- 1Department of Pathology, Children's Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Clinical Laboratory, Molecular Epidemiology Laboratory, Harbin Medical University Cancer Hospital, Harbin, China
- 3Department of Pathology, Anhui Provincial Children's Hospital, Hefei, China
- 4Department of Hematology, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, China
- 5Department of Pediatric Surgery, Guangzhou Institute of Pediatrics, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
Background: Neuroblastoma, a neuroendocrine tumor, stems from the developing sympathetic nervous system. Previous genome-wide association studies (GWASs) have discovered a number of neuroblastoma susceptibility genes in Caucasians including LIM domain only 1 (LMO1).
Objective: We conducted a three-center case-control study including 313 cases and 716 controls with the purpose to evaluate the association between five GWAS-identified LMO1 variants (rs110419 A>G, rs4758051 G>A, rs10840002 A>G, rs204938 A>G, and rs2168101 G>T) and neuroblastoma susceptibility in eastern Chinese children.
Methods: Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of the associations. False positive report possibility (FPRP) analysis was performed to check whether significant results were noteworthy.
Results: Significant associations with neuroblastoma risk were found for four (rs110419, rs4758051, rs10840002, and rs2168101) out of the five polymorphisms. Combined analysis demonstrated that carriers of 4–5 protective genotypes had a significantly decreased risk of neuroblastoma in comparison those with 0–3 protective genotypes (adjusted OR = 0.51, 95% CI = 0.39–0.68, P < 0.0001). Haplotype analysis of the five SNPs yield four significant haplotypes associated with neuroblastoma susceptibility.
Conclusion: In conclusion, we confirmed LMO1 polymorphisms may reduce neuroblastoma risk in eastern Chinese populations.
Introduction
Neuroblastoma is the most frequently diagnosed solid tumor outside of cranium in childhood, especially in the first year of life (1–3). A median age at diagnosis of this disease is about 17 months. Neuroblastoma is a type of neuroendocrine tumor, originating from the developing sympathetic nervous system. Its incidence rate varies worldwide, affecting ~8–14 individuals per million in Western countries (4) and 7.7 per million in China (5). Neuroblastoma is a group of heterogeneous diseases. Majority of tumors (65%) occur in the abdomen, followed by the neck, pelvis, and chest (2). The clinic outcomes are age-dependent, varying greatly from spontaneous regression of tumor in infants to unfavorable prognosis in older children even after intensive multimodality treatments. Neuroblastoma patients are traditionally categorized into low-, intermediate-, and high-risk groups. To more accurately group patients, the International Neuroblastoma Risk Group (INRG) classification has yield 16 risk groups, by integrating clinical and molecular characteristics, such as age at diagnosis, tumor stage, histopathology, as well as genetic signatures (6). Environmental factors for neuroblastoma are not well-established. Environmental exposures, such as exposures to drugs and hair dyes during maternity and pregnancy, may increase the risk of the disease, to a small extent (7).
Neuroblastoma is genetically heterogeneous (8). Lately, intensive research has led to dramatic discoveries of some causal heritable mutations in familial neuroblastoma (9). These hallmark advances include mutations in the anaplastic lymphoma kinase (ALK) receptor tyrosine kinase gene and inactivating mutations in Paired-like homeobox 2b (PHOX2B), a transcription factor essentially regulating neural crest development. However, it should be noted that familial subset only constitutes up to 10% of neuroblastoma cases. More studies are needed to further define the genetic basis of neuroblastoma, particularly for sporadic cases. Previous genome-wide association studies (GWASs) have discovered 9 neuroblastoma susceptibility genes in Caucasians, including CASC15, BARD1, LIN28B, LMO1, DUSP12, IL31RA, DDX4, HSD17B12, and HACE1 (10–12).
LIM domain only 1 (LMO1) at 11p15.4 belongs to LMO gene family, all members of which (LMO1-4) are involved in carcinogenesis. The name of LIM domain originated from the LIN-11, ISL-1, and MEC-3 proteins in Caenorhabditis elegans (13, 14). LMO encodes a cysteine-rich transcriptional regulator composed of two zinc finger LIM domains but little other sequence (10, 15). Gene amplification (duplication) was observed in 12.4% of 701 neuroblastoma patients with primary tumor (10). A number of neuroblastoma susceptibility loci have been identified in this gene by GWAS. LMO1 rs110419 A>G, rs4758051 G>A, rs10840002 A>G, and rs204938 A>G were identified in a GWAS with 2,251 neuroblastoma patients and 6,097 controls of European ancestry (10). The rs2168101 G>T located in the LMO1 super-enhancer was also reported to modify neuroblastoma susceptibility by the same research group (15). In order to investigate the association between these LMO1 polymorphisms and neuroblastoma in eastern Chinese children, we performed a multi-center study with 313 cases and 716 controls recruited from three regions of East China.
Methods and Materials
Study Population
We carried out a multi-center case control study with 313 cases and 762 controls by incorporating participants from Jiangsu (158 cases and 426 controls, Supplemental Table 1) and Anhui (119 cases and 264 controls, Supplemental Table 2) provinces, as well as Wenzhou area (36 cases and 72 controls, Supplemental Table 3) (16, 17). The detailed descriptions of participants were elaborated in previous studies. All cases had primary tumors that were histologically confirmed to be neuroblastoma. Age and sex-matched controls were separately collected in each participating center. Each participant or his/her guardian was obligated to sign informed consent before blood sample collection. Approvals of the study protocols were issued by individual Institutional Review Board of participating centers.
Polymorphism Selection and Genotyping
SNPs in the LMO1 gene were selected by literature review. In total, five SNPs in the LMO1 gene (rs110419, rs4758051, rs10840002, rs204938, rs2168101) were analyzed. The first four SNPs were identified in a GWAS with 2,251 neuroblastoma patients and 6,097 controls of European ancestry (10). The last one is a SNP located in the LMO1 super-enhancer, which was also reported to modify neuroblastoma susceptibility by the same research group (15).
Genomic DNAs were extracted from blood samples, quantified, and following standardized procedures (18–21). TaqMan assay was performed for SNP genotyping with allele-specific probes tagged with the fluorescent dyes VIC and FAM, respectively (22–24). All assays were run in 384-well plates on the ABI 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA). Quality control was applied as reported before (25–27).
Statistical Analysis
Departure from Hardy-Weinberg equilibrium (HWE) was examined for each SNP using a good-of-fit χ2 in control subjects. The two-sided χ2 test was used to compare the differences in the categorical variables between cases and controls, including age, gender, sites of origin, and genotype frequency. The strength of association of tested SNPs and neuroblastoma was assessed by conducting logistic regression analysis to calculate odds ratios (ORs) and 95% confidence intervals (CIs). We also carried out the false positive report probability (FPRP) analysis to check whether significant results are noteworthy as described previously (28). Briefly, three parameters were employed to determine FPRP values, prior probability π representing a true association between the SNP and a disease, statistical power, and P-value (29). A FPRP threshold of 0.2 was preset for this study. We chose P < 0.05 as a standard of statistical significance. All P-values were two-sided. Statistics were completed with STATA (version 11.0; Stata Corporation, College Station, TX).
Results
Population Characters
A total of 313 cases and 762 controls were recruited for this association study (Table 1). Prior to study, case and controls were subjected to frequency matching to ensure there was no significant difference in the age (P = 0.823) and gender (P = 0.610) between cases and controls. Neuroblastoma may stem from different sites of the body. Based on sites of origin, cases were divided into adrenal gland (68, 21.73%), retroperitoneal region (126, 40.26%), mediastinum (99, 31.63%), and other region (20, 6.39%) groups.
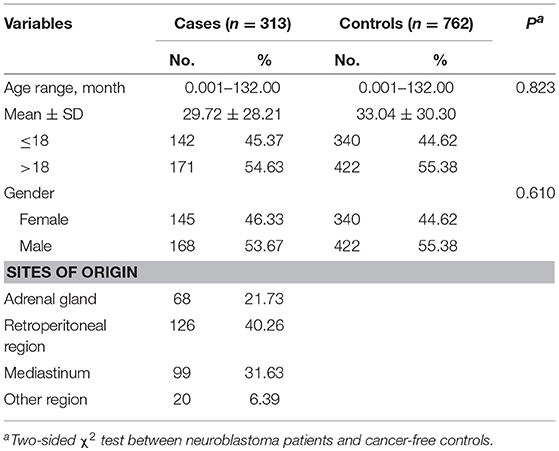
Table 1. Demographic characteristics of neuroblastoma patients and cancer-free controls for eastern Chinese children.
Association Between LMO1 Polymorphisms and Neuroblastoma Susceptibility
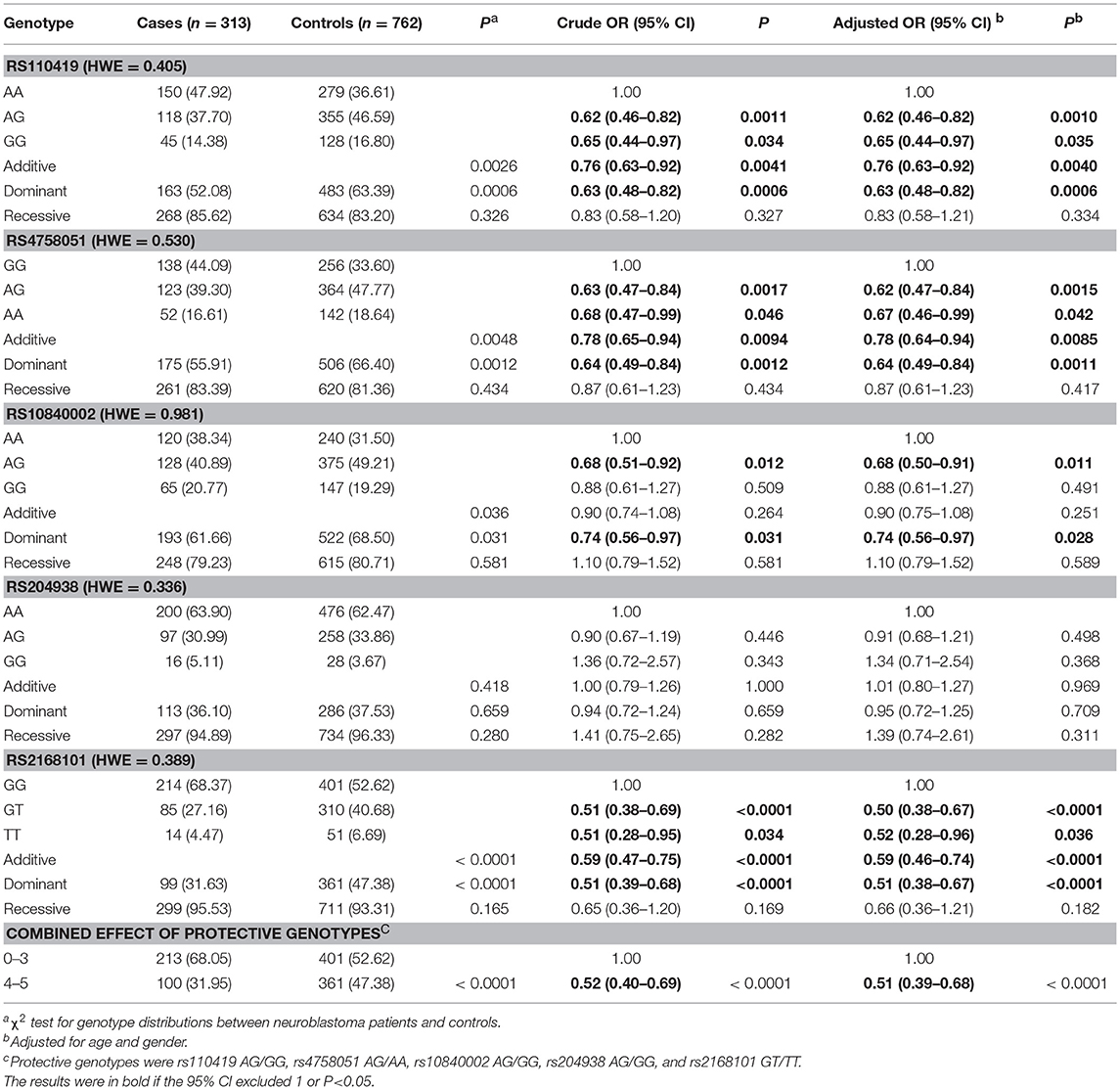
No deviation from HWE was found for any studied SNPs (P = 0.405 for rs110419, P = 0.530 for rs4758051, P = 0.981 for rs10840002, P = 0.336 for rs204938, P = 0.389 for rs2168101). Single locus analysis indicated that four out of the five polymorphisms were significantly associated with neuroblastoma risk (Table 2). First, LMO1 rs110419 was shown to significantly decrease the risk of neuroblastoma (heterogeneous: adjusted OR = 0.62, 95% CI = 0.46–0.82; homogenous: adjusted OR = 0.65, 95% CI = 0.44–0.97; additive: adjusted OR = 0.76, 95% CI = 0.63–0.92; dominant: adjusted OR = 0.63, 95% CI = 0.48–0.82). Second, a protective association with neuroblastoma susceptibility was also found for rs4758051 (heterogeneous: adjusted OR = 0.62, 95% CI = 0.47–0.84; homogenous: adjusted OR = 0.67, 95% CI = 0.46–0.99; additive: adjusted OR = 0.78, 95% CI = 0.64–0.94; dominant: adjusted OR = 0.64, 95% CI = 0.49–0.84). Third, the rs10840002 conferred decreased susceptibility to neuroblastoma (heterogeneous: adjusted OR = 0.68, 95% CI = 0.50–0.91; dominant: adjusted OR = 0.74, 95% CI = 0.56–0.97). Finally, we found that rs2168101 was significantly associated with a decreased neuroblastoma risk (heterogeneous: adjusted OR = 0.50, 95% CI = 0.38–0.67; homogenous: adjusted OR = 0.52, 95% CI = 0.28–0.96; additive: adjusted OR = 0.59, 95% CI = 0.46–0.74; dominant: adjusted OR = 0.51, 95% CI = 0.38–0.67). Genotypes with variant alleles were referred to as protective genotypes. While the five SNPs were combined, we found that carriers of 4–5 protective genotypes had a significantly decreased risk of neuroblastoma at an OR of 0.51 (95% CI = 0.39–0.68).
Stratified Analysis
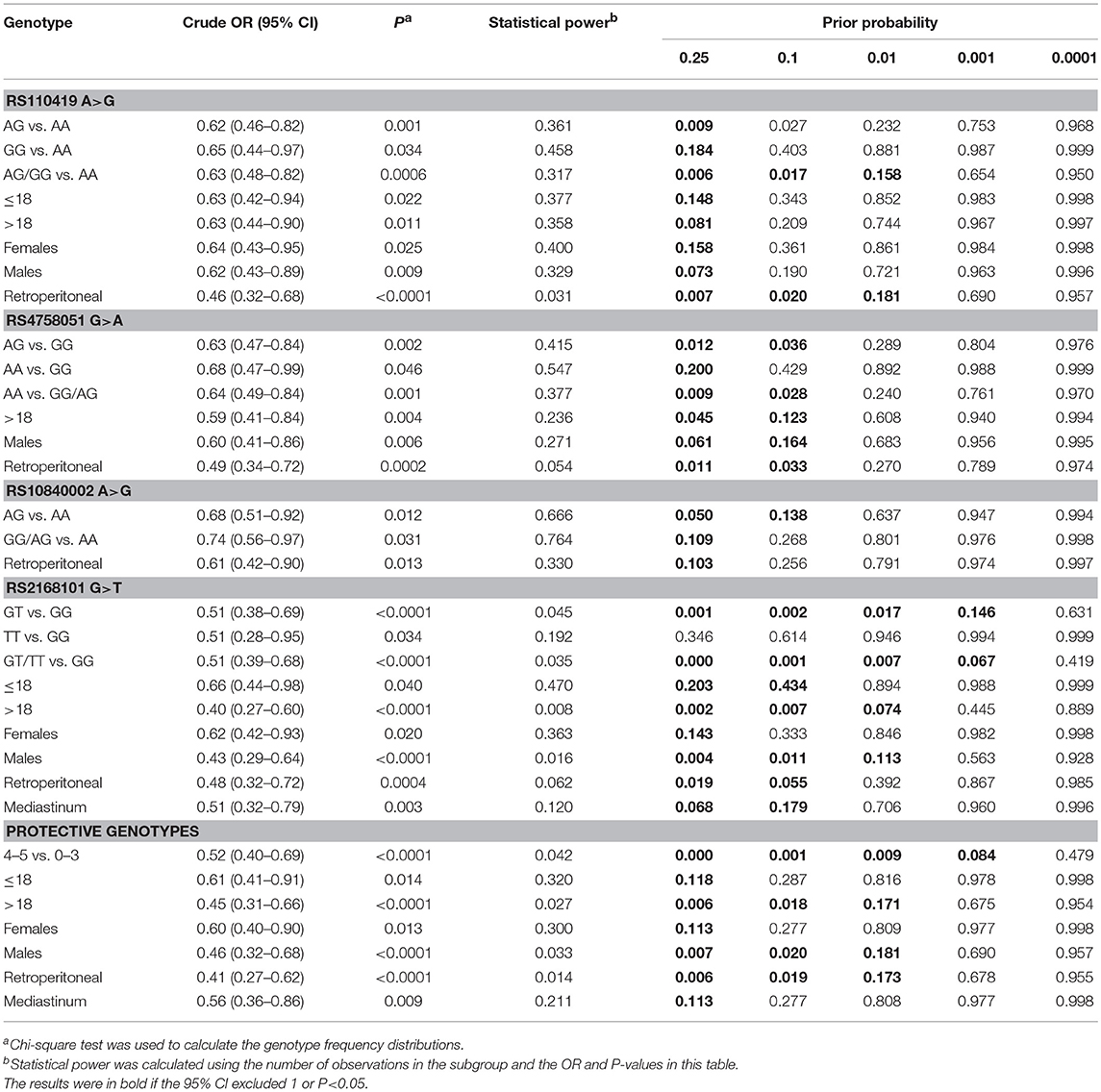
Significant SNPs were further subjected to stratified analysis by age, gender, and sites of origin (Table 3). Protective association between SNPs and neuroblastoma risk were observed in all age- and gender-groups for rs110419, rs2168101, and combined analysis, but only in children >18 and males for rs4758051. In term of sites of origin, all SNPs and combination were shown to decrease the risk of develop tumor in retroperitoneal region. Additionally, children carrying rs2168101 or 4–5 protective genotypes were also less likely develop tumor in mediastinum. For rs10840002, significant result only remained in the group of retroperitoneal region.
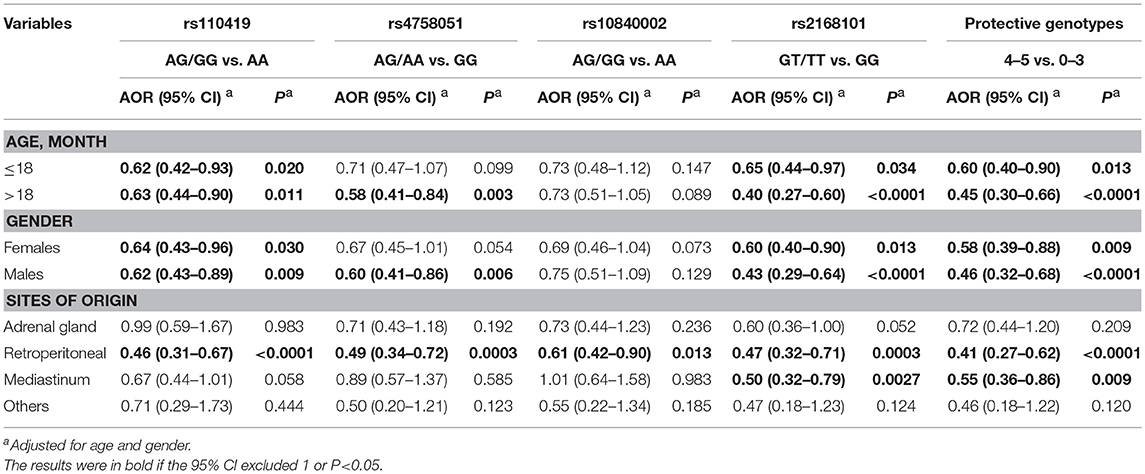
Table 3. Stratification analysis for the association between protective genotypes and neuroblastoma susceptibility.
FPRP Analysis
Results of association between genetic variants and diseases may be subjected to false positivity. Here, we performed FPRP analysis to interrogate our significant findings (Table 4). An FPRP noteworthiness value below 0.2 was prespecified for each association (29). When prior probability of 0.1 was adopted, significant association for rs110419 A>G (AG/GG vs. AA) remained noteworthy, so were results for retroperitoneal subgroup. All results for rs4758051 G>A were deserving of attention, with an exception of homogeneous model (AA vs. GG). Noteworthy results were also found for the rs10840002 A>G (AG vs. AA) and the rs2168101 G>T except for homogeneous model (TT vs. GG) and females. In the combined analysis, findings for 4–5 vs. 0–3 protective genotypes, children>18, males and retroperitoneal subgroup could be called noteworthy.
LMO1 Haplotypes and Neuroblastoma Risk
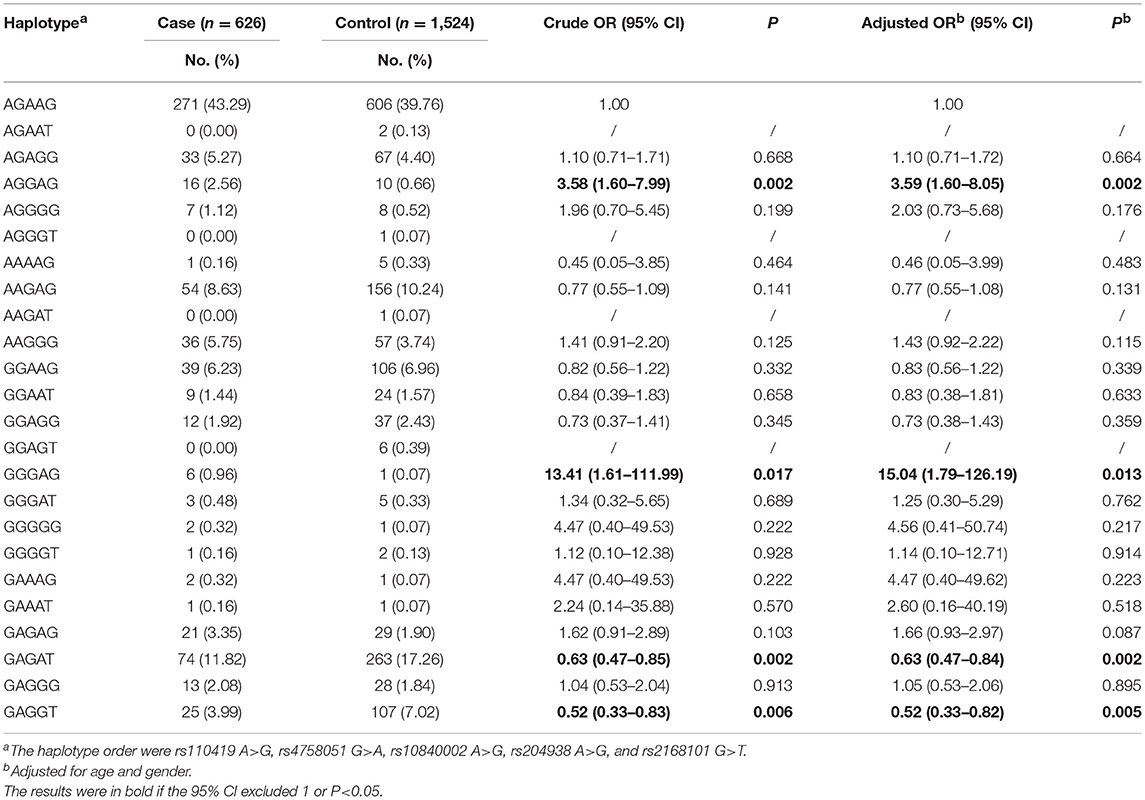
We next further explored whether the haplotypes of the five LMO1 SNPs would modify neuroblastoma risk. As presented in Table 5, 19 LMO1 haplotypes were available for analysis. When compared to reference haplotype AGAAG, four haplotypes, AGGAG, GGGAG, GAGAT, and GAGGT were significantly associated with altered neuroblastoma risk.
Discussion
The replication study is the golden method for the validation of the genetic associations. Therefore, we performed this multi-center case control study to examine the role of five GWAS-identified neuroblastoma susceptibility polymorphisms in Chinese children recruited from East China. We confirmed that four variants (rs110419, rs4758051, rs10840002, and rs2168101) in the LMO1 gene were associated with a decreased risk of neuroblastoma in our study population.
Functions of LMO family member remain to be clarified. These proteins consist of two LIM domains that can interact with a variety of proteins to regulate cancer-related cellular events including cell cycle, self-renewal, and metastasis (14). LMO1–4 are engaged in a broad spectrum of developmental processes, and found to be involved in the initiation or the progression of T cell leukemia, breast cancer as well as neuroblastoma, to date (30, 31). LIM domain is characterized as a form of zinc finger mediating protein-protein interacting, but not binding DNA sequences (13, 14). They may modulate transcriptional processes by nucleating and regulating transcription factor complexes (14).
LMO1 at 11p15.4 was identified as a neuroblastoma susceptibility locus in a previous GWAS (10). The association between LMO1 SNPs with neuroblastoma susceptibility was replicated in several independent cohorts from US, UK, and Italy (10). Clinical analysis indicated that the risk alleles of LMO1 (rs110419 A, rs4758051 G, rs10840002 A, rs204938 C) were in significant association with metastatic and high-risk neuroblastoma. Genotype and phenotype correlation analysis revealed that rs110419 A (risk allele) was significantly associated with elevated LMO1 expression. LMO1 somatic copy number gain also led to increased mRNA and protein expression accordingly. In vitro study further showed that loss- or gain-of-function of LMO1 suppressed and promoted proliferation of neuroblastoma cell lines, respectively (10). The rs2168101 polymorphism is positioned in a super-enhancer of LMO1. The G allele is a risk allele that constitutes a conserved GATA box to facilitate transcription factor binding (e.g., GATA3). The variant A allele eradicates GATA3 binding site and associates with a decrease in the LMO1 expression levels in neuroblastoma primary tumor. These biological findings are consistent with the protective association of with neuroblastoma susceptibility (15).
A recent study reported that transgenic expression of LMO1 alone failed to induce tumorigenesis in a zebra fish model, but acted as a synergist of MYCN, an oncogene amplified in ~20% of neuroblastoma (32). Eighty percent of transgenic fish with overexpression of both LMO1 and MYCN developed tumors by 24 weeks of age in comparison with 20–30% of the fish expressing MYCN alone. LMO1 cooperated with MYCN to induce abnormal proliferation of sympathoadrenal cells in the inter-renal gland and enhance metastasis. Moreover, LMO1 was found to confer human neuroblastoma cells invasive and migratory attributes. RNA sequencing (RNA-seq) and gene set enrichment analysis (GSEA) indicated that matrisome, extracellular matrix-associated proteins, integrins coding signature genes were enriched in the neuroblastoma cell lines expression high levels of LMO1. Later on, a mechanistic study using chromatin immunoprecipiation and DNA sequencing demonstrated that in neuroblastoma, LMO1 could affect LIMSI (LIM and senescent cell antigen-like domains 1), Ras suppressor protein 1 (RSU1), and relaxin 2 (RLN2) gene, and regulate a carcinogenic LIMSI/integin-linked kinase pathway (33).
Since LMO1 was established as a neuroblastoma risk gene, the association between LMO1 SNPs and neuroblastoma susceptibility has been replicated in several different ethnic groups (34–39). The first four LMO1 SNPs were tested for association in 390 African-American neuroblastoma patients and 2,500 controls; however, no significant association was observed (34). Moreover, the two most significant GWAS-identified SNPs in LMO1 (intronic rs110419 and intergenic rs4758051) were further replication in 370 cases and 809 controls from Italy and significant association was detected for rs110419 (35). Lu et al examine 11 LMO1 SNPs including rs110419 and rs204938 in a study population of 244 neuroblastoma patients and 305 healthy controls from North China but no significant association were found for these two SNPs (36). LMO1 rs110419 A>G, rs4758051 G>A, rs10840002 A>G, and rs204938 A>G) were analyzed in 256 neuroblastoma cases and 531 controls accrued from South China. Only rs110419 was associated with a decreased neuroblastoma risk (37). Replication study with 118 cases and 281 controls from North China reported negative association of neuroblastoma risk with rs4758051 and rs10840002. Moreover, the association between the LMO1 super-enhancer polymorphism rs2168101 and a decreased neuroblastoma risk was validated in the southern and northern Chinese population alone as well as in the combined case series (39).
As shown in literature review and our results above, association results between the same variant and disease varies among different ethnicities, even among different China Han groups from different geographical regions. Several reasons may affect association results and cause discrepancies, including relative modest effects of the SNPs, limited sample sizes of case control studies, different genetic backgrounds among different ethnicities, as well as different environmental exposure and life styles among different geographical regions. Therefore, our findings should be explained cautiously, and cannot be extrapolated to other populations prior to validation studies.
Limitations of this study should be discussed. First, although we included samples from three independent medical centers, sample size in this study is still relative small due to low incidence of this disease. Second, due to the retrospective nature of the study, many precious environmental factors that participants exposed to were not available for further analysis. Finally, functional analysis should be performed to investigate underlying biological mechanisms of LMO1 SNPs' protective effects on neuroblastoma.
In conclusion, we found that four LMO1 SNPs were associated with a decreased neuroblastoma risk in eastern China populations. Our findings warrant further validation in large well-designed studies.
Author Contributions
JH and HW conceived and designed the study. FH, YT, CZ, JD, YW, and HZ collected the samples and information. LH, JZ, and JH performed the experiment, analyzed the data and prepared the tables, and wrote the manuscript. All authors reviewed the manuscript. In addition, all authors have read and approved the manuscript.
Funding
This study was funded by grants from the Pearl River S&T Nova Program of Guangzhou (No: 201710010086).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00468/full#supplementary-material
References
1. Matthay K, Maris J, Schleiermacher G, Nakagawara A, Mackall C, Diller L, et al. Neuroblastoma. Nat Rev Dis Primers (2016) 2:16078. doi: 10.1038/nrdp.2016.78
2. Maris J, Hogarty M, Bagatell R. Neuroblastoma. Lancet (2007) 369:2106–20. doi: 10.1016/S0140-6736(07)60983-0
3. Bernstein M, Leclerc J, Bunin G, Brisson L, Robison L, Shuster J, et al. A population-based study of neuroblastoma incidence, survival, and mortality in North America. J Clin Oncol. (1992) 10:323–9. doi: 10.1200/JCO.1992.10.2.323
4. Spix C, Pastore G, Sankila R, Stiller C, Steliarova-Foucher E. Neuroblastoma incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur Cancer J. (2006) 42:2081–91. doi: 10.1016/j.ejca.2006.05.008
5. Bao P, Li K, Wu C, Huang Z, Wang C, Xiang Y, et al. [Recent incidences and trends of childhood malignant solid tumors in Shanghai, 2002-2010]. Zhonghua Er Ke Za Zhi (2013) 51:288–94. doi: 10.3760/cma.j.issn.0578-1310.2013.04.010
6. Cohn S, Pearson A, London W, Monclair T, Ambros P, Brodeur G, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol. (2009) 27:289–97. doi: 10.1200/JCO.2008.16.6785
7. De Roos A, Teschke K, Savitz D, Poole C, Grufferman S, Pollock B, et al. Parental occupational exposures to electromagnetic fields and radiation and the incidence of neuroblastoma in offspring. Epidemiology (2001) 12:508–17. doi: 10.1097/00001648-200109000-00008
8. Ritenour L, Randall M, Bosse K, Diskin S. Genetic susceptibility to neuroblastoma: current knowledge and future directions. Cell Tissue Res. (2018) 372:287–307. doi: 10.1007/s00441-018-2820-3
9. Deyell R, Attiyeh E. Advances in the understanding of constitutional and somatic genomic alterations in neuroblastoma. Cancer Genet. (2011) 204:113–21. doi: 10.1016/j.cancergen.2011.03.001
10. Wang K, Diskin S, Zhang H, Attiyeh E, Winter C, Hou C, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature (2011) 469:216–20. doi: 10.1038/nature09609
11. Nguyen B, Diskin S, Capasso M, Wang K, Diamond M, Glessner J, et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. PLoS Genet. (2011) 7:e1002026. doi: 10.1371/journal.pgen.1002026
12. Capasso M, Devoto M, Hou C, Asgharzadeh S, Glessner J, Attiyeh E, et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. (2009) 41:718–23. doi: 10.1038/ng.374
13. Matthews J, Bhati M, Lehtomaki E, Mansfield R, Cubeddu L, Mackay J. It takes two to tango: the structure and function of LIM, RING, PHD and MYND domains. Curr Pharm Des. (2009) 15:3681–96. doi: 10.2174/138161209789271861
14. Bach I. The LIM domain: regulation by association. Mech Dev. (2000) 91:5–17. doi: 10.1016/S0925-4773(99)00314-7
15. Oldridge D, Wood A, Weichert-Leahey N, Crimmins I, Sussman R, Winter C, et al. Genetic predisposition to neuroblastoma mediated by a LMO1 super-enhancer polymorphism. Nature (2015) 528:418–21. doi: 10.1038/nature15540
16. Wang J, Zhuo Z, Chen M, Zhu J, Zhao J, Zhang J, et al. RAN/RANBP2 polymorphisms and neuroblastoma risk in Chinese children: a three-center case-control study. Aging (2018) 10:808–18. doi: 10.18632/aging.101429
17. Zhou H, Zhuo Z, Chen S, Zhao J, Mo Y, Zhang J, et al. Polymorphisms in MYCN gene and neuroblastoma risk in Chinese children: a 3-center case-control study. Cancer Manag Res. (2018) 10:1807–16. doi: 10.2147/CMAR.S168515
18. Zhu J, Jia W, Wu C, Fu W, Xia H, Liu G, et al. Base excision repair gene polymorphisms and wilms tumor susceptibility. Ebiomedicine (2018) 33:88–93. doi: 10.1016/j.ebiom.2018.06.018
19. He J, Wang F, Zhu J, Zhang Z, Zou Y, Zhang R, et al. The TP53 gene rs1042522 C>G polymorphism and neuroblastoma risk in Chinese children. Aging (2017) 9:852–9. doi: 10.18632/aging.101196
20. He J, Zou Y, Wang T, Zhang R, Yang T, Zhu J, et al. Genetic variations of GWAS-identified genes and neuroblastoma susceptibility: a replication study in Southern Chinese children. Transl Oncol. (2017) 10:936–41. doi: 10.1016/j.tranon.2017.09.008
21. He J, Zou Y, Liu X, Zhu J, Zhang J, Zhang R, et al. Association of common genetic variants in pre-micrornas and neuroblastoma susceptibility: a two-center study in Chinese children. Mol Ther Nucleic Acids (2018) 11:1–8. doi: 10.1016/j.omtn.2018.01.003
22. Chang J, Tian J, Yang Y, Zhong R, Li J, Zhai K, et al. A rare missense variant in TCF7L2 associates with colorectal cancer risk by interacting with a GWAS-identified regulatory variant in the MYC enhancer. Cancer Res. (2018) 78:5164–72. doi: 10.1158/0008-5472.CAN-18-0910
23. Li J, Chang J, Tian J, Ke J, Zhu Y, Yang Y, et al. A rare variant P507L in TPP1 interrupts TPP1-TIN2 interaction, influences telomere length, and confers colorectal cancer risk in Chinese population. Cancer Epidemiol Biomarkers Prev. (2018) 27:1029–35. doi: 10.1158/1055-9965.EPI-18-0099
24. Zou D, Lou J, Ke J, Mei S, Li J, Gong Y, et al. Integrative expression quantitative trait locus-based analysis of colorectal cancer identified a functional polymorphism regulating SLC22A5 expression. Eur J Cancer (2018) 93:1–9. doi: 10.1016/j.ejca.2018.01.065
25. Zhu J, Wang M, He J, Zhu M, Wang JC, Jin L, et al. Polymorphisms in the AKT1 and AKT2 genes and oesophageal squamous cell carcinoma risk in an Eastern Chinese population. J Cell Mol Med. (2016) 20:666–77. doi: 10.1111/jcmm.12750
26. Zhu J, Wang M, Zhu M, He J, Wang JC, Jin L, et al. Associations of PI3KR1 and mTOR polymorphisms with esophageal squamous cell carcinoma risk and gene-environment interactions in Eastern Chinese populations. Sci Rep. (2015) 5:8250. doi: 10.1038/srep08250
27. He J, Qiu LX, Wang MY, Hua RX, Zhang RX, Yu HP, et al. Polymorphisms in the XPG gene and risk of gastric cancer in Chinese populations. Hum Genet. (2012) 131:1235–44. doi: 10.1007/s00439-012-1152-8
28. He J, Wang MY, Qiu LX, Zhu ML, Shi TY, Zhou XY, et al. Genetic variations of mTORC1 genes and risk of gastric cancer in an Eastern Chinese population. Mol Carcinog. (2013) 52(Suppl. 1):E70–9. doi: 10.1002/mc.22013
29. Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. (2004) 96:434–42. doi: 10.1093/jnci/djh075
30. Matthews J, Lester K, Joseph S, Curtis D. LIM-domain-only proteins in cancer. Nat Rev Cancer (2013) 13:111–22. doi: 10.1038/nrc3418
31. Beuten J, Gelfond J, Piwkham D, Pollock B, Winick N, Collier A, et al. Candidate gene association analysis of acute lymphoblastic leukemia identifies new susceptibility locus at 11p15 (LMO1). Carcinogenesis (2011) 32:1349–53. doi: 10.1093/carcin/bgr091
32. Zhu S, Zhang X, Weichert-Leahey N, Dong Z, Zhang C, Lopez G, et al. LMO1 synergizes with MYCN to promote neuroblastoma initiation and metastasis. Cancer Cell (2017) 32:310–23.e5. doi: 10.1016/j.ccell.2017.08.002
33. Saeki N, Saito A, Sugaya Y, Amemiya M, Ono H, Komatsuzaki R, et al. Chromatin immunoprecipitation and DNA sequencing identified a LIMS1/ILK pathway regulated by LMO1 in neuroblastoma. Cancer Genomics Proteomics (2018) 15:165–74. doi: 10.21873/cgp.20074
34. Latorre V, Diskin S, Diamond M, Zhang H, Hakonarson H, Maris J, et al. Replication of neuroblastoma SNP association at the BARD1 locus in African-Americans. Cancer Epidemiol Biomarkers Prev. (2012) 21:658–63. doi: 10.1158/1055-9965.EPI-11-0830
35. Capasso M, Diskin S, Totaro F, Longo L, De Mariano M, Russo R, et al. Replication of GWAS-identified neuroblastoma risk loci strengthens the role of BARD1 and affirms the cumulative effect of genetic variations on disease susceptibility. Carcinogenesis (2013) 34:605–11. doi: 10.1093/carcin/bgs380
36. Lu J, Chu P, Wang H, Jin Y, Han S, Han W, et al. Candidate Gene Association Analysis of Neuroblastoma in Chinese Children Strengthens the Role of LMO1. PLoS ONE (2015) 10:e0127856. doi: 10.1371/journal.pone.0127856
37. He J, Zhong W, Zeng J, Zhu J, Zhang R, Wang F, et al. LMO1 gene polymorphisms contribute to decreased neuroblastoma susceptibility in a Southern Chinese population. Oncotarget (2016) 7:22770–8. doi: 10.18632/oncotarget.8178
38. Zhang J, Lin H, Wang J, He J, Zhang D, Qin P, et al. polymorphisms reduce neuroblastoma risk in Chinese children: a two-center case-control study. Oncotarget (2017) 8:65620–6. doi: 10.7150/jca.25973
Keywords: LMO1, polymorphism, neuroblastoma, susceptibility, eastern Chinese Han
Citation: He L, Zhu J, Han F, Tang Y, Zhou C, Dai J, Wang Y, Zhou H, He J and Wu H (2018) LMO1 Gene Polymorphisms Reduce Neuroblastoma Risk in Eastern Chinese Children: A Three-Center Case-Control Study. Front. Oncol. 8:468. doi: 10.3389/fonc.2018.00468
Received: 04 August 2018; Accepted: 04 October 2018;
Published: 23 October 2018.
Edited by:
Rimas J. Orentas, Seattle Children's Research Institute, United StatesReviewed by:
Lei Song, National Cancer Institute (NCI), United StatesHema Dave, Children's National Health System, United States
Copyright © 2018 He, Zhu, Han, Tang, Zhou, Dai, Wang, Zhou, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing He, aGVqaW5nMTk4Mzc0QGdtYWlsLmNvbQ==
Haiyan Wu, bmNod2h5QDE2My5jb20=
† These authors have contributed equally to this work
 Lili He1†
Lili He1† Jinhong Zhu
Jinhong Zhu Jing He
Jing He