- 1Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Gynecological Oncology, Cancer Hospital Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Obstetrics and Gynecology, Beijing Chao-Yang Hospital, Beijing, China
- 4Department of Obstetrics and Gynecology, China-Japan Friendship Hospital, Beijing, China
- 5Department of Obstetrics and Gynecology, Chinese PLA General Hospital, Beijing, China
Introduction: With the expansion of value-based medicine, we explore whether using type III hysterectomy to treat low-risk, early-stage cervical cancer constitutes overtreatment. In present study, we evaluate the midterm safety and postoperative quality of life of patients who underwent type II hysterectomy vs. type III hysterectomy with systematic lymphadenectomy for low-risk early-stage cervical cancer (International Federation of Gynecology and Obstetrics (FIGO) IA2-IB1; maximum tumor diameter < 2 cm).
Patients and methods: The main study was a multicenter, phase III, randomized controlled trial (NCT02368574, https://www.clinicaltrials.gov/ct2/show/NCT02368574). Patients meeting the criteria were randomly divided into type II and type III hysterectomy groups between 2015 and 2018. Midterm outcomes were analyzed at 36 months after the first eligible patient was enrolled. The primary end point was disease-free survival, and the secondary end point was postoperative quality of life.
Results: A total of 97 patients were preliminarily enrolled, 93 of whom were included in the final analysis. The general information of the two groups did not differ. The 2-year DFS rate in the type II group was 100% compared with 97.9% in the type III group (P > 0.05). Compared to the type III group, the patients who underwent type II hysterectomy showed a shorter surgical time (163 ± 18.8 min vs. 226 ± 16.4 min, P = 0.014), less intraoperative blood loss (174 ± 27.7 ml vs. 268 ± 37.4 ml, P = 0.047), less postoperative urinary retention (5/46 vs. 11/47 cases, P = 0.109), and milder bladder injuries. The postoperative symptom experience scores of the type II group were significantly lower than those of the type III group. Moreover, the postoperative sexual/vaginal functioning and lubrication scores of the type II group were significantly lower than those of the type III group in subgroup analyses of patients who did not undergo postoperative chemoradiotherapy. Sexual apprehension scores were increased postoperatively in both groups.
Conclusion: Based on the midterm analysis, the two groups show considerable security within 2 years after surgery, but long-term security requires further analysis. Type II hysterectomy can effectively reduce the surgical time and intraoperative blood loss, decrease postoperative complications, and improve the quality of life of early-stage cervical cancer patients.
Introduction
Cervical cancer is the most common malignant gynecologic tumor and severely affects the mental and physical health of women. Although effective screening has significantly reduced the incidence of advanced cervical cancer in the last 20 years, the incidence of early-stage cervical cancer in young women shows an increasing trend (1). The standard surgical method for stage IA2-IB1 cervical cancer is radical (type III) hysterectomy with systematic lymphadenectomy. The 24th International Federation of Gynecology and Obstetrics (FIGO) conference reported that the 5-year survival rates of stage IA2 and IB1 cervical squamous cell carcinoma patients were 99.1 and 92.3%, respectively. In addition, these rates among adenocarcinoma patients were 97.1 and 94.2%, respectively (2).
The Piver–Rutledge–Smith classification published in 1974 which includes the class I–V category, has gained substantial popularity (3). Piver class I hysterectomy aims to ensure removal of all cervical tissue. Piver class II hysterectomy and Piver class III hysterectomy are modified radical hysterectomy and radical hysterectomy procedures, respectively. Piver class IV hysterectomy aims to completely remove all periureteral tissue, with more extensive excision of the perivaginal tissues and excision of the internal iliac vessels along this part of the pelvic wall. The objective of Piver class V hysterectomy is to remove central recurrent cancer involving portions of the distal ureter or bladder, although this procedure is no longer used. Recently, many updated versions of hysterectomy based on the Piver–Rutledge–Smith classification have been proposed. The advanced Querleu and Morrow surgical classification system, which was proposed in 2008, represents an updated version of the Piver classification (4) and describes four types of radical hysterectomy (types A, B, C, and D), with a few additional subtypes, such as subtype C1, which includes nerve preservation, and subtype C2, which does not include preservation of autonomic nerves. Type B corresponds to Piver class II hysterectomy, or modified radical hysterectomy, and type C corresponds to Piver class III hysterectomy, or radical hysterectomy. The advanced National Comprehensive Cancer Network (NCCN) guidelines recommend type B and type C hysterectomy as the surgical methods for cervical cancer patients with stage IA2 and IB1 disease. However, to distinguish patients requiring subtype C1 hysterectomy, the Piver classification was used in the present study. Standard radical hysterectomy (Piver type III hysterectomy) requires resection of parametrial tissues close to the pelvic wall and the upper 1/3 or 1/2 of the vagina to ensure negative margins and surgical thoroughness (5). Because the surgical range is large, various intraoperative and postoperative complications occur frequently, such as bleeding, other organ injuries, urinary retention, and dyspareunia. These complications substantially compromise postoperative sexual, bladder, and physiological functions of patients and severely affect their quality of life (6, 7). However, in clinical practice, the risks of parametrial tissue infiltration and lymph node (LN) metastasis in low-risk, early-stage cervical cancer patients (FIGO stage: IA2-IB1; maximum tumor diameter < 2 cm) are very low (2, 8, 9).
With changes in medical models and the expansion of value-based medicine, using traditional Piver type III hysterectomy as the standard treatment method for low-risk, early-stage cervical cancer has been challenged. Does the use of type III hysterectomy to treat low-risk, early-stage cervical cancer constitute overtreatment (6)? Can modified radical hysterectomy (Piver type II hysterectomy) with a reduced surgical range be used to minimize postoperative complications and improve the quality of life of patients? No clinical studies have provided strong medical evidence to answer these questions. Therefore, we comprehensively, systematically, and scientifically evaluated the clinical value of type II/type III hysterectomy with systematic lymphadenectomy to identify an appropriate surgical method for low-risk, early-stage cervical cancer patients.
Recently, the Laparoscopic Approach to Cervical Cancer (LACC) study reported that laparoscopic or robot-assisted radical hysterectomy was associated with lower rates of disease-free survival and overall survival than open abdominal radical hysterectomy among women with early-stage cervical cancer (10). However, the prospective study lacks some relevant data, such as tumor size in 1/3 of the cases and information regarding paraventricular and vaginal involvement in 7–10% of the cases, and only 39.5% of the cases reached the 4.5-year follow-up end point. In addition, the 2019 NCCN guidelines, version 2, suggest that laparotomy, laparoscopy, or robotic laparoscopy is an acceptable radical hysterectomy approach, and laparoscopic radical hysterectomy has been demonstrated to be associated with more favorable morbidity profiles, lower costs of care, and comparable survival relative to abdominal radical hysterectomy through decades of research (11–14). Therefore, Piver II hysterectomy and Piver III hysterectomy were performed through laparoscopy in the initial of present study.
Patients and Methods
General Information
Patients diagnosed with cervical cancer (FIGO stage: IA2 and IB1, maximum tumor diameter < 2 cm) in five research centers were enrolled in a multicenter, phase III, randomized controlled trial (ClinicalTrials.gov identifier: NCT02368574) between March 2015 and March 2018. The estimated number of enrolled participants for the final analysis was as least 180 with a noninferiority margin of 10%, an alpha error of 0.05, and a power for 0.8. Significance different survival time between two groups is defined as the early endpoint. The patients were randomly divided into type II and type III hysterectomy groups by the Interactive Web Response System (IWRS). The five research centers include: Peking Union Medical College Hospital, Cancer Hospital Chinese Academy of Medical Sciences, Beijing Chao-Yang Hospital, Chinese PLA General Hospital, and China-Japan Friendship Hospital.
Inclusion Criteria
(1) 18–60 years old; (2) FIGO stage IA2–IB1; (3) MRI examination showing a maximum tumor diameter < 2 cm and a depth of interstitial infiltrates < 50%; (4) Histological diagnosis of squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma; and (5) Signed informed consent.
Exclusion Criteria
(1) High-risk histological types; (2) CT or MRI evaluation indicative of LN-positive disease; (3) A history of neoadjuvant chemotherapy; (4) Pregnancy; (5) Strong desire to retain fertility; (6) Surgical contraindications; (7) Previous history of intestinal obstruction, recurrent pelvic inflammatory disease, pelvic tuberculosis, or pelvic or abdominal tumor surgery; (8) Previous history of bladder or ureteral surgery, previous urinary retention, urinary incontinence or fecal incontinence; and (9) No written informed consent. All patients underwent MRI or contrast-enhanced CT, and the depth of interstitial infiltrates was reviewed by two radiologists at least in the study group until the patients could be enrolled. Pathology and radiology were reviewed in each individual center, and the MRI or CT data will be saved for regular centralized sampling inspection.
Surgical Methods
Piver Type II Hysterectomy
Piver type II hysterectomy (3) is also known as modified radical hysterectomy, and the corresponding surgical range is wider than that of type I extrafascial total hysterectomy, with resection of more parametrial tissues and preservation of the blood supply to the distal ureter and bladder. Separation of the ureter started from the ureteral tunnel, the entire vesicouterine ligament was preserved, and 1/2 of the uterosacral ligament and 1–2 cm of the vagina were resected. In addition, the pelvic LNs were dissected. Type II total hysterectomy was performed laparoscopically (Supplementary Figure 1A).
Piver Type III Hysterectomy
Piver type III hysterectomy (3), which is also known as radical hysterectomy, is the traditional surgical method for the treatment of early-stage cervical cancer. This extended hysterectomy technique requires opening the lateral bladder fossa and lateral rectal fossa. The uterine artery was ligated at the beginning of the internal iliac artery, the ureteral tunnel was completely freed, and the ureter was pushed down to the ureterovesical junction. Next, all ligaments and connective tissues joining the anterior and posterior sides and the bilateral sides of the uterus were separated and resected. The uterosacral ligament was resected close to the sacrum, and the cardinal ligament was resected close to the pelvic lateral wall. After the paravaginal connective tissues were all resected, the top 1/3 or 1/2 of the vagina was resected, and the resection margin was 3–4 cm from the cervical lesion. In addition, the pelvic LNs were dissected. Type III hysterectomy was performed through a laparoscope (Supplementary Figure 1B).
Quality Assurance of Surgery
According to the Standard Operating Procedures (SOPs) of our study, all surgical procedures were videotaped and postoperative specimens with scale plate were photographed and uploaded to our EDC system (Supplementary Figure 1). A quality-control team consisted of five gynecological oncologists was established to evaluate the surgical procedures.
Observation Indicators
The basic information of the patients was statistically analyzed. Eligibility screening, quality of life assessments, and urodynamic examinations were completed 6 weeks before surgery. Intraoperative indicators included the surgical time, intraoperative blood loss, and other organ and blood vessel injuries. Perioperative indicators included intestinal obstruction, infection, thromboembolic diseases, and urinary retention. The postoperative follow-up included a regular follow-up, urodynamic examinations at 6 months postoperatively, and quality of life assessments at 6, 12, and 24 months postoperatively using the Chinese version of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire—Cervical Cancer Module (QLQ-CX24), the Female Sexual Distress Scale (FSDS), and the Female Sexual Functioning Index (FSFI) (15–17). These questionnaires have been tested and corrected in Asian countries (including China).
Statistical Analysis
Quantitative data were compared by a t-test or the Wilcoxon rank sum test. Qualitative data were analyzed using the chi-square test or Fisher's exact test. Survival was calculated using the Kaplan-Meier method. ANOVA with a post hoc test and Fisher's exact test were also used for comparisons among groups. Midterm results were analyzed 36 months after the first eligible patient was enrolled. The primary end point was disease-free survival (DFS), and the secondary end point was postoperative quality of life. Patients with positive LNs intraoperatively or on frozen section received standard treatment according to the advanced NCCN guidelines and were included in the final statistical analysis as a subgroup of the experimental group according to SOPs.
Results
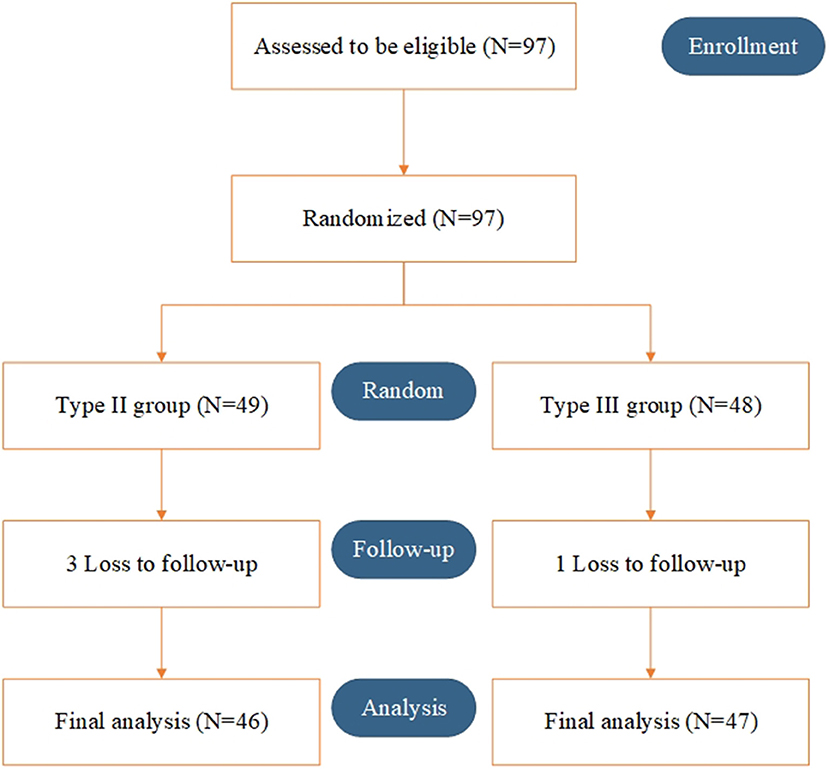
Between March 2015 and March 2018, a total of 97 patients were randomly enrolled. Overall, 49 and 48 patients underwent type II and type III hysterectomy with systematic lymphadenectomy, respectively. Three patients in the type II group and 1 patient in the type III group were lost to follow-up. Ninety-three patients (46 in the type II group and 47 in the type III group) were included in the final analysis (Figure 1).
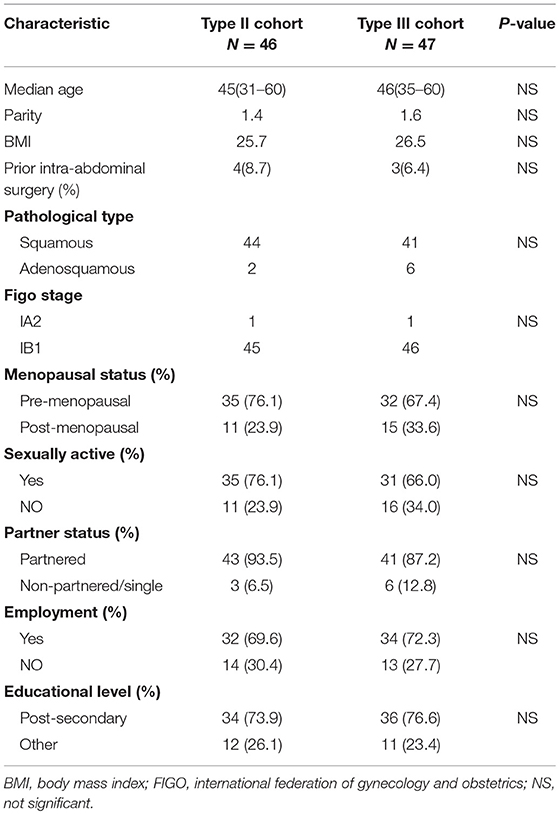
The general information of the patients is shown in Table 1. Age, gravidity and parity history, body mass index (BMI), tumor stage, menstrual status, sexual life, marital status, working condition, education level, and hypogastric surgical history (including previous surgery related to intestinal obstruction, recurrent pelvic inflammatory disease, pelvic tuberculosis, pelvic, and abdominal tumors or the bladder/ureter) were not significantly different between the two groups (P > 0.05).
The comparison of surgery-related information between the two groups is shown in Table 2. The results show that the hospitalization time did not differ between the two groups. However, compared to the type III group, the surgical time was significantly shorter (163 ± 18.8 min vs. 226 ± 16.4 min, P = 0.014) and the intraoperative blood loss was significantly less (174 ± 27.7 ml vs. 268 ± 37.4 ml, P = 0.047) in the type II group. A total of three patients experienced intraoperative complications, including two patients with intraoperative large blood vessel injuries (one in each group) and one patient with a ureteral injury (type II). In the type II group, 1 case of parametrial infiltration and 1 case of pelvic LN metastasis combined with myometrial invasion were noted. Four cases of pelvic LN metastasis were noted in the type III group, 2 of which were accompanied by myometrial infiltration (P = 0.413). These six patients subsequently received postoperative radiotherapy and chemotherapy. The mean postoperative first aerofluxus times in the type II and type III groups were 1.8 and 2 days (P = 0.803), and the mean times to the first bowel movement were 3.3 and 3.6 days, respectively (P = 0.841). Five patients had perioperative complications, including four patients with infection (one patient in the type II group and three patients in the type III group) and one patient with a thromboembolic event (the type II group). Urinary retention within 14 days postoperatively occurred in 5/46 and 11/47 cases in the type II and type III hysterectomy groups, respectively (P = 0.109). Twenty-two of 93 (23.7%) patients had lymphovascular space invasion (LVSI) after surgery and received postoperative radiotherapy and chemotherapy, 10 of whom were in the type II group and 12 of whom were in the type III group (P = 0.667). The rates of intraoperative complications, parametrial infiltration, LVSI, pelvic LN metastasis, and myometrial infiltration were not significantly different between the two groups.
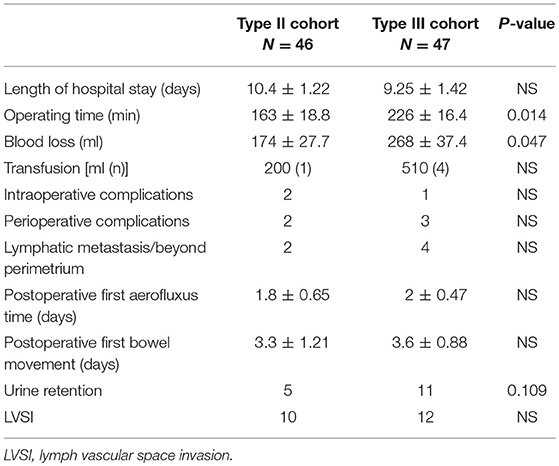
Table 2. Comparison of surgical characteristics in patients treated with Type II and Type III hysterectomy cohort.
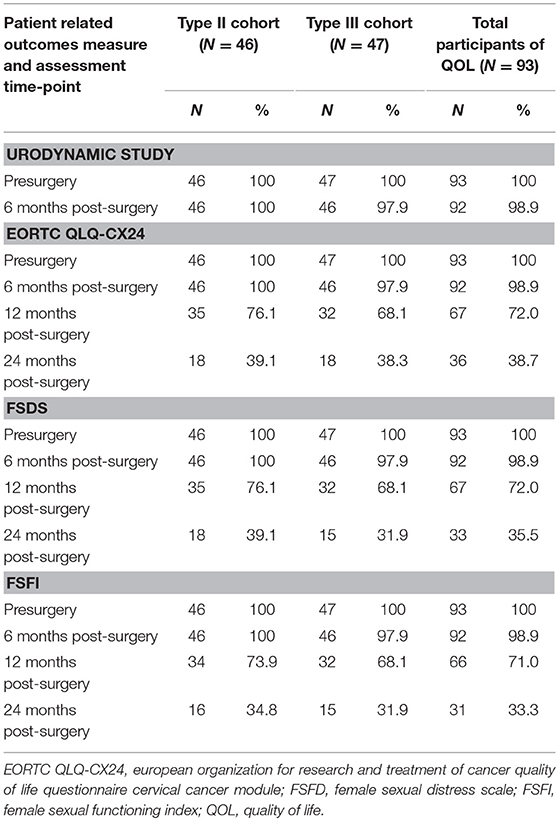
The follow-up was performed until August 2018. The median follow-up time was 28 (6–38) months in the midterm analysis. During the follow-up period, only one DFS event (2.1%), recurrence at 4 months postoperatively, occurred in the type III group, while no DFS events were observed in the type II group. The corresponding 2-year DFS rates were 97.9 and 100%, respectively. The remaining 92 patients all attended a regular follow-up (Figure 2). All patients completed preoperative urodynamic examinations and the QLQ-CX24, FSDS, and FSFI questionnaires. More than 95% of the patients completed urodynamic examinations and questionnaires regarding quality of life at 6 months postoperatively. The percentages of patients who completed the QLQ-CX24, FSDS, and FSFI questionnaires at 12 months postoperatively were 72, 72, and 71%, respectively, and these percentages were 38.7, 35.5, and 33.3 at 24 months postoperatively, respectively (Table 3).
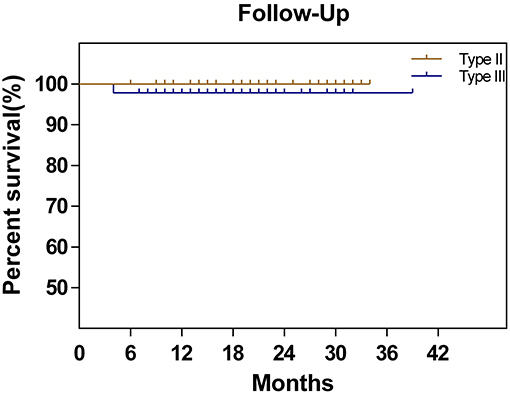
Figure 2. Disease-free survival in patients with cervical cancer treated by type II hysterectomy or type III hysterectomy.
The urodynamic detection results are shown in Table 4. All indicators in the preoperative urodynamic examinations were not significantly different between the two groups. At 6 months postoperatively, the maximum cystometric capacity of the patients was decreased in the type III group compared to that in the type II group (322.6 ± 44.7 ml vs. 438.8 ± 33.1 ml, P = 0.040). Other indicators were not different between the two groups. However, in a subgroup analysis, the maximum cystometric capacity and the maximum detrusor pressure (Pdet-max) of the patients in the type III group were both significantly decreased compared to those in the type II group (360.4 ± 32.7 ml vs. 446.8 ± 27.9 ml, P = 0.048; 24.4 ± 3.0 vs. 34.9 ± 4.1, P = 0.045, respectively) at 6 months postoperatively. The maximum flow rate (Qmax) in the patients in the type III group was decreased, although not significantly, compared to that in the type II group (16.2 ± 2.2 vs. 23.5 ± 3.4, P > 0.05) (Table 5).
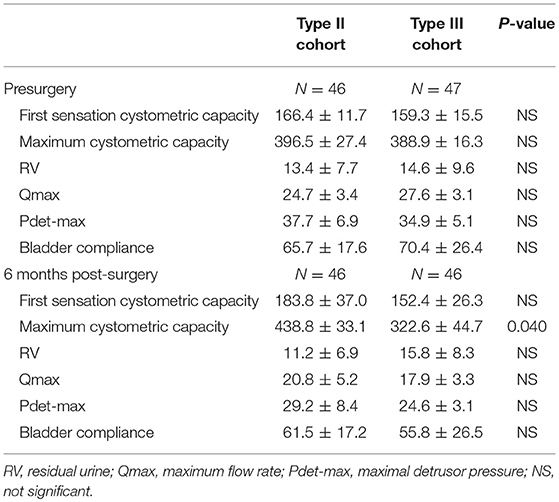
Table 4. Comparison of urodynamic study in patients treated with Type II and Type III hysterectomy cohort.
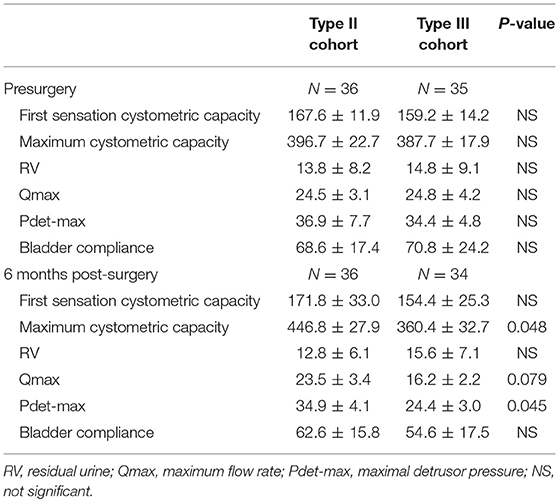
Table 5. Subgroup analysis of urodynamic study in patients treated with Type II and Type III hysterectomy cohort without postoperative chemoradiotherapy.
The preoperative baseline scores on the QLQ-CX24, FSDS, and FSFI assessments were similar between the two groups (Table 6). At 6 and 12 months postoperatively, the symptom experience scores on the QLQ-CX24 in the type II group were significantly decreased compared to those in the type III group (15.76 ± 2.83 vs. 25.18 ± 3.56, P = 0.042; 8.38 ± 2.36 vs. 16.56 ± 3.33, P = 0.046, respectively). Within the first 6 months after surgery, 75% of the patients reported having no sexual life, indicating reduced responses to sexual/vaginal functioning, sexual activity, and sexual enjoyment. The differences in symptom experience scores between the two groups were not statistically significant at 24 months (7.24 ± 0.76 vs. 9.85 ± 1.13, P = 0.064). The FSDS and FSFI scores in the type II group and type III group were 38.28 ± 4.87 and 22.67 ± 2.37 compared with 37.77 ± 3.39 and 26.53 ± 3.82 at 6 months postoperatively (P > 0.05), 36.28 ± 4.27 and 12.84 ± 2.66 compared with 40.11 ± 5.33 and 11.82 ± 2.26 at 12 months postoperatively (P > 0.05), and 41.75 ± 6.13 and 14.11 ± 2.05 compared with 44.46 ± 5.06 and 10.88 ± 2.32 at 24 months postoperatively, respectively (P > 0.05) (Table 7).
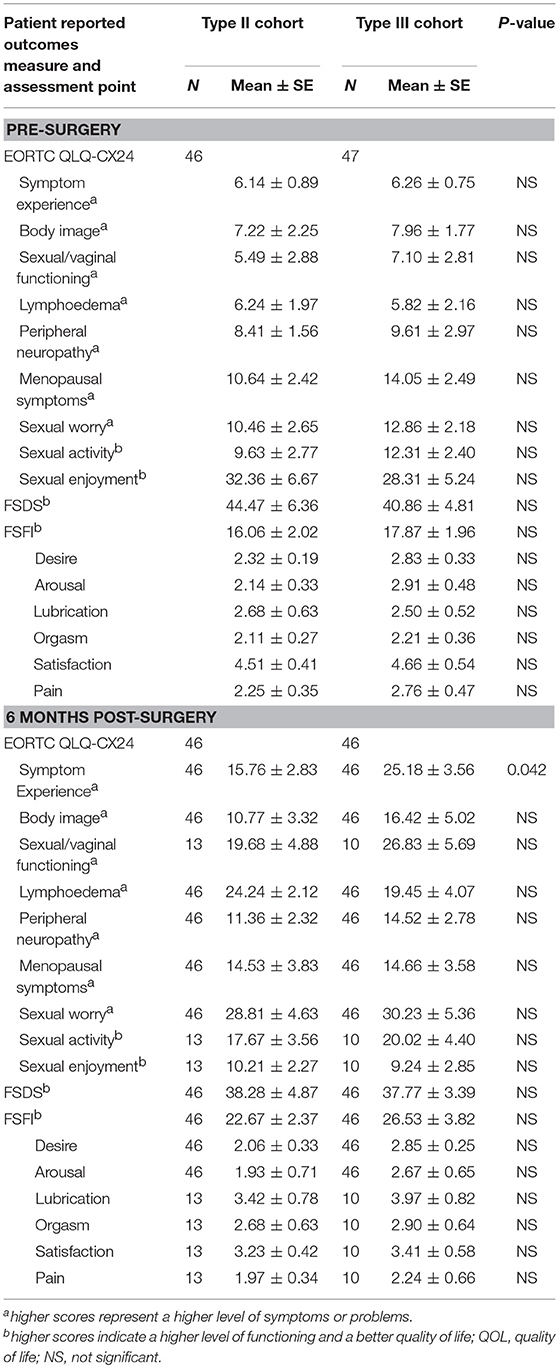
Table 6. Mean scores for each patient reported outcomes assessment between Type II and Type III hysterectomy cohort at pre-surgery and 6 months post-surgery.
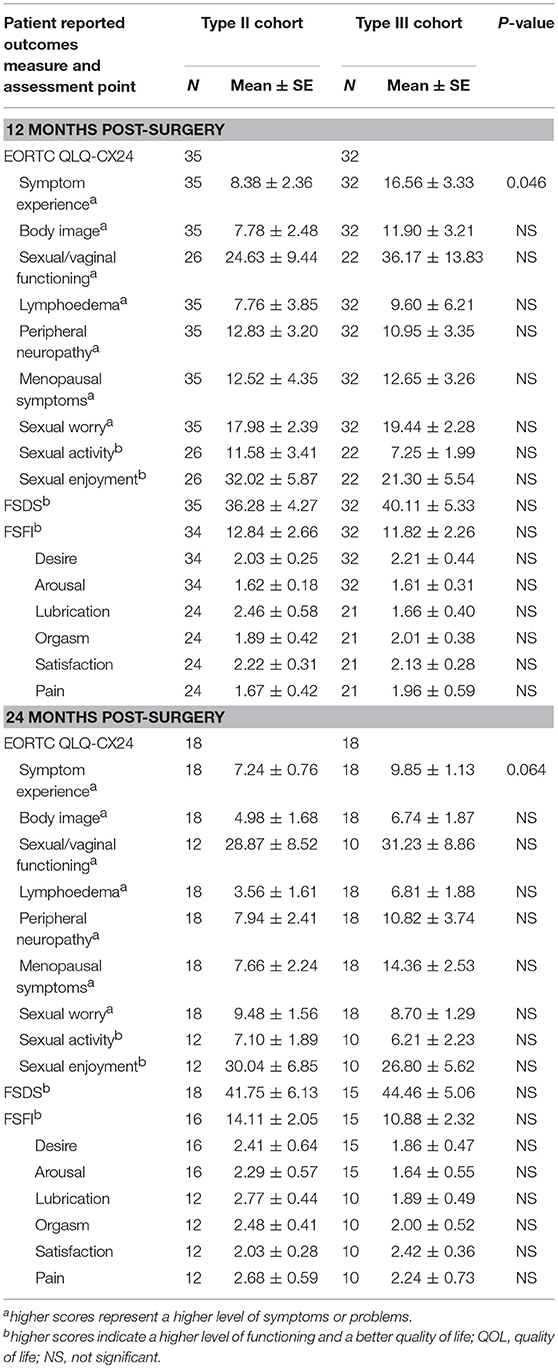
Table 7. Mean scores for each patient reported outcomes assessment between Type II and Type III hysterectomy cohort at 12 and 24 months post-surgery.
In a subgroup analysis, the symptom experience scores on the QLQ-CX24 in the type II group were also significantly decreased compared to those in the type III group (16.54 ± 2.73 vs. 23.63 ± 2.62, P = 0.036), and no differences in the other dimensions of the QLQ-CX24 were noted at 6 months postoperatively. The FSDS and FSFI scores in the type II group were 40.70 ± 6.87 and 21.67 ± 2.47 compared with 37.64 ± 5.54 and 22.86 ± 3.52 in the type III group, respectively (P > 0.05) (Table 8).
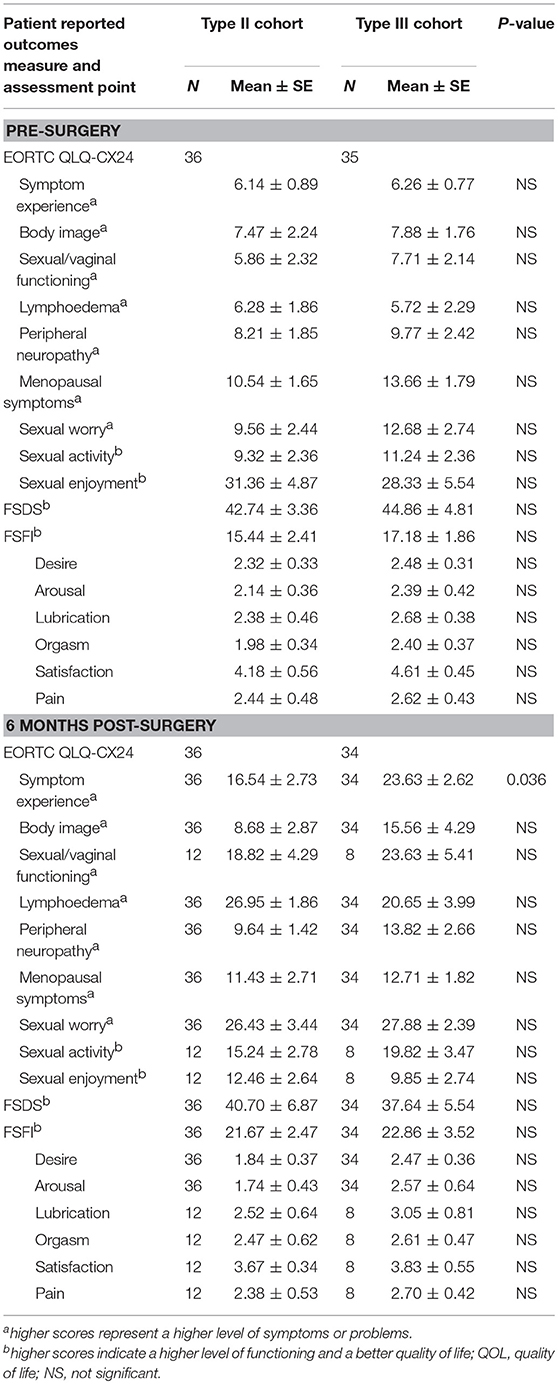
Table 8. Mean scores between Type II and Type III hysterectomy subgroup without postoperative chemoradiotherapy at Pre-surgery and 12 months post-surgery.
At 12 months postoperatively, the symptom experience scores in the type II and type III groups were 7.69 ± 1.78 and 13.62 ± 2.23, respectively (P = 0.045). The sexual/vaginal functioning scores on the QLQ-CX24 in the type II group were significantly lower than those in the type III group (20.85 ± 3.38 vs. 33.74 ± 4.79, P = 0.036), while scores on the other dimensions of the QLQ-CX24 were not different between the two groups. The total FSDS and FSFI scores in the type II group were 41.43 ± 4.18 and 13.47 ± 1.16 compared with 40.20 ± 6.84 and 13.86 ± 1.44 in the type III group, respectively (P > 0.05). However, the lubrication score on the FSFI scale in the type II group was significantly higher than that in the type III group (2.79 ± 0.29 vs. 1.42 ± 0.52, P = 0.032) (Table 9).
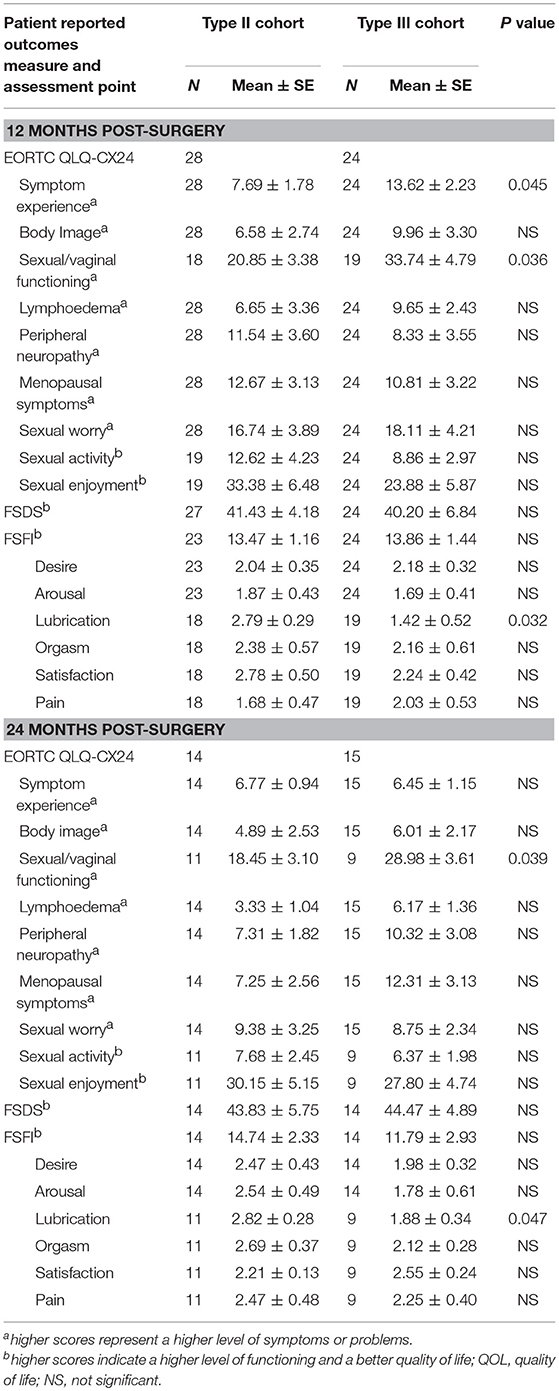
Table 9. Mean scores between Type II and Type III hysterectomy subgroup without postoperative chemoradiotherapy at 12 and 24 months post-surgery.
At 24 months postoperatively, the sexual/vaginal functioning score on the QLQ-CX24 in the type II group was significantly lower than that in the type III group (18.45 ± 3.10 vs. 28.98 ± 3.61, P = 0.039). The FSDS score in the type II group was 43.83 ± 5.75, which was not different from the score of 44.47 ± 4.89 in the type III group (P > 0.05). The total FSFI score was not different between the two groups (14.74 ± 2.33 vs. 11.79 ± 2.93, P > 0.05), but the lubrication score on the FSFI questionnaire in the type II group was higher than that in the type III group (2.82 ± 0.28 vs. 1.88 ± 0.34, P = 0.047). However, because fewer patients completed the questionnaire in this period, the validity should be further analyzed (Table 9).
For all patients, the mean sexual apprehension scores on the QLQ-CX24 at 6 and 12 months postoperatively were 29.52 ± 4.88 and 18.74 ± 2.51, respectively, a significant increase compared with the score of 11.66 ± 2.27 before surgery (P = 0.001, P = 0.038, respectively). However, the score recovered to 9.09 ± 1.43 at 24 months postoperatively. The postoperative sexual/vaginal functioning scores on the QLQ-CX24 in both groups increased after surgery (P < 0.05). The overall FSDS and FSFI scores were not different pre- and post-surgery (Figure 3).
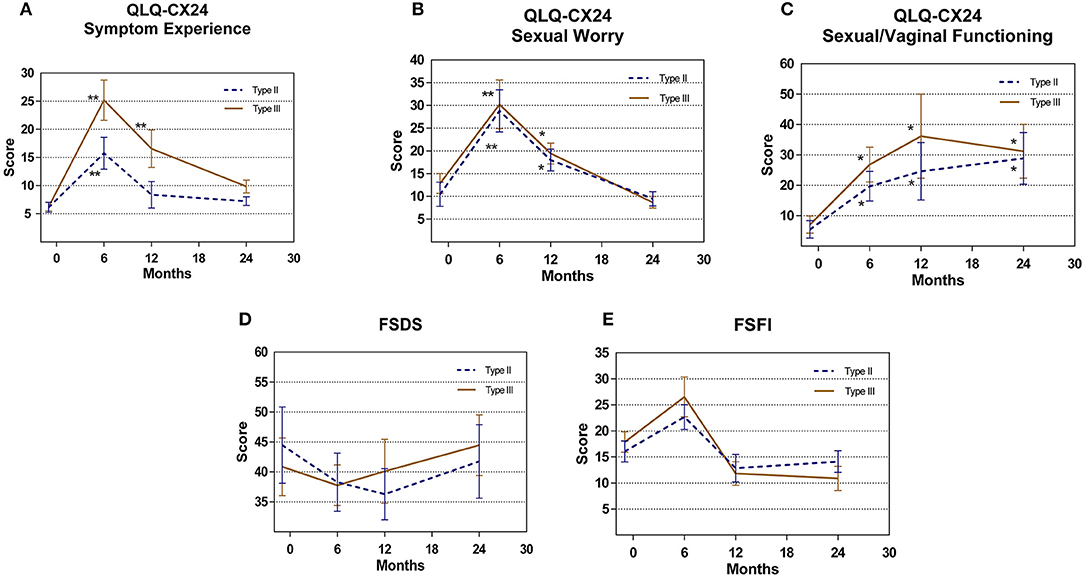
Figure 3. Patient related outcomes measure (A) QLQ-CX24 Symptom Experience (B) QLQ-CX24 Sexual Worry (C) QLQ-CX24 Sexual/Vaginal Functioning (D) FSDS (E) FSFI comparison for type II vs. type III group. *P < 0.05, **P < 0.01.
Discussion
For early-stage cervical cancer patients with FIGO IA2-IB1 stage disease, the NCCN currently recommends radical hysterectomy combined with systematic lymphadenectomy. However, the surgical range is too large, which severely affects the quality of life of patients (18, 19).
Van Meurs et al. noted that no parametrial infiltration occurred in 1,063 patients with stage IA2 cervical cancer. However, 4.8% of the patients had LN metastasis and the recurrence rate was 3.6% (19). Covens et al. retrospectively analyzed 842 patients with stage IB1 cervical cancer and noted that the risk of parametrial infiltration in low-risk patients (tumor diameter < 2 cm, interstitial infiltration depth < 10 mm, and negative pelvic LNs) was 0.6% (20). Our previous multicenter study indicated that among 1,123 patients with stage IA2 and IB1 (maximum tumor diameter < 2 cm) cervical cancer, the parametrial and vaginal involvement rates were both 0.2% (2/1,123), the uterine isthmus involvement rate was 1.8% (20/1,123), and the pelvic LN metastasis rate was 6.1% (69/1,123) (21, 22). Moreover, the JCOG0806-A study retrospectively analyzed patients with stage IB1 cervical cancer and reported that the 5-year overall survival rate was 95.8% and that pathological parametrial involvement was observed in only 1.9% of low-risk patients (2). To evaluate the security of modified radical hysterectomy, Xie analyzed 86 patients with early-stage cervical cancer (IB1-IAl) who underwent modified vaginal radical hysterectomy and system lymphadenectomy and reported that the recurrence rate and overall survival rate were 3.57 and 97.62% within 46 months, respectively (23). In 2013, the Gynecologic Cancer Study Group of the Japan Clinical Oncology Group (JCOG) initiated a multicenter study to evaluate the efficacy of Piver II hysterectomy in FIGO Stage IB1 low-risk cervical cancer patients (JCOG1101), and we look forward to their reports (24).
The present study systematically evaluated the safety and quality of life associated with type II and type III hysterectomy with systematic lymphadenectomy in the treatment of stage IA2-IB1 cervical cancer patients with a maximum tumor diameter < 2 cm. The results showed that the surgical time and intraoperative blood loss in the type II group was significantly lower than those in the type III group, mainly because of the reduced surgical range and minimal blood vessel injuries (25). The present study showed that the effects of these two surgical methods on postoperative aerofluxus and defecation functions were not different. However, different effects on urinary function were found. The number of patients with urinary retention within 14 days postoperatively was lower in the type II group than that in the type III group, which may be associated with bladder retroflexion caused by pelvic nerve injury and the larger resection range of uterine, vaginal, and parametrial tissues with type III hysterectomy (26). The positive rate of LVSI in low-risk, early-stage cervical cancer has been reported to be 10.3–45%. The positive rate of LVSI was 23.7% in the present study, which was the major risk factor for postoperative chemoradiotherapy (26, 27).
To effectively evaluate changes in bladder function and quality of life among patients, we excluded possible confounding factors (patients aged >60 years and those with other diseases) to control biases. Moreover, postoperative chemoradiotherapy was also identified as a confounding factor (28); therefore, we performed subgroup analyses in the two groups without postoperative chemoradiotherapy. The maximum cystometric capacity and Pdet-max of the patients in the type II group were significantly higher at 6 months postoperatively, suggesting that the detrusor function of the patients in the type III group had different degrees of injury, which caused a reduction in the Qmax and affected urinary function. Chen et al. reported that the number of patients with detrusor instability after surgery significantly increased, which may be associated with local sympathetic and parasympathetic nerve injury (29). The subsequent study by Todo on nerve-sparing surgery also confirmed this point (30).
Jensen et al. reported that the lack of sexual interest among cervical cancer patients after radical hysterectomy persisted for 2 years (31). The present study showed that most patients had lower sexual activity overall after surgery, which may be due to sexual apprehension within 2 years postoperatively. Therefore, we suggest that psychological factors are important and affect postoperative quality of life and sexual function. The sexual enjoyment and desire scores were not significantly different in our study, which may be related to the conservative nature of Asian females in response of sexual function-related questions (15, 17).
Furthermore, better sexual/vaginal functioning and lubrication were noted among the patients in the type II group, and symptoms in the type II group were significantly attenuated according to the subgroup analysis, which may be due to resection of less parametrial and vaginal tissues and decreased rates of pelvic nerve injury, scar fibrosis, and vaginal blood circulation disorders in the type II group (32, 33). The symptom experience scores in the type III group were significantly increased compared to those in the type II group at 6 and 12 months postoperatively, and these scores recovered at 24 months postoperatively, possibly due to recovery from surgical trauma and postoperative complications (34, 35). In addition to the differences in other indicators in all patients before and after surgery, we considered that this finding may have been observed because other indicators, such as sexual apprehension, sexual activity, and sexual enjoyment, were mainly associated with the psychological and neuroendocrine factors of malignant oncology and related surgery, which are rarely influenced by the type of radical hysterectomy (31). Moreover, the ovaries are not routinely removed in both types of surgical procedure, and menopausal symptoms were therefore not significantly different because such symptoms are mainly regulated by ovarian hormones, which can be affected by different surgery types. Similarly, the postoperative lymphedema score was significantly higher than the preoperative score in all patients, which was mainly related to pelvic lymphadenectomy (36). Therefore, no differences in surgical types were observed. The scores for sexual activity, sexual enjoyment, orgasm, and satisfaction dimensions were not different between the two groups at each time point postoperatively, which is inconsistent with the conclusion reported by Francesco Plotti (37). It should be noted that we performed an overall analysis of the low-risk, early-stage cervical cancer population rather than patients who were young and sexually active. In addition, baseline scoring of the preoperative quality of life and sexual life status of all patients was performed to control for differences between the groups before surgery. Moreover, the characteristics (including ethnic factors, educational background, physical factors, and psychological factors) of patients may differ across studies. The enrolled patients were all Chinese in our study, which may also reflect a distinguishing characteristic of our study population. The 2-year survival rates of the type II and type III groups were 100 and 97.6%, respectively, which is consistent with the rates reported in the literature (38, 39). The security of type II hysterectomy combined with systematic lymphadenectomy in the treatment of low-risk, early-stage cervical cancer patients was not worse than that for the type III hysterectomy patients within 2 years after surgery. Certainly, more patients will be enrolled in our next study.
Conclusion
Overall, based on the midterm analysis, type II hysterectomy effectively decreased the surgical time and intraoperative blood loss, reduced bladder complications, increased patients' quality of life, and showed favorable security in low-risk, early-stage cervical cancer within 2 years after surgery. However, we should also focus more on the postoperative apprehension of patients.
Ethics Statement
Ethical approval: All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments (the name and affiliation of the ethics committee that approved this study: The Institutional Ethics Committee of Peking Union Medical College Hospital, CAMS Chinese Academy of Medical Sciences, No. S-785 2015. Each institution received Institutional Review Board (IRB) approval).
Written informed consent was obtained from all participants included in the study.
Author Contributions
DC and KS: protocol/project development and clinical data provider; HS: data collection, data analysis, manuscript writing, and the follow-up; JY, YX, FF, LW, ZZ, BL, and LS: clinical data providers.
Funding
This study was funded by the Beijing Science and Technology Plan Project [D151100001915004] (KS) and [CAMS Initiative for Innovative Medicine CAMS-2018-I2M-1-002] (KS).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00568/full#supplementary-material
Supplementary Figure 1. The photograph of specimens for quality assurance of surgery. (A) Piver type II hysterectomy. (B) Piver type III hysterectomy.
References
1. McGraw SL, Ferrante JM. Update on prevention and screening of cervical cancer. World J Clin Oncol. (2014) 5:744–52. doi: 10.5306/wjco.v5.i4.744
2. Kato T, Takashima A, Kasamatsu T, Nakamura K, Mizusawa J, Nakanishi T, et al. Clinical tumor diameter and prognosis of patients with FIGO stage IB1 cervical cancer (JCOG0806-A). Gynecol Oncol. (2015) 137:34–9. doi: 10.1016/j.ygyno.2015.01.548
3. Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. (1974) 44:265–72.
4. Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol. (2008) 9:297–303. doi: 10.1016/S1470-2045(08)70074-3
5. Meigs JV. Radical hysterectomy with bilateral dissection of the pelvic lymph nodes for cancer of the cervix (the Wertheim, Reis, Clark, Wertheim-Meigs operation). Surg Clin North Am. (1956): 1083–116. doi: 10.1016/S0039-6109(16)34948-9
6. Buchanan T, Pierce JY, Graybill W, Kohler M, Creasman W. Why do we continue to overtreat stage Ia carcinoma of the cervix? Am J Obstet Gynecol. (2017) 217:413–7. doi: 10.1016/j.ajog.2017.05.020
7. Wang X, Chen C, Liu P, Li W, Wang L, Liu Y. The morbidity of sexual dysfunction of 125 Chinese women following different types of radical hysterectomy for gynaecological malignancies. Arch Gynecol Obstet. (2018) 297:459–66. doi: 10.1007/s00404-017-4625-0
8. Mikami M, Aoki Y, Sakamoto M, Shimada M, Takeshima N, Fujiwara H, et al. Current surgical principle for uterine cervical cancer of stages Ia2, Ib1, and IIa1 in Japan. Int J Gynecol Cancer (2013) 23:1655–62. doi: 10.1097/IGC.0000000000000005
9. Horn LC, Bilek K, Fischer U, Einenkel J, Hentschel B. A cut-off value of 2 cm in tumor size is of prognostic value in surgically treated FIGO stage IB cervical cancer. Gynecol Oncol. (2014) 134:42–6. doi: 10.1016/j.ygyno.2014.04.011
10. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. (2018) 379:1895–904. doi: 10.1056/NEJMoa1806395
11. Kim JH, Kim K, Park SJ, Lee JY, Kim K, Lim MC, et al. Comparative effectiveness of abdominal versus laparoscopic radical hysterectomy for cervical cancer in the postdissemination era. Cancer Res Treat. (2018). doi: 10.4143/crt.2018.120. [Epub ahead of print].
12. Mendivil AA, Rettenmaier MA, Abaid LN, Brown JV III, Micha JP, Lopez KL, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol. (2016) 25:66–71. doi: 10.1016/j.suronc.2015.09.004
13. Gil-Moreno A, Carbonell-Socias M, Salicru S, Centeno-Mediavilla C, Franco-Camps S, Colas E, et al. Radical hysterectomy: efficacy and safety in the dawn of minimally invasive techniques. J Minim Invasive Gynecol. (2018). doi: 10.1016/j.jmig.2018.06.007. [Epub ahead of print].
14. Corrado G, Vizza E, Legge F, Pedone Anchora L, Sperduti I, Fagotti A, et al. Comparison of different surgical approaches for stage IB1 cervical cancer patients: a multi-institution study and a review of the literature. Int J Gynecol Cancer (2018) 28:1020–8. doi: 10.1097/IGC.0000000000001254
15. Shin DW, Ahn E, Kim YM, Kang S, Kim BG, Seong SJ, et al. Cross-cultural application of the Korean version of the European Organization for Research and Treatment of Cancer quality of life questionnaire cervical cancer module. Oncology (2009) 76:190–8. doi: 10.1159/000201571
16. Bae JH, Han CS, Kang SH, Shim KS, Kim JJ, Moon DG. Development of a Korean version of the Female Sexual Distress Scale. J Sex Med. (2006) 3:1013–7. doi: 10.1111/j.1743-6109.2006.00328.x
17. Sun X, Li C, Jin L, Fan Y, Wang D. Development and validation of Chinese version of female sexual function index in a Chinese population-a pilot study. J Sex Med. (2011) 8:1101–11. doi: 10.1111/j.1743-6109.2010.02171.x
18. Vistad I, Fossa SD, Dahl AA. A critical review of patient-rated quality of life studies of long-term survivors of cervical cancer. Gynecol Oncol. (2006) 102:563–72. doi: 10.1016/j.ygyno.2006.03.050
19. van Meurs H, Visser O, Buist MR, Ten Kate FJ, van der Velden J. Frequency of pelvic lymph node metastases and parametrial involvement in stage IA2 cervical cancer: a population-based study and literature review. Int J Gynecol Cancer (2009) 19:21–6. doi: 10.1111/IGC.0b013e318197f3ef
20. Covens A, Rosen B, Murphy J, Laframboise S, DePetrillo AD, Lickrish G, et al. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecol Oncol. (2002) 84:145–9. doi: 10.1006/gyno.2001.6493
21. Bai H, Yuan F, Wang H, Chen J, Cui Q, Shen K. The potential for less radical surgery in women with stage IA2-IB1 cervical cancer. Int J Gynaecol Obstet. (2015) 130:235–40. doi: 10.1016/j.ijgo.2015.03.042
22. Bai H, Cao D, Yuan F, Wang H, Xiao M, Chen J, et al. Accuracy of conization procedure for predicting pathological parameters of radical hysterectomy in stage Ia2-Ib1 (< / = 2 cm) cervical cancer. Sci Rep. (2016) 6:25992. doi: 10.1038/srep25992
23. Xie QH, Deng KX, Zheng YH, Wang H, Huang XB, Liu XC. Modified radical vaginal hysterectomy for cervical cancer treatment. Eur J Gynaecol Oncol. (2015) 36:554–9. doi: 10.12892/ejgo2703.2015
24. Kunieda F, Kasamatsu T, Arimoto T, Onda T, Toita T, Shibata T, et al. Non-randomized confirmatory trial of modified radical hysterectomy for patients with tumor diameter 2 cm or less FIGO Stage IB1 uterine cervical cancer: Japan Clinical Oncology Group Study (JCOG1101). Jpn J Clin Oncol. (2015) 45:123–6. doi: 10.1093/jjco/hyu168
25. Piver MS, Lee JY. The 21st century role of Piver type II hysterectomy in FIGO stage IA, IB cervical cancer: a personal perspective. Eur J Gynaecol Oncol. (2008) 29:109–13.
26. Uccella S, Laterza R, Ciravolo G, Volpi E, Franchi M, Zefiro F, et al. A comparison of urinary complications following total laparoscopic radical hysterectomy and laparoscopic pelvic lymphadenectomy to open abdominal surgery. Gynecol Oncol. (2007) 107(1 Suppl. 1):S147–9. doi: 10.1016/j.ygyno.2007.07.027
27. Magrina JF, Goodrich MA, Lidner TK, Weaver AL, Cornella JL, Podratz KC. Modified radical hysterectomy in the treatment of early squamous cervical cancer. Gynecol Oncol. (1999) 72:183–6. doi: 10.1006/gyno.1998.5245
28. Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. (1999) 340:1383–9. doi: 10.1056/NEJM199905063401802
29. Chen GD, Lin LY, Wang PH, Lee HS. Urinary tract dysfunction after radical hysterectomy for cervical cancer. Gynecol Oncol. (2002) 85:292–7. doi: 10.1006/gyno.2002.6614
30. Todo Y, Kuwabara M, Watari H, Ebina Y, Takeda M, Kudo M, et al. Urodynamic study on postsurgical bladder function in cervical cancer treated with systematic nerve-sparing radical hysterectomy. Int J Gynecol Cancer (2006) 16:369–75. doi: 10.1111/j.1525-1438.2006.00345.x
31. Jensen PT, Groenvold M, Klee MC, Thranov I, Petersen MA, Machin D. Early-stage cervical carcinoma, radical hysterectomy, and sexual function. A longitudinal study. Cancer (2004) 100:97–106. doi: 10.1002/cncr.11877
32. Benedetti-Panici P, Zullo MA, Plotti F, Manci N, Muzii L, Angioli R. Long-term bladder function in patients with locally advanced cervical carcinoma treated with neoadjuvant chemotherapy and type 3–4 radical hysterectomy. Cancer (2004) 100:2110–7. doi: 10.1002/cncr.20235
33. Maas CP, ter Kuile MM, Laan E, Tuijnman CC, Weijenborg PT, Trimbos JB, et al. Objective assessment of sexual arousal in women with a history of hysterectomy. BJOG (2004) 111:456–62. doi: 10.1111/j.1471-0528.2004.00104.x
34. Pieterse QD, Maas CP, ter Kuile MM, Lowik M, van Eijkeren MA, Trimbos JB et al. An observational longitudinal study to evaluate miction, defecation, and sexual function after radical hysterectomy with pelvic lymphadenectomy for early-stage cervical cancer. Int J Gynecol Cancer (2006) 16:1119–29. doi: 10.1111/j.1525-1438.2006.00461.x
35. Zullo MA, Manci N, Angioli R, Muzii L, Panici PB. Vesical dysfunctions after radical hysterectomy for cervical cancer: a critical review. Crit Rev Oncol Hematol. (2003) 48:287–93. doi: 10.1016/S1040-8428(03)00125-2
36. Werngren-Elgstrom M, Lidman D. Lymphoedema of the lower extremities after surgery and radiotherapy for cancer of the cervix. Scand J Plastic Reconstr Surg Hand Surg. (1994) 28:289–93. doi: 10.3109/02844319409022014
37. Plotti F, Nelaj E, Sansone M, Antonelli E, Altavilla T, Angioli R, et al. Sexual function after modified radical hysterectomy (Piver II/Type B) vs. classic radical hysterectomy (Piver III/Type C2) for early stage cervical cancer. A prospective study. J Sex Med. (2012) 9:909–17. doi: 10.1111/j.1743-6109.2011.02581.x
38. Yang YC, Chang CL. Modified radical hysterectomy for early Ib cervical cancer. Gynecol Oncol. (1999) 74:241–4. doi: 10.1006/gyno.1999.5434
Keywords: cervical cancer, modified radical hysterectomy, early stage, quality of life, security
Citation: Sun H, Cao D, Shen K, Yang J, Xiang Y, Feng F, Wu L, Zhang Z, Ling B and Song L (2018) Piver Type II vs. Type III Hysterectomy in the Treatment of Early-Stage Cervical Cancer: Midterm Follow-up Results of a Randomized Controlled Trial. Front. Oncol. 8:568. doi: 10.3389/fonc.2018.00568
Received: 30 August 2018; Accepted: 13 November 2018;
Published: 28 November 2018.
Edited by:
Rebecca Stone, Johns Hopkins Medicine, United StatesReviewed by:
Stephanie Adele Sullivan, VCU Medical Center, United StatesSuzana Arenhart Pessini, Universidade Federal do Rio Grande do Sul (UFRGS), Brazil
Copyright © 2018 Sun, Cao, Shen, Yang, Xiang, Feng, Wu, Zhang, Ling and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongyan Cao, Y2FvZG9uZ3lhbjIwMDgwMDFAMTYzLmNvbQ==
Keng Shen, c2hlbmtlbmdAcHVtY2guY24=
 Hengzi Sun
Hengzi Sun Dongyan Cao
Dongyan Cao Keng Shen
Keng Shen Jiaxin Yang1
Jiaxin Yang1