherapy have been explored, such as intratumoral (IT) or peritumoral DCs' administration, cytokines (IL-3, IL-12, TNF-α), as well as CTLA-4 blockade administration, some with promising results (22). Other combinations consist of virus, dendritic cell-based vaccines, and TLR agonists (83).
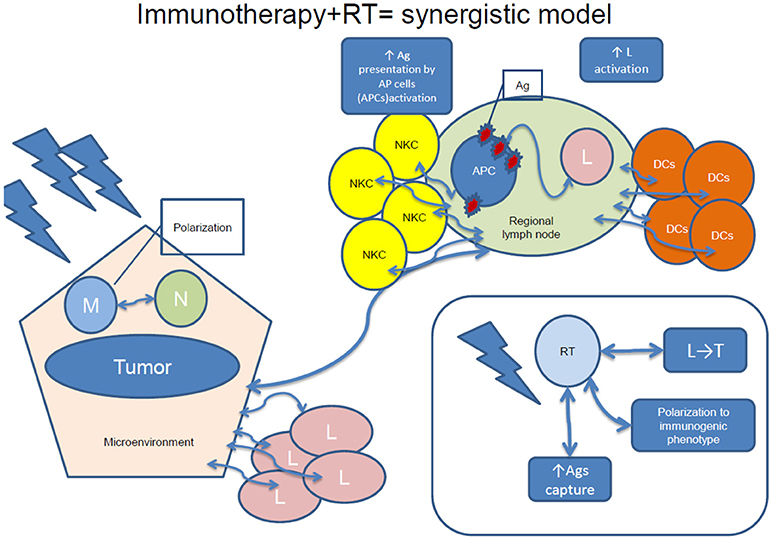
Figure 2. A synergistic model of immunotherapy and RT. NKC, natural killer cells; Ag, antigen; APC, antigen-presenting cells; L, lymphocytes; DCs, dendritic cells; M, macrophages; RT, radiotherapy; N, neutrophils; L, regional lymph node; T, tumor.
Another potential benefit of the association of RT and immunotherapy relates to the considerable evidence suggesting that RT can have inhibitory effects on tumor cells outside of the irradiation field. Formenti et al. describe four such events: (a) responses of non-irradiated tissues due to signals from irradiated cells (bystander effect), (b) effects of irradiation to the whole body (consisting of RT producing host-dependent inflammation), (c) effects of RT in tumor microenvironment, promoting phenomena outside of the treatment field, and finally (d) abscopal effects. All of these effects seem to involve the immune system (94, 95, 99–101). It remains nevertheless complex to anticipate systemic RT effects, since similar doses can provoke either pro or anti-tumorigenic effects depending upon the context. For instance, both pro- and anti-tumorigenic immune effects has been described with low doses (< 4 Gy) of irradiation (100, 102), while doses provoking cell death, such as high ablative doses of irradiation induce danger signals and activate an adaptive immune response (103) and at the same time activate immunosuppressive signals such as TGF-β1 (104).
An interplay between the primary tumor and the metastases seems to exist: in some cases, the enhancement of metastatic growth after the removal of the primary breast tumor has been observed, which was reversed when surgical removal occurred after irradiation (105–107). Although this effect remains somehow controversial in the literature and has not been extensively evaluated, it reinforces the concept of associating radiotherapy and immunotherapy in BC, a disease known par excellence to be of a strong local and systematic component (108).
Practical Considerations of the Association
Combining the appropriate RT regimen (dose, fractionation, volume) with the appropriate immunotherapy in an appropriate schedule would therefore theoretically be locally and systemically highly effective. The real challenge today is related to our ability to determine what is the appropriate schedule in each and every tumor, capable of providing reproducible effects.
Preclinical data have shown maximum RT- immunotherapy interactions with SBRT fractions such as 6-8 Gy delivered in one to 3 fractions (83). Although immunostimulatory effects can be observed with doses as low as 0.5–0.94 Gy (109), many preclinical studies have shown that doses >7Gy increase local interferon production, which enhances antigen presentation in tumor cells (110–112). A single dose of 10 Gy has been shown to increase the efficacy of adoptive T-cell transfer in vivo (113). Generally, it has been shown that hypo-fractionated SBRT (single fraction of 10–24 Gy) provokes massive immunogenic release of antigens and DAMP ligands as well as stimulates TLRs on antigen-presenting cells for several days (51, 113, 114).
Notably, Dewan et al. have evaluated three different radiotherapy schemas (1 × 20 Gy, 3 × 8 Gy, and 5 × 6 Gy) in combination with a monoclonal antibody against CTLA-4 and have shown that 3 × 8 Gy was the most immunogenic combination, enriching tumors with active CD8+ T cells and associated to an effect on distant lesions, being either an abscopal effect or a direct effect of CTLA-4 monoclonal antibody (115). In the study by Verbugge and colleagues, immunotherapy and RT showed enhanced curative capacity of RT combined with α-CD137 and α-PD-1 antibodies in AT-3 tumors (corresponding to the triple negative subtype) with doses of 12 Gy (116), although various regimens (1 × 12 Gy, 4 × 4 Gy, or 4 × 5 Gy) were investigated. Finally, Filatenkov et al. has compared the immune modulation of 1 × 30 Gy with 10 × 3 Gy or 30 Gy + 10 × 3 Gy and has shown that 1 × 30 Gy was the most efficient regimen in terms of MDSCs decrease and CD8+ T-cell infiltration (117).
Emerging Clinical Evidence and Hypotheses
The idea of combining irradiation and immunotherapy to enhance the host's immune response to tumor has been clinically evaluated in melanoma, where enhanced responses (17%) to ipilimumab were seen after irradiation of a single lesion in multimetastatic patients (118). A proof-of-principle clinical study of GM-CSF and RT managed to show the production of objective abscopal responses in oligometastatic disease, using concurrent RT of 35 Gy in 10 fractions (119). The strategy of employing high doses in small tumor volumes with tight margins seems to be the most interesting from an immunostimulatory point of view. Another recent proof-of-principle study used stereotactic body RT (SBRT) to stimulate immunity in metastatic solid tumors, associated with pembrolizumab, with doses ranging from 30 to 50 Gy in 3–5 fractions and observed that interferon-γ associated genes from tumor biopsies after SBRT were related to abscopal responses (120). Recently, Jatoi and colleagues have formulated the hypothesis that RT exerts an abscopal effect eradicating micrometastasis in the early setting of BC, which is manifested by its constant effect on distant-metastasis decrease in studies of adjuvant RT (121).
Combining Immunotherapy and RT in BC
Preclinical Evidence
Within BC, a preclinical study has explored the potential immunomodulatory effect of irradiation and BC (51), while the interplay between irradiation and immunotherapy has been validated in the preclinical study by Verbugge et al. (122). In mice bearing orthotopically implanted TNBC tumors, the combination of RT and immunotherapy, consisting of mAbs to CD137, CD40 and PD-1 resulted in the rejection of AT-3 and 4T1.2 tumors (122). The key innate immune cells critical to the antitumor effects of radio-immunotherapy have been those expressing CD137 and/or PD-1 and that persisted within the irradiated tumors, notably CD8+ and NK cells that expressed CD137. CD8+ T cells were essential for the therapeutic effect. In this study, anti-CD137 alone or in combination with α-CD40 treatment significantly enhanced RT-induced tumor shrinkage. It was observed that once the mice were cured of primary tumors, the growth of secondary tumors was impaired, suggesting the development of immunologic memory (122).
In another preclinical study, high-dose, ablative RT dramatically increased T-cell priming in lymphoid nodes and resulted into eradication of the primary tumor or distant metastasis also in a CD8+ T cell-dependent way, in mice harboring the 4T1 cell line (a TN cell line) (123). This phenomenon was greatly amplified by local immunotherapy (123). The same group had previously shown that targeting a primary breast tumor (4T1 cell line) with immunotherapy (“Ad-LIGHT, a name derived from “homologous to lymphotoxins, that shows inducible expression, and competes with herpes simplex virus glycoprotein D for herpes virus entry mediator,” a receptor expressed by T lymphocytes) can result into eradication of distant metastases (124).
In another preclinical study, evaluating topical imiquimod (a TLR-7 agonist) and local RT in the TSA murine model of BC with cutaneous metastases, both complete regression of treated lesions and improved distant control and survival were observed (125). This was further shown in nude transgenic mice when imiquimod was associated to IL-10 antibodies, possibly suggesting independence from innate immune response (126).
An overview of ongoing clinical studies in the BC setting is presented in Table 2. Most of them combine RT and immunotherapy in the metastatic setting, often in TNBC. On single study is testing permbrolizumab and preoperative RT in the early setting. All of the studies are early phase and test tolerance of the association, with several testing efficacy in terms of local and distant control, in search of an abscopal effect. The preoperative study tests standard treatment delay and TILs increase (127). In the same spirit, a recent study used radiofrequency ablation and/or single dose preoperative ipilimumab in patients with BC who would anyway undergo mastectomy (91). They observed sustained (persisting at 30 days after mastectomy) immunological responses, such as peripheral elevation in Th-1 type cytokines, activated and proliferating T cells, both CD4+ and CD8+, as suggested by high Inducible T-cell COStimulator (ICOS)+ and Ki-67, respectively, along with T-reg-associated T-eff cells intratumorally (91).
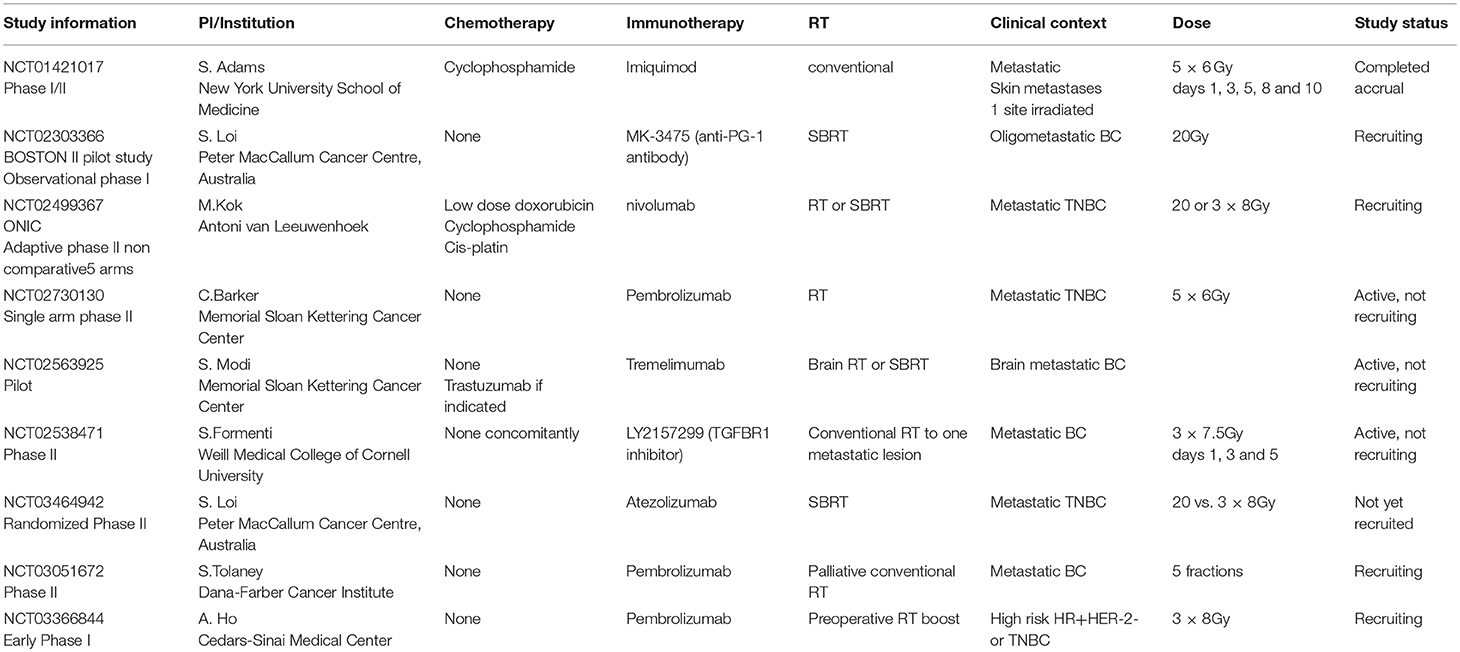
Table 2. Summary of ongoing clinical studies associating RT and immunotherapy in the BC setting (Source: clinicaltrials.gov accessed 03.05.2018).
Triple Negative BC (TNBC), Radiotherapy and Immunotherapy
Triple negative BC (TNBC) remains, along with HR-/HER2 positive, among the subtypes with the most aggressive biology, associated to an increased risk of locoregional recurrence and distant failure (15). However, in contrary to the HR−/HER2+ subtype, systemic treatments have not yet been adequately developed for TNBC and it represents par excellence the BC subtype lacking therapeutical targets (16). It represents an ideal target for the combination of RT and immunotherapy, given that this subtype is the most immunogenic among BC subtypes (78) and that the presence of tumor infiltrating lymphocytes (TILs) within the tumors of patients with early invasive TNBC has been associated with improved prognosis (44).
The association of radiotherapy and immunotherapy has gained extensive attention in the last few years (119, 128) and might be of particular interest in the context of TNBC as this subtype is the most immunogenic among BC subtypes (78).
As already specified above, the median percentage of TILs in TNBC patients is 20% (129) and a 10% increase in intratumoral and stromal TILs translates into a 15 and 17% reduction of risk for recurrence or death and 17 and 27% reduction of risk for death, respectively, in data from the BIG-02-98 study (33). Therefore, if any of the investigational treatments increases sTILs' levels in tumors of TNBC patients, this is expected to be beneficial for those patients. A study of preoperative immunostimulatory SBRT associated to immunotherapy (a toll-like receptor agonist) in the setting of TNBC with the objective to increase TILs is currently in preparation in our Institution. A study with the same objective in a population of TNBC or high risk Luminal non-HER-2 positive patients has recently started accrual in the US (Principal Investigator: Alice Ho, Trial number: NCT03366844), combining preoperative RT and pembrolizumab. Hopefully, these studies will prove the principle of the possibility of TILs increase in this population, susceptible to micrometastatic disease opening the road to larger-scale studies testing outcomes in terms of distant control with the association of RT and immunotherapy.
Conclusions
The clinical relevance of BC heterogeneity when designing studies in the era of introduction of immunotherapy is pivotal. The combination of radiotherapy and immunotherapy is promising and particularly relevant for immunogenic BC subtypes, such as TNBC or HER-2 positive BC. The availability of a valid biomarker, such as TILs, makes studies of association of immunotherapy and RT very appealing in this context. Combinations of established modalities are nowadays tailored to BC heterogeneity and, accordingly, the design of modern studies of BC requires a careful selection of BC subtypes that are likely to benefit from tailored experimental approaches with a strong translational background. Little is known on the intrinsic properties of radiosensitivity within the BC subtypes (15, 33). Insights on this information will optimize the RT-immunotherapy associations and are expected to lead to tailored studies in selected populations.
The timing of the introduction of immunotherapy with or without immune-stimulatory RT seems to be important, since, the least the tumor burden, the more efficient these treatments are expected to be. Therefore, optimal RT-immunotherapy studies should ideally be designed in the early or oligometastatic setting.
The optimum sequence of RT and immunotherapy remains to be found, as is the ideal dose, fractionation and volume of irradiation. The optimum immunotherapeutic agents to combine with RT are also under investigation. The accumulation of data is rapid and hopefully meaningful insights will be available within the next few years.
In our mind, an ideal RT-immunotherapy study in the setting of BC, should take into account the following elements: (a) focus into subtypes with high mutational load, (b) introduce the combination in early disease or early in the oligometastatic setting, with the scope of micrometastasis eradication by a abscopal effect, (c) use, if possible, fractionated irradiation on the primary, unresected tumor or on the most active metastasis, with fractions in the order of 8 Gy, (d) combine RT with TLR- agonists, that enhance dendritic activation and permit enhanced cross-presentation to T-cells (which permits to take advantage of the major immunogenic effect of irradiation, being the liberation of TAAs) or with the combination of check-point inhibitors and TLR-agonists in order to assist activated T-cells to unmask and kill tumor cells and e. evaluate biomarkers, such as TILs or immune gene signatures, to detect early immune activation, which might otherwise be undetectable in the clinical setting (given that the abscopal effect remains hard to reproduce). These strategies might permit to transform not only the role of RT in BC but also, hopefully and most importantly, might transform not only the role of RT in BC but also, hopefully and most importantly, bring a great therapeutic benefit to patients.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Punglia RS, Morrow M, Winer EP, Harris JR. Local therapy and survival in breast cancer. N Engl J Med. (2007) 356:2399–405. doi: 10.1056/NEJMra065241
2. Halsted WS. I. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. (1907) 46:1–19. doi: 10.1097/00000658-190707000-00001
3. Fisher B. Biological and clinical considerations regarding the use of surgery and chemotherapy in the treatment of primary breast cancer. Cancer (1977) 40:574–87. doi: 10.1002/1097-0142(197707)40:1+<574::AID-CNCR2820400724>3.0.CO;2-O
4. Fisher B. Laboratory and clinical research in breast cancer–a personal adventure: the David A. Karnofsky memorial lecture. Cancer Res. (1980) 40:3863–74.
5. Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol. (1994) 12:2229–34. doi: 10.1200/JCO.1994.12.10.2229
7. Perou CM, Sorlie T, Eisen MB, M, van de Rijn, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature (2000) 406:747–52. doi: 10.1038/35021093
8. Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature (2012) 486:346–52. doi: 10.1038/nature10983
9. Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. (2009) 20:1319–29. doi: 10.1093/annonc/mdp322
10. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
11. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. (2011) 22:1736–47. doi: 10.1093/annonc/mdr304
12. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. (2009) 101:736–50. doi: 10.1093/jnci/djp082
13. Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. (2011) 5:5–23. doi: 10.1016/j.molonc.2010.11.003
14. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295 (2006) 2492–502. doi: 10.1001/jama.295.21.2492
15. Tsoutsou PG, Vozenin MC, Durham AD, Bourhis J. How could breast cancer molecular features contribute to locoregional treatment decision making? Crit Rev Oncol Hematol. (2017) 110:43–48. doi: 10.1016/j.critrevonc.2016.12.006
16. Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, et al. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. (2013) 31:3083–90. doi: 10.1200/JCO.2012.46.1574
17. Curigliano G, Burstein HJ, P Winer, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. (2017) 28:1700–12. doi: 10.1093/annonc/mdx308
18. Rossi L, Stevens D, Pierga J-Y, Lerebours F, Reyal F, Robain M, et al. Impact of adjuvant chemotherapy on breast cancer survival: a real-world population. PLoS ONE (2015) 10:e0132853. doi: 10.1371/journal.pone.0132853
19. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Early Breast Cancer Trialists' Collaborative, Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet (2005) 366:2087–106. doi: 10.1016/S0140-6736(05)67887-7
20. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. (2002) 3:991–8. doi: 10.1038/ni1102-991
21. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. (2007) 25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609
22. Roses RE, Xu M, Koski GK, Czerniecki BJ. Radiation therapy and Toll-like receptor signaling: implications for the treatment of cancer. Oncogene (2008) 27:200–7. doi: 10.1038/sj.onc.1210909
23. Disis ML. Mechanism of Action of Immunotherapy. Semin Oncol. (2014) 41(Suppl. 5):S3–13. doi: 10.1053/j.seminoncol.2014.09.004
24. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Investig. (2015) 125:3335–7. doi: 10.1172/JCI83871
25. Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Rad Oncol Biol Phys. (2005) 63:655–66. doi: 10.1016/j.ijrobp.2005.06.032
26. Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. (2002) 188:22–32. doi: 10.1034/j.1600-065X.2002.18803.x
27. Radvanyi LG. Tumor-infiltrating lymphocyte therapy: addressing prevailing questions. Cancer J. (2015) 21:450–64. doi: 10.1097/PPO.0000000000000162
28. Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest. (2006) 35:459–83. doi: 10.1080/08820130600803429
29. Molgora M, Supino D, Mavilio D, Santoni A, Moretta L, Mantovani A, et al. The yin-yang of the interaction between myelomonocytic cells and NK cells. Scan J Immunol. (2018) e12705. doi: 10.1111/sji.12705
30. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature Med. (2013) 19:1423–37. doi: 10.1038/nm.3394
31. Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. (2016) 13:228–41. doi: 10.1038/nrclinonc.2015.215
32. Degnim AC, Brahmbhatt RD, Radisky DC, Hoskin TL, Stallings-Mann M, Laudenschlager M, et al. Immune cell quantitation in normal breast tissue lobules with and without lobulitis. Breast Cancer Res Treat (2014) 144:539–49. doi: 10.1007/s10549-014-2896-8
33. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. (2013) 31:860–7. doi: 10.1200/JCO.2011.41.0902
34. Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor (2015) 2-positive and triple-negative primary breast cancers. J Clin Oncol. 33:983–91. doi: 10.1200/JCO.2014.58.1967
35. Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. (2014) 32:2959–66. doi: 10.1200/JCO.2013.55.0491
36. Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer (1992) 28A:859–64. doi: 10.1016/0959-8049(92)90134-N
37. Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO (2014) 25:1536–43. doi: 10.1093/annonc/mdu191
38. Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. (2014) 25:1544–50. doi: 10.1093/annonc/mdu112
39. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. (2015) 26:259–71. doi: 10.1093/annonc/mdu450
40. Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat (2014) 148:467–76. doi: 10.1007/s10549-014-3185-2
41. Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE (2016) 11:e0152500. doi: 10.1371/journal.pone.0152500
42. Loi S. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer. J Clin Oncol. (2014) 32:2935–7. doi: 10.1200/JCO.2014.56.7677
43. Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. (1992) 12:1463–6.
44. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. (2011) 29:1949–55. doi: 10.1200/JCO.2010.30.5037
45. Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. (2008) 14:5158–65. doi: 10.1158/1078-0432.CCR-07-4756
46. Kitano A, Ono M, Yoshida M, Noguchi E, Shimomura A, Shimoi T, et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open (2017) 2:e000150. doi: 10.1136/esmoopen-2016-000150
47. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. (2013) 123:2873–92. doi: 10.1172/JCI67428
48. Schalper KA. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer. Oncoimmunology (2014) 3:e29288. doi: 10.4161/onci.29288
49. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell (2015) 160:48–61. doi: 10.1016/j.cell.2014.12.033
50. Wang Y, Waters J, Leung ML, Unruh A, Roh W, Shi X, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature (2014) 512:155–60. doi: 10.1038/nature13600
51. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. (2007) 13:1050–9. doi: 10.1038/nm1622
52. Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. (2014) 20:5995–6005. doi: 10.1158/1078-0432.CCR-14-1622
53. Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. (2014) 16:432. doi: 10.1186/s13058-014-0432-8
54. Rathore AS, Kumar S, Konwar R, Makker A, Negi MP, Goel MM. CD3+, CD4+ & CD8+ tumour infiltrating lymphocytes (TILs) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian J Med Res (2014) 140:361–9.
55. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer (2011) 105:93–103. doi: 10.1038/bjc.2011.189
56. Boivin G, Kalambaden P, Faget J, Rusakiewicz S, Montay-Gruel P, Meylan E, et al. Cellular composition and contribution of tertiary lymphoid structures to tumor immune infiltration and modulation by radiation therapy. Front Oncol. (2018) 8:256. doi: 10.3389/fonc.2018.00256
57. Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. (2018) 19:40–50. doi: 10.1016/S1470-2045(17)30904-X
58. Vonderheide RH, Domchek SM, Clark AS. Immunotherapy for breast cancer: what are we missing? Clin Cancer Res. (2017) 23:2640–6. doi: 10.1158/1078-0432.CCR-16-2569
59. Montagna E, Vingiani A, Maisonneuve P, Cancello G, Contaldo F, Pruneri G, et al. Unfavorable prognostic role of tumor-infiltrating lymphocytes in hormone-receptor positive, HER2 negative metastatic breast cancer treated with metronomic chemotherapy. Breast (2017) 34:83–88. doi: 10.1016/j.breast.2017.05.009
60. Chung YR, Kim HJ, Jang MH, Park SY. Prognostic value of tumor infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res Treat. (2017) 161:409–420. doi: 10.1007/s10549-016-4072-9
61. Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: a systematic review. JAMA Oncol. (2016) 2:1354–60. doi: 10.1001/jamaoncol.2016.1061
62. Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. (2012) 124:192–8. doi: 10.1016/j.ygyno.2011.09.039
63. Conejo-Garcia JR, Benencia F, Courreges MC, Gimotty PA, Khang E, Buckanovich RJ, et al. Ovarian carcinoma expresses the NKG2D ligand Letal and promotes the survival and expansion of CD28- antitumor T cells. Cancer Res. (2004) 64:2175–82. doi: 10.1158/0008-5472.CAN-03-2194
64. Tsoutsou PG, Bourhis J, Coukos G. Tumor-infiltrating lymphocytes in triple-negative breast cancer: a biomarker for use beyond prognosis? J Clin Oncol. (2015) 33:1297–8. doi: 10.1200/JCO.2014.59.2808
65. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science (2011) 331:1565–70. doi: 10.1126/science.1203486
66. Mozaffari F, Lindemalm C, Choudhury A, Granstam-Bjorneklett H, Helander I, Lekander M, et al. NK-cell and T-cell functions in patients with breast cancer: effects of surgery and adjuvant chemo- and radiotherapy. Br J Cancer (2007) 97:105–11. doi: 10.1038/sj.bjc.6603840
67. Soliman H. Developing an effective breast cancer vaccine. Cancer Control (2010) 17:183–90. doi: 10.1177/107327481001700307
68. Soliman H. Immunotherapy strategies in the treatment of breast cancer. Cancer Control (2013) 20:17–21. doi: 10.1177/107327481302000104
69. Mayer IA, Dent R, Tan T, Savas P, Loi S. Novel Targeted Agents and Immunotherapy in Breast Cancer. Am Soc Clin Oncol Educ Book (2017) 37:65–75. doi: 10.14694/EDBK_175631
70. Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 Expression in triple negative breast cancer. Cancer Immunol Res. (2014) 2:361–370. doi: 10.1158/2326-6066.CIR-13-0127
71. Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. (2016) 34:2460–7. doi: 10.1200/JCO.2015.64.8931
72. Emens LA, Braiteh FS, Cassier P, Delord J-P, Eder JP, Fasso M, et al. Abstract 2859: Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). Cancer Res. (2015) 75:2859. doi: 10.1158/1538-7445.AM2015-2859
73. Adams S, Diamond JR, Hamilton EP, Pohlmann PR, Tolaney SM, Molinero L, et al. Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. (2016) 34:1009–1009. doi: 10.1200/JCO.2016.34.15_suppl.1009
74. Kok M, Horlings HM, van de Vijver K, Wiersma T, Russell N, Voorwerk L, et al. LBA14Adaptive phase II randomized non-comparative trial of nivolumab after induction treatment in triple negative breast cancer: TONIC-trial. Ann Oncol. (2017) 28:mdx440.006. doi: 10.1093/annonc/mdx440.006
75. Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer (2006) 94:259–67. doi: 10.1038/sj.bjc.6602930
76. Barok M, Isola J, Palyi-Krekk Z, Nagy P, Juhasz I, Vereb G, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. (2007) 6:2065–72. doi: 10.1158/1535-7163.MCT-06-0766
77. Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. (2008) 26:1789–96. doi: 10.1200/JCO.2007.14.8957
78. Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. (2009) 27:4685–92. doi: 10.1200/JCO.2008.20.6789
79. Hamilton E, Blackwell K, Hobeika AC, Clay TM, Broadwater G, Ren XR, et al. Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected]. J Transl Med. (2012) 10:28. doi: 10.1186/1479-5876-10-28
80. Pusztai L, Karn T, Safonov A, Abu-Khalaf MM, Bianchini G. New Strategies in Breast Cancer: Immunotherapy. Clin Cancer Res. (2016) 22:2105–10. doi: 10.1158/1078-0432.CCR-15-1315
81. Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. (2015) 21:1128–38. doi: 10.1038/nm.3944
82. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (2015) 348:69–74. doi: 10.1126/science.aaa4971
83. Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. (2017) 67:65–85. doi: 10.3322/caac.21358
84. Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the Game in Immunotherapy. Trends Cancer (2016) 2:286–294. doi: 10.1016/j.trecan.2016.05.002
85. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol (2015) 1:1325–32. doi: 10.1001/jamaoncol.2015.2756
86. Demaria S, Pilones KA, Vanpouille-Box C, Golden EB, Formenti SC. The optimal partnership of radiation and immunotherapy: from preclinical studies to clinical translation. Rad Res. (2014) 182:170–81. doi: 10.1667/RR13500.1
87. Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Rad Oncol Biol Phys. (2012) 84:879–80. doi: 10.1016/j.ijrobp.2012.06.020
88. Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Instit. (2013) 105:256–65. doi: 10.1093/jnci/djs629
89. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev. Cancer (2018) 18:313–22. doi: 10.1038/nrc.2018.6
90. Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. (2014) 4:325. doi: 10.3389/fonc.2014.00325
91. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nature reviews. Clin Oncol. (2017) 14:365–79. doi: 10.1038/nrclinonc.2016.211
92. Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Instit. (1979) 63:1229–35.
93. Demaria S, Formenti SC. Radiotherapy effects on anti-tumor immunity: implications for cancer treatment. Front Oncol. (2013) 3:128. doi: 10.3389/fonc.2013.00128
94. Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res. (2008) 10:215. doi: 10.1186/bcr2160
95. Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. (2009) 10:718–26. doi: 10.1016/S1470-2045(09)70082-8
96. McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Rad Res. (2004) 162:1–19. doi: 10.1667/RR3196
97. Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci USA. (1989) 86:10104–7. doi: 10.1073/pnas.86.24.10104
98. Finkelstein SE, Fishman M. Clinical opportunities in combining immunotherapy with radiation therapy. Front Oncol. (2012) 2:169. doi: 10.3389/fonc.2012.00169
99. Formenti SC. Immunological aspects of local radiotherapy: clinical relevance. Discov Med (2010) 9:119–24.
100. Morganti AG, Mignogna S, Caravatta L, Deodato F, Macchia G, Plantamura NM, et al. FOLFIRI-bevacizumab and concurrent low-dose radiotherapy in metastatic colorectal cancer: preliminary results of a phase I-II study. J Chemother (2014) 26:353–8. doi: 10.1179/1973947813Y.0000000163
101. Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res. (2008) 68:450–6. doi: 10.1158/0008-5472.CAN-07-3050
102. Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. (2007) 14:1237–43. doi: 10.1038/sj.cdd.4402148
103. Ewan KB, Henshall-Powell RL, Ravani SA, Pajares MJ, Arteaga C, Warters R, et al. Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res. (2002) 62:5627–31.
104. Fisher B, Gunduz N, Coyle J, Rudock C, Saffer E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. (1989) 49:1996–2001.
105. Fisher B, Saffer E, Gunduz N, Coyle J, Rudock C. Serum growth factor following primary tumor removal and the inhibition of its production by preoperative therapy. Prog Clin Biol Res. (1990) 354A:47–60.
106. Fisher B, Saffer E, Rudock C, Coyle J, Gunduz N. Effect of local or systemic treatment prior to primary tumor removal on the production and response to a serum growth-stimulating factor in mice. Cancer Res. (1989) 49:2002–4.
107. Fisher B. The evolution of paradigms for the management of breast cancer: a personal perspective. Cancer Res. (1992) 52:2371–83.
108. Cao M, Cabrera R, Xu Y, Liu C, Nelson D. Different radiosensitivity of CD4(+)CD25(+) regulatory T cells and effector T cells to low dose gamma irradiation in vitro. Int J Radiat Biol. (2011) 87:71–80. doi: 10.3109/09553002.2010.518208
109. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. (2011) 71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820
110. Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. (2011) 208:2005–16. doi: 10.1084/jem.20101159
111. Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. (2008) 180:3132–9.
112. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. (2006) 203:1259–71. doi: 10.1084/jem.20052494
113. Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. (2004) 64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525
114. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. (2009) 15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265
115. Verbrugge I, Gasparini A, Haynes NM, Hagekyriakou J, Galli M, Stewart TJ, et al. The curative outcome of radioimmunotherapy in a mouse breast cancer model relies on mTOR signaling. Radiat Res. (2014) 182:219–29. doi: 10.1667/RR13511.1
116. Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. (2015) 21:3727–39. doi: 10.1158/1078-0432
117. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature (2015) 520:373–7. doi: 10.1038/nature14292
118. Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. (2015) 16:795–803. doi: 10.1016/S1470-2045(15)00054-6
119. Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. (2018) 36:1611–18. doi: 10.1200/JCO.2017.76.2229
120. Jatoi I, Benson JR, Kunkler I. Hypothesis: can the abscopal effect explain the impact of adjuvant radiotherapy on breast cancer mortality? NPJ Breast Cancer (2018) 4:8. doi: 10.1038/s41523-018-0061-y
121. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood (2009) 114:589–95. doi: 10.1182/blood-2009-02-206870
122. Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. (2012) 72:3163–74. doi: 10.1158/0008-5472.CAN-12-0210
123. Yu P, Lee Y, Wang Y, Liu X, Auh S, Gajewski TF, et al. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol. (2007) 179:1960–8.
124. Dewan MZ, Vanpouille-Box C, Kawashima N, DiNapoli S, Babb JS, Formenti SC, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res. (2012) 18:6668–78. doi: 10.1158/1078-0432.CCR-12-0984
125. Lu H, Wagner WM, Gad E, Yang Y, Duan H, Amon LM, et al. Treatment failure of a TLR-7 agonist occurs due to self-regulation of acute inflammation and can be overcome by IL-10 blockade. J Immunol. (2010) 184:5360–7. doi: 10.4049/jimmunol.0902997
126. Vacchelli E, Bloy N, Aranda F, Buque A, Cremer I, Demaria S, et al. Trial Watch: Immunotherapy plus radiation therapy for oncological indications. Oncoimmunology (2016) 5:e1214790. doi: 10.1080/2162402X.2016.1214790
127. McArthur HL, Diab A, Page DB, Yuan J, Solomon SB, Sacchini V, et al. A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling. Clin Cancer Res. (2016) 22:5729–37. doi: 10.1158/1078-0432.CCR-16-0190
128. Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. (2016) 249–56. doi: 10.1093/annonc/mdv571
Keywords: breast cancer, subtypes, immunotherapy, radiotherapy, TILs
Citation: Tsoutsou PG, Zaman K, Martin Lluesma S, Cagnon L, Kandalaft L and Vozenin M-C (2018) Emerging Opportunities of Radiotherapy Combined With Immunotherapy in the Era of Breast Cancer Heterogeneity. Front. Oncol. 8:609. doi: 10.3389/fonc.2018.00609
Received: 25 May 2018; Accepted: 28 November 2018;
Published: 12 December 2018.
Edited by:
Radka Stoyanova, University of Miami, United StatesReviewed by:
Andrea Riccardo Filippi, Policlinico San Matteo Fondazione (IRCCS), ItalyEllen Ackerstaff, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2018 Tsoutsou, Zaman, Martin Lluesma, Cagnon, Kandalaft and Vozenin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pelagia G. Tsoutsou, cGVsYWdpYS50c291dHNvdUBoLW5lLmNo; cGVsYWdpYS50c291dHNvdUBjaHV2LmNo
 Pelagia G. Tsoutsou
Pelagia G. Tsoutsou Khalil Zaman4
Khalil Zaman4 Laurene Cagnon
Laurene Cagnon Lana Kandalaft
Lana Kandalaft Marie-Catherine Vozenin
Marie-Catherine Vozenin