- 1Department of Biology, University of Pisa, Pisa, Italy
- 2Interdepartmental Research Center Nutrafood “Nutraceuticals and Food for Health”, University of Pisa, Pisa, Italy
Diabetic retinopathy (DR) is a common complication of diabetes and constitutes a major cause of vision impairment and blindness in the world. DR has long been described exclusively as a microvascular disease of the eye. However, in recent years, a growing interest has been focused on the contribution of neuroretinal degeneration to the pathogenesis of the disease, and there are observations suggesting that neuronal death in the early phases of DR may favor the development of microvascular abnormalities, followed by the full manifestation of the disease. However, the mediators that are involved in the crosslink between neurodegeneration and vascular changes have not yet been identified. According to our hypothesis, vascular endothelial growth factor (VEGF) could probably be the most important connecting link between the death of retinal neurons and the occurrence of microvascular lesions. Indeed, VEGF is known to play important neuroprotective actions; therefore, in the early phases of DR, it may be released in response to neuronal suffering, and it would act as a double-edged weapon inducing both neuroprotective and vasoactive effects. If this hypothesis is correct, then any retinal stress causing neuronal damage should be accompanied by VEGF upregulation and by vascular changes. Similarly, any compound with neuroprotective properties should also induce VEGF downregulation and amelioration of the vascular lesions. In this review, we searched for a correlation between neurodegeneration and vasculopathy in animal models of retinal diseases, examining the effects of different neuroprotective substances, ranging from nutraceuticals to antioxidants to neuropeptides and others and showing that reducing neuronal suffering also prevents overexpression of VEGF and vascular complications. Taken together, the reviewed evidence highlights the crucial role played by mediators such as VEGF in the relationship between retinal neuronal damage and vascular alterations and suggests that the use of neuroprotective substances could be an efficient strategy to prevent the onset or to retard the development of DR.
Introduction
Diabetes is a disease affecting a growing number of people worldwide. It is expected to increase to a little <700 million by 2045, with almost half of diabetics suffering from the slowly progressive type 2 diabetes, which in many cases remains undiagnosed (Cho et al., 2018). Type 2 diabetes is the main cause of diabetes in the population aged 40–74 years, although there is an increasing number of people aged <40 suffering from this form of the disease (Pantalone et al., 2015). Untreated or poorly controlled diabetes may lead to the appearance of serious complications, including diabetic retinopathy (DR). DR is the most common complication of diabetes and the leading cause of preventable visual impairment in the working age population in developed countries. It is also one of the main causes of blindness worldwide. In 2010, it has been estimated that about 95 million people suffered from a form of DR (Leasher et al., 2016). Due to the increasing number of diabetic people and the increased life expectancy, these numbers are expected to rise in the near future.
DR is a multifactorial progressive disease characterized by an extremely complex pathogenesis involving different factors and a variety of pathophysiologic mechanisms. Hyperglycemia represents a link between diabetes and DR complications. Indeed, prolonged high glucose levels damage the retina, inducing metabolic changes that result in dysregulation of a number of mediators, including growth factors, neurotrophic factors, cytokines/chemokines, vasoactive agents, and inflammatory and adhesion molecules. The altered retinal microenvironment is responsible for the appearance and the progression of extended vascular lesions and cell death (Qian and Ripps, 2011; Ola et al., 2012; Tarr et al., 2013; Abcouwer and Gardner, 2014).
DR has often been regarded as a purely vascular disorder of the retina. Clinically, it is classified as non-proliferative, characterized by microvascular damage, including blood-retina barrier (BRB) breakdown, basement membrane thickening, leukocyte adhesion, occurrence of acellular capillaries, capillary degeneration, pericyte loss; or proliferative, where neoangiogenesis phenomena are observed and new blood vessels are formed. These neovessels may generate a mechanic traction, causing retinal detachment and consequent blindness (Stitt et al., 2013). The key factor involved in pathologic vascular changes, from microvascular damage to neoangiogenesis, is vascular endothelial growth factor (VEGF). Consequently, DR treatments are mainly based on intraocular delivery of anti-VEGF molecules; however, the intravitreal administration of anti-VEGF drugs has several drawbacks, not the least of which is the fact that, due to the short half-life of the drug, frequent intraocular injections are necessary, generating different side effects, such as endophthalmitis and cataracts (Simo et al., 2014; Duh et al., 2017; Zhao and Singh, 2018). In addition, anti-VEGF drugs are used in mid to late stages of DR—when the vascular phenotype becomes evident, the disease is well-established, and vision has been significantly affected. Therefore, new alternative approaches to the current standard are urgently required to develop effective and early treatment options that may counteract the progression of DR at stages preceding the appearance of an evident vessel damage or vessel proliferation.
In addition to, and in contrast with, the view of DR as a purely vascular pathology, several investigations have studied the involvement and the role of retinal neurons in the disease. Indeed, since neurons are the most fragile and demanding cellular elements in the retina, it is conceivable that they are the first to be affected by damage when the microenvironment composition is drastically changed. Consistent with this hypothesis, a large amount of data has been collected in recent years, confirming that considerable damage of retinal neurons is present in early stages of DR (Antonetti et al., 2006; Hernandez and Simo, 2012; Zhang et al., 2013; Jindal, 2015; Simo and Hernandez, 2015; Hernandez et al., 2016b) and that DR may be considered a neurodegenerative disease of the retina (Barber, 2003).
Summarizing the evidence, one can say that both retinal neurons and vessels are affected in DR; therefore, the question is what kind of relationship, if any, exists between neuronal and vascular damage in DR. A first possibility is that there is no relationship and that neurons on one side and vascular elements on the other independently respond to the alterations caused by high glucose. Only at late stages of the disease, when proliferating vessels cause retinal detachment, the vascular pathology would affect neuronal function and survival. This hypothesis seems unlikely because neuronal, glial, and vascular cells are known to be intimately connected in the neurovascular unit, and recently reviewed evidence indicates that glial, neural, and microvascular dysfunctions are interdependent and intimately involved in the development of DR (Hammes, 2018; Simo et al., 2018). In this line, the American Diabetes Association has defined DR as a tissue-specific neurovascular complication involving progressive disruption of the interdependence between multiple cell types in the retina (Solomon et al., 2017; Simo et al., 2018). In particular, the function of the neurovascular unit is precociously affected in DR often before microvascular complications can be appreciated (see Simo and Hernandez, 2015, for references). Therefore, we favor the hypothesis that in DR, retinal neurons are primarily affected and their reaction to stress induces the vascular complications. Supporting this hypothesis, there are observations suggesting that brain damage, together with the activation of death pathways, also stimulates protective mechanisms mediated by chemical signals derived from the injured brain itself (Iadecola and Anrather, 2011). In the case of DR, one of these signals is likely to be represented by VEGF, which would be released by the retina in the early phases of the disease as an immediate response to neuronal stress. Indeed, this growth factor not only is a powerful inducer of vascular responses but is also known to exert important neuroprotective actions in the retina (Azzouz et al., 2004; Saint-Geniez et al., 2008; Romano et al., 2012; Beazley-Long et al., 2013; Foxton et al., 2013; Casini et al., 2014; Hombrebueno et al., 2015; Amato et al., 2016). Consistent with this view, glutamate excitotoxicity, one of the major causes of retinal neuronal death in DR, has been reported to upregulate VEGF production in diabetic retinas (Cervantes-Villagrana et al., 2010), while inhibition of NMDA receptors resulted in decreased vitreoretinal VEGF in diabetic rats (Kusari et al., 2010). In general, it is interesting to note that in studies analyzing VEGF in DR models after treatment with neuroprotectants, a decrease in apoptotic markers is often associated with a decrease in VEGF expression and/or release (see for instance Amato et al., 2016, 2018b).
In summary, our hypothesis is that, in early DR, VEGF is expressed and released to protect retinal neurons. In this phase, VEGF would not act as a proangiogenic but as a prosurvival factor. Then, a prolonged upregulation of VEGF would lead to microvascular lesions and, further on, to the full manifestation of the pathology (Figure 1). If our hypothesis is correct, then any retinal stress causing neuronal damage should be accompanied by increased VEGF expression and/or release and by vascular changes. Similarly, any compound with neuroprotective properties should also induce VEGF downregulation and amelioration of the vascular lesions. The present review examined a variety of studies in models of DR and of other retinal diseases to highlight the co-occurrence of neuronal damage and VEGF upregulation (with the appearance of vascular lesions) as well as the concomitant neuroprotection and VEGF downregulation (with the amelioration of vascular lesions) in response to neuroprotective treatments.
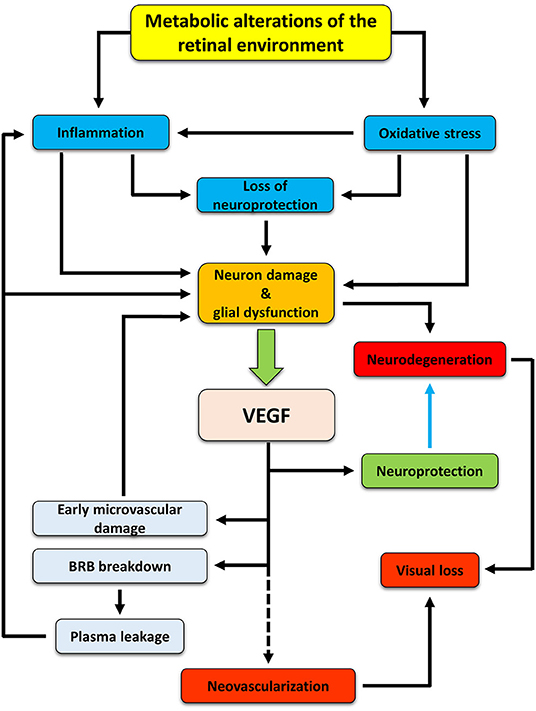
Figure 1. Hypothetic cascade of events occurring in the retina during diabetes and leading to the development of DR. Hyperglycemia induces metabolic changes in the retinal milieu, leading to oxidative stress and inflammation. Oxidative and inflammatory processes cause damages to neuron and glial cells both directly and indirectly by inducing alterations in the production and release of neurotrophic factors. As a consequence, neurodegenerative processes are activated. In an attempt to protect themselves, suffering neurons would trigger production and release of VEGF, mainly by Müller cells, that may act as a neuroprotectant, thus counteracting neurodegeneration (blue arrow). However, if in the early phases of DR VEGF may act as a neuroprotective factor, its prolonged release triggers vascular damages (which, in turn, may reinforce in a different fashion inflammation and retinal damage), ultimately leading to new vessel proliferation. If untreated, neurodegenerative and neovascular processes concur to visual dysfunction, finally leading to vision loss.
Methodology and Definitions
We considered different compounds belonging to different molecular classes but sharing the characteristic of protecting retinal neurons from a variety of stressing conditions. For each compound, a possible correlation between neuroprotective effects and the effects on VEGF expression/release or on vasculopathy has been considered. For the sake of simplicity, only in vivo and ex vivo studies have been reviewed. A compound was considered “neuroprotective” when it induced a decrease of oxidative stress, inflammation, or apoptotic markers or if it induced an amelioration of retinal function as evaluated, for instance, with electroretinogram (ERG). It was considered “vasoprotective” when it reduced VEGF expression/release, BRB leakage, or vascular lesions (including basement membrane thickening, leukocyte adhesion, occurrence of acellular capillaries, capillary degeneration, pericyte loss). For each compound, papers are first reviewed that documented either neuroprotective or vasoprotective effects of the compound. Then, we considered the papers in which both neuroprotective and vasoprotective effects were documented in the same experimental samples.
Nutraceuticals
The term “nutraceutical” indicates a food (or part of a food) that can provide health benefits, including the prevention and/or treatment of a disease (Brower, 1998). Nutraceuticals are effective antioxidants since they may act as direct scavengers of reactive oxygen species or they may induce the expression of antioxidant enzymes (Milatovic et al., 2016). They may also exert anti-inflammatory effects by inhibiting pathways linked to the production of inflammatory mediators, including those activated by the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Aggarwal et al., 2009). These compounds can be used as natural dietary supplements and therefore can be easily administered, are readily available, and are not likely to induce collateral side effects (Chauhan et al., 2013). Nutraceuticals are known to display neuroprotective effects due to their antioxidant and anti-inflammatory properties and to protect the retina from the vascular damage typical of DR (Rossino and Casini, 2019).
Curcumin
Curcumin is a yellowish polyphenolic substance constituting the major active compound of Curcuma longa. It is largely known for its antioxidant and anti-inflammatory properties (Hewlings and Kalman, 2017), and it may have therapeutic potential for retinal diseases (Wang et al., 2013).
Some studies reported beneficial effects of curcumin on the side of retinal neuroprotection. For instance, in rats with streptozotocin (STZ)-induced diabetes (a model of type 1 diabetes), curcumin inhibited retinal oxidative stress, protected Müller cells, and prevented the downregulation of glutamine synthetase, the enzyme involved in glutamate detoxification and recycling, thus protecting the retinal neurons from glutamate excitotoxicity (Zuo et al., 2013). On the other hand, there are data documenting an inhibitory effect of curcumin on diabetes-induced VEGF upregulation in diabetic rat retinas (Mrudula et al., 2007).
Other studies investigated the neuroprotective actions of curcumin together with its effects on VEGF expression and/or retinal vascular lesions. In particular, in STZ diabetic rats, oral curcumin administrations significantly reduced retinal oxidative stress, inflammation, thinning of the retina, and apoptosis, inhibiting, at the same time, VEGF upregulation and thickening of retinal capillary basement membrane (Kowluru and Kanwar, 2007; Gupta et al., 2011; Yang et al., 2018). Similarly, in a rat model of retinal ischemia-reperfusion, curcumin administered with the food inhibited NF-κB activation, with a consequent decrease of pro-inflammatory cytokines, and protected retinal neurons from apoptosis, while it also reduced the retinal capillary degeneration induced by the ischemic treatment (Wang L. et al., 2011).
Resveratrol
Resveratrol is a polyphenol found in different plants, such as grapes, peanuts, and berries. Similar to curcumin, it possesses important antioxidant properties (Gerszon et al., 2014).
There are studies reporting neuroprotective effects, while other investigations describe vasoprotective actions of resveratrol in retinal diseases. Indeed, orally administered resveratrol has been reported to decrease oxidative stress, NF-κB activation, and apoptosis in diabetic rat or mouse retinas (Kim et al., 2010; Soufi et al., 2012). On the other hand, additional studies in mice with STZ-induced diabetes documented the efficacy of resveratrol in decreasing diabetes-induced retinal VEGF upregulation, pericyte loss, and BRB breakdown (Kim et al., 2012).
Different studies have reported concomitant protective effects of resveratrol against diabetes-induced retinal inflammation or apoptosis of retinal cells on one side and VEGF overexpression, BRB leakage, or leukocyte adhesion on the other (Kubota et al., 2011; Sohn et al., 2016; Chen Y. et al., 2019). Similarly, in a mouse model of endotoxin-induced uveitis, resveratrol led to significant and dose-dependent suppression of oxidative stress, NF-κB activation, and leukocyte adhesion (Kubota et al., 2009).
Carotenoids
The carotenoids lutein and zeaxantin are the main constituents of oranges, yellow fruits, and dark green leafy vegetables. Together with meso-zeaxanthin, they form the macular pigment of primate eyes and prevent oxidative damage to the retina (Jia et al., 2017).
Likely due to its antioxidant properties, lutein is a recognized protective agent in the retina. In particular, in models of DR or of light-induced retinal degeneration, lutein was reported to preserve neurotrophin levels, protect retinal cells from apoptosis, and prevent both the oxidative stress and functional visual impairment caused by the disease (Sasaki et al., 2010, 2012; Hu et al., 2012; Ozawa et al., 2012).
Several papers have reported an effect of lutein and zeaxantin favoring both retinal cell protection and retinal function on one hand and inhibition of VEGF increase and vascular lesions on the other. Indeed, in retinas of STZ rats, zeaxantin inhibited the diabetes-induced oxidative stress as well as the upregulation of VEGF and intercellular adhesion molecule-1 (ICAM-1), an indicator of leukocyte adhesion (Kowluru et al., 2008). In addition, in the rat STZ model, a nutritional supplement containing lutein, zeaxantin, and other nutrients preserved retinal function, as evaluated with ERG, and at the same time reduced the diabetes-induced increase of NF-κB activation and interleukin-1β (IL-1β) expression, while it decreased VEGF and capillary degeneration (Kowluru et al., 2014). Similarly, in an obesity-induced high-fat diet rat model, lutein and zeaxantin, or meso-zeaxantin, reduced oxidative stress by promoting the expression of antioxidant enzymes and inhibited NF-κB activation, while they also inhibited VEGF and ICAM-1 upregulation and vascular pathology (Orhan et al., 2016; Tuzcu et al., 2017).
Catechins
Green tea is a popular beverage rich in catechin, epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate. Among these, epigallocatechin gallate is the most abundant catechin in green tea and possesses antioxidant and anti-inflammatory activities (Chu et al., 2017).
Catechins have been shown to exert powerful anti-inflammatory effects in the retinas of STZ rats by decreasing NF-κB activation and the production of inflammatory factors, such as tumor necrosis factor α (TNFα), IL-6, and IL-1β (Wang N. et al., 2018). In addition, epicatechin has been shown to exert neuroprotective effects in retinas of diabetic rats likely by reducing the production of the precursor form of nerve growth factor (Al-Gayyar et al., 2011). On the vascular side, recent observations reported an effect of epigallocatechin-3-gallate in reducing vascular leakage and permeability in an in vivo model of VEGF-induced BRB breakdown (Lee et al., 2014).
There is evidence of concomitant neuroprotective and vasoprotective effects of catechins in rat models of DR. In particular, orally administered green tea was observed to protect the diabetic retina against oxidative stress and promote glutamate uptake by Müller cells. It also preserved retina functionality, as demonstrated by ERG responses, and reduced BRB permeability, as demonstrated by reduced downregulation of occludin, a tight junction protein of the BRB (Silva et al., 2013). In addition, green tea was observed to prevent not only the diabetes-induced decrease of antioxidant enzymes and the increase of TNFα but also VEGF upregulation and the increase of retinal capillary basement membrane thickness (Kumar et al., 2012a).
Hesperetin
Hesperetin is a flavonoid polyphenol that is commonly present in citrus fruits and has been reported to exert antioxidant effects in diabetic retinas (Rossino and Casini, 2019).
In rodent models of retinal ischemia-reperfusion, hesperetin displayed potent neuroprotective actions as it prevented oxidative stress and apoptosis and preserved retinal layer thickness (Kara et al., 2014; Shimouchi et al., 2016). In addition, in retinas of STZ-treated rats, hesperetin administrations significantly reduced VEGF overexpression, BRB leakage, and pathologic vascular changes (Kumar et al., 2012b).
There is only one report in the literature concerning neuroprotective and vasoprotective effects of hesperetin in the same experimental material. In retinas of STZ-induced diabetic rats, orally administered hesperetin was effective in promoting antioxidant enzyme expression and preventing the increase of the pro-inflammatory cytokines TNFα and IL-1β and of apoptotic markers, while a protective effect of limiting the increase of basement membrane thickness was also reported (Kumar et al., 2013).
Other Nutraceuticals
There are a number of additional nutraceuticals that have been sporadically reported to exert both neuroprotective and vasoprotective effects, mostly in models of DR.
Quercetin, a common flavonoid polyphenol found in vegetables and fruits, has been reported to protect the diabetic retina from oxidative stress, inflammation, and histopathologic changes (Kumar et al., 2014; Ola et al., 2017) probably by promoting the expression of neurotrophic factors (Ola et al., 2017). Notably, quercetin has also been reported to prevent diabetes-induced retinal VEGF upregulation (Chen B. et al., 2017). Chrysin, another natural flavonoid, is found in herbs and honeycomb. It may exert neuroprotective effects since it has been shown recently to protect retinal photoreceptors by maintaining valid retinoid visual cycle-related components in the retinal pigment epithelium of diabetic rats (Kang et al., 2018). It has also been observed to inhibit VEGF upregulation, BRB leakage, and vascular lesions in the retinas of diabetic db/db mice (Kang et al., 2016). Anthocyanins constitute a further class of flavonoids, which are responsible for the red or blue color of plants, fruits, and flowers. Blueberry anthocyanins have been observed to protect diabetic rat retinas from oxidative stress and decrease VEGF levels in these same retinas (Song et al., 2016), while a Vaccinium myrtillus extract, containing large amounts of anthocyanins, reduced VEGF expression and preserved BRB integrity in retinas of STZ rats (Kim et al., 2015).
Different compounds have been described to exert at the same time neuroprotective and vasoprotective effects. For instance, eriodictyol, one of the most abundant dietary flavonoids, administered to STZ rats inhibited the retinal expression of the pro-inflammatory cytokine TNFα, while it also decreased the retinal levels of VEGF and of ICAM-1 and suppressed BRB breakdown (Bucolo et al., 2012). In both an ex vivo mouse model of retinal oxidative stress and the in vivo STZ rat model, Lisosan G, a fermented powder obtained from organic whole grains, has been described recently to exert powerful antioxidant, antiapoptotic, and anti-inflammatory actions. It also preserved retinal function, as evaluated with ERG. Concurrently, it inhibited upregulation of retinal VEGF and prevented BRB breakdown (Amato et al., 2018b). Similarly, an ethanolic extract of Morus alba leaves displaying high free radical scavenging activity reduced oxidative stress, inflammation, apoptosis, and VEGF expression in retinas of STZ rats (Mahmoud et al., 2017). Also, the traditional Chinese prescription Tang Wang Ming Mu granule has been found to protect diabetic rat retinas from oxidative stress and inflammation reducing at the same time retinal VEGF levels and vascular changes (Chen M. et al., 2017). Finally, kaempferol, a flavonol found in tea, broccoli, apples, strawberries, and beans, protected rat retinas from sodium iodate-induced retinal degeneration by reducing histopathologic changes and apoptosis, while it also reduced the upregulated VEGF protein expression (Du et al., 2018).
Taken together, these studies with nutraceuticals documented an action of these compounds that was at the same time both neuroprotective and vasoprotective. Indeed, the data, prevalently obtained in rodent models of DR, revealed that nutraceuticals, acting as antioxidant and/or as anti-inflammatory agents, not only are effective in reducing retinal neurodegeneration but also prevent the deleterious increase of VEGF levels and consequent vascular lesions.
Antioxidants
The nutraceuticals discussed above display important antioxidant capabilities, but other antioxidant compounds have been also described, which may act in retinal diseases and protect both retinal neurons and vessels.
Calcium Dobesilate
Calcium dobesilate (CaD) is an oxygen free radical scavenger (Brunet et al., 1998; Szabo et al., 2001). It is considered a vasoprotective drug, and it has been approved for the treatment of DR in several countries for many years (Tejerina and Ruiz, 1998; Berthet et al., 1999); however, it has not been widely used in clinical practice. In effect, CaD exerts multifaceted actions contrasting neurovascular unit impairment, and therefore, it can be considered a good candidate drug for targeting the early stages of DR. In particular, the effects of CaD in DR have been recently reviewed, and they include (i) reduction of capillary permeability and consequent BRB leakage; (ii) inhibition of endothelial cell apoptosis; (iii) antioxidant activity and protection against reactive oxygen species; and (iv) inhibition of the expression of VEGF and ICAM-1 (Zhang et al., 2015).
In retinas of db/db mice (a model of type 2 diabetes) and in retinas of STZ rats, CaD significantly reduced biomarkers of oxidative stress and NF-κB activation with consequent decrease of pro-inflammatory cytokines, such as TNFα, IL-1β, IL-6, and IL-8 (Bogdanov et al., 2017; Voabil et al., 2017). In addition, in STZ rats, CaD reduced vascular leakage and VEGF expression (Rota et al., 2004). Both neuroprotective and vasoprotective effects of CaD have been described in diabetic retinas. Indeed, in retinas of STZ rats, in addition to protective effects against oxidative stress, inflammation, and retinal thinning, CaD has been reported to exert inhibitory effects against diabetes-induced BRB breakdown, downregulation of tight junction protein expression, increased VEGF and ICAM-1 expression, and leukocyte adhesion (Leal et al., 2010). In retinas of db/db diabetic mice, CaD significantly decreased diabetes-induced oxidative stress and retinal cell apoptosis. In addition, it reduced glutamate extracellular concentration, by preventing glutamate transporter downregulation, and improved ERG responses. At the same time, CaD inhibited VEGF upregulation and vascular leakage (Sola-Adell et al., 2017).
Other Antioxidants
It is clear that suppression of antioxidant defenses is deleterious to the retina. For this reason, recent studies have focused on the ability of some antioxidant compounds to regulate antioxidant gene expression, such as the nuclear factor erythroid-2-related factor 2 (Nrf2) activator dh404 and a DNA methyltransferase (DNMT) inhibitor.
Nrf2 is a redox-sensitive transcription factor that is kept in a latent state until an increase in free radical concentration releases Nrf2, which enters the cell nucleus and initiates the transcription of antioxidant genes (Di Marco et al., 2015). Boosting Nrf2 with a specific activator increases the transcription of antioxidant genes and therefore may protect tissues from oxidative damage. In retinas of STZ rats, the Nrf2 activator dh404 has been reported not only to decrease oxidative stress and the expression of inflammatory mediators but also to prevent VEGF upregulation and vascular leakage (Deliyanti et al., 2018).
It has been observed that DNA methylation may be involved in the regulation of gene expression in the retina during the progression of DR (Kowluru et al., 2015; Mishra and Kowluru, 2016, 2019). In particular, DNMT inhibitors may favor the expression of antioxidant genes. Indeed, in diabetic rat retinas, DNMT inhibition restored antioxidant enzyme expression and, in parallel, also prevented the diabetes-induced increase of VEGF and of ICAM-1 expression (Xie et al., 2019).
These observations on the effects of antioxidant compounds in models of DR indicate that reduction of oxidative stress is accompanied by positive effects on the vascular pathology and therefore favors both neuroprotective and vasoprotective actions.
Neuropeptides
Neuropeptides are short to medium amino acid chains, which function primarily as complementary signals to “classic” neurotransmitters to fine-tune neurotransmission (Hokfelt et al., 2003). Some of them have been found to be important for the regulation of cell death/survival in different neuronal systems, where they express important neuroprotective properties (Catalani et al., 2017; Reglodi et al., 2017; Chen X.Y. et al., 2019). Neuropeptides and their receptors are widely expressed in mammalian retinas, where they exert multifaceted functions both during development and in the mature animal (Bagnoli et al., 2003). In particular, some of them may exert important roles in retinal diseases (Gabriel, 2013; Cervia et al., 2019).
Glucagon-Like Peptide-1
Glucagon-like peptide-1 (GLP-1) is known as a hormone secreted by the gastrointestinal tract in response to food, stimulating insulin and inhibiting glucagon secretion (Drucker and Nauck, 2006). GLP-1 has also been recognized as a neuropeptide. Indeed, GLP-1 and its receptor GLP-1R are expressed in the brain, where they influence multiple neural circuits modulating feeding behavior and reward (Smith et al., 2019). Both GLP-1 and GLP-1R are expressed in mammalian retinas (Zhang et al., 2009; Zhang Y. et al., 2011; Hernandez et al., 2016a; Cai et al., 2017; Hebsgaard et al., 2018).
Neuroprotective effects of GLP-1R activation have been demonstrated in a rat model of optic nerve crush, where intravitreal implants of beads with genetically modified cells producing GLP-1 decreased apoptosis and promoted survival of retinal ganglion cells (Zhang R. et al., 2011), and in diabetic rats, where exendin-4, an analog of GLP-1, protected from oxidative stress from apoptotic cell death and ameliorated retinal function as assessed with ERG (Zhang et al., 2009; Zhang Y. et al., 2011; Fan et al., 2014b; Zeng et al., 2016; Cai et al., 2017; Cervia et al., 2019). Most importantly, both neuroprotective and vasoprotective effects of GLP-1 agonists have been described in models of retinal diseases. For instance, in a rat model of retinal ischemia-reperfusion, exendin-4 suppressed inflammatory gene expression and reduced BRB permeability (Goncalves et al., 2016). Strong evidence for a double action of GLP-1 as a neuroprotectant and vasoprotectant also comes from studies in models of DR. Indeed, recent studies in rodent retinas have reported that GLP-1 or GLP-1R agonists may exert a neuroprotective action since they improved retinal function, as assessed with ERG, protected retinal cells from death, reduced oxidative stress and IL-1β expression, and inhibited the increase of extracellular glutamate. At the same time, these compounds induced vasoprotection since they decreased VEGF levels, preserved the expression of tight junction proteins of the BRB, reduced BRB leakage, and inhibited the increase of ICAM-1 levels (Fan et al., 2014a; Hernandez et al., 2016a; Sampedro et al., 2019).
Similar to GLP-1R agonists, inhibitors of dipeptidyl peptidase 4 (DPP4, the GLP-1 degrading enzyme) have been tested for their potential use in DR treatments. The data of different studies indicated that DPP4 inhibitors, such as linagliptin, saxagliptin, or sitagliptin, efficiently increase retinal GLP-1 levels and that this increase, in rodent models of DR, is correlated with reduced oxidative stress, inflammation (as assessed by IL-1β levels), extracellular glutamate levels and neuronal apoptosis and with preservation of retinal function. At the same time, DPP4 inhibitors induced amelioration of different vascular features, including diabetes-induced changes in the subcellular distribution of the tight junction proteins occludin, claudin-5, and zonula occludens-1; BRB breakdown; ICAM-1 upregulation; pericyte loss; and formation of acellular capillaries (Goncalves et al., 2012, 2014; Dietrich et al., 2016; Hernandez et al., 2017).
Somatostatin
Somatostatin (somatotropin release inhibiting factor, SRIF) is expressed in the retina, together with its five receptor subtypes (named sst1-5), where they express important physiological functions (Casini et al., 2005; Cervia et al., 2008a). Low vitreous levels and low intraocular production of SRIF have been found in patients with diabetic macular edema, chronic uveitis macular edema, and quiescent intraocular inflammation (Simo et al., 2007; Fonollosa et al., 2012), suggesting that SRIF alterations may be directly involved in the pathogenesis of these conditions. In addition, a variety of experimental observations suggested that SRIF may exert powerful neuroprotective effects in different retinal diseases (Cervia et al., 2008a; Cervia and Casini, 2013; Hernandez et al., 2014; Wang et al., 2017).
The SRIF analog pasireotide and SRIF receptor agonists targeting the sst2 or sst5 receptors were found to significantly protect rat retinal neurons in in vivo models of AMPA excitotoxicity (Kiagiadaki and Thermos, 2008; Kiagiadaki et al., 2010; Kokona et al., 2012). In addition, in a retinal ischemia-reperfusion mouse model, SRIF mediated the neuroprotective and anti-inflammatory effects of capsaicin, a selective agonist for transient receptor potential vanilloid type-1, a ligand-gated non-selective cation channel (Wang et al., 2017). Moreover, in retinas of STZ rats, topical SRIF administrations prevented glutamate accumulation, apoptosis, and ERG abnormalities (Hernandez et al., 2013). Similarly, studies in ex vivo ischemic retinas of mice or rats reported that SRIF, its analogs, or the constitutive activation of the sst2 receptor significantly preserved retinal neurons from ischemia-induced morphological changes and apoptosis (Catalani et al., 2007; Cervia et al., 2008b; Kokona et al., 2012). Moreover, in retinal explants in which hypoxic conditions induced the expression of apoptotic markers, the sst2-preferring SRIF analog octreotide reduced apoptotic signals (Dal Monte et al., 2012). Finally, in ex vivo explants of mouse retinas treated with high glucose, octreotide prevented apoptosis of retinal neurons, likely stimulating an increase of the autophagic flux (Amato et al., 2018a). Interestingly, a study in ex vivo ischemic mouse retinas reported that acute ischemia induces a sudden increase in VEGF release from neurons, suggesting that VEGF may represent a stress signal released by retinal neurons when their integrity is threatened. Supporting this view, the neuroprotective SRIF analog octreotide reduced VEGF release from ischemic retinas (Cervia et al., 2012).
Some studies reported concomitant effects of SRIF, or its analogs, on neuroprotection and vasoprotection. For instance, in a mouse model of retinal ischemia-reperfusion, octreotide has been reported to protect from oxidative stress, inflammation (as assessed by NF-κB activation), and neuronal death, while it also significantly reduced ICAM-1 expression, indicating decreased leukocyte adhesion (Wang et al., 2015). In ex vivo ischemic mouse retinas, octreotide inhibited the ischemia-induced increase of oxidative stress, glutamate levels, apoptosis, and VEGF expression (D'alessandro et al., 2014). Similar observations were reported in ex vivo mouse retinal explants challenged with high glucose, oxidative stress, or advanced glycation end products (AGE), toxic products that accumulate under hyperglycemic conditions and that are likely to play an important role in the pathogenesis of DR. In particular, these studies showed that protecting retinal neurons from diabetic stress also reduces VEGF expression and release, while inhibiting VEGF leads to exacerbation of apoptosis (Amato et al., 2016). Therefore, the retina in early DR may release VEGF as a prosurvival factor, and a neuroprotective agent such as octreotide may decrease the need of VEGF production by the retina, therefore limiting the vasculopathy associated with VEGF upregulation.
Angiotensin
The renin-angiotensin system (RAS) is involved in the regulation of blood pressure. Angiotensin I (AngI) is generated from the proteolytic cleavage of angiotensinogen, a reaction catalyzed by the enzyme renin. AngI is further processed by angiotensin-converting enzyme (ACE) and ACE2 to angiotensin II (AngII), the main effector of the RAS, acting at the angiotensin type 1 and type 2 receptors (AT1R and AT2R) (Fletcher et al., 2010). A local RAS is present in the retina, where RAS components have been localized to different retinal cell types, including retinal neurons and Müller cells (Wilkinson-Berka et al., 2012). A variety of studies have shown that reduction of AngII expression or blockade of AT1R on the one hand, or stimulation of ACE2 on the other, may reduce the retinal damage occurring in retinal pathologies, such as glaucoma, retinal ischemia, autoimmune uveitis, or DR (Cervia et al., 2019).
Several investigations have provided evidence for a neuroprotective role exerted by AT1R inhibitors in different models of retinal diseases. For instance, inhibitors like valsartan, losartan, or candesartan were effective in attenuating light-induced retinal damage in mice by reducing oxidative stress and improving ERG responses (Narimatsu et al., 2014). Similarly, candesartan prevented ganglion cell loss, thinning of the retina, and ERG deficits in a retinal excitotoxicity mouse model (Semba et al., 2014). In mice with increased intraocular pressure, used as models of glaucoma or ischemia-reperfusion, AT1R blockade reduced oxidative stress, inhibited the increase of extracellular glutamate, and mitigated ganglion cell loss (Yang et al., 2009; Fujita et al., 2012; Liu et al., 2012; Quigley et al., 2015). In rats or mice with STZ-induced diabetes, blockers of AT1R, in addition to protecting the retina from oxidative stress, apoptotic cell death, and histopathologic damage (Silva et al., 2009; Ola et al., 2013; Thangaraju et al., 2014), also preserved mitochondrial integrity, increased the expression of neurotrophic factors, and improved functional ERG responses (Silva et al., 2009; Ozawa et al., 2011; Ola et al., 2013). In experimental models of mouse autoimmune uveitis or endotoxin-induced uveitis, the delivery of different formulations of ACE2 and/or its product Ang(1-7) or the administration of an ACE2 activator reduced retinal inflammation (Qiu et al., 2014, 2016; Shil et al., 2014) and prevented histologic damage as well as ERG abnormalities (Qiu et al., 2016). Similarly, both in an experimental glaucoma model and in STZ rats, retinal ganglion cells were protected from apoptotic cell death by the administration of an ACE2 activator (Foureaux et al., 2013, 2015). On the vascular side, in retinas of STZ rats evidence was provided of an inhibitory effect on VEGF expression and on leukocyte adhesion exerted by a prorenin receptor blocker (Satofuka et al., 2009).
There are a few studies documenting concomitant effects of the Ang system both on the side of neuroprotection and on that of vasoprotection. One study reported that in diabetic rats, increased plasma prorenin levels exacerbated the expression of inflammatory cytokines, retinal apoptotic cell death, as well as the formation of acellular capillaries, while a prorenin receptor blocker significantly reduced these effects (Batenburg et al., 2014). Similarly, adeno-associated virus-mediated gene delivery of ACE2 or Ang(1-7) significantly reduced diabetes-induced oxidative damage, inflammation, retinal vascular leakage, and formation of acellular capillaries in both diabetic mice and rats (Verma et al., 2012).
These data support the possibility that the documented neuroprotective actions of AngII blockade or ACE2 stimulation also influence the vascular pathology and induce an amelioration of the vascular traits (VEGF upregulation, acellular capillaries, leukocyte adhesion, BRB breakdown) especially in models of DR.
Pituitary Adenylate Cyclase-Activating Polypeptide and Vasoactive Intestinal Peptide
Pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) belong to the same peptide superfamily, which also includes secretin and glucagon. The PACAP receptors can be classified into two groups: PACAP receptor 1 (PAC1), which binds PACAP with higher affinity than VIP, and vasoactive intestinal polypeptide receptors (VPAC1 and VPAC2), which bind PACAP and VIP with similar affinities (Vaudry et al., 2009).
PACAP and PAC1R have been detected in the retina, where they are involved in neurotransmission, neuromodulation, and, mostly, neuroprotective functions (Nakamachi et al., 2012). VPAC2 expression has also been reported in the mouse retina (Harmar et al., 2004). The retinoprotective effects of PACAP have been the subject of a variety of studies, and these data have been excellently reviewed (Atlasz et al., 2010; Nakamachi et al., 2012; Shioda et al., 2016). Further evidence has been provided by more recent studies. Indeed, PACAP has been shown to inhibit apoptosis and promote survival of retinal ganglion cells in different models of retinal injury (Lakk et al., 2015; Ye et al., 2019). In addition, intravitreal or topical administrations of PACAP or a PAC1 agonist to ischemic retinas in vivo ameliorated ERG responses, prevented inflammation, and reduced the thinning of retinal layers and the loss of cells in the ganglion cell layer (Danyadi et al., 2014; Vaczy et al., 2016; Werling et al., 2017; Atlasz et al., 2018). Similarly, PACAP intraocular delivery in rats with STZ-induced diabetes protected the retina from apoptosis and maintained retinal synaptic integrity (Szabadfi et al., 2014, 2016). PACAP was also demonstrated to contrast the diabetes-induced modifications of the expression of hypoxia-inducible factors (HIFs), among which HIF-1 is the main regulator of VEGF expression (D'amico et al., 2015).
Both neuroprotective and vasoprotective effects of PACAP have been documented in retinas of STZ rats and in ischemic retinas in vivo, where PACAP reduced thinning of retinal layers and prevented the expression of both inflammatory cytokines and VEGF (Werling et al., 2016; D'amico et al., 2017b). In addition, in an ex vivo model of retinal ischemia, PACAP effectively decreased oxidative stress, glutamate accumulation, inflammatory mediators, and apoptosis. At the same time, it also decreased VEGF expression, which was upregulated in the ischemic retina (D'alessandro et al., 2014). Finally, in ex vivo retinal explants stressed with high glucose, oxidative stress, or AGE, the strong PACAP antiapoptotic effects were paralleled by inhibition of the stress-induced increase of VEGF expression and release (Amato et al., 2016).
VIP is expressed in the retina in a population of amacrine cells (Perez De Sevilla Muller et al., 2019). It has been reported to reduce retinal neurodegeneration caused by ischemia-reperfusion injury, promoting an antioxidant effect (Tuncel et al., 1996). The neuroprotective effects of VIP may be mediated by activity-dependent neurotrophic protein (ADNP) (Bassan et al., 1999; Zusev and Gozes, 2004; Giladi et al., 2007). Indeed, both ADNP and an 8-amino acid peptide derived from ADNP (referred to as NAP) display important neuroprotective activities (Magen and Gozes, 2014). Interestingly, NAP seems to exert protective effects against both the neural and the vascular pathology induced by DR, as it reduced inflammation (D'amico et al., 2019) and apoptosis (Scuderi et al., 2014) as well as the levels of the α subunit of HIF-1 (HIF-1α) and VEGF in retinas of rats with STZ-induced diabetes (D'amico et al., 2017a).
Other Peptides
α-Melanocyte-stimulating hormone (α-MSH) is a widely-distributed 13-amino acid peptide derived from proteolytic cleavage of proopiomelanocortin (Wardlaw, 2011). It acts at five subtypes of G protein-coupled receptors designated MC1R to MC5R (Yang, 2011). α-MSH protected the rat retina from both functional and structural damage induced by ischemia-reperfusion (Varga et al., 2013), suppressed inflammation and maintained retinal structure in a mouse model of experimental autoimmune uveitis (Edling et al., 2011), and protected photoreceptors from degeneration in a rat model of retinal dystrophy (Naveh, 2003). In a rat model of STZ-induced diabetes, intravitreal injections of α-MSH reduced oxidative stress, inflammation, and apoptosis, while they also inhibited the expression of ICAM-1 (Zhang et al., 2014), indicating reduced leukostasis. In early diabetic retinas, α-MSH also reduced inflammation, ameliorated ERG responses, and reduced retinal thinning, while it inhibited BRB breakdown and vascular leakage, likely acting at MC4R (Cai et al., 2018).
Endothelin (ET) is a potent vasoconstrictor composed of three isoforms designated ET-1, ET-2, and ET-3, whose actions are mediated by the ET type A receptor (ETRA) and ET type B receptor (ETRB) (Davenport et al., 2016). There are indications that ET activity may be involved in DR, and evidence has been provided that ET inhibition may ameliorate the pathologic signs of DR. Indeed, an ETRA antagonist has been reported to block the diabetes-induced upregulation of both VEGF and ICAM-1 in retinas of STZ rats (Masuzawa et al., 2006), while other observations have described positive effects of ETR inhibition on both neuronal and vascular changes seen in DR. For instance, ETRA and/or ETRB inhibitors reduced retinal thinning, the number of apoptotic cells, and the levels of the pro-inflammatory cytokine TNFα in diabetic rat retinas, and at the same time they also reduced pericyte loss, capillary degeneration, vascular leakage, and the levels of both VEGF and ICAM-1 (Chou et al., 2014; Alrashdi et al., 2018; Bogdanov et al., 2018).
Erythropoietin (EPO) stimulates erythroid progenitor cell and early erythroblast maturation and is mainly used in anemia treatment (Jelkmann, 2013). EPO is expressed in the retina (Hernandez et al., 2006; Fu et al., 2008), where it exerts well-documented neuroprotective functions (Kilic et al., 2005; Chung et al., 2009; Colella et al., 2011; Chang et al., 2013). Both neuroprotective and vasoprotective actions of EPO have been described. Indeed, in retinas of STZ rats, EPO was reported to significantly decrease oxidative stress, apoptotic neurodegeneration, and retinal thinning on one hand, and VEGF upregulation, BRB breakdown, and pericyte loss on the other (Zhang et al., 2008; Wang et al., 2010; Wang Q. et al., 2011). Similarly, carbamylated erythropoietin, an EPO derivative, protected diabetic rat retinas from retinal thinning, neuron apoptosis, and functional deficits as evaluated with ERG, while it also reduced VEGF upregulation and vascular leakage (Liu et al., 2015).
In addition to the peptides cited above, some evidence exists for an effect of a few other peptides that is both neuroprotective and vasoprotective. Indeed, antioxidative, anti-inflammatory, and/or antiapoptotic actions together with effects preventing VEGF upregulation and/or BRB breakdown have been described for the peptides growth hormone-releasing hormone (Thounaojam et al., 2017), insulin (Rong et al., 2018), melatonin (Djordjevic et al., 2018), substance P (D'alessandro et al., 2014), and vasoinhibins (Garcia et al., 2008; Arredondo Zamarripa et al., 2014).
Together, these data demonstrate that, similar to the nutraceuticals and antioxidants reviewed above, the powerful neuroprotective effects exerted by different classes of neuropeptides also result in VEGF downregulation and attenuation of the vascular damage in various models of retinal disease.
Other Factors
The urokinase-type plasminogen activator (uPA) receptor (uPAR) is a glycosylphosphatidylinositol-anchored receptor activated by uPA. uPAR lacks a transmembrane domain, however, it can activate intracellular signaling pathways through lateral interactions with other cell surface receptors, including integrins, G-protein–coupled receptors, and receptor tyrosine kinases, thus forming a system that is involved in many pathological processes, including retinal diseases (Cammalleri et al., 2019b). Recently, the inhibition of the uPAR system has been found to be effective in slowing down cone degeneration and visual dysfunction in a mouse model of retinitis pigmentosa (Cammalleri et al., 2019a). Of interest for this review, in two different models of DR, the STZ rat model mimicking type 1 diabetes (Navaratna et al., 2008; Cammalleri et al., 2017b) and the Torii rat model mimicking type 2 diabetes (Cammalleri et al., 2017a), inhibiting the uPAR system not only ameliorated diabetes-induced ERG dysfunction and reduced inflammation and apoptosis but also resulted in inhibition of VEGF upregulation and BRB breakdown.
Brimonidine is an α2 adrenergic agonist with extensively documented neuroprotective effects in a variety of models of retinal disease (see for instance Guo et al., 2015; Marangoz et al., 2018). In addition, in retinas of rats with STZ-induced diabetes, it has been reported to induce a significant decrease of VEGF expression and of BRB breakdown to levels similar to those observed in control rats (Kusari et al., 2010). Furthermore, in a mouse model of ischemic optic neuropathy, topically applied brimonidine not only reduced oxidative stress and ganglion cell loss but also decreased HIF-1α and VEGF expression (Goldenberg-Cohen et al., 2009).
Peroxisome proliferator-activated receptor α (PPARα), a hormone-activated nuclear receptor, is known as an important modulator of lipid metabolism (Pyper et al., 2010), which also possesses anti-inflammatory and antioxidant properties (Li et al., 2005; Simo and Hernandez, 2009). The PPARα agonist fenofibrate has been used clinically as a triglyceride-lowering drug. However, it seems that downregulation of PPARα in the retina plays a major role in the pathogenesis of DR (Hu et al., 2013), and two independent perspective clinical trials demonstrated that fenofibrate had unprecedented therapeutic effects in DR (Keech et al., 2007; Chew et al., 2010). Emerging evidence suggests that fenofibrate exerts a broad range of beneficial effects on diabetic complications acting against oxidative stress, inflammation, cell death, and angiogenesis (Noonan et al., 2013). In particular, in rodent models of DR, different studies documented the protective effects of fenofibrate or another PPARα agonist against oxidative stress, inflammation, retinal cell death, and decreased retinal function on one hand, and VEGF upregulation, vascular leakage, thickening of capillary basement membrane, ICAM-1 expression, and leukostasis on the other (Chen et al., 2013; Deng et al., 2017; Li et al., 2018; Liu et al., 2018, 2019; Wang N. et al., 2018; Qiu et al., 2019).
Acetaldehyde dehydrogenase 2 is a rate-limiting enzyme for alcohol metabolism, which has been shown to exert neuroprotective effects (Deza-Ponzio et al., 2018). In retinas of STZ rats, it has been reported to promote antioxidant enzyme activity, reduce the expression of proinflammatory cytokines, ameliorate ERG, and significantly reduce VEGF expression (He et al., 2018).
The last example, in this review, of a neuroprotective factor that also ameliorates vascular changes in retinal disease is not concerned with a compound but involves a procedure. Indeed, it is known that ischemic conditioning can be considered a form of protection against ischemic injury through the initiation of endogenous protective mechanisms (Heusch, 2013; Li et al., 2017). As expected, in retinas of STZ rats, ischemic conditioning produced anti-inflammatory and antioxidant effects, and it also protected ganglion cells from death. Interestingly, diabetic retinas treated with ischemic conditioning also showed a significantly downregulated VEGF protein expression (Ren et al., 2018).
Conclusion
The experimental data summarized in this review of the literature clearly indicate that, in a variety of experimental models of retinal disease, a neuroprotective treatment is efficacious in preventing the vascular changes that are usually associated with the disease (see Table 1 for a comprehensive summary of the data). Although the participation of VEGF is crucial in the early stages of DR, we cannot exclude the participation of some other important molecules, such as the neuronal guidance cues, including ephrins, netrin, and semaphorins, which are also released early by damaged neurons and may promote or attenuate the development of DR (Moran et al., 2016). These molecules are highly expressed in the retina and vitreous of patients with advanced DR (Umeda et al., 2004; Liu et al., 2011; Cerani et al., 2013; Dejda et al., 2014), and their inhibition, like that of VEGF, could reveal an efficient method to reduce aberrant growth of retinal vessels. The possibility exists that treatments with neuroprotectants, in addition to reducing VEGF expression/release, also limit the release of these molecules, thereby exerting a multitarget effect that results in efficient protection from microvascular damage and subsequent development of advanced stages of DR.
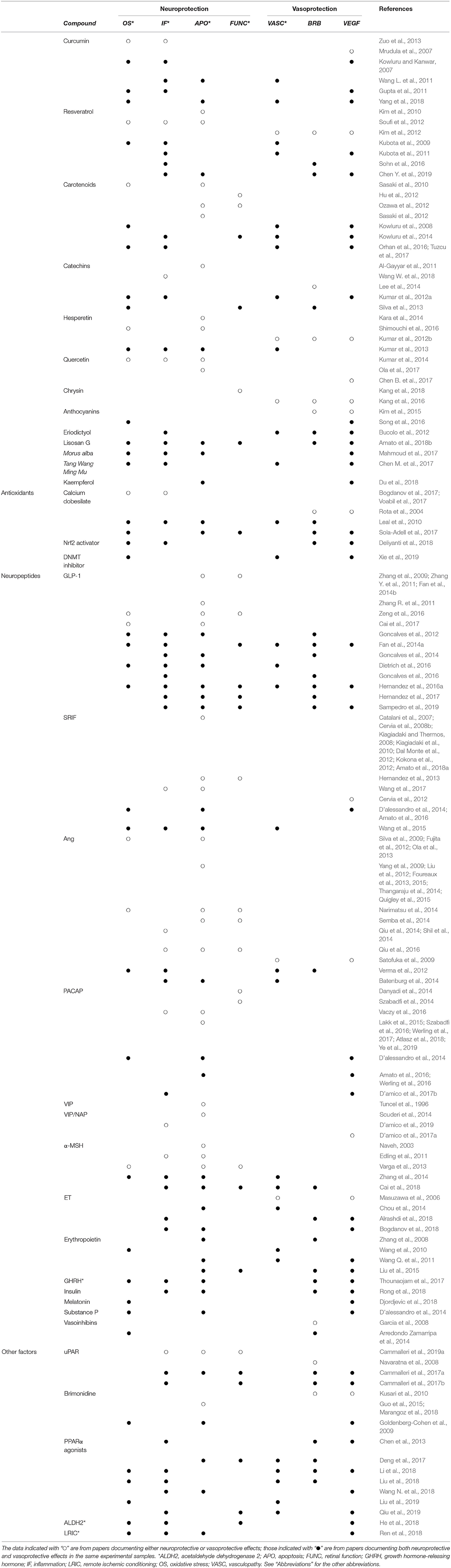
Table 1. Summary of the neuroprotective and vasoprotective effects of different compounds in models of retinal disease.
The fact that neuroprotection may limit vascular pathology could be explained assuming that the reviewed compounds may trigger two types of independent, parallel responses: one finalized to neuroprotection and the other affecting the mechanisms regulating VEGF expression and/or release. This might be the case, for instance, for SRIF. Indeed, for the SRIF analog octreotide, there is evidence of an effect, reducing oxidative stress and glutamate release (Dal Monte et al., 2003; D'alessandro et al., 2014), and of a regulatory action on the intracellular mechanisms for VEGF expression (Dal Monte et al., 2009, 2010; Mei et al., 2012). However, most compounds listed in this review are primarily antioxidant and anti-inflammatory substances, which are likely to exert their primary effects on retinal neurons. Therefore, it appears that protecting retinal neurons from stress reduces the probability of VEGF upregulation and the consequent vascular damage. This evidence indicates that treatments with natural substances (nutraceuticals or neuropeptides) during early phases of DR may represent the basis for efficacious therapies of DR that do not impact on the patients' quality of life and that may have only little or no side effects.
Author Contributions
MR and GC collected the references. MR, MD, and GC organized the layout of the paper and wrote the manuscript.
Funding
This research was supported by the Italian Ministry of University and Research (FFABR 2017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACE, Angiotensin-converting enzyme; ADNP, Activity-dependent neurotrophic protein; AGE, Advanced glycation end products; AngI, Angiotensin I; AngII, Angiotensin II; AT1R, Angiotensin type 1 receptor; AT2R, Angiotensin type 2 receptor; BRB, Blood-retina barrier; CaD, Calcium dobesilate; DNMT, DNA methyltransferase; DPP4, Dipeptidyl peptidase 4; DR, Diabetic retinopathy; EPO, Erythropoietin; ERG, Electroretinogram; ET, Endothelin; ETRA, ET type A receptor; ETRB, ET type B receptor; GLP-1, Glucagon-like peptide-1; HIF, Hypoxia-inducible factor; HIF-1α, α subunit of HIF-1; ICAM-1, Intercellular adhesion molecule-1; IL-1β, Interleukin-1β; IL-6, Interleukin-6; NF-κB, Nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, Nuclear factor erythroid-2-related factor 2; PACAP, Pituitary adenylate cyclase-activating polypeptide; PAC1, PACAP receptor 1; PPARα, Peroxisome proliferator-activated receptor α; RAS, Renin-angiotensin system; SRIF, Somatotropin release inhibiting factor (or somatostatin); sst, Somatostatin receptor subtype; STZ, Streptozotocin; TNFα, Tumor necrosis factor α; uPA, Urokinase-type plasminogen activator; uPAR, Urokinase-type plasminogen activator receptor; VEGF, Vascular endothelial growth factor; VIP, Vasoactive intestinal peptide; VPAC1, Vasoactive intestinal peptide type 1 receptor; VPAC2, Vasoactive intestinal peptide type 2 receptor; α-MSH, α-Melanocyte-stimulating hormone.
References
Abcouwer, S. F., and Gardner, T. W. (2014). Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 1311, 174–190. doi: 10.1111/nyas.12412
Aggarwal, B. B., Van Kuiken, M. E., Iyer, L. H., Harikumar, K. B., and Sung, B. (2009). Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp. Biol. Med. 234, 825–849. doi: 10.3181/0902-MR-78
Al-Gayyar, M. M., Matragoon, S., Pillai, B. A., Ali, T. K., Abdelsaid, M. A., and El-Remessy, A. B. (2011). Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes [corrected]. Diabetologia 54, 669–680. doi: 10.1007/s00125-010-1994-3
Alrashdi, S. F., Deliyanti, D., and Wilkinson-Berka, J. L. (2018). Intravitreal administration of endothelin type A receptor or endothelin type B receptor antagonists attenuates hypertensive and diabetic retinopathy in rats. Exp. Eye Res. 176, 1–9. doi: 10.1016/j.exer.2018.06.025
Amato, R., Biagioni, M., Cammalleri, M., Dal Monte, M., and Casini, G. (2016). VEGF as a survival factor in ex vivo models of early diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 57, 3066–3076. doi: 10.1167/iovs.16-19285
Amato, R., Catalani, E., Dal Monte, M., Cammalleri, M., Di Renzo, I., Perrotta, C., et al. (2018a). Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacol. Res. 128, 167–178. doi: 10.1016/j.phrs.2017.09.022
Amato, R., Rossino, M. G., Cammalleri, M., Locri, F., Pucci, L., Dal Monte, M., et al. (2018b). Lisosan G protects the retina from neurovascular damage in experimental diabetic retinopathy. Nutrients 10:E1932. doi: 10.3390/nu10121932
Antonetti, D. A., Barber, A. J., Bronson, S. K., Freeman, W. M., Gardner, T. W., Jefferson, L. S., et al. (2006). Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 55, 2401–2411. doi: 10.2337/db05-1635
Arredondo Zamarripa, D., Diaz-Lezama, N., Melendez Garcia, R., Chavez Balderas, J., Adan, N., Ledesma-Colunga, M. G., et al. (2014). Vasoinhibins regulate the inner and outer blood-retinal barrier and limit retinal oxidative stress. Front. Cell Neurosci. 8:333. doi: 10.3389/fncel.2014.00333
Atlasz, T., Szabadfi, K., Kiss, P., Racz, B., Gallyas, F., Tamas, A., et al. (2010). Pituitary adenylate cyclase activating polypeptide in the retina: focus on the retinoprotective effects. Ann. N. Y. Acad. Sci. 1200, 128–139. doi: 10.1111/j.1749-6632.2010.05512.x
Atlasz, T., Werling, D., Song, S., Szabo, E., Vaczy, A., Kovari, P., et al. (2018). Retinoprotective effects of TAT-bound vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide. J. Mol. Neurosci. 68, 397–407. doi: 10.1007/s12031-018-1229-5
Azzouz, M., Ralph, G. S., Storkebaum, E., Walmsley, L. E., Mitrophanous, K. A., Kingsman, S. M., et al. (2004). VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429, 413–417. doi: 10.1038/nature02544
Bagnoli, P., Dal Monte, M., and Casini, G. (2003). Expression of neuropeptides and their receptors in the developing retina of mammals. Histol. Histopathol. 18, 1219–1242. doi: 10.14670/HH-18.1219
Barber, A. J. (2003). A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 283–290. doi: 10.1016/S0278-5846(03)00023-X
Bassan, M., Zamostiano, R., Davidson, A., Pinhasov, A., Giladi, E., Perl, O., et al. (1999). Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 72, 1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x
Batenburg, W. W., Verma, A., Wang, Y., Zhu, P., Van Den Heuvel, M., Van Veghel, R., et al. (2014). Combined renin inhibition/(pro)renin receptor blockade in diabetic retinopathy–a study in transgenic (mREN2)27 rats. PLoS ONE 9:e100954. doi: 10.1371/journal.pone.0100954
Beazley-Long, N., Hua, J., Jehle, T., Hulse, R. P., Dersch, R., Lehrling, C., et al. (2013). VEGF-A165b is an endogenous neuroprotective splice isoform of vascular endothelial growth factor A in vivo and in vitro. Am. J. Pathol. 183, 918–929. doi: 10.1016/j.ajpath.2013.05.031
Berthet, P., Farine, J. C., and Barras, J. P. (1999). Calcium dobesilate: pharmacological profile related to its use in diabetic retinopathy. Int. J. Clin. Pract. 53, 631–636.
Bogdanov, P., Simo-Servat, O., Sampedro, J., Sola-Adell, C., Garcia-Ramirez, M., Ramos, H., et al. (2018). Topical administration of bosentan prevents retinal neurodegeneration in experimental diabetes. Int. J. Mol. Sci. 19:E3578. doi: 10.3390/ijms19113578
Bogdanov, P., Sola-Adell, C., Hernandez, C., Garcia-Ramirez, M., Sampedro, J., Simo-Servat, O., et al. (2017). Calcium dobesilate prevents the oxidative stress and inflammation induced by diabetes in the retina of db/db mice. J. Diabetes Complications 31, 1481–1490. doi: 10.1016/j.jdiacomp.2017.07.009
Brower, V. (1998). Nutraceuticals: poised for a healthy slice of the healthcare market? Nat. Biotechnol. 16, 728–731. doi: 10.1038/nbt0898-728
Brunet, J., Farine, J. C., Garay, R. P., and Hannaert, P. (1998). Angioprotective action of calcium dobesilate against reactive oxygen species-induced capillary permeability in the rat. Eur. J. Pharmacol. 358, 213–220. doi: 10.1016/S0014-2999(98)00604-9
Bucolo, C., Leggio, G. M., Drago, F., and Salomone, S. (2012). Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 84, 88–92. doi: 10.1016/j.bcp.2012.03.019
Cai, S., Yang, Q., Hou, M., Han, Q., Zhang, H., Wang, J., et al. (2018). Alpha-melanocyte-stimulating hormone protects early diabetic retina from blood-retinal barrier breakdown and vascular leakage via MC4R. Cell Physiol. Biochem. 45, 505–522. doi: 10.1159/000487029
Cai, X., Li, J., Wang, M., She, M., Tang, Y., Li, H., et al. (2017). GLP-1 treatment improves diabetic retinopathy by alleviating autophagy through GLP-1R-ERK1/2-HDAC6 signaling pathway. Int. J. Med. Sci. 14, 1203–1212. doi: 10.7150/ijms.20962
Cammalleri, M., Dal Monte, M., Locri, F., Marsili, S., Lista, L., De Rosa, M., et al. (2017a). Diabetic retinopathy in the spontaneously diabetic torii rat: pathogenetic mechanisms and preventive efficacy of inhibiting the urokinase-type plasminogen activator receptor system. J. Diabetes Res. 2017:2904150. doi: 10.1155/2017/2904150
Cammalleri, M., Dal Monte, M., Locri, F., Pecci, V., De Rosa, M., Pavone, V., et al. (2019a). The urokinase-type plasminogen activator system as drug target in retinitis pigmentosa: new pre-clinical evidence in the rd10 mouse model. J. Cell Mol. Med. 23, 5176–5192. doi: 10.1111/jcmm.14391
Cammalleri, M., Dal Monte, M., Pavone, V., De Rosa, M., Rusciano, D., and Bagnoli, P. (2019b). The uPAR system as a potential therapeutic target in the diseased eye. Cells 8:E925. doi: 10.3390/cells8080925
Cammalleri, M., Locri, F., Marsili, S., Dal Monte, M., Pisano, C., Mancinelli, A., et al. (2017b). The urokinase receptor-derived peptide UPARANT recovers dysfunctional electroretinogram and blood-retinal barrier leakage in a rat model of diabetes. Invest. Ophthalmol. Vis. Sci. 58, 3138–3148. doi: 10.1167/iovs.17-21593
Casini, G., Catalani, E., Dal Monte, M., and Bagnoli, P. (2005). Functional aspects of the somatostatinergic system in the retina and the potential therapeutic role of somatostatin in retinal disease. Histol. Histopathol. 20, 615–632. doi: 10.14670/HH-20.615
Casini, G., Dal Monte, M., Fornaciari, I., Filippi, L., and Bagnoli, P. (2014). The beta-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog. Retin Eye Res. 42, 103–129. doi: 10.1016/j.preteyeres.2014.06.001
Catalani, E., Cervia, D., Martini, D., Bagnoli, P., Simonetti, E., Timperio, A. M., et al. (2007). Changes in neuronal response to ischemia in retinas with genetic alterations of somatostatin receptor expression. Eur. J. Neurosci. 25, 1447–1459. doi: 10.1111/j.1460-9568.2007.05419.x
Catalani, E., De Palma, C., Perrotta, C., and Cervia, D. (2017). Current evidence for a role of neuropeptides in the regulation of autophagy. Biomed. Res. Int. 2017:5856071. doi: 10.1155/2017/5856071
Cerani, A., Tetreault, N., Menard, C., Lapalme, E., Patel, C., Sitaras, N., et al. (2013). Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab. 18, 505–518. doi: 10.1016/j.cmet.2013.09.003
Cervantes-Villagrana, A. R., Garcia-Roman, J., Gonzalez-Espinosa, C., and Lamas, M. (2010). Pharmacological inhibition of N-methyl d-aspartate receptor promotes secretion of vascular endothelial growth factor in muller cells: effects of hyperglycemia and hypoxia. Curr. Eye Res. 35, 733–741. doi: 10.3109/02713683.2010.483312
Cervia, D., and Casini, G. (2013). The Neuropeptide systems and their potential role in the treatment of mammalian retinal ischemia: a developing story. Curr. Neuropharmacol. 11, 95–101. doi: 10.2174/1570159X11311010011
Cervia, D., Casini, G., and Bagnoli, P. (2008a). Physiology and pathology of somatostatin in the mammalian retina: a current view. Mol. Cell Endocrinol. 286, 112–122. doi: 10.1016/j.mce.2007.12.009
Cervia, D., Catalani, E., and Casini, G. (2019). Neuroprotective peptides in retinal disease. J. Clin. Med. 8:E1146. doi: 10.3390/jcm8081146
Cervia, D., Catalani, E., Dal Monte, M., and Casini, G. (2012). Vascular endothelial growth factor in the ischemic retina and its regulation by somatostatin. J. Neurochem. 120, 818–829. doi: 10.1111/j.1471-4159.2011.07622.x
Cervia, D., Martini, D., Ristori, C., Catalani, E., Timperio, A. M., Bagnoli, P., et al. (2008b). Modulation of the neuronal response to ischaemia by somatostatin analogues in wild-type and knock-out mouse retinas. J. Neurochem. 106, 2224–2235. doi: 10.1111/j.1471-4159.2008.05556.x
Chang, Z. Y., Yeh, M. K., Chiang, C. H., Chen, Y. H., and Lu, D. W. (2013). Erythropoietin protects adult retinal ganglion cells against NMDA-, trophic factor withdrawal-, and TNF-alpha-induced damage. PLoS ONE 8:e55291. doi: 10.1371/journal.pone.0055291
Chauhan, B., Kumar, G., Kalam, N., and Ansari, S. H. (2013). Current concepts and prospects of herbal nutraceutical: a review. J. Adv. Pharm. Technol. Res. 4, 4–8. doi: 10.4103/2231-4040.107494
Chen, B., He, T., Xing, Y., and Cao, T. (2017). Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Exp. Ther. Med. 14, 6022–6026. doi: 10.3892/etm.2017.5275
Chen, M., Lv, H., Gan, J., Ren, J., and Liu, J. (2017). Tang Wang Ming Mu granule attenuates diabetic retinopathy in type 2 diabetes rats. Front. Physiol. 8:1065. doi: 10.3389/fphys.2017.01065
Chen, X. Y., Du, Y. F., and Chen, L. (2019). Neuropeptides exert neuroprotective effects in Alzheimer's disease. Front. Mol. Neurosci. 11:493. doi: 10.3389/fnmol.2018.00493
Chen, Y., Hu, Y., Lin, M., Jenkins, A. J., Keech, A. C., Mott, R., et al. (2013). Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62, 261–272. doi: 10.2337/db11-0413
Chen, Y., Meng, J., Li, H., Wei, H., Bi, F., Liu, S., et al. (2019). Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp. Eye Res. 181, 356–366. doi: 10.1016/j.exer.2018.11.023
Chew, E. Y., Ambrosius, W. T., Davis, M. D., Danis, R. P., Gangaputra, S., Greven, C. M., et al. (2010). Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 363, 233–244. doi: 10.1056/NEJMoa1001288
Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., Da Rocha Fernandes, J. D., Ohlrogge, A. W., et al. (2018). IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281. doi: 10.1016/j.diabres.2018.02.023
Chou, J. C., Rollins, S. D., Ye, M., Batlle, D., and Fawzi, A. A. (2014). Endothelin receptor-A antagonist attenuates retinal vascular and neuroretinal pathology in diabetic mice. Invest. Ophthalmol. Vis. Sci. 55, 2516–2525. doi: 10.1167/iovs.13-13676
Chu, C., Deng, J., Man, Y., and Qu, Y. (2017). Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed. Res. Int. 2017:5615647. doi: 10.1155/2017/5615647
Chung, H., Lee, H., Lamoke, F., Hrushesky, W. J., Wood, P. A., and Jahng, W. J. (2009). Neuroprotective role of erythropoietin by antiapoptosis in the retina. J. Neurosci. Res. 87, 2365–2374. doi: 10.1002/jnr.22046
Colella, P., Iodice, C., Di Vicino, U., Annunziata, I., Surace, E. M., and Auricchio, A. (2011). Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum. Mol. Genet. 20, 2251–2262. doi: 10.1093/hmg/ddr115
Dal Monte, M., Latina, V., Cupisti, E., and Bagnoli, P. (2012). Protective role of somatostatin receptor 2 against retinal degeneration in response to hypoxia. Naunyn Schmiedebergs Arch. Pharmacol. 385, 481–494. doi: 10.1007/s00210-012-0735-1
Dal Monte, M., Petrucci, C., Cozzi, A., Allen, J. P., and Bagnoli, P. (2003). Somatostatin inhibits potassium-evoked glutamate release by activation of the sst(2) somatostatin receptor in the mouse retina. Naunyn Schmiedebergs Arch. Pharmacol. 367, 188–192. doi: 10.1007/s00210-002-0662-7
Dal Monte, M., Ristori, C., Cammalleri, M., and Bagnoli, P. (2009). Effects of somatostatin analogues on retinal angiogenesis in a mouse model of oxygen-induced retinopathy: involvement of the somatostatin receptor subtype 2. Invest. Ophthalmol. Vis. Sci. 50, 3596–3606. doi: 10.1167/iovs.09-3412
Dal Monte, M., Ristori, C., Videau, C., Loudes, C., Martini, D., Casini, G., et al. (2010). Expression, localization, and functional coupling of the somatostatin receptor subtype 2 in a mouse model of oxygen-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 51, 1848–1856. doi: 10.1167/iovs.09-4472
D'alessandro, A., Cervia, D., Catalani, E., Gevi, F., Zolla, L., and Casini, G. (2014). Protective effects of the neuropeptides PACAP, substance P and the somatostatin analogue octreotide in retinal ischemia: a metabolomic analysis. Mol. Biosyst. 10, 1290–1304. doi: 10.1039/c3mb70362b
D'amico, A. G., Maugeri, G., Bucolo, C., Saccone, S., Federico, C., Cavallaro, S., et al. (2017a). Nap interferes with hypoxia-inducible factors and VEGF expression in retina of diabetic rats. J. Mol. Neurosci. 61, 256–266. doi: 10.1007/s12031-016-0869-6
D'amico, A. G., Maugeri, G., Rasa, D., Federico, C., Saccone, S., Lazzara, F., et al. (2019). NAP modulates hyperglycemic-inflammatory event of diabetic retina by counteracting outer blood retinal barrier damage. J. Cell Physiol. 234, 5230–5240. doi: 10.1002/jcp.27331
D'amico, A. G., Maugeri, G., Rasa, D. M., Bucolo, C., Saccone, S., Federico, C., et al. (2017b). Modulation of IL-1beta and VEGF expression in rat diabetic retinopathy after PACAP administration. Peptides 97, 64–69. doi: 10.1016/j.peptides.2017.09.014
D'amico, A. G., Maugeri, G., Reitano, R., Bucolo, C., Saccone, S., Drago, F., et al. (2015). PACAP modulates expression of hypoxia-inducible factors in streptozotocin-induced diabetic rat retina. J. Mol. Neurosci. 57, 501–509. doi: 10.1007/s12031-015-0621-7
Danyadi, B., Szabadfi, K., Reglodi, D., Mihalik, A., Danyadi, T., Kovacs, Z., et al. (2014). PACAP application improves functional outcome of chronic retinal ischemic injury in rats-evidence from electroretinographic measurements. J. Mol. Neurosci. 54, 293–299. doi: 10.1007/s12031-014-0296-5
Davenport, A. P., Hyndman, K. A., Dhaun, N., Southan, C., Kohan, D. E., Pollock, J. S., et al. (2016). Endothelin. Pharmacol. Rev. 68, 357–418. doi: 10.1124/pr.115.011833
Dejda, A., Mawambo, G., Cerani, A., Miloudi, K., Shao, Z., Daudelin, J. F., et al. (2014). Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J. Clin. Invest. 124, 4807–4822. doi: 10.1172/JCI76492
Deliyanti, D., Alrashdi, S. F., Tan, S. M., Meyer, C., Ward, K. W., De Haan, J. B., et al. (2018). Nrf2 activation is a potential therapeutic approach to attenuate diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 59, 815–825. doi: 10.1167/iovs.17-22920
Deng, G., Moran, E. P., Cheng, R., Matlock, G., Zhou, K., Moran, D., et al. (2017). Therapeutic effects of a novel agonist of peroxisome proliferator-activated receptor alpha for the treatment of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 58, 5030–5042. doi: 10.1167/iovs.16-21402
Deza-Ponzio, R., Herrera, M. L., Bellini, M. J., Virgolini, M. B., and Herenu, C. B. (2018). Aldehyde dehydrogenase 2 in the spotlight: the link between mitochondria and neurodegeneration. Neurotoxicology 68, 19–24. doi: 10.1016/j.neuro.2018.06.005
Di Marco, E., Jha, J. C., Sharma, A., Wilkinson-Berka, J. L., Jandeleit-Dahm, K. A., and De Haan, J. B. (2015). Are reactive oxygen species still the basis for diabetic complications? Clin. Sci. 129, 199–216. doi: 10.1042/CS20150093
Dietrich, N., Kolibabka, M., Busch, S., Bugert, P., Kaiser, U., Lin, J., et al. (2016). The DPP4 inhibitor linagliptin protects from experimental diabetic retinopathy. PLoS ONE 11:e0167853. doi: 10.1371/journal.pone.0167853
Djordjevic, B., Cvetkovic, T., Stoimenov, T. J., Despotovic, M., Zivanovic, S., Basic, J., et al. (2018). Oral supplementation with melatonin reduces oxidative damage and concentrations of inducible nitric oxide synthase, VEGF and matrix metalloproteinase 9 in the retina of rats with streptozotocin/nicotinamide induced pre-diabetes. Eur. J. Pharmacol. 833, 290–297. doi: 10.1016/j.ejphar.2018.06.011
Drucker, D. J., and Nauck, M. A. (2006). The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705. doi: 10.1016/S0140-6736(06)69705-5
Du, W., An, Y., He, X., Zhang, D., and He, W. (2018). Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid. Med. Cell Longev. 2018:1610751. doi: 10.1155/2018/1610751
Duh, E. J., Sun, J. K., and Stitt, A. W. (2017). Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2:93751. doi: 10.1172/jci.insight.93751
Edling, A. E., Gomes, D., Weeden, T., Dzuris, J., Stefano, J., Pan, C., et al. (2011). Immunosuppressive activity of a novel peptide analog of alpha-melanocyte stimulating hormone (alpha-MSH) in experimental autoimmune uveitis. J. Neuroimmunol. 236, 1–9. doi: 10.1016/j.jneuroim.2011.04.015
Fan, Y., Liu, K., Wang, Q., Ruan, Y., Ye, W., and Zhang, Y. (2014a). Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp. Eye Res. 127, 104–116. doi: 10.1016/j.exer.2014.05.004
Fan, Y., Liu, K., Wang, Q., Ruan, Y., Zhang, Y., and Ye, W. (2014b). Exendin-4 protects retinal cells from early diabetes in Goto-Kakizaki rats by increasing the Bcl-2/Bax and Bcl-xL/Bax ratios and reducing reactive gliosis. Mol. Vis. 20, 1557–1568.
Fletcher, E. L., Phipps, J. A., Ward, M. M., Vessey, K. A., and Wilkinson-Berka, J. L. (2010). The renin-angiotensin system in retinal health and disease: its influence on neurons, glia and the vasculature. Prog. Retin. Eye Res. 29, 284–311. doi: 10.1016/j.preteyeres.2010.03.003
Fonollosa, A., Coronado, E., Catalan, R., Gutierrez, M., Macia, C., Zapata, M. A., et al. (2012). Vitreous levels of somatostatin in patients with chronic uveitic macular oedema. Eye 26, 1378–1383. doi: 10.1038/eye.2012.161
Foureaux, G., Nogueira, B. S., Coutinho, D. C., Raizada, M. K., Nogueira, J. C., and Ferreira, A. J. (2015). Activation of endogenous angiotensin converting enzyme 2 prevents early injuries induced by hyperglycemia in rat retina. Braz. J. Med. Biol. Res. 48, 1109–1114. doi: 10.1590/1414-431x20154583
Foureaux, G., Nogueira, J. C., Nogueira, B. S., Fulgencio, G. O., Menezes, G. B., Fernandes, S. O., et al. (2013). Antiglaucomatous effects of the activation of intrinsic Angiotensin-converting enzyme 2. Invest. Ophthalmol. Vis. Sci. 54, 4296–4306. doi: 10.1167/iovs.12-11427
Foxton, R. H., Finkelstein, A., Vijay, S., Dahlmann-Noor, A., Khaw, P. T., Morgan, J. E., et al. (2013). VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am. J. Pathol. 182, 1379–1390. doi: 10.1016/j.ajpath.2012.12.032
Fu, Q. L., Wu, W., Wang, H., Li, X., Lee, V. W., and So, K. F. (2008). Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue retinal ganglion cells after chronic ocular hypertension. Cell Mol. Neurobiol. 28, 317–329. doi: 10.1007/s10571-007-9155-z
Fujita, T., Hirooka, K., Nakamura, T., Itano, T., Nishiyama, A., Nagai, Y., et al. (2012). Neuroprotective effects of angiotensin II type 1 receptor (AT1-R) blocker via modulating AT1-R signaling and decreased extracellular glutamate levels. Invest. Ophthalmol. Vis. Sci. 53, 4099–4110. doi: 10.1167/iovs.11-9167
Gabriel, R. (2013). Neuropeptides and diabetic retinopathy. Br. J. Clin. Pharmacol. 75, 1189–1201. doi: 10.1111/bcp.12003
Garcia, C., Aranda, J., Arnold, E., Thebault, S., Macotela, Y., Lopez-Casillas, F., et al. (2008). Vasoinhibins prevent retinal vasopermeability associated with diabetic retinopathy in rats via protein phosphatase 2A-dependent eNOS inactivation. J. Clin. Invest. 118, 2291–2300. doi: 10.1172/JCI34508
Gerszon, J., Rodacka, A., and Puchała, M. (2014). Antioxidant properties of resveratrol and its protective effects in neurodegenerative diseases. Med. J. Cell Biol. 4, 97–117. doi: 10.2478/acb-2014-0006
Giladi, E., Hill, J. M., Dresner, E., Stack, C. M., and Gozes, I. (2007). Vasoactive intestinal peptide (VIP) regulates activity-dependent neuroprotective protein (ADNP) expression in vivo. J. Mol. Neurosci. 33, 278–283. doi: 10.1007/s12031-007-9003-0
Goldenberg-Cohen, N., Dadon-Bar-El, S., Hasanreisoglu, M., Avraham-Lubin, B. C., Dratviman-Storobinsky, O., Cohen, Y., et al. (2009). Possible neuroprotective effect of brimonidine in a mouse model of ischaemic optic neuropathy. Clin. Exp. Ophthalmol. 37, 718–729. doi: 10.1111/j.1442-9071.2009.02108.x
Goncalves, A., Leal, E., Paiva, A., Teixeira Lemos, E., Teixeira, F., Ribeiro, C. F., et al. (2012). Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabetes Obes. Metab. 14, 454–463. doi: 10.1111/j.1463-1326.2011.01548.x
Goncalves, A., Lin, C. M., Muthusamy, A., Fontes-Ribeiro, C., Ambrosio, A. F., Abcouwer, S. F., et al. (2016). Protective effect of a GLP-1 analog on ischemia-reperfusion induced blood-retinal barrier breakdown and inflammation. Invest. Ophthalmol. Vis. Sci. 57, 2584–2592. doi: 10.1167/iovs.15-19006
Goncalves, A., Marques, C., Leal, E., Ribeiro, C. F., Reis, F., Ambrosio, A. F., et al. (2014). Dipeptidyl peptidase-IV inhibition prevents blood-retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochim. Biophys. Acta 9:25. doi: 10.1016/j.bbadis.2014.04.013
Guo, X., Namekata, K., Kimura, A., Noro, T., Azuchi, Y., Semba, K., et al. (2015). Brimonidine suppresses loss of retinal neurons and visual function in a murine model of optic neuritis. Neurosci. Lett. 592, 27–31. doi: 10.1016/j.neulet.2015.02.059
Gupta, S. K., Kumar, B., Nag, T. C., Agrawal, S. S., Agrawal, R., Agrawal, P., et al. (2011). Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 27, 123–130. doi: 10.1089/jop.2010.0123
Hammes, H. P. (2018). Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia 61, 29–38. doi: 10.1007/s00125-017-4435-8
Harmar, A. J., Sheward, W. J., Morrison, C. F., Waser, B., Gugger, M., and Reubi, J. C. (2004). Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology 145, 1203–1210. doi: 10.1210/en.2003-1058
He, M., Long, P., Yan, W., Chen, T., Guo, L., Zhang, Z., et al. (2018). ALDH2 attenuates early-stage STZ-induced aged diabetic rats retinas damage via Sirt1/Nrf2 pathway. Life Sci. 215, 227–235. doi: 10.1016/j.lfs.2018.10.019
Hebsgaard, J. B., Pyke, C., Yildirim, E., Knudsen, L. B., Heegaard, S., and Kvist, P. H. (2018). Glucagon-like peptide-1 receptor expression in the human eye. Diabetes Obes. Metab. 20, 2304–2308. doi: 10.1111/dom.13339
Hernandez, C., Bogdanov, P., Corraliza, L., Garcia-Ramirez, M., Sola-Adell, C., Arranz, J. A., et al. (2016a). Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 65, 172–187. doi: 10.2337/db15-0443
Hernandez, C., Bogdanov, P., Sola-Adell, C., Sampedro, J., Valeri, M., Genis, X., et al. (2017). Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 60, 2285–2298. doi: 10.1007/s00125-017-4388-y
Hernandez, C., Dal Monte, M., Simo, R., and Casini, G. (2016b). Neuroprotection as a therapeutic target for diabetic retinopathy. J. Diabetes Res. 2016:9508541. doi: 10.1155/2016/9508541
Hernandez, C., Fonollosa, A., Garcia-Ramirez, M., Higuera, M., Catalan, R., Miralles, A., et al. (2006). Erythropoietin is expressed in the human retina and it is highly elevated in the vitreous fluid of patients with diabetic macular edema. Diabetes Care 29, 2028–2033. doi: 10.2337/dc06-0556
Hernandez, C., Garcia-Ramirez, M., Corraliza, L., Fernandez-Carneado, J., Farrera-Sinfreu, J., Ponsati, B., et al. (2013). Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 62, 2569–2578. doi: 10.2337/db12-0926
Hernandez, C., and Simo, R. (2012). Neuroprotection in diabetic retinopathy. Curr. Diab. Rep. 12, 329–337. doi: 10.1007/s11892-012-0284-5
Hernandez, C., Simo-Servat, O., and Simo, R. (2014). Somatostatin and diabetic retinopathy: current concepts and new therapeutic perspectives. Endocrine 46, 209–214. doi: 10.1007/s12020-014-0232-z
Heusch, G. (2013). Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381, 166–175. doi: 10.1016/S0140-6736(12)60916-7
Hewlings, S. J., and Kalman, D. S. (2017). Curcumin: a review of its' effects on human health. Foods 6:E92. doi: 10.3390/foods6100092
Hokfelt, T., Bartfai, T., and Bloom, F. (2003). Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2, 463–472. doi: 10.1016/S1474-4422(03)00482-4
Hombrebueno, J. R., Ali, I. H., Xu, H., and Chen, M. (2015). Sustained intraocular VEGF neutralization results in retinal neurodegeneration in the Ins2(Akita) diabetic mouse. Sci. Rep. 5:18316. doi: 10.1038/srep18316
Hu, C. K., Lee, Y. J., Colitz, C. M., Chang, C. J., and Lin, C. T. (2012). The protective effects of Lycium barbarum and Chrysanthemum morifolum on diabetic retinopathies in rats. Vet. Ophthalmol. 2, 65–71. doi: 10.1111/j.1463-5224.2012.01018.x
Hu, Y., Chen, Y., Ding, L., He, X., Takahashi, Y., Gao, Y., et al. (2013). Pathogenic role of diabetes-induced PPAR-alpha down-regulation in microvascular dysfunction. Proc. Natl. Acad. Sci. U.S.A. 110, 15401–15406. doi: 10.1073/pnas.1307211110
Iadecola, C., and Anrather, J. (2011). Stroke research at a crossroad: asking the brain for directions. Nat. Neurosci. 14, 1363–1368. doi: 10.1038/nn.2953
Jelkmann, W. (2013). Physiology and pharmacology of erythropoietin. Transfus Med. Hemother. 40, 302–309. doi: 10.1159/000356193
Jia, Y. P., Sun, L., Yu, H. S., Liang, L. P., Li, W., Ding, H., et al. (2017). The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules 22:E610. doi: 10.3390/molecules22040610
Jindal, V. (2015). Neurodegeneration as a primary change and role of neuroprotection in diabetic retinopathy. Mol. Neurobiol. 51, 878–884. doi: 10.1007/s12035-014-8732-7
Kang, M. K., Lee, E. J., Kim, Y. H., Kim, D. Y., Oh, H., Kim, S. I., et al. (2018). Chrysin ameliorates malfunction of retinoid visual cycle through blocking activation of AGE-RAGE-ER stress in glucose-stimulated retinal pigment epithelial cells and diabetic eyes. Nutrients 10:E1046. doi: 10.3390/nu10081046
Kang, M. K., Park, S. H., Kim, Y. H., Lee, E. J., Antika, L. D., Kim, D. Y., et al. (2016). Dietary compound chrysin inhibits retinal neovascularization with abnormal capillaries in db/db mice. Nutrients 8:782. doi: 10.3390/nu8120782
Kara, S., Gencer, B., Karaca, T., Tufan, H. A., Arikan, S., Ersan, I., et al. (2014). Protective effect of hesperetin and naringenin against apoptosis in ischemia/reperfusion-induced retinal injury in rats. ScientificWorldJournal 30:797824. doi: 10.1155/2014/797824
Keech, A. C., Mitchell, P., Summanen, P. A., O'day, J., Davis, T. M., Moffitt, M. S., et al. (2007). Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370, 1687–1697. doi: 10.1016/S0140-6736(07)61607-9
Kiagiadaki, F., Savvaki, M., and Thermos, K. (2010). Activation of somatostatin receptor (sst 5) protects the rat retina from AMPA-induced neurotoxicity. Neuropharmacology 58, 297–303. doi: 10.1016/j.neuropharm.2009.06.028
Kiagiadaki, F., and Thermos, K. (2008). Effect of intravitreal administration of somatostatin and sst2 analogs on AMPA-induced neurotoxicity in rat retina. Invest. Ophthalmol. Vis. Sci. 49, 3080–3089. doi: 10.1167/iovs.07-1644
Kilic, U., Kilic, E., Soliz, J., Bassetti, C. I., Gassmann, M., and Hermann, D. M. (2005). Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating ERK-1/-2. FASEB J. 19, 249–251. doi: 10.1096/fj.04-2493fje
Kim, J., Kim, C. S., Lee, Y. M., Sohn, E., Jo, K., and Kim, J. S. (2015). Vaccinium myrtillus extract prevents or delays the onset of diabetes–induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 66, 236–242. doi: 10.3109/09637486.2014.979319
Kim, Y. H., Kim, Y. S., Kang, S. S., Cho, G. J., and Choi, W. S. (2010). Resveratrol inhibits neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II activity in diabetic mouse retina. Diabetes 59, 1825–1835. doi: 10.2337/db09-1431
Kim, Y. H., Kim, Y. S., Roh, G. S., Choi, W. S., and Cho, G. J. (2012). Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 90, 1755–3768. doi: 10.1111/j.1755-3768.2011.02243.x
Kokona, D., Mastrodimou, N., Pediaditakis, I., Charalampopoulos, I., Schmid, H. A., and Thermos, K. (2012). Pasireotide (SOM230) protects the retina in animal models of ischemia induced retinopathies. Exp. Eye Res. 103, 90–98. doi: 10.1016/j.exer.2012.08.005
Kowluru, R. A., and Kanwar, M. (2007). Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 4, 1743–7075. doi: 10.1186/1743-7075-4-8
Kowluru, R. A., Kowluru, A., Mishra, M., and Kumar, B. (2015). Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 48, 40–61. doi: 10.1016/j.preteyeres.2015.05.001
Kowluru, R. A., Menon, B., and Gierhart, D. L. (2008). Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest. Ophthalmol. Vis. Sci. 49, 1645–1651. doi: 10.1167/iovs.07-0764
Kowluru, R. A., Zhong, Q., Santos, J. M., Thandampallayam, M., Putt, D., and Gierhart, D. L. (2014). Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. 11, 1743–7075. doi: 10.1186/1743-7075-11-8
Kubota, S., Kurihara, T., Mochimaru, H., Satofuka, S., Noda, K., Ozawa, Y., et al. (2009). Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest. Ophthalmol. Vis. Sci. 50, 3512–3519. doi: 10.1167/iovs.08-2666
Kubota, S., Ozawa, Y., Kurihara, T., Sasaki, M., Yuki, K., Miyake, S., et al. (2011). Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest. Ophthalmol. Vis. Sci. 52, 9142–9148. doi: 10.1167/iovs.11-8041
Kumar, B., Gupta, S. K., Nag, T. C., Srivastava, S., and Saxena, R. (2012a). Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. 47, 103–108. doi: 10.1159/000330051
Kumar, B., Gupta, S. K., Nag, T. C., Srivastava, S., Saxena, R., Jha, K. A., et al. (2014). Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 125, 193–202. doi: 10.1016/j.exer.2014.06.009
Kumar, B., Gupta, S. K., Srinivasan, B. P., Nag, T. C., Srivastava, S., and Saxena, R. (2012b). Hesperetin ameliorates hyperglycemia induced retinal vasculopathy via anti-angiogenic effects in experimental diabetic rats. Vascul. Pharmacol. 57, 201–207. doi: 10.1016/j.vph.2012.02.007
Kumar, B., Gupta, S. K., Srinivasan, B. P., Nag, T. C., Srivastava, S., Saxena, R., et al. (2013). Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc. Res. 87, 65–74. doi: 10.1016/j.mvr.2013.01.002
Kusari, J., Zhou, S. X., Padillo, E., Clarke, K. G., and Gil, D. W. (2010). Inhibition of vitreoretinal VEGF elevation and blood-retinal barrier breakdown in streptozotocin-induced diabetic rats by brimonidine. Invest. Ophthalmol. Vis. Sci. 51, 1044–1051. doi: 10.1167/iovs.08-3293
Lakk, M., Denes, V., and Gabriel, R. (2015). Pituitary adenylate cyclase-activating polypeptide receptors signal via phospholipase c pathway to block apoptosis in newborn rat retina. Neurochem. Res. 40, 1402–1409. doi: 10.1007/s11064-015-1607-0
Leal, E. C., Martins, J., Voabil, P., Liberal, J., Chiavaroli, C., Bauer, J., et al. (2010). Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes 59, 2637–2645. doi: 10.2337/db09-1421
Leasher, J. L., Bourne, R. R., Flaxman, S. R., Jonas, J. B., Keeffe, J., Naidoo, K., et al. (2016). Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care 39, 1643–1649. doi: 10.2337/dc15-2171
Lee, H. S., Jun, J. H., Jung, E. H., Koo, B. A., and Kim, Y. S. (2014). Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 19, 12150–12172. doi: 10.3390/molecules190812150
Li, J., Wang, P., Chen, Z., Yu, S., and Xu, H. (2018). Fenofibrate ameliorates oxidative stress-induced retinal microvascular dysfunction in diabetic rats. Curr. Eye Res. 43, 1395–1403. doi: 10.1080/02713683.2018.1501072
Li, S., Gokden, N., Okusa, M. D., Bhatt, R., and Portilla, D. (2005). Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am. J. Physiol. Renal Physiol. 289, F469–F480. doi: 10.1152/ajprenal.00038.2005
Li, X., Ren, C., Li, S., Han, R., Gao, J., Huang, Q., et al. (2017). Limb remote ischemic conditioning promotes myelination by upregulating PTEN/Akt/mTOR signaling activities after chronic cerebral hypoperfusion. Aging Dis. 8, 392–401. doi: 10.14336/AD.2016.1227
Liu, J., Xia, X., Xiong, S., Le, Y., and Xu, H. (2011). Intravitreous high expression level of netrin-1 in patients with proliferative diabetic retinopathy. Eye Sci. 26, 85–90, 120. doi: 10.3969/j.issn.1000-4432.2011.02.017
Liu, Q., Zhang, F., Zhang, X., Cheng, R., Ma, J. X., Yi, J., et al. (2018). Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol Cell Biochem. 445, 105–115. doi: 10.1007/s11010-017-3256-x
Liu, Q., Zhang, X., Cheng, R., Ma, J. X., Yi, J., and Li, J. (2019). Salutary effect of fenofibrate on type 1 diabetic retinopathy via inhibiting oxidative stress-mediated Wnt/beta-catenin pathway activation. Cell Tissue Res. 376, 165–177. doi: 10.1007/s00441-018-2974-z
Liu, X., Zhu, B., Zou, H., Hu, D., Gu, Q., Liu, K., et al. (2015). Carbamylated erythropoietin mediates retinal neuroprotection in streptozotocin-induced early-stage diabetic rats. Graefes Arch. Clin. Exp. Ophthalmol. 253, 1263–1272. doi: 10.1007/s00417-015-2969-3
Liu, Y., Hirooka, K., Nishiyama, A., Lei, B., Nakamura, T., Itano, T., et al. (2012). Activation of the aldosterone/mineralocorticoid receptor system and protective effects of mineralocorticoid receptor antagonism in retinal ischemia-reperfusion injury. Exp. Eye Res. 96, 116–123. doi: 10.1016/j.exer.2011.12.012
Magen, I., and Gozes, I. (2014). Davunetide: peptide therapeutic in neurological disorders. Curr. Med. Chem. 21, 2591–2598. doi: 10.2174/0929867321666140217124945
Mahmoud, A. M., Abd El-Twab, S. M., and Abdel-Reheim, E. S. (2017). Consumption of polyphenol-rich Morus alba leaves extract attenuates early diabetic retinopathy: the underlying mechanism. Eur. J. Nutr. 56, 1671–1684. doi: 10.1007/s00394-016-1214-0
Marangoz, D., Guzel, E., Eyuboglu, S., Gumusel, A., Seckin, I., Ciftci, F., et al. (2018). Comparison of the neuroprotective effects of brimonidine tartrate and melatonin on retinal ganglion cells. Int. Ophthalmol. 38, 2553–2562. doi: 10.1007/s10792-017-0768-z
Masuzawa, K., Goto, K., Jesmin, S., Maeda, S., Miyauchi, T., Kaji, Y., et al. (2006). An endothelin type A receptor antagonist reverses upregulated VEGF and ICAM-1 levels in streptozotocin-induced diabetic rat retina. Curr. Eye Res. 31, 79–89. doi: 10.1080/02713680500478923
Mei, S., Cammalleri, M., Azara, D., Casini, G., Bagnoli, P., and Dal Monte, M. (2012). Mechanisms underlying somatostatin receptor 2 down-regulation of vascular endothelial growth factor expression in response to hypoxia in mouse retinal explants. J. Pathol. 226, 519–533. doi: 10.1002/path.3006
Milatovic, D., Zaja-Milatovic, S., and Gupta, R. C. (2016). “Oxidative stress and excitotoxicity: antioxidants from nutraceuticals,” in Nutraceuticals, ed R. C. Gupta (Amsterdam; Boston, MA; Heidelberg; London; New York, NY; Oxford; San Diego, CA; San Francisco, CA; Singapore; Sydney, NSW; Tokyo: Elsevier), 401–413. doi: 10.1016/B978-0-12-802147-7.00029-2
Mishra, M., and Kowluru, R. A. (2016). The role of DNA methylation in the metabolic memory phenomenon associated with the continued progression of diabetic retinopathy. Invest Ophthalmol. Vis. Sci. 57, 5748–5757. doi: 10.1167/iovs.16-19759
Mishra, M., and Kowluru, R. A. (2019). DNA methylation-a potential source of mitochondria DNA base mismatch in the development of diabetic retinopathy. Mol. Neurobiol. 56, 88–101. doi: 10.1007/s12035-018-1086-9
Moran, E. P., Wang, Z., Chen, J., Sapieha, P., Smith, L. E., and Ma, J. X. (2016). Neurovascular cross talk in diabetic retinopathy: pathophysiological roles and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 311, H738–H749. doi: 10.1152/ajpheart.00005.2016
Mrudula, T., Suryanarayana, P., Srinivas, P. N., and Reddy, G. B. (2007). Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem. Biophys. Res. Commun. 361, 528–532. doi: 10.1016/j.bbrc.2007.07.059
Nakamachi, T., Matkovits, A., Seki, T., and Shioda, S. (2012). Distribution and protective function of pituitary adenylate cyclase-activating polypeptide in the retina. Front. Endocrinol. 3:145. doi: 10.3389/fendo.2012.00145
Narimatsu, T., Ozawa, Y., Miyake, S., Nagai, N., and Tsubota, K. (2014). Angiotensin II type 1 receptor blockade suppresses light-induced neural damage in the mouse retina. Free Radic. Biol. Med. 71, 176–185. doi: 10.1016/j.freeradbiomed.2014.03.020
Navaratna, D., Menicucci, G., Maestas, J., Srinivasan, R., Mcguire, P., and Das, A. (2008). A peptide inhibitor of the urokinase/urokinase receptor system inhibits alteration of the blood-retinal barrier in diabetes. FASEB J. 22, 3310–3317. doi: 10.1096/fj.08-110155
Naveh, N. (2003). Melanocortins applied intravitreally delay retinal dystrophy in Royal College of Surgeons rats. Graefes Arch. Clin. Exp. Ophthalmol. 241, 1044–1050. doi: 10.1007/s00417-003-0781-y
Noonan, J. E., Jenkins, A. J., Ma, J. X., Keech, A. C., Wang, J. J., and Lamoureux, E. L. (2013). An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 62, 3968–3975. doi: 10.2337/db13-0800
Ola, M. S., Ahmed, M. M., Abuohashish, H. M., Al-Rejaie, S. S., and Alhomida, A. S. (2013). Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem. Res. 38, 1572–1579. doi: 10.1007/s11064-013-1058-4
Ola, M. S., Ahmed, M. M., Shams, S., and Al-Rejaie, S. S. (2017). Neuroprotective effects of quercetin in diabetic rat retina. Saudi J. Biol. Sci. 24, 1186–1194. doi: 10.1016/j.sjbs.2016.11.017
Ola, M. S., Nawaz, M. I., Siddiquei, M. M., Al-Amro, S., and Abu El-Asrar, A. M. (2012). Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes Complic. 26, 56–64. doi: 10.1016/j.jdiacomp.2011.11.004
Orhan, C., Akdemir, F., Tuzcu, M., Sahin, N., Yilmaz, I., Deshpande, J., et al. (2016). Mesozeaxanthin protects retina from oxidative stress in a rat model. J. Ocul. Pharmacol. Ther. 32, 631–637. doi: 10.1089/jop.2015.0154
Ozawa, Y., Kurihara, T., Tsubota, K., and Okano, H. (2011). Regulation of posttranscriptional modification as a possible therapeutic approach for retinal neuroprotection. J. Ophthalmol. 2011:506137. doi: 10.1155/2011/506137
Ozawa, Y., Sasaki, M., Takahashi, N., Kamoshita, M., Miyake, S., and Tsubota, K. (2012). Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 18, 51–56. doi: 10.2174/138161212798919101
Pantalone, K. M., Hobbs, T. M., Wells, B. J., Kong, S. X., Kattan, M. W., Bouchard, J., et al. (2015). Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res. Care 3:2015-000093. doi: 10.1136/bmjdrc-2015-000093
Perez De Sevilla Muller, L., Solomon, A., Sheets, K., Hapukino, H., Rodriguez, A. R., and Brecha, N. C. (2019). Multiple cell types form the VIP amacrine cell population. J. Comp. Neurol. 527, 133–158. doi: 10.1002/cne.24234
Pyper, S. R., Viswakarma, N., Yu, S., and Reddy, J. K. (2010). PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucleic Recept. Signal. 16:08002. doi: 10.1621/nrs.08002
Qian, H., and Ripps, H. (2011). Neurovascular interaction and the pathophysiology of diabetic retinopathy. Exp. Diabetes Res. 2011:693426. doi: 10.1155/2011/693426
Qiu, F., Meng, T., Chen, Q., Zhou, K., Shao, Y., Matlock, G., et al. (2019). Fenofibrate-loaded biodegradable nanoparticles for the treatment of experimental diabetic retinopathy and neovascular age-related macular degeneration. Mol. Pharm. 16, 1958–1970. doi: 10.1021/acs.molpharmaceut.8b01319
Qiu, Y., Shil, P. K., Zhu, P., Yang, H., Verma, A., Lei, B., et al. (2014). Angiotensin-converting enzyme 2 (ACE2) activator diminazene aceturate ameliorates endotoxin-induced uveitis in mice. Invest. Ophthalmol. Vis. Sci. 55, 3809–3818. doi: 10.1167/iovs.14-13883
Qiu, Y., Tao, L., Zheng, S., Lin, R., Fu, X., Chen, Z., et al. (2016). AAV8-mediated angiotensin-converting enzyme 2 gene delivery prevents experimental autoimmune uveitis by regulating MAPK, NF-kappaB and STAT3 pathways. Sci. Rep. 6:31912. doi: 10.1038/srep31912
Quigley, H. A., Pitha, I. F., Welsbie, D. S., Nguyen, C., Steinhart, M. R., Nguyen, T. D., et al. (2015). Losartan treatment protects retinal ganglion cells and alters scleral remodeling in experimental glaucoma. PLoS ONE 10:e0141137. doi: 10.1371/journal.pone.0141137
Reglodi, D., Renaud, J., Tamas, A., Tizabi, Y., Socias, S. B., Del-Bel, E., et al. (2017). Novel tactics for neuroprotection in Parkinson's disease: role of antibiotics, polyphenols and neuropeptides. Prog. Neurobiol. 155, 120–148. doi: 10.1016/j.pneurobio.2015.10.004
Ren, C., Wu, H., Li, D., Yang, Y., Gao, Y., Jizhang, Y., et al. (2018). Remote ischemic conditioning protects diabetic retinopathy in streptozotocin-induced diabetic rats via anti-inflammation and antioxidation. Aging Dis. 9, 1122–1133. doi: 10.14336/AD.2018.0711
Romano, M. R., Biagioni, F., Besozzi, G., Carrizzo, A., Vecchione, C., Fornai, F., et al. (2012). Effects of bevacizumab on neuronal viability of retinal ganglion cells in rats. Brain Res. 10, 55–63. doi: 10.1016/j.brainres.2012.08.014
Rong, X., Ji, Y., Zhu, X., Yang, J., Qian, D., Mo, X., et al. (2018). Neuroprotective effect of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel on diabetic retinopathy in rats. Int. J. Nanomed. 14, 45–55. doi: 10.2147/IJN.S184574
Rossino, M. G., and Casini, G. (2019). Nutraceuticals for the treatment of diabetic retinopathy. Nutrients 11:E771. doi: 10.3390/nu11040771
Rota, R., Chiavaroli, C., Garay, R. P., and Hannaert, P. (2004). Reduction of retinal albumin leakage by the antioxidant calcium dobesilate in streptozotocin-diabetic rats. Eur. J. Pharmacol. 495, 217–224. doi: 10.1016/j.ejphar.2004.05.019
Saint-Geniez, M., Maharaj, A. S., Walshe, T. E., Tucker, B. A., Sekiyama, E., Kurihara, T., et al. (2008). Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS ONE 3:e3554. doi: 10.1371/journal.pone.0003554
Sampedro, J., Bogdanov, P., Ramos, H., Sola-Adell, C., Turch, M., Valeri, M., et al. (2019). New insights into the mechanisms of action of topical administration of GLP-1 in an experimental model of diabetic retinopathy. J. Clin. Med. 8:E339. doi: 10.3390/jcm8030339
Sasaki, M., Ozawa, Y., Kurihara, T., Kubota, S., Yuki, K., Noda, K., et al. (2010). Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 53, 971–979. doi: 10.1007/s00125-009-1655-6
Sasaki, M., Yuki, K., Kurihara, T., Miyake, S., Noda, K., Kobayashi, S., et al. (2012). Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 23, 423–429. doi: 10.1016/j.jnutbio.2011.01.006
Satofuka, S., Ichihara, A., Nagai, N., Noda, K., Ozawa, Y., Fukamizu, A., et al. (2009). (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes 58, 1625–1633. doi: 10.2337/db08-0254
Scuderi, S., D'amico, A. G., Castorina, A., Federico, C., Marrazzo, G., Drago, F., et al. (2014). Davunetide (NAP) protects the retina against early diabetic injury by reducing apoptotic death. J. Mol. Neurosci. 54, 395–404. doi: 10.1007/s12031-014-0244-4
Semba, K., Namekata, K., Guo, X., Harada, C., Harada, T., and Mitamura, Y. (2014). Renin-angiotensin system regulates neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 17:296. doi: 10.1038/cddis.2014.296
Shil, P. K., Kwon, K. C., Zhu, P., Verma, A., Daniell, H., and Li, Q. (2014). Oral delivery of ACE2/Ang-(1-7) bioencapsulated in plant cells protects against experimental uveitis and autoimmune uveoretinitis. Mol. Ther. 22, 2069–2082. doi: 10.1038/mt.2014.179
Shimouchi, A., Yokota, H., Ono, S., Matsumoto, C., Tamai, T., Takumi, H., et al. (2016). Neuroprotective effect of water-dispersible hesperetin in retinal ischemia reperfusion injury. Jpn. J. Ophthalmol. 60, 51–61. doi: 10.1007/s10384-015-0415-z
Shioda, S., Takenoya, F., Wada, N., Hirabayashi, T., Seki, T., and Nakamachi, T. (2016). Pleiotropic and retinoprotective functions of PACAP. Anat Sci. Int. 91, 313–324. doi: 10.1007/s12565-016-0351-0
Silva, K. C., Rosales, M. A., Biswas, S. K., Lopes De Faria, J. B., and Lopes De Faria, J. M. (2009). Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes 58, 1382–1390. doi: 10.2337/db09-0166
Silva, K. C., Rosales, M. A., Hamassaki, D. E., Saito, K. C., Faria, A. M., Ribeiro, P. A., et al. (2013). Green tea is neuroprotective in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 54, 1325–1336. doi: 10.1167/iovs.12-10647
Simo, R., Carrasco, E., Fonollosa, A., Garcia-Arumi, J., Casamitjana, R., and Hernandez, C. (2007). Deficit of somatostatin in the vitreous fluid of patients with diabetic macular edema. Diabetes Care 30, 725–727. doi: 10.2337/dc06-1345
Simo, R., and Hernandez, C. (2009). Advances in the medical treatment of diabetic retinopathy. Diabetes Care 32, 1556–1562. doi: 10.2337/dc09-0565
Simo, R., and Hernandez, C. (2015). Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin Eye Res. 48, 160–180. doi: 10.1016/j.preteyeres.2015.04.003
Simo, R., Stitt, A. W., and Gardner, T. W. (2018). Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 61, 1902–1912. doi: 10.1007/s00125-018-4692-1
Simo, R., Sundstrom, J. M., and Antonetti, D. A. (2014). Ocular Anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 37, 893–899. doi: 10.2337/dc13-2002
Smith, N. K., Hackett, T. A., Galli, A., and Flynn, C. R. (2019). GLP-1: molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 128, 94–105. doi: 10.1016/j.neuint.2019.04.010
Sohn, E., Kim, J., Kim, C. S., Lee, Y. M., and Kim, J. S. (2016). Extract of polygonum cuspidatum attenuates diabetic retinopathy by inhibiting the high-mobility group box-1 (HMGB1) signaling pathway in streptozotocin-induced diabetic rats. Nutrients 8:140. doi: 10.3390/nu8030140
Sola-Adell, C., Bogdanov, P., Hernandez, C., Sampedro, J., Valeri, M., Garcia-Ramirez, M., et al. (2017). Calcium dobesilate prevents neurodegeneration and vascular leakage in experimental diabetes. Curr. Eye Res. 42, 1273–1286. doi: 10.1080/02713683.2017.1302591
Solomon, S. D., Chew, E., Duh, E. J., Sobrin, L., Sun, J. K., Vanderbeek, B. L., et al. (2017). Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care 40, 412–418. doi: 10.2337/dc16-2641
Song, Y., Huang, L., and Yu, J. (2016). Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 301, 1–6. doi: 10.1016/j.jneuroim.2016.11.001
Soufi, F. G., Mohammad-Nejad, D., and Ahmadieh, H. (2012). Resveratrol improves diabetic retinopathy possibly through oxidative stress - nuclear factor kappaB - apoptosis pathway. Pharmacol. Rep. 64, 1505–1514. doi: 10.1016/S1734-1140(12)70948-9
Stitt, A. W., Lois, N., Medina, R. J., Adamson, P., and Curtis, T. M. (2013). Advances in our understanding of diabetic retinopathy. Clin. Sci. 125, 1–17. doi: 10.1042/CS20120588
Szabadfi, K., Reglodi, D., Szabo, A., Szalontai, B., Valasek, A., Setalo, G. Jr., et al. (2016). Pituitary adenylate cyclase activating polypeptide, a potential therapeutic agent for diabetic retinopathy in rats: focus on the vertical information processing pathway. Neurotox Res. 29, 432–446. doi: 10.1007/s12640-015-9593-1
Szabadfi, K., Szabo, A., Kiss, P., Reglodi, D., Setalo, G. Jr., Kovacs, K., et al. (2014). PACAP promotes neuron survival in early experimental diabetic retinopathy. Neurochem. Int. 64, 84–91. doi: 10.1016/j.neuint.2013.11.005
Szabo, M. E., Haines, D., Garay, E., Chiavaroli, C., Farine, J. C., Hannaert, P., et al. (2001). Antioxidant properties of calcium dobesilate in ischemic/reperfused diabetic rat retina. Eur. J. Pharmacol. 428, 277–286. doi: 10.1016/S0014-2999(01)01196-7
Tarr, J. M., Kaul, K., Chopra, M., Kohner, E. M., and Chibber, R. (2013). Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013:343560. doi: 10.1155/2013/343560
Tejerina, T., and Ruiz, E. (1998). Calcium dobesilate: pharmacology and future approaches. Gen. Pharmacol. 31, 357–360. doi: 10.1016/S0306-3623(98)00040-8
Thangaraju, P., Chakrabarti, A., Banerjee, D., Hota, D., Tamilselvan Bhatia, A., and Gupta, A. (2014). Dual blockade of Renin Angiotensin system in reducing the early changes of diabetic retinopathy and nephropathy in a diabetic rat model. N. Am. J. Med. Sci. 6, 625–632. doi: 10.4103/1947-2714.147978
Thounaojam, M. C., Powell, F. L., Patel, S., Gutsaeva, D. R., Tawfik, A., Smith, S. B., et al. (2017). Protective effects of agonists of growth hormone-releasing hormone (GHRH) in early experimental diabetic retinopathy. Proc. Natl. Acad. Sci. U.S.A. 114, 13248–13253. doi: 10.1073/pnas.1718592114
Tuncel, N., Basmak, H., Uzuner, K., Tuncel, M., Altiokka, G., Zaimoglu, V., et al. (1996). Protection of rat retina from ischemia-reperfusion injury by vasoactive intestinal peptide (VIP): the effect of VIP on lipid peroxidation and antioxidant enzyme activity of retina and choroid. Ann. N.Y. Acad. Sci. 805, 489–498. doi: 10.1111/j.1749-6632.1996.tb17509.x
Tuzcu, M., Orhan, C., Muz, O. E., Sahin, N., Juturu, V., and Sahin, K. (2017). Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol. 17:129. doi: 10.1186/s12886-017-0524-1
Umeda, N., Ozaki, H., Hayashi, H., and Oshima, K. (2004). Expression of ephrinB2 and its receptors on fibroproliferative membranes in ocular angiogenic diseases. Am. J. Ophthalmol. 138, 270–279. doi: 10.1016/j.ajo.2004.04.006
Vaczy, A., Reglodi, D., Somoskeoy, T., Kovacs, K., Lokos, E., Szabo, E., et al. (2016). The protective role of PAC1-receptor agonist maxadilan in BCCAO-induced retinal degeneration. J. Mol. Neurosci. 60, 186–194. doi: 10.1007/s12031-016-0818-4
Varga, B., Gesztelyi, R., Bombicz, M., Haines, D., Szabo, A. M., Kemeny-Beke, A., et al. (2013). Protective effect of alpha-melanocyte-stimulating hormone (alpha-MSH) on the recovery of ischemia/reperfusion (I/R)-induced retinal damage in a rat model. J. Mol. Neurosci. 50, 558–570. doi: 10.1007/s12031-013-9998-3
Vaudry, D., Falluel-Morel, A., Bourgault, S., Basille, M., Burel, D., Wurtz, O., et al. (2009). Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 61, 283–357. doi: 10.1124/pr.109.001370
Verma, A., Shan, Z., Lei, B., Yuan, L., Liu, X., Nakagawa, T., et al. (2012). ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol. Ther. 20, 28–36. doi: 10.1038/mt.2011.155
Voabil, P., Liberal, J., Leal, E. C., Bauer, J., Cunha-Vaz, J., Santiago, A. R., et al. (2017). Calcium dobesilate is protective against inflammation and oxidative/nitrosative stress in the retina of a type 1 diabetic rat model. Ophthalmic Res. 58, 150–161. doi: 10.1159/000478784
Wang, J., Sun, Z., Shen, J., Wu, D., Liu, F., Yang, R., et al. (2015). Octreotide protects the mouse retina against ischemic reperfusion injury through regulation of antioxidation and activation of NF-kappaB. Oxid. Med. Cell Longev. 2015:970156. doi: 10.1155/2015/970156
Wang, J., Tian, W., Wang, S., Wei, W., Wu, D., Wang, H., et al. (2017). Anti-inflammatory and retinal protective effects of capsaicin on ischaemia-induced injuries through the release of endogenous somatostatin. Clin. Exp. Pharmacol. Physiol. 44, 803–814. doi: 10.1111/1440-1681.12769
Wang, L., Li, C., Guo, H., Kern, T. S., Huang, K., and Zheng, L. (2011). Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS ONE 6:e23194. doi: 10.1371/journal.pone.0023194
Wang, L. L., Sun, Y., Huang, K., and Zheng, L. (2013). Curcumin, a potential therapeutic candidate for retinal diseases. Mol. Nutr. Food Res. 57, 1557–1568. doi: 10.1002/mnfr.201200718
Wang, N., Zou, C., Zhao, S., Wang, Y., Han, C., and Zheng, Z. (2018). Fenofibrate exerts protective effects in diabetic retinopathy via inhibition of the ANGPTL3 pathway. Invest. Ophthalmol. Vis. Sci. 59, 4210–4217. doi: 10.1167/iovs.18-24155
Wang, Q., Gorbey, S., Pfister, F., Hoger, S., Dorn-Beineke, A., Krugel, K., et al. (2011). Long-term treatment with suberythropoietic Epo is vaso- and neuroprotective in experimental diabetic retinopathy. Cell Physiol. Biochem. 27, 769–782. doi: 10.1159/000330085
Wang, Q., Pfister, F., Dorn-Beineke, A., Vom Hagen, F., Lin, J., Feng, Y., et al. (2010). Low-dose erythropoietin inhibits oxidative stress and early vascular changes in the experimental diabetic retina. Diabetologia 53, 1227–1238. doi: 10.1007/s00125-010-1727-7
Wang, W., Zhang, Y., Jin, W., Xing, Y., and Yang, A. (2018). Catechin weakens diabetic retinopathy by inhibiting the expression of NF-kappaB signaling pathway-mediated inflammatory factors. Ann. Clin. Lab Sci. 48, 594–600.
Wardlaw, S. L. (2011). Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur. J. Pharmacol. 660, 213–219. doi: 10.1016/j.ejphar.2010.10.107
Werling, D., Banks, W. A., Salameh, T. S., Kvarik, T., Kovacs, L. A., Vaczy, A., et al. (2017). Passage through the ocular barriers and beneficial effects in retinal ischemia of topical application of PACAP1-38 in rodents. Int. J. Mol. Sci. 18:E675. doi: 10.3390/ijms18030675
Werling, D., Reglodi, D., Banks, W. A., Salameh, T. S., Kovacs, K., Kvarik, T., et al. (2016). Ocular delivery of PACAP1-27 protects the retina from ischemic damage in rodents. Invest. Ophthalmol. Vis. Sci. 57, 6683–6691. doi: 10.1167/iovs.16-20630
Wilkinson-Berka, J. L., Agrotis, A., and Deliyanti, D. (2012). The retinal renin-angiotensin system: roles of angiotensin II and aldosterone. Peptides 36, 142–150. doi: 10.1016/j.peptides.2012.04.008
Xie, M. Y., Yang, Y., Liu, P., Luo, Y., and Tang, S. B. (2019). 5-aza-2′-deoxycytidine in the regulation of antioxidant enzymes in retinal endothelial cells and rat diabetic retina. Int. J. Ophthalmol. 12, 1–7. doi: 10.18240/ijo.2019.01.01
Yang, F., Yu, J., Ke, F., Lan, M., Li, D., Tan, K., et al. (2018). Curcumin alleviates diabetic retinopathy in experimental diabetic rats. Ophthalmic Res. 60, 43–54. doi: 10.1159/000486574
Yang, H., Hirooka, K., Fukuda, K., and Shiraga, F. (2009). Neuroprotective effects of angiotensin II type 1 receptor blocker in a rat model of chronic glaucoma. Invest. Ophthalmol. Vis. Sci. 50, 5800–5804. doi: 10.1167/iovs.09-3678
Yang, Y. (2011). Structure, function and regulation of the melanocortin receptors. Eur. J. Pharmacol. 660, 125–130. doi: 10.1016/j.ejphar.2010.12.020
Ye, D., Shi, Y., Xu, Y., and Huang, J. (2019). PACAP attenuates optic nerve crush-induced retinal ganglion cell apoptosis via activation of the CREB-Bcl-2 pathway. J. Mol. Neurosci. 16, 019–01309. doi: 10.1007/s12031-019-01309-9
Zeng, Y., Yang, K., Wang, F., Zhou, L., Hu, Y., Tang, M., et al. (2016). The glucagon like peptide 1 analogue, exendin-4, attenuates oxidative stress-induced retinal cell death in early diabetic rats through promoting Sirt1 and Sirt3 expression. Exp. Eye Res. 151, 203–211. doi: 10.1016/j.exer.2016.05.002
Zhang, J., Wu, Y., Jin, Y., Ji, F., Sinclair, S. H., Luo, Y., et al. (2008). Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest. Ophthalmol. Vis. Sci. 49, 732–742. doi: 10.1167/iovs.07-0721
Zhang, L., Dong, L., Liu, X., Jiang, Y., Zhang, X., Li, X., et al. (2014). α-Melanocyte-stimulating hormone protects retinal vascular endothelial cells from oxidative stress and apoptosis in a rat model of diabetes. PLoS ONE 9:e93433. doi: 10.1371/journal.pone.0093433
Zhang, R., Zhang, H., Xu, L., Ma, K., Wallrapp, C., and Jonas, J. B. (2011). Neuroprotective effect of intravitreal cell-based glucagon-like peptide-1 production in the optic nerve crush model. Acta Ophthalmol. 89, 1755–3768. doi: 10.1111/j.1755-3768.2010.02044.x
Zhang, X., Liu, W., Wu, S., Jin, J., Li, W., and Wang, N. (2015). Calcium dobesilate for diabetic retinopathy: a systematic review and meta-analysis. Sci. China Life Sci. 58, 101–107. doi: 10.1007/s11427-014-4792-1
Zhang, X., Wang, N., Barile, G. R., Bao, S., and Gillies, M. (2013). Diabetic retinopathy: neuron protection as a therapeutic target. Int. J. Biochem. Cell Biol. 45, 1525–1529. doi: 10.1016/j.biocel.2013.03.002
Zhang, Y., Wang, Q., Zhang, J., Lei, X., Xu, G. T., and Ye, W. (2009). Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 247, 699–706. doi: 10.1007/s00417-008-1004-3
Zhang, Y., Zhang, J., Wang, Q., Lei, X., Chu, Q., Xu, G. T., et al. (2011). Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest. Ophthalmol. Vis. Sci. 52, 278–285. doi: 10.1167/iovs.09-4727
Zhao, Y., and Singh, R. P. (2018). The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context 7:212532. doi: 10.7573/dic.212532
Zuo, Z. F., Zhang, Q., and Liu, X. Z. (2013). Protective effects of curcumin on retinal Muller cell in early diabetic rats. Int. J. Ophthalmol. 6, 422–424. doi: 10.3980/j.issn.2222-3959.2013.04.02
Keywords: nutraceuticals, antioxidants, neuropeptides, vascular endothelial growth factor, blood-retina barrier
Citation: Rossino MG, Dal Monte M and Casini G (2019) Relationships Between Neurodegeneration and Vascular Damage in Diabetic Retinopathy. Front. Neurosci. 13:1172. doi: 10.3389/fnins.2019.01172
Received: 23 August 2019; Accepted: 16 October 2019;
Published: 08 November 2019.
Edited by:
Abel Santamaria, National Institute of Neurology and Neurosurgery (INNN), MexicoReviewed by:
Nataliya G. Kolosova, Institute of Cytology and Genetics (RAS), RussiaJosé Carlos Rivera, Université de Montréal, Canada
Copyright © 2019 Rossino, Dal Monte and Casini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Dal Monte, bWFzc2ltby5kYWxtb250ZSYjeDAwMDQwO3VuaXBpLml0; Giovanni Casini, Z2lvdmFubmkuY2FzaW5pJiN4MDAwNDA7dW5pcGkuaXQ=
 Maria Grazia Rossino
Maria Grazia Rossino Massimo Dal Monte
Massimo Dal Monte Giovanni Casini
Giovanni Casini