- Neuroradiology Division, Department of Radiology, Stanford University, Stanford, CA, United States
A long-standing goal of translational neuroscience is the ability to noninvasively deliver therapeutic agents to specific brain regions with high spatiotemporal resolution. Focused ultrasound (FUS) is an emerging technology that can noninvasively deliver energy up the order of 1 kW/cm2 with millimeter and millisecond resolution to any point in the human brain with Food and Drug Administration-approved hardware. Although FUS is clinically utilized primarily for focal ablation in conditions such as essential tremor, recent breakthroughs have enabled the use of FUS for drug delivery at lower intensities (i.e., tens of watts per square centimeter) without ablation of the tissue. In this review, we present strategies for image-guided FUS-mediated pharmacologic neurointerventions. First, we discuss blood–brain barrier opening to deliver therapeutic agents of a variety of sizes to the central nervous system. We then describe the use of ultrasound-sensitive nanoparticles to noninvasively deliver small molecules to millimeter-sized structures including superficial cortical regions and deep gray matter regions within the brain without the need for blood–brain barrier opening. We also consider the safety and potential complications of these techniques, with attention to temporal acuity. Finally, we close with a discussion of different methods for mapping the ultrasound field within the brain and describe future avenues of research in ultrasound-targeted drug therapies.
Introduction
Focused Ultrasound as a Potential Modality for Noninvasive Neurointervention
Neuropsychiatric diseases have emerged as one of the largest public health threats today, contributing to an estimated 57% of years lived with disability in the United States from 1990 to 2016 (Mokdad et al., 2018). Treatment of these conditions and other brain disorders is limited by several factors. First, the cytoarchitecture and connectivity of brain regions change significantly every few millimeters (Amunts and Zilles, 2015), and many neuropsychiatric disorders are thought to be mediated by a subset of these different brain areas, demanding a need for focal techniques that can target these specific regions. Second, the blood–brain barrier (BBB) limits the passage of many therapeutics of interest to the brain. Finally, because the brain is a sensitive organ that is only directly accessible by procedures requiring general anesthesia and/or craniotomy, routine direct application of drugs to specific brain targets is many times infeasible at the moment.
Focused ultrasound (FUS) is an emerging technology that offers promising strategies to address these issues. Today in the clinic, ultrasound is most often used diagnostically, where a transducer fires ultrasound pulses into the tissue and records returning echoes in order to image the structures within. With the use of transmit focusing (i.e., geometrical and/or electronic), it is possible to noninvasively direct over 1 kW/cm2 of acoustic intensity to precisely lesion a specific target site, without substantial energy deposition within the intervening regions between the transducer and the target or regions beyond the focus, an idea initially developed in the 50s by William Fry (Figure 1A; Fry, 1958; Kennedy, 2005). Food and Drug Administration-approved FUS transducers can achieve focusing with millimeter and millisecond resolution anywhere in the brain, whether it be a deep or a cortical structure (Figure 1B; Jolesz, 2009; Liu et al., 2014; Ghanouni et al., 2015).
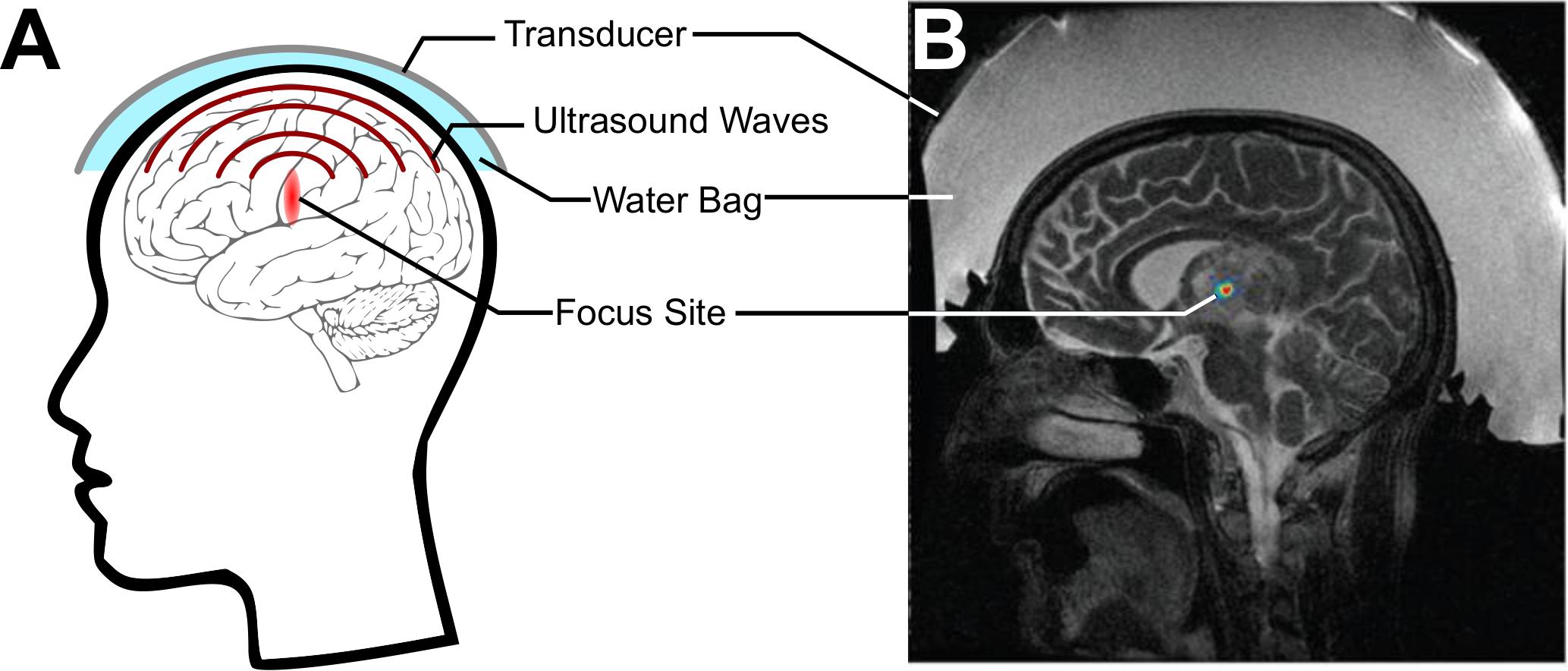
Figure 1. Focused ultrasound (FUS) for noninvasively delivering acoustic energy to the brain. (A) Schematic of FUS use. A transducer is coupled to the skin using a water bag and delivers ultrasound waves to a focus within the brain. (B) Simulation of energy deposited by a commercial MRI-guided FUS system (Exablate 4000, Insightec, Haifa, Israel) through the skull. Adapted from Vyas et al. (2016). Reprinted with permission from the American Association of Physicists in Medicine.
The predominant clinical use of this technology is for high-intensity FUS operated in a continuous-wave mode to deliver energy to a tightly focused brain region in a completely noninvasive manner. This approach is currently used in the clinic to thermally ablate specific regions of the brain for conditions such as essential tremor and Parkinson’s Disease (Lipsman et al., 2013; Magara et al., 2014; Elias et al., 2016). However, the same FUS systems can be operated in pulsed-wave mode and at lower intensities to enable local drug delivery within the brain. In this review, we will first introduce a number of methods for drug delivery to the brain and then discuss applications of FUS to achieve targeted delivery, namely by temporarily opening the BBB or by directly releasing pharmacologic agents from carrier particles within millimeter-sized structures. We will then close by presenting current techniques that are available for mapping the FUS field within the brain to confirm treatment efficacy and safety, highlighting the need for methods that are well-suited for these low-intensity non-ablative applications.
Non-ultrasound Methods for Drug Delivery to the Brain
Various methods for delivering drugs directly to the brain have been proposed. One example is osmotic blood–brain barrier opening (BBBO), where a cannula is introduced percutaneously through the arteries to target a cerebral artery. Hyperosmolar mannitol is then injected through the cannula, causing an osmotic shift that disrupts the endothelial cells that partially form the BBB (Burkhardt et al., 2012). Disadvantages of this method include the need for general anesthesia and a high rate of adverse events, including seizures after osmotic BBBO (Marchi et al., 2007). Furthermore, osmotic BBBO covers a wide region of the brain, often opening the BBB across an entire hemisphere in large animals (Joshi et al., 2011), which could be seen as positive or negative depending on the scenario.
Another method being evaluated in small animals currently is laser interstitial thermotherapy (LITT). Here, a fiber optic is introduced into the brain to deliver laser light into a tumor site, improving the blood–tumor barrier permeability to chemotherapeutic agents (Salehi et al., 2019). However, this method requires neurosurgery for a burr-hole craniotomy and insertion of a fiber optic within the brain, limiting the potential indications for this procedure.
Finally, there exist several methods for directly delivering drugs past the BBB. For example, polymer wafers containing the drug of interest can be implanted directly within the brain tissue to slowly release drug into the surrounding cerebrospinal fluid (Brem et al., 1991). One meta-study associated this technique with a 42.7% complication rate, including cerebrospinal fluid leak, infection, cerebral edema, and seizures (Bregy et al., 2013). Another related method is convection-enhanced delivery, where a cannula is stereotactically introduced within the brain and mini-pumps help distribute the drug within the site by convection. Some of these cannulae (up to 68%) can be misplaced, limiting their efficacy (Sampson et al., 2010).
The invasive nature of implanting a foreign object within the brain and the relatively limited efficacy of these methods vs. their complications highlight the urgent need for noninvasive ways to deliver drugs to the brain without requiring general anesthesia or invasive procedures.
Ultrasound-Based Methods for Drug Delivery to the Brain
Focused Ultrasound-Mediated Blood–Brain Barrier Opening
The Blood–Brain Barrier as a Challenge for Drug Delivery to the Brain
At the time of writing, the only ultrasound-mediated method for drug delivery to the brain in clinical trials is FUS-mediated BBBO (Carpentier et al., 2015, 2016; Mainprize et al., 2019). The BBB is formed by a combination of endothelial cells, pericytes, neurons, and astrocytes, all connected with tight junctions and other intercellular connections to form a neurovascular unit that prevents the passage of most small and large molecules, including even water (Abbott et al., 2006). For a more complete description of the BBB (Figure 2A), please refer to recent reviews on the topic by Abbott et al. (2006) and Sweeney et al. (2018b). Physiologically, the BBB serves as a physical barrier that forces most molecular transport through specialized channels, effectively restricting molecular traffic to specific molecules necessary for proper brain function (Abbott et al., 2006). Typically, for passive diffusion across the BBB, a molecule must be both small (<600 Da) and hydrophobic (Norinder and Haeberlein, 2002; Geldenhuys et al., 2015). Otherwise, a compound would need to take advantage of passage through specialized transporters in the BBB to reach the central nervous system (Pardridge, 2005, 2012). Because the BBB is estimated to block 98% of all small-molecule drugs and effectively all foreign large-molecule therapeutics (e.g., monoclonal antibodies), the BBB is considered one of the largest bottlenecks for the development of neuropsychiatric and neuro-oncologic therapies (Pardridge, 2005). Thus, there is a pressing clinical need for methods that can noninvasively, safely, and reversibly open the BBB in and around the target to enable the temporary passage of therapeutic agents to the target. Here, we describe ultrasound-mediated BBBO as a promising technique that meets many of these criteria.
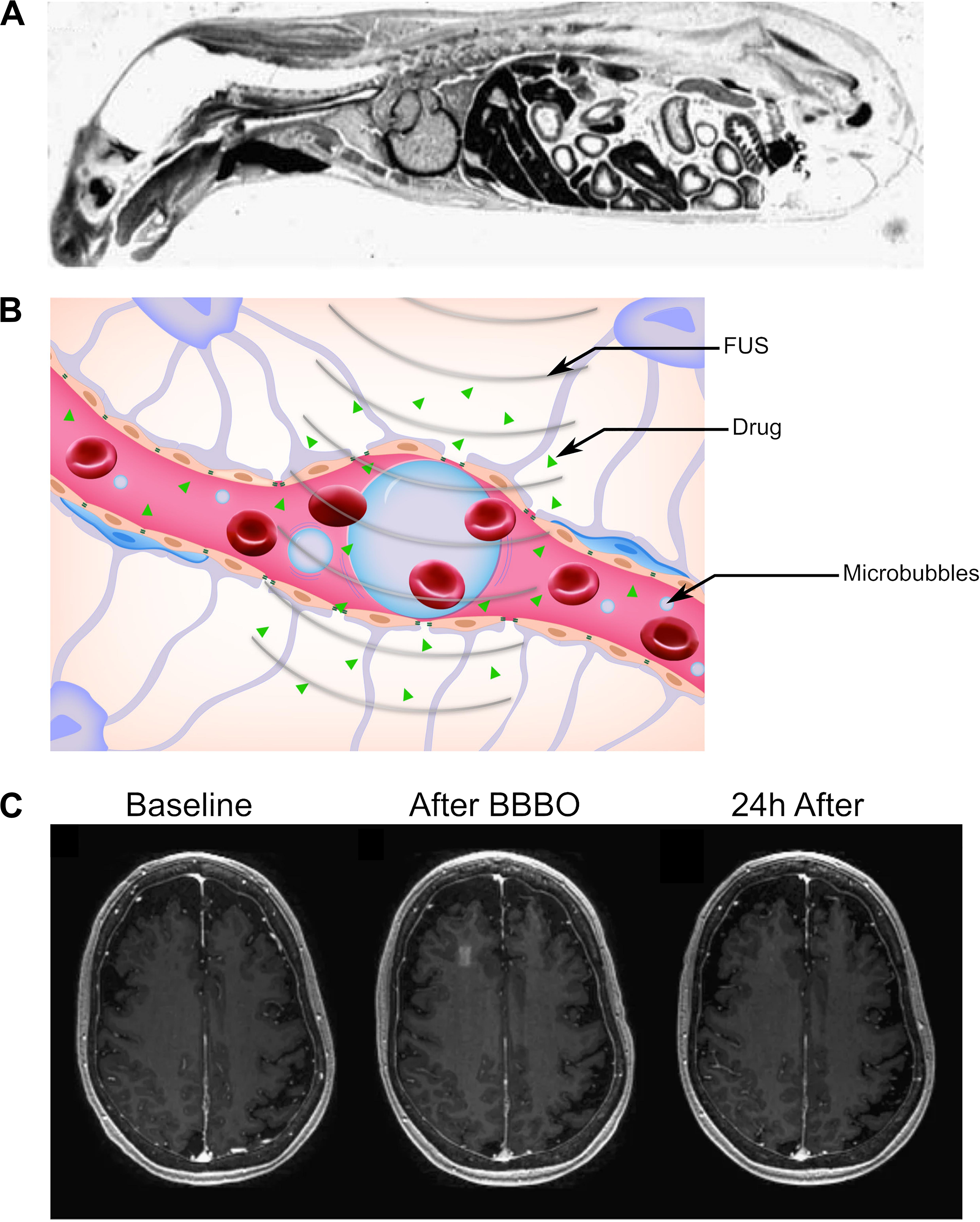
Figure 2. Reversible blood–brain barrier opening (BBBO) with focused ultrasound (FUS). (A) Sagittal autoradiography image of a rat following intravenous administration of radiolabeled histamine demonstrates the efficience of exclusion of certain agents from the central nervous system by the blood–brain barrier. Adapted from Pardridge et al. (1986). Reprinted with permission from The American College of Physicians. (B) Schematic of BBBO with FUS. Microbubbles (blue) are injected into the bloodstream and are activated by FUS. This causes the spaces between pericytes and astrocytes to open up, enabling delivery of the therapeutic agent (green) past the BBB. (C) T1-weighted gadolinium MR images for a patient before (left), immediately after (center), and 24 h after (right) FUS-mediated BBBO. Adapted from Lipsman et al. (2018). Reprinted under Creative Commons License.
Focused Ultrasound and Microbubble-Mediated Blood–Brain Barrier Opening
In its current form, ultrasound-mediated BBBO is achieved by first intravenously injecting microbubbles and shortly thereafter applying short low-pressure (<1 MPa) ultrasound pulses specifically to the target site (Figure 2B; Hynynen et al., 2001). Microbubbles were originally designed to be contrast agents for ultrasound imaging (Chong et al., 2018). They are micrometer-sized particles that consist of a shell (usually either lipid or protein) encapsulating a gaseous core (typically perfluoropropane or sulfur hexafluoride) (Christiansen et al., 1994; Sontum, 2008). Upon sonication with typical BBBO parameters, microbubbles undergo small, stable oscillations, a phenomenon referred to as stable cavitation (Bader and Holland, 2013; Vignon et al., 2013). These oscillations radiate pressure to the surrounding fluid, causing the mechanical formation of pores within the endothelium and opening the tight junctions that form the BBB (Sheikov et al., 2004; Tung et al., 2011). The BBBO can then be visualized with T1-weighted magnetic resonance (MR) imaging after administration of a gadolinium-based contrast agent (Figure 2C).
A wide variety of clinically available and custom microbubbles have been used for BBBO. The most commonly used clinical agents are Optison (Choi et al., 2007), Definity (Baseri et al., 2010), and Sonovue (Fan et al., 2014), whose diameters range from 2.5 to 5 μm. Although these microbubbles might vary in performance at lower pressures (≤0.3 MPa), at higher pressures, these differences are minimized, indicating that beyond some parameter optimization, different microbubbles are functionally equivalent (Wang et al., 2014).
The volume of the affected region depends on the ultrasound focus size and the sonication parameters, highlighting that millimeter-level resolution is achievable with currently clinically available hardware (Ghanouni et al., 2015). After sonication, it has been estimated that the BBB remains open for 24–72 h (Hynynen et al., 2001; Konofagou et al., 2012), with MR-resolved measures of BBBO (e.g., Ktrans) having half-lives of 2–5 h (Park et al., 2012; Chai et al., 2014; Chu et al., 2016).
Agents delivered via ultrasound-mediated BBBO include small-molecule drugs (Hynynen et al., 2001; Park et al., 2012; Aryal et al., 2013, 2014), monoclonal antibodies (Kinoshita et al., 2006a, b), gene delivery vectors (both nonviral and viral) (Hynynen, 2008; Lin et al., 2015; Szablowski et al., 2018), and even stem cells (Burgess et al., 2011). The size of molecules allowed to pass through the pores created with BBBO depends primarily on the peak negative pressure of the ultrasound pulses, with molecules up to 2,000 kDa in size passing through at higher pressures (Chen and Konofagou, 2014). However, BBBO at high enough pressures to allow passage of molecules or cells larger than 500 kDa also led to microhemorrhage on histologic evaluation (Burgess et al., 2011; Chen and Konofagou, 2014). Nonetheless, it is important to note that extravasation of red blood cells after ultrasound-mediated BBBO was not correlated with long-term neural damage (McDannold et al., 2005).
Clinical Applications of Blood–Brain Barrier Opening
Currently, the majority of FUS-mediated BBBO trials being conducted in humans are for delivering chemotherapeutic agents to treat brain tumors (Table 1). One approach for clinical BBBO involves the implantation of an unfocused ultrasound transducer within the skull to routinely perform BBBO before chemotherapy administration (Carpentier et al., 2016), whereas another approach uses a noninvasive transducer to perform MR-guided sonication (Mainprize et al., 2019). It is important to note that these studies are often uncontrolled, have less than a dozen subjects, and are primarily powered to evaluate safety and whether BBBO successfully occurred, without assessing the efficacy of ultrasound-mediated BBBO for drug delivery to achieve tumor control.
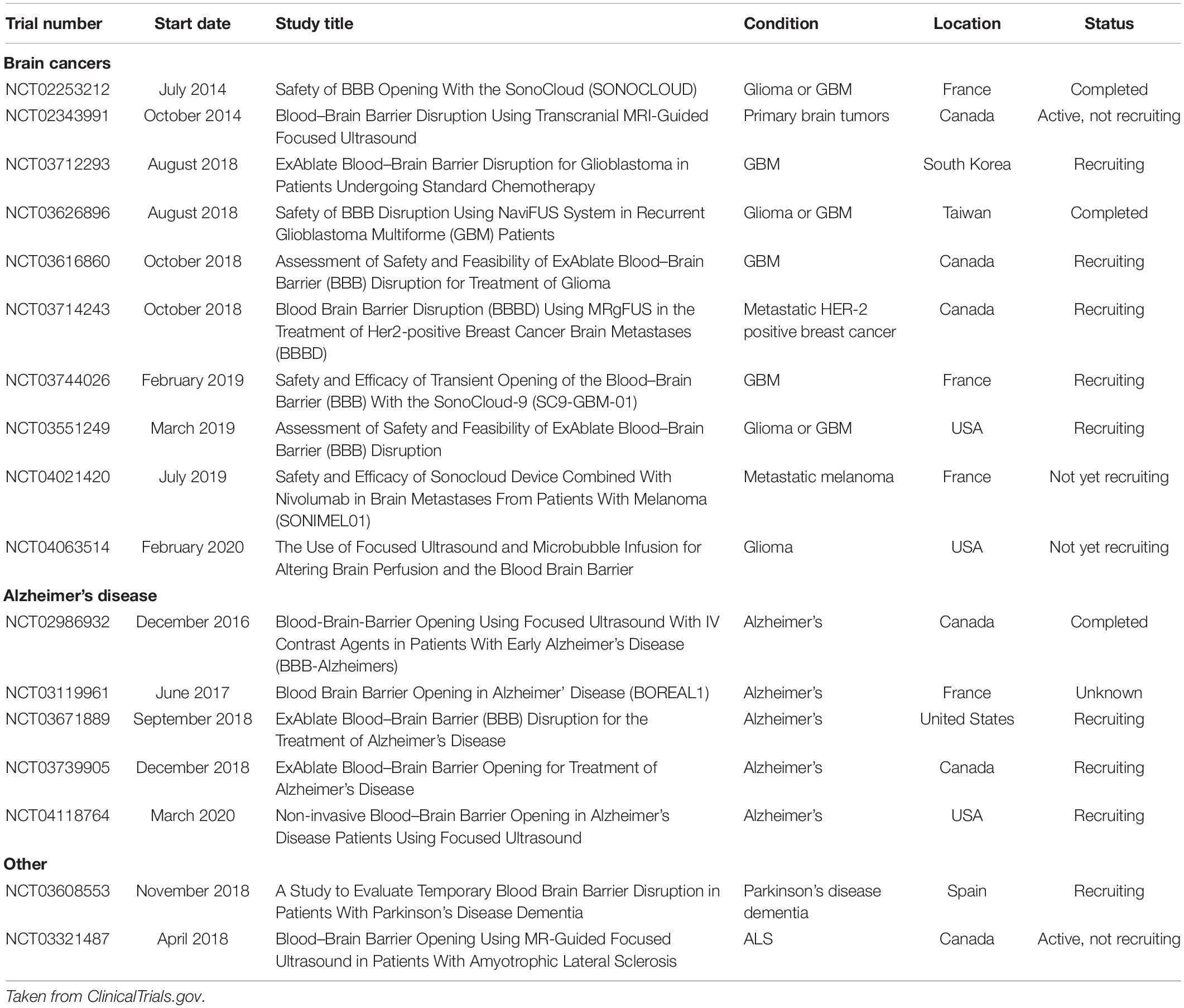
Table 1. Clinical trials evaluating ultrasound-mediated blood-brain barrier opening in humans at the time of publication.
One other exciting application of BBBO is for the direct treatment of Alzheimer’s disease. Preclinical studies have suggested that ultrasound-mediated BBBO could lead to amyloid plaque clearance in preclinical models even without the administration of other therapeutic agents (Jordão et al., 2013; Burgess et al., 2014; Leinenga and Götz, 2015). A recent Phase I safety and feasibility trial in five patients demonstrated no clinically severe adverse events along with no clinically significant worsening in cognitive performance 3 months after BBBO (Lipsman et al., 2018). Further studies are required to establish the mechanism of BBBO for improving Alzheimer’s and to validate and/or refine its use in humans. The use of BBBO to deliver is also actively being investigated in other diseases, such as amyotrophic lateral sclerosis (Abrahao et al., 2019) and Parkinson’s disease (Lin et al., 2016; LeWitt et al., 2019). Again, these preliminary studies were designed to evaluate the safety of BBBO in these patients without actually delivering therapeutic agents through the disrupted BBB. Future trials could be focused on evaluating the efficacy of drug delivery with this method, along with therapeutic efficacy.
In Table 1, we report current and past clinical trials evaluating ultrasound-mediated BBBO in humans (ClinicalTrials.gov, 2020).
Safety Considerations of Blood–Brain Barrier Opening
FUS-mediated BBBO is not without risk. The most well-studied adverse effects of FUS-mediated BBBO are the acute complications that arise immediately after sonication, which include microhemorrhage formation (erythrocytic extravasation) and vacuolation of the pericytes and surrounding cells, even at the typical low pressures used (Hynynen et al., 2005; McDannold et al., 2005; Liu et al., 2008; Baseri et al., 2010). At higher pressures, microbubble-enhanced ablation can also occur (McDannold N. J. et al., 2006; McDannold et al., 2013). Hemorrhage can be detected after sonication on MRI using T2∗w or susceptibility-weighted imaging (Liu et al., 2008). Nonetheless, as discussed later, there is a pressing need for real-time monitoring to avoid complications during sonication.
Beyond these acute effects, the potential for adverse effects of FUS-mediated BBBO in the long term is less well understood or agreed upon. A single session of BBBO was shown to have no effects at least a week beyond treatment, to the resolution of that preclinical analysis (McDannold et al., 2005). Furthermore, there are a number of studies that have performed repeated BBBO sessions for at least 1 month, which found no changes in either MRI, histology, or cognitive testing in nonhuman primates (McDannold et al., 2012; Downs et al., 2015; Horodyckid et al., 2017). However, it is important to note that these BBBO sessions were conducted once every 2 weeks or longer. A contrasting study recently found that repeated weekly BBBO sessions in rats over 6 weeks at the same site led to cortical atrophy, persistent BBB disruption, ventricular size increase, and hyperphosphorylated tau protein buildup at the target site, consistent with neurodegeneration (Figure 3; Kovacs et al., 2018). It is important to note that these effects were not observed with repeated sessions in humans that occurred every 4 weeks with an unfocused transducer, albeit with similar acoustic power (Carpentier et al., 2016). Nonetheless, caution should be taken, especially because histological analysis has revealed sterile inflammation immediately after sonication that persists up to 1 week (Kovacs et al., 2017). Given that neurodegenerative diseases have been closely linked with BBB breakdown (Sweeney et al., 2018a; Nation et al., 2019), further investigation of the long-term effects of repeated BBBO sessions is warranted for the treatment of chronic neurologic conditions without the morbidity and risk–benefit considerations of cancer. Additionally, consideration needs to be given as to how many of these potential effects are due to the permeabilization of the BBB generally vs. the use of ultrasound-induced microbubble cavitation specifically.
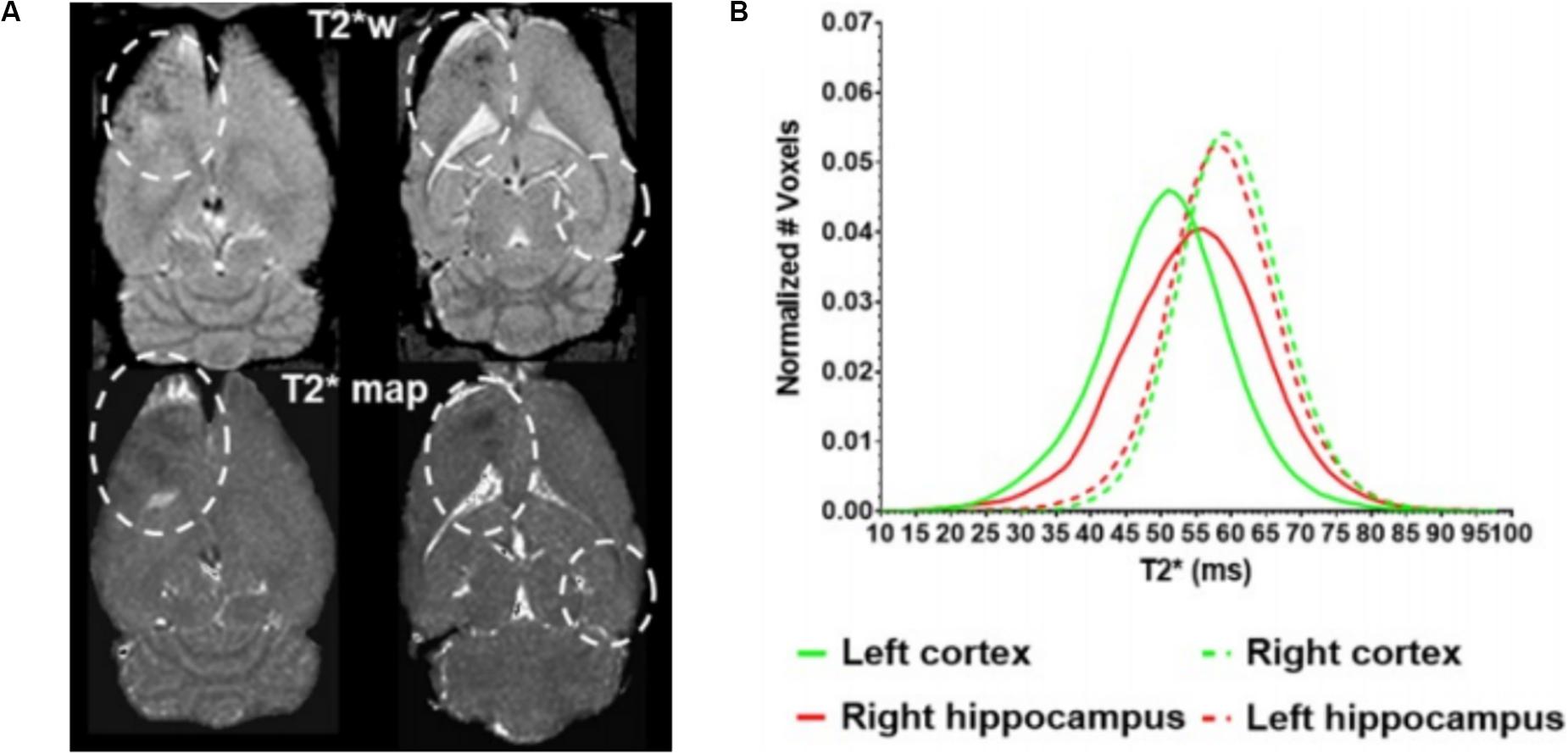
Figure 3. Long-term cortical atrophy after repeated focused ultrasound (FUS)-mediated blood–brain barrier opening sessions. (A) Representative T2* images demonstrating long-term effects of 6 weekly BBBO sessions in cortical and deep structures of the brain (white dashed lines). (B) Quantification of distribution of T2* times at the targeted sites (solid lines) vs. the contralateral site (dashed lines). Adapted from Kovacs et al. (2018). Reprinted under Creative Commons License.
Focused Ultrasound and Nanoparticle-Mediated Drug Uncaging
Focal Noninvasive Drug Delivery With Ultrasonic Drug Uncaging
As discussed earlier, most work regarding drug delivery in the brain using FUS is centered around BBBO for the delivery of agents that do not normally cross the intact BBB. However, decades of pharmacologic inquiry have yielded libraries of small molecules that are known to normally cross the BBB (Alavijeh et al., 2005; Pardridge, 2012) and are known to have specific action at any of a variety of receptors of importance (Kim et al., 2009; Machado-Vieira et al., 2017). However, these molecules may have adverse effects due to drug action outside the target area in the brain or body, or at the wrong time with respect to the rest of therapy (Haddad and Dursun, 2008). One exciting emerging technology for targeted delivery of drugs that do cross the BBB is the use of ultrasound-sensitive nanoparticles that release their drug payload specifically upon sonication (Airan, 2017). In this application, drug-loaded nanoparticles are intravenously administered, and then, the drug is released (or uncaged) with ultrasound in the intravascular blood volume of the target brain region. The drug then diffuses across the intact BBB into the parenchyma (Figure 4A). These nanoparticles are typically structured as nanoemulsions, with a coat of surfactant such as an amphiphilic block copolymer that encapsulates an ultrasound-sensitive core, typically a liquid perfluorocarbon. The hydrophilic component of the surfactant faces the aqueous medium, whereas the hydrophobic component binds the drug payload and emulsifies the perfluorocarbon droplet. Our group has found that this platform is generalizable to a wide range of hydrophobic drugs, with similar release characteristics and nanoparticle properties regardless of the drug’s identity (Zhong et al., 2019). This criterion allows encapsulation of virtually any drug that is small and hydrophobic, and therefore most drugs of neuropsychiatric interest, as these are the chemical features of drugs that can cross the intact BBB (Norinder and Haeberlein, 2002; Weksler et al., 2005; Geldenhuys et al., 2015).
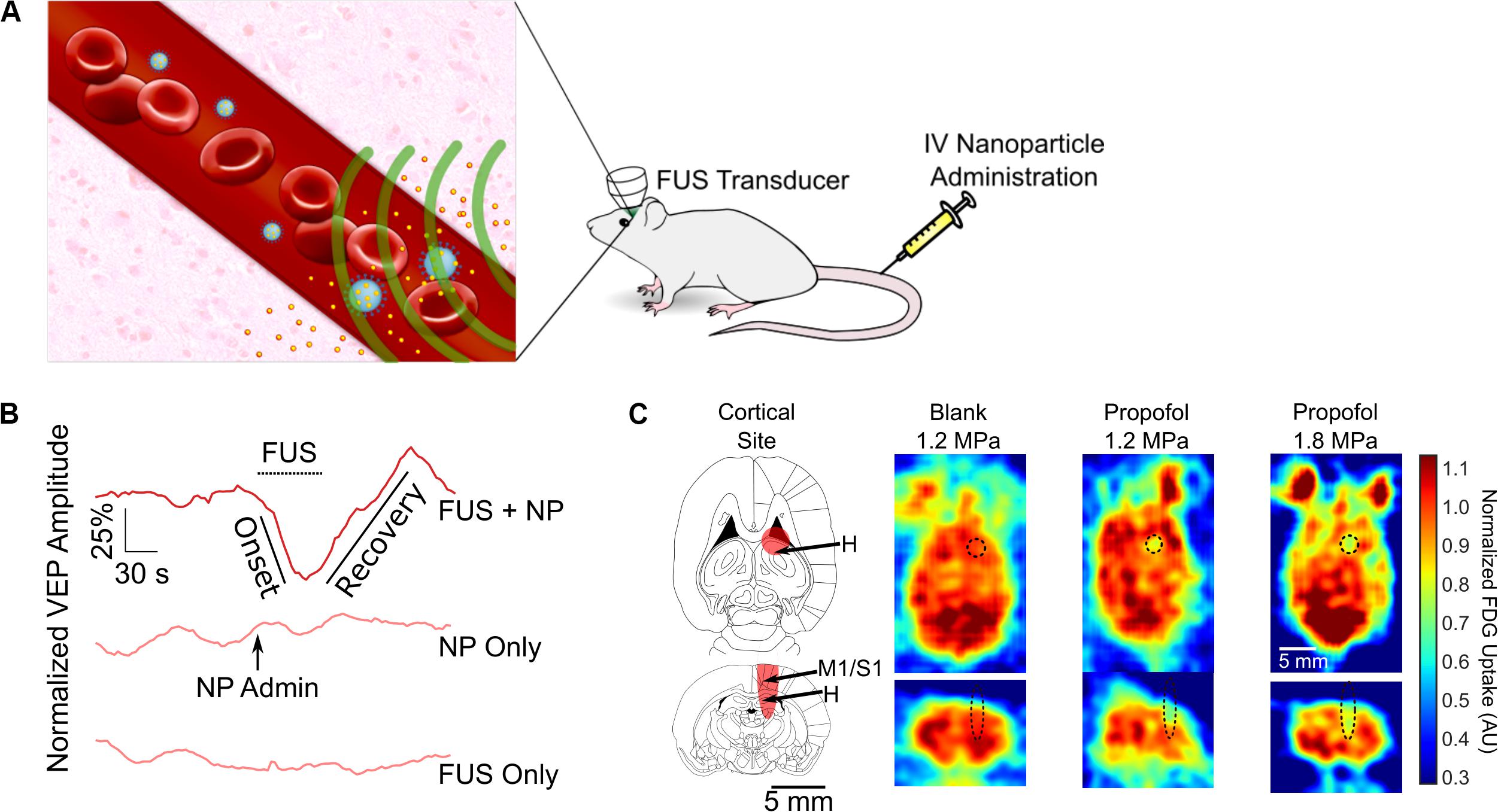
Figure 4. Ultrasonic drug uncaging for spatiotemporally precise neuromodulation. (A) Schematic of ultrasonic drug uncaging. Nanoparticles (blue) are administered intravenously, where they are selectively activated by focused ultrasound (FUS) (green). The activated nanoparticles then release their drug (yellow), and the freed drug then diffuses across an intact blood–brain barrier (BBB) into the brain parenchyma (pink). (B) Uncaging propofol in the visual cortex silences visually evoked potentials (VEPs), with intensity recovering seconds after ultrasound ceases. (C) Fluorodeoxyglucose-positron emission tomography demonstrates that the neuromodulation induced by propofol uncaging is spatially limited to the ultrasound focus (black oval). Adapted from Wang et al. (2018). Reprinted with permission from Elsevier.
Notably, the nanoparticles used for ultrasonic drug uncaging can be activated using short low/moderate-intensity ultrasound pulses, namely 1–2 MPa in situ at 650 kHz with pulse lengths of 50–100 ms and a pulse repetition frequency of 1 Hz (Airan, 2017; Airan et al., 2017; Wang et al., 2018). These parameters theoretically only lead to a transient 0.1°C temperature increase within the targeted brain region (Wang et al., 2018). This is in contrast to the continuous mode, high-intensity ultrasound protocols required to raise the tissue temperature in order to activate drug release from heat-gated systems like thermosensitive liposomes (Nardecchia et al., 2019). Given the limitations on being able to effectively heat the brain outside the center of the cranium (Odéen et al., 2014) and the risk of heat shock of the brain parenchyma with thermosensitive liposome gating, nanoparticle-mediated ultrasonic drug uncaging is more practically feasible for brain applications.
Most previous work with ultrasound-sensitive nanoparticles have been centered around delivering chemotherapeutics to tumors outside the central nervous system (Rapoport et al., 2009; Fabiilli et al., 2010). In these applications, the nanoparticle uncaging was intended to be completed after the particles were collected within the tumor, taking advantage of the enhanced permeability and retention effect (Rapoport, 2012). In brain applications, because the nanoparticle size (∼300–450 nm) precludes transit across the BBB, the uncaging and delivery occur intravascularly as the uncaged drug diffuses into the brain parenchyma (Figure 4A). Given the types of drugs that are best delivered via ultrasonic drug uncaging, the noninvasive mechanism of delivery, and the high spatiotemporal resolution achieved by FUS, ultrasonic drug uncaging has great potential for neuropsychiatric therapy.
Spatiotemporally Precise Neuromodulation With Ultrasonic Drug Uncaging
The use of ultrasonic drug uncaging for spatiotemporally precise neuromodulation was first proposed with the use of nanoparticles loaded with propofol, an anesthetic agent. Preliminary work showed that sonication of propofol-loaded nanoparticles was sufficient to stop seizure activity in the rat, although this work did not fully demonstrate the spatiotemporal resolution of the achieved neuromodulation (Airan et al., 2017). Recently, our group demonstrated by using electrophysiologic recordings and positron emission tomography functional imaging, that the spatiotemporal resolution of neuromodulation is strictly limited by the sonication focus and the kinetics of the uncaged drug, effectively achieving noninvasive neuromodulation with millimeter and second-level resolution for the case of propofol (Figures 4B,C; Wang et al., 2018). With further analysis, we demonstrated that we were able to visualize whole-brain changes that occurred during focal pharmacologic activity at the sonication site, enabling causative mapping of functional networks in the brain with resolutions and a depth of penetration for the causal manipulation that was previously unattainable with noninvasive methods (Wang et al., 2018). As used in combination with positron emission tomography imaging in Wang et al. (2018), ultrasonic drug uncaging could certainly be combined in future efforts with other functional imaging modalities such as functional MRI (Davis et al., 1998), functional ultrasound (Macé et al., 2011), or photoacoustic imaging (Yao et al., 2013). Because ultrasonic drug uncaging does not require any invasive or irreversible procedures such as gene therapy, it is an attractive noninvasive neuromodulation method that could potentially be translated into the clinic. As stated before, ultrasonic drug uncaging is generalizable to excitatory, inhibitory, and neuromodulatory neuropsychiatric drugs (Zhong et al., 2019), enabling selection for the therapeutic effects of these powerful drugs while minimizing off-target effects. Indeed, recently, Lea-Banks et al. (2020) used nanoparticles loaded with pentobarbital to selectively anesthetize part of the rat motor cortex in awake motor tasks.
Other potential uses for ultrasonic drug uncaging include focal treatment of vascular pathologies. Calcium channel blockers such as nicardipine have been encapsulated successfully in these nanoparticles and have been shown to be able to selectively dilate parts of the aorta based on where the uncaging ultrasound transducer was placed (Zhong et al., 2019). Budding applications of this work include the treatment of cerebral vasospasm, a common highly morbid complication of subarachnoid hemorrhage after cerebral aneurysm rupture (Condette-Auliac et al., 2001).
Safety Considerations of Ultrasonic Drug Uncaging
It has been hypothesized that ultrasound-sensitive nanoparticles effectively undergo vaporization after exposure to sonication, changing into a gaseous bubble akin to microbubbles used for BBBO (Rapoport, 2012). Theoretically, this would mean that ultrasonic drug uncaging could potentially disrupt the BBB or lead to other forms of cavitation-induced parenchymal injury. However, high-speed microscopy and acoustic recordings by our group have shown that our formulation of these nanoparticles does not undergo vaporization or cavitation during sonication, highlighting their safety in this regard (Zhong et al., 2019). Furthermore, repeated sonication of animals treated with these nanoparticles (upward of two to three times per week for a month) at the same site led to no discernable changes on histology or MRI (Wang et al., 2018).
Our current compositions of these ultrasound-sensitive nanoparticles are made of ingredients that have been approved for human administration by the Food and Drug Administration (Robbin and Eisenfeld, 1998; Makadia and Siegel, 2011). However, a common feature of nanoparticles and microbubbles, in general, is the risk of a hypersensitivity-like reaction upon intravenous administration in humans (Szebeni et al., 2007, 2018; Moghimi, 2018). This reaction is characterized by dyspnea, hypotension, angioedema, and generalized urticaria, similar to anaphylaxic reactions (Moghimi, 2018). It is currently believed that this reaction is not a true anaphylaxis and is mediated through complement and/or macrophage activation and can be controlled through reducing the size of the nanoparticles (which also reduces the sensitivity to ultrasound), changing the shape of the nanoparticle to be less spherical (which has yet to be achieved with ultrasound-sensitive nanoparticles) or slowing the rate of infusion (Moghimi, 2018; Szebeni et al., 2018).
In Table 2, we summarize the features of various methods for drug delivery to the brain, including ultrasound-based and non-ultrasound-based interventions.
Noninvasively Visualizing the Ultrasound Field Within the Brain for Guidance
The increasing popularity of transcranial FUS applications has propelled the development of noninvasive imaging technologies to fulfill the twofold need for treatment guidance and monitoring.
First, the focusing accuracy must be verified before the treatment. Attenuation and phase distortions are introduced in the propagating ultrasound waves by the acoustically heterogenous skull bone (Clement and Hynynen, 2002), which undermine the focus quality and increase the risk for nonspecific energy deposition in off target brain areas. Moreover, the inherent acoustic propagation properties of the skull are patient-specific and vary significantly between subjects (Vyas et al., 2016). However, if multielement FUS arrays are available, phase correction and adaptive focusing techniques can be applied to restore the desired focusing accuracy, given that the transcranial pressure field can be visualized reliably and noninvasively.
Second, it is paramount to monitor safety parameters and treatment outcomes in real time. To ensure safe and effective ultrasonic exposure levels, real-time monitoring systems have been implemented based on indirect measures of the deposited ultrasonic energy.
In this section, we will review a number of imaging techniques developed to provide focusing quality feedback based on thermal and mechanical acoustic effects in addition to real-time controllers allowing for online safety monitoring. We will limit our analysis to technologies relevant to low-intensity FUS pharmacologic neurointervention.
Passive Cavitation Monitoring
Originally developed for monitoring high-intensity FUS therapy, passive cavitation detection techniques have been successfully adapted for safety monitoring in applications such as BBBO, which utilize lower ultrasound intensities in combination with intravenously administered gas-filled microbubble contrast agents. When placed within a FUS field, these microbubbles undergo characteristic nonlinear oscillating behaviors (referred to as cavitation), depending upon the ultrasound parameters, including the fundamental frequency of the ultrasound pulse (f0), the pulse length, and the applied acoustic pressure. Scattered acoustic emissions generated from cavitation events are detected by passive ultrasound receivers, and the received ultrasonic signals present distinct spectral features. These features carry information on the location, strength, and nature of the cavitation activity. More specifically, cavitation phenomena can be distinguished as inertial or non-inertial. Inertial cavitation is characterized by abrupt particle collapse leading to the production of broadband ultrasonic emissions. This situation is linked to vascular endothelial and parenchymal damage and, therefore, is largely undesired in FUS neurointervention (Hwang et al., 2006). In contrast, non-inertial cavitation is characterized by stable microbubble oscillations induced by relatively weaker ultrasonic energy deposited within the ultrasound focus (Marmottant and Hilgenfeldt, 2003). Such oscillations present harmonic (2f0, 3f0, …), subharmonic (1/2f0), and ultraharmonic (3/2f0, 5/2f0, …) spectral components in the detected ultrasonic signals (Sun et al., 2012).
Using passive broadband receivers, usually referred to as passive cavitation detectors, the cavitation activity can be monitored by assessing the spectral content of the received ultrasonic signals. In several studies, the occurrence of broadband emissions from inertial cavitation events was correlated with neurovascular damage and red blood cell extravasation, as confirmed by post hoc MRI and histologic findings (Tung et al., 2010; Arvanitis et al., 2012). Conversely, harmonic, subharmonic, or ultraharmonic emissions from non-inertial cavitation could predict effective and reversible BBBO (McDannold N. et al., 2006; Sun et al., 2015). Importantly, multiple real-time safety monitoring systems have been developed based on the online assessment of harmonic, subharmonic, ultraharmonic, and broadband emissions (O’Reilly and Hynynen, 2012; Huang et al., 2017; Sun et al., 2017). This approach was used in recent BBBO clinical trials in amyotrophic lateral sclerosis and Alzheimer’s disease (Lipsman et al., 2018; Abrahao et al., 2019).
Although inexpensive and simple to implement, single detectors can only monitor cavitation activity at the focal region and are unable to spatially resolve different cavitation sources, limiting its ability to directly visualize the total treatment volume (Gyöngy and Coussios, 2010). By integrating multielement receive arrays into the FUS system, on the other hand, spatial maps of cavitation activity can be created from the received broadband signals, allowing for the spatial discrimination of cavitation sources. Based on the multielement passive approach, there have been several implementations of integrated custom and clinical ultrasound imaging arrays with FUS transducers to transcranially monitor BBBO and vascular damage in rodents and in nonhuman primates (Gateau et al., 2011; Arvanitis et al., 2013; Deng et al., 2016; Crake et al., 2018; Figures 5A,B).
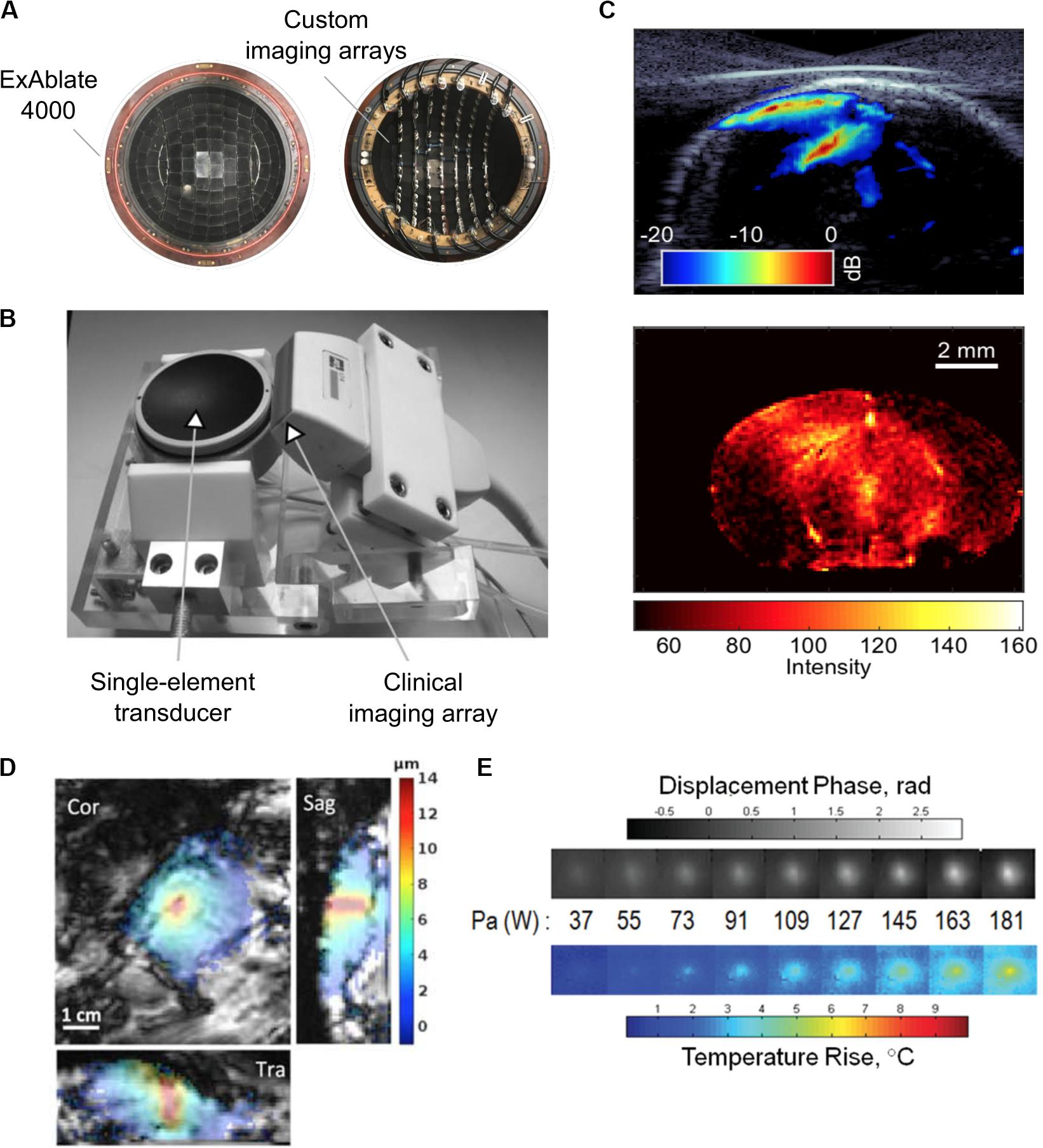
Figure 5. (A) Experimental 128-element imaging array (5 MHz) integrated into the 230 kHz hemispherical transducer of an InSightec ExAblate 4000 MRgFUS system for three-dimensional cavitation imaging and skull localization (Crake et al., 2018). © Institute of Physics and Engineering in Medicine. Reproduced by permission of the Institute of Physics Publishing. All rights reserved. (B) Conventional ultrasound imaging array (5 MHz) integrated with a single-element therapy transducer (660 kHz) for two-dimensional cavitation imaging (Gateau et al., 2011). Reprinted with permission from the Institute of Electrical and Electronics Engineers. (C) (Top) Cavitation image overlaid with a B-mode image obtained during an ultrasound-mediated blood–brain barrier opening (BBBO) experiment (Burgess et al., 2018). (Bottom) © Institute of Physics and Engineering in Medicine. Reproduced by permission of the Institute of Physics Publishing. All rights reserved. (D) Example of magnetic resonance-acoustic radiation force imaging (MR-ARFI) displacement image in ex vivo porcine brain. The images indicate the focused ultrasound (FUS) energy distribution (de Bever et al., 2018). Reprinted with permission from John Wiley and Sons. (E) Displacement maps and temperature rise measured with a modified MR-ARFI sequence for increasing acoustic power (Kaye et al., 2013). Reprinted with permission from John Wiley and Sons.
In typical BBBO sonication protocols, long sonication pulses make it challenging to use conventional pulse-echo ultrasound imaging techniques for passive cavitation imaging, as the exact time at which a cavitation event occurs is unknown. Numerous passive beamforming algorithms have been developed that rely on the receiver spatial information only to create the cavitation maps (Farny et al., 2009; Salgaonkar et al., 2009; Gyöngy and Coussios, 2010; Haworth et al., 2017). Using beamforming techniques conventionally used in pulse-echo imaging, a recent study used short FUS pulses for BBBO to create cavitation images with improved resolution (Burgess et al., 2018; Figure 5C). Interestingly, another study developed a skull localization and registration routine based entirely on ultrasound data, toward the implementation of an ultrasound-guided FUS platform (Crake et al., 2018). For a comprehensive review of methods for treatment monitoring and control, see Jones and Hynynen (2019).
Magnetic Resonance Thermometry
MR thermometry offers a possible solution to monitor and guide FUS treatment and to visualize the therapy beam in applications where acoustic feedback from cavitating particles is not available. This technique can noninvasively detect temperature changes in water-containing tissues based on the temperature-dependent proton resonance frequency shift (Chung et al., 1996; McDannold, 2005; Rieke and Pauly, 2008). This approach has proven useful as a pretreatment tool to control ultrasound exposure and to verify the accuracy of the targeting, achieving consistent localization of the sonication profile without apparent tissue damage or BBB disruption, as confirmed by follow-up MRI and histological findings (Hynynen et al., 1997). Importantly, by analyzing the spatial profile of the temperature change, the shape of the ultrasound beam can be inferred, and possible focusing errors or aberrations introduced by the skull can be compensated for before the treatment begins. MR thermometry was combined with passive cavitation monitoring for safety control in recent preclinical and clinical BBBO studies (Huang et al., 2017; Lipsman et al., 2018; Abrahao et al., 2019). Although this technique is routinely used for monitoring of thermal therapies such as low-temperature hyperthermia and ablation (Elias et al., 2013; Lipsman et al., 2013), it has limited effectiveness for real-time safety monitoring in low-power FUS applications due to the low rate of heating involved (< 0.5°C) in these applications.
Acoustic Radiation Force Imaging
In absorbing tissues, low-power ultrasound pulses exert an acoustic radiation force (ARF) at the focus that moves the tissue away from its resting position along the direction of propagation. McDannold and Maier (2008) demonstrated that the induced longitudinal displacement is linearly proportional to the acoustic power and can be encoded by the phase of the MR signal. Dedicated motion-sensitive MRI sequences have been implemented using displacement-encoding gradients to create displacement maps. These maps provide an indirect measurement of the in situ pressure field (Figure 5D). This approach has been tested in vivo in rats (Larrat et al., 2010a) and pigs (Hertzberg et al., 2010) and has been optimized to increase the technique sensitivity and to reduce scanning time and heat deposition (Kaye et al., 2011). Currently, the temperature rise induced by magnetic resonance-acoustic radiation force imaging (MR-ARFI) pulse sequences is below 1°C and is considered safe (Table 3 and Figure 5E). However, it is worth specifying that reported ultrasound parameters in MR-ARF imaging studies vary significantly.
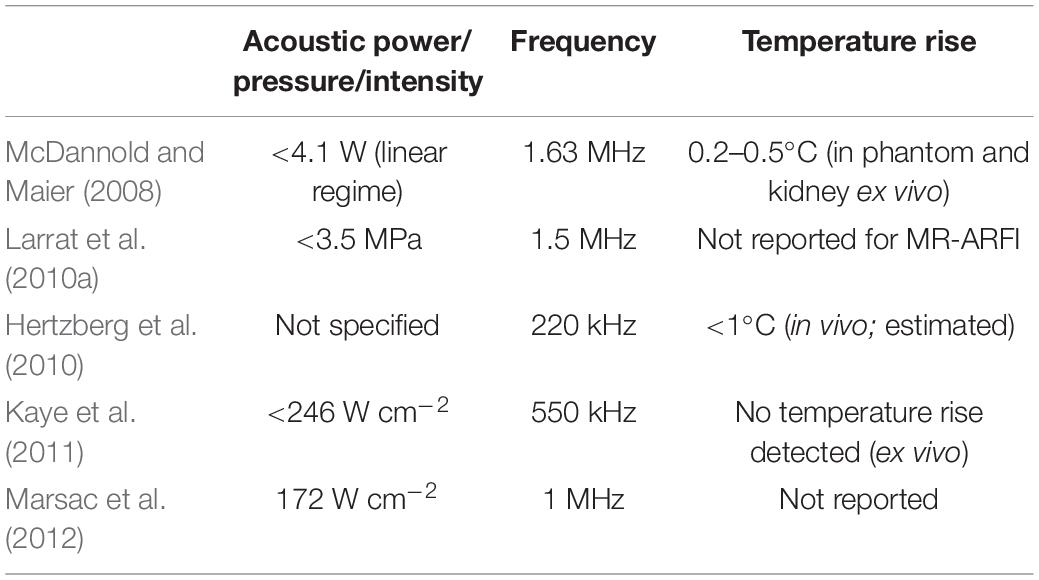
Table 3. Reported sonication parameters for selected publications investigating the use of magnetic resonance-acoustic radiation force imaging (MR-ARFI).
Based on MR-ARFI measurements, adaptive focusing techniques have also been developed for correcting phase aberrations to restore the focus sharpness at the target location (Hertzberg et al., 2010; Larrat et al., 2010b; Marsac et al., 2012; Vyas et al., 2012; Kaye and Pauly, 2013). Also, radiation force imaging has been combined with MR thermometry to include safety monitoring capabilities (Kaye and Pauly, 2013; Paquin et al., 2013; de Bever et al., 2018). Currently, the focus intensities needed for MR-ARFI are typically higher than those used for either BBBO or nanoparticle uncaging (Larrat et al., 2010a; Kaye and Pauly, 2013).
Conclusion
In summary, FUS is an emerging technology that holds great potential for designing noninvasive, targeted pharmacologic neurointerventions with millimeter resolution. We have discussed the use of FUS for opening the BBB to deliver a wide range of large and small molecules, along with the potential safety issues associated with repeated BBBO. We have also reviewed recently developed techniques for directly delivering pharmacologic neuromodulatory agents that normally cross the BBB using ultrasonic drug uncaging. Finally, we provided an overview of the use of passive cavitation mapping, MR thermometry, and MR-ARFI for directly monitoring the sonication field during treatment. It should be noted that these monitoring techniques are mostly limited to use either with microbubbles or with sonication powers higher than those typically used for drug delivery. There is therefore an acute need for the development of more sensitive methods for real-time monitoring of the sonication field during low-intensity applications like ultrasound-mediated drug delivery. Other potential future directions include further investigation of the long-term effects of repeated BBBO and the clinical translation of ultrasonic drug uncaging.
Author Contributions
JW and TD wrote the initial draft of the manuscript and prepared the figures. All authors contributed to manuscript revisions, read, and approved the submitted version.
Funding
This work would not have been possible without the generous funding support of several programs. JW was supported by a Stanford Wu Tsai Neurosciences Institute Mark and Mary Stevens Interdisciplinary Graduate Fellowship and F30 Ruth L. Kirschstein National Research Service Award (NRSA; 1 F30 MH119763-01A1). TD was supported by a Stanford School of Medicine Dean’s Postdoctoral Fellowship.
Conflict of Interest
Patent applications have been filed on the nanoparticles described for ultrasonic drug uncaging (17-163 – Provisional application with Stanford University; RDA and PCT/US2017/033226 with Johns Hopkins University; RDA).
Acknowledgments
We would like to thank Amy Thomas for assistance in figure preparation.
References
Abbott, N. J., Rönnbäck, L., and Hansson, E. (2006). Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53. doi: 10.1038/nrn1824
Abrahao, A., Meng, Y., Llinas, M., Huang, Y., Hamani, C., Mainprize, T., et al. (2019). First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 10:4373. doi: 10.1038/s41467-019-12426-9
Airan, R. (2017). Neuromodulation with nanoparticles. Science 357, 465–465. doi: 10.1126/science.aao1200
Airan, R. D., Meyer, R. A., Ellens, N. P. K., Rhodes, K. R., Farahani, K., Pomper, M. G., et al. (2017). Noninvasive targeted transcranial neuromodulation via focused ultrasound gated drug release from nanoemulsions. Nano Lett. 17, 652–659. doi: 10.1021/acs.nanolett.6b03517
Alavijeh, M. S., Chishty, M., Qaiser, M. Z., and Palmer, A. M. (2005). Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. Neurotherapeutics 2, 554–571. doi: 10.1602/neurorx.2.4.554
Amunts, K., and Zilles, K. (2015). Architectonic mapping of the human brain beyond Brodmann. Neuron 88, 1086–1107. doi: 10.1016/j.neuron.2015.12.001
Arvanitis, C. D., Livingstone, M. S., and McDannold, N. (2013). Combined ultrasound and MR imaging to guide focused ultrasound therapies in the brain. Phys. Med. Biol. 58, 4749–4761. doi: 10.1088/0031-9155/58/14/4749
Arvanitis, C. D., Livingstone, M. S., Vykhodtseva, N., and McDannold, N. (2012). Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PLoS One 7:e45783. doi: 10.1371/journal.pone.0045783
Aryal, M., Arvanitis, C. D., Alexander, P. M., and McDannold, N. (2014). Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 72, 94–109. doi: 10.1016/j.addr.2014.01.008
Aryal, M., Vykhodtseva, N., Zhang, Y.-Z., Park, J., and McDannold, N. (2013). Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood–tumor and blood–brain barriers improve outcomes in a rat glioma model. J. Control. Release 169, 103–111. doi: 10.1016/j.jconrel.2013.04.007
Bader, K. B., and Holland, C. K. (2013). Gauging the likelihood of stable cavitation from ultrasound contrast agents. Phys. Med. Biol. 58, 127–144. doi: 10.1088/0031-9155/58/1/127
Baseri, B., Choi, J. J., Tung, Y.-S., and Konofagou, E. E. (2010). Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med. Biol. 36, 1445–1459. doi: 10.1016/j.ultrasmedbio.2010.06.005
Bregy, A., Shah, A. H., Diaz, M. V., Pierce, H. E., Ames, P. L., Diaz, D., et al. (2013). The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 13, 1453–1461. doi: 10.1586/14737140.2013.840090
Brem, H., Mahaley, M. S., Vick, N. A., Black, K. L., Schold, S. C., Burger, P. C., et al. (1991). Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J. Neurosurg. 74, 441–446. doi: 10.3171/jns.1991.74.3.0441
Burch, P. A., Grossman, S. A., and Reinhard, C. S. (1988). Spinal cord penetration of intrathecally administered cytarabine and methotrexate: a quantitative autoradiographic study. J. Natl. Cancer Inst. 80, 1211–1216. doi: 10.1093/jnci/80.15.1211
Burgess, A., Ayala-Grosso, C. A., Ganguly, M., Jordão, J. F., Aubert, I., and Hynynen, K. (2011). Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One 6:e27877. doi: 10.1371/journal.pone.0027877
Burgess, A., Dubey, S., Yeung, S., Hough, O., Eterman, N., Aubert, I., et al. (2014). Alzheimer disease in a mouse model: MR imaging–guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 273, 736–745. doi: 10.1148/radiol.14140245
Burgess, M. T., Apostolakis, I., and Konofagou, E. E. (2018). Power cavitation-guided blood brain barrier opening with focused ultrasound and microbubbles. Phys. Med. Biol. 63:065009. doi: 10.1088/1361-6560/aab05c
Burkhardt, J.-K., Riina, H., Shin, B. J., Christos, P., Kesavabhotla, K., Hofstetter, C. P., et al. (2012). Intra-arterial delivery of bevacizumab after blood-brain barrier disruption for the treatment of recurrent glioblastoma: progression-free survival and overall survival. World Neurosurg. 77, 130–134. doi: 10.1016/j.wneu.2011.05.056
Carpentier, A., Canney, M., Vignot, A., Horodyckid, C., Goldwirt, L., Leclercq, D., et al. (2015). Temporary disruption of the blood-brain barrier using an implantable ultrasound system for recurrent glioblastoma patients under IV carboplatin chemotherapy: initial phase 1/2a clinical trial observations. J. Ther. Ultrasound 3:O14. doi: 10.1186/2050-5736-3-S1-O14
Carpentier, A., Canney, M., Vignot, A., Reina, V., Beccaria, K., Horodyckid, C., et al. (2016). Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 8:343re2. doi: 10.1126/scitranslmed.aaf6086
Chai, W.-Y., Chu, P.-C., Tsai, M.-Y., Lin, Y.-C., Wang, J.-J., Wei, K.-C., et al. (2014). Magnetic-resonance imaging for kinetic analysis of permeability changes during focused ultrasound-induced blood–brain barrier opening and brain drug delivery. J. Control. Release 192, 1–9. doi: 10.1016/j.jconrel.2014.06.023
Chen, H., and Konofagou, E. E. (2014). The size of blood–brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow Metab. 34, 1197–1204. doi: 10.1038/jcbfm.2014.71
Chen, M. Y., Lonser, R. R., Morrison, P. F., Governale, L. S., and Oldfield, E. H. (1999). Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J. Neurosurg. 90, 315–320. doi: 10.3171/jns.1999.90.2.0315
Choi, J. J., Pernot, M., Small, S. A., and Konofagou, E. E. (2007). Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 33, 95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018
Chong, W. K., Papadopoulou, V., and Dayton, P. A. (2018). Imaging with ultrasound contrast agents: current status and future. Abdom. Radiol. 43, 762–772. doi: 10.1007/s00261-018-1516-1
Christiansen, C., Kryvi, H., Sontum, P., and Skotland, T. (1994). Physical and biochemical characterization of Albunex, a new ultrasound contrast agent consisting of air-filled albumin microspheres suspended in a solution of human albumin. Biotechnol. Appl. Biochem. 19, 307–320. doi: 10.1111/j.1470-8744.1994.tb00300.x
Chu, P.-C., Chai, W.-Y., Tsai, C.-H., Kang, S.-T., Yeh, C.-K., and Liu, H.-L. (2016). Focused ultrasound-induced blood-brain barrier opening: association with mechanical index and cavitation index analyzed by dynamic contrast-enhanced magnetic-resonance imaging. Sci. Rep. 6:33264. doi: 10.1038/srep33264
Chung, A. H., Hynynen, K., Colucci, V., Oshio, K., Cline, H. E., and Jolesz, F. A. (1996). Optimization of spoiled gradient-echo phase imaging for in vivo localization of a focused ultrasound beam. Magn. Reson. Med. 36, 745–752. doi: 10.1002/mrm.1910360513
Clement, G. T., and Hynynen, K. (2002). Correlation of ultrasound phase with physical skull properties. Ultrasound Med. Biol. 28, 617–624. doi: 10.1016/S0301-5629(02)00503-3
ClinicalTrials.gov (2020). Search of: Blood Brain Barrier Ultrasound - List Results - ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/ct2/results?term=blood+brain+barrier+ultrasound&draw=2&rank=9#rowId8 (accessed April 16, 2020).
Condette-Auliac, S., Bracard, S., Anxionnat, R., Schmitt, E., Lacour, J. C., Braun, M., et al. (2001). Vasospasm after subarachnoid hemorrhage. Stroke 32, 1818–1824. doi: 10.1161/01.STR.32.8.1818
Crake, C., Brinker, S. T., Coviello, C. M., Livingstone, M. S., and McDannold, N. J. (2018). A dual-mode hemispherical sparse array for 3D passive acoustic mapping and skull localization within a clinical MRI guided focused ultrasound device. Phys. Med. Biol. 63:065008. doi: 10.1088/1361-6560/aab0aa
Davis, T. L., Kwong, K. K., Weisskoff, R. M., and Rosen, B. R. (1998). Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. U.S.A. 95, 1834–1839. doi: 10.1073/pnas.95.4.1834
de Bever, J., Odéen, H., Hofstetter, L., and Parker, D. (2018). Simultaneous MR thermometry and acoustic radiation force imaging using interleaved acquisitions. Magn. Reson. Med. 79, 1515–1524. doi: 10.1002/mrm.26827
Deng, L., O’Reilly, M. A., Jones, R. M., An, R., and Hynynen, K. (2016). A multi-frequency sparse hemispherical ultrasound phased array for microbubble-mediated transcranial therapy and simultaneous cavitation mapping. Phys. Med. Biol. 61, 8476–8501. doi: 10.1088/0031-9155/61/24/8476
Downs, M. E., Buch, A., Sierra, C., Karakatsani, M. E., Chen, S., Konofagou, E. E., et al. (2015). Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One 10:e0125911. doi: 10.1371/journal.pone.0125911
Elias, W. J., Huss, D., Voss, T., Loomba, J., Khaled, M., Zadicario, E., et al. (2013). A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 369, 640–648. doi: 10.1056/NEJMoa1300962
Elias, W. J., Lipsman, N., Ondo, W. G., Ghanouni, P., Kim, Y. G., Lee, W., et al. (2016). A randomized trial of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 375, 730–739. doi: 10.1056/NEJMoa1600159
Fabiilli, M. L., Haworth, K. J., Sebastian, I. E., Kripfgans, O. D., Carson, P. L., and Fowlkes, J. B. (2010). Delivery of chlorambucil using an acoustically-triggered, perfluoropentane emulsion. Ultrasound Med. Biol. 36, 1364–1375. doi: 10.1016/j.ultrasmedbio.2010.04.019
Fan, C.-H., Liu, H.-L., Ting, C.-Y., Lee, Y.-H., Huang, C.-Y., Ma, Y.-J., et al. (2014). Submicron-bubble-enhanced focused ultrasound for blood–brain barrier disruption and improved CNS drug delivery. PLoS One 9:e96327. doi: 10.1371/journal.pone.0096327
Farny, C. H., Holt, R. G., and Roy, R. A. (2009). Temporal and spatial detection of HIFU-induced inertial and hot-vapor cavitation with a diagnostic ultrasound system. Ultrasound Med. Biol. 35, 603–615. doi: 10.1016/j.ultrasmedbio.2008.09.025
Fry, F. J. (1958). Precision high intensity focusing ultrasonic machines for surgery. Am. J. Phys. Med. 37, 152–156.
Gateau, J., Aubry, J. F., Pernot, M., Fink, M., and Tanter, M. (2011). Combined passive detection and ultrafast active imaging of cavitation events induced by short pulses of high-intensity ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 517–532. doi: 10.1109/TUFFC.2011.1836
Geldenhuys, W. J., Mohammad, A. S., Adkins, C. E., and Lockman, P. R. (2015). Molecular determinants of blood–brain barrier permeation. Ther. Deliv. 6, 961–971. doi: 10.4155/tde.15.32
Ghanouni, P., Pauly, K. B., Elias, W. J., Henderson, J., Sheehan, J., Monteith, S., et al. (2015). Transcranial MR-guided focused ultrasound: a review of the technology and neuro applications. AJR Am. J. Roentgenol. 205, 150–159. doi: 10.2214/AJR.14.13632
Gyöngy, M., and Coussios, C.-C. (2010). Passive cavitation mapping for localization and tracking of bubble dynamics. J. Acoust. Soc. Am. 128, EL175–EL180. doi: 10.1121/1.3467491
Haddad, P. M., and Dursun, S. M. (2008). Neurological complications of psychiatric drugs: clinical features and management. Hum. Psychopharmacol. 23, S15–S26. doi: 10.1002/hup.918
Haworth, K. J., Bader, K. B., Rich, K. T., Holland, C. K., and Mast, T. D. (2017). Quantitative frequency-domain passive cavitation imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 64, 177–191. doi: 10.1109/TUFFC.2016.2620492
Hertzberg, Y., Volovick, A., Zur, Y., Medan, Y., Vitek, S., and Navon, G. (2010). Ultrasound focusing using magnetic resonance acoustic radiation force imaging: application to ultrasound transcranial therapy. Med. Phys. 37, 2934–2942. doi: 10.1118/1.3395553
Horodyckid, C., Canney, M., Vignot, A., Boisgard, R., Drier, A., Huberfeld, G., et al. (2017). Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J. Neurosurg. 126, 1351–1361. doi: 10.3171/2016.3.JNS151635
Huang, Y., Alkins, R., Schwartz, M. L., and Hynynen, K. (2017). Opening the blood-brain barrier with MR imaging-guided focused ultrasound: preclinical testing on a trans-human skull porcine model. Radiology 282, 123–130. doi: 10.1148/radiol.2016152154
Hwang, J. H., Tu, J., Brayman, A. A., Matula, T. J., and Crum, L. A. (2006). Correlation between inertial cavitation dose and endothelial cell damage in vivo. Ultrasound Med. Biol. 32, 1611–1619. doi: 10.1016/j.ultrasmedbio.2006.07.016
Hynynen, K. (2008). Ultrasound for drug and gene delivery to the brain. Adv. Drug Deliv. Rev. 60, 1209–1217. doi: 10.1016/j.addr.2008.03.010
Hynynen, K., McDannold, N., Sheikov, N. A., Jolesz, F. A., and Vykhodtseva, N. (2005). Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 24, 12–20. doi: 10.1016/j.neuroimage.2004.06.046
Hynynen, K., McDannold, N., Vykhodtseva, N., and Jolesz, F. A. (2001). Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits. Radiology 220, 640–646. doi: 10.1148/radiol.2202001804
Hynynen, K., Vykhodtseva, N. I., Chung, A. H., Sorrentino, V., Colucci, V., and Jolesz, F. A. (1997). Thermal effects of focused ultrasound on the brain: determination with MR imaging. Radiology 204, 247–253. doi: 10.1148/radiology.204.1.9205255
Jolesz, F. A. (2009). MRI-guided focused ultrasound surgery. Annu. Rev. Med. 60, 417–430. doi: 10.1146/annurev.med.60.041707.170303
Jones, R. M., and Hynynen, K. R. (2019). Advances in acoustic monitoring and control of focused ultrasound-mediated increases in blood-brain barrier permeability. Br. J. Radiol. 92:20180601. doi: 10.1259/bjr.20180601
Jordão, J. F., Thévenot, E., Markham-Coultes, K., Scarcelli, T., Weng, Y.-Q., Xhima, K., et al. (2013). Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 248, 16–29. doi: 10.1016/j.expneurol.2013.05.008
Joshi, S., Ergin, A., Wang, M., Reif, R., Zhang, J., Bruce, J. N., et al. (2011). Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: implications for chemotherapy. J. Neurooncol. 104, 11–19. doi: 10.1007/s11060-010-0466-4
Kaye, E. A., Chen, J., and Pauly, K. B. (2011). Rapid MR-ARFI method for focal spot localization during focused ultrasound therapy. Magn. Reson. Med. 65, 738–743. doi: 10.1002/mrm.22662
Kaye, E. A., and Pauly, K. B. (2013). Adapting MRI acoustic radiation force imaging for in vivo human brain focused ultrasound applications. Magn. Reson. Med. 69, 724–733. doi: 10.1002/mrm.24308
Kennedy, J. E. (2005). High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 5, 321–327. doi: 10.1038/nrc1591
Kim, D. G., Kim, K. H., Seo, Y. J., Yang, H., Marcusson, E. G., Son, E., et al. (2016). Anti-miR delivery strategies to bypass the blood-brain barrier in glioblastoma therapy. Oncotarget 7, 29400–29411. doi: 10.18632/oncotarget.8837
Kim, D. H., Maneen, M. J., and Stahl, S. M. (2009). Building a better antipsychotic: receptor targets for the treatment of multiple symptom dimensions of schizophrenia. Neurotherapeutics 6, 78–85. doi: 10.1016/j.nurt.2008.10.020
Kinoshita, M., McDannold, N., Jolesz, F. A., and Hynynen, K. (2006a). Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc. Natl. Acad. Sci. U.S.A. 103, 11719–11723. doi: 10.1073/pnas.0604318103
Kinoshita, M., McDannold, N., Jolesz, F. A., and Hynynen, K. (2006b). Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 340, 1085–1090. doi: 10.1016/j.bbrc.2005.12.112
Konofagou, E. E., Tung, Y.-S., Choi, J., Deffieux, T., Baseri, B., and Vlachos, F. (2012). Ultrasound-induced blood-brain barrier opening. Curr. Pharm. Biotechnol. 13, 1332–1345.
Kovacs, Z. I., Kim, S., Jikaria, N., Qureshi, F., Milo, B., Lewis, B. K., et al. (2017). Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. U.S.A. 114, E75–E84. doi: 10.1073/pnas.1614777114
Kovacs, Z. I., Tu, T.-W., Sundby, M., Qureshi, F., Lewis, B. K., Jikaria, N., et al. (2018). MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain. Theranostics 8, 4837–4855. doi: 10.7150/thno.24512
Larrat, B., Pernot, M., Aubry, J. F., Dervishi, E., Sinkus, R., Seilhean, D., et al. (2010a). MR-guided transcranial brain HIFU in small animal models. Phys. Med. Biol. 55, 365–388. doi: 10.1088/0031-9155/55/2/003
Larrat, B., Pernot, M., Montaldo, G., Fink, M., and Tanter, M. (2010b). MR-guided adaptive focusing of ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 57, 1734–1747. doi: 10.1109/TUFFC.2010.1612
Lea-Banks, H., O’Reilly, M. A., Hamani, C., and Hynynen, K. (2020). Localized anesthesia of a specific brain region using ultrasound-responsive barbiturate nanodroplets. Theranostics 10, 2849–2858. doi: 10.7150/thno.41566
Leinenga, G., and Götz, J. (2015). Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 7:278ra33. doi: 10.1126/scitranslmed.aaa2512
LeWitt, P. A., Lipsman, N., and Kordower, J. H. (2019). Focused ultrasound opening of the blood–brain barrier for treatment of Parkinson’s disease. Mov. Disord. 34, 1274–1278. doi: 10.1002/mds.27722
Lin, C.-Y., Hsieh, H.-Y., Chen, C.-M., Wu, S.-R., Tsai, C.-H., Huang, C.-Y., et al. (2016). Non-invasive, neuron-specific gene therapy by focused ultrasound-induced blood-brain barrier opening in Parkinson’s disease mouse model. J. Control. Release 235, 72–81. doi: 10.1016/j.jconrel.2016.05.052
Lin, C.-Y., Hsieh, H.-Y., Pitt, W. G., Huang, C.-Y., Tseng, I.-C., Yeh, C.-K., et al. (2015). Focused ultrasound-induced blood-brain barrier opening for non-viral, non-invasive, and targeted gene delivery. J. Control. Release 212, 1–9. doi: 10.1016/j.jconrel.2015.06.010
Lipsman, N., Meng, Y., Bethune, A. J., Huang, Y., Lam, B., Masellis, M., et al. (2018). Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 9:2336.
Lipsman, N., Schwartz, M. L., Huang, Y., Lee, L., Sankar, T., Chapman, M., et al. (2013). MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 12, 462–468. doi: 10.1016/S1474-4422(13)70048-6
Liu, H.-L., Jan, C.-K., Chu, P.-C., Hong, J.-C., Lee, P.-Y., Hsu, J.-D., et al. (2014). Design and experimental evaluation of a 256-channel dual-frequency ultrasound phased-array system for transcranial blood–brain barrier opening and brain drug delivery. IEEE Trans. Biomed. Eng. 61, 1350–1360. doi: 10.1109/tbme.2014.2305723
Liu, H.-L., Wai, Y.-Y., Chen, W.-S., Chen, J.-C., Hsu, P.-H., Wu, X.-Y., et al. (2008). Hemorrhage detection during focused-ultrasound induced blood-brain-barrier opening by using susceptibility-weighted magnetic resonance imaging. Ultrasound Med. Biol. 34, 598–606. doi: 10.1016/j.ultrasmedbio.2008.01.011
Macé, E., Montaldo, G., Cohen, I., Baulac, M., Fink, M., and Tanter, M. (2011). Functional ultrasound imaging of the brain. Nat. Methods 8, 662–664.
Machado-Vieira, R., Henter, D. I., and Zarate, C. A. Jr. (2017). New targets for rapid antidepressant action. Prog. Neurobiol. 152, 21–37. doi: 10.1016/j.pneurobio.2015.12.001
Magara, A., Bühler, R., Moser, D., Kowalski, M., Pourtehrani, P., and Jeanmonod, D. (2014). First experience with MR-guided focused ultrasound in the treatment of Parkinson’s disease. J. Ther. Ultrasound 2:11. doi: 10.1186/2050-5736-2-11
Mainprize, T., Lipsman, N., Huang, Y., Meng, Y., Bethune, A., Ironside, S., et al. (2019). Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci. Rep. 9:321.
Makadia, H. K., and Siegel, S. J. (2011). Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397. doi: 10.3390/polym3031377
Marchi, N., Angelov, L., Masaryk, T., Fazio, V., Granata, T., Hernandez, N., et al. (2007). Seizure-promoting effect of blood? Brain barrier disruption. Epilepsia 48, 732–742. doi: 10.1111/j.1528-1167.2007.00988.x
Marmottant, P., and Hilgenfeldt, S. (2003). Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 423, 153–156. doi: 10.1038/nature01613
Marsac, L., Chauvet, D., Larrat, B., Pernot, M., Robert, B., Fink, M., et al. (2012). MR-guided adaptive focusing of therapeutic ultrasound beams in the human head. Med. Phys. 39, 1141–1149. doi: 10.1118/1.3678988
McDannold, N. (2005). Quantitative MRI-based temperature mapping based on the proton resonant frequency shift: review of validation studies. Int. J. Hyperthermia 21, 533–546. doi: 10.1080/02656730500096073
McDannold, N., Arvanitis, C. D., Vykhodtseva, N., and Livingstone, M. S. (2012). Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 72, 3652–3663. doi: 10.1158/0008-5472.CAN-12-0128
McDannold, N., and Maier, S. E. (2008). Magnetic resonance acoustic radiation force imaging. Med. Phys. 35, 3748–3758. doi: 10.1118/1.2956712
McDannold, N., Vykhodtseva, N., Raymond, S., Jolesz, F. A., and Hynynen, K. (2005). MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 31, 1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010
McDannold, N., Zhang, Y.-Z., Power, C., Jolesz, F., and Vykhodtseva, N. (2013). Nonthermal ablation with microbubble-enhanced focused ultrasound close to the optic tract without affecting nerve function. J. Neurosurg. 119, 1208–1220. doi: 10.3171/2013.8.JNS122387
McDannold, N. J., Vykhodtseva, N. I., and Hynynen, K. (2006). Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology 241, 95–106. doi: 10.1148/radiol.2411051170
McDannold, N., Vykhodtseva, N., and Hynynen, K. (2006). Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys. Med. Biol. 51, 793–807. doi: 10.1088/0031-9155/51/4/003
Moghimi, S. M. (2018). Nanomedicine safety in preclinical and clinical development: focus on idiosyncratic injection/infusion reactions. Drug Discov. Today 23, 1034–1042. doi: 10.1016/j.drudis.2017.11.006
Mokdad, A. H., Ballestros, K., Echko, M., Glenn, S., Olsen, H. E., Mullany, E., et al. (2018). The state of US Health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA 319, 1444–1472. doi: 10.1001/jama.2018.0158
Nardecchia, S., Sánchez-Moreno, P., de Vicente, J., Marchal, J. A., and Boulaiz, H. (2019). Clinical trials of thermosensitive nanomaterials: an overview. Nanomater. (Basel) 9:191. doi: 10.3390/nano9020191
Nation, D. A., Sweeney, M. D., Montagne, A., Sagare, A. P., D’Orazio, L. M., Pachicano, M., et al. (2019). Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276. doi: 10.1038/s41591-018-0297-y
Norinder, U., and Haeberlein, M. (2002). Computational approaches to the prediction of the blood–brain distribution. Adv. Drug Deliv. Rev. 54, 291–313. doi: 10.1016/s0169-409x(02)00005-4
Odéen, H., de Bever, J., Almquist, S., Farrer, A., Todd, N., Payne, A., et al. (2014). Treatment envelope evaluation in transcranial magnetic resonance-guided focused ultrasound utilizing 3D MR thermometry. J. Ther. Ultrasound 2:19. doi: 10.1186/2050-5736-2-19
O’Reilly, M. A., and Hynynen, K. (2012). Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions–based controller. Radiology 263, 96–106. doi: 10.1148/radiol.11111417
Paquin, R., Vignaud, A., Marsac, L., Younan, Y., Lehéricy, S., Tanter, M., et al. (2013). Keyhole acceleration for magnetic resonance acoustic radiation force imaging (MR ARFI). Magn. Reson. Imaging 31, 1695–1703. doi: 10.1016/j.mri.2013.07.011
Pardridge, W. M. (2005). The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2, 3–14. doi: 10.1007/bf03206638
Pardridge, W. M. (2012). Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 32, 1959–1972. doi: 10.1038/jcbfm.2012.126
Pardridge, W. M., Oldendorf, W. H., Cancilla, P., and Frank, H. J. (1986). Blood-brain barrier: interface between internal medicine and the brain. Ann. Intern. Med. 105, 82–95. doi: 10.7326/0003-4819-105-1-82
Park, J., Zhang, Y., Vykhodtseva, N., Jolesz, F. A., and McDannold, N. J. (2012). The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J. Control. Release 162, 134–142. doi: 10.1016/j.jconrel.2012.06.012
Rapoport, N. (2012). Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 4, 492–510. doi: 10.1002/wnan.1176
Rapoport, N. Y., Kennedy, A. M., Shea, J. E., Scaife, C. L., and Nam, K.-H. (2009). Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release 138, 268–276. doi: 10.1016/j.jconrel.2009.05.026
Rieke, V., and Pauly, K. B. (2008). MR thermometry. J. Magn. Reson. Imaging 27, 376–390. doi: 10.1002/jmri.21265
Robbin, M. L., and Eisenfeld, A. J. (1998). Perflenapent emulsion: a US contrast agent for diagnostic radiology–multicenter, double-blind comparison with a placebo. EchoGen Contrast Ultrasound Study Group. Radiology 207, 717–722. doi: 10.1148/radiology.207.3.9609895
Salehi, A., Paturu, M., Caine, M., Klein, R., and Kim, A. H. (2019). Laser therapy enhances blood-brain barrier and blood-tumor barrier permeability in a mouse model of glioblastoma. Neurosurgery 66:nyz310_635. doi: 10.1093/neuros/nyz310_635
Salgaonkar, V. A., Datta, S., Holland, C. K., and Mast, T. D. (2009). Passive cavitation imaging with ultrasound arrays. J. Acoust. Soc. Am. 126, 3071–3083.
Sampson, J. H., Archer, G., Pedain, C., Wembacher-Schröder, E., Westphal, M., Kunwar, S., et al. (2010). Poor drug distribution as a possible explanation for the results of the PRECISE trial. J. Neurosurg. 113, 301–309. doi: 10.3171/2009.11.JNS091052
Sheikov, N., McDannold, N., Vykhodtseva, N., Jolesz, F., and Hynynen, K. (2004). Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 30, 979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010
Sontum, P. C. (2008). Physicochemical Characteristics of SonazoidTM, A New Contrast Agent for Ultrasound Imaging. Ultrasound Med. Biol. 34, 824–833. doi: 10.1016/j.ultrasmedbio.2007.11.006
Sun, T., Jia, N., Zhang, D., and Xu, D. (2012). Ambient pressure dependence of the ultra-harmonic response from contrast microbubbles. J. Acoust. Soc. Am. 131, 4358–4364. doi: 10.1121/1.4707512
Sun, T., Samiotaki, G., Wang, S., Acosta, C., Chen, C. C., and Konofagou, E. E. (2015). Acoustic cavitation-based monitoring of the reversibility and permeability of ultrasound-induced blood-brain barrier opening. Phys. Med. Biol. 60, 9079–9094. doi: 10.1088/0031-9155/60/23/9079
Sun, T., Zhang, Y., Power, C., Alexander, P. M., Sutton, J. T., Aryal, M., et al. (2017). Closed-loop control of targeted ultrasound drug delivery across the blood-brain/tumor barriers in a rat glioma model. Proc. Natl. Acad. Sci. U.S.A 114, E10281–E10290.
Sweeney, M. D., Sagare, A. P., and Zlokovic, B. V. (2018a). Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150. doi: 10.1038/nrneurol.2017.188
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., and Zlokovic, B. V. (2018b). Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78. doi: 10.1152/physrev.00050.2017
Szablowski, J. O., Lee-Gosselin, A., Lue, B., Malounda, D., and Shapiro, M. G. (2018). Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng. 2, 475–484. doi: 10.1038/s41551-018-0258-2
Szebeni, J., Alving, C. R., Rosivall, L., Bünger, R., Baranyi, L., Bedöcs, P., et al. (2007). Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J. Liposome Res. 17, 107–117. doi: 10.1080/08982100701375118
Szebeni, J., Simberg, D., González-Fernández, Á., Barenholz, Y., and Dobrovolskaia, M. A. (2018). Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotech. 13, 1100–1108. doi: 10.1038/s41565-018-0273-1
Tung, Y. S., Vlachos, F., Choi, J. J., Deffieux, T., Selert, K., and Konofagou, E. E. (2010). In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys. Med. Biol. 55, 6141–6155. doi: 10.1088/0031-9155/55/20/007
Tung, Y.-S., Vlachos, F., Feshitan, J. A., Borden, M. A., and Konofagou, E. E. (2011). The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J. Acoust. Soc. Am. 130, 3059–3067. doi: 10.1121/1.3646905
Vignon, F., Shi, W. T., Powers, J. E., Everbach, E. C., Liu, J., Gao, S., et al. (2013). Microbubble cavitation imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 60, 661–670. doi: 10.1109/TUFFC.2013.2615
Vyas, U., Ghanouni, P., Halpern, C. H., Elias, J., and Pauly, K. B. (2016). Predicting variation in subject thermal response during transcranial magnetic resonance guided focused ultrasound surgery: comparison in seventeen subject datasets. Med. Phys. 43, 5170–5180. doi: 10.1118/1.4955436
Vyas, U., Kaye, E., and Pauly, K. B. (2012). Transcranial phase aberration correction using beam simulations and MR-ARFI. AIP Conf. Proc. 1503, 185–190. doi: 10.1063/1.4769941
Wang, J. B., Aryal, M., Zhong, Q., Vyas, D. B., and Airan, R. D. (2018). Noninvasive ultrasonic drug uncaging maps whole-brain functional networks. Neuron 100, 728–738.e7. doi: 10.1016/j.neuron.2018.10.042
Wang, S., Samiotaki, G., Olumolade, O., Feshitan, J. A., and Konofagou, E. E. (2014). Microbubble type and distribution dependence of focused ultrasound-induced blood–brain barrier opening. Ultrasound Med. Biol. 40, 130–137. doi: 10.1016/j.ultrasmedbio.2013.09.015
Weksler, B. B., Subileau, E. A., Perrière, N., Charneau, P., Holloway, K., Leveque, M., et al. (2005). Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874. doi: 10.1096/fj.04-3458fje
Yao, J., Xia, J., Maslov, K. I., Nasiriavanaki, M., Tsytsarev, V., Demchenko, A. V., et al. (2013). Noninvasive photoacoustic computed tomography of mouse brain metabolism in vivo. Neuroimage 64, 257–266. doi: 10.1016/j.neuroimage.2012.08.054
Keywords: focused ultrasound, drug delivery, neurointervention, neuromodulation, nanotechnology, blood–brain barrier
Citation: Wang JB, Di Ianni T, Vyas DB, Huang Z, Park S, Hosseini-Nassab N, Aryal M and Airan RD (2020) Focused Ultrasound for Noninvasive, Focal Pharmacologic Neurointervention. Front. Neurosci. 14:675. doi: 10.3389/fnins.2020.00675
Received: 25 November 2019; Accepted: 02 June 2020;
Published: 14 July 2020.
Edited by:
Victor Frenkel, University of Maryland, Baltimore, United StatesReviewed by:
Vassiliy Tsytsarev, University of Maryland, College Park, United StatesGraeme F. Woodworth, University of Maryland, Baltimore, United States
Copyright © 2020 Wang, Di Ianni, Vyas, Huang, Park, Hosseini-Nassab, Aryal and Airan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raag D. Airan, cmFpcmFuQHN0YW5mb3JkLmVkdQ==
†These authors have contributed equally to this work
 Jeffrey B. Wang
Jeffrey B. Wang Tommaso Di Ianni
Tommaso Di Ianni Daivik B. Vyas
Daivik B. Vyas Zhenbo Huang
Zhenbo Huang Sunmee Park
Sunmee Park Muna Aryal
Muna Aryal Raag D. Airan
Raag D. Airan