
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 16 July 2020
Sec. Neuropharmacology
Volume 14 - 2020 | https://doi.org/10.3389/fnins.2020.00629
 Georg A. Petroianu1,2*
Georg A. Petroianu1,2* Dietrich E. Lorke1,2
Dietrich E. Lorke1,2The use of dopamine receptor blockers for chronic singultus treatment is based—at least partially—on circular thinking: chlorpromazine is FDA-approved for hiccups, chlorpromazine is a neuroleptic, neuroleptics are dopamine receptor blockers, and therefore hiccup is due to dopaminergic dysfunction. Chlorpromazine interacts with high affinity with a multitude of receptors and ion channels. This promiscuity is the basis for many of the therapeutic effects and adverse drug reactions of this drug. While an involvement of dopamine is certain, it is by no means clear that dopaminergic dysfunction is the hallmark of singultus. The common denominator of most remedies for transient hiccup is their ability to activate the vagus nerve. Both afferent and efferent vagal activity and the central integration of the Xth cranial nerve function are modulated, inter alia, via serotonergic mechanisms; beneficial (therapeutic) effects for hiccup are to be expected from serotonin (5-HT) receptor subtype ligands that enhance vagal activity. Taken together, it appears that the ability to increase vagus output is mainly associated with 5-HT1A, 5-HT3, and 5-HT7 agonists and with 5-HT2C antagonists. The plausibility of the serotonergic singultus hypothesis is examined against available pharmacokinetic, pharmacodynamic, and clinical data for a number of drugs.
Hiccup (Latin, singultus) is generated by an involuntary contraction of the diaphragm followed by closure of the glottis. The inspired air meeting a closed glottis causes the typical hiccup sound. Hiccupping of extended duration can be incapacitating (Petroianu, 2019).
Most classifications use arbitrary time limits to categorize the phenomenon. Brief episodes of hiccupping are physiologic. The point of transition to a pathologic form is not well defined. The longer the duration of the hiccupping, the less amenable it will be to interventions. An episode lasting longer than a week is considered chronic while resistance to sequential therapy using three different drugs warrants the use of the label obstinate (Petroianu, 2019).
Hiccup is not a disease but a symptom. The situation most commonly encountered is that of hiccup of idiopathic origin. In this context, “idiopathic” describes one’s inability to demonstrate, rather than the absence of, an organic origin.
Probably only a few drugs in the Physician’s Desk Reference have not been tried in the therapy of singultus, and anyone who looks hard enough at the literature will be able to find anecdotal support for the use of almost any drug. In contrast, only a few drug categories (benzodiazepines, barbiturates, alcohol, and steroids) are well-established hiccup inducers (Petroianu, 2019).
Prevalence of chronic obstinate singultus was estimated in Germany in the 1990s at 1:103–1:105, with an overwhelming elderly male preponderance (Petroianu and Brunnengraber, 1992).
The introduction of chlorpromazine into clinical practice in the early 1950s had a major impact on psychiatry. The drug revolutionized the discipline and established the field of psycho-pharmacology (Laborit et al., 1952; Ban, 2007). The success of chlorpromazine as an antipsychotic (neuroleptic) combined with the fact that it was far superior to the (very) few other central nervous system (CNS) drugs available at the time (morphine, hyoscine, and quinidine) led to its use for a multitude of conditions (Ey and Faure, 1956).
One of the conditions chlorpromazine was tested for was chronic (obstinate) hiccup, and positive case results were reported by various groups (Moyer et al., 1954; Stewart and Redeker, 1954; Davignon et al., 1955; Friedgood and Ripstein, 1955; Garipuy and Raymond, 1956; Guiang and Leones-Guiang, 1957).
The manufacturer Smith Kline and French advertised “another dramatic use of Thorazine: to stop intractable hiccups (often after the first dose) in 56 out of 62 patients in seven different studies,” and the United States Food and Drug Administration (FDA) approved chlorpromazine for the treatment of hiccups (Thorazine advertisements, 1954, 1955).
Chlorpromazine established itself as a successful hiccup treatment, even after attributing some of the reported success rate to a difficult-to-quantify placebo effect (Friedman, 1996).
While chlorpromazine efficacy for chronic hiccup treatment is generally accepted, the mechanism of action is unclear and not necessarily identical with the antipsychotic mechanism of action. Chlorpromazine has a rich pharmacology with at least if not greater affinity for a range of other targets.
This promiscuity is the basis for many of the therapeutic effects and adverse drug reactions (ADRs) of this drug. The antipsychotic usefulness of the drug is related to its ability to block dopamine and serotonin (5-HT) receptors. Among the more relevant ADRs to be named are orthostatic hypotension (α-adrenergic blockade), dry mouth, urinary retention, and other signs and symptoms of parasympathetic inhibition (muscarinic cholinergic blockade), Parkinson’s-like symptoms, decrease in libido and increase in plasma prolactin levels (dopaminergic blockade), sedation and weight gain (histaminergic blockade), weight gain, and anhedonia (5-HT2C serotonergic blockade), and QT prolongation (inhibition of the human ether-a-go-go-related gene = hERG potassium channel). While the affinity of chlorpromazine for hERG channels is low (high Ki), the ability to block other sites and induce ADRs is comparable or even higher than its affinity for the sites associated with the antipsychotic response.
The assumption that dopamine receptor blockers must be the pillar for treatment of hiccups is—at least partially—based on circular thinking: chlorpromazine is FDA-approved for hiccups, chlorpromazine is a typical neuroleptic, neuroleptics are dopamine receptor blockers, and therefore hiccup is a manifestation of dopaminergic dysfunction.
• Reports of dopaminergic agents (amantadine, levodopa, pergolide, piribedil, and pramipexole) inducing hiccups (Launois et al., 1993; Bagheri et al., 1999; Sharma et al., 2006).
• Reports of selective anti-dopaminergic agents (haloperidol) being able to control hiccups (Korczyn, 1971; Scarnati, 1979; Ives et al., 1985).
• Reports of failure of anti-dopaminergic agents to control hiccups (Schuchmann and Browne, 2007).
• Reports of anti-dopaminergic agents (perphenazine) inducing hiccups (Miyaoka and Kamijima, 1999; Cheng et al., 2011).
• Reports of dopaminergic agonists (amantadine, apomorphine, pergolide, pramipexole, piribedil, levodopa, ropinrole) used to treat hiccup (Welsh, 1904; Garrick, 1917; Askenasy et al., 1988; Martinez-Ruiz et al., 2004; Sharma et al., 2006; Lester et al., 2007; Gerschlager and Bloem, 2009; Coletti Moja, 2010).
• Reports of failure of selective anti-dopaminergic agents (haloperidol) to control hiccups (Nishikawa et al., 2015).
• Discrepancies between the incidence of use of dopaminergic agonists and the incidence of hiccup, although these might be due to underreporting (Stegmeier-Petroianu and Petroianu, 2008; Miwa and Kondo, 2010).
Taken together, the evidence indicates that while an involvement of dopamine as a neurotransmitter in the hiccup reflex circuitry is certain, it is by no means clear that dopaminergic overactivity is the common denominator of hiccups, and therefore dopaminergic blockade must not necessarily be the main thrust of therapeutic attempts.
Hiccupping is a physiologic occurrence during intrauterine life (Miller and Petroianu, 2016). It has been proposed that hiccup is an essential and universal primitive reflex that may recur, like other primitive reflexes, in adult life (Ingiulla, 1962; Dunn, 1977; Fuller, 1990; Steger et al., 2015). The suggested hiccup reflex arc consists of vagal, phrenic, and sympathetic afferents, a hiccup center in the upper spinal cord/brainstem region, and efferents that elicit a contraction of the diaphragm and the external intercostal muscles along the phrenic and intercostal nerves, as well as, immediately thereafter, a closure of the glottis via the vagus nerve, whose motor fibers travel with the recurrent laryngeal nerve to the larynx (Askenasy, 1992; Friedman, 1996; Steger et al., 2015).
In the adult, this primitive reflex is suppressed (Oshima et al., 1998; Straus et al., 2003). Reappearance is explained either by the loss of inhibition from hierarchically higher structures or by a surge in input from the periphery.
Many therapeutic strategies, with the GABAB receptor agonist baclofen and the α2-δ ligands (gabapentin, pregabalin) being the most successful ones (Burke et al., 1988; Lance and Bassil, 1989; Ramirez and Graham, 1992; Guelaud et al., 1995; Petroianu et al., 1997, 2000; Petroianu, 1998; Jatzko et al., 2007), non-specifically reduce neurotransmitter release; their unquestionable success in chronic hiccup treatment does not, however, allow any inference as to the specific neurotransmitters and their receptors involved in the assumed hiccup reflex circuitry (black-box approach).
More contributory to the understanding of the pathophysiology is a look at therapies empirically established for the suppression of the so-called occasional or transient hiccup. These have recently been reviewed, and the author concludes that the common denominator of most, if not all of these homegrown remedies is their ability to activate the vagus nerve, as evidenced by their additional ability to terminate paroxysmal supraventricular tachycardias (Petroianu, 2013, 2015, 2020). Among the best-known “vagal maneuvers” are the oculo-cardiac reflex (Dagnini-Aschner), carotid sinus massage, the Valsalva maneuver, and ice ingestion. While usually effective in terminating bouts of acute hiccup, they are mostly ineffective in cases of hiccupping that have been present for an extended period, probably due to insufficiently sustained vagus nerve activation (Petroianu, 2015). The successful use of vagus nerve electrical stimulation for chronic intractable hiccups has been reported (Payne et al., 2005; Longatti et al., 2010) as have been failures of this approach (Grewal et al., 2018). The rationale for stimulating the left vagus nerve is that it innervates the AV node of the heart so as to have less of an effect on heart rate than the right vagus, which innervates the SA node (Carreno and Frazer, 2017).
Nucleus tractus solitarius (NTS) receives general visceral afferent information. According to Jordan “the NTS can be considered the brainstem equivalent of the dorsal horn” (Jordan, 2005). The NTS is involved in a number of reflex mechanisms (gag, carotid sinus, cough, and vomiting reflex).
Nucleus ambiguus (NA) is in the medullary reticular formation. The nerve fibers originating from the NA are efferent visceral motor fibers that provide motor innervation for swallowing and phonation. This vagal nucleus innervates most striated muscles of the pharynx and larynx. The NA also contains the majority (≈90%) of preganglionic cholinergic parasympathetic neurons that innervate postganglionic parasympathetic neurons in the heart.
Dorsal vagal nucleus (DVN) sends parasympathetic visceral efferent fibers to thoracic and abdominal viscera.
The activation of cardiac vagal outflow by afferents involves a multi-synaptic pathway within the brainstem. Cardiorespiratory afferents terminate within the NTS. Neurotransmitters used by vagal afferents include peptides such as substance P and calcitonin gene-related peptide and the excitatory amino acid transmitter glutamate (Carreno and Frazer, 2017). Within this nucleus, the information is processed and integrated before passing to the output neurons located within the DVN and NA (Skinner et al., 2002; Jordan, 2005).
5-HT interacts with the autonomic nervous system, in particular its parasympathetic component (vagus nerve) at several levels.
Intravenous administration of 5-HT lowers the heart rate. Since 5-HT is barely able to cross the blood–brain barrier, this is likely an effect in the periphery. The intra-cerebro-ventricular injection (rat) of 5-HT has minor effects on the mean arterial blood pressure but produces a decrease in heart rate (Sévoz-Couche et al., 2000; Villalon and Centurion, 2007; Davisson et al., 2014). However, 5-HT can also cause generalized sympatho-excitation by stimulation of receptors at the site of sympathetic control (rostral ventrolateral medulla) and of receptor-mediated catecholamine release from adrenomedullary chromaffin cells. Neither systemic application nor intra-cerebro-ventricular injection allows identification of the specific receptor subtype involved (Figure 1).
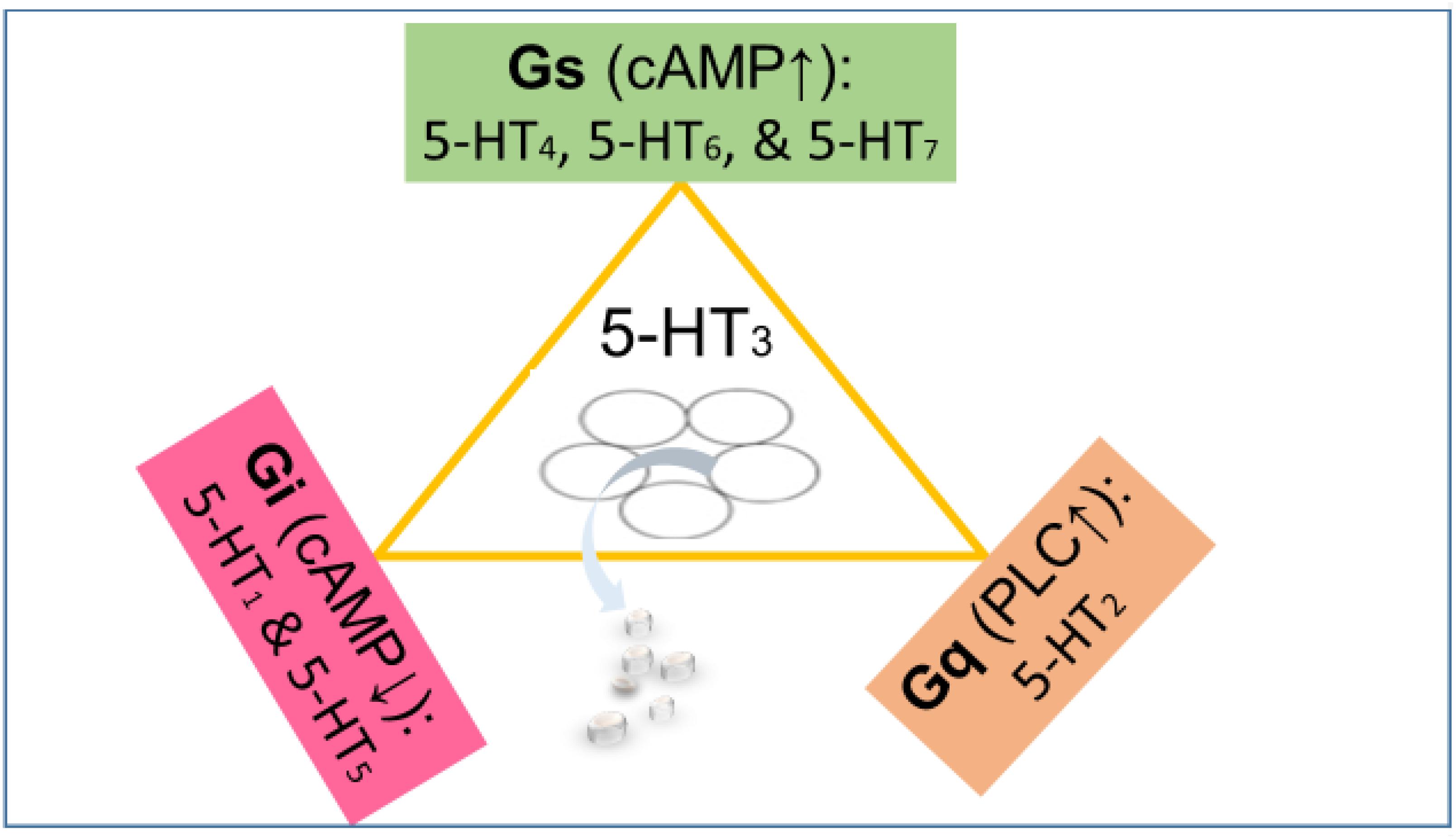
Figure 1. The 5-HT3 receptor (center) is an excitatory cation channel (mainly Na+ and Ca2+), belonging to the cys-loop superfamily of ligand-gated ion channels closely related by homology to the nicotinic acetylcholine receptor. All other serotonin receptors are G-protein-coupled (GPCRs). The 5-HT subtypes 4, 6, and 7 are coupled to stimulatory G proteins (Gs) responsible for increasing the cyclic AMP concentration. The subtypes 1 and 5 are coupled to inhibitory G proteins (Gi) responsible for lowering the cyclic AMP concentration. The 5-HT2 receptors couple to Gq proteins activating phospholipase C (PLC) and ultimately increasing Ca2+ concentration (Kaumann and Levy, 2006). The effect of the activation of various serotonin receptors depends not only on the G protein they are coupled to but also on their localization. For instance, a serotonin receptor coupled to Gs localized on a GABAergic neuron will enhance inhibition, while a serotonin receptor coupled to Gi localized on a GABAergic neuron will reduce inhibition (inhibition of inhibition).
5-HT1A receptors (Gi) inhibit adenyl cyclase as their principal signaling mechanism (Kaumann and Levy, 2006). They are located both pre-synaptically and post-synaptically (Stahl, 2015; Svob Strac et al., 2016). Pre-synaptic 5-HT1A auto-receptors located on cell bodies, when stimulated, lead to inhibition of firing of 5-HT neurons and are key components of a negative feedback loop (inhibitory auto-receptors), while pre-synaptic hetero-receptors located on GABAergic neurons reduce neurotransmitter release. By blocking 5-HT1A auto-receptors at doses that are selective for them over post-synaptic 5-HT1A receptors, it is possible to disinhibit 5-HT release. Stimulation of post-synaptic 5-HT1A receptors on GABAergic neurons leads to hyperpolarization and reduced inhibition.
Adding complexity, the 5-HT1A functions as a hub receptor in a number of iso- and hetero-receptor dimerizations (Borroto-Escuela et al., 2017). Receptor–receptor interaction (cross talk inhibition) takes place in the 5-HT iso-receptor complexes described (5-HT1A-5-HT7 and 5-HT1A-5-HT2A; Renner et al., 2012; Borroto-Escuela et al., 2016, 2017).
Present knowledge indicates that activation of 5-HT1A hetero-receptors enhances vagal activity by disinhibition of glutamatergic neurons [reduction of (inhibitory) GABA release]. Transgenic mice overexpressing 5-HT1A receptors show prolonged episodes of bradycardia, and 5-HT1A agonists induce bradycardia (Ramage, 1990; Jordan, 2005; Audero et al., 2008; Ramage and Villalon, 2008; Restrepo et al., 2010). 5-HT1A receptor agonists produce miosis in humans (Yu et al., 2004). Measurement of pupil size seems to provide a valuable and sensitive index of 5-HT1A receptor function (Fanciullacci et al., 1995).
5-HT1A gene knockout animals showed increased fear and sympatho-activation under experimental conditions (Klemenhagen et al., 2006).
In conclusion, stimulation of 5-HT1A receptors causes central sympatho-inhibition and an increase in cardiac vagal drive (Ramage, 1990).
5-HT2A receptors [Gq; activation of phospholipase C→inositol triphosphate (IP3↑) and diacylglycerol (DAG↑)] are expressed widely throughout the CNS and periphery (Hoyer et al., 2002). This is the main excitatory receptor subtype among the metabotropic 5-HT receptors. The receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD; later, it came back to prominence, because it was also found to be mediating, at least partly, the action of many antipsychotic drugs. Age-related reduction in the density of 5-HT2A receptors is correlated with cognitive decline (Hasselbalch et al., 2008), and 5-HT2A receptors are decreased in the prefrontal cortex of patients with Alzheimer’s disease (Lorke et al., 2006). In the periphery, it is highly expressed in platelets, cardiovascular system, fibroblasts, and neurons of the peripheral nervous system. Calcium entry through glutamate responsive NMDA channels subsequent to 5-HT2A receptor activation dramatically affects both pre-synaptic and post-synaptic excitability of neurons in the DVN (Huang and Pickel, 2003; Svob Strac et al., 2016). Jordan assigns 5-HT2A receptors a vagal activator effect (Jordan, 2005). In contrast, others expressed the view that activation of 5-HT2A leads to inhibition of parasympathetic synaptic transmission (Chang et al., 2017).
5-HT2B receptors (Gq) are located both centrally and in the periphery. Agonists have been associated with endocardial fibrous tissue proliferation and (nor-fenfluramine) valvulopathy (Zanettini et al., 2007; Andersohn and Garbe, 2009); antagonists lack (up to now) a clear therapeutic application. While Jordan (2005) ascribes 5-HT2B receptors a role in vagal activity, this does not appear to be prominent. Nevertheless, in NTS neurons receiving vagal afferent inputs, using ligands selective for the different 5-HT2 receptor subtypes, it was observed that activation of 5-HT2A and 5-HT2B receptors had predominantly excitatory effects while activation of 5-HT2C receptors predominantly reduced neuronal firing.
5-HT2C receptors (Gq) are structurally similar to 5-HT2A receptors, and the two coexist in many brain regions and on the same neurons. Functionally, 5-HT2A and 5-HT2C are mostly antagonists. They play opposing facilitative and inhibitory roles. 5-HT2C activation inhibits neurotransmitter (dopamine) release. Feeding, social interaction, sexual activity, and drugs (caffeine, nicotine, amphetamine, morphine, cocaine) all induce dopamine release, which is subject to inhibition by 5-HT2C. 5-HT2C activation inhibits NTS neurons (vagal activity; Sévoz-Couche et al., 2000; Jordan, 2005).
5-HT3 receptors are the only ionotropic serotonin receptors. 5-HT3 receptors are located (mainly) on sensory vagal nerve endings and play a vital role for vagal afferent input from the gastrointestinal tract, lungs, and heart. The central terminals of vagal afferents exhibit 5-HT3 receptors that function to increase glutamatergic synaptic transmission to second-order neurons of the NTS within the brainstem (Browning, 2015). Experimental compounds with 5-HT3 blocking properties increase the heart rate by decreasing vagal afferent input and efferent output; this is compatible with data showing that 5-HT3 receptors excite vagal afferent neurons by a glutamate-dependent mechanism (Jordan, 2005; Ramage and Villalon, 2008). Blockade of these receptors by 5-HT3 antagonists (setrons) is used clinically for control of emesis (Svob Strac et al., 2016).
5-HT4 receptors (Gs; couple positively to adenylyl cyclase) control acetylcholine release; 5-HT4 antagonists have been proposed to treat an overactive bladder (Brudeli et al., 2013) while agonists (-pride) are gastro-kinetic agents. For a number of years (until its removal from the market due to concerns related to QT prolongation), cisapride was (more or less) successfully used to treat hiccups as part of a combination therapy with baclofen or gabapentin and omeprazole (Petroianu et al., 1997; 1998; 2000; 2004).
5-HT5 receptors (Gi protein coupled) are virtually unexplored due to lack of selective ligands (Pithadia and Jain, 2009).
5-HT6 receptors are Gs protein coupled and mediate excitatory neurotransmission. 5-HT6 receptors are expressed almost exclusively in the brain. Despite the 5-HT6 receptor having a functionally excitatory action (Gs), it is largely co-localized with GABAergic neurons and produces an overall inhibition of brain activity (Yun and Rhim, 2011).
More recently, it was recognized that 5-HT6 receptors modulate primarily GABA and glutamate levels, modulating the secondary release of other neurotransmitters (Khoury et al., 2018). Most interestingly, it was recently demonstrated that 5-HT6 receptor antagonism reduces defecation in rats (Hagsäter et al., 2019). This finding suggests an involvement of this receptor in the control of parasympathetic activity.
5-HT7 receptors (Gs protein coupled) are expressed both centrally and in the periphery (Svob Strac et al., 2016). Many—if not all—atypical antipsychotic drugs are also antagonists at this receptor. Defining the influence of this receptor on the vagus nerve is difficult, due to both species differences and the lack of selective agonists and antagonists. There is indication that 5-HT7 receptor protein is localized on vagal nerve fibers and that 5-HT2 and 5-HT7 receptors have opposite effects on vagal activity (García-Pedraza et al., 2017). Initial studies indicate that, in rodents, central 5-HT7 receptors play a facilitatory role in the reflex activation of vagal outflow to the heart (Kellett et al., 2005) and that blocking either 5-HT1A or 5-HT7 receptors attenuates bradycardias (i.e., increases heart rate), indicating that both subtypes have the ability to activate the vagus nerve (Jordan, 2005; Ramage and Villalon, 2008). It was speculated that vagus activation was mediated by 5-HT7 receptors located in the NTS (Jordan, 2005; Kellett et al., 2005). In contrast, García-Pedraza et al. (2017) report that 5-HT7 receptor activation suppresses the vagally induced bradycardia, suggesting the opposite, i.e., an inhibitory role of 5-HT7 receptors upon vagal activity. Moreover, the same group could demonstrate that 5-HT7 activation also stimulates the sympathetic outflow (García-Pedraza et al., 2013). Hernández-Abreu et al. (2020) reported that blockade of 5-HT2 receptors uncovers 5-HT7 receptors’ ability to inhibit the sympathetic drive in pithed rats, involving hyperpolarization due to the opening of ATP-sensitive K+ channels.
Complexity is added by 5-HT1A-5-HT7 co-expression and heterodimer formation. Hetero-dimerization (cross talk inhibition) decreases the ability of the 5-HT1A receptor to induce hyperpolarization (Renner et al., 2012).
Taken together, it appears that the ability to increase vagus (efferent) output is associated with 5-HT1A, 5-HT3, 5-HT4, and possibly 5-HT7 agonists and with 5-HT2C antagonists. The role of 5-HT2A receptors is not clearly established (Figure 2).
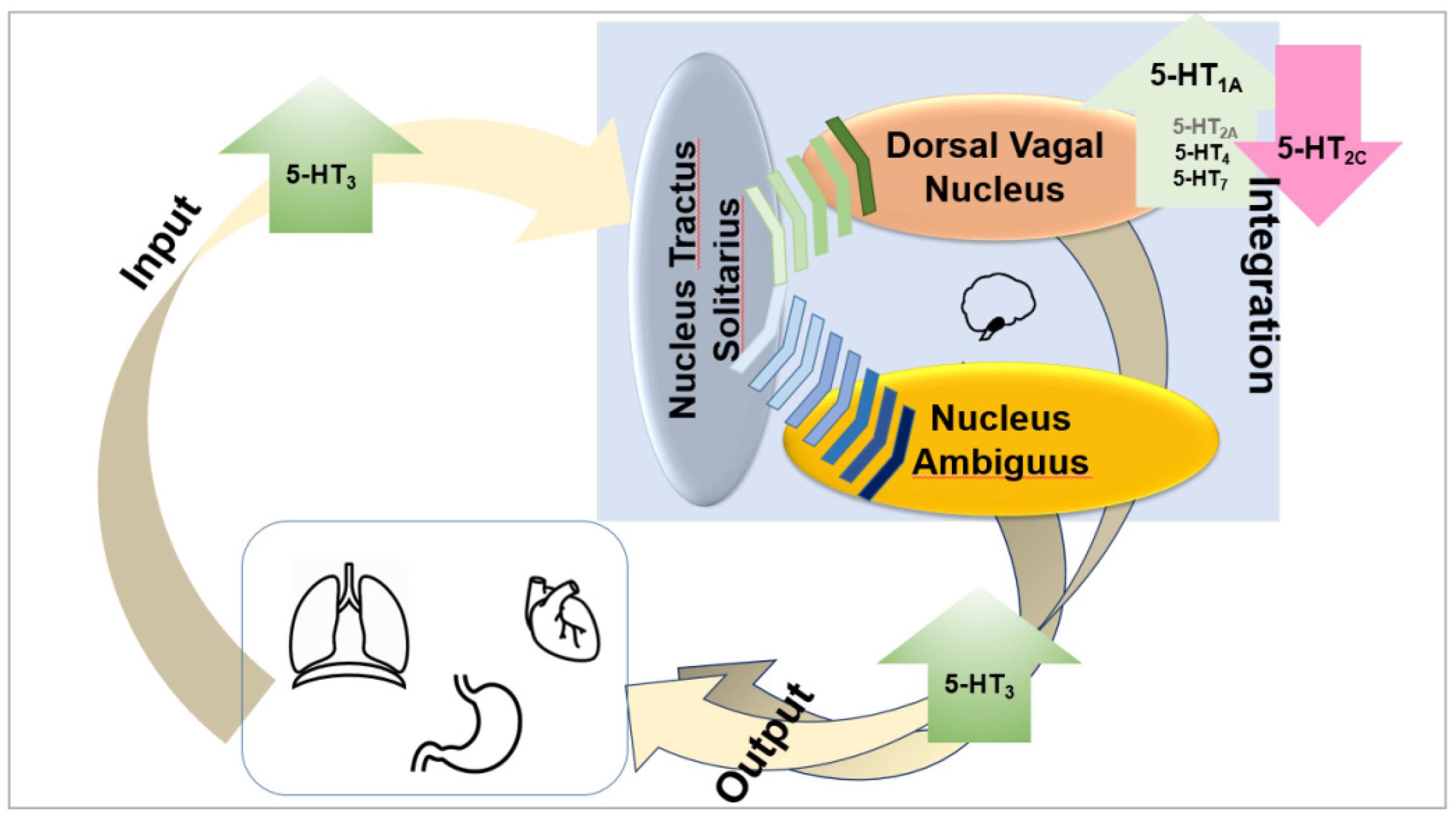
Figure 2. Taken together, it appears that the ability to increase vagus (efferent) output is associated with 5-HT1A, 5-HT3, and 5-HT4 agonists and with 5-HT2C antagonists. The role of 5-HT7 and 5-HT2A receptors is not clearly established, but it appears possible that they also enhance vagal output.
Vagal mechanisms are operational in the occasional (transient) hiccup and most probably also in chronic singultus. Serotonergic neurotransmission is intricately related to vagal activity. Modulation of serotonergic neurotransmission influences vagal activity, offering possible explanations for the facilitation of singultus (vagal inhibition) by some drugs as well as for the ability of other compounds to suppress hiccups (vagal activation). We will discuss the effects of drugs that either stimulate or block 5-HT receptors upon vagal activity and hiccups. We hope the review will add to the understanding of the phenomenon singultus and possibly trigger a rethinking of the underlying biology of this condition.
Activation of 5-HT1A receptors enhances vagal activity; therefore, 5-HT1A agonists could be useful in the control of chronic hiccups.
Flibanserin (approved for the treatment of premenopausal women with hypoactive sexual desire disorder) acts as a full agonist of the 5-HT1A receptor and, with lower affinity, as an antagonist of the 5-HT2A receptor (Borsini et al., 2002). To our knowledge, no effect of flibanserin on singultus has been reported.
Tandospirone was successfully employed for the treatment of intractable hiccups (Takahashi et al., 2004). However, other mechanisms and receptors may also come into play, since a major metabolite of tandospirone, 1-(2-pyrimidinyl)-piperazine (1-PP), is a centrally acting α2-adrenergic antagonist (Blier et al., 1991; Onizuka et al., 2002). Central α2 antagonism increases 5-HT release and availability (Scheibner et al., 2001). Therefore, the central α2-adrenergic antagonist mirtazapine (atypical antidepressant), can be considered an indirect 5-HT1A receptor agonist. By additionally antagonizing 5-HT2 and 5-HT3 receptors (Anttila and Leinonen, 2001), thereby funneling most of its action to the 1A subtype, mirtazapine mainly activates the 5-HT1A receptor; it has also been successfully used for the treatment of intractable singultus (de Boer et al., 1996; Chung et al., 2008).
In contrast, no beneficial effect upon singultus has been reported for buspirone, a tandospirone-like drug, which is a weaker partial 5-HT1A agonist that has been available for nearly 30 years and is widely used. It has even been associated—albeit very rarely—with causing hiccups (<1/1000 patients; Silverman et al., 2014; Healthy Place, 2017).
However, these differential effects of tandospirone and buspirone upon singultus may be due to differences in efficacies between these two compounds. Tandospirone and buspirone have similar affinities for the 5-HT1A receptor (≈20–30 nM), but different efficacies. Buspirone has an efficacy (Emax) of 20–50% at this receptor (as compared with 5-HT), while tandospirone has a higher Emax of 80%, closer to that of a full agonist (Yabuuchi et al., 2004; Sumiyoshi et al., 2007).
Typical (haloperidol) and atypical (aripiprazole, olanzapine, risperidone, and brexpiprazole) antipsychotics also interact with the 5-HT1A receptor. Aripiprazole is a partial 5-HT1A agonist with comparable if not higher affinity (≈1–10 nM) than the spirones (5-HT1A partial agonists), its efficacy being similar to tandospirone (Emax = 70%) (Shapiro et al., 2003; Davies et al., 2004).
In contrast, olanzapine, risperidone, and haloperidol have much lower affinities for this receptor (two orders of magnitude) and comparative efficacy (Emax) in the low negative values (−15, −20, and −10, respectively), indicating antagonist/inverse agonist profiles (Newman-Tancredi et al., 2005).
Aripiprazole shows the strongest association of any antipsychotic drugs with hiccup induction. In contrast, olanzapine seems to be a successful antipsychotic agent in the context of singultus suppression (Alderfer and Arciniegas, 2006; Rizzo et al., 2014). There is an abundance of reports on hiccups associated with aripiprazole treatment (Behere et al., 2007; Ginsberg, 2007; Ray et al., 2009; De Filippis et al., 2015; Sakalli Kani et al., 2015; Caloro et al., 2016; Kutuk et al., 2016) and on persistent hiccups associated with switching antipsychotic treatment from risperidone to aripiprazole (Yeh, 2011), from a typical antipsychotic drug of the thioxanthene class to aripiprazole (Duvarci and Yilmaz, 2013), and from olanzapine to aripiprazole (Hori and Nakamura, 2014). In a patient treated with aripiprazole, singultus persisted, despite trials of metoclopramide and chlorpromazine; remission of hiccups occurred after discontinuation of aripiprazole (Silverman et al., 2014).
Pindolol, a beta blocker with nanomolar affinity at 5-HT1A receptors (Ki ≈ 10–30 nM) exhibits antagonist properties showing preferential action on somatodendritic 5-HT1A auto-receptors with an efficacy of ≈20% relative to the endogenous agonist. Despite the functional antagonist profile, the drug to our knowledge has not been associated with hiccup induction (Celada et al., 2004).
Aripiprazole, buspirone, pindolol, and tandospirone, having similar effects upon the 5-HT1A receptor, but quite different effects on singultus, do not provide support to the assumption that 5-HT1A receptors play a pivotal role in the pathogenesis of hiccups. Equally unsupportive is the finding that inverse agonists/antagonists such as olanzapine do not seem to induce hiccups and, on the contrary, can be quite useful in treating them.
Takahashi et al. (2004) suggested that 5-HT1A agonists suppress hiccups by inhibiting phrenic nerve activity, while Silverman et al. (2014) proposed that 5-HT1A partial agonists (functional antagonists) promote singultus by enhancing phrenic nerve motor activity.
Chlorpromazine (Ki ≈ 840 nM), for all practical purposes, has no effect at the 5-HT1A receptor (Yonemura et al., 1998).
Therapeutic efficacy of atypical antipsychotics and their metabolites depends on their high affinity (single digit nanomolar Ki) for and antagonist activity at this receptor subtype. As elaborated above, atypical antipsychotics can either induce or suppress hiccups, due to their affinity to other 5-HT receptor subtypes. With 5-HT2A antagonism being a class-defining property of atypical antipsychotics, the influence of the 5-HT2A receptor upon singultus pathogenesis can therefore not be easily evaluated. For comparison, chlorpromazine has a Ki ≈ 10 nM for this receptor.
Most typical (including chlorpromazine) and atypical antipsychotics are antagonists or inverse agonists at this receptor. In contrast, the previously mentioned atypical aripiprazole binds with nanomolar affinity at 5-HT2C receptors (Ki ≈ 15–180 nM) and exhibits partial agonist properties with an efficacy Emax ≈ 80% relative to the endogenous agonist. Lorcaserin, marketed for weight loss, is the only selective 5-HT2C receptor agonist clinically available (Ki ≈ 15 nM; Emax ≈ 40%). There are no reports regarding lorcaserin and hiccup. Pimavanserin, marketed for Parkinson’s psychosis, is a 5-HT2A and 5-HT2C receptor antagonist (Ki ratio ≈ 1: 40; Stahl, 2016b). There are no reports regarding pimavanserin and singultus.
Antagonists (-setrons) are potent and highly selective competitive inhibitors with negligible affinity for other receptors. They are rapidly absorbed and penetrate the blood–brain barrier easily. Antiemetic efficacy results from a simultaneous action at peripheral and central 5-HT3 receptors.
Blockage of 5-HT3 receptors in the periphery reduces the activity of vagal afferents and would thus decrease efferent output; blocking 5-HT3 receptors centrally would also reduce efferent output. A single anecdotal mentioning of a negative impact of setrons on a patient with chronic hiccup has been published in 1992 (Petroianu and Brunnengraber, 1992). There are numerous anecdotal reports in the non-scientific literature claiming that setrons cause hiccups (Wilkes, 2007; Kantrowitz, 2009; MylanPharmaceuticals, 2017; Pharmacorama, 2017). Chlorpromazine has a very low affinity for this receptor (Yonemura et al., 1998). Taken together, these data suggest that 5-HT3 antagonists may facilitate singultus.
Initial studies suggest that activation of 5-HT7 receptors increases efferent vagal activity (Jordan, 2005; Kellett et al., 2005); more recent reports, however, indicate the opposite (García-Pedraza et al., 2017). Most antipsychotics (chlorpromazine, clozapine, risperidone, ziprasidone, paliperidone, pimozide, and amisulpride) are antagonists at the 5-HT7 receptors.
Nishikawa and his colleagues reported on a patient with intractable hiccups where haloperidol failed to provide relief, while in contrast, risperidone completely abolished the singultus shortly after administration (Nishikawa et al., 2015). While both haloperidol and risperidone are antagonists with comparably low nanomolar affinity at D2 receptors, only risperidone blocks 5-HT2C receptors and has an affinity, at least one order of magnitude higher (lower Ki) than haloperidol at 5-HT2A and 5-HT7 receptors (Roth et al., 1994; Amato et al., 2015). The authors conclude that the ability of risperidone to suppress hiccups versus the failure of haloperidol to do so indicates that the serotonergic system may play a role in the pathophysiology of some hiccup forms (Nishikawa et al., 2015). Notwithstanding such therapeutic successes, there are also case reports of risperidone inducing singultus (Cheng and Tsai, 2015).
Pimozide acts as an antagonist at D2-like receptors and the 5-HT7 receptor; it has the highest affinity of all the typical antipsychotic agents tested for the 5-HT7 receptor (Ki < 1 nM; Roth et al., 1994). Pimozide is anecdotally reported to be clinically used to control not only nausea and vomiting but also intractable hiccups (DrugInfoSys, 2016). However, there are also reports on pimozide causing hiccups (Merck, 2017).
Amisulpride, a benzamide antagonist of the dopamine D2 and D3 receptors and an antagonist of the 5-HT2B and 5-HT7 receptors has been reported to induce singultus in a schizophrenic patient not sufficiently controlled on paliperidone (Cheng and Tsai, 2015). Paliperidone (9-OH-risperidone) has a receptor profile very similar to risperidone, with the difference of a lower affinity (antagonist) at the 5-HT1A receptor. Compared with amisulpride, the affinity of the two for 5-HT7 is very similar (≈10 nM). Amisulpride has, however, no effect on other 5-HT receptors except 5-HT2B (15 nM; Abbas et al., 2009).
Aripiprazole, while commonly described as a partial agonist at 5-HT7, has a low intrinsic activity (Emax ≈ 2%), and hence is a functional antagonist of this receptor.
In summary, the observed pharmacological effects of medications upon hiccup can be attributed to a variety of receptors, including the 5-HT7 type. As long as there are no selective ligands of the 5-HT7 receptor that are clinically available, it is difficult to draw conclusions about the role of this receptor upon hiccup. Available data, however, suggest an influence of 5-HT7 receptors upon singultus pathogenesis and therapy.
The overlap between maneuvers used to terminate paroxysmal supraventricular tachycardia, a not uncommon cardiac arrhythmia, and those employed to terminate bouts (paroxysms) of hiccups is striking. It suggests that activation of efferent vagal fibers can be therapeutic in both instances. While coincidence is obviously not proof of causality, it warrants nevertheless further investigations.
Taken together, it appears that the ability to increase vagus (efferent) output is associated with 5-HT1A, 5-HT3, and 5-HT4 agonists and with 5-HT2C antagonists. The role of 5-HT7 receptors is not clearly established, but it appears possible that they also enhance vagal output.
Chlorpromazine does not display the ideal required profile for a vagus activator (Table 1).
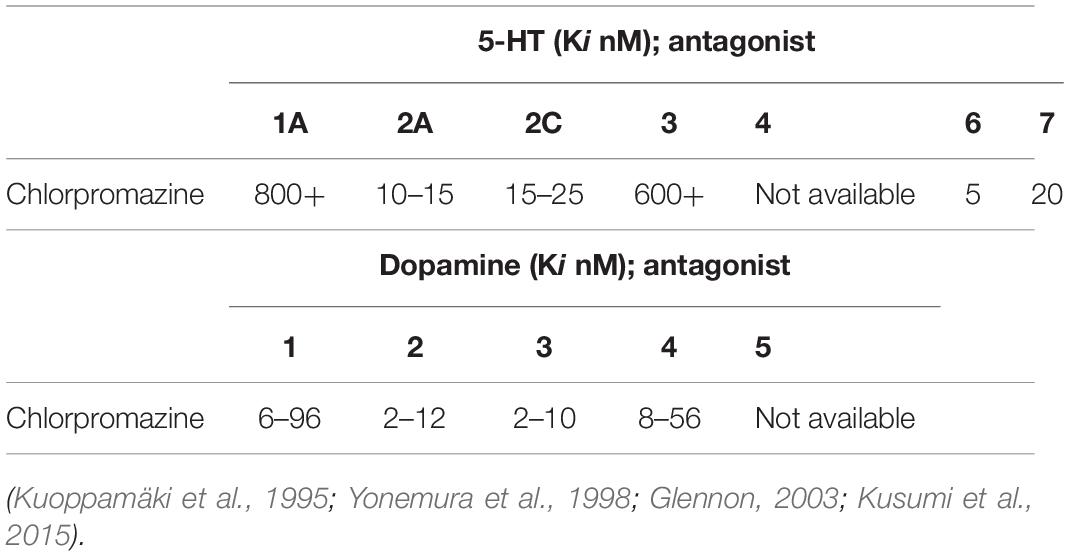
Table 1. Chlorpromazine, while without effect at 5-HT1A, 5-HT3, and 5-HT4 receptors, is a 5-HT2A, 5-HT2C, 5-HT6, and 5-HT7 antagonist and thus theoretically—at least from a serotonergic perspective—does not display the ideal required profile for a vagus activator. Affinities for the dopaminergic receptors are also provided.
A review of the various drug actions does not warrant a definitive conclusion at this time. While painfully aware of the limitations of comparing receptor affinities/intrinsic activity values—even more so when obtained from different sources using different methodologies—and inferring biological effects based on such data, it is still the only practical option available (de Bartolomeis et al., 2015; Das et al., 2016; Stahl, 2016a). We nevertheless hope our work might add to the understanding of the phenomenon singultus and possibly trigger a rethinking of the underlying biology of this condition.
GP and DL drafted the manuscript and both approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbas, A. I., Hedlund, P. B., Huang, X. P., Tran, T. B., Meltzer, H. Y., and Roth, B. L. (2009). Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology 205, 119–128. doi: 10.1007/s00213-009-1521-8
Alderfer, B. S., and Arciniegas, D. B. (2006). Treatment of intractable hiccups with olanzapine following recent severe traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 18, 551–562.
Amato, D., Pum, M. E., Groos, D., Lauber, A. C., Huston, J. P., Carey, R. J., et al. (2015). Neuropharmacology of light-induced locomotor activation. Neuropharmacology 95, 243–251. doi: 10.1016/j.neuropharm.2015.03.023
Andersohn, F., and Garbe, E. (2009). Cardiac and noncardiac fibrotic reactions caused by ergot-and nonergot-derived dopamine agonists. Mov. Disord. 24, 129–133. doi: 10.1002/mds.22385
Anttila, S. A., and Leinonen, E. V. (2001). A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 7, 249–264. doi: 10.1111/j.1527-3458.2001.tb00198.x
Askenasy, J. J., Boiangiu, M., and Davidovitch, S. (1988). Persistent hiccup cured by amantadine. N. Engl. J. Med. 318:711. doi: 10.1056/nejm198803173181118
Askenasy, J. J. (1992). About the mechanism of hiccup. Eur. Neurol. 32, 159–163. doi: 10.1159/000116815
Audero, E., Coppi, E., Mlinar, B., Rossetti, T., Caprioli, A., Banchaabouchi, M. A., et al. (2008). Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science 321, 130–133. doi: 10.1126/science.1157871
Bagheri, H., Cismondo, S., and Montastruc, J. L. (1999). Drug-induced hiccup: a review of the France pharmacologic vigilance database. Therapie 54, 35–39.
Ban, T. A. (2007). Fifty years chlorpromazine: a historical perspective. Neuropsychiatr. Dis. Treat. 3, 495–500.
Behere, R. V., Venkatasubramanian, G., Naveen, M. N., and Gangadhar, B. N. (2007). Aripiprazole-induced hyponatremia: a case report. J. Clin. Psychiatry 68, 640–641. doi: 10.4088/jcp.v68n0423g
Blier, P., Curet, O., Chaput, Y., and de Montigny, C. (1991). Tandospirone and its metabolite, 1-(2-pyrimidinyl)-piperazine–II. Effects of acute administration of 1-PP and long-term administration of tandospirone on noradrenergic neurotransmission. Neuropharmacology 30, 691–701. doi: 10.1016/0028-3908(91)90176-c
Borroto-Escuela, D. O., DuPont, C. M., Li, X., Savelli, D., Lattanzi, D., Srivastava, I., et al. (2017). Disturbances in the FGFR1-5-HT1A heteroreceptor complexes in the raphe-hippocampal 5-HT system develop in a genetic rat model of depression. Front. Cell. Neurosci. 11:309. doi: 10.3389/fncel.2017.00309
Borroto-Escuela, D. O., Tarakanov, A. O., and Fuxe, K. (2016). FGFR1–5 HT1A heteroreceptor complexes: implications for understanding and treating major depression. Trends Neurosci. 39, 5–15. doi: 10.1016/j.tins.2015.11.003
Borsini, F., Evans, K., Jason, K., Rohde, F., Alexander, B., and Pollentier, S. (2002). Pharmacology of flibanserin. CNS Drug Rev. 8, 117–142.
Browning, K. N. (2015). Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front. Neurosci. 9:413. doi: 10.3389/fnins.2015.00413
Brudeli, B., Moltzau, L. R., Nguyen, C. H., Andressen, K. W., Nilsen, N. O., Levy, F. O., et al. (2013). Synthesis and pharmacological properties of a new hydrophilic and orally bioavailable 5-HT4 antagonist. Eur. J. Med. Chem. 64, 629–637. doi: 10.1016/j.ejmech.2013.03.060
Burke, A. M., White, A. B., Brill, N. (1988). Baclofen for intractable hiccups. N. Engl. J. Med. 319:1354. doi: 10.1056/nejm198811173192017
Caloro, M., Pucci, D., Calabro, G., de Pisa, E., Mancinelli, I., Rosini, E., et al. (2016). Development of hiccup in male patients hospitalized in a psychiatric ward: is it specifically related to the aripiprazole-benzodiazepine combination? Clin. Neuropharmacol. 39, 67–72. doi: 10.1097/wnf.0000000000000129
Carreno, F. R., and Frazer, A. (2017). Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 14: 716–727 doi: 10.1007/s13311-017-0537-8
Celada, P., Puig, M., Amargos-Bosch, M., Adell, A., and Artigas, F. (2004). The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J. Psychiatry Neurosci. 29, 252–265.
Chang, C. C., Fang, W. H., Chang, H. A., Chang, T. C., Shyu, J. F., and Huang, S. Y. (2017). Serotonin 2A receptor (5-HT2A) gene promoter variant interacts with chronic perceivedstress to modulate resting parasympathetic activity in humans. Psychoneuroendocrinology 76, 119–126. doi: 10.1016/j.psyneuen.2016.11.015
Cheng, C. M., and Tsai, S. J. (2015). Persistent hiccups associated with switching from paliperidone to amisulpride. Psychiatry Clin. Neurosci. 69:383. doi: 10.1111/pcn.12239
Cheng, Y. M., Lin, W. A., and Yang, H. N. (2011). Risperidone-induced hiccups in a youth with down syndrome. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 641–642. doi: 10.1016/j.pnpbp.2010.09.001
Chung, S., Oh, Y. M., and Yang, J. C. (2008). Persistent hiccups successfully treated with mirtazapine. Eur. Neuropsychopharmacol. 18:343.
Coletti Moja, M. (2010). Hiccups associated with non-ergoline dopamine agonists in Parkinson’s disease. Mov. Disord. 25, 1292–1313.
Das, S., Barnwal, P., Winston, A. B., Mondal, S., and Saha, I. (2016). Brexpiprazole: so far so good. Ther. Adv. Psychopharmacol. 6, 39–54. doi: 10.1177/2045125315614739
Davies, M. A., Sheffler, D. J., and Roth, B. L. (2004). Aripiprazole: a novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 10, 317–336. doi: 10.1111/j.1527-3458.2004.tb00030.x
Davignon, A., Lemieux, G., and Genest, J. (1955). Chlorpromazine in the treatment of stubborn hiccup. Union Med. Can. 84, 282–284.
Davisson, R. L., Bates, J. N., Johnson, A. K., and Lewis, S. J. (2014). Effects of intracerebroventricular injections of 5-HT on systemic vascular resistances of conscious rats. Microvasc. Res. 95, 116–123. doi: 10.1016/j.mvr.2014.08.002
de Bartolomeis, A., Tomasetti, C., and Iasevoli, F. (2015). Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs 29, 773–799. doi: 10.1007/s40263-015-0278-3
de Boer, T. H., Nefkens, F., van Helvoirt, A., and van Delft, A. M. (1996). Differences in modulation of noradrenergic and serotonergic transmission by the alpha-2 adrenoceptor antagonists, mirtazapine, mianserin and idazoxan. J. Pharmacol. Exp. Ther. 277, 852–860.
De Filippis, S., Ranieri, V., Cuomo, I., Zingaretti, P., Kotzalidis, G. D., Telesforo, C. L., et al. (2015). Hiccup with aripiprazole plus benzodiazepines resolving with pregabalin and/or benzodiazepine switch/discontinuation: four case reports. J. Clin. Psychopharmacol. 35, 195–197. doi: 10.1097/jcp.0000000000000292
DrugInfoSys (2016). Pimozide. Available online at: http://www.druginfosys.com/drug.aspx?drugcode=574&type=1 (accessed June 28, 2020).
Duvarci, I., and Yilmaz, M. (2013). Persistent hiccups after switching from zuclopenthixol to ariprazole. Bull. Clin. Psychopharmacol. 23, 89–90. doi: 10.5455/bcp.20120419125722
Ey, H., and Faure, H. (1956). Various methods for the use of chlorpromazine in psychiatric therapy, and their indications. Encephale 45, 361–371.
Fanciullacci, M., Sicuteri, R., Alessandri, M., and Geppetti, P. (1995). Buspirone, but not sumatriptan, induces miosis in humans: relevance for a serotoninergic pupil control. Clin. Pharmacol. Therap. 57, 349–355. doi: 10.1016/0009-9236(95)90161-2
Friedgood, C. E., and Ripstein, C. B. (1955). Chlorpromazine (thorazine) in the treatment of intractable hiccups. J. Am. Med. Assoc. 157, 309–310.
García-Pedraza, J. A., Garcia, M., Martin, M. L., Eleno, N., and Moran, A. (2017). Chronic sarpogrelate treatment reveals 5-HT7 receptor in the serotonergic inhibition of the rat vagal bradycardia. J. Cardiovasc. Pharmacol. 69, 13–22. doi: 10.1097/fjc.0000000000000433
García-Pedraza, J. Á, García, M., Martín, M. L., Gómez-Escudero, J., Rodríguez-Barbero, A., Román, L. S., et al. (2013). Peripheral 5-HT1D and 5-HT7 serotonergic receptors modulate sympathetic neurotransmission in chronic sarpogrelate treated rats. Eur. J. Pharmacol. 714, 65–73. doi: 10.1016/j.ejphar.2013.05.045
Gerschlager, W., and Bloem, B. R. (2009). Hiccups associated with levodopa in Parkinson’s disease. Mov. Disord. 24, 621–622. doi: 10.1002/mds.22395
Glennon, R. A. (2003). Higher-end serotonin receptors: 5-HT5, 5-HT6, and 5-HT7. J. Med. Chem. 46, 2795–2812. doi: 10.1021/jm030030n
Grewal, S. S., Adams, A. C., and Van Gompel, J. J. (2018). Vagal nerve stimulation for intractable hiccups is not a panacea: a case report and review of the literature. Intern. J. Neurosci. 128, 1114–1117. doi: 10.1080/00207454.2018.1486307
Guelaud, C., Similowski, T., Bizec, J. L., Cabane, J., Whitelaw, W. A., and Derenne, J. P. (1995). Baclofen therapy for chronic hiccup. Eur. Respir. J. 8, 235–237. doi: 10.1183/09031936.95.08020235
Guiang, R. V., and Leones-Guiang, M. A. (1957). Intractable hiccups in subarachnoid hemorrhage relieved by chlorpromazine. J. Philipp. Med. Assoc. 33, 40–41.
Hagsäter, S. M., Lisinski, A., and Eriksson, E. (2019). 5-HT6 receptor antagonism reduces defecation in rat: a potential treatment strategy for irritable bowel syndrome with diarrhea. Eur. J. Pharmacol. 864:172718. doi: 10.1016/j.ejphar.2019.172718
Hasselbalch, S. G., Madsen, K., Svarer, C., Pinborg, L. H., Holm, S., Paulson, O. B., et al. (2008). Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol. Aging 29, 1830–1838. doi: 10.1016/j.neurobiolaging.2007.04.011
Hernández-Abreu, O. I., García-Pedraza, J. Á, Rivera-Mancilla, E., Villanueva-Castillo, B., Morán, A., García-Domingo, M., et al. (2020). Blockade of 5-HT2 receptors with sarpogrelate uncovers 5-HT7 receptors inhibiting the tachycardic sympathetic drive in pithed rats. Clin. Exp. Pharmacol. Physiol. 47, 403–411. doi: 10.1111/1440-1681.13227
Hori, H., and Nakamura, J. (2014). Hiccups associated with switching from olanzapine to aripiprazole in a patient with paranoid schizophrenia. Clin. Neuropharmacol. 37, 88–89. doi: 10.1097/wnf.0000000000000032
Hoyer, D., Hannon, J. P., and Martin, G. R. (2002). Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 71, 533–554. doi: 10.1016/s0091-3057(01)00746-8
Huang, J., and Pickel, V. M. (2003). Ultrastructural localization of serotonin 2A and N-methyl-D-aspartate receptors in somata and dendrites of single neurons within rat dorsal motor nucleus of the vagus. J. Compar. Neurol. 455, 270–280. doi: 10.1002/cne.10497
Ives, T. J., Fleming, M. F., Weart, C. W., and Bloch, D. (1985). Treatment of intractable hiccups with intramuscular haloperidol. Am. J. Psychiatry 142, 1368–1369. doi: 10.1176/ajp.142.11.1368
Jatzko, A., Stegmeier-Petroianu, A., and Petroianu, G. A. (2007). Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J. Pain Sympt. Manag. 33, 756–760. doi: 10.1016/j.jpainsymman.2006.09.026
Jordan, D. (2005). Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp. Physiol. 90, 175–181. doi: 10.1113/expphysiol.2004.029058
Kantrowitz, M. (2009). Chemo Hiccups. Available online at: http://www.kantrowitz.com/cancerpoints/hiccups.html (accessed June 28, 2020).
Kaumann, A. J., and Levy, F. O. (2006). 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 111, 674–706. doi: 10.1016/j.pharmthera.2005.12.004
Kellett, D. O., Ramage, A. G., and Jordan, D. (2005). Central 5-HT7 receptors are critical for reflex activation of cardiac vagal drive in anaesthetized rats. J. Physiol. 563, 319–331. doi: 10.1113/jphysiol.2004.076521
Khoury, R., Grysman, N., Gold, J., Patel, K., and Grossberg, G. T. (2018). The role of 5 HT6-receptor antagonists in Alzheimer’s disease: an update. Expert. Opin. Investig. Drugs 27, 523–533. doi: 10.1080/13543784.2018.1483334
Klemenhagen, K. C., Gordon, J. A., David, D. J., Hen, R., and Gross, C. T. (2006). Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology 31, 101–111. doi: 10.1038/sj.npp.1300774
Kuoppamäki, M., Pälvimäki, E. P., Hietala, J., and Syvälahti, E. (1995). Differential regulation of rat 5-HT 2A and 5-HT 2C receptors after chronic treatment with clozapine, chlorpromazine and three putative atypical antipsychotic drugs. Neuropsychopharmacology 13, 139–150. doi: 10.1016/0893-133x(95)00049-j
Kusumi, I., Boku, S., and Takahashi, Y. (2015). Psychopharmacology of atypical antipsychotic drugs: From the receptor binding profile to neuroprotection and neurogenesis. Psychiatr. Clin. Neurosci. 69, 243–258. doi: 10.1111/pcn.12242
Kutuk, M. O., Tufan, A. E., Guler, G., Yildirim, V., and Toros, F. (2016). Persistent hiccups due to aripiprazole in an adolescent with obsessive compulsive disorder responding to dose reduction and rechallenge. Oxf. Med. Case Rep. 2016, 66–67. doi: 10.1093/omcr/omw017
Laborit, H., Huguenard, P., and Alluame, R. (1952). Un nouveau stabilisateur vegetatif (le 4560 R.P.). Presse Med. 60, 206–208.
Lance, J. W., and Bassil, G. T. (1989). Familial intractable hiccup relieved by baclofen. Lancet 2, 276–277. doi: 10.1016/s0140-6736(89)90460-1
Launois, S., Bizec, J. L., Whitelaw, W. A., Cabane, J., and Derenne, J. P. (1993). Hiccup in adults: an overview. Eur. Respir. J. 6, 563–575.
Lester, J., Raina, G. B., Uribe-Roca, C., and Micheli, F. (2007). Hiccup secondary to dopamine agonists in Parkinson’s disease. Mov. Disord. 22, 1667–1668. doi: 10.1002/mds.21583
Longatti, P., Basaldella, L., Moro, M., Ciccarino, P., and Franzini, A. (2010). Refractory central supratentorial hiccup partially relieved with vagus nerve stimulation. J. Neurol. Neurosurg. Psychiatr. 81, 821–822. doi: 10.1136/jnnp.2009.179929
Lorke, D. E., Lu, G., Cho, E., and Yew, D. T. (2006). Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci. 7:36. doi: 10.1186/1471-2202-7-36
Martinez-Ruiz, M., Fernandez Riestra, F., and Quesada Rubio, R. (2004). Pramipexole for intractable hiccups. Med. Clin. 123:679.
Merck (2017). EMEND (Aprepitant). Available from: https://www.merck.com/product/usa/pi_circulars/e/emend/emend_pi.pdf.6/08/2017 (accessed June 24, 2020).
Miller, C. C., and Petroianu, G. A. (2016). Singultus foetalis and Dr. Alfons Mermann. J. Hist. Neurosci. 25, 420–422. doi: 10.1080/0964704x.2015.1077017
Miwa, H., and Kondo, T. (2010). Hiccups in Parkinson’s disease: an overlooked non-motor symptom? Parkinsonism Relat. Disord. 16, 249–251. doi: 10.1016/j.parkreldis.2009.12.004
Miyaoka, H., and Kamijima, K. (1999). Perphenazine-induced hiccups. Pharmacopsychiatry 32:81. doi: 10.1055/s-2007-979198
Moyer, J. H., Kent, B., Knight, R. W., Morris, G., Dizon, M., Rogers, S., et al. (1954). Clinical studies of an anti-emetic agent, chlorpromazine. Am. J. Med. Sci. 228, 174–189. doi: 10.1097/00000441-195408000-00007
Newman-Tancredi, A., Assie, M. B., Leduc, N., Ormiere, A. M., Danty, N., and Cosi, C. (2005). Novel antipsychotics activate recombinant human and native rat serotonin 5-HT1A receptors: affinity, efficacy and potential implications for treatment of schizophrenia. Int. J. Neuropsychopharmacol. 8, 341–356. doi: 10.1017/s1461145704005000
Nishikawa, T., Araki, Y., and Hayashi, T. (2015). Intractable hiccups (singultus) abolished by risperidone, but not by haloperidol. Ann. Gen. Psychiatry 14:13. doi: 10.1186/s12991-015-0051-5
Onizuka, J., Murata, T., Omori, M., and Wada, Y. (2002). Effectiveness of serotonin (5-HT)1A receptor agonist in a patient with psychogenic apneusis. J. Clin. Psychopharmacol. 22, 334–337. doi: 10.1097/00004714-200206000-00017
Oshima, T., Sakamoto, M., Tatsuta, H., and Arita, H. (1998). GABAergic inhibition of hiccup-like reflex induced by electrical stimulation in medulla of cats. Neurosci. Res. 30, 287–293. doi: 10.1016/s0168-0102(98)00011-x
Payne, B. R., Tiel, R. L., Payne, M. S., and Fisch, B. (2005). Vagus nerve stimulation for chronic intractable hiccups, Case report. J. Neurosurg. 102, 935–937. doi: 10.3171/jns.2005.102.5.0935
Petroianu, G. (1998). Idiopathic chronic hiccup (ICH): phrenic nerve block is not the way to go. Anesthesiology 89, 1284–1285. doi: 10.1097/00000542-199811000-00045
Petroianu, G., and Brunnengraber, R. (1992). Wenn schluckauf zur plage wird: gibt es eine rationale therapie? Pharm. Ztg. 137, 1891–1896.
Petroianu, G., Hein, G., Petroianu, A., Bergler, W., and Rufer, R. (1997). Idiopathic chronic hiccup: combination therapy with cisapride, omeprazole, and baclofen. Clin. Ther. 19, 1031–1038. doi: 10.1016/s0149-2918(97)80055-0
Petroianu, G., Hein, G., Stegmeier-Petroianu, A., Bergler, W., and Rufer, R. (2000). Gabapentin “add-on therapy” for idiopathic chronic hiccup (ICH). J. Clin. Gastroenterol. 30, 321–324. doi: 10.1097/00004836-200004000-00025
Petroianu, G. A. (2013). Treatment of singultus by traction on the tongue: an eponym revised. J. Hist. Neurosci. 22, 183–190. doi: 10.1080/0964704x.2012.728389
Petroianu, G. A. (2015). Treatment of hiccup by vagal maneuvers. J. Hist. Neurosci. 24, 123–136. doi: 10.1080/0964704x.2014.897133
Petroianu, G. A. (2000). When singultus becomes an illness. General practice references for hiccup. MMW Fortschr. Med. 142(49–50), 4–8.
Petroianu, G. A. (2004). Chronic idiopathic singultus: is there life after cisapride? N. Z. Med. J. 117(1188):U756.
Petroianu, G. A. (2020). Singultus, paper-bag ventilation, and hypercapnia. J. Hist. Neurosci. doi: 10.1080/0964704X.2019.1708161 [Epub ahead of print],
Pharmacorama (2017). Serotonin Antagonists. Available online at: http://www.pharmacorama.com/en/Sections/Serotonin_5.php (accessed June 28, 2020).
Pithadia, A. B., and Jain, S. M. (2009). 5-Hydroxytryptamine receptor subtypes and their modulators with therapeutic potentials. J. Clin. Med. Res. 1:72.
Ramage, A. G., and Villalon, C. M. (2008). 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol. Sci. 29, 472–481. doi: 10.1016/j.tips.2008.06.009
Ramage, A. G. (1990). Influence of 5-HT1A receptor agonists on sympathetic and parasympathetic nerve activity. J. Cardiovasc. Pharmacol. 15, S75–S85.
Ramirez, F. C., and Graham, D. Y. (1992). Treatment of intractable hiccup with baclofen: results of a double-blind randomized, controlled, cross-over study. Am. J. Gastroenterol. 87, 1789–1791.
Ray, P., Zia Ul Haq, M., and Nizamie, S. H. (2009). Aripiprazole-induced hiccups: a case report. Gen. Hosp. Psychiatry 31, 382–384.
Renner, U., Zeug, A., Woehler, A., Niebert, M., Dityatev, A., Dityateva, G., et al. (2012). Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 125, 2486–2499. doi: 10.1242/jcs.101337
Restrepo, B., Martin, M. L., Roman, L. S., and Moran, A. (2010). Peripheral 5-HT1A and 5-HT7 serotonergic receptors modulate parasympathetic neurotransmission in long-term diabetic rats. Exp. Diabetes Res. 2010:686734.
Rizzo, C., Vitale, C., and Montagnini, M. (2014). Management of intractable hiccups: an illustrative case and review. Am. J. Hosp. Palliat. Care 31, 220–224. doi: 10.1177/1049909113476916
Roth, B. L., Craigo, S. C., Choudhary, M. S., Uluer, A., Monsma, F. J. Jr., and Shen, Y., et al. (1994). Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 268, 1403–1410.
Sakalli Kani, A., Ocek, T., Aksoy-Poyraz, C., Turan, S., and Duran, A. (2015). Aripiprazole-induced acute hiccups: a case report. J. Neuropsychiatr. Clin. Neurosci. 27:e60. doi: 10.1176/appi.neuropsych.13100307
Scarnati, R. A. (1979). Intractable hiccup (singultus): report of case. J. Am. Osteopath. Assoc. 79, 127–129.
Scheibner, J., Trendelenburg, A. U., Hein, L., and Starke, K. (2001). α2-Adrenoceptors modulating neuronal serotonin release: a study in α2-adrenoceptor subtype-deficient mice. Br. J. Pharmacol. 132, 925–933. doi: 10.1038/sj.bjp.0703882
Schuchmann, J. A., and Browne, B. A. (2007). Persistent hiccups during rehabilitation hospitalization: three case reports and review of the literature. Am. J. Phys. Med. Rehabil. 86, 1013–1018. doi: 10.1097/phm.0b013e3181152152
Sévoz-Couche, C., Spyer, K. M., and Jordan, D. (2000). Inhibition of rat nucleus tractus solitarius neurones by activation of 5-HT2C receptors. Neuroreport 11, 1785–1790. doi: 10.1097/00001756-200006050-00038
Shapiro, D. A., Renock, S., Arrington, E., Chiodo, L. A., Liu, L. X., Sibley, D. R., et al. (2003). Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28, 1400–1411. doi: 10.1038/sj.npp.1300203
Sharma, P., Morgan, J. C., and Sethi, K. D. (2006). Hiccups associated with dopamine agonists in Parkinson disease. Neurology 66: 774. doi: 10.1212/01.wnl.0000201267.78431.f0
Silverman, M. A., Leung, J. G., and Schak, K. M. (2014). Aripiprazole-associated hiccups: a case and closer look at the association between hiccups and antipsychotics. J. Pharm. Pract. 27, 587–590. doi: 10.1177/0897190014544797
Skinner, M. R., Ramage, A. G., and Jordan, D. (2002). Modulation of reflexly evoked vagal bradycardias by central 5-HT1A receptors in anaesthetized rabbits. Br. J. Pharmacol. 137, 861–873. doi: 10.1038/sj.bjp.0704941
Stahl, S. M. (2015). Modes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): enhancing serotonin release by combining serotonin (5HT) transporter inhibition with actions at 5HT receptors (5HT1A, 5HT1B, 5HT1D, 5HT7 receptors). CNS Spectr. 20, 93–97. doi: 10.1017/s1092852915000139
Stahl, S. M. (2016a). Mechanism of action of brexpiprazole: comparison with aripiprazole. CNS Spectr. 21, 1–6. doi: 10.1017/s1092852915000954
Stahl, S. M. (2016b). Mechanism of action of pimavanserin in Parkinson’s disease psychosis: targeting serotonin 5HT2A and 5HT2C receptors. CNS Spectr. 21, 271–275. doi: 10.1017/s1092852916000407
Steger, M., Schneemann, M., and Fox, M. (2015). Systemic review: the pathogenesis and pharmacological treatment of hiccups. Alim. Pharm. Ther. 42, 1037–1050. doi: 10.1111/apt.13374
Stegmeier-Petroianu, A., and Petroianu, G. A. (2008). Hiccups and dopamine. Am. J. Health Syst. Pharm. 65, 2092–2094. doi: 10.2146/ajhp080128
Stewart, B. L., and Redeker, A. G. (1954). Emesis and hiccough; treatment with chlorpromazine. Calif. Med. 81, 203–205.
Straus, C., Vasilakos, K., Wilson, R. J. A., Oshima, T., Zelter, M., Derenne, J. P. et al. (2003). A phylogenetic hypothesis for the origin of hiccough. Bioessays 25, 182–188. doi: 10.1002/bies.10224
Sumiyoshi, T., Park, S., Jayathilake, K., Roy, A., Ertugrul, A., and Meltzer, H. Y. (2007). Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr. Res. 95, 158–168. doi: 10.1016/j.schres.2007.06.008
Svob Strac, D., Pivac, N., and Muck-Seler, D. (2016). The serotonergic system and cognitive function. Transl. Neurosci. 7, 35–49.
Takahashi, T., Murata, T., Omori, M., Tagaya, M., and Wada, Y. (2004). Successful treatment of intractable hiccups with serotonin (5-HT)1A receptor agonist. J. Neurol. 251, 486–487. doi: 10.1007/s00415-004-0377-4
Villalon, C. M. and Centurion, D. (2007). Cardiovascular responses produced by 5-hydroxytriptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedebergs Arch. Pharmacol. 376, 45–63. doi: 10.1007/s00210-007-0179-1
Yabuuchi, K., Tagashira, R., and Ohno, Y. (2004). Effects of tandospirone, a novel anxiolytic agent, on human 5-HT1A receptors expressed in Chinese hamster ovary cells (CHO cells). Biogen. Amines 18, 319–328. doi: 10.1163/1569391041501933
Yeh, Y. W. (2011). Persistent hiccups associated with switching from risperidone to aripiprazole in a schizophrenic patient with cerebral palsy. Clin. Neuropharmacol. 34, 135–136. doi: 10.1097/wnf.0b013e31822046bc
Yonemura, K., Miyanaga, K., and Machiyama, Y. (1998). Profiles of the affinity of antipsychotic drugs for neurotransmitter receptors and their clinical implication. Kitakanto Med. J. 48, 87–102. doi: 10.2974/kmj.48.87
Yu, B. N., Wang, A., Zhou, G., Zhang, W., Hu, D. L., Li, Q. and Zhou, H. H. (2004). T102C genetic polymorphism of the 5-HT2A receptor in Chinese hypertensive patients and healthy controls. Clin. Exp. Pharmacol. Physiol. 31, 847–849. doi: 10.1111/j.1440-1681.2004.04124.x
Yun, H. M., and Rhim, H. (2011). The serotonin-6 receptor as a novel therapeutic target. Exp. Neurobiol. 20, 159–168. doi: 10.5607/en.2011.20.4.159
Keywords: singultus, hiccup, vagal maneuver, serotonin, aripiprazole, buspirone
Citation: Petroianu GA and Lorke DE (2020) The Role of Serotonin in Singultus: A Review. Front. Neurosci. 14:629. doi: 10.3389/fnins.2020.00629
Received: 25 March 2020; Accepted: 22 May 2020;
Published: 16 July 2020.
Edited by:
Nick Andrews, Harvard Medical School, United StatesReviewed by:
Kate McDonnell-Dowling, Harvard Medical School, United StatesCopyright © 2020 Petroianu and Lorke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georg A. Petroianu, Z2VvcmcucGV0cm9pYW51QGt1LmFjLmFl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.