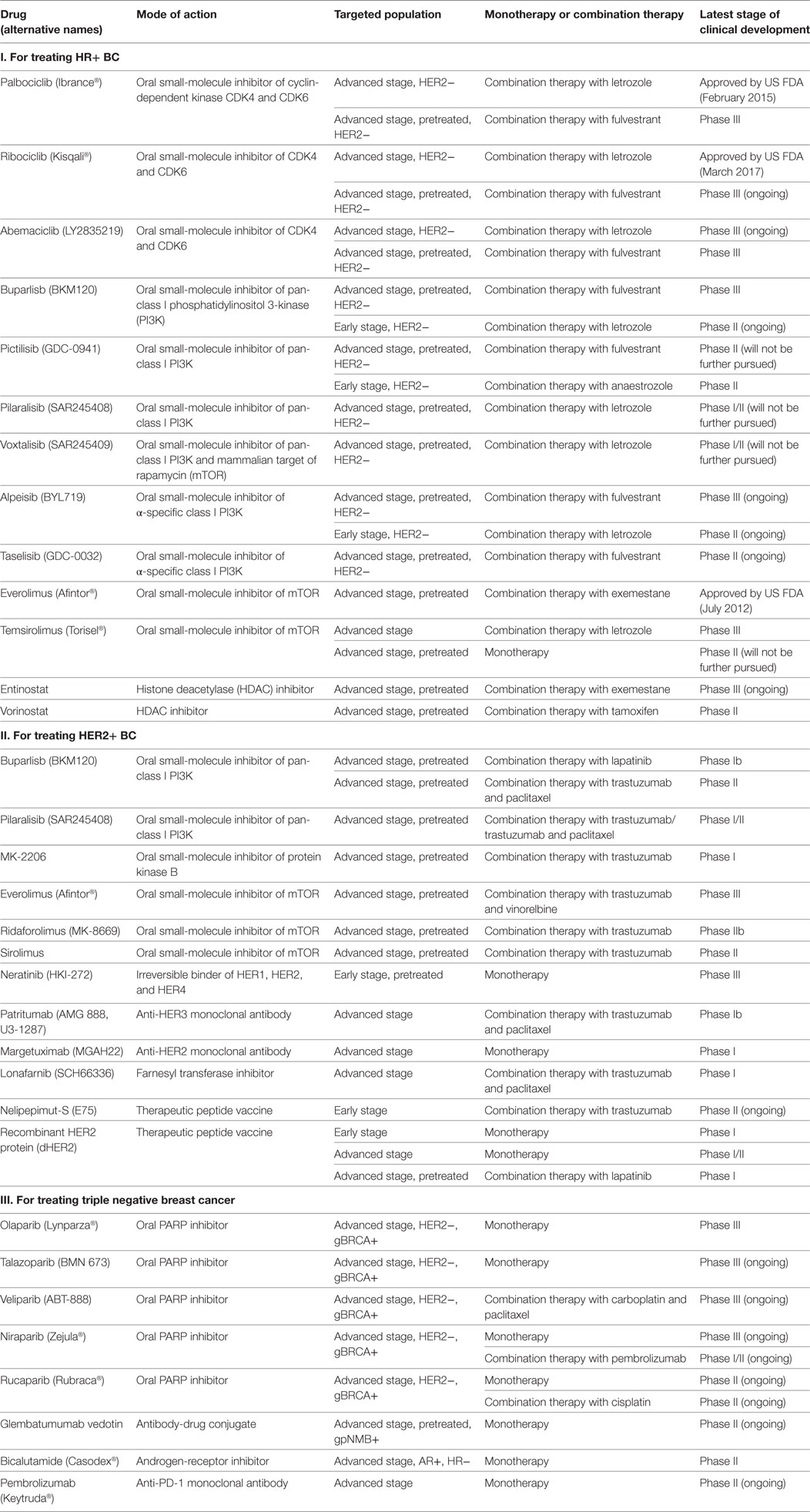
- 1Faculty of Medicine, School of Pharmacy, The Chinese University of Hong Kong, Hong Kong, Hong Kong
- 2Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong, Hong Kong
Breast cancer (BC) is the most common malignancy in women. It is classified into a few major molecular subtypes according to hormone and growth factor receptor expression. Over the past few years, substantial advances have been made in the discovery of new drugs for treating BC. Improved understanding of the biologic heterogeneity of BC has allowed the development of more effective and individualized approach to treatment. In this review, we provide an update about the current treatment strategy and discuss the various emerging novel therapies for the major molecular subtypes of BC. A brief account of the clinical development of inhibitors of poly(ADP-ribose) polymerase, cyclin-dependent kinases 4 and 6, phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin pathway, histone deacetylation, multi-targeting tyrosine kinases, and immune checkpoints for personalized treatment of BC is included. However, no targeted drug has been approved for the most aggressive subtype—triple negative breast cancer (TNBC). Thus, we discuss the heterogeneity of TNBC and how molecular subtyping of TNBC may help drug discovery for this deadly disease. The emergence of drug resistance also poses threat to the successful development of targeted therapy in various molecular subtypes of BC. New clinical trials should incorporate advanced methods to identify changes induced by drug treatment, which may be associated with the upregulation of compensatory signaling pathways in drug resistant cancer cells.
Introduction
Breast cancer (BC) is the most commonly diagnosed and the second leading cause of cancer-related deaths among women worldwide (1). One of the major challenges for its treatment is its heterogeneous nature, which determines the therapeutic options (2). By evaluating a few biomarkers, including the presence of hormone receptors (HRs), excess levels of human epidermal growth factor receptor 2 (HER2) protein, and/or extra copies of the HER2 gene (3, 4), BC is classified into four major molecular subtypes: (i) luminal A (HR+/HER2−); (ii) HER2+; (iii) luminal B (HR+/HER2+); and (iv) triple negative (TNBC; HR−/HER2−; also overlap with the basal-like subtype). Each of these subtypes has different risk factors for incidence, therapeutic response, disease progression, and preferential organ sites of metastases.
Luminal BC is positive for HR [estrogen receptor (ER) and progesterone receptor (PR)]. It is subdivided into two subgroups (A and B). Luminal A subgroup (HR+/HER2−) is usually slow-growing and less aggressive than other subtypes. They are more responsive to hormonal interventions (5). Luminal B subgroup (HR+/HER2+) is further defined by its high expression of Ki67 (a proliferation marker) or HER2. Luminal B usually has a poorer prognosis than luminal A (5). HER2+ BC has overexpression or amplification of the HER2/ERBB2 oncogene and may be treated with anti-HER2 therapies. Basal-like BC lacks HR and HER2, so they are also known as triple negative breast cancer (TNBC). Most BC patients (84%) have HR+ diseases, which includes 71% from HR+/HER− (luminal A) and 12% from HR+/HER2+ (luminal B). Only 5% of BC patients are HER2+ but HR−. TNBC makes up the remaining 12% of the total patient population (6).
Current Treatment Regimens and Novel Therapies for Different Subtypes of BC
Luminal BC (HR+ BC)
Current Treatment Regimens
Luminal BC, which is also hormone receptor positive (HR+), represents the vast majority (60–80%) of BC cases in developed countries (6) and this patient population is increasing in premenopausal women (7, 8).
For HR+ BC, endocrine therapy is the mainstay for treatment, which works by blocking the effects of hormone or lowering the hormone level. Currently available drugs include (i) tamoxifen, a prodrug that blocks estrogen uptake by the ER; (ii) aromatase inhibitors (letrozole, anastrozole, and exemestane), which suppress the conversion of androgens to estrogens, thus resulting in estrogen depletion; (iii) luteinizing hormone-releasing hormone analogs (goserelin and leuprolide), which suppress the production of hormone from the ovary; and (iv) fulvestrant (a selective ER degrader), which is suitable for BC patients refractory to previous hormonal therapy. Sequential administration of endocrine treatments are recommended until there is a need for rapid response or evidence of clinical resistance, when chemotherapy will be indicated (9).
Since endocrine drugs work by different mechanisms, they are generally used in combination for better anticancer efficacy. However, conflicting results have been reported (10–12). It is generally believed that patients with endocrine therapy-naïve advanced BC and those with highly endocrine-sensitive tumors may benefit the most from combination endocrine therapy (13).
Novel Therapies
Metastatic HR+ BC may develop resistance to standard hormonal therapies, which was mediated by genomic alterations in the ER and/or upregulation of other signaling pathways. Therefore, the development of new agents has aimed at reversing resistance to hormonal therapies (Table 1).
Cyclin-Dependent Kinases 4 and 6 (CDK4/6) Inhibitors
Among the emerging therapies, CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) have attracted the most attention. CDK4/6 regulate cell cycle progression by their reversible interaction with cyclin D1. Approximately 29 and 14% of patients with HR+/HER2− BC were found to have amplification of cyclin D1 and CDK4, respectively. Importantly, even when hormonal resistance developed, the tumors still depend on CDK4/6-cyclin D1 for proliferation (14). Therefore, more pronounced G1-S cell cycle arrest was observed in HR+/HER2− BC after treatment with combination of hormonal therapy and CDK4/6 inhibitor (15). CDK4/6 inhibitors work by blocking the phosphorylation of retinoblastoma protein, thereby downregulating E2F-response genes to mediate G1-S arrest. They also dephosphorylate the transcription factor Forkhead box protein M1 to inhibit cell proliferation (15).
Palbociclib and ribociclib have received FDA approval for combination with aromatase inhibitor as first-line treatment of HR+/HER2− advanced BC. They were shown to significantly improve median PFS by 10 months (16) and PFS rate by 20% after 18 months (17), respectively, compared to letrozole alone. On the other hand, abemaciclib is still under phase III investigation (NCT02246621). As second-line treatment in combination with fulvestrant in HR+/HER2− advanced BC, palbociclib and abemaciclib were demonstrated to significantly prolong median PFS by 5 months (18) and 7 months (19), respectively, compared to fulvestrant alone. Ribociclib is in phase III investigation (NCT02422615). Although all three CDK4/6 inhibitors worked through similar mechanism, abemaciclib exhibited a higher monotherapy response rate and induced less neutropenia, which may be related to its superior CDK4 inhibition (20).
Inhibitors Targeting Phosphatidylinositol 3-Kinase (PI3K)/Protein Kinase B (Akt)/Mammalian Target of Rapamycin (mTOR) Pathway
Aberrant activation of the PI3K–Akt–mTOR signaling pathway is known to contribute to hormonal resistance (21). This pathway is activated in over 70% BC, with the PI3K catalytic subunit p110α (PIK3CA) being one of the most frequently mutated and/or amplified genes (22). Combination therapies targeting both HR and PI3K/Akt/mTOR pathways have been evaluated to reverse hormone resistance (21).
PI3K Inhibitors. The combination of PI3K inhibitors with aromatase inhibitor has been used as second-line treatment for HR+/HER− advanced BC. While buparlisib (a pan-class I PI3K inhibitor) has been shown to significantly improve PFS, especially in those who also have PIK3CA mutation, buparlisib (23), pictillisib (24), pilaralisib (25), and voxtalisib (also an mTOR inhibitor) (25) did not give rise to significant clinical benefit due to high toxicities. The more selective and less toxic α-specific PI3K inhibitors (alpeisib and taselisib), currently in phase III trials (NCT02437318 and NCT02340221), were found to exhibit promising efficacy, particularly in BC patients who had PIK3CA mutation (26, 27).
As neoadjuvant treatment in combination with letrozole or anastrozole for HR+/HER2− early BC, both pictillisib (28) and taselisib (29) were found to enhance antitumor effects irrespective of PIK3CA status. Buparlisb and alpelisib are under phase II investigation (NCT01923168).
mTOR Inhibitors. Everolimus has received US FDA approval for HR+ advanced BC in combination with exemestane after treatment failure with letrozole or anastrozole (30). However, temsirolimus failed to show any clinical benefits either as first-line treatment in combination with letrozole (31) or as second-line therapy as a single agent (32) in advanced HR+ BC.
Histone Deacetylase (HDAC) Inhibitors. Hormonal resistance is also caused by histone deacetylation-mediated loss of ER expression in ER+ patients (33). This may be reversed by HDAC inhibitors, which upregulated expression of ERα and aromatase and inhibited growth factor signaling pathways (34). As second-line treatment for HR+ advanced BC [phase III (NCT02115282)], both entinostat and vorinostat exhibited superior anticancer activity in combination with exemestane (35) and tamoxifen (36) respectively, compared to exemestane/tamoxifen alone.
Steroid Sulfatase Inhibitors. Steroid sulfatase is a key enzyme regulating the conversion of inactive sulfate-conjugated steroids to active and estrogenic non-conjugated forms (37). The expression level and enzyme activity of steroid sulfatase were found to be remarkably increased in ERα-positive BC (38). Thus, inhibition of steroid sulfatase represents a logical approach for reducing estrogenic steroids that may stimulate BC growth. A recent phase II trial showed that combination of irosustat (first-generation steroid sulfatase inhibitor) and aromatase inhibitor was well-tolerated and resulted in clinical benefit (39). Another novel dual-acting steroid sulfatase inhibitor (SR16157), which directly inhibits steroid sulfatase and releases a selective ERα modulator, has been evaluated in hormone-dependent BC (40).
HER2+ BC
Current Treatment Regimens
For HER2+ BC, several molecular targeted agents have been approved to be used alone or in combination with standard chemotherapy. They include (i) trastuzumab (anti-HER2 monoclonal antibody); (ii) pertuzumab (anti-HER2 monoclonal antibody with a different binding site on HER2 than trastuzumab); (iii) ado-trastuzumab emtansine, an antibody-cytotoxic agent conjugate consisting of trastuzmab linked with a small-molecule microtubule inhibitor (emtansine); and (iv) lapatinib, a dual tyrosine kinase inhibitor (TKI) that interrupts both HER2 and epidermal growth factor receptor (EGFR) pathways. BC patients are tested for HER2 gene amplification or protein overexpression to determine whether they would benefit from anti-HER2 therapy.
In early-stage HER2-positive BC, neoadjuvant treatment with a combination of chemotherapy and anti-HER2 targeted therapy is currently the standard regimen (41). This is followed by surgery, radiotherapy, and another 12-month HER2-targeted therapy. Endocrine adjuvant therapy may also be added depending on specific cancer biology. With the introduction of HER2-targeted therapies over the past 15 years, the median overall survival (OS) of patients with HER2+ advanced BC has increased substantially from approximately 20 months to currently about 5 years (42, 43).
Novel Therapies
The emergence of primary and acquired resistance to trastuzumab is severely limiting its clinical utility in HER2+ BC. Elucidation of the resistance mechanisms and discovery of targeted agents and immunotherapies have resulted in improved treatment outcomes (Table 1).
PI3K/Akt/mTOR Inhibitors
Combinations of PI3K/Akt/mTOR inhibitors with trastuzumab have been studied to overcome trastuzumab resistance mediated by aberrant activation of the pathway. Pan-class I PI3K inhibitors (buparlisib and pilaralisib), when combined with lapatinib (44), trastuzumab (45), or trastuzumab and paclitaxel (46), were found to demonstrate promising efficacy and safety in patients with pretreated HER2+ advanced BC. Akt inhibitor (MK-2206) was found to exhibit favorable antitumor activities when combined with trastuzumab (47) or trastuzumab and paclitaxel (48) in pretreated patients with HER2+ advanced BC. As for the mTOR inhibitor, the combination of everolimus (an mTOR inhibitor) with trastuzumab and vinorelbine did not significantly improve clinical outcome in pretreated HER+ BC patients (49). However, the combination was found to produce better anticancer activity than trastuzumab alone in HER+ patients who are also HR− (49). On the other hand, the combination of two newer mTOR inhibitors, ridaforolimus (50) and sirolimus (51), with trastuzumab have also shown promising activity in refractory HER2+ BC.
Inhibitors Targeting HER-Family Receptors
Growth factor ligands of HER-family receptors [HER1 (EGFR), HER3, or HER4] are known to inhibit the anticancer effect of trastuzumab (52). Moreover, overexpression of HER2/HER3 heterodimers, which are more active than other heterodimers or homodimers formed by HER family (53), have been reported to cause trastuzumab resistance. Therefore, a broader inhibition of HER-family receptors may elicit greater anticancer effect than trastuzumab alone.
Multi-Targeting TKIs. Neratinib, an irreversible TKI of HER1/HER2/HER4, has been reported to significantly improve the 2-year invasive disease-free survival after trastuzumab-based adjuvant therapy in HER2+ BC (54).
Monoclonal Antibodies. Patritumab (anti-HER3 monoclonal antibody) showed promising antitumor activity in preclinical study through inhibiting the formation of HER2/HER3 heterodimers. It was found to exhibit favorable efficacy and acceptable tolerability in patients with HER2+ advanced BC (55). Margetuximab (targeting HER2) was well-tolerated and it demonstrated promising single-agent activity in a first-in-man phase I trial in HER2+ advanced BC (56). Further clinical trials are ongoing to investigate its usefulness as a single agent (NCT02492711) or in combination with pembrolizumab (NCT02689284) (56).
Antibody-Drug Conjugate (ADC). Trastuzumab emtansine is an ADC that incorporates the HER2-targeting activity of trastuzumab with the cytotoxicity of a microtubule-inhibitory drug (57). It is approved for second-line treatment in trastuzumab/lapatinib-relapsed/refractory HER2+ BC (58, 59).
Farnesyl Transferase Inhibitors (FTI). Lonafarnib, as a specific FTI, inhibits Ras function by farnesylation. Although RAS mutations are not common (<2%) in BC, Ras protein and its downstream effectors are often activated due to overexpression of upstream signaling molecules (e.g., HER2) (60). Recently, a phase I trial showed that the addition of lonafarnib to trastuzumab and paclitaxel therapy exhibited superior antitumor activities in HER2+ advanced BC (61).
Immunotherapy. Nelipepimut-S is a short peptide (HER2/neu 369–377, KIFGSLAFL) from the extracellular domain of HER2 (62). It was investigated as a vaccine to prevent clinical recurrence in high-risk BC patients (63). The combination use of nelipepimut-S and trastuzumab in HER2+ early BC is now studied in a phase IIb trial (NCT02297698). Another protein vaccine, recombinant HER2 protein (dHER2) was also found to exhibit immunogenicity to induce T-cell-mediated cytotoxicity in HER2+ early BC patients as an adjuvant treatment (64), in HER2+ advanced BC patients (65) as a single agent and in HER2+ advanced BC patients refractory to trastuzumab+ lapatinib (66).
Triple Negative Breast Cancer
Current Treatment Regimens
Triple negative breast cancer is more aggressive and difficult to treat than HR+ and HER2+ BC. For TNBC, standard chemotherapy remains the mainstay of treatment. Interestingly, TNBC is the BC subtype with the most complete response to chemotherapy (22%). However, their recurrence and metastasis rates are higher than those carrying non-TNBC tumors (67). The median OS for patients with metastatic TNBC is about 9–12 months with conventional cytotoxic agents. The lack of ER, PR, and HER2 expression precludes the use of targeted therapies in advanced TNBC, and the only approved systemic treatment option is chemotherapy [usually taxanes, anthracycline, and platinum drugs (68)] with or without bevacizumab [a recombinant humanized monoclonal antibody against vascular endothelial growth factor (VEGF)]. Given the suboptimal treatment outcome with chemotherapy, new targeted therapies for TNBC are badly needed.
Novel Therapies
Among all BC subtypes, TNBC has the fewest therapeutic options due to the lack of well-defined molecular target(s). Identification of new therapeutic targets and development of effective targeted agents is urgently needed. Table 1 and Figure 1 summarize the promising agents currently in clinical development for TNBC.
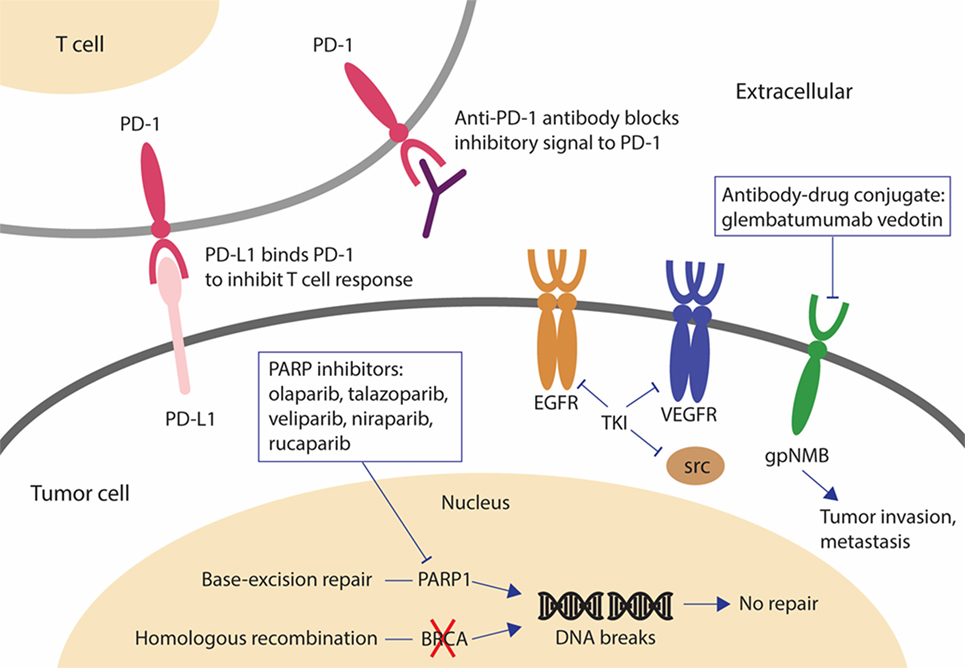
Figure 1. Novel drugs under investigation for triple negative breast cancer (TNBC). PARP inhibitors are effective in BRCA-mutated breast cancer (BC). When BRCA function is absent and PARP is inhibited, cancer cells are unable to repair DNA damage by homologous recombination or base-excision repair and cell death results. The antibody-drug conjugate, glembatumumab vedotin, may be effective in gpNMB-overexpressing BC by releasing the cytotoxic drug into gpNMB-expressing tumor cells, resulting in a targeted-cell killing effect. Tyrosine kinase inhibitors against EGFR, VEGFR, and SRC have been investigated for the treatment of TNBC because these signaling receptors mediating cancer cell growth are overexpressed or dysregulated in TNBC. The monoclonal antibody, pembrolizumab, may be effective regardless of PD-L1 expression by inducing an immune response to kill cancer cells. Abbreviations: PARP, poly(ADP-ribose) polymerase; gpNMB, glycoprotein NMB; AR, androgen receptor; DHT, dihydrotestosterone; PD-1, programmed cell death 1 receptor; PD-L1, ligand of programmed cell death 1 receptor; EGFR, epidermal growth factor receptor.
Poly(ADP-ribose) Polymerase (PARP) Inhibitors
The most important advancement toward understanding the complex heterogeneity of TNBC is probably the discovery of a subgroup of sporadic TNBC that shares the homologous repair deficiency characteristic with BRCA1/2-mutated BC. Drug combination regimens are thus proposed by incorporating PARP inhibtors or the DNA-targeting platinum drug (carboplatin) (69, 70) to standard chemotherapy (71, 72).
The PARP enzyme repairs DNA single-strand breaks whereas the BRCA1/BRCA2 genes encode tumor-suppressor proteins that repair DNA double-strand breaks through homologous recombination. PARP inhibitors have showed promising clinical activities in patients bearing germline BRCA1/BRCA2 mutation (gBRCA+), presumably by synthetic lethality from unresolved DNA damage and by replication arrest caused by physical obstruction of DNA replication forks (73).
Olaparib has proceeded the furthest in clinical development. In a phase III trial, it improved median PFS by 2.8 months and lowered the risk of disease progression/death by 42% compared to standard chemotherapy (71). Talazoparib, currently in phase III trial (NCT01945775), has the greatest preclinical potency due to its strong binding to DNA by trapping PARP–DNA complexes (74). It demonstrated encouraging antitumor activities as a single agent in advanced gBRCA+ BC (75). Veliparib combined with carboplatin and paclitaxel, though failed to prolong PFS in gBRCA+ BC (76), is being investigated in phase III trial (NCT02163694) in advanced gBRCA+ BC (77). Niraparib (phase III, NCT01905592) and rucaparib (phase II, NCT02505048) are being investigated in gBRCA+ advanced BC patients as monotherapy and also in combination with chemotherapy (niraparib: phase I/II, NCT02657889; rucaparib: phase II, NCT01074970).
The use of PARP inhibitors or carboplatin in TNBC is usually determined by three DNA-based homologous recombination deficiency scores, which are highly correlated with genetic defects in BRCA1/2 (78). However, none of these agents is effective in treating all TNBC because TNBC can be further divided into at least six subclasses [basal-like (BL1 and BL2), an immunomodulatory, a mesenchymal, a mesenchymal stem-like, and a luminal androgen receptor subtype], each of which has its own molecular features and unique drug sensitivity (79–81). The identification and characterization of clinically relevant molecular biomarkers of drug responsiveness is needed to further refine this treatment strategy.
Anti-Angiogenic Agents
The intra-tumoral expression of VEGF, a key angiogenic factor, is known to be remarkably higher in TNBC than in non-TNBC BC (82). Bevacizumab (anti-VEGF monoclonal antibody) suppresses tumor neovasculature growth and inhibits metastasis. In metastatic TNBC (phase III), the addition of bevacizumab to first-line chemotherapy (docetaxel) has been shown to increase response rate (placebo plus docetaxel: 46% versus bevacizumab plus docetaxel: 64%) and median PFS (placebo plus docetaxel: 8.1 months versus bevacizumab plus docetaxel: 10.0 months) (HR, 0.67; P < 0.001) (83, 84). Importantly, combination of bevacizumab with docetaxel did not affect significantly the overall safety profile of the regimen.
EGFR Inhibitors
Epidermal growth factor receptor is overexpressed in TNBC. Numerous phase II studies have recently evaluated the efficacy of cetuximab (anti-EGFR monoclonal antibody) in combination with cisplatin in metastatic TNBC (85, 86). While only modest objective response rate (ORR) was observed (ORR = 20% for cisplatin plus cetuximab versus 10% for cisplatin alone), cisplatin plus cetuximab resulted in longer median PFS (3.7 versus 1.5 months) and median OS (12.9 versus 9.4 months) compared with cisplatin alone. Current effort is being made to identify a sub-population of TNBC patients that may be more likely to respond to EGFR inhibitors (87). Favorable response may be correlated with lower expression of alpha-crystallin B chain, higher expression of PTEN, and lack of KRAS expression in the tumors (87).
SRC Inhibitors
SRC is a non-receptor signaling kinase downstream of several growth factor receptors (EGFR, IGF-1R, PDGFR, and HGFR), which is/are deregulated in TNBC. Dasatinib (inhibitor of multiple tyrosine kinases including SRC), when tested as a single agent for TNBC in a prospective, open label, phase II trial (CA180059), has shown disappointing result (88). Objective response rate (ORR) was only 4.7%. Median PFS was 8.3 weeks. Higher dose (100 mg BID) was associated with treatment interruption, dose reduction, and serious adverse events (88). However, in cell line studies, when dasatinib was combined with cetuximab (anti-EGFR monoclonal antibody) and cisplatin, synergistic anticancer activity in a panel of TNBC cell lines was observed (89). The three-drug combination produced more pronounced induction of apoptosis and inhibition of EGFR and MAPK phosphorylation than either single or two-drug combination (89). Moreover, cancer cell migration and invasion was also significantly inhibited by dasatinib alone treatment or dasatinib-containing combination treatment in TNBC cell lines (89). Therefore, clinical studies may be warranted to investigate the use of dasatinib-containing combinations in TNBC patients whose tumors co-overexpressed both EGFR and c-Src.
Monoclonal Antibodies
Glembatumumab vedotin is a monoclonal antibody-cytotoxic drug conjugate designed to target glycoprotein NMB-overexpressing (gpNMB+) TNBC (90). gpNMB is a transmembrane protein associated with tumor invasion and metastasis and it is overexpressed in 40% of TNBC (91). On gpNMB+ advanced TNBC patients (phase II trial), a significantly improved PFS and OS by glembatumumab vedotin was observed compared to the treatment of physician’s choice (92).
Immunotherapies
Pembrolizumab is a human monoclonal IgG4-ĸ antibody against the programmed cell death 1 receptor (PD-1). It demonstrated clinical efficacy and safety in patients with advanced TNBC. PD-1 prevents autoimmunity by suppressing T cells and thus preventing the immune system from killing cancer cells. While patients with PD-L1 (a ligand of PD-1)-positive advanced TNBC were selected for investigation in a phase Ib study (93), the antitumor activity of pembrolizumab appeared to be independent of PD-L1 expression according to another ongoing phase II study (94). Importantly, pembrolizumab also showed durable antitumor activity in patients with heavily pretreated metastatic TNBC (94).
Conclusion
With the advancements in the chemotherapy for BC, the mortality rate from BC is decreasing in the last decade. Targeting ER has proved one of the most powerful treatment modalities against HR+ BC (95). Moreover, the success of the biological drugs such as anti-HER2 monoclonal antibody (96) also highlighted the feasibility and significance of the molecular targeting approach in BC therapy. However, metastasizing TNBC remains a deadly disease with limited treatment options. In recent years, the molecular mechanisms driving the heterogeneous treatment response in BC are better elucidated. This has fueled the development of novel targeted agents, including inhibitors of PARP, CDK4/6, PI3K/AKT/mTOR, multiple kinases, or immune checkpoint, for the treatment of specific molecular subtypes of BC. Treatment options should be tailored to individual patient accordingly.
Author Contributions
CT, MW, WC, and KT: design, collection of data, manuscript, editing, approval of final version, and accountability.
Conflict of Interest Statement
The authors declare that the manuscript was prepared in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADC, antibody-drug conjugate; Akt, protein kinase B; AR, androgen receptor; BC, breast cancer; CDK4/6, cyclin-dependent kinases 4 and 6; EGFR, epidermal growth factor receptor; ER, estrogen receptor; FOXM1, Forkhead box protein M1; HDAC, histone deacetylase; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; mTOR, mammalian target of rapamycin; PARP, poly(ADP-ribose) polymerase; PD-1, programmed cell death 1 receptor; PD-L1, ligand of programmed cell death 1 receptor; PI3K, phosphatidylinositol 3-kinase; PIK3CA, PI3K catalytic subunit p110α; PR, progesterone receptor; Rb, retinoblastoma protein; TNBC, triple negative breast cancer; VEGF, vascular endothelial growth factor.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin (2017) 67:7–30. doi:10.3322/caac.21387
3. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists Guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med (2010) 134:e48–72. doi:10.1043/1543-2165-134.7.e48
4. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American pathologists clinical practice guideline update. J Clin Oncol (2013) 31:3997–4013. doi:10.1200/JCO.2013.50.9984
5. Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, Polikoff J, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev (2012) 21:1848–55. doi:10.1158/1055-9965.EPI-12-0474
6. American Cancer Society. Breast Cancer Facts & Figures 2017–18. Atlanta: American Cancer Society, Inc. (2017).
7. Anderson W, Katki H, Rosenberg P. Incidence of breast cancer in the United States: current an future trends. J Natl Cancer Inst (2011) 103:1397–402. doi:10.1093/jnci/djr257
8. Sledge G, Mamounas E, Hortobagyi G, Burstein HJ, Goodwin PJ, Wolff AC. Past, present and future challenges in breast cancer treatment. J Clin Oncol (2014) 32:1979–86. doi:10.1200/JCO.2014.55.4139
9. Reinert T, Barrios CH. Optimal management of hormone receptor positive metastatic breast cancer in 2016. Ther Adv Med Oncol (2015) 7:304–20. doi:10.1177/1758834015608993
10. Michaud LB, Jones KL, Buzdar AU. Combination endocrine therapy in the management of breast cancer. Oncologist (2001) 6:538–46. doi:10.1634/theoncologist.6-6-538
11. Bergh J, Jonsson P, Lidbrink E, Trudeau M, Eiermann W, Brattstrom D, et al. Fact: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol (2012) 30:1919–25. doi:10.1200/JCO.2011.38.1095
12. Mehta R, Barlow W, Albain K, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med (2012) 367:435–44. doi:10.1056/NEJMoa1201622
13. Johnston S, Kilburn L, Ellis P, Dodwell D, Cameron D, Hayward L, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicenter, phase 3 randomized trial. Lancet Oncol (2013) 14:989–98. doi:10.1016/S1470-2045(13)70322-X
14. Shah AN, Cristofanilli M. The Growing role of CDK4/6 inhibitors in treating hormone receptor-positive advanced breast cancer. Curr Treat Options Oncol (2017) 18:6. doi:10.1007/s11864-017-0443-7
15. Xu H, Yu S, Liu Q, Yuan X, Mani S, Pestell RG, et al. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J Hematol Oncol (2017) 10:97. doi:10.1186/s13045-017-0467-2
16. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med (2016) 375:1925–36. doi:10.1056/NEJMoa1607303
17. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med (2016) 375:1738–48. doi:10.1056/NEJMoa1609709
18. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol (2016) 17:425–39. doi:10.1016/S1470-2045(15)00613-0
19. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol (2017) 35:2875–84. doi:10.1200/JCO.2017.73.7585
20. Barroso-Sousa R, Shapiro GI, Tolaney SM. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care (2016) 11:167–73. doi:10.1159/000447284
21. Miller TW, Hennessy BT, González-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor–positive human breast cancer. J Clin Invest (2010) 120:2406–13. doi:10.1172/JCI41680
22. Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res (2011) 13:224. doi:10.1186/bcr3039
23. Di Leo A, Seok Lee K, Ciruelos E, Lonning P, Janni W, O-Regan R, et al. Abstract S4-07: BELLE-3: a phase III study of buparlisib + fulvestrant in postmenopausal women with HR+, HER2–, aromatase inhibitor-treated, locally advanced or metastatic breast cancer, who progressed on or after mTOR inhibitor-based treatment. Cancer Res (2017) 77:S4–07. doi:10.1158/1538-7445.SABCS16-S4-07
24. Krop IE, Mayer IA, Ganju V, Dickler M, Johnston S, Morales S, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol (2016) 17:811–21. doi:10.1016/S1470-2045(16)00106-6
25. Blackwell K, Burris H, Gomez P, Lynn Henry N, Isakoff S, Campana F, et al. Phase I/II dose-escalation study of PI3K inhibitors pilaralisib or voxtalisib in combination with letrozole in patients with hormone-receptor-positive and HER2-negative metastatic breast cancer refractory to a non-steroidal aromatase inhibitor. Breast Cancer Res Treat (2015) 154:287–97. doi:10.1007/s10549-015-3615-9
26. Perez EA. Treatment strategies for advanced hormone receptor-positive and human epidermal growth factor 2-negative breast cancer: the role of treatment order. Drug Resist Updat (2016) 24:13–22. doi:10.1016/j.drup.2015.11.001
27. Dickler MN, Saura C, Richards D, Krop I, Cervantes A, Bedard PL, et al. Phase II study of taselisib (GDC-0032) in combination with fulvestrant in patients with HER2-negative, hormone receptor-positive advanced breast cancer. Clin Cancer Res (2018). doi:10.1158/1078-0432.CCR-18-0613
28. Schmid P, Pinder SE, Wheatley D, Macaskill J, Zammit C, Hu J, et al. Phase II randomized preoperative window-of-opportunity study of the PI3K inhibitor pictilisib plus anastrozole compared with anastrozole alone in patients with estrogen receptor–positive breast cancer. J Clin Oncol (2016) 34:1987–94. doi:10.1200/JCO.2015.63.9179
29. Saura C, de Azambuja E, Hlauschek D, Oliveira M, Zardavas D, Jallitsch-Halper A, et al. LBA10_PR-primary results of LORELEI: a phase II randomized, double-blind study of neoadjuvant letrozole (LET) plus taselisib versus LET plus placebo (PLA) in postmenopausal patients (pts) with ER+/HER2-negative early breast cancer (EBC). Ann Oncol (2017) 28:.001–.440. doi:10.1093/annonc/mdx440.001
30. Baselga J, Campone M, Piccart M, Burris HA III, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med (2012) 366:520–9. doi:10.1056/NEJMoa1109653
31. Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, Chow L, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol (2013) 31:195–202. doi:10.1200/JCO.2011.38.3331
32. Fleming GF, Ma CX, Huo D, Sattar H, Tretiakova M, Lin L, et al. Phase II trial of temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat (2012) 136:355–63. doi:10.1007/s10549-011-1910-7
33. Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res (2001) 61:7025–9.
34. Sabnis GJ, Goloubeva O, Chumsri S, Nguyen N, Sukumar S, Brodie AM, et al. Functional activation of the estrogen receptor-α and aromatase by the HDAC inhibitor, entinostat, sensitizes of ER-negative tumors to letrozole. Cancer Res (2011) 71:1893–903. doi:10.1158/0008-5472.CAN-10-2458
35. Yardley DA, Ismail-Khan RR, Melichar B, Lichinitser M, Munster PN, Klein PM, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol (2013) 31:2128–35. doi:10.1200/JCO.2012.43.7251
36. Munster PN, Thurn KT, Thomas S, Raha P, Lacevic M, Miller A, et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer (2011) 104:1828–35. doi:10.1038/bjc.2011.156
37. Purohit A, Woo LW, Potter BV. Steroid sulfatase: a pivotal player in estrogen synthesis and metabolism. Mol Cell Endocrinol (2011) 340:154–60. doi:10.1016/j.mce.2011.06.012
38. Stanway SJ, Delavault P, Purohit A, Woo LW, Thurieau C, Potter BV, et al. Steroid sulfatase: a new target for the endocrine therapy of breast cancer. Oncologist (2007) 12:370–4. doi:10.1634/theoncologist.12-4-370
39. Palmieri C, Stein RC, Liu X, Hudson E, Nicholas H, Sasano H, et al. IRIS study: a phase II study of the steroid sulfatase inhibitor irosutat when added to an aromatase inhibitor in ER-positive breast cancer patients. Breast Cancer Res Treat (2017) 165:343–53. doi:10.1007/s10549-017-4328-z
40. Rasmussen LM, Zaveri NT, Stenvang J, Peters RH, Lykkesfeldt AE. A novel dual-target steroid sulfatase inhibitor and antiestrogen: SR16157, a promising agent for the therapy of breast cancer. Breast Cancer Res Treat (2007) 106:191–203. doi:10.1007/s10549-007-9494-y
41. Wuerstlein R, Harbeck N. Neoadjuvant therapy for HER2-positive breast cancer. Rev Recent Clin Trials (2017) 12:81–92. doi:10.2174/1574887112666170202165049
42. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med (2001) 344:783–92. doi:10.1056/NEJM200103153441101
43. Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med (2012) 366:109–19. doi:10.1056/NEJMoa1113216
44. Guerin M, Rezai K, Isambert N, Campone M, Autret A, Pakradouni J, et al. PIKHER2: A phase IB study evaluating buparlisib in combination with lapatinib in trastuzumab-resistant HER2-positive advanced breast cancer. Eur J Cancer (2017) 86:28–36. doi:10.1016/j.ejca.2017.08.025
45. Saura C, Bendell J, Jerusalem G, Su S, Ru Q, De Buck S, et al. Phase Ib study of buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on trastuzumab-based therapy. Clin Cancer Res (2014) 20:1935–45. doi:10.1158/1078-0432.CCR-13-1070
46. Tolaney S, Burris H, Gartner E, Mayer IA, Saura C, Maurer M, et al. Phase I/II study of pilaralisib (SAR245408) in combination with trastuzumab or trastuzumab plus paclitaxel in trastuzumab-refractory HER2-positive metastatic breast cancer. Breast Cancer Res Treat (2015) 149:151–61. doi:10.1007/s10549-014-3248-4
47. Hudis C, Swanton C, Janjigian YY, Lee R, Sutherland S, Lehman R, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res (2013) 15:R110. doi:10.1186/bcr3577
48. Chien AJ, Cockerill A, Fancourt C, Schmidt E, Moasser MM, Rugo HS, et al. A phase 1b study of the Akt-inhibitor MK-2206 in combination with weekly paclitaxel and trastuzumab in patients with advanced HER2-amplified solid tumor malignancies. Breast Cancer Res Treat (2016) 155:521–30. doi:10.1007/s10549-016-3701-7
49. Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol (2015) 16:816–29. doi:10.1016/S1470-2045(15)00051-0
50. Seiler M, Ray-Coquard I, Melichar B, Yardley DA, Wang RX, Dodion PF, et al. Oral ridaforolimus plus trastuzumab for patients with HER2+ trastuzumab-refractory metastatic breast cancer. Clin Breast Cancer (2015) 15:60–5. doi:10.1016/j.clbc.2014.07.008
51. Acevedo-Gadea C, Hatzis C, Chung G, Fishbach N, Lezon-Geyda K, Zelterman D, et al. Sirolimus and trastuzumab combination therapy for HER2-positive metastatic breast cancer after progression on prior trastuzumab therapy. Breast Cancer Res Treat (2015) 150:157–67. doi:10.1007/s10549-015-3292-8
52. Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res (2006) 8:215. doi:10.1186/bcr1612
53. Hettman T, Schneider M, Blum S, Hartmann S, Hendrich M, Moor R, et al. Abstract B161: U3-1287 (AMG 888), a fully human anti-HER3 mAb, demonstrates preclinical efficacy in HER2+ and HER2- breast cancer models. Mol Cancer Ther (2009) 8:B161–161. doi:10.1158/1535-7163.TARG-09-B161
54. Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2016) 17:367–77. doi:10.1016/S1470-2045(15)00551-3
55. Mukai H, Saeki T, Aogi K, Naito Y, Matsubara N, Shigekawa T, et al. Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Cancer Sci (2016) 107:1465–70. doi:10.1111/cas.13017
56. Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol (2017) 28:855–61. doi:10.1093/annonc/mdx002
57. Dieras V, Bachelot T. The success story of trastuzumab emtansine, a targeted therapy in HER2-positive breast cancer. Target Oncol (2014) 9:111–22. doi:10.1007/s11523-013-0287-4
58. Reichert JM. Antibodies to watch in 2013. Mid-year update. MAbs (2013) 5:513–7. doi:10.4161/mabs.24990
59. Lianos GD, Vlachos K, Zoras O, Katsios C, Cho WC, Roukos DH. Potential of antibody-drug conjugates and novel therapeutics in breast cancer management. Onco Targets Ther (2014) 7:491–500. doi:10.2147/OTT.S34235
60. Smith CA, Pollice AA, Gu LP, Brown KA, Singh SG, Janocko LE, et al. Correlations among p53, Her-2/neu, and ras overexpression and aneuploidy by multiparameter flow cytometry in human breast cancer: evidence for a common phenotypic evolutionary pattern in infiltrating ductal carcinomas. Clin Cancer Res (2000) 6:112–26.
61. Milojkovic Kerklaan B, Dieras V, Le Tourneau C, Mergui-Roelvink M, Huitema AD, Rosing H, et al. Phase I study of lonafarnib (SCH66336) in combination with trastuzumab plus paclitaxel in Her2/neu overexpressing breast cancer: EORTC study 16023. Cancer Chemother Pharmacol (2013) 71:53–62. doi:10.1007/s00280-012-1972-1
62. Fisk B, Blevins TL, Wharton JT, Ionannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med (1995) 181:2109–17. doi:10.1084/jem.181.6.2109
63. Mittendorf EA, Clifton GT, Holmes JP, Schneble E, van Echo D, Ponniah S, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol (2014) 25:1735–42. doi:10.1093/annonc/mdu211
64. Limentani SA, Campone M, Dorval T, Curigliano G, de Boer R, Vogel C, et al. A non-randomized dose-escalation phase I trial of a protein-based immunotherapeutic for the treatment of breast cancer patients with HER2-overexpressing tumors. Breast Cancer Res Treat (2016) 156:319–30. doi:10.1007/s10549-016-3751-x
65. Curigliano G, Romieu G, Campone M, Dorval T, Duck L, Canon JL, et al. A phase I/II trial of the safety and clinical activity of a HER2-protein based immunotherapeutic for treating women with HER2-positive metastatic breast cancer. Breast Cancer Res Treat (2016) 156:301–10. doi:10.1007/s10549-016-3750-y
66. Hamilton E, Blackwell K, Hobeika AC, Clay TM, Broadwater G, Ren XR, et al. Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition. J Tran Med (2012) 10:28. doi:10.1186/1479-5876-10-28
67. Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol (2008) 26:1275–81. doi:10.1200/JCO.2007.14.4147
68. Berrada N, Delaloge S, Andre F. Treatment of triple-negative metastatic breast cancer: toward individualized targeted treatments or chemosensitization? Ann Oncol (2010) 21(Suppl 7):vii30–5. doi:10.1093/annonc/mdq279
69. Castrellon AB, Pidhorecky I, Valero V, Raez LE. The role of carboplatin in the neoadjuvant chemotherapy treatment of triple negative breast cancer. Oncol Rev (2017) 11:324. doi:10.4081/oncol.2017.324
70. Konecny GE, Kristeleit RS. PARP inhibitors for BRCA1/2 mutated and sporadic ovarian cancer: current practice and future directions. Br J Cancer (2016) 115:1157–73. doi:10.1038/bjc.2016.311
71. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med (2017) 377:523–33. doi:10.1056/NEJMoa1706450
72. Telli ML, Hellyer J, Audeh W, Jensen KC, Bose S, Timms KM, et al. Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer. Breast Cancer Res Treat (2018) 168:625–30. doi:10.1007/s10549-017-4624-7
73. Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature (2005) 434:913–7. doi:10.1038/nature03443
75. Turner NC, Telli ML, Rugo HS, Mailliez A, Ettl J, Grischke E-M. Final results of a phase 2 study of talazoparib (TALA) following platinum or multiple cytotoxic regimens in advanced breast cancer patients (pts) with germline BRCA1/2 mutations (ABRAZO). J Clin Oncol (2017) 35(15_suppl):1007. doi:10.1200/JCO.2017.35.15_suppl.1007
76. AbbVie’s Veliparib and Chemotherapy Combo Trial Failed in Early Triple Negative Breast Cancer. Breast Cancer News. BioNews Services, LLC (2017). Available from: https://breastcancer-news.com/2017/04/25/veliparib-chemotherapy-combo-failed-triple-negative-breast-cancer/ (Accessed: December 26, 2017).
77. Han HS, Dieras V, Robson M, Palacova M, Marcom PK, Jager A, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol (2018) 29:154–61. doi:10.1093/annonc/mdx505
78. Timms KM, Abevich V, Hughes E, Neff C, Reid J, Morris B, et al. Association of BRCA1/2 defects with genomic scores predictice of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res (2014) 16:475. doi:10.1186/s13058-014-0475-x
79. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest (2011) 121:2750–67. doi:10.1172/JCI45014
80. Prat A, Parker JS, Karqinova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res (2010) 12:R68. doi:10.1186/bcr2635
81. Russnes HG, Vollan HKM, Linqiaerde OC, Krasnitz A, Lundin P, Naume B, et al. Genomic architecture characterizes tumor progression paths and fate in breast cancer patients. Sci Transl Med (2010) 2:38ra47. doi:10.1126/scitranslmed.3000611
82. Linderholm BK, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtio J, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol (2009) 20:1639–46. doi:10.1093/annonc/mdp062
83. Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol (2010) 28:3239–47. doi:10.1200/JCO.2008.21.6457
84. Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol (2011) 29:1252–60. doi:10.1200/JCO.2010.28.0982
85. Baselga J, Gomez P, Greil R, Braga S, Climent MA, Wardley AM, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol (2013) 31:2586–92. doi:10.1200/JCO.2012.46.2408
86. Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol (2012) 30:2615–23. doi:10.1200/JCO.2010.34.5579
87. Tomao F, Papa A, Zaccarelli E, Rossi L, Caruso D, Minozzi M, et al. Triple-negative breast cancer: new perspectives for targeted therapies. Onco Targets Ther (2015) 8:177–93. doi:10.2147/OTT.S67673
88. Finn RS, Bengala C, Ibrahim N, Roche H, Sparano J, Strauss LC, et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clin Cancer Res (2011) 17:6905–13. doi:10.1158/1078-0432.CCR-11-0288
89. Kim EM, Mueller K, Gartner E, Boerner J. Dasatinib is synergistic with cetuximab and cisplatin in triple-negative breast cancer cells. J Surg Res (2013) 185:231–9. doi:10.1016/j.jss.2013.06.041
90. Rose AAN, Biondini M, Curiel R, Siegel PM. Targeting GPNMB with glembatumumab vedotin: current developments and future opportunities for the treatment of cancer. Pharmacol Ther (2017) 179:127–41. doi:10.1016/j.pharmthera.2017.05.010
91. Rose AA, Grosset AA, Dong Z, Russo C, Macdonald PA, Bertos NR, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res (2010) 16:2147–56. doi:10.1158/1078-0432.CCR-09-1611
92. Yardley DA, Weaver R, Melisko ME, Saleh MN, Arena FP, Forero A, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB-expressing breast cancer. J Clin Oncol (2015) 33:1609–19. doi:10.1200/JCO.2014.56.2959
93. Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol (2016) 34:2460–7. doi:10.1200/JCO.2015.64.8931
94. Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J Clin Oncol (2017) 35(15_suppl):1008. doi:10.1200/JCO.2017.35.15_suppl.1008
95. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med (1998) 339:1609–18. doi:10.1056/NEJM199811263392207
96. Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomized controlled trial. Lancet Oncol (2011) 12:236–44. doi:10.1016/S1470-2045(11)70033-X
Keywords: breast cancer, cyclin-dependent kinases 4 and 6 inhibitors, hormone receptor, human epidermal growth factor receptor 2, poly(ADP-ribose) polymerase inhibitor, programmed cell death protein 1, trastuzumab, triple negative breast cancer
Citation: Tong CWS, Wu M, Cho WCS and To KKW (2018) Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 8:227. doi: 10.3389/fonc.2018.00227
Received: 03 April 2018; Accepted: 01 June 2018;
Published: 14 June 2018
Edited by:
Francois X. Claret, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Maria Rosa Ciriolo, Università degli Studi di Roma Tor Vergata, ItalyChun Hei Antonio Cheung, National Cheng Kung University, Taiwan
Copyright: © 2018 Tong, Wu, Cho and To. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William C. S. Cho, Y2hvY3MmI3gwMDA0MDtoYS5vcmcuaGs=;
Kenneth K. W. To, a2VubmV0aHRvJiN4MDAwNDA7Y3Voay5lZHUuaGs=
 Christy W. S. Tong1
Christy W. S. Tong1 Kenneth K. W. To
Kenneth K. W. To