
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 02 July 2018
Sec. Cancer Epidemiology and Prevention
Volume 8 - 2018 | https://doi.org/10.3389/fonc.2018.00250
This article is part of the Research Topic EBV-Associated Carcinomas: Presence, Role and Prevention Strategies View all 10 articles
 Hamda Al-Thawadi1†
Hamda Al-Thawadi1† Lina Ghabreau2,3†
Lina Ghabreau2,3† Tahar Aboulkassim4
Tahar Aboulkassim4 Amber Yasmeen4
Amber Yasmeen4 Semir Vranic1
Semir Vranic1 Gerald Batist4,5
Gerald Batist4,5 Ala-Eddin Al Moustafa1,3,5,6*
Ala-Eddin Al Moustafa1,3,5,6*
Epstein–Barr virus (EBV) has been recently shown to be co-present with high-risk human papillomaviruses (HPVs) in human cervical cancer; thus, these oncoviruses play an important role in the initiation and/or progression of this cancer. Accordingly, our group has recently viewed the presence and genotyping distribution of high-risk HPVs in cervical cancer in Syrian women; our data pointed out that HPVs are present in 42/44 samples (95%). Herein, we aim to explore the co-prevalence of EBV and high-risk HPVs in 44 cervical cancer tissues from Syrian women using polymerase chain reaction, immunohistochemistry, and tissue microarray analyses. We found that EBV and high-risk HPVs are co-present in 15/44 (34%) of the samples. However, none of the samples was exclusively EBV-positive. Additionally, we report that the co-expression of LMP1 and E6 genes of EBV and high-risk HPVs, respectively, is associated with poorly differentiated squamous cell carcinomas phenotype; this is accompanied by a strong and diffuse overexpression of Id-1 (93% positivity), which is an important regulator of cell invasion and metastasis. These data imply that EBV and HPVs are co-present in cervical cancer samples in the Middle East area including Syria and their co-presence is associated with a more aggressive cancer phenotype. Future investigations are needed to elucidate the exact role of EBV and HPVs cooperation in cervical carcinogenesis.
Cervical cancer is the fourth most common malignancy among women worldwide with approximately 528,000 new cases and 266,000 deaths each year estimated by the World Health Organization. Notably, most cervical cancer deaths (87%) occur in the developing countries. Currently, it is well known that the majority of cancer deaths are the result of metastasis, either directly due to tumor involvement of critical organs or indirectly due to therapeutic resistance and the inability of available therapy to control tumor progression (1). On the other hand, it is estimated that approximately 20% of human cancers could be linked to oncoviruses infection including Epstein–Barr virus (EBV) and high-risk human papillomaviruses (HPVs) especially types 16, 18, and 33 (2–4). EBV is a human gammaherpesvirus that infects more than 90% of the human adult population. Acute infection with EBV can cause infectious mononucleosis, and its latent state can lead to several types of human B-cell lymphomas and carcinomas, especially nasopharyngeal (5, 6).
Today, it is well established that high-risk of HPVs infections are important etiological factors in the development of human cervical cancer; as more than 96% of cervical cancers are positive for high-risk HPVs especially types 16, 18, 31, 33, and 35 worldwide including the Middle East region (3, 7). Moreover, accumulating evidence suggests that persistent infection with these viruses is necessary for cervical precursors to evolve into invasive carcinomas (8). Accordingly, we have explored the presence of high-risk HPVs in cervical cancer in Syrian women; our study revealed that 95% of our samples are positive for HPVs; more significantly, we noted that the most frequent high-risk HPV types in Syrian women are 33, 16, 18, 45, 52, 58, and 35, in descending order. Furthermore, the expression of E6 onco-protein of high-risk HPVs was found to be correlated with the overexpression of Id-1, which is a member of the inhibitor of DNA-binding (Id) proteins (9).
Id proteins constitute a family of highly preserved transcriptional controllers that play critical roles during normal development and in the maintenance of homeostasis in human tissue (10). The main biological properties of Id proteins are inhibition of differentiation and conservation of the self-renewal capability and multipotency of stem cells (11). Id proteins are overexpressed in several human carcinomas (11, 12). More specifically, Id-1 protein expression is directly involved in cancer initiation and/or progression in different types of human malignancies including cervical (9, 13–15). On the other hand, it has been pointed out that LMP1 onco-protein of EBV upregulates Id-1 expression in nasopharyngeal immortalized and cancer cells (16, 17); however, the association between EBV onco-proteins and Id-1 in human carcinomas, including cervical is not clear.
Earlier studies have indicated that EBV is frequently present in human cervical cancer tissues, suggesting EBV is associated with the development of cervical cancer (18). Moreover, it has been shown that the co-occurrence of EBV and high-risk HPVs in cervical tissues is more frequent in patients with high-grade squamous intraepithelial lesions in comparison with low-grade lesions (19). Thus, the presence of EBV in high-grade cervical lesions and cancer could suggest a possible cooperation between EBV and HPV in human cervical carcinogenesis; however, there are no studies regarding the co-presence of EBV and HPVs in the Middle East region.
Therefore, in this study, we evaluated the co-presence of these viruses and their association with Id-1 expression in cervical cancers in Syrian women. Our study pointed out that EBV and high-risk HPVs are co-present in 34% of our samples; more significantly, we noted that the co-incidence of these viruses is associated with poorly differentiated squamous cell carcinomas, which is accompanied with Id-1 overexpression.
Formalin fixed paraffin embedded blocks of cervical cancer were obtained from 44 Syrian patients with an average age of 57.25 years. Paraffin-embedded cervical tumor tissues were obtained from the Department of Pathology, Faculty of Medicine at the University of Aleppo, Syria. The specimens and data used in this study were approved by the Ethics Committee of the Faculty of Medicine of Aleppo University, Syria. Five micrograms of purified genomic DNA (Qiagen GmbH, Hilden, Germany), from each sample, was analyzed for EBV and HPV by polymerase chain reaction (PCR) using specific primers for LMP1 and EBNA1 as well as E6/E7 of HPV types 16, 18, 31, 33, 35, 45, 51, 52, and 58, while, primers for GAPDH gene were used as an internal control (Tables 1 and 2). This analysis was performed as previously described by our group (9, 20).
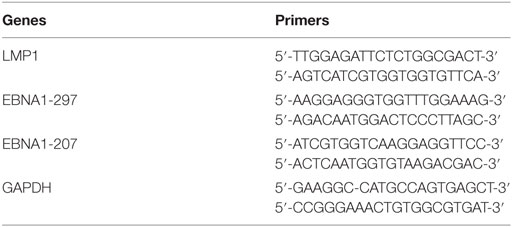
Table 1. The specific primer sets for LMP1 and EBNA1 genes of Epstein–Barr virus used for polymerase chain reaction (PCR) amplification.
The TMA construction was achieved as illustrated previously by our group (21, 22). Briefly, cervical cancer samples were embedded into a virgin paraffin TMA block using a manual tissue arrayer (Beecher Instruments, Silver Spring, MD, USA). Each block was assembled without previous knowledge of linked clinical or pathological staging information. Two TMA cores of 1.0 mm in diameter were sampled from a cohort of 44 block tissue samples of Syrian patients diagnosed with cervical carcinomas. Afterward, 4 µm sections were cut and stained with hematoxylin and eosin on the initial slides to verify the histological diagnosis. Next, slides of the completed blocks were used for immunohistochemistry (IHC) analysis.
Immunohistochemistry procedures examining the expression of LMP1, E6, and Id-1 were carried out using standard practices as follows. To analyze the protein expression patterns of LMP1, E6, and Id-1 in TMA slides, each one was deparaffinized in graded alcohol, rehydrated, and boiled (microwave) in 10 mM citrate buffer (pH 6.0) for antigen retrieval. Then, TMA slides were incubated for 35 min at 37°C with primary monoclonal and polyclonal antibodies for LMP1 of EBV and E6 of HPV as well as Id-1 (clone 1–4, clone C1P5, sc-488, from Dako and Calbiochem, Canada; as well as Santa Cruz Biotechnology, USA, respectively) using an automated immunostainer (Ventana Medical System, Tuscon, AZ, USA). The automated Ventana Medical System uses an indirect biotin–avidin system with a universal biotinylated immunoglobulin secondary antibody. Afterward, slides were counterstained with hematoxylin prior to mounting; staining procedures were completed according to the manufacturer’s recommendations. Negative controls were obtained by omitting specific primary antibody for LMP1 and E6 as well as specific blocking peptides from Santa Cruz Biotechnology and antibody for Id-1 protein. Following IHC, two independent observers examined all TMA slides. The tumors were considered positive for LMP1, E6, and Id-1 onco-proteins if cancer cells exhibited positivity ≥1%. In case of LMP1 protein expression (EBV), we also evaluated the presence of viral infection in tumor-infiltrating lymphocytes and stromal cells. All IHC assays were evaluated using the Olympus light microscope (BX53); the slides were evaluated under magnifications 2×, 4×, 10×, and 20×.
Statistical evaluations were done using IBM SPSS Statistics (version 22; SPSS Inc., Chicago, IL, USA) and R. Data were calculated as nonparametric files. We utilized χ2 test with Yates correction to assess the significance of the association between cancer aggressiveness, Id-1 expression, and the co-presence of EBV and high-risk HPVs. Analysis of variance (ANOVA) test was used to analyze the differences among the group means.
We have recently explored the presence of high-risk HPVs in a cohort of 44 cervical cancer samples from Syrian women. Our previous study revealed that 42 (95.45%) of the 44 samples are high-risk HPVs positive and all cases were infected with more than one HPV type. Moreover, these data revealed that the most prevalent high-risk HPV types are 33 (24+/44), 16 (21+/44), 18 (18+/44), 45 (17+/44), 52 (13+/44), 58 (11+/44), 35 (9+/44), 51 (7+/44), and 31 (5+/44) (9) [for methodology used for PCR assay, please refer to Ref. (6)]. Herein, we further investigated the co-presence of EBV and high-risk HPVs in our 44 samples by PCR and IHC analysis using specific primers for LMP1 and EBNA1 as well as E6/E7 genes of EBV and HPVs, respectively (Table 1; Figure S1 in Supplementary Material) and monoclonal antibodies for LMP1 and E6, as described in the Section “Materials and Methods.”
We found that 15 (34%) of the 44 samples are positive (≥1% positive cancer cells) for both EBV and high-risk HPVs (Table 2; Figures 1A–D). None of the cases was exclusively EBV positive while two cases were both HPV and EBV negative. In addition, we found no statistically significant association between the various HPV types and EBV co-infection in cervical cancer samples (p > 0.05).
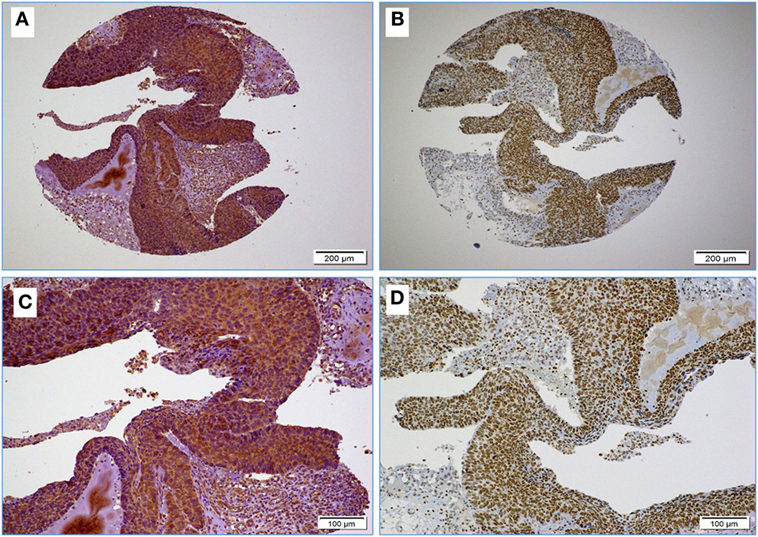
Figure 1. (A,B) Images reflect the diffused and strong cervical cancer cell positivity for high-risk HPV (E6 onco-protein) (A) and Epstein–Barr virus (EBV) (LMP1 protein) (B) (10× magnification); images (C,D) High-risk HPV and EBV positivity at higher magnification (D); as shown, EBV positivity is clear in some stromal cells and tumor infiltrating lymphocytes (arrows) (D) (20× magnification).
Next, we assessed the association between the co-presence of these viruses and tumor phenotype in our samples using TMA methodology. Our data indicate that the co-expression of the LMP1 and E6 onco-proteins of EBV and high-risk HPVs, respectively, is associated with poorly differentiated squamous cell carcinoma form (Figure 2) in comparison with HPVs positive cases alone as well as negative cases for both, EBV and HPVs (p < 0.0001, respectively). On the other hand, we noted that the expression of LMP1 is located in cervical squamous cell carcinomas and frequently in stromal cells in addition to tumor infiltrating lymphocytes (Figure 1D); however, E6 of HPV, in general, is detected in cancer cells while the stromal and inflammatory cells (tumor infiltrating lymphocytes) are consistently negative (Figures 1D and 2).
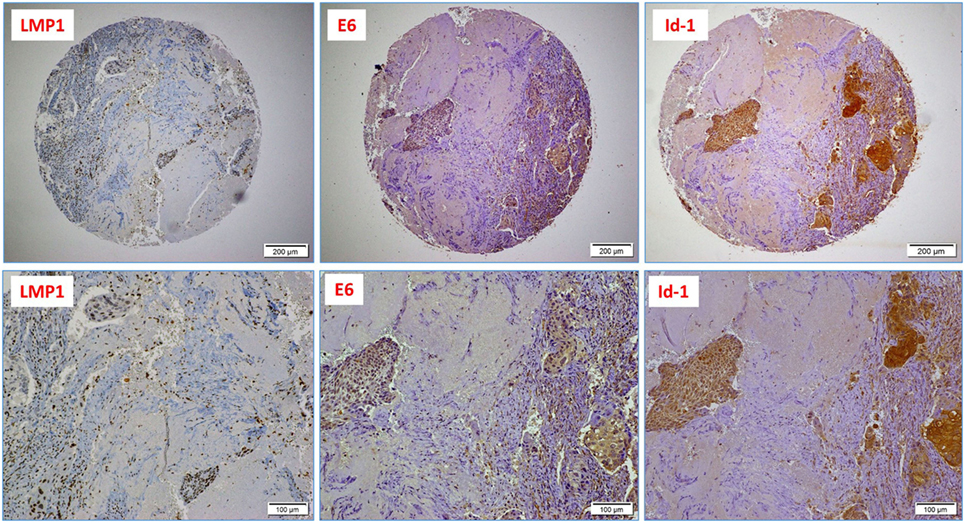
Figure 2. A case of poorly differentiated (high-grade, non-keratinizing) cervical carcinoma: upper images highlight the presence of Epstein–Barr virus (EBV) (LMP1 protein), high-risk HPV (E6 onco-protein), and a diffused Id-1 protein expression (10× magnification); lower images are respective high-power images (20× magnification); note the presence of EBV-positive tumor infiltrating lymphocytes (arrows).
Finally, we explored the association between the presence of EBV and HPVs with Id-1 overexpression in our Syrian samples by IHC. Using a 1% cutoff for positivity, Id-1 protein expression was observed in 41/44 cases (93%); diffuse and strong Id-1 expression (>50% cancer cells positive) was predominantly observed in high-grade (poorly differentiated) carcinomas (Figure 2). Moreover, we found that the co-expression of LMP1 and E6 (of EBV and HPV, respectively) is associated with diffuse and strong Id-1 overexpression in all invasive squamous cell carcinomas including high-grade carcinomas (p = 0.001) (Figure 2). In particular, the association between HPV (E6) and diffuse Id-1 (>50% cancer cells) was strong (p < 0.0001). ANOVA test for overall significance confirmed the observed differences between the subgroups (HPV+/EBV+ vs. HPV+ alone) and Id-1 status (p < 0.0001).
In this investigation, we explored, for the first time, the co-presence of EBV and high-risk HPVs in human cervical cancer and the role of this co-incidence with cancer phenotype in the conventional Middle East region. While, one study from North Africa pointed out that EBV and high-risk HPVs are co-present in 67.2% of cervical cancer cases in Algerian women (23). Herein, it is important to highlight that infection with, at least one high-risk HPV alone, is necessary but not sufficient to provoke cervical cancer initiation, additional oncovirus infection, and/or host genetic changes are required to drive neoplastic transformation and consequently lead to tumor formation (24, 25). In our investigation, we demonstrated that EBV is co-present with high-risk HPVs in 34% of cervical cancer cases in the Syrian population. Accordingly, a recent meta-analysis study of 25 investigations regarding the presence of EBV in human cervical cancer revealed that EBV is present in 43.63% of samples from cancer patients in comparison with 19% of samples from healthy people or patients with cervical intraepithelial neoplasia grade 1 (CIN) (27.34%) or CIN grade 2/3 (34.67%) (19). More significantly, co-infection with EBV and HPV is present in most of the cases, which display a similar phenotype of EBV infection (19); moreover, EBV infection is associated with differentiation (grade) of cervical epithelial cells (18). On the other hand, it has been pointed out that cervical carcinomas are four times more likely to occur among EBV-positive patients as compared with patients without EBV infection (19), which suggests a strong cooperation between EBV and HPVs in cervical carcinogenesis and possibly other human carcinomas (5). This concurs with our findings regarding the co-presence of EBV and high-risk HPVs and their association with cervical carcinomas in all positive cases, all of which are high-grade invasive cancers. Likewise, we have recently reported that EBV and high-risk HPVs are co-present in 32% of human breast cancer samples and their co-presence is associated with high-grade breast carcinomas and positive axillary lymph nodes (22).
On the other hand, it is important to highlight that EBV onco-proteins’ expression in cervical tissues is still controversial. Using in situ techniques for the detection of viral genomes or gene expressions, few investigations showed that EBV is present in cervical carcinoma cells (23, 26–28). However, others studies reported EBV localization in infiltrating lymphoid cells next to cervical carcinomas and concluded that EBV infection could not play a specific role in cervical carcinogenesis (29, 30). Interestingly, our study revealed that the expression of LMP1 protein is present in cervical squamous cell carcinomas and occasionally in the stroma as well as in tumor infiltrating lymphocytes; LMP1 is co-present with E6 onco-protein of high-risk HPVs in cervical carcinoma cells in most cases.
Concerning the association between the two oncoviruses (EBV and HPV) and Id-1 gene, which is overexpressed in several human carcinomas, it has been reported that LMP1 onco-protein of EBV upregulates the expression of Id-1 but not FoxO3a in human Hodgkin’s lymphoma cells (31). Likewise, in nasopharyngeal carcinoma, LMP1 induces an upregulation of Id-1 via FoxO3a inactivation (32). However, there are no studies regarding the EBV onco-proteins and Id-1 in human cervical cancer. In our present report, we demonstrate for the first time, the co-expression of LMP1 and E6 of EBV and high-risk HPVs, respectively, which is associated with Id-1 overexpression in human cervical cancer samples. However, herein, it is important to highlight that few investigations, including one from our lab, have pointed out that the presence of E6/E7 of high-risk HPVs is linked with Id-1 overexpression in human cervical cancer cells (9, 15, 33). More significantly, we have demonstrated that E6/E7 onco-proteins of HPV type 16 bind and active Id-1 promotor in human breast cancer cells; in parallel, we reported that Id-1 is the main regulator of cell invasion and metastasis induced by E6/E7 onco-proteins in these cancer cells (34). Accordingly, it is possible that EBV and high-risk HPV cooperate to upregulate the expression of Id-1 in human cervical cancer, which could enhance rapidly the progression of this cancer into invasive and metastatic form.
Nevertheless, further studies are necessary to clarify the role and pathogenesis of the co-presence of EBV and HPVs in human cervical carcinomas; especially since EBV and HPVs vaccines are presently under clinical trial and available, respectively (35–37). This is an important step, which could possibly limit cervical cancer initiation as well as its progression to a metastatic form, thereby decreasing cancer-related deaths especially in developing countries where cervical cancer is still the second major cause of death among women.
Finally, it is important to highlight that our investigation, in the Syrian population, is limited to a small number of cases located in a single region of Syria; therefore, it is essential to perform other studies of a larger number of cases from different regions in this country combined with several studies from the Middle East in general.
HA-T, SV, and AEA conceived the study. LG provided the samples and analyzed these data. HA-T, SV, TA, AY, GB, and AEA analyzed the data. All authors wrote and approved final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Mrs. A. Kassab for her critical reading of the manuscript. This work was supported by Qatar University grants # GCC-2017-002 QU/KU and QUCG-CMED-2018\2019-3.
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fonc.2018.00250/full#supplementary-material.
Figure S1. Representative polymerase chain reaction reactions for LMP1 of Epstein–Barr virus (EBV) in four cervical cancer samples. Chronic B leukemia cells were used as a positive control (PC); human normal cervical cells were utilized as negative control (NC).
1. Al Moustafa AE, Yasmeen A, Ghabreau L, Mohamed AH, Achkhar A. Brain metastases progression of breast cancer. In: Gunduz M, Gunduz E, editors. Breast Cancer – Carcinogenesis, Cell Growth and Signalling Pathways. InTech (2011). p. 87–108.
2. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol (2012) 13(6):607–15. doi:10.1016/S1470-2045(12)70137-7
3. Al Moustafa AE, Al-Awadhi R, Missaoui N, Adam I, Durusoy R, Ghabreau L, et al. Human papillomaviruses-related cancers. Presence and prevention strategies in the Middle east and north African regions. Hum Vaccin Immunother (2014) 10(7):1812–21. doi:10.4161/hv.28742
4. Al Moustafa AE, Farhan S, Cyprian F, Al-Antary N, Yasmeen A. High-risk human papillomaviruses and Epstein-Barr virus presence and crosstalk in human oral carcinogenesis, development oral cancer: risk factors & prevention strategies. In: Ala-Eddin, Al Moustafa, editors. Development of Oral Cancer. Cham: Springer International Publishing (2017). p. 83–94.
5. Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S, Al Moustafa AE. Epstein-Barr virus and human papillomaviruses interactions and their roles in the initiation of EMT and cancer progression: a brief review. Front Oncol (2018) 8:111. doi:10.3389/fonc.2018.00111
6. Elgui de Oliveira D, Muller-Coan BG, Pagano JS. Viral carcinogenesis beyond malignant transformation: EBV in the progression of human cancers. Trends Microbiol (2016) 24(8):649–64. doi:10.1016/j.tim.2016.03.008
7. Al Moustafa AE, Ghabreau L, Akil N, Rastam S, Alachkar A, Yasmeen A. High-risk HPVs and human carcinomas in the Syrian population. Front Oncol (2014) 4:68. doi:10.3389/fonc.2014.00068
8. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer (2007) 121(3):621–32. doi:10.1002/ijc.22527
9. Darnel AD, Wang D, Ghabreau L, Yasmeen A, Sami S, Akil N, et al. Correlation between the presence of high-risk human papillomaviruses and Id gene expression in Syrian women with cervical cancer. Clin Microbiol Infect (2010) 16(3):262–6. doi:10.1111/j.1469-0691.2009.02774.x
11. Ling F, Kang B, Sun XH. Id proteins: small molecules, mighty regulators. Curr Top Dev Biol (2014) 110:189–216. doi:10.1016/B978-0-12-405943-6.00005-1
12. Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci U S A (2007) 104(49):19506–11. doi:10.1073/pnas.0709185104
13. Castanon E, Soltermann A, Lopez I, Roman M, Ecay M, Collantes M, et al. The inhibitor of differentiation-1 (Id1) enables lung cancer liver colonization through activation of an EMT program in tumor cells and establishment of the pre-metastatic niche. Cancer Lett (2017) 402:43–51. doi:10.1016/j.canlet.2017.05.012
14. Gumireddy K, Li A, Kossenkov AV, Cai KQ, Liu Q, Yan J, et al. ID1 promotes breast cancer metastasis by S100A9 regulation. Mol Cancer Res (2014) 12(9):1334–43. doi:10.1158/1541-7786.MCR-14-0049
15. Schindl M, Oberhuber G, Obermair A, Schoppmann SF, Karner B, Birner P. Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res (2001) 61(15):5703–6.
16. Hau PM, Tsang CM, Yip YL, Huen MS, Tsao SW. Id1 interacts and stabilizes the Epstein-Barr virus latent membrane protein 1 (LMP1) in nasopharyngeal epithelial cells. PLoS One (2011) 6(6):e21176. doi:10.1371/journal.pone.0021176
17. Li HM, Man C, Jin Y, Deng W, Yip YL, Feng HC, et al. Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int J Cancer (2006) 119(7):1567–76. doi:10.1002/ijc.22032
18. Vranic S, Cyprian FS, Akhtar S, Al Moustafa AE. The role of Epstein-Barr virus (EBV) in cervical cancer: a brief update. Front Oncol (2018) 8:113. doi:10.3389/fonc.2018.00113
19. de Lima MAP, Neto PJN, Lima LPM, Goncalves Junior J, Teixeira Junior AG I, Teodoro PP, et al. Association between Epstein-Barr virus (EBV) and cervical carcinoma: a meta-analysis. Gynecol Oncol (2018) 148(2):317–28. doi:10.1016/j.ygyno.2017.10.005
20. Aboulkassim T, Yasmeen A, Akil N, Batist G, Al Moustafa AE. Incidence of Epstein-Barr virus in Syrian women with breast cancer: a tissue microarray study. Hum Vaccin Immunother (2015) 11(4):951–5. doi:10.1080/21645515.2015.1009342
21. Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer (2008) 99(3):404–7. doi:10.1038/sj.bjc.6604503
22. Al Moustafa AE, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother (2016) 12(7):1936–9. doi:10.1080/21645515.2016.1139255
23. Khenchouche A, Sadouki N, Boudriche A, Houali K, Graba A, Ooka T, et al. Human papillomavirus and Epstein-Barr virus co-infection in cervical carcinoma in Algerian women. Virol J (2013) 10:340. doi:10.1186/1743-422X-10-340
24. Al Moustafa AE, Foulkes WD, Benlimame N, Wong A, Yen L, Bergeron J, et al. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene (2004) 23(2):350–8. doi:10.1038/sj.onc.1207148
25. Al Moustafa AE, Kassab A, Darnel A, Yasmeen A. High-risk HPV/ErbB-2 interaction on E-cadherin/catenin regulation in human carcinogenesis. Curr Pharm Des (2008) 14(22):2159–72. doi:10.2174/138161208785740216
26. Kim NR, Lin Z, Kim KR, Cho HY, Kim I. Epstein-Barr virus and p16INK4A methylation in squamous cell carcinoma and precancerous lesions of the cervix uteri. J Korean Med Sci (2005) 20(4):636–42. doi:10.3346/jkms.2005.20.4.636
27. Santos NB, Villanova FE, Andrade PM, Ribalta J, Focchi J, Otsuka AY, et al. Epstein-Barr virus detection in invasive and pre-invasive lesions of the uterine cervix. Oncol Rep (2009) 21(2):403–5. doi:10.3892/or_00000236
28. Abudoukadeer A, Niyazi M, Aikula A, Kamilijian M, Sulaiman X, Mutailipu A, et al. Association of EBV and HPV co-infection with the development of cervical cancer in ethnic Uyghur women. Eur J Gynaecol Oncol (2015) 36(5):546–50.
29. Aromseree S, Pientong C, Swangphon P, Chaiwongkot A, Patarapadungkit N, Kleebkaow P, et al. Possible contributing role of Epstein-Barr virus (EBV) as a cofactor in human papillomavirus (HPV)-associated cervical carcinogenesis. J Clin Virol (2015) 73:70–6. doi:10.1016/j.jcv.2015.10.015
30. Shoji Y, Saegusa M, Takano Y, Hashimura M, Okayasu I. Detection of the Epstein-Barr virus genome in cervical neoplasia is closely related to the degree of infiltrating lymphoid cells: a polymerase chain reaction and in situ hybridization approach. Pathol Int (1997) 47(8):507–11. doi:10.1111/j.1440-1827.1997.tb04532.x
31. Ikeda JI, Wada N, Nojima S, Tahara S, Tsuruta Y, Oya K, et al. ID1 upregulation and FoxO3a downregulation by Epstein-Barr virus-encoded LMP1 in Hodgkin’s lymphoma. Mol Clin Oncol (2016) 5(5):562–6. doi:10.3892/mco.2016.1012
32. Lo AK, Dawson CW, Lo KW, Yu Y, Young LS. Upregulation of Id1 by Epstein-Barr virus-encoded LMP1 confers resistance to TGFbeta-mediated growth inhibition. Mol Cancer (2010) 9:155. doi:10.1186/1476-4598-9-155
33. Prates J, Franco-Salla GB, Dinarte Dos Santos AR, da Silva WA Jr, da Cunha BR, Tajara EH, et al. ANXA1Ac(2)(-)(2)(6) peptide reduces ID1 expression in cervical carcinoma cultures. Gene (2015) 570(2):248–54. doi:10.1016/j.gene.2015.06.021
34. Yasmeen A, Bismar TA, Kandouz M, Foulkes WD, Desprez PY, Al Moustafa AE. E6/E7 of HPV type 16 promotes cell invasion and metastasis of human breast cancer cells. Cell Cycle (2007) 6(16):2038–42. doi:10.4161/cc.6.16.4555
35. Rajcani J, Banati F, Szenthe K, Szathmary S. The potential of currently unavailable herpes virus vaccines. Expert Rev Vaccines (2018) 17(3):239–48. doi:10.1080/14760584.2018.1425620
36. Luxembourg A, Moeller E. 9-Valent human papillomavirus vaccine: a review of the clinical development program. Expert Rev Vaccines (2017) 16(11):1119–39. doi:10.1080/14760584.2017.1383158
Keywords: Epstein–Barr virus, high-risk human papillomaviruses, cervical cancer, Syrian women, cancer phenotype
Citation: Al-Thawadi H, Ghabreau L, Aboulkassim T, Yasmeen A, Vranic S, Batist G and Al Moustafa A-E (2018) Co-Incidence of Epstein–Barr Virus and High-Risk Human Papillomaviruses in Cervical Cancer of Syrian Women. Front. Oncol. 8:250. doi: 10.3389/fonc.2018.00250
Received: 11 April 2018; Accepted: 19 June 2018;
Published: 02 July 2018
Edited by:
Ali A. Sultan, Weill Cornell Medicine, QatarReviewed by:
Kin Ming (Clement) Tsui, Sidra Medical and Research Center, QatarCopyright: © 2018 Al-Thawadi, Ghabreau, Aboulkassim, Yasmeen, Vranic, Batist and Al Moustafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ala-Eddin Al Moustafa, aalmoustafa@qu.edu.qa, ala-eddin.almoustafa@mcgill.ca
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.