
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Synaptic Neurosci., 11 February 2020
Volume 12 - 2020 | https://doi.org/10.3389/fnsyn.2020.00005
This article is part of the Research TopicMethods for Synaptic InterrogationView all 20 articles
Synaptic transmission between neurons is the basic mechanism for information processing in cortical microcircuits. To date, paired recording from synaptically coupled neurons is the most widely used method which allows a detailed functional characterization of unitary synaptic transmission at the cellular and synaptic level in combination with a structural characterization of both pre- and postsynaptic neurons at the light and electron microscopic level. In this review, we will summarize the many applications of paired recordings to investigate synaptic function and structure. Paired recordings have been used to study the detailed electrophysiological and anatomical properties of synaptically coupled cell pairs within a synaptic microcircuit; this is critical in order to understand the connectivity rules and dynamic properties of synaptic transmission. Paired recordings can also be adopted for quantal analysis of an identified synaptic connection and to study the regulation of synaptic transmission by neuromodulators such as acetylcholine, the monoamines, neuropeptides, and adenosine etc. Taken together, paired recordings from synaptically coupled neurons will remain a very useful approach for a detailed characterization of synaptic transmission not only in the rodent brain but also that of other species including humans.
To understand local neuronal microcircuits in the brain, it is necessary to know the morphological and electrophysiological properties of both the pre- and postsynaptic neurons, the synaptic connection type(s) and their structure-function relationship. However, in many studies of synaptic transmission the identity of the pre- and postsynaptic neuron is not well or not at all characterized. This is because of the relatively unspecific stimulation protocols (e.g., extracellular stimulation) often used to investigate synaptic connectivity, which generally do not allow to determine the structural and functional properties of the presynaptic neuron. Paired recordings together with intracellular staining by markers such as biocytin/neurobiotin and/or fluorescent dyes are better suited for studying local neuronal microcircuits. This technique permits a simultaneous, correlated characterization of the structural and functional properties of a synaptic connection.
Monosynaptic connections between identified neurons have been investigated in both cortical and subcortical brain regions using paired recordings in acute brain slices (Malinow, 1991; Mason et al., 1991; Buhl et al., 1994; Deuchars et al., 1994; Bolshakov and Siegelbaum, 1995; Miles et al., 1996; Stratford et al., 1996; Geiger et al., 1997; Markram et al., 1997a; Thomson and Deuchars, 1997; Feldmeyer et al., 1999; Gupta et al., 2000; Tamas et al., 2000, 2003; Holmgren et al., 2003; Szabadics et al., 2006; Helmstaedter et al., 2008; Olah et al., 2009, for reviews, see Miles and Poncer, 1996; Debanne et al., 2008; Feldmeyer and Radnikow, 2016). Sharp microelectrodes were initially used in these experiments (Mason et al., 1991; Buhl et al., 1994; Deuchars et al., 1994). However, electrophysiological recordings with sharp microelectrodes have several limitations, e.g., the electrical noise is high and the membrane seal poor, the approach is generally blind and thus the inter-somatic distance between pre- and postsynaptic neurons not well controlled (Brette and Destexhe, 2012). Later, patch pipettes were employed in order to measure synaptic responses with a higher signal-to-noise ratio and an improved temporal resolution. A significant advance was the use of infrared differential interference contrast optics (Dodt and Zieglgansberger, 1990) that significantly improved the visual identification of neurons in acute brain slices (Stuart et al., 1993) so that it became possible to obtain recordings from synaptic connections between visually identified neurons.
An advantage of paired recordings is the fact that functional characterization can be combined with the morphological and/or molecular analysis at both the light and electron microscopic level (Deuchars et al., 1994; Markram et al., 1997a, 1998b; Reyes et al., 1998; Feldmeyer et al., 2002, 2006; Silver et al., 2003; Tamas et al., 2003; Kapfer et al., 2007; Silberberg and Markram, 2007; Helmstaedter et al., 2008). After histochemical processing, the expression of specific marker proteins of the synaptically connected neuron pair can be determined, in a subsequent step the somatodendritic and axonal morphologies recovered and then reconstructed in three spatial dimensions. This will allow a quantitative analysis of morphological features such as orientation, branching pattern, spatial length density etc. These parameters could provide a basis for an objective classification of pre- and postsynaptic neurons in a specific synaptic connection. Furthermore, paired recordings also permit the identification of synaptic contacts of unitary synaptic connections using a combination of light and electron microscopy. In addition to this detailed analysis of the synaptic transmission at a defined neuronal microcircuit paired recordings also allow the study of quantal properties of identified synapses and the modulation of synaptic transmission by neurotransmitters such as acetylcholine, noradrenaline, dopamine, serotonin, and adenosine.
The most crucial step for paired recordings in acute brain slices is to find a sufficiently stable synaptic connection so that a detailed analysis of its structural and functional properties is possible. This step depends on several important factors which will be discussed here in brief (for more details, see Radnikow et al., 2012; Feldmeyer and Radnikow, 2016). First, it is important to determine the optimal procedure for preparing brain slices so that the axo-dendritic branches of both pre- and postsynaptic neuron for the synaptic connection under study is well preserved. A suitable slice thickness needs to be determined depending on the recording configuration (whole-cell with patch pipettes or intracellular with sharp microelectrodes); an increase in the slice thickness may significantly increase the connection probability and the quantification of synapse number per connection (Thomson and Lamy, 2007; Stepanyants et al., 2009). Second, the composition of solutions used during the slicing and incubation needs to be adjusted carefully according to the age of animals and type of species. Several slicing and incubation solutions for adult and senescent animal and human brain tissue are available under http://www.brainslicemethods.com/ (Ting et al., 2014, 2018a,b). Finally, the connection probability of different neuron types is highly variable (from 5 to 70%) depending on both the presynaptic axonal projection and the postsynaptic dendritic arborization (Thomson and Lamy, 2007; Lefort et al., 2009; Fino et al., 2013; Pfeffer et al., 2013; Jiang et al., 2015; Markram et al., 2015; Radnikow et al., 2015; Seeman et al., 2018; Jouhanneau and Poulet, 2019). Therefore, choosing the appropriate strategy, either a random patch or a “searching” protocol (Qi et al., 2015), is critical for the success of paired recordings. Paired recordings from synaptically coupled neurons allow a wide variety of functional and structural analysis. The most relevant issues will be described below.
The synaptic strength (or weight) is a key parameter to characterize the efficacy of a synaptic connection. It reflects whether the synaptic connection has a strong or weak influence on postsynaptic output. It is measured as the peak amplitude of postsynaptic potentials (PSPs) evoked by presynaptic action potentials (APs). For excitatory synaptic connections in the neocortex, the PSP amplitude is not normally distributed but skewed toward lower values (~0.5 mV) with a long tail with higher values (>2 mV) (Figures 1A,B) (Markram et al., 1997a; Feldmeyer et al., 1999, 2002, 2006; Sjostrom et al., 2001; Holmgren et al., 2003; Lefort et al., 2009). It has been shown by theoretical analysis that this synaptic weight distribution can be understood through optimization of information storage in neuronal networks (Brunel et al., 2004; Varshney et al., 2006; Barbour et al., 2007). It has also been suggested that the high-amplitude connections represent rare, strong connections that mediate stimulus-specific response amplification in cortical microcircuits (Cossell et al., 2015).
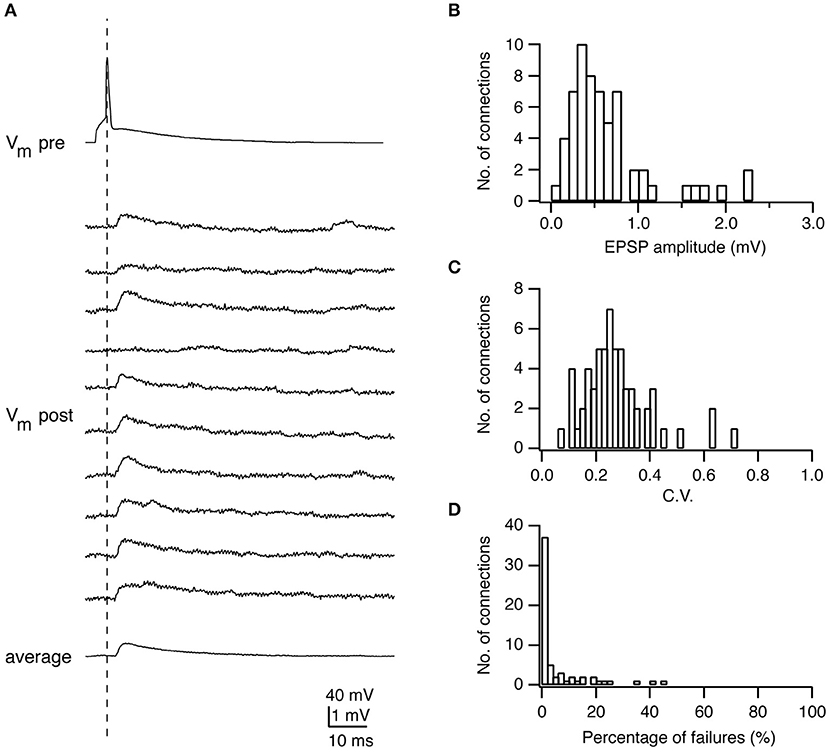
Figure 1. Electrophysiological characterization of synaptic connections using paired recordings. (A) Paired recordings from a synaptic connection established between a presynaptic L4 spiny neuron and a postsynaptic L2/3 pyramidal cell. Top, a presynaptic AP; Middle, ten successive EPSPs in response to a presynaptic AP; Bottom, the average EPSP. (B) Histogram of the EPSP amplitudes in L4-L2/3 connections (n = 64). (C) Histogram of the coefficients of variation (C.V.s) of EPSPs in L4-L2/3 connections (n = 64). (D) Histogram of the failure rate (in %) in L4-L2/3 connections (n = 64). Figure has been adapted from Feldmeyer et al. (2002) with permission.
The time course of postsynaptic response is another important determinant of the computational power of a synaptic connection and significantly affects the synaptic integration in postsynaptic neurons. Long-lasting PSPs show a stronger summation while brief postsynaptic responses are necessary to achieve a high temporal fidelity for repetitive synaptic inputs. Quantitatively, the time course of excitatory or inhibitory PSPs (EPSPs/IPSPs) is described by its 20–80% rise time, decay time constant and half-width. It should be noted that the EPSP/IPSP time course is shaped by (low-pass) dendritic filtering due to the distance between the recording site (normally at the soma) and the synapse location (Rall, 1967).
The latency is defined as the time difference between the peak of presynaptic AP and the beginning of the PSP. The size and variation of latencies determines the time window of integration of the synaptic response. Many factors such as the fine structure of the pre- and postsynaptic sites, the release probability of neurotransmitters, and the passive and/or active electrophysiological properties of both pre- and postsynaptic neurons affect the latency in synaptic transmission.
The reliability is an important property of a synaptic connection, which characterizes the extent of the PSP variability. Synaptic reliability and variability are sensitive to recording conditions, e.g., the temperature and Ca2+ concentration in the recording solution. The reliability of synaptic transmission increases with the increasing temperature (Hardingham and Larkman, 1998; Volgushev et al., 2004) and Ca2+ concentration (Rozov et al., 2001; Silver et al., 2003) due to enhanced transmitter release. To determine this parameter, an AP is elicited in the presynaptic neuron resulting in an EPSP or IPSP in the postsynaptic neuron (Figures 1A, 4A). Between 50 and 100 sweeps are recorded to determine the mean amplitude of the synaptic response (Figures 1B, 4B) and its variance. A frequently used measure for the reliability is the coefficient of variation (CV) which is defined as:
where is the variance of the PSP amplitude, the variance of the membrane potential fluctuation, and μPSP the mean PSP amplitude (Figure 1C). The variance of the PSP is corrected by subtracting the membrane potential variance, which includes membrane potential noise (i.e., from random ion channel openings) and electrical noise introduced by the recording equipment. CVPSP is a surrogate measure for the release probability of transmitters. However, this measure is only indirect and a detailed quantal analysis (see below) is needed to determine its actual value.
The failure rate is defined as the frequency with which a synapse fails to respond to a presynaptic AP (Figure 1D). In general, synaptic connections with a low neurotransmitter release probability (e.g., synapses formed by L6A cortico-thalamic pyramidal neurons) (Yang et al., 2019) and/or few synaptic contacts (e.g., synapse formed between parallel fibers from granule cells and Purkinje cell dendrites) (Isope and Barbour, 2002) show a significant number of failures. However, failures may not be apparent despite a relatively low release probability when the number of synaptic contacts is sufficiently large. Under this condition it is likely that vesicle release would occur at least at a small fraction of synaptic contacts; hence, no failures would be observed. This is in accordance with findings in a number of paired recording studies in acute cortical slices that generally report a low failure rate of synaptic transmission (Atzori et al., 2001; Koester and Johnston, 2005; Feldmeyer et al., 2006; Frick et al., 2008; Lefort et al., 2009).
Changes in the strength of the synaptic response are critical for the flexibility and plasticity of synaptic function. For monosynaptic connections, paired recordings have shown that, during the delivery of multiple stimuli at short time intervals, the size of the postsynaptic responses can become either larger or smaller, a phenomenon known as short-term facilitation or depression, respectively. When the release probability is low during the initial presynaptic AP, PSP facilitation is likely to occur. This is likely to results from an increase in the Ca2+ concentration in the presynaptic terminal with each successive presynaptic AP which will lead to successively larger PSPs (i.e., an increase in release probability). After some time the release probability and hence the PSP amplitude will decrease again because of a depletion of the readily releasable pool of synaptic vesicles (see below). Short-term synaptic depression, on the other hand, occurs when the initial release probability is high, i.e., when many synaptic vesicles are released during the first presynaptic AP. This then results in a transient depletion of synaptic vesicle from the readily releasable pool (Zucker and Regehr, 2002; Rizzoli and Betz, 2004, 2005). Whether a synaptic connection shows short-term facilitation or depression depends on the pre- and/or postsynaptic neuron identity (Markram et al., 1998b; Reyes et al., 1998; Scanziani et al., 1998; Gupta et al., 2000; Koester and Johnston, 2005; Ma et al., 2012) (Figure 3). By eliciting a pair (or train) of APs in the presynaptic neuron at a fixed interval (e.g., 100 ms) and measuring the amplitude of the postsynaptic response, the paired-pulse ratio (PPR) is calculated as PPR = PSP2/PSP1. The PPR is commonly used to characterize short-term synaptic plasticity and specifies whether the initial release probability is high or low. Although the PPR is widely used, it is not sufficient to unmask the interplay between release, depression and facilitation (Dittman et al., 2000). There is some ambiguity in using the PPR to determine depression/facilitation dynamics in the case of strongly facilitating synapses. In these synapses, PPR might be small for the first two PSPs and gradually becomes larger during repetitive presynaptic stimulation (Markram et al., 1998a). For such cases a train of frequency-dependent APs elicited in the presynaptic neuron is more appropriate to be adopted for measuring the postsynaptic response.
Synaptic function is also affected by retrograde messengers (e.g., glutamate, GABA, endocannabinoid) released from postsynaptic dendrites (Zilberter et al., 2005). Paired recordings between layer 2/3 pyramidal cells and bitufted interneurons showed that the dendritic GABA release depresses excitatory transmission via presynaptic metabotropic GABAB receptors in the rat neocortex (Zilberter et al., 1999). For the inhibitory transmission, depolarization-induced suppression of inhibition (DSI) was found widely in different cortical areas including the hippocampus (Wilson and Nicoll, 2001), cerebellum (Kreitzer and Regehr, 2001), and neocortex (Trettel and Levine, 2003). DSI has been shown to be caused by the postsynaptic deporalization-induced dendritic release of endocannabinoids, which diffuse retrogradely to presynaptic axonal terminals where they bind to cannabinoid 1 receptors to reduce the GABA release.
It should be noted that there are some differences between in vitro acute brain slice (or ex vivo) and in vivo recording conditions. Therefore, the property of synaptic transmission studied in vitro may be different from that in vivo condition. A prominent difference is the extracellular Ca2+ concentration which is ~1.2–1.3 mM free Ca2+ in the cerebrospinal fluid (Heinemann et al., 1977; Massimini and Amzica, 2001; Crochet et al., 2005; Borst, 2010) but 2 mM Ca2+ compound in a standard extracellular perfusion solution. Because calcium salts do not fully dissociate the free Ca2+ concentration in the extracellular fluid will be lower than the absolute CaCl2 concentration [or any other calcium salt this is substituted for CaCl2 (e.g., Ca(CH3SO3)2)]. An absolute CaCl2 concentration of 2 mM amounts to 1.7 mM free Ca2+ (as can be measured with an ion-selective electrode and/or calculated from the dissociation constant). Thus, compared to the in vitro condition, the PSP amplitude and reliability will be lower and the failure rate higher under in vivo condition because of the reduced synaptic release probability. In addition, the short-term synaptic plasticity is likely to change from strong depression to no change or weak facilitation. In addition, the membrane conductance of neocortical neurons is high in vivo because of the intense synaptic bombardment, which rarely appears under in vitro conditions (Destexhe et al., 2003). Therefore, the time course of PSPs recorded in vivo is also different from that in vitro, e.g., the decay of PSPs is faster in vivo than in vitro because of enhanced membrane conductances.
Long-term synaptic changes such as long-term potentiation (LTP) and depression (LTD) have been considered as the cellular mechanism of learning and memory (Huganir and Nicoll, 2013). Paired recordings have been widely adopted to investigate the LTP and LTD and uncover their induction conditions and mechanisms (Malinow, 1991; Arancio et al., 1995; Bolshakov and Siegelbaum, 1995; Liao et al., 1995; Markram et al., 1997b; Bi and Poo, 1998; Egger et al., 1999; Montgomery et al., 2001). For example, the postsynaptic insertion of AMPA receptors has been considered to be the molecular basis of LTP induction. Spike-timing-dependent plasticity (STDP) is one Hebbian type of long-term synaptic plasticity. Its induction depends on the precise timing of pre- and postsynaptic AP firing. Paired recordings between layer 5 pyramidal cells showed that if a presynaptic neuron fires earlier (e.g., +10 ms) than its postsynaptic neuron, LTP will be induced. Otherwise, if the presynaptic neuron fires later (e.g., −10 ms) than its postsynaptic neuron, LTD will develop (Markram et al., 1997b; Bi and Poo, 1998, 2001; Abbott and Nelson, 2000). However, this rule does not apply to synaptic connections established between layer 4 spiny neurons. Whether presynaptic neurons fire earlier or later (e.g., ±10 ms) than postsynaptic neurons LTD will always be induced because of presynaptic metabotropic glutamate receptor activation (Egger et al., 1999).
In addition to chemical synapses, synaptic coupling can also occur via electrical synapses or gap junctions, in particular between immature neurons and interneurons of the same type. Paired recordings are also feasible to record from neurons coupled via gap junctions and to characterize their electrical properties such as the coupling coefficient and junctional conductance (Galarreta and Hestrin, 1999; Gibson et al., 1999). When combining with the biocytin labeling, the morphological properties of gap junctions can be studied at both light and electron microscopic levels as described below (Tamas et al., 2000).
Paired (or multiple) recordings allow to study the organization principles of neuronal networks and shed light on their fundamental features. Previous connectivity studies suggest that neuronal networks are not randomly connected but may have a fine-scale specificity of connectivity (Song et al., 2005; Brown and Hestrin, 2009; Yu et al., 2009; Ko et al., 2011; Perin et al., 2011; Jiang et al., 2013; Cossell et al., 2015). For example, it was demonstrated that two excitatory neurons are more likely to be connected if they share a common neighbor, the so-called “common neighbor rule,” in neuronal networks of cortical layers 2/3 and 5 (Song et al., 2005; Ko et al., 2011; Perin et al., 2011). The preference of connection formation between two excitatory neurons also depends on their long-range axonal targets (Brown and Hestrin, 2009), developing origins (Yu et al., 2009) and orientation selectivities (Ko et al., 2011).
For a detailed characterization of the morphological properties of synaptic connections, an optimal biocytin filling and a careful histochemical processing are of major importance. We have optimized these procedures in our laboratory (see Marx et al., 2012; Radnikow et al., 2012; Qi et al., 2015; Feldmeyer and Radnikow, 2016).
Following histochemical processing biocytin-labeled neuronal cell pairs are inspected under the light microscope using a 100× or a 50× oil immersion objective. Oil immersion objectives with a high numerical aperture (= 1.4) have to be used in order to focus throughout the entire slice thickness (~300 μm). Computer-assisted 3D neuronal reconstructions are made using the Neurolucida® system (Microbrightfield). This is a neuroanatomical reconstruction system for tracing the neuronal somatodendritic and axonal branches in all three dimensions (3D). Tracing is normally done manually; automatic or semi-automatic tracing approaches are often not applicable because of the dense and profuse branching of the dendritic branches and in particular axonal collaterals of the pre- and postsynaptic neurons (Figure 2A). Dendrites and axons are traced at high resolution, i.e., with 0.5–1.0 μm step size in z-direction. Furthermore, frequent alignments in the x, y, and z-dimensions of the neurons are required.
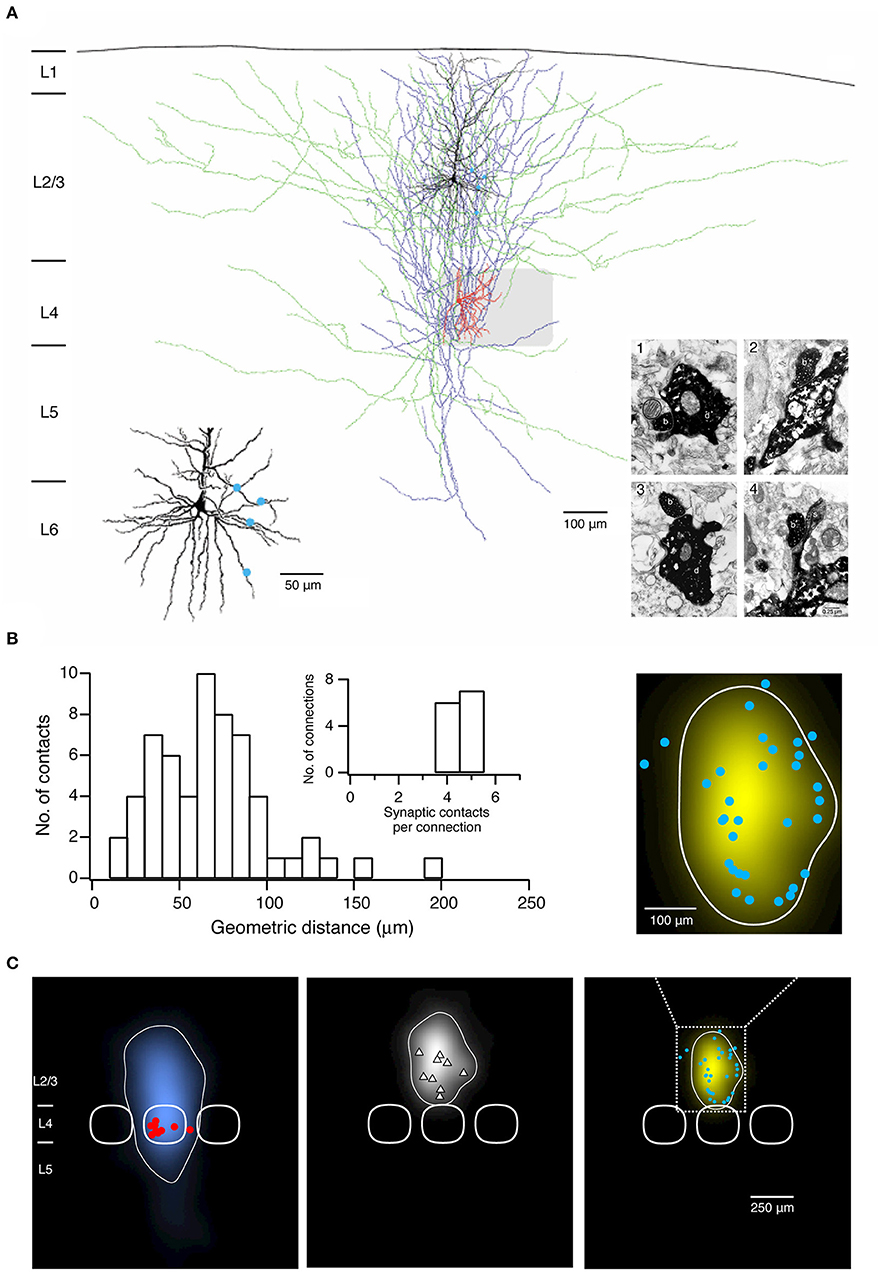
Figure 2. Morphological characterization of synaptic connections using paired recordings in combination with biocytin fillings. (A) Morphological reconstruction of a synaptically coupled cell pair between a L4 spiny stellate cell and a L2/3 pyramidal neuron. The somatodendritic and axonal compartments of the presynaptic spiny stellate cell are drawn in red and blue, respectively. The somatodendritic and axonal compartments of the postsynaptic L2/3 pyramidal neuron are drawn in black and green, respectively. The gray square represents the L4 barrel where the spiny stellate cell is located. Left inset, four putative synaptic contacts established by the axon of the L4 spiny stellate cell with the dendrites of the L2/3 pyramidal neuron are marked by blue dots. Right inset, electron micrographs of the synaptic contacts. All four synaptic contacts which were identified with the light microscope were confirmed at the electron microscopic level. The axonal boutons (b) of the L4 spiny stellate cell established synaptic contacts on dendritic shafts (d) in contacts 1–3 while on a dendritic spine in contact 4 of the L2/3 pyramidal neuron. (B) Histogram of the geometric distances from the somata of putative synaptic contacts in 13 L4 spiny neuron-L2/3 pyramidal cell pairs. Inset, distribution of number of synaptic contacts per connection. (C) 2D maps of axonal (left) and dendritic (middle) “length density” of synaptically coupled L4 spiny neurons and L2/3 pyramidal cells (n = 9), aligned with respect to the barrel center. The predicted innervation domain (right) of L2/3 dendrites by L4 axons is given by the product of the L4 axonal density and the L2/3 dendritic density. Contours (thin lines) enclosing 80% of the integrated density are superimposed. Positions of L4 spiny neuron sonata (red dots), L2/3 pyramidal cell sonata (white triangles), putative synaptic contacts (cyan dots), and outlines of barrels (thick lines) are indicated symbolically. Inset, zoom in the predicted innervation domain superimposed by putative synaptic contacts. (A,B) have been adapted from Feldmeyer et al. (2002) with permission and (C) from Lubke et al. (2003) with permission.
To identify synaptic contacts formed between the pre- and postsynaptic neurons a light microscope with the highest magnification [e.g., 1000×, 100× objective (oil immersion) and 10× eyepiece] is used. Putative synaptic contacts are defined as locations where a presynaptic axonal bouton comes near or overlaps with a dendritic spine or shaft of the postsynaptic neuron at the same focus (Figure 2A). Then, the spatial distribution of putative synaptic contacts on postsynaptic somatodendritic compartments can be determined (Figure 2B). In order to verify putative synaptic contacts identified under a light microscope a subsequent electron microscopic (EM) analysis is required (Markram et al., 1997a; Feldmeyer et al., 2002); under EM pre- and postsynaptic axonal boutons and dendritic spines or shafts, respectively, can be identified unambiguously (Figure 2A).
A quantitative morphological analysis of reconstructed neurons can be performed using the Neuroexplorer® (Microbrightfield) software. This software extracts parameters including the length of axonal and dendritic branches, the degree of arborization, the orientation etc., which can be used to classify neuronal cell types, e.g., by using the cluster analysis. Furthermore, morphological data about the axonal and dendritic arborization of the pre- and postsynaptic neurons can be further processed to calculate axonal and dendritic length 'density maps' (Figure 2C) (Lubke et al., 2003; Narayanan et al., 2015). These “density maps” could reflect a general pattern of axonal or dendritic length distribution across the layers and columns. By calculating the product of the presynaptic axonal density with the postsynaptic dendritic density, the average 'innervation domains' can be determined (Figure 2C). Such 'innervation domains' delineate the probability distribution of synaptic contacts for an identified synaptic microcircuit (Lubke et al., 2003; Stepanyants and Chklovskii, 2005).
In addition to biocytin labeling alone, a combination with immunofluorescent staining is also possible, e.g., for specific molecular marker proteins such as Ca2+-binding protein/neuropeptide like parvalbumin, somatostatin, vasoactive intestinal polypeptide (VIP), cholecystokinin (CCK) or transcription factor like Fez2, CTIP2, Foxp2 for different inhibitory and excitatory neuron types, respectively (Figures 3A,C). For this, the neuron is filled with biocytin and a biocytin-conjugated fluorescent dye during the electrophysiological recording (e.g., Alexa Fluor 594) so that it is easily distinguished from other neurons after paraformaldehyde fixation. In a second step, immunofluorescent staining is performed after brief period of fixation (<1 day) using a primary antibody for the marker protein and a secondary antibody coupled to a fluorosphore. Finally, the neuron is permanently stained via the biocytin-horseradish peroxidase (HRP) reaction in which diaminobenzidine (DAB) is converted in a dark brownish precipitate. This allows high resolution morphological reconstructions of the labeled neurons (Figure 3C). It should be noted, however, that this multiple staining protocol may compromise the efficiency and quality of the biocytin-HRP staining to some extent, especially when the waiting time between fluorescence imaging and DAB processing is too long, making reconstructions of the neuronal morphology less reliable.
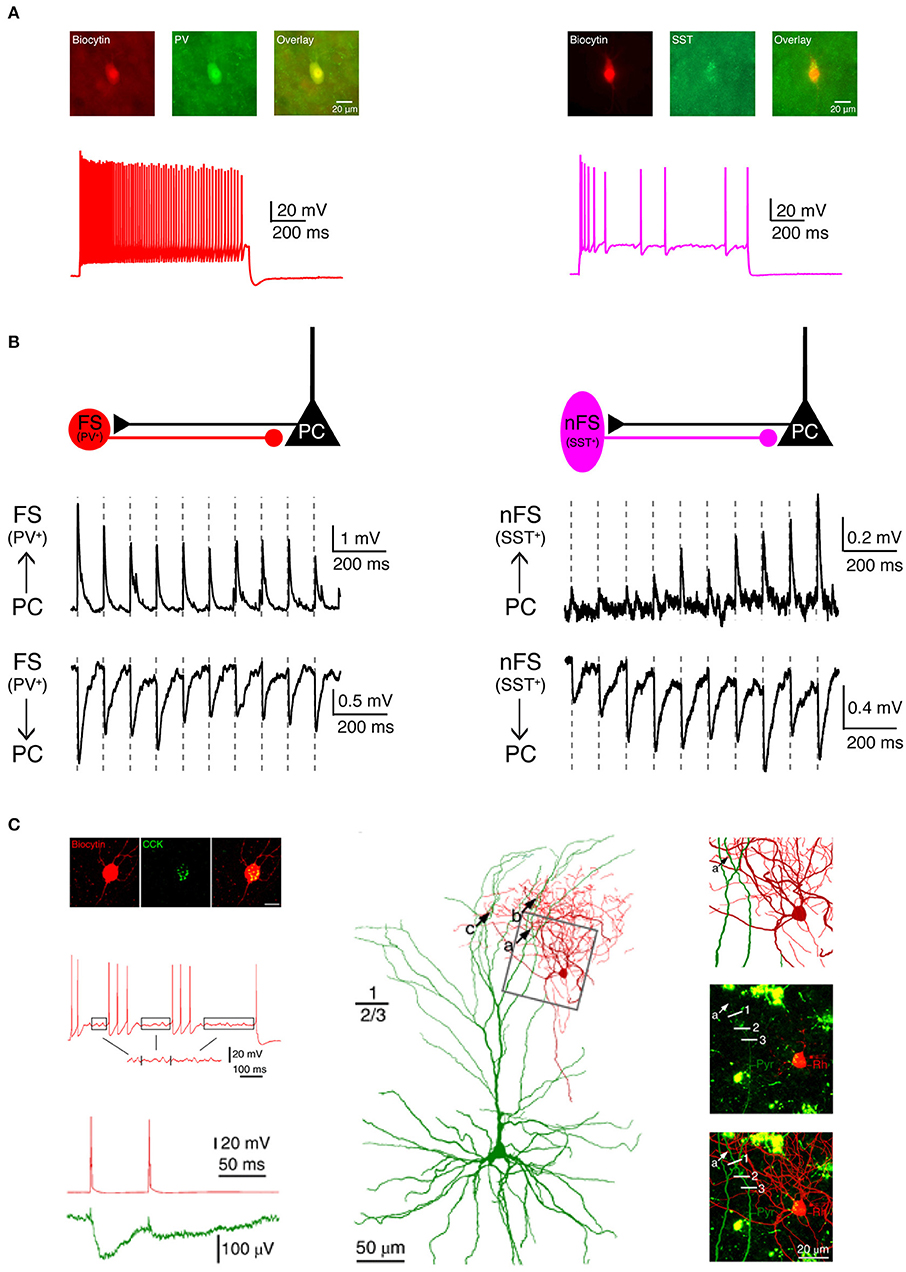
Figure 3. Electrophysiological, morphological and molecular characterization of synaptic connections by combining paired recordings with immuno-fluorescent stainings for specific marker proteins. (A) Two main types of GABAergic interneurons in the neocortex are PV+ fast spiking interneurons (left, red) which express the Ca2+-binding protein parvalbumin (PV) and SST+ non-fast spiking interneurons (right, violet) which express the neuropeptide somatostatin (SST). (B) Two interneuron types form synaptic connections with different characteristics. Left, PV+ fast spiking interneurons receive initially strong but quickly depressing EPSPs from neighboring excitatory neurons. At the same time, they produce depressing IPSPs in synaptically connected neighboring excitatory neurons. Right, SST+ non-fast spiking interneurons, in contrast, receive initially weak and gradually facilitating EPSPs from neighboring excitatory neurons and in turn elicit facilitating IPSPs in their target excitatory neurons. (C) Boldog et al. identified a specialized human cortical GABAergic cell type, the so-called L1 rosehip cell (RC). L1 RCs express cholecystokinin (CCK), but not PV, SST, or other molecular markers. L1 RCs exhibit an intermittent non-fast spiking firing pattern with subthreshold membrane potential oscillations (boxed segments). By combining paired recordings with Ca2+ imaging the authors were able to demonstrate that L1 RCs establish inhibitory synapses onto apical dendritic tufts of L2/3 pyramidal cells to regulate the AP backpropagation in a segment-specific manner. Electrical signals and morphologies of L1 RCs are in red and those of L2/3 pyramidal cells in green. (A,B) have been adapted from Feldmeyer et al. (2018) with permission and (C) from Boldog et al. (2018) with permission.
As described above, postsynaptic responses in postsynaptic neurons induced by presynaptic neuronal firing fluctuate in amplitude with time; in some trials the presynaptic AP may even fail to elicit a PSP. These fluctuations have been interpreted in the framework of the quantal analysis of synaptic transmission. Quantal analysis extracts the basic functional properties of synapses from postsynaptic responses using statistical models based on some assumptions (for review, see Korn and Faber, 1991). It can give an insight into the function of synapses and identify the locus of changes in synaptic strength (Stevens, 1993). Three parameters are adopted to describe the synaptic properties: the number of release sites (N), the release probability (p), and the amplitude of postsynaptic response following a single vesicle release—the quantum (q). The size of postsynaptic response and its variability are determined by these quantal parameters. Presynaptic modulation is related to p (i.e., the release probability), while postsynaptic changes (i.e., in the number of postsynaptic receptors etc.) are related to q. The formation of new contacts would be related to a change in N. In addition, an increase in p from zero at existing release sites in so-called “silent” synapses could also be treated as an increase in N. In the past years, paired recordings in different preparations including the neocortex, hippocampus, striatum, and cerebellum have been extensively used to uncover the values for parameters N, p, and q of synaptic connections (Bekkers and Stevens, 1990; Malinow and Tsien, 1990; Larkman et al., 1991; Gulyas et al., 1993; Isaac et al., 1995; Liao et al., 1995; Scheuss et al., 2002; Silver et al., 2003; Koos et al., 2004; Biro et al., 2005; Saviane and Silver, 2006; Bremaud et al., 2007; Hardingham et al., 2010; Huang et al., 2010; Molnar et al., 2016).
Using the frog neuromuscular junction preparation, del Castillo and Katz (Del Castillo and Katz, 1954) found that several peaks appear in the PSP amplitude histogram. Later, it has been shown that the number of peaks matched the number of anatomical synaptic contacts and the location of peaks is always multiple of that in the miniature PSP amplitude histogram, which led to postulate of the “one-site/one-vesicle” hypothesis (Del Castillo and Katz, 1954; Korn et al., 1981). However, at most synapses the PSP amplitude histogram displays no clear peaks. Therefore, more sophisticated methods have been introduced so that quantal analysis can be applied more generally. Clements and Silver developed the variance-mean (V-M) analysis of synaptic transmission, also called multiple probability fluctuation analysis, MPFA (Clements and Silver, 2000). The variance and mean are calculated from the fluctuation of PSP amplitudes in response to a presynaptic AP. A fundamental feature of this method is that it explores the fluctuation of synaptic responses at different p (induced by altering the extracellular Ca2+ concentration) (Figure 4C), therefore it can provide more information about the underlying synaptic mechanisms because of multiple points in V-M plot. Assuming that the vesicle release follows a binomial model, a plot of the variance vs. the mean of synaptic responses at different p displays a parabolic relationship. From the V-M plot, the values for N, p, and q can be estimated (Figure 4D). Scheuss and Neher further extended the application of the V-M analysis to the synaptic response during a train of APs (Scheuss and Neher, 2001). Instead of changing p by altering extracellular [Ca2+], this method allows to sample from a dynamic p, i.e., the PSP amplitude variation during AP train in the presynaptic neuron (Figures 4E,F). In this way, the experimental protocol is simplified because prolonged recordings are not necessary. Therefore, this approach is more readily usable.
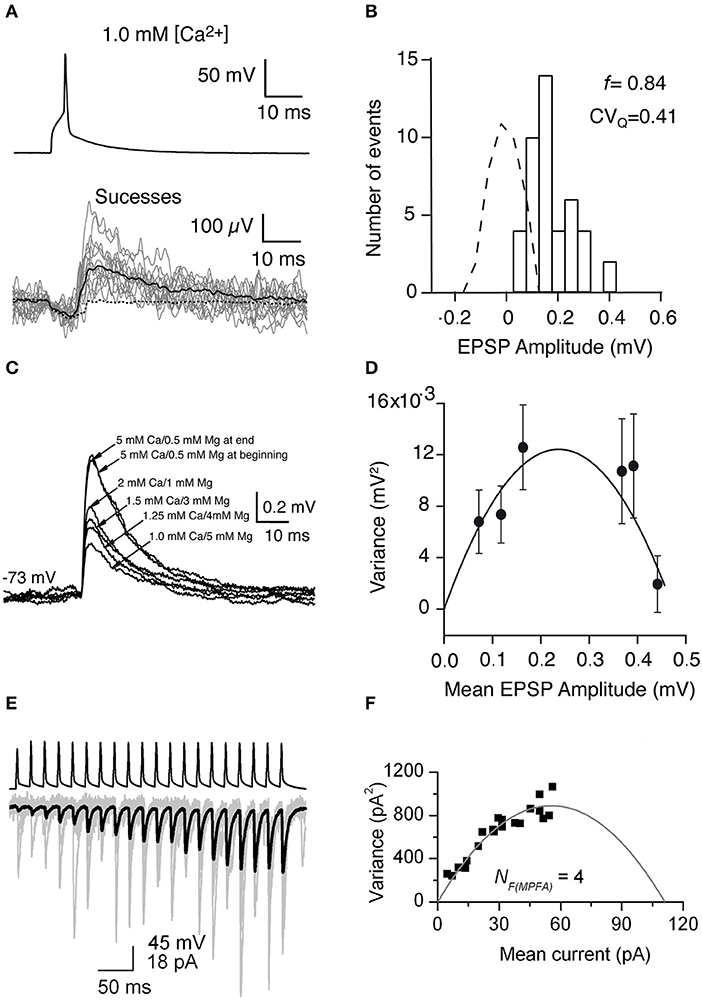
Figure 4. Uncovering quantal properties of synaptic transmission between identified cortical neurons. (A) Top, the single AP evoked by a brief suprathreshold depolarizing current pulse. Bottom, 14 individual EPSPs (gray traces), the mean of the 44 EPSP successes (black solid line), and the mean of the 279 failures (black dashed line) recorded at −72 mV in 1 mM [Ca2+] and 5 mM [Mg2+]. (B) Histogram of EPSP amplitude and scaled baseline noise (dashed line). For EPSP recordings from this specific synaptic connection, the failure rate (f) is 0.84 and the coefficient of variation of the quantal EPSP amplitude (CVQ) is 0.41 which was calculated from the background-subtracted variance. (C) Mean EPSPs recorded in different extracellular Ca2+ and Mg2+ concentrations at a postsynaptic membrane potential of −73 mV. (D) Relationship between the variance of the EPSP amplitude which was corrected for background variance and mean EPSP amplitude for a synaptically coupled L4-L2/3 cell pair. Each data point shows a different release probability condition. Error bars indicate the theoretical standard error in the estimate of the variance. Solid line shows the fit to a multinomial model with q = 0.09 mV, NF = 5.25, and α = 19,800. (E) A brief train of 20 APs (top) in a presynaptic CA1 pyramidal cell evoke facilitating EPSCs in an oriens-alveus interneuron. Individual EPSCs are shown in gray and the averaged EPSC in black. (F) Relationship between the variance values of the postsynaptic responses which were calculated at each AP of the train and the mean current. A multinomial quantal model was fitted to the data, resulting in an NF(MPFA) of 4, and a q of 24.7 pA. (A–D) have been adapted from Silver et al. (2003) with permission and (E,F) from Biro et al. (2005) with permission.
In addition to the aforementioned univesicular release hypothesis (UVR), a multivesicular release hypothesis (MVR) has been proposed, where several vesicles are released at a single synaptic site. Recent studies in the neocortex of rodents and humans have supplied controversial evidence regarding uni- and multivesicular release. It has been reported that synaptic connections between layer 4 excitatory neurons and layer 2/3 pyramidal cells in the rat barrel cortex exhibit the UVR (Silver et al., 2003). In contrast, synaptic connections between layer 4 excitatory neurons exhibit either UVR in the primary visual cortex or MVR in the primary somatosensory cortex of mice (Huang et al., 2010). Synaptic connections between layer 5B pyramidal cells also exhibit MVR in the developing and adult somatosensory cortex of rats (Rollenhagen et al., 2018; Barros-Zulaica et al., 2019). Depending on the species, synaptic connections between pyramidal cells and interneurons exhibit either UVR in the rat neocortex or MVR in the human neocortex (Molnar et al., 2016). Therefore, transmitter release at different synaptic connections can be mediated by UVR or MVR depending on the synapse type, the cortical area and the species.
Given that synaptic transmission between individual neuron pairs is the basic unit in information processing in the brain, it is crucial to understand how synaptic transmission is dynamically regulated by neuromodulators. Neuromodulator receptors are ubiquitously distributed in the brain and can be found on both dendrites and axon terminals of excitatory and inhibitory neurons (Marder, 2012). Most neuromodulators, such as acetylcholine, norepinephrine, dopamine, serotonin etc., are synthesized by a relatively small population of neurons located in several distinct nuclei in the basal forebrain, midbrain or brainstem. These neuromodulator-releasing neurons have long-range axonal afferents that project to many cortical areas. Once released from their axon terminals, neuromodulators can diffuse over substantial distances and act on receptors remote from their release sites (a mechanism termed “volume transmission”) (Zoli et al., 1999; Agnati et al., 2010). Other neuromodulators, such as adenosine and different types of neuropeptides (e.g., VIP, Neuropeptide Y), are locally synthesized and released by neurons and/or glial cells during neuronal network activity. Synaptic transmission between synaptically coupled neurons are constantly under the influence of neuromodulators. The effect of these neuromodulators can change the function and dynamics of cortical microcircuits in a differential way because the receptor types and their distribution may differ in pre- and postsynaptic neurons. The effects of neuromodulators can be studied by bath-application of the specific neuromodulator, their agonists and antagonists. In this way, the exact concentration of applied compounds at equilibrium is known and hence pharmacological approaches, including dose-response relationships can be applied easily to dissect the molecular mechanisms of neuromodulator effects. Bath-application of neuromodulators at different concentrations might correspond to physiological concentrations of neuromodulatory release at different brain states. For example, in the neocortex, the acetylcholine concentration changes dramatically during sleep, wakefulness, arousal and sustained attention (Himmelheber et al., 2000; Teles-Grilo Ruivo et al., 2017). It is worth noting that the concentration of bath-applied agonists needs to be carefully adjusted in the physiologically meaningful range, e.g., 1–10 μM for acetylcholine. Excessive concentrations (>100 μM for acetylcholine) should be avoided in order not to distort the quantification of the synaptic effects of neuromodulators. The effects of neuromodulators can also be studied by local puff-application of the neuromodulator itself or one of its agonists/antagonists; however, with this method the actual concentration of the neuromodulator is not known. In this way transient components of the response can be detected; this is not possible when using bath-application. By combining local puff-application of neuromodulator agonists with bath-application of neuromodulator antagonists, the subtypes of neuromodulator receptors can be determined pharmacologically. Recently, optogenetic stimulation of specific types of neuromodulator afferents (e.g., cholinergic afferents from the basal forebrain) has been applied to detect synaptic responses to the endogenous release of neuromodulators (Hedrick and Waters, 2015; Urban-Ciecko et al., 2018). Below, acetylcholine and adenosine are chosen as examples to illustrate the regulation of synaptic transmission by neuromodulators.
Acetylcholine (ACh) plays an important role in arousal, attention and vigilance. In the neocortex, ACh is released mainly from axonal boutons of neurons located in the nucleus basalis of Meynert in the basal forebrain. Cholinergic afferent terminals are distributed at high density throughout the cortical layers (Kalmbach et al., 2012). It has been proposed that most of the intra-cortical ACh is not released at synaptic contacts but rather diffusely into the extracellular space, i.e., by volume transmission. However, some evidence suggests that phasic release exists ubiquitously in the cortical cholinergic system (Sarter et al., 2009). The effects of ACh in the neocortex are mediated by two types of ACh receptors, the G-protein-coupled muscarinic AChRs (mAChRs) and the nicotinic AChR ion channels (nAChRs). It has been shown that ACh affects excitatory synaptic transmission by causing either a reduction or an increase in the release probability. An ACh-induced reduction in release probability has been shown through paired recordings of excitatory L4-L4 (Figures 5A,B) and L4-L2/3 (Figures 5C,D) synaptic connections in the rat barrel cortex (Eggermann and Feldmeyer, 2009) which exhibited a decreased EPSP amplitude and increased failure rate, variability and PPR. M4 mAChRs located in presynaptic L4 axonal terminals caused the suppression of synaptic release probably by decreasing the open probability of presynaptic Ca2+ channels. Such a suppressive effect of ACh was also found in excitatory connections established by L2/3 and L5 pyramidal neurons (Levy et al., 2006, 2008). In layer 6, the ACh effect on synaptic transmission depends on the presynaptic neuron type: ACh decreases the synaptic release probability of L6 cortico-cortical pyramidal neurons to other excitatory and inhibitory neurons via activating the presynaptically located M4 mAChRs. In contrast, ACh enhances the synaptic transmission originating from L6A cortico-thalamic pyramidal neurons via activating the α4/β2 nAChRs located at presynaptic axonal terminals (Yang et al., 2019). A similar nicotinic enhancement effect of ACh was found both in vitro and in vivo at synaptic connections between L2 pyramidal neurons and somatostatin-expressing interneurons (Urban-Ciecko et al., 2018).
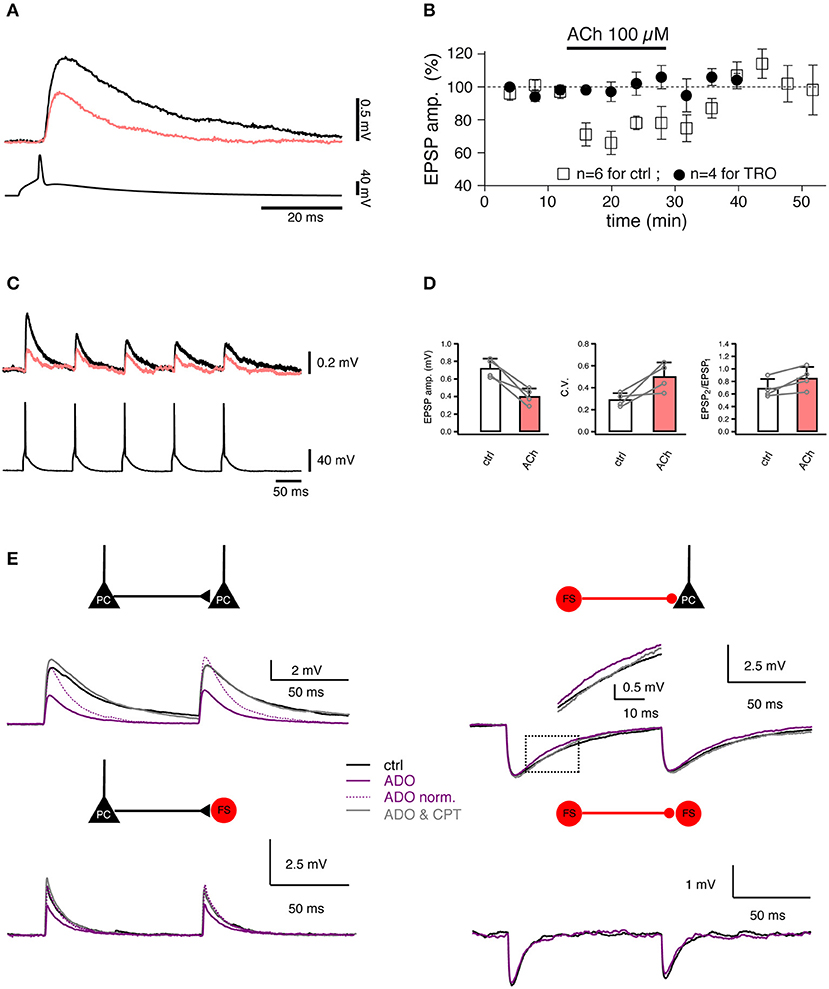
Figure 5. Studying the regulation of synaptic transmission by neuromodulators. (A) Paired recordings from a synaptic connection between two L4 spiny neurons. Bath-applied acetylcholine (ACh, 100 μM) reduces the EPSP amplitude (coral trace). (B) Time course of the ACh effect on the first EPSP amplitude [open boxes, control; filled circles, in the presence of 1 μM tropicamide (TRO), a selective M4 muscarinic acetylcholine receptor antagonist]. (C) Paired recordings from a synaptic connection between a L4 spiny neuron and a L2/3 pyramidal cell. A train of five APs elicited in a presynaptic L4 spiny neuron (bottom) evoked EPSPs in a postsynaptic L2/3 pyramidal cell (top) in the control condition (black) and in the presence of 100 μM ACh (coral). (D) Summary of the effects in L4-to-L2/3 connections (n = 4) in the control condition (black open box) and in the presence of 100 μM ACh (coral filled box). Left, the EPSP amplitude. Middle, the coefficient of variation (C.V.). Right, the paired-pulse ratio. Open circles are values for individual connections, connecting lines indicate the direction of change. Error bars indicate the standard deviation. (E) Paired recordings from synaptic connections formed between L4 spiny neurons, between L4 spiny neurons and L4 interneurons, and between L4 interneurons show that adenosine (ADO) differentially modulate the excitatory and inhibitory synaptic transmission. Overlay of average EPSPs recorded under three recording conditions: control (black), 100 μM adenosine (purple), and 100 μM adenosine plus 5 μM 8-cyclopentyltheophylline (CPT), a specific adenosine A1 receptor antagonist (gray) are shown for four connection types. (A–D) have been adapted from Eggermann and Feldmeyer (2009) with permission and (E) from Qi et al. (2017) with permission.
In contrast to ACh, adenosine is an endogenous neuromodulator which is generated during high neuronal activity, e.g., by the intra- and extracellular metabolism of adenosine triphosphate. Adenosine has been suggested to play an important role in the sleep homeostasis (Porkka-Heiskanen et al., 1997, 2000). Recently, the effect of adenosine on synaptic transmission has been assessed using paired recordings (Kerr et al., 2013; Qi et al., 2017). Adenosine induces a suppression of the neurotransmitter release probability at intralaminar L2/3, L4, and L5 and translaminar L4-L2/3 excitatory connections. The adenosine effect is most likely mediated by A1 adenosine receptors located in presynaptic axonal terminals; they induce a reduction in the open probability of presynaptic Ca2+ channels involved in triggering the release of neurotransmitters. This effect is already apparent at low endogenous concentrations of adenosine (~1 μM) which are tonically released (Qi et al., 2017). In contrast, adenosine has a much smaller effect on inhibitory synaptic transmission onto excitatory neurons: here, only the IPSP time course is altered due to activation of postsynaptically located A1 adenosine receptors. There is no effect on inhibitory synaptic transmission onto interneurons (Figure 5E).
In addition to ACh and adenosine, a synapse type-dependent neuromodulation has also been found for other neuromodulators such as dopamine. Paired recordings from pyramidal cells and interneurons in ferret prefrontal cortex showed that dopamine depresses excitatory transmission between two pyramidal cells through D1 receptor actions at a presynaptic site (Gao et al., 2001) but has no effect on excitatory transmission between pyramidal cells and fast-spiking (FS) interneurons (Gao and Goldman-Rakic, 2003). In addition, dopamine differentially modulates inhibition of pyramidal cells from FS vs. non-FS interneurons. Dopamine decreases release of GABA onto pyramidal cells through effects on presynaptic D1 receptors on axonal terminals of FS interneurons, whereas inhibition from non-FS interneurons onto pyramidal cells is enhanced, presumably owing to a postsynaptic effect (Gao et al., 2003). Similarly, differential modulatory effects of dopamine on different types of synaptic transmission in the medial prefrontal cortex (Dembrow et al., 2010; Dembrow and Johnston, 2014) and neostriatum (Tecuapetla et al., 2007, 2009) have also been found. In summary, the effect of neuromodulators on synaptic transmission depends on the synapse type which is determined by both presynaptic and postsynaptic neuronal identities.
Paired recordings from synaptically coupled excitatory and/or inhibitory neurons are a powerful technique to investigate the structure-function relationship of synaptic microcircuits at the subcellular, cellular, and network level. It allows the simultaneous electrophysiological, morphological and/or molecular analysis of both the pre- and postsynaptic neurons in synaptic connections. This is as yet difficult if not impossible for other techniques using extracellular (electrical or optical) stimulation of presynaptic neurons, see e.g., Crochet et al. (2005) and Pala and Petersen (2018). In addition, long-time stable paired recordings permit an in-depth characterization of a defined unitary synaptic connection using, e.g., the quantal analysis. Furthermore, agonist and/or antagonist can be applied readily to neurons in slice preparations (and even spatially focussed), which allows studying the effects of neuromodulators on the synaptic transmission. However, to appreciate the insight obtained from paired recordings in brain slices, one needs to be aware of several shortcomings.
A major disadvantage of slice preparations is the often substantial truncation of axonal branches so that only parts of the axon are reserved in the 300–400 μm-thick brain slice. For some pyramidal cell types, the degree of truncation could be up to 90% when taking into account projections to other cortical or subcortical areas (Stepanyants et al., 2009; Narayanan et al., 2015). Therefore, the slice preparation is not suited for the study of synaptic connections between neurons whose cell bodies are more than >300 μm in the lateral direction. For studying synaptic connections between neurons with inter-soma distances >500 μm within the same column, e.g., translaminar L2/3-to-L5 or L4-to-L6 connections (Reyes and Sakmann, 1999; Qi and Feldmeyer, 2016), paired recordings in the slice preparation is still usable when the slicing procedure is optimized. However, local axonal projections, in particular those of interneurons are generally recovered with a relatively low degree of truncation (~10% or less) (Koelbl et al., 2015; Emmenegger et al., 2018) because of their limited horizontal and vertically projections (see Movie S1). Synaptic connections involving these neuron types can therefore be characterized with high accuracy and reliability and their connectivity estimates are largely correct. Except for these local synaptic connections, absolute values for connectivity ratios between two neuron types obtained in slice preparations are highly questionable, in particular for those with large inter-somatic distances such as translaminar or non-local intralaminar synaptic connections. This problem is even more prominent when slicing procedures have not been optimized for a given synaptic connection at a defined developmental stage. Another problem for connectivity estimates is that distal synaptic contacts, e.g., those on the apical tuft dendrites of pyramidal neurons, may escape detection (Williams and Stuart, 2002, 2003). When recorded at the soma the amplitude of their synaptic response is very small and therefore likely to be obscured by electrical noise. However, this type of problem is not confined to the paired recording approach but could also arise in other techniques adopted to study the synaptic connectivity.
In recent years light-induced activation of neurons by photo-release of caged glutamate (Callaway and Katz, 1993) or by activation of channelrhodopsin-2 channels expressed in different neuronal compartments, e.g., soma, dendrites (Boyden et al., 2005), or axonal terminals (Petreanu et al., 2007) has been used to investigate neuronal microcircuits on a larger scale. However, it is so far not possible to identify the detailed structural properties of presynaptic neurons with these optical approaches. Furthermore, the number and location of synaptic contacts for a synaptic connection cannot be identified. Paired recordings, however, allow a detailed characterization of both pre- and postsynaptic neurons and their synaptic contacts in a synaptic connection. This is of paramount importance because many studies have demonstrated that both GABAergic interneurons and glutamatergic excitatory neurons in the neocortex are highly diverse with respect to their morphologies and synaptic properties. Therefore, the identification of both pre- and postsynaptic neurons is necessary for a deep characterization of a synaptic connection.
To enhance the success rate of recording synaptic connections in local neuronal microcircuits, the number of simultaneously recorded neurons (n) has been increased from dual (2), triple (3), quadruple (4), octuple (8) up to 12 (Thomson et al., 2002; Song et al., 2005; Kampa et al., 2006; Brown and Hestrin, 2009; Lefort et al., 2009; Yu et al., 2009; Ko et al., 2011; Perin et al., 2011; Rieubland et al., 2014; Jiang et al., 2015; Guzman et al., 2016; Peng et al., 2017; Hemberger et al., 2019). Multiple (n > 2) recordings may yield more synaptic connections because the number of potential synaptic connections (m) established between n neurons increases steeply with increasing n: m = n × (n−1). However, multiple recordings especially when n > 4 have several shortcomings compared to paired recordings. First, the mechanical stability will decrease the more electrodes are placed together in the recordings chamber while the electrical noise of the recording will increase substantially because of capacitive coupling (electrical “cross-talk”) in multichannel electrophysiology experiments. This is particularly problematic when the two recorded signals are not of similar amplitude as is the case in paired recordings (presynaptic AP vs. small postsynaptic response) (Nelson et al., 2017). It is likely to decrease the probability of successful, high resolution recordings from a large number of neurons. In addition, the quality of the measured signals (i.e., the signal to-noise ratio of the recordings) will also deteriorate so that the detection of small PSPs (10–20 μV) is severely compromised (Seeman et al., 2018). Furthermore, the time for recording from an individual synaptic connections will be relatively short, i.e., the characterization of this connection limited because of the restricted overall total recording time for all possible synaptic connections. Therefore, a detailed functional characterization of the properties of unitary PSPs (e.g., quantal analysis) is very difficult. Moreover, when biocyin is added to pipettes during multiple recordings, many neurons will be stained in the same slice after the histochemical processing, which makes a reliable and complete reconstruction of neuronal morphology (including both the dendritic and axonal branches) extremely complicated if not impossible, especially when more than two interneurons with a dense axonal plexus are involved. Finally, the estimate of connectivity ratios for all connection types using multiple recordings in the same slice preparation is likely to be unreliable in particular for translaminar or non-local intralaminar synaptic connections because the slicing procedure is optimal only for a few specific connection types (mainly the local ones) but not for the majority. This problem could be overcome in paired recordings through optimizing the slicing procedure for specific types of synaptic connections. Despite of aforementioned shortcomings that exist so far, multiple recordings show great promise for future high-throughput analysis of cortical microcircuits in rodent and more precious human brains (Peng et al., 2019).
Not only cortical inhibitory but also excitatory neurons show a high diversity (Zeng and Sanes, 2017). To directly target specific neuronal subpopulations, paired recordings have been conducted in acute brain slices from transgenic animals where one specific or several populations of neurons are labeled by fluorescent groups (e.g., GFP, YFP, tdTomato etc.) as in transgenic, knock-in animals or via viral infection (Pfeffer et al., 2013; Seeman et al., 2018). Paired recordings can be combined easily with other cutting-edge techniques, such as optogenetics, Ca2+ imaging, activity-dependent immediate early gene expression and pseudorabies virus retrograde tracing etc. (Wickersham et al., 2007; Yassin et al., 2010; Ko et al., 2011; Jouhanneau et al., 2014; Lee et al., 2014; Cossell et al., 2015; Morgenstern et al., 2016). More recently, the paired recording approach has also been adopted to record from synaptically coupled neurons in the intact brain of anesthetized mice (Jouhanneau et al., 2015, 2018). Paired recordings have also been used to investigate the functional and structural properties of synapses in surgically dissected human brain slices (Molnar et al., 2008; Testa-Silva et al., 2010, 2014; Boldog et al., 2018; Seeman et al., 2018). For human tissue, paired recording in slices is still the only method of choice to study the functional neuronal microcircuits in preparations from human brains. Therefore, paired recordings will remain an important approach for studying neuronal microcircuits in different brain regions and species.
GQ generated the figures. GQ and DF wrote the manuscript with comments and suggestions from DY and CD.
This work was supported by the Helmholtz Society and the European Union's Horizon 2020 Research, Innovation Programme under Grant Agreement No. 785907 (Human Brain Project SGA2; to DF).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Werner Hucko for help with immunohistochemical staining and Valerie Wiener for help with morphological reconstruction.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnsyn.2020.00005/full#supplementary-material
Movie S1. Rotation of a 3D morphologically reconstructed parvalbumin+ (PV+), fast-spiking interneuron in layer 4 of rat barrel cortex. The somatodendritic compartment is shown in red and the axon in cyan. Borders between different layers are also shown.
Abbott, L. F., and Nelson, S. B. (2000). Synaptic plasticity: taming the beast. Nat. Neurosci. 3(Suppl.), 1178–1183. doi: 10.1038/81453
Agnati, L. F., Guidolin, D., Guescini, M., Genedani, S., and Fuxe, K. (2010). Understanding wiring and volume transmission. Brain Res. Rev. 64, 137–159. doi: 10.1016/j.brainresrev.2010.03.003
Arancio, O., Kandel, E. R., and Hawkins, R. D. (1995). Activity-dependent long-term enhancement of transmitter release by presynaptic 3',5'-cyclic GMP in cultured hippocampal neurons. Nature 376, 74–80. doi: 10.1038/376074a0
Atzori, M., Lei, S., Evans, D. I., Kanold, P. O., Phillips-Tansey, E., Mcintyre, O., et al. (2001). Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat. Neurosci. 4, 1230–1237. doi: 10.1038/nn760
Barbour, B., Brunel, N., Hakim, V., and Nadal, J. P. (2007). What can we learn from synaptic weight distributions? Trends Neurosci. 30, 622–629. doi: 10.1016/j.tins.2007.09.005
Barros-Zulaica, N., Rahmon, J., Chindemi, G., Perin, R., Markram, H., Muller, E., et al. (2019). Estimating the readily-releasable vesicle pool size at synaptic connections in the neocortex. Front. Synaptic. Neurosci. 11:29. doi: 10.3389/fnsyn.2019.00029
Bekkers, J. M., and Stevens, C. F. (1990). Presynaptic mechanism for long-term potentiation in the hippocampus. Nature 346, 724–729. doi: 10.1038/346724a0
Bi, G., and Poo, M. (2001). Synaptic modification by correlated activity: Hebb's postulate revisited. Annu. Rev. Neurosci. 24, 139–166. doi: 10.1146/annurev.neuro.24.1.139
Bi, G. Q., and Poo, M. M. (1998). Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 18, 10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998
Biro, A. A., Holderith, N. B., and Nusser, Z. (2005). Quantal size is independent of the release probability at hippocampal excitatory synapses. J. Neurosci. 25, 223–232. doi: 10.1523/JNEUROSCI.3688-04.2005
Boldog, E., Bakken, T. E., Hodge, R. D., Novotny, M., Aevermann, B. D., Baka, J., et al. (2018). Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat. Neurosci. 21, 1185–1195. doi: 10.1038/s41593-018-0205-2
Bolshakov, V. Y., and Siegelbaum, S. A. (1995). Regulation of hippocampal transmitter release during development and long-term potentiation. Science 269, 1730–1734. doi: 10.1126/science.7569903
Borst, J. G. (2010). The low synaptic release probability in vivo. Trends Neurosci. 33, 259–266. doi: 10.1016/j.tins.2010.03.003
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., and Deisseroth, K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268. doi: 10.1038/nn1525
Bremaud, A., West, D. C., and Thomson, A. M. (2007). Binomial parameters differ across neocortical layers and with different classes of connections in adult rat and cat neocortex. Proc. Natl. Acad. Sci. U.S.A. 104, 14134–14139. doi: 10.1073/pnas.0705661104
Brette, R., and Destexhe, A. (2012). “Intracellular recording,” in Handbook of Neural Activity Measurement, eds R. Brette and A. Destexhe (Cambridge: Cambridge University Press), 44–91.
Brown, S. P., and Hestrin, S. (2009). Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature 457, 1133–1136. doi: 10.1038/nature07658
Brunel, N., Hakim, V., Isope, P., Nadal, J. P., and Barbour, B. (2004). Optimal information storage and the distribution of synaptic weights: perceptron versus Purkinje cell. Neuron 43, 745–757. doi: 10.1016/S0896-6273(04)00528-8
Buhl, E. H., Halasy, K., and Somogyi, P. (1994). Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368, 823–828. doi: 10.1038/368823a0
Callaway, E. M., and Katz, L. C. (1993). Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl. Acad. Sci. U.S.A. 90, 7661–7665. doi: 10.1073/pnas.90.16.7661
Clements, J. D., and Silver, R. A. (2000). Unveiling synaptic plasticity: a new graphical and analytical approach. Trends Neurosci. 23, 105–113. doi: 10.1016/S0166-2236(99)01520-9
Cossell, L., Iacaruso, M. F., Muir, D. R., Houlton, R., Sader, E. N., Ko, H., et al. (2015). Functional organization of excitatory synaptic strength in primary visual cortex. Nature 518, 399–403. doi: 10.1038/nature14182
Crochet, S., Chauvette, S., Boucetta, S., and Timofeev, I. (2005). Modulation of synaptic transmission in neocortex by network activities. Eur. J. Neurosci. 21, 1030–1044. doi: 10.1111/j.1460-9568.2005.03932.x
Debanne, D., Boudkkazi, S., Campanac, E., Cudmore, R. H., Giraud, P., Fronzaroli-Molinieres, L., et al. (2008). Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat. Protoc. 3, 1559–1568. doi: 10.1038/nprot.2008.147
Del Castillo, J., and Katz, B. (1954). Quantal components of the end-plate potential. J. Physiol. 124, 560–573. doi: 10.1113/jphysiol.1954.sp005129
Dembrow, N., and Johnston, D. (2014). Subcircuit-specific neuromodulation in the prefrontal cortex. Front. Neural Circuits 8:54. doi: 10.3389/fncir.2014.00054
Dembrow, N. C., Chitwood, R. A., and Johnston, D. (2010). Projection-specific neuromodulation of medial prefrontal cortex neurons. J. Neurosci. 30, 16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010
Destexhe, A., Rudolph, M., and Pare, D. (2003). The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 4, 739–751. doi: 10.1038/nrn1198
Deuchars, J., West, D. C., and Thomson, A. M. (1994). Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. J. Physiol. 478, 423–435. doi: 10.1113/jphysiol.1994.sp020262
Dittman, J. S., Kreitzer, A. C., and Regehr, W. G. (2000). Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J. Neurosci. 20, 1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000
Dodt, H. U., and Zieglgansberger, W. (1990). Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 537, 333–336. doi: 10.1016/0006-8993(90)90380-T
Egger, V., Feldmeyer, D., and Sakmann, B. (1999). Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat. Neurosci. 2, 1098–1105. doi: 10.1038/16026
Eggermann, E., and Feldmeyer, D. (2009). Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc. Natl. Acad. Sci. U.S.A. 106, 11753–11758. doi: 10.1073/pnas.0810062106
Emmenegger, V., Qi, G., Wang, H., and Feldmeyer, D. (2018). Morphological and functional characterization of non-fast-spiking GABAergic interneurons in layer 4 microcircuitry of rat barrel cortex. Cereb. Cortex 28, 1439–1457. doi: 10.1093/cercor/bhx352
Feldmeyer, D., Egger, V., Lubke, J., and Sakmann, B. (1999). Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel' of developing rat somatosensory cortex. J. Physiol. 521, 169–190. doi: 10.1111/j.1469-7793.1999.00169.x
Feldmeyer, D., Lubke, J., and Sakmann, B. (2006). Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J. Physiol. 575, 583–602. doi: 10.1113/jphysiol.2006.105106
Feldmeyer, D., Lubke, J., Silver, R. A., and Sakmann, B. (2002). Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J. Physiol. 538, 803–822. doi: 10.1113/jphysiol.2001.012959
Feldmeyer, D., Qi, G., Emmenegger, V., and Staiger, J. F. (2018). Inhibitory interneurons and their circuit motifs in the many layers of the barrel cortex. Neuroscience 368, 132–151. doi: 10.1016/j.neuroscience.2017.05.027
Feldmeyer, D., and Radnikow, G. (2016). “Paird recordings from synaptically coupled neurones in acute neocortical slices,” in Neuromethods, ed A. Korngreen (New York, NY: Humana Press), 171–191.
Fino, E., Packer, A. M., and Yuste, R. (2013). The logic of inhibitory connectivity in the neocortex. Neuroscientist 19, 228–237. doi: 10.1177/1073858412456743
Frick, A., Feldmeyer, D., Helmstaedter, M., and Sakmann, B. (2008). Monosynaptic connections between pairs of L5A pyramidal neurons in columns of juvenile rat somatosensory cortex. Cereb. Cortex 18, 397–406. doi: 10.1093/cercor/bhm074
Galarreta, M., and Hestrin, S. (1999). A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402, 72–75. doi: 10.1038/47029
Gao, W. J., and Goldman-Rakic, P. S. (2003). Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc. Natl. Acad. Sci. U.S.A. 100, 2836–2841. doi: 10.1073/pnas.262796399
Gao, W. J., Krimer, L. S., and Goldman-Rakic, P. S. (2001). Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc. Natl. Acad. Sci. U.S.A. 98, 295–300. doi: 10.1073/pnas.98.1.295
Gao, W. J., Wang, Y., and Goldman-Rakic, P. S. (2003). Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J. Neurosci. 23, 1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003
Geiger, J. R., Lubke, J., Roth, A., Frotscher, M., and Jonas, P. (1997). Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 18, 1009–1023. doi: 10.1016/S0896-6273(00)80339-6
Gibson, J. R., Beierlein, M., and Connors, B. W. (1999). Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402, 75–79. doi: 10.1038/47035
Gulyas, A. I., Miles, R., Sik, A., Toth, K., Tamamaki, N., and Freund, T. F. (1993). Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature 366, 683–687. doi: 10.1038/366683a0
Gupta, A., Wang, Y., and Markram, H. (2000). Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science 287, 273–278. doi: 10.1126/science.287.5451.273
Guzman, S. J., Schlogl, A., Frotscher, M., and Jonas, P. (2016). Synaptic mechanisms of pattern completion in the hippocampal CA3 network. Science 353, 1117–1123. doi: 10.1126/science.aaf1836
Hardingham, N. R., and Larkman, A. U. (1998). Rapid report: the reliability of excitatory synaptic transmission in slices of rat visual cortex in vitro is temperature dependent. J. Physiol. 507, 249–256. doi: 10.1111/j.1469-7793.1998.249bu.x
Hardingham, N. R., Read, J. C., Trevelyan, A. J., Nelson, J. C., Jack, J. J., and Bannister, N. J. (2010). Quantal analysis reveals a functional correlation between presynaptic and postsynaptic efficacy in excitatory connections from rat neocortex. J. Neurosci. 30, 1441–1451. doi: 10.1523/JNEUROSCI.3244-09.2010
Hedrick, T., and Waters, J. (2015). Acetylcholine excites neocortical pyramidal neurons via nicotinic receptors. J. Neurophysiol. 113, 2195–2209. doi: 10.1152/jn.00716.2014
Heinemann, U., Lux, H. D., and Gutnick, M. J. (1977). Extracellular free calcium and potassium during paroxsmal activity in the cerebral cortex of the cat. Exp. Brain Res. 27, 237–243. doi: 10.1007/BF00235500
Helmstaedter, M., Staiger, J. F., Sakmann, B., and Feldmeyer, D. (2008). Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. J. Neurosci. 28, 8273–8284. doi: 10.1523/JNEUROSCI.5701-07.2008
Hemberger, M., Shein-Idelson, M., Pammer, L., and Laurent, G. (2019). Reliable sequential activation of neural assemblies by single pyramidal cells in a three-layered cortex. Neuron 104, 353–369.e5. doi: 10.1016/j.neuron.2019.07.017
Himmelheber, A. M., Sarter, M., and Bruno, J. P. (2000). Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res. Cogn. Brain Res. 9, 313–325. doi: 10.1016/S0926-6410(00)00012-4
Holmgren, C., Harkany, T., Svennenfors, B., and Zilberter, Y. (2003). Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J. Physiol. 551, 139–153. doi: 10.1113/jphysiol.2003.044784
Huang, C. H., Bao, J., and Sakaba, T. (2010). Multivesicular release differentiates the reliability of synaptic transmission between the visual cortex and the somatosensory cortex. J. Neurosci. 30, 11994–12004. doi: 10.1523/JNEUROSCI.2381-10.2010
Huganir, R. L., and Nicoll, R. A. (2013). AMPARs and synaptic plasticity: the last 25 years. Neuron 80, 704–717. doi: 10.1016/j.neuron.2013.10.025
Isaac, J. T., Nicoll, R. A., and Malenka, R. C. (1995). Evidence for silent synapses: implications for the expression of LTP. Neuron 15, 427–434. doi: 10.1016/0896-6273(95)90046-2
Isope, P., and Barbour, B. (2002). Properties of unitary granule cell–>Purkinje cell synapses in adult rat cerebellar slices. J. Neurosci. 22, 9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002
Jiang, X., Shen, S., Cadwell, C. R., Berens, P., Sinz, F., Ecker, A. S., et al. (2015). Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350:aac9462. doi: 10.1126/science.aac9462
Jiang, X., Wang, G., Lee, A. J., Stornetta, R. L., and Zhu, J. J. (2013). The organization of two new cortical interneuronal circuits. Nat. Neurosci. 16, 210–218. doi: 10.1038/nn.3305
Jouhanneau, J. S., Ferrarese, L., Estebanez, L., Audette, N. J., Brecht, M., Barth, A. L., et al. (2014). Cortical fosGFP expression reveals broad receptive field excitatory neurons targeted by POm. Neuron 84, 1065–1078. doi: 10.1016/j.neuron.2014.10.014
Jouhanneau, J. S., Kremkow, J., Dorrn, A. L., and Poulet, J. F. (2015). In vivo monosynaptic excitatory transmission between layer 2 cortical pyramidal neurons. Cell. Rep. 13, 2098–2106. doi: 10.1016/j.celrep.2015.11.011
Jouhanneau, J. S., Kremkow, J., and Poulet, J. F. A. (2018). Single synaptic inputs drive high-precision action potentials in parvalbumin expressing GABA-ergic cortical neurons in vivo. Nat. Commun. 9:1540. doi: 10.1038/s41467-018-03995-2
Jouhanneau, J. S., and Poulet, J. F. A. (2019). Multiple two-photon targeted whole-cell patch-clamp recordings from monosynaptically connected neurons in vivo. Front. Synaptic Neurosci. 11:15. doi: 10.3389/fnsyn.2019.00015
Kalmbach, A., Hedrick, T., and Waters, J. (2012). Selective optogenetic stimulation of cholinergic axons in neocortex. J. Neurophysiol. 107, 2008–2019. doi: 10.1152/jn.00870.2011
Kampa, B. M., Letzkus, J. J., and Stuart, G. J. (2006). Cortical feed-forward networks for binding different streams of sensory information. Nat. Neurosci. 9, 1472–1473. doi: 10.1038/nn1798
Kapfer, C., Glickfeld, L. L., Atallah, B. V., and Scanziani, M. (2007). Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci. 10, 743–753. doi: 10.1038/nn1909
Kerr, M. I., Wall, M. J., and Richardson, M. J. (2013). Adenosine A1 receptor activation mediates the developmental shift at layer 5 pyramidal cell synapses and is a determinant of mature synaptic strength. J. Physiol. 591, 3371–3380. doi: 10.1113/jphysiol.2012.244392
Ko, H., Hofer, S. B., Pichler, B., Buchanan, K. A., Sjostrom, P. J., and Mrsic-Flogel, T. D. (2011). Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91. doi: 10.1038/nature09880
Koelbl, C., Helmstaedter, M., Lubke, J., and Feldmeyer, D. (2015). A barrel-related interneuron in layer 4 of rat somatosensory cortex with a high intrabarrel connectivity. Cereb. Cortex 25, 713–725. doi: 10.1093/cercor/bht263
Koester, H. J., and Johnston, D. (2005). Target cell-dependent normalization of transmitter release at neocortical synapses. Science 308, 863–866. doi: 10.1126/science.1100815
Koos, T., Tepper, J. M., and Wilson, C. J. (2004). Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J. Neurosci. 24, 7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004
Korn, H., and Faber, D. S. (1991). Quantal analysis and synaptic efficacy in the CNS. Trends. Neurosci. 14, 439–445. doi: 10.1016/0166-2236(91)90042-S
Korn, H., Triller, A., Mallet, A., and Faber, D. S. (1981). Fluctuating responses at a central synapse: n of binomial fit predicts number of stained presynaptic boutons. Science 213, 898–901. doi: 10.1126/science.6266015
Kreitzer, A. C., and Regehr, W. G. (2001). Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J. Neurosci. 21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001
Larkman, A., Stratford, K., and Jack, J. (1991). Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature 350, 344–347. doi: 10.1038/350344a0
Lee, A. T., Gee, S. M., Vogt, D., Patel, T., Rubenstein, J. L., and Sohal, V. S. (2014). Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron 81, 61–68. doi: 10.1016/j.neuron.2013.10.031
Lefort, S., Tomm, C., Floyd Sarria, J. C., and Petersen, C. C. (2009). The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316. doi: 10.1016/j.neuron.2008.12.020
Levy, R. B., Reyes, A. D., and Aoki, C. (2006). Nicotinic and muscarinic reduction of unitary excitatory postsynaptic potentials in sensory cortex; dual intracellular recording in vitro. J. Neurophysiol. 95, 2155–2166. doi: 10.1152/jn.00603.2005
Levy, R. B., Reyes, A. D., and Aoki, C. (2008). Cholinergic modulation of local pyramid-interneuron synapses exhibiting divergent short-term dynamics in rat sensory cortex. Brain Res. 1215, 97–104. doi: 10.1016/j.brainres.2008.03.067
Liao, D., Hessler, N. A., and Malinow, R. (1995). Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375, 400–404. doi: 10.1038/375400a0
Lubke, J., Roth, A., Feldmeyer, D., and Sakmann, B. (2003). Morphometric analysis of the columnar innervation domain of neurons connecting layer 4 and layer 2/3 of juvenile rat barrel cortex. Cereb. Cortex 13, 1051–1063. doi: 10.1093/cercor/13.10.1051
Ma, Y., Hu, H., and Agmon, A. (2012). Short-term plasticity of unitary inhibitory-to-inhibitory synapses depends on the presynaptic interneuron subtype. J. Neurosci. 32, 983–988. doi: 10.1523/JNEUROSCI.5007-11.2012
Malinow, R. (1991). Transmission between pairs of hippocampal slice neurons: quantal levels, oscillations, and LTP. Science 252, 722–724. doi: 10.1126/science.1850871
Malinow, R., and Tsien, R. W. (1990). Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature 346, 177–180. doi: 10.1038/346177a0
Marder, E. (2012). Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11. doi: 10.1016/j.neuron.2012.09.010
Markram, H., Lubke, J., Frotscher, M., Roth, A., and Sakmann, B. (1997a). Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. 500, 409–440. doi: 10.1113/jphysiol.1997.sp022031
Markram, H., Lubke, J., Frotscher, M., and Sakmann, B. (1997b). Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215. doi: 10.1126/science.275.5297.213
Markram, H., Muller, E., Ramaswamy, S., Reimann, M. W., Abdellah, M., Sanchez, C. A., et al. (2015). Reconstruction and simulation of neocortical microcircuitry. Cell 163, 456–492. doi: 10.1016/j.cell.2015.09.029
Markram, H., Pikus, D., Gupta, A., and Tsodyks, M. (1998a). Potential for multiple mechanisms, phenomena and algorithms for synaptic plasticity at single synapses. Neuropharmacology 37, 489–500. doi: 10.1016/S0028-3908(98)00049-5
Markram, H., Wang, Y., and Tsodyks, M. (1998b). Differential signaling via the same axon of neocortical pyramidal neurons. Proc. Natl. Acad. Sci. U.S.A. 95, 5323–5328. doi: 10.1073/pnas.95.9.5323
Marx, M., Gunter, R. H., Hucko, W., Radnikow, G., and Feldmeyer, D. (2012). Improved biocytin labeling and neuronal 3D reconstruction. Nat. Protoc. 7, 394–407. doi: 10.1038/nprot.2011.449
Mason, A., Nicoll, A., and Stratford, K. (1991). Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J. Neurosci. 11, 72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991
Massimini, M., and Amzica, F. (2001). Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. J. Neurophysiol. 85, 1346–1350. doi: 10.1152/jn.2001.85.3.1346
Miles, R., and Poncer, J. C. (1996). Paired recordings from neurones. Curr. Opin. Neurobiol. 6, 387–394. doi: 10.1016/S0959-4388(96)80124-3
Miles, R., Toth, K., Gulyas, A. I., Hajos, N., and Freund, T. F. (1996). Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16, 815–823. doi: 10.1016/S0896-6273(00)80101-4
Molnar, G., Olah, S., Komlosi, G., Fule, M., Szabadics, J., Varga, C., et al. (2008). Complex events initiated by individual spikes in the human cerebral cortex. PLoS Biol. 6:e222. doi: 10.1371/journal.pbio.0060222
Molnar, G., Rozsa, M., Baka, J., Holderith, N., Barzo, P., Nusser, Z., et al. (2016). Human pyramidal to interneuron synapses are mediated by multi-vesicular release and multiple docked vesicles. Elife 5:e18167. doi: 10.7554/eLife.18167.008
Montgomery, J. M., Pavlidis, P., and Madison, D. V. (2001). Pair recordings reveal all-silent synaptic connections and the postsynaptic expression of long-term potentiation. Neuron 29, 691–701. doi: 10.1016/S0896-6273(01)00244-6
Morgenstern, N. A., Bourg, J., and Petreanu, L. (2016). Multilaminar networks of cortical neurons integrate common inputs from sensory thalamus. Nat. Neurosci. 19, 1034–1040. doi: 10.1038/nn.4339
Narayanan, R. T., Egger, R., Johnson, A. S., Mansvelder, H. D., Sakmann, B., De Kock, C. P., et al. (2015). Beyond columnar organization: cell type- and target layer-specific principles of horizontal axon projection patterns in rat vibrissal cortex. Cereb. Cortex 25, 4450–4468. doi: 10.1093/cercor/bhv053
Nelson, M. J., Valtcheva, S., and Venance, L. (2017). Magnitude and behavior of cross-talk effects in multichannel electrophysiology experiments. J. Neurophysiol. 118, 574–594. doi: 10.1152/jn.00877.2016
Olah, S., Fule, M., Komlosi, G., Varga, C., Baldi, R., Barzo, P., et al. (2009). Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281. doi: 10.1038/nature08503
Pala, A., and Petersen, C. C. (2018). State-dependent cell-type-specific membrane potential dynamics and unitary synaptic inputs in awake mice. Elife 7:e35869. doi: 10.7554/eLife.35869.020
Peng, Y., Barreda Tomas, F. J., Klisch, C., Vida, I., and Geiger, J. R. P. (2017). Layer-specific organization of local excitatory and inhibitory synaptic connectivity in the rat presubiculum. Cereb. Cortex 27, 2435–2452. doi: 10.1093/cercor/bhx049
Peng, Y., Mittermaier, F. X., Planert, H., Schneider, U. C., Alle, H., and Geiger, J. R. P. (2019). High-throughput microcircuit analysis of individual human brains through next-generation multineuron patch-clamp. Elife 8:e48178. doi: 10.7554/eLife.48178.sa2
Perin, R., Berger, T. K., and Markram, H. (2011). A synaptic organizing principle for cortical neuronal groups. Proc. Natl. Acad. Sci. U.S.A. 108, 5419–5424. doi: 10.1073/pnas.1016051108
Petreanu, L., Huber, D., Sobczyk, A., and Svoboda, K. (2007). Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668. doi: 10.1038/nn1891
Pfeffer, C. K., Xue, M., He, M., Huang, Z. J., and Scanziani, M. (2013). Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076. doi: 10.1038/nn.3446
Porkka-Heiskanen, T., Strecker, R. E., and Mccarley, R. W. (2000). Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99, 507–517. doi: 10.1016/S0306-4522(00)00220-7
Porkka-Heiskanen, T., Strecker, R. E., Thakkar, M., Bjorkum, A. A., Greene, R. W., and Mccarley, R. W. (1997). Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276, 1265–1268. doi: 10.1126/science.276.5316.1265
Qi, G., and Feldmeyer, D. (2016). Dendritic target region-specific formation of synapses between excitatory layer 4 neurons and layer 6 pyramidal cells. Cereb. Cortex 26, 1569–1579. doi: 10.1093/cercor/bhu334
Qi, G., Radnikow, G., and Feldmeyer, D. (2015). Electrophysiological and morphological characterization of neuronal microcircuits in acute brain slices using paired patch-clamp recordings. J. Vis. Exp. 95:e52358. doi: 10.3791/52358
Qi, G., Van Aerde, K., Abel, T., and Feldmeyer, D. (2017). Adenosine differentially modulates synaptic transmission of excitatory and inhibitory microcircuits in layer 4 of rat barrel cortex. Cereb. Cortex 27, 4411–4422. doi: 10.1093/cercor/bhw243
Radnikow, G., Gunter, R. H., Marx, M., and Feldmeyer, D. (2012). “Morpho-functional mapping of cortical networks in brain slice preparations using paired electrophysiological recordings,” in Neuronal Network Analysis Neuromethods, Vol. 67, eds T. Fellin and M. Halassa (New York, NY: Humana Press), 405–431.
Radnikow, G., Qi, G., and Feldmeyer, D. (2015). “Synaptic microcircuits in the barrel cortex,” in Sensorimotor Integration in the Whisker System, eds P. Krieger and A. Groh. (New York, NY: Springer), 59–108.
Rall, W. (1967). Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J. Neurophysiol. 30, 1138–1168. doi: 10.1152/jn.1967.30.5.1138
Reyes, A., Lujan, R., Rozov, A., Burnashev, N., Somogyi, P., and Sakmann, B. (1998). Target-cell-specific facilitation and depression in neocortical circuits. Nat. Neurosci. 1, 279–285. doi: 10.1038/1092
Reyes, A., and Sakmann, B. (1999). Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J. Neurosci. 19, 3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999
Rieubland, S., Roth, A., and Hausser, M. (2014). Structured connectivity in cerebellar inhibitory networks. Neuron 81, 913–929. doi: 10.1016/j.neuron.2013.12.029
Rizzoli, S. O., and Betz, W. J. (2004). The structural organization of the readily releasable pool of synaptic vesicles. Science 303, 2037–2039. doi: 10.1126/science.1094682
Rizzoli, S. O., and Betz, W. J. (2005). Synaptic vesicle pools. Nat. Rev. Neurosci. 6, 57–69. doi: 10.1038/nrn1583
Rollenhagen, A., Ohana, O., Satzler, K., Hilgetag, C. C., Kuhl, D., and Lubke, J. H. R. (2018). Structural properties of synaptic transmission and temporal dynamics at excitatory layer 5B synapses in the adult rat somatosensory cortex. Front. Synaptic. Neurosci. 10:24. doi: 10.3389/fnsyn.2018.00024
Rozov, A., Burnashev, N., Sakmann, B., and Neher, E. (2001). Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J. Physiol. 531, 807–826. doi: 10.1111/j.1469-7793.2001.0807h.x
Sarter, M., Parikh, V., and Howe, W. M. (2009). Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat. Rev. Neurosci. 10, 383–390. doi: 10.1038/nrn2635
Saviane, C., and Silver, R. A. (2006). Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature 439, 983–987. doi: 10.1038/nature04509
Scanziani, M., Gahwiler, B. H., and Charpak, S. (1998). Target cell-specific modulation of transmitter release at terminals from a single axon. Proc. Natl. Acad. Sci. U.S.A. 95, 12004–12009. doi: 10.1073/pnas.95.20.12004
Scheuss, V., and Neher, E. (2001). Estimating synaptic parameters from mean, variance, and covariance in trains of synaptic responses. Biophys. J. 81, 1970–1989. doi: 10.1016/S0006-3495(01)75848-1
Scheuss, V., Schneggenburger, R., and Neher, E. (2002). Separation of presynaptic and postsynaptic contributions to depression by covariance analysis of successive EPSCs at the calyx of Held synapse. J. Neurosci. 22, 728–739. doi: 10.1523/JNEUROSCI.22-03-00728.2002
Seeman, S. C., Campagnola, L., Davoudian, P. A., Hoggarth, A., Hage, T. A., Bosma-Moody, A., et al. (2018). Sparse recurrent excitatory connectivity in the microcircuit of the adult mouse and human cortex. Elife 7:e37349. doi: 10.7554/eLife.37349.032
Silberberg, G., and Markram, H. (2007). Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53, 735–746. doi: 10.1016/j.neuron.2007.02.012
Silver, R. A., Lubke, J., Sakmann, B., and Feldmeyer, D. (2003). High-probability uniquantal transmission at excitatory synapses in barrel cortex. Science 302, 1981–1984. doi: 10.1126/science.1087160
Sjostrom, P. J., Turrigiano, G. G., and Nelson, S. B. (2001). Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32, 1149–1164. doi: 10.1016/S0896-6273(01)00542-6
Song, S., Sjostrom, P. J., Reigl, M., Nelson, S., and Chklovskii, D. B. (2005). Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3:e68. doi: 10.1371/journal.pbio.0030068
Stepanyants, A., and Chklovskii, D. B. (2005). Neurogeometry and potential synaptic connectivity. Trends Neurosci. 28, 387–394. doi: 10.1016/j.tins.2005.05.006
Stepanyants, A., Martinez, L. M., Ferecsko, A. S., and Kisvarday, Z. F. (2009). The fractions of short- and long-range connections in the visual cortex. Proc. Natl. Acad. Sci. U.S.A. 106, 3555–3560. doi: 10.1073/pnas.0810390106
Stevens, C. F. (1993). Quantal release of neurotransmitter and long-term potentiation. Cell 72(Suppl), 55–63. doi: 10.1016/S0092-8674(05)80028-5
Stratford, K. J., Tarczy-Hornoch, K., Martin, K. A., Bannister, N. J., and Jack, J. J. (1996). Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature 382, 258–261. doi: 10.1038/382258a0
Stuart, G. J., Dodt, H. U., and Sakmann, B. (1993). Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 423, 511–518. doi: 10.1007/BF00374949
Szabadics, J., Varga, C., Molnar, G., Olah, S., Barzo, P., and Tamas, G. (2006). Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311, 233–235. doi: 10.1126/science.1121325
Tamas, G., Buhl, E. H., Lorincz, A., and Somogyi, P. (2000). Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat. Neurosci. 3, 366–371. doi: 10.1038/73936
Tamas, G., Lorincz, A., Simon, A., and Szabadics, J. (2003). Identified sources and targets of slow inhibition in the neocortex. Science 299, 1902–1905. doi: 10.1126/science.1082053
Tecuapetla, F., Carrillo-Reid, L., Bargas, J., and Galarraga, E. (2007). Dopaminergic modulation of short-term synaptic plasticity at striatal inhibitory synapses. Proc. Natl. Acad. Sci. U.S.A. 104, 10258–10263. doi: 10.1073/pnas.0703813104
Tecuapetla, F., Koos, T., Tepper, J. M., Kabbani, N., and Yeckel, M. F. (2009). Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J. Neurosci. 29, 8977–8990. doi: 10.1523/JNEUROSCI.6145-08.2009
Teles-Grilo Ruivo, L. M., Baker, K. L., Conway, M. W., Kinsley, P. J., Gilmour, G., Phillips, K. G., et al. (2017). Coordinated acetylcholine release in prefrontal cortex and hippocampus is associated with arousal and reward on distinct timescales. Cell. Rep. 18, 905–917. doi: 10.1016/j.celrep.2016.12.085
Testa-Silva, G., Verhoog, M. B., Goriounova, N. A., Loebel, A., Hjorth, J., Baayen, J. C., et al. (2010). Human synapses show a wide temporal window for spike-timing-dependent plasticity. Front. Synaptic. Neurosci. 2:12. doi: 10.3389/fnsyn.2010.00012
Testa-Silva, G., Verhoog, M. B., Linaro, D., De Kock, C. P., Baayen, J. C., Meredith, R. M., et al. (2014). High bandwidth synaptic communication and frequency tracking in human neocortex. PLoS Biol. 12:e1002007. doi: 10.1371/journal.pbio.1002007
Thomson, A. M., and Deuchars, J. (1997). Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cereb. Cortex 7, 510–522. doi: 10.1093/cercor/7.6.510
Thomson, A. M., and Lamy, C. (2007). Functional maps of neocortical local circuitry. Front. Neurosci. 1, 19–42. doi: 10.3389/neuro.01.1.1.002.2007
Thomson, A. M., West, D. C., Wang, Y., and Bannister, A. P. (2002). Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb. Cortex 12, 936–953. doi: 10.1093/cercor/12.9.936
Ting, J. T., Daigle, T. L., Chen, Q., and Feng, G. (2014). Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 1183, 221–242. doi: 10.1007/978-1-4939-1096-0_14
Ting, J. T., Kalmbach, B., Chong, P., De Frates, R., Keene, C. D., Gwinn, R. P., et al. (2018a). A robust ex vivo experimental platform for molecular-genetic dissection of adult human neocortical cell types and circuits. Sci. Rep. 8:8407. doi: 10.1038/s41598-018-26803-9
Ting, J. T., Lee, B. R., Chong, P., Soler-Llavina, G., Cobbs, C., Koch, C., et al. (2018b). Preparation of acute brain slices using an optimized N-Methyl-D-glucamine protective recovery method. J. Vis. Exp. 132:e53825. doi: 10.3791/53825
Trettel, J., and Levine, E. S. (2003). Endocannabinoids mediate rapid retrograde signaling at interneuron right-arrow pyramidal neuron synapses of the neocortex. J. Neurophysiol. 89, 2334–2338. doi: 10.1152/jn.01037.2002
Urban-Ciecko, J., Jouhanneau, J. S., Myal, S. E., Poulet, J. F. A., and Barth, A. L. (2018). Precisely timed nicotinic activation drives SST inhibition in neocortical circuits. Neuron 97, 611–625 e615. doi: 10.1016/j.neuron.2018.01.037
Varshney, L. R., Sjostrom, P. J., and Chklovskii, D. B. (2006). Optimal information storage in noisy synapses under resource constraints. Neuron 52, 409–423. doi: 10.1016/j.neuron.2006.10.017
Volgushev, M., Kudryashov, I., Chistiakova, M., Mukovski, M., Niesmann, J., and Eysel, U. T. (2004). Probability of transmitter release at neocortical synapses at different temperatures. J. Neurophysiol. 92, 212–220. doi: 10.1152/jn.01166.2003
Wickersham, I. R., Lyon, D. C., Barnard, R. J., Mori, T., Finke, S., Conzelmann, K. K., et al. (2007). Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647. doi: 10.1016/j.neuron.2007.01.033
Williams, S. R., and Stuart, G. J. (2002). Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science 295, 1907–1910. doi: 10.1126/science.1067903
Williams, S. R., and Stuart, G. J. (2003). Role of dendritic synapse location in the control of action potential output. Trends Neurosci. 26, 147–154. doi: 10.1016/S0166-2236(03)00035-3
Wilson, R. I., and Nicoll, R. A. (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592. doi: 10.1038/35069076
Yang, D., Gunter, R. H., Qi, G., Radnikow, G., and Feldmeyer, D. (2019). Cell type-Specific modulation of layer 6A excitatory microcircuits by acetylcholine in rat barrel Cortex. BioRxiv. 701318. doi: 10.1101/701318
Yassin, L., Benedetti, B. L., Jouhanneau, J. S., Wen, J. A., Poulet, J. F., and Barth, A. L. (2010). An embedded subnetwork of highly active neurons in the neocortex. Neuron 68, 1043–1050. doi: 10.1016/j.neuron.2010.11.029
Yu, Y. C., Bultje, R. S., Wang, X., and Shi, S. H. (2009). Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458, 501–504. doi: 10.1038/nature07722
Zeng, H., and Sanes, J. R. (2017). Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci. 18, 530–546. doi: 10.1038/nrn.2017.85
Zilberter, Y., Harkany, T., and Holmgren, C. D. (2005). Dendritic release of retrograde messengers controls synaptic transmission in local neocortical networks. Neuroscientist 11, 334–344. doi: 10.1177/1073858405275827
Zilberter, Y., Kaiser, K. M., and Sakmann, B. (1999). Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron 24, 979–988. doi: 10.1016/S0896-6273(00)81044-2
Zoli, M., Jansson, A., Sykova, E., Agnati, L. F., and Fuxe, K. (1999). Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci. 20, 142–150. doi: 10.1016/S0165-6147(99)01343-7
Keywords: paired recordings, synaptic connection, structure-function analysis, quantal analysis, neuromodulation
Citation: Qi G, Yang D, Ding C and Feldmeyer D (2020) Unveiling the Synaptic Function and Structure Using Paired Recordings From Synaptically Coupled Neurons. Front. Synaptic Neurosci. 12:5. doi: 10.3389/fnsyn.2020.00005
Received: 30 September 2019; Accepted: 22 January 2020;
Published: 11 February 2020.
Edited by:
Christiaan P. J. De Kock, Vrije Universiteit Amsterdam, NetherlandsReviewed by:
Jean-Sébastien Jouhanneau, Helmholtz Association of German Research Centers (HZ), GermanyCopyright © 2020 Qi, Yang, Ding and Feldmeyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanxiao Qi, Zy5xaUBmei1qdWVsaWNoLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.