- 1Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States
- 2Department of Computer Science, Dartmouth College, Hanover, NH, United States
- 3National Center for PTSD, White River Junction, VT, United States
- 4Department of Psychiatry, Dartmouth–Hitchcock Medical Center, Lebanon, NH, United States
- 5Department of Psychology, The Ohio State University, Columbus, OH, United States
As smartphone usage has become increasingly prevalent in our society, so have rates of depression, particularly among young adults. Individual differences in smartphone usage patterns have been shown to reflect individual differences in underlying affective processes such as depression (Wang et al., 2018). In the current study, a positive relationship was identified between smartphone screen time (e.g., phone unlock duration) and resting-state functional connectivity (RSFC) between the subgenual cingulate cortex (sgCC), a brain region implicated in depression and antidepressant treatment response, and regions of the ventromedial/orbitofrontal cortex (OFC), such that increased phone usage was related to stronger connectivity between these regions. This cluster was subsequently used to constrain subsequent analyses looking at individual differences in depressive symptoms in the same cohort and observed partial replication in a separate cohort. Similar analyses were subsequently performed on metrics of circadian rhythm consistency showing a negative relationship between connectivity of the sgCC and OFC. The data and analyses presented here provide relatively simplistic preliminary analyses which replicate and provide an initial step in combining functional brain activity and smartphone usage patterns to better understand issues related to mental health. Smartphones are a prevalent part of modern life and the usage of mobile sensing data from smartphones promises to be an important tool for mental health diagnostics and neuroscience research.
Introduction
Smartphone usage has become nearly ubiquitous in daily life at a time when depression rates are concurrently rising, particularly among college students. Smartphones contain a variety of sensors that can allow researchers to passively measure various behaviors of the phone’s user. Previous research has linked smartphone usage to self-reported depressive symptoms (Matar and Jaalouk, 2017; Twenge et al., 2018; Wang et al., 2018). In parallel, depressive symptoms have been linked to brain connectivity using resting-state functional connectivity (RSFC) MRI (Greicius et al., 2007). The current manuscript has multiple goals. First, is to provide a proof-of-concept for linking passive mobile smartphone sensing technologies to brain connectivity measures that have also been linked to self-reported depressive symptoms. Second is to replicate these initial findings in a separate cohort. Third, is to identify preliminary links between a key behavior inferred from sensing (e.g., smartphone screen time or circadian rhythm consistency) and brain connectivity metrics. Fourth, is to briefly describe a variety of methods which could be used to combine results across these various data types in the future.
Depression Assessment
Depressive disorders affect over 300 million people worldwide and is currently ranked as the single largest contributor to global disability (Ustün et al., 2004; World Health Organization, 2018). Despite this, the diagnosis of depression has remained largely unchanged; further, a reliable means of identifying individuals at risk of becoming depressed remains absent. Psychology, psychiatry and neuroscience have long relied up self-reported surveys and in-person interviews to measure symptoms, diagnose mental health disorders and identify appropriate treatment strategies (Horwitz et al., 2016). As a result of staggering fiscal and personal costs inflicted at both individual and societal levels, clinicians and researchers set out to redefine the way mental disorders are conceptualized in hopes of creating innovative identification and prevention strategies. The aforementioned aims have been synthesized in a research framework known as RDoC (Research Domain Criteria). RDoC’s objective is to incorporate information across all planes of analysis ranging from cellular level data to person level self-report survey data to provide of a holistic picture of mental disorders (NIMH). A core principle within the RDoC framework is the notion that neuroscience will inform future psychiatric classification schemes; in other words, aid in moving toward the establishment of a neural biomarker for depression. Thus, of great importance is understanding the complete range of human behavior (and neurological functioning) from typical to atypical (Insel et al., 2010). The Patient Health Questionnaire (PHQ, with two, four, eight and nine question versions) is a reliable, short survey which has been validated in clinical settings and can be used to assess self-reported symptoms of depression that cause significant impairment and subjective distress (Kroenke et al., 2001, 2009a,b; Cameron et al., 2008), an approach in keeping within the RDoC research framework, seeking to explain individual variance in symptoms across domains, constructs, and units of analysis. Future methods to accurately diagnose depression may hold promise with the inclusion of techniques that capitalize on the passive collection of behavioral data through mobile sensors (e.g., smartphones).
Passive Sensing
Passive sensing using mobile smartphone technology allows for the assessment of daily activities by the smartphone user without continual effort on their part. This increases the frequency with which data can be collected and is less vulnerable to self-report bias, which is often a problem in prompted surveys (Rosenman et al., 2011; Ben-Zeev et al., 2015). Smartphone ownership has increased steadily over the last decade, with over 75% of the United States population owning one (Smith, 2017). In parallel, depression rates have increased over the last decade (Twenge et al., 2018). While it is unlikely that smartphone ownership by itself has prompted increased rates of depression, has perhaps facilitated increased access to and usage of social network platforms (Kross et al., 2013). Prevalence of both smartphone ownership and depression rates are often reported as being higher in college-age students (Eisenberg et al., 2013; Nielsen.com, 2016). Screen time, e.g., the amount of time that the screen is unlocked and being used is a relatively simple metric to calculate that has been previously related to depressive symptoms by multiple groups through either passive sensing or self-reported surveys (Twenge et al., 2018; Wang et al., 2018). Screen time and unlock duration will be used interchangeably henceforth.
Depression has been linked to a variety of metrics available from smartphone sensing applications including amount of stationary time, GPS patterns, phone usage and conversation patterns, among others (Burns et al., 2011; Canzian and Musolesi, 2015; Saeb et al., 2015; Mehrotra et al., 2017; Wang et al., 2018). The higher amplitude circadian rhythms as measured by accelerometer are associated with reduced chances of major depressive disorder and other negative mental health outcomes (Lyall et al., 2018). Saeb et al. (2015) determined that circadian movement (regularity in 24-h patterns), mobility between favorite locations and location variance were all negatively correlated with depressive symptoms, while phone usage was positively correlated with depressive symptoms. Using smartphone passive sensing, distance between locations visited and a routine index, or the reliability of the locations visited on a day-to-day basis were related to depressive symptoms (Canzian and Musolesi, 2015). Links between features such as location category (home, car, office etc.) and depression, with further accuracy in prediction when adding context, such as if the individual is alone, with other people (particularly friends) or current physical exertion status (Burns et al., 2011). Self-reported happiness has been linked to decreased phone usage in the subsequent hour (Mehrotra et al., 2017). While several groups have started to characterize traits linked to depression, phone usage and circadian rhythms are the ones that are most prominent in the current literature.
Resting-State Functional Connectivity
Blood-oxygenation-level dependent (BOLD) functional magnetic resonance imaging (fMRI) is a non-invasive way to study activity in the human brain. Changes in BOLD signal are highly correlated with changes in neuronal activity in the local area, particularly local field potentials (Logothetis et al., 2001). RSFC measures the relationship between the time-courses of different regions, often by using the correlation of the time-series. While connectivity across the whole brain, or “functional connectome” is fairly similar across individuals, there are small individual differences in connectivity between individuals which can be reliably observed across time. There are a variety of factors which may potentially influence RSFC, including genetics, experiences across the lifetime and current physiological and emotional state (Shehzad et al., 2009; Birn et al., 2013; Patriat et al., 2013; Zuo et al., 2014; Poldrack et al., 2015; Richiardi et al., 2015; Sinclair et al., 2015).
Depression and Neuroimaging
Resting-state functional connectivity has been used successfully to distinguish between healthy controls and depressed individuals, even going so far as to distinguish between subtypes of depressed individuals (Greicius et al., 2007; Berman et al., 2013; Kaiser et al., 2015; Drysdale et al., 2016). Task-based studies of self-referential processing have revealed that the sgCC is preferentially involved in processing valenced self-referential information (Moran et al., 2006; Somerville et al., 2006). Additionally, this region has been associated with antidepressant treatment response, and an area proximal to this has been used as a site of deep-brain stimulation for treatment-resistant depression (Mayberg et al., 2005; Holtzheimer, 2012).
Combing RSFC and Mobile Smartphone Passive-Sensing Technology
There are a wide-variety of approaches that can be taken when combining high-dimensional data from multiple modalities. We wanted to answer the following question: do smartphone sensing features previously identified as being related to depression show correlations with RSFC from a region previously identified to have aberrant connectivity in depressed individuals? A targeted approach was used, selecting screen time with mobile smartphone (e.g., unlock duration), a feature previously shown to be linked to depressive symptoms (Saeb et al., 2015; Twenge et al., 2018; Wang et al., 2018) and a brain area, the subgenual cingulate cortex (sgCC) which has previously been identified as having aberrant RSFC in depressed individuals, and more recently has been used as a target for deep brain stimulation for treatment resistant depression (Mayberg et al., 2005; Greicius et al., 2007; Holtzheimer, 2012). Furthermore, if there are regions identified in the passive-sensing unlock duration analysis and RSFC analysis, do these regions also show similar connectivity patterns when looking at the same correlations with brief surveys of self-reported depressive symptoms (PHQ-2, 4 and 8)? We expect that they would. Alternatively, depression may be a summation of multiple factors and may be better understood by interrogating passive-sensing mobile technology and neuroimaging than self-reported scales. As a secondary analysis, other passive-sensing features similar to those previously reported by other groups to be indicative of depression were explored, specifically, circadian rhythms in both movement and number of locations visited. Keeping within the RDoC matrix, a variety of units of analysis including brain connectivity with fMRI (physiological), passive-sensing of phone usage (behavioral) and both computer-based and phone-based depression scales (self-report) were assessed.
Materials and Methods
Study Design
In the current study two separate cohorts of first-year undergraduate students were enrolled and analyzed separately for test-retest comparison. Individuals were enrolled in three study components: neuroimaging, smartphone sensing/EMA and online surveys. Three modified versions of the PHQ-9 were used: PHQ-2/4/8. PHQ-8 is the same as PHQ-9 with the suicide ideation question removed. This question was removed before administration because the survey results are not monitored in real-time. PHQ-4 is a four-question survey which includes two questions from the PHQ-8 and two from the GAD-7 as to assess both depressive and anxiety related symptoms (Kroenke et al., 2009a,b). They are used because of their brief form. They may miss some of the nuances that the other inventories pick up on but have been found to have high internal reliability (Cronbach’s Alpha > 0.8) and are correlated with diagnoses of clinically relevant depression (Cameron et al., 2008; Khubchandani et al., 2016). PHQ-2 is used as a super-brief form of the PHQ-8 that is slightly more specific to depressive symptoms by excluding the GAD-related questions (Arroll et al., 2010).
Individuals completed an online survey to assess study eligibility (safe for MRI per Dartmouth Brain Imaging Center guidelines, no contraindications that would lead to MRI signal loss, and owned an Android or iOS smartphone compatible with StudentLife). If an individual was eligible and interested in participating in the study, she or he completed a battery of online surveys, including the PHQ-8 through REDCap (Harris et al., 2009). Individuals were then scanned during the academic term and had the StudentLife application (Wang et al., 2014) installed on their phone at or near the time of scanning. In Cohort 1, StudentLife data was collected from the time of scanning until the end of the term. In Cohort 2, StudentLife data was collected from the time of scanning and data collection is currently ongoing but the data presented here is only from their first term in college.
StudentLife
A smartphone application, StudentLife is used in the current study to collect a variety of data about smartphone usage and mood from participants. The application is installed on a participant’s phone (iOS or Android) and collects data from the GPS, microphone, accelerometer and lock/unlock status among others. Data from StudentLife is uploaded to a secure server whenever a participant is both using WiFi and charging their phone, which they were encouraged to do daily. Data from these sensors are processed on the server to create variables that assesses the day-to-day and week-by-week impact of workload on stress, sleep, activity, mood, sociability, mental well-being, and academic performance of students (Wang et al., 2014). The workflow of the current study includes data collected through StudentLife, MRI scanning sessions and self-reported surveys (Figure 1). Unlock duration is a measurement of time that the phone is unlocked and the screen is on, calculated as the time between the user unlocking the phone and the user either manually relocking the phone or autolocking due to disuse (iOS default of 30 seconds, Android default vary by manufacturer). Notification and system services do not influence the measurement of unlock duration. While not an absolute measurement of phone usage it is the closest approximation implemented in StudentLife. In Cohort 1, unlock duration (phone usage) was continually sampled, providing coverage 100% of the time. This was decreased in Cohort 2 to help conserve battery usage. In Cohort 2, phones were remotely triggered every 10 min, sampling 1 min every 10 min period (minimum 10% temporal coverage), unless conversation was detected during the 1-min sampling period, in which case sampling was extended up to 3 min for a maximum of 30% temporal coverage.
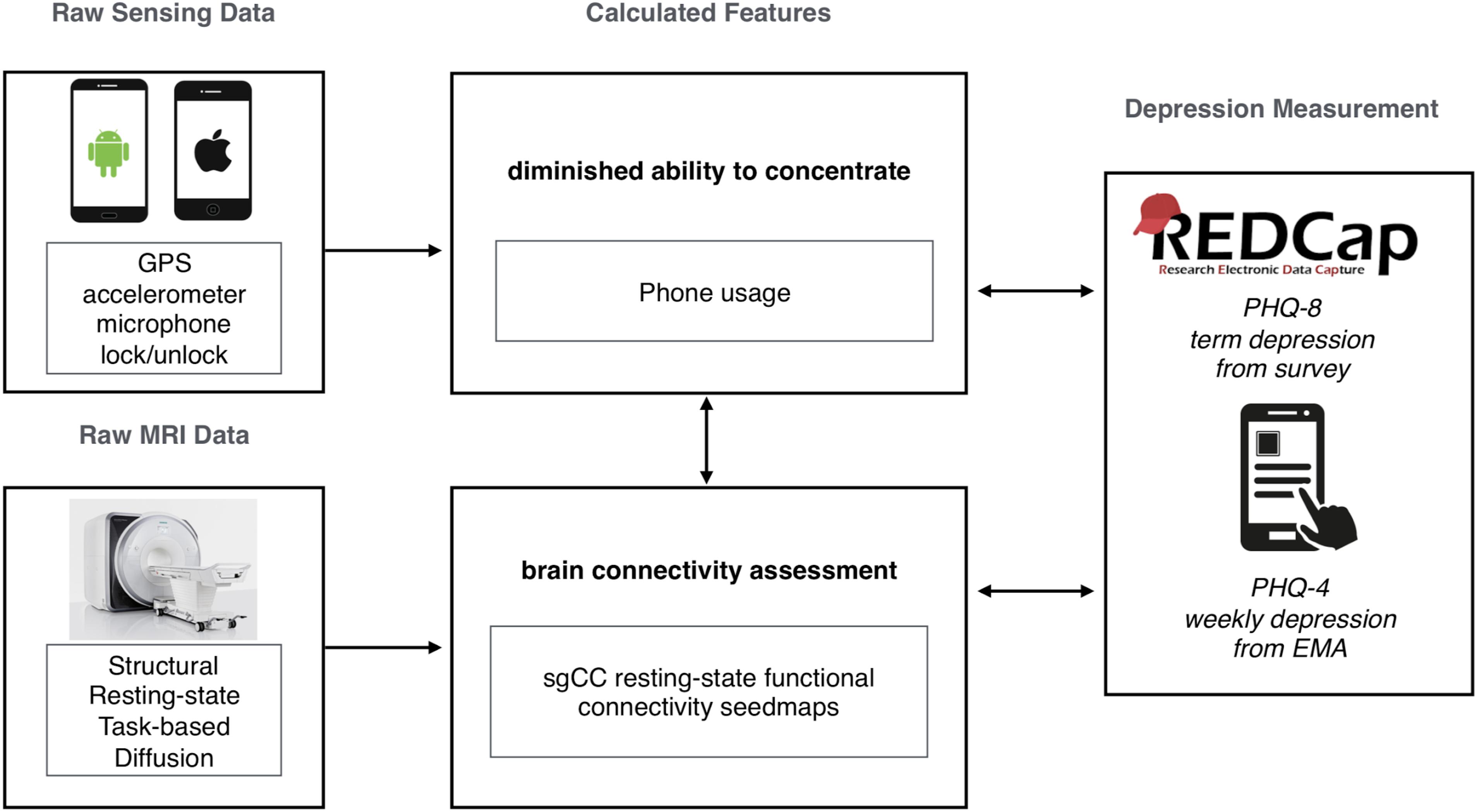
Figure 1. Summary graphic of the study workflow in the current study, showing raw data collection from both smartphones (StudentLife, passive sensing) and MRI (resting-state functional connectivity, sgCC seed-based analysis). Calculated features were selected based on previous research. Survey data was collected with both online (REDCap, PHQ-8) and smartphone (StudentLife, Ecological Momentary Assessments, PHQ-2/4) sources.
Ecological Momentary Assessments
Students were prompted once a week within the StudentLife application during the term to complete a few short surveys as Ecological Momentary Assessments EMA, one of which was PHQ-4 (Shiffman et al., 2008). In the current study PHQ-4 was collected weekly as an EMA PHQ-4 is a modified, shorter version of the PHQ-8 which in four questions provides a glimpse of depressive and anxious symptoms (two questions related to each, with the two depression questions comprising the PHQ-2).
Calculation of Circadian Similarity
As part of the StudentLife app, many feature estimates are calculated for each of the following time-epochs: 9 am – 6 pm (day), 6 pm – 12 am (evening), 12 am – 9 am (night). Accordingly, the relative occurrences of behaviors within each epoch can be estimated and analyzed alongside their daily totals as features. Similarity of day-to-day variation in these feature values across these three time periods were calculated using intra-class correlation, or ICC (Shrout and Fleiss, 1979) which was slightly modified to still run with missing values, by changing mean and summation operations to the equivalent NaN operator in MATLAB. Only individuals with more than 20 days of data for a given feature were included.
Several motion features such as time spent walking, biking, running, or in car are calculated, there is some variance in how they are calculated between Android and iOS. The feature with the most similarity across platforms, which allows for the retention of the greatest number of subjects is the feature “time still,” which is a relatively simple metric which is calculated by how much time the phone is still or not moving. This was broken into three time-epochs as mentioned above and the similarity of activity cycles (or lack thereof) across days was calculated using ICC and termed Circadian Stillness Similarity.
Previous research has focused on frequency of visits to known places and the interaction with depression. Within the constraints of the currently processed data, these features could not be calculated exactly, but instead the number of unique locations visited during each time-epoch was calculated (Wang et al., 2014). The reliability of how many locations a person visited through the three epochs each day was calculated with ICC and termed Circadian Location Number Similarity.
Subjects
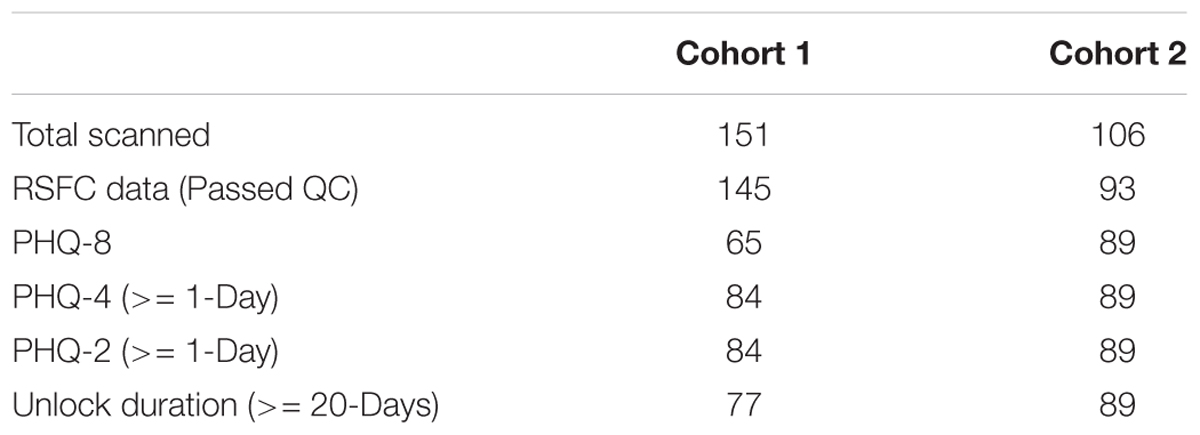
Subjects were first-year undergraduate students recruited from the Dartmouth College community. Cohort 1 included 151 subjects (94 female, mean age = 19.59, std = 1.69, range = 18–28) which were all scanned during their first year at Dartmouth and followed for the subsequent academic term. Cohort 2 included 106 subjects (75 female, mean age = 18.25, std = 0.63, range = 18–22) which were all scanned during the first academic term of their first year at Dartmouth. In Cohort 2, one subject was removed from the study for having an incompatible phone and one MRI session was stopped due to not reporting a permanent top retainer.
See Table 1 for a summary of the number of individuals included in each analysis, grouped by Cohort. Subjects were only included in each analysis if they met the minimum number of time-points for smartphone-based StudentLife data and each analysis and had RSFC that passed quality control (see RSFC analysis methods section below for further details). Subjects had normal or corrected-to-normal visual acuity. The Committee for the Protection of Human Subjects at Dartmouth College approved this study. Each subject provided written informed consent in accordance with guidelines set by the above-mentioned committee and received either course credit or monetary compensation for participating in the study.
RSFC Data Collection
Apparatus
Cohort 1 imaging was performed on a Philips Intera Achieva 3-Tesla scanner (Philips Medical Systems, Bothell, WA, United States). Cohort 2 imaging was performed on a Siemens MAGNETOM Prisma 3-Tesla scanner (Siemens Medical Solutions, Malvern, PA, United States). Data for both cohorts was collected using a 32-channel phased array head coil. During scanning, participants viewed a white fixation cross on a black background projected on a screen positioned at the head end of the scanner bore, which participants viewed through a mirror mounted on top of the head coil.
Cohort 1 Imaging
Anatomic images were acquired using a high-resolution 3-D magnetization-prepared rapid gradient echo sequence (MP-RAGE; 160 sagittal slices; TE, 4.6 ms; TR, 9.9 ms; flip angle, 8°; voxel size, 1 × 1 × 1 mm). Resting-state functional images were collected using T2∗-weighted fast field echo, echo planar functional imaging sensitive to BOLD contrast (TR = 2500 ms; TE = 35 ms; flip angle = 90°; 3 × 3 mm in-plane resolution; sense factor of 2). Functional scanning was performed in one or two runs; during each run, 240 brain volumes (36 slices, 3.5 mm slice thickness, 0.5 mm skip between slices) were acquired, allowing complete brain coverage. As such, each participant completed between 10 and 20 min of RSFC scanning.
Cohort 2 Imaging
Anatomic images were acquired using a high-resolution 3-D magnetization-prepared rapid gradient echo sequence (MP-RAGE; 192 sagittal slices; TE, 2.32 ms; TR, 2300 ms; flip angle, 8°; voxel size, 1 × 1 × 1 mm) with a Grappa 2 acceleration factor. Resting-state functional images were collected using T2∗-weighted fast field echo, echo planar functional imaging sensitive to BOLD contrast (TR = 1190 ms; TE = 32 ms; flip angle = 63°; 2.4 × 2.4 mm in-plane resolution; SMS factor of 4). Functional scanning was performed in one or two runs; during each run, 605 volumes (46 slices, 3 mm slice thickness, no skip between slices) were acquired, allowing complete brain coverage. As such, each participant completed 12 or 24 min of RSFC scanning. Initial data acquisition and conversion to BIDS for cohort 2 was facilitated by the ReproIn specification and tools (ReproNim project NIH-NIBIB P41 EB019936) and organized into BIDS format with datalad (Gorgolewski et al., 2016; Halchenko et al., 2017).
RSFC Analyses
All processing was performed using a standard previously published processing stream (Power et al., 2014) with two exceptions: frame-displacement (FD) threshold was set to 0.25 mm (instead of 0.2 mm) and 36 motion parameters (instead of 24) were used for motion regression. Functional images were preprocessed to reduce artifacts, including: (i) slice-timing correction, (ii) rigid body realignment to correct for head movement within and across runs, (iii) within-run intensity normalization such that the intensity of all voxels and volumes achieved a mode value of 1000 scale with 10 units equal to ∼1% signal change, (iv) transformation to a standardized atlas space (3 mm isotropic voxels) based on (Talairach and Tournoux, 1988), (v) frame censoring, (vi) nuisance regression (excluding censored frames), (vii) interpolation, and (viii) bandpass filtering (0.009 < f < 0.08Hz) following Power et al. (2014) and using exactly the same processing stream as Huckins et al. (2019). Final correlation calculations between time-courses were calculated based upon uncensored frames. Preprocessing steps i-v were completed using custom scripts which call 4dfp Tools1. Steps specific to resting-state functional-connectivity processing (vi-x) were completed using custom MATLAB (Version R2012b, by MathWorks, Natick, MA, United States) scripts.
Nuisance Regressors
To control for motion, a Volterra expansion (Friston et al., 1996) with 36 motion parameters was used. This expansion includes motion, motion squared, motion at the previous two frames, and motion in the previous two frames squared. Tissue-based nuisance regressors were calculated by taking the mean signal across voxels within each of the following individual masks from FreeSurfer2 (Dale et al., 1999; Desikan et al., 2006): an eroded (up to 4x) ventricular mask for the cerebrospinal fluid, an eroded white matter mask for the white matter signal, and a whole-brain mask for global signal. When eroded masks included no voxels, lesser erosions were progressive considered until a mask with qualifying voxels was identified. This occurred infrequently for white-matter masks while erosions of 1 were often used for CSF masks. The first derivative for each tissue regressor, as calculated by the difference from the current from to the previous frame, was also included.
Volume Censoring and Data Retention
Movement of the head from one volume to the next (FD) was calculated by the sum of the absolute values of the differentiated realignment values (x, y, z, pitch, roll, yaw) at each time-point (Power et al., 2012). A frame displacement threshold of 0.25mm was used. Volumes with motion above the frame displacement threshold were identified and replaced after multiple regressions but prior to frequency filtering. Spectral decomposition of the uncensored data was performed and used to reconstitute (stage vii: interpolation) data at censored time-points. The frequency content of uncensored data was calculated with a least squares spectral analyses for non-uniformly sampled data (Mathias et al., 2004) based upon the Lomb-Scargle periodogram (Lomb, 1976). Segments of data with less than 5 contiguous volumes below the FD threshold were flagged for censoring. Functional runs were only included in the final analysis if the run contained 50 or more uncensored frames. Only subjects with at least 5 min of uncensored data across runs were included in the current study. Consistent with Power et al. (2014), only uncensored volumes were used when calculating temporal correlations.
Neurosynth Analysis and Subgenual Cingulate Cortex Seedmaps
To identify an unbiased sgCC seed to create voxelwise functional seed maps, an automated meta-analysis was performed using Neurosynth for the term “subgenual” (Yarkoni et al., 2011). sgCC seed maps were created from a 4mm spherical seed placed at 0, 25, -10 (MNI coordinates), which was the peak of the term “subgenual” as of February 17th, 2017 and are centered around BA 25. The mean time-course from this seed was correlated with the time-course from every voxel within the brain. These seed maps, i.e., maps of resting-state connectivity from the subgenual region, were produced for each individual that passed quality control (more than 5 min of uncensored frames, see above for more details).
Combining Data
Since the version of the StudentLife application used in the current study generates 182 features automatically, and with RSFC it is possible to generate thousands of features, it is necessary to minimize the number of features compared given the relatively small size of the Cohorts (N < 100). To minimize the number of features inspected, unlock duration was the only feature inspected given its simplicity to calculate and previously identified relationship with PHQ-8 (Wang et al., 2018). While many features were automatically calculated, unlock duration (e.g., screen time) was first targeted as a simple feature both to calculate and to conceptualize as it can be considered a proxy for total phone screen time.
For all surveys analyzed here, one time-point was sufficient for a subject to be included in the current analyses. If there were multiple responses to ecological momentary assessments (EMAs, e.g., surveys prompted by the application) over the course of the term those responses were averaged. Individuals were included in the passive sensing unlock duration analysis if they had 20 days of quality data with more than 16 h of quality unlock duration data for each day that was included.
Group Analyses and Statistics
Subgenual cingulate cortex seedmaps from Cohort 1 were correlated with unlock duration sampled from smartphone usage with the StudentLife application. For each analysis, the degrees of freedom was N-2, with N being the number of subjects which is listed in Table 1. Results from the unlock duration and sgCC correlational analysis from Cohort 1 were volume corrected to account for multiple comparisons using AFNI’s 3dClustSim ACF function. Results from the sgCC/unlock duration analysis were used to restrict the regions investigated in further analyses. Given the proof-of-concept and exploratory nature of the current work, clusters are marked as having passed volume-correction or not.
Visualization
All results were transformed into MNI space (Montreal Neurological Institute) and mapped onto the Conte69 template for volume-based slices or inflated surfaces for visualization (Van Essen et al., 2012). Group results were visualized in Connectome Workbench Version 1.1.1 (Marcus et al., 2010).
Results
Self-Reported Depression Measures
Depression symptomatology severity was assessed pre-scan with an online survey using PHQ-8 and during the term using the StudentLife application to administer the PHQ-4 (which contains the PHQ-2). PHQ-8 distributions were similar between Cohort 1 and Cohort 2 (mean = 4.77, 4.52; SEM = 0.58, 0.47, respectively). Depression severity (as categorized by Kroenke et al., 2001) revealed that in both Cohorts a large portion of individuals had minimal depressive symptoms (56.9 and 62.9%, respectively), leaving roughly 40 percent of individuals with a range of depressive symptoms (Supplementary Table S1). PHQ-4 distributions where also similar between Cohort 1 and Cohort 2 (mean = 2.52, 2.09; SEM = 0.24, 0.18, respectively). PHQ-2 distributions where also similar between Cohort 1 and Cohort 2 (mean = 0.77, 0.80; SEM = 0.13, 0.10, respectively). Density figures for all self-reported depression symptoms can be found in the Supplementary Figure S1.
Passive Sensing Features Correlated With sgCC Connectivity
In Cohort 1 exploratory whole-brain analyses of the correlation between unlock duration and sgCC seedmaps identified a large cluster (584 voxels, 15,768mm3) in the ventromedial prefrontal cortex with a positive linear relationship (Figure 2 and Supplementary Figure S2A). This cluster extended from the ventral striatum to medial frontal orbitofrontal cortex (OFC) and dorsally to medial prefrontal cortex. Information about subpeaks within this cluster can be found in Table 2. To determine if these results replicated in Cohort 2, the cluster identified in Cohort 1 was used as a mask and voxels which showed a significant positive relationship between unlock duration and sgCC connectivity in Cohort 2 were identified. This analysis identified a cluster with the peak located at -6, 51, -18 (MNI coordinates, peak T = 2.94, voxel extent = 42, volume-corrected to p < 0.05) (Supplementary Figure S2B).
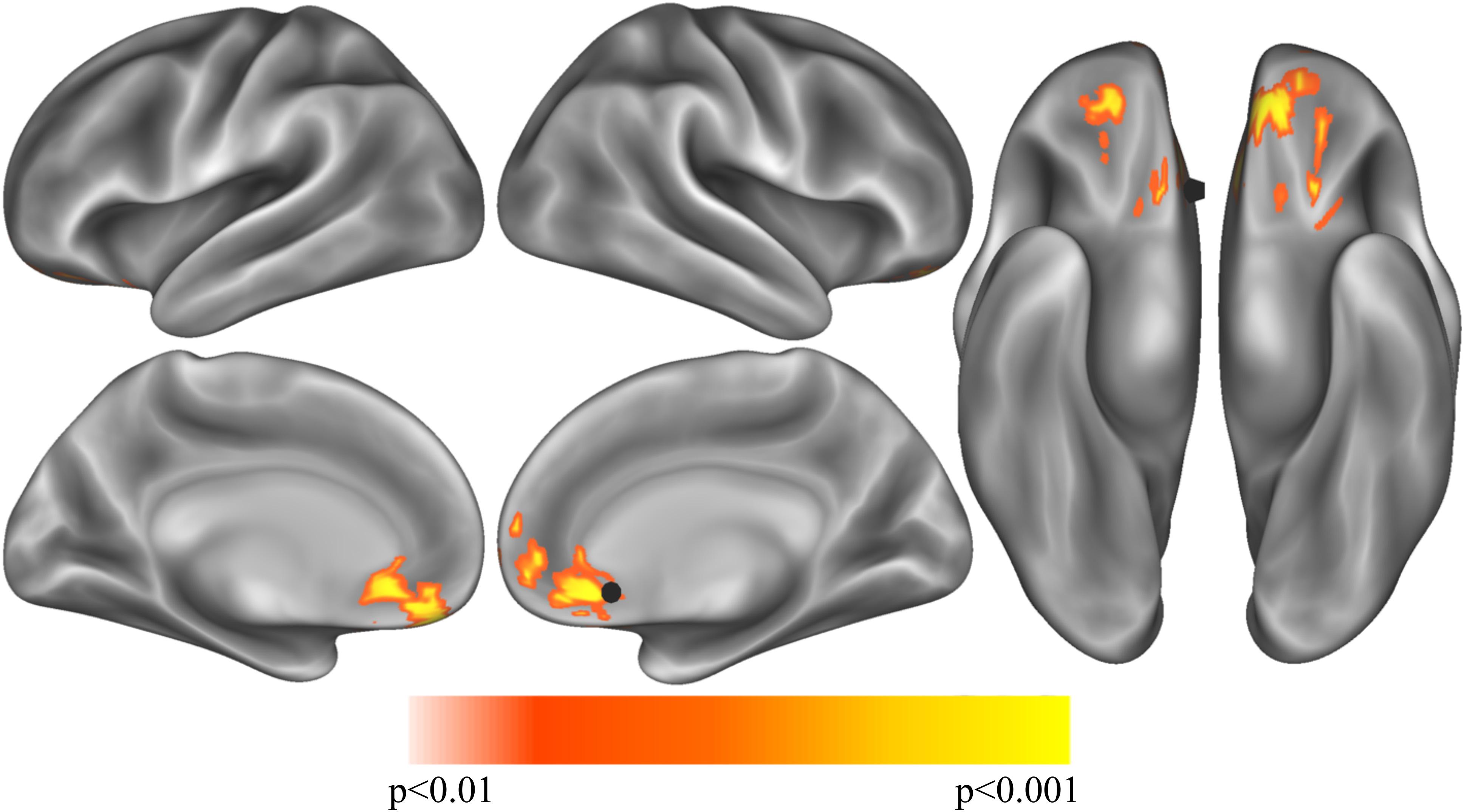
Figure 2. Exploratory analysis correlation sgCC RSFC seedmaps correlated with mean unlock duration identified a cluster with a positive relationship to unlock duration in the ventromedial prefrontal cortex (p < 0.01, volume corrected using ACF to p < 0.001) shown on inflated lateral (top left), medial (bottom left) and ventral (right) cortical surfaces. The sgCC seed is represented as a black 10 mm sphere, larger than the 4 mm sphere used to create the seedmaps for visualization purposes.
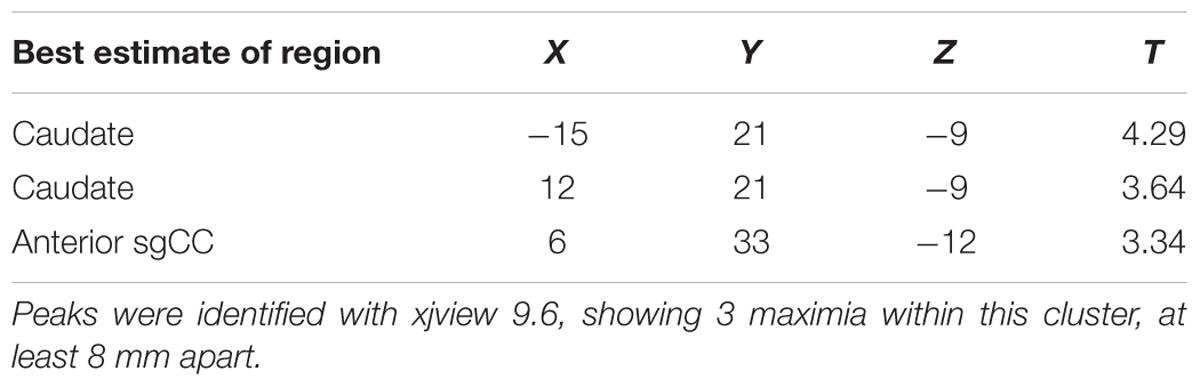
Table 2. Exploratory analysis correlation sgCC RSFC seedmaps correlated with mean unlock duration (smartphone screen time) identified one cluster in the ventromedial prefrontal cortex (p < 0.01, volume corrected using AFNI’s ACF to p < 0.001, k > 449, voxel extent = 548).
Two features estimating the reliability of day-to-day activity patterns, including phone motion measured as how long the phone is still at three different time epochs throughout the day and the number of locations an individual visits per time epoch were subsequently analyzed. Circadian Stillness Similarity derived from phone stillness across the three daily time-epochs did not identify any significant regions in Cohort 1 (N = 77) after volume-correction within the prefrontal mask from unlock duration used in other analyses in the main text. Cohort 2 (N = 89) did, however, identify a small cluster (MNI = 12, 45, -12; t = 2.73; 31 voxels) in right medial OFC which was negatively correlated with circadian similarity. In other words, individuals with daily movements patterns that were more similar had less connectivity between sgCC and medial OFC. Similar results were observed for Circadian Location Number Similarity, where no clusters passed volume correction in Cohort 1, but a small cluster (MNI = -9, 45, -12; t = -2.54; 31 voxels) was found in left medial OFC (not shown given similarity with Supplementary Figure S5). Between the two analyses there were 7 voxels which overlapped.
Self-Reported Depression Symptoms Correlated With sgCC Connectivity
Previous research (Wang et al., 2018) identified a relationship between depressive symptoms and unlock duration. To determine if depressive symptoms and unlock duration had overlap in the brain connectivity (seed based subgenual RSFC) regressions for both computer-based pre-screening (PHQ-8), phone based post-scanning (PHQ-2/4 as EMA) were performed. Results from each of these analyses were masked with the cluster identified in Cohort 1’s sgCC/unlock duration analysis.
PHQ-8 computer-based surveys correlated with sgCC connectivity maps identified clusters with a positive relationship with sgCC connectivity in both Cohorts and identified a cluster which overlapped between the two. Cohort 1 revealed one cluster at which passed volume-correction -21, 42, -12 (peak T = 3.19, voxel extent = 63, volume corrected to p < 0.05), 24, 51, -9 (peak T = 2.55, voxel extent = 15, did not pass volume correction) (Figure 3 and Table 3). In the PHQ-8 analysis of Cohort 2, results were further masked by the cluster which passed volume-correction in the Cohort 1 PHQ-8 analysis (63 voxels), identifying 1 significant cluster in Cohort 2, located at -15, 33, -12 (peak T = 2.98, voxel extent = 8, volume corrected to p < 0.05). In addition to identifying a cluster with overlap between the both Cohorts for the PHQ-8 analysis, qualitative visual inspection suggests proximal cortical regions in both cohorts meeting a voxelwise threshold of p < 0.05, with regions proximal to the mask having overlap at a threshold of p < 0.05 and increased overlap, including right OFC at a more liberal threshold of p < 0.1.
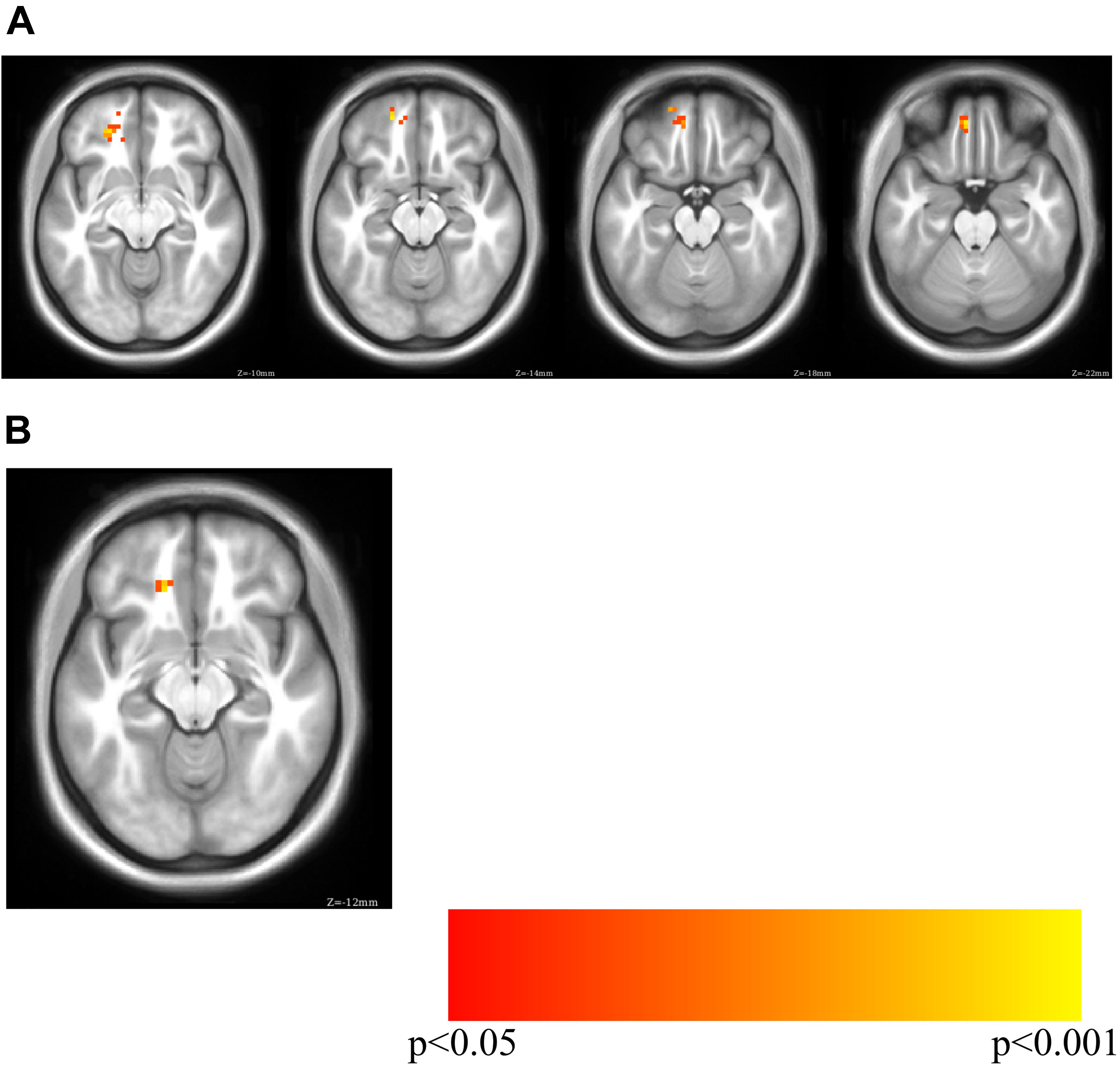
Figure 3. PHQ-8 regression for sgCC connectivity seedmaps for (A) Cohort 1 (MNI Z of –10 to –22 in steps of 4) and (B) overlap between Cohort 1 and Cohort 2 (MNI Z of –12). Cohort 1 PHQ-8 results were masked with the volume-corrected cluster identified in the Cohort 1 phone usage analysis (unlock duration) and Cohort 2 PHQ-8 results were masked with the PHQ-8 results from Cohort 1.
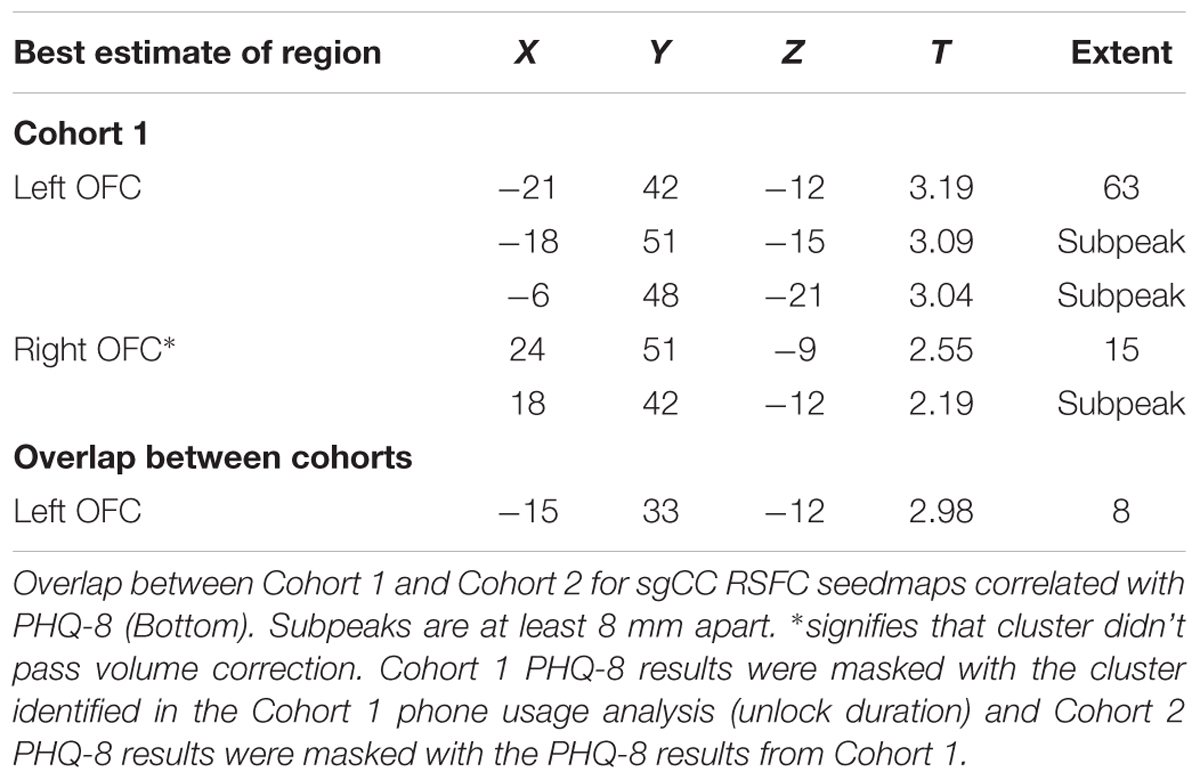
Table 3. Results for the correlation of sgCC RSFC seedmaps with PHQ-8, masked by phone screen time results.
PHQ-4 EMAs correlated with sgCC connectivity maps identified peaks in Cohort 1 and 2, but there was no overlap in the clusters between the Cohorts (Supplementary Figure S3 and Table S2). In Cohort 1 no significant clusters were identified when PHQ-4 was masked with Cohort 1 unlock duration. As Cohort 1 didn’t identify any regions which passed volume-correction, there was no overlap of significant volume-corrected regions between Cohort 1 and Cohort 2 for PHQ-2 (Supplementary Figure S4). As such, Cohort 2 results were masked with the Cohort 1 unlock duration cluster which identified one significant cluster with the peak at -15, 30, -12 (peak T = 3.71, voxel extent = 41, volume corrected to p < 0.05). Two clusters were identified that didn’t pass volume correction were also identified at -9, 51, -18 (peak T = 2.87, voxel extent = 28, volume correction ns) and 24, 39, -15 (peak T = 1.87, voxel extent = 9, volume correction ns).
PHQ-4 includes two anxiety questions, so the subsequent analysis was restricted to the two questions related to depressive symptoms which comprise the PHQ-2. As Cohort 1 didn’t identify any regions which passed volume-correction, there was no overlap of significant volume-corrected regions between Cohort 1 and Cohort 2 for PHQ-2. As such, Cohort 2 results were masked with the Cohort 1 Unlock Duration cluster which identified 1 cluster which passed volume correction, with the peak at -18, 30, -12 (peak T = 3.81, voxel extent = 60, volume corrected to p < 0.05). One cluster was identified that didn’t pass volume correction with peak at -9, 42, -27 (peak T = 2.93, voxel extent = 40, ns).
Overlap Across Analyses
Given the similarity of regions found across the PHQ analyses in Cohort 2, the overlap between the results of PHQ 2/4 masked by the Cohort 1 unlock duration was investigated, with 39 voxels out of the 41 voxels identified in the PHQ-2 analysis overlapping with the PHQ-4 analysis. The overlap between Cohort 2 PHQ-2, 4 and 8 identified 11 voxels, which are located around the peaks of the PHQ-8 analysis.
Discussion
The current manuscript is provided as a proof-of-concept example of how passive smartphone metrics, active smartphone-based surveys of mental health and computer-based surveys of mental health with brain connectivity measures can be linked. Specifically, RSFC between the subgenual cingulate cortex, a region previously implicated in depression, and nearby ventral prefrontal regions, was strongly related to unlock duration, such that more connectivity was associated with more screen time, which has been implicated as being related to self-reported depressive symptoms. The link between RSFC and individual differences has long been established but extending that and combining it with an individual’s behavior inferred from smartphone sensors provides exciting new directions. While the results presented here are a relatively simple analysis of complex, highly dimensional data, methods are discussed which could be used in the future to combine these highly multivariate and complex datasets in exciting ways.
Phone-related screen time, defined here as the amount of time a phone is unlocked, or unlock duration, has previously been shown to be related to self-reported depression levels (Twenge et al., 2018; Wang et al., 2018). An exploratory analysis in Cohort 1 of the correlation between unlock duration and sgCC seedmaps identified a large cluster which extended from the anterior caudate to medial frontal OFC and dorsally to medial prefrontal cortex, a result which was replicated in Cohort 2 with a smaller voxel extent, even though the sampling rate for screen time was greatly reduced, reducing our sensitivity to pick up individual differences in phone usage for this cohort. Next, to determine if depressive symptoms showed a similar pattern of connectivity between sgCC and ventral prefrontal cortex the cluster from Cohort 1’s unlock duration analysis was used as a mask with PHQ-8, a commonly used survey to assess depressive symptoms in the general population. Two small clusters of overlap were identified in the left OFC, one of them neighboring voxels that were identified to replicate in the unlock duration analysis between the Cohorts. While these clusters are not large and would not necessarily survive volume correction on their own, observing similar regions across Cohorts and analyses suggests that there is a link between depressive symptoms and related behaviors and sgCC-OFC connectivity, particularly left OFC that should be further investigated. The PHQ-4, which contains two depression questions and two anxiety questions, did not show the same robust relationship across both Cohorts, with no voxels overlapping, although Cohort 2 identified a cluster in the left OFC which overlapped with results observed with PHQ-8 in both Cohorts. Connectivity between the sgCC seed (BA 25), located at 0, 25, -10 and the left OFC region around -15, 33, -12 shows a consistent relationship between self-reported depressive symptoms and screen time, which has previously been associated with depression. Increased connectivity between sgCC, a region involved in processing of valenced information about the self (Moran et al., 2006) and OFC, which is involved in valuation and reward processing has been linked increased depressive symptoms and screen time across both Cohorts. Similar results were observed with PHQ-2, which only contains the two questions directly related to mood. It seems quite plausible that regions involved in valence processing related to the concept of self and a more general reward valuation processing region would have increased connectivity in individuals with higher depressive symptoms.
Individuals in Cohort 2 with daily movement routines which were more similar from day-to-day exhibited less connectivity between sgCC and medial OFC. This is the opposite direction of a correlation that unlock duration and PHQ depression surveys identified, which is expected in light of results by Lyall et al. (2018), where individuals that exhibited activity patterns with reliable rest/activity cycles were less likely to be depressed. Similarly, individuals with more similarity in locations visited, meaning consistent day-to-day schedules had less connectivity between sgCC and medial OFC, which in the current study is associated with lower depression levels. The current work used very large time epochs and could be investigated in more depth with future modifications to the StudentLife application and feature generation pipeline to perform finer grained analyses. Similarly, extending StudentLife to calculate frequently visited locations such as Burns et al. (2011) could prove fruitful. In summary, in the current dataset the regularity in the number of locations visited (as measured by GPS) and regularity in the time that the phone is not moving are both negatively correlated with connectivity between the sgCC and medial OFC.
We have shown that RSFC of the brain, as measured with MRI, in two separate Cohorts of individuals, with two separate MRI’s and two separate versions of the StudentLife application and three separate passive-sensing feature show similarity in the results observed. The cluster identified with the unlock duration analysis covered an extent similar to that of the limbic network previously identified (Yeo et al., 2011; Choi et al., 2012). Due to the constraints we imposed on the analysis, all of the subsequent results were within this area, but noticeably, many of the results were proximal to the left OFC, which is also a member of a set of nodes which are commonly activated during reward processing and can form their own preferentially coupled system (Huckins et al., 2018) and is identified as a peak of the term “reward” in reverse-inference meta-analyses using Neurosynth (Yarkoni et al., 2011).
Limitations and Future Directions
The current work is a first-pass at analyzing longitudinal multi-cohort, multimodality data and has several limitations. There are several ways in which future research may provide a more comprehensive survey of the relationships between the diverse set of features provided from passive smartphone sensing, functional brain connectivity measures and self-reported measures of depression or other mental health metrics. The relatively small number of clinically depressed individual in the current sample weighs the results heavily on the RSFC and passive-sensing features from those individuals. Test-retest within the moderately sized samples allows for identification of factors with reliable cross-cohort replicability in RSFC both and passive-sensing features. Ideally, similar sensing features could be collected across many sites, allow for identification and characterization of depressive subtypes that span across passive-sensing and RSFC as has been done by Drysdale et al. (2016) with RSFC and survey data. Diagnosis of depression by neuroimaging techniques such as RSFC MRI could potentially be cost prohibitive in a medical setting. With that said, the medical costs associated with untreated depression accounts for $26.1 billion per year with a total economic loss about $83 billion in just the United States alone (Greenberg et al., 2003). As noted in the current Cohorts, roughly 40 percent of participants had mild depressive symptoms or worse as measured.
In the current study, particularly Cohort 2 in which data quality was actively monitored, a relatively large portion of individuals from those scanned was retained (see Table 1). The sample sizes used here would have been considered relatively large several years ago. Increased sample sizes in the current study would help future analyses given the large number of features from both passive mobile smartphone sensing and RSFC. An outstanding question is if long-term changes in depressive symptoms can be better predicted by RSFC or smartphone sensing metrics at the initiation of the study or if changes in either of these over time parallel depressive symptoms. Ideally to assess this a large number of individuals would be tracked over multiple years. In the second Cohort our working group aims to track them over multiple years while eventually increasing the number of individuals enrolled. Furthermore, including multiple sites, as the ABCD study does (Volkow et al., 2017), would increase applicability to a wider population. Multiple research sites are currently collecting MRI data, self-reported surveys and smartphone sensing metrics. An unresolved issue is what, exactly, is the optimal approach to analyze the huge amounts of multivariate data produced by these methods.
Application Changes Between Cohorts
In the current study, unlock duration data collection changed between the cohorts. In Cohort 1, unlock duration was continually sampled, while in Cohort 2 unlock duration was adaptively sampled between 10 and 30% of the time. This change was instituted to optimize battery life, a primary limitation to users being willing to keep the StudentLife app on their phone. By decreasing the amount of time sampled from 100% to 10–30%, our ability to accurately estimate unlock duration may decrease slightly as evidenced by an observed decrease in peak effect (T-value) and voxel extent. As with all passive and active smartphone features, the ability to collect data must be weighed against the invasiveness to the user experience, either through app prompts or decreased battery life and phone speed.
Feature Selection and Calculation
In the current study, initial analyses focused on unlock duration as a proxy for general phone usage then investigated the similarity of individuals circadian rhythms from day to day and how each of these was related to brain connectivity from a region known to be involved in depression and many cognitive functions. Unlock duration on its own in very unlikely to be an optimal feature to predict depression and this is where generating and testing a variety of higher-level features may prove fruitful. Identifying changes in features from day-to-day or week-to-week may increase predictability, such as an increase in unlock duration could be associated with increases in depression within an individual (Wang et al., 2018). Variability or stability of passive-sensing features may also be able to predict individual differences in depression. Ideally, a template of passive-sensing features for non-depressed individuals could be created and deviance from this template could be calculated as a sort of depression-index or propensity score. This high-level feature could then be linked to deviance of brain connectivity patterns from non-depressed individuals. Critically, future work should select features that reflect not just phone usage and other standard passive-sensing metrics, but build upon the current sensing literature related to depression (Burns et al., 2011; Canzian and Musolesi, 2015; Saeb et al., 2015; Lyall et al., 2018; Wang et al., 2018) and calculate higher level features which are likely to better reflect nuances in behavioral differences across individuals.
Temporal Factors Related to School
The demands of the academic term provide a generally applicable path of stress which is shaped over the term. Avoiding, or potentially purposefully collecting MRI data during finals, which may be particularly stressful, or during popular social weekends may lead to changes in stress levels, sleep patterns and other variables which could alter connectivity patterns and self-reported behavioral data that would have otherwise been observed. In the study herein, attempts were made to scan before finals and avoid well-known “party weekends.” Future studies may be able to capitalize on temporal differences in stress and depression levels by scanning at these peak times of stress or sleep deprivation and comparing that data to less stressful times, such as the beginning of the term.
Functional Differences and Alignment Across Individuals
Resting-state functional connectivity shows robust and relatively reliable connectivity across large groups of individuals across methods (Yeo et al., 2011; Gordon et al., 2016). Meanwhile there are individual differences in the cortical extent of large-scale functional regions across individuals and even the network membership of these regions can vary (Gordon et al., 2017). Furthermore, critical to identifying group and individual differences is acquiring a large quantity of high-quality data (Gratton et al., 2018). Defining networks on an individual basis will likely help in the pursuit of the individual differences in brain connectivity that underlie depression. Variability in RSFC has been observed at the functional parcel level, but what about at finer resolutions? While a departure of traditional anatomical alignment methods, hyperalignment is a method which attempts to align brain based on similar response patterns in high-dimensional space (Guntupalli et al., 2016). While this method originated using time-locked dynamic stimuli such as a movie, it has recently been applied to RSFC as connectivity hyperalignment (CHA), which revealed both coarse-scale, areal structure as previously observed, along with fine-scale structure which was previously inaccessible. Applying CHA to RSFC data will hopefully allow for increased ability to discern individual differences in depression and other mental-health metrics.
Voxelwise Resting-State Functional Connectivity
A relatively simple first-pass method is to target specific region and feature pairs. If there are a priori hypotheses related to the topic of interest it may be possible to look at connectivity from one region using seed maps or between a small number of regions and relate them to specific passive-sensing features. As shown here this is plausible but even correlating seed maps with 1 sensing variable leads to potential multiple comparisons issues based on the 50,000+ voxels in the brain using a 3 mm3 voxel size. Recent statistical simulations have suggested an increased false-positive rate associated with older versions of 3dClustSim, a function of AFNI (Cox et al., 2017). Indeed, the authors of 3dClustSim now suggest using a different algorithm with the same program, the autocorrelation function (ACF) with a high p-value threshold per voxel to minimize the possibility of false-positives. In some datasets, at lower p-value thresholds ACF requires a much larger voxel-extent than the old version of 3dClustSim. The increased voxel-extent may make it less likely to identify smaller functional regions in a whole-brain regression using a lower per-voxel p-value threshold (p < 0.05). This evolution of methods decreases the rate of the false-positives which is critical but requires a larger expected functional region, a very strong effect size or a very large number of participants. Across all possible methods presented here there are a variety of factors which should be taken into consideration to decrease false positive rates. Having a large number of subjects to draw data will increase the portion of the population sampled.
If possible having two distinct Cohorts to analyze then looking for overlap in results between the Cohorts would decrease false positives due to random sampling, Cohort specific variance, and further increase the total size of individuals sampled. The above factors apply to most any study. With passive smartphone mobile sensing there are many features which can be measured or computed based on the intersection of multiple features. For example, “phone unlock duration” is a very simple metric, which measures the time that the smartphone was unlocked. This can be further broken down into location specific features, such as “phone unlock duration at dorm” or “phone unlock duration at study places” by looking at the intersection of location on a geo-tagged campus and “phone unlock duration.” Given the large number of initial features that can be calculated, along with the nearly endless number of meta-features that could potentially be generated, making sure that the feature is relatively straightforward to calculate and interpret should be at the forefront of anyone analyzing passive-mobile phone sensing features. Features that are difficult to calculate or interpret could easily be embedded with unforeseen confounds. Furthermore, such features should be validated to make sure they are measuring the effect or phenomena they are supposed to in an accurate manner.
Typically, only features with sensing data from many days should be used to get a more stable estimate of that features’ value. While putting a sensing application of many students’ phones may seem like a plausible method for maximizing data collect, there are a variety of factors which can lead to reduced data collection, potentially rendering an individual’s sensing data unusable. Phone operating system (OS) updates can often change application permission or render the sensing application completely useless. To avoid this beta testing should be done as early as possible and new versions of the application that are compatible with the latest OS pushed to participants. Participant non-compliance or attrition is another important factor to consider. Individuals may delete the application, limit its permissions within the OS or otherwise limit the researcher’s’ ability to accurately measure data. Clearly, it is the individual’s choice to continue to participate in any study, particularly one where large amounts of data are being collected (anonymously) on their habits. It may be difficult for the researcher to determine if the individual has deleted the application or simply not uploaded their data in while. Finally, a rate of attrition is expected in all longitudinal studies and some individuals may simply decide that they do not wish to continue their participation in the study.
Whole-Brain and Network-Based Connectivity
A possible method to deal with the large number of comparisons related to voxelwise or whole-brain connectivity is to simply look at connectivity between a set of predefined regions or parcellation (Power et al., 2011; Yeo et al., 2011; Poldrack et al., 2015; Gordon et al., 2016; Huckins et al., 2018). Connectivity between each pair of regions can be correlated with the sensing feature of interest. Unfortunately, many of the commonly used parcellations have many nodes, which increases the total number of comparisons in a non-linear manner as the number of nodes increases. The number of comparisons can soon approach the number of comparisons evident when using voxelwise seed maps without methods such as voxel extent to appropriately correct for the associated multiple comparisons.
A simple but perhaps relatively unsophisticated sophisticated method is to calculate mean connectivity within a functional system or network. The system or network would be determined off of data driven approach such community detection using a random walk technique like InfoMap (Rosvall and Bergstrom, 2008) or regions identified as being part of a coherent functional system using another method or even searching Neurosynth.org for a term of interest. In this approach, the mean of all Fisher r-to-z transformed correlation values between nodes of interest is calculated. For example, mean connectivity within the Cingulo-Opercular network would be calculated between all nodes or parcels belonging to that network. Between-network or system connectivity can also be calculated by taking the mean of all pairwise connections between the two networks of interest. This can greatly reduce the number of total connections observed, thus reducing the multiple comparisons problem mentioned under the whole-brain connectivity section. One drawback to this method is that it is not selective about which connections it is using in the calculation – specifically, that it may be and probably is including connections that are not physiologically or psychologically relevant.
A plausible may to reduce the number of connections by selections ones that are likely to be “real,” such that information may actually travel through that connection on the neural level, even if not on a first-order or even second-order synapse. Multiple approaches have been taken to identify meaningful connections. Within or between networks there are likely to be positive and negative correlations, which then somewhat cancel out. One could take the absolute value of each connection before averaging across the network, but this would introduce bias in any connections with a distribution of correlation values that included positive and negative values. Values of correlation, or connectivity measures in the brain vary by orders of magnitude. Identifying a multiscale network backbone that accounts for important connections within and between communities, regardless of the connectivity strength would be a method to decrease the number of connections analyzed. One way of identifying the network backbone is to use the z-value from each connection as the weight, or amount of information that could travel between the two brain regions that the connectivity was estimated from. A group did just this (Serrano et al., 2009), identifying connections which are statistically relevant across multiple scales of connectivity, work which has been extended non-parametrically (Foti et al., 2011). By identifying the network backbone for each individual (Huckins et al., 2019), it may be plausible to identify a variety of subcategories or continuums of depression along which different symptom severities fall for each individual, along with passive smartphone monitoring will allow for greater insight into interactions of behavioral, self-report and physiological RDoC matrix criteria.
Wrangling High Dimensional Data
A variety of techniques can be used to extract information from data that are both longitudinal and high-dimensional; that is, situations where the data are collected from participants at multiple time points and the number of covariates begins to approach, or even surpasses the number of subjects in the dataset (Wang et al., 2012; Cheng et al., 2014; Zipunnikov et al., 2014; Chu et al., 2016).
As has been mentioned repeatedly above, both with resting-state and passive smartphone sensing there are a large quantity of features and analyses that can be generated. In the current study we chose features that were reasonable based on previous data but are unlikely to be the optimal features that describe the relationship between depression, passive mobile sensing and brain connectivity. Multiple approaches could be taken with data from both sources. One approach which would greatly decrease the number of features that were necessary including trying to create a singular propensity metric, or biomarker of depression for both the resting-state fMRI data and a separate one for the sensing data then observing the relationship between the two. Alternatively, data reduction techniques such as independent component analysis could be applied to each group then the relationship between them could be measured. Many researchers have taken a “risk” or “propensity” score approach, where they generate models which contain predictive variables (gender, substance use, family history) pertinent to the outcome of interest and use the propensity score as a regressor when doing analyses at the group or individual difference level (Stuart, 2010; Hansen et al., 2012). This could be applied to smartphone data, but only once appropriate sensor features, and model have been calculated. By creating a unitary risk feature multiple comparison issues can be greatly mitigated. Data reduction techniques that account for variance that is common between two data modalities such as joint ICA, parallel ICA and CCA-Joint ICA, which has been implemented for combining high-dimension data across fMRI and genetic data (FusionICA, available from http://mialab.mrn.org/software/fit/).
Unresolved Questions About Directionality and Timing
In the current sample, resting-state fMRI data is from 1 time-point while mobile smartphone sensing data is dynamic and data is collected over a longer period of time. An unresolved question is if changes in fMRI data across multiple sessions reflects or predict changes in smartphone usage. Likely a more sensitive measure would be to do the reverse – using changes in smartphone usage, which is continuously monitored, to predict when there may be changes in brain connectivity as measured by fMRI. Changes in depressive symptoms have been successfully predicted with passive smartphone features (Wang et al., 2018), and may be useful for signaling when an individual should be referred to clinical services or brought in for a subsequent fMRI session. Longitudinal penalized functional regression is a method designed to deal with multiple timepoints of both exposure and outcomes (Goldsmith et al., 2012) which may help provide insight into the temporal association between brain connectivity, depression and phone usage.
Moderating Factors of RSFC
Resting-state functional connectivity has repeatedly been shown to be relatively stable across individuals and time, displaying similar network structure across thousands of individuals. While similar network structure and connectivity patterns are observed between sites, preprocessing methods, and Cohorts, differences between individuals are observed across individual differences in personality, affect and current mood have been related to alterations in RSFC. Furthermore, individual differences in the network structure on an individual level have been observed. Properly mapping individual differences in networks across the cortex would allow for better cross-subject alignment. The network assignment of particular regions may in itself be linked to depressive symptoms, while lining up networks would allow for the proper comparison of networks across individuals. Additionally, the current state physiological state an individual is in, such as food satiety or caffeination status can influence their mood (Rogers and Lloyd, 1994) and has also been shown to influence an individual’s brain connectivity (Poldrack et al., 2015). While there are a variety of factors that can influence RSFC, reliable individual differences across brain disorders have been observed in previous studies and here. As the predictive accuracy of RSFC or other neuroimaging methods increases the field may move closer to using MRI as a biomarker of depression, as has been done with physical pain (Atlas et al., 2010; Wager et al., 2013).
Conclusion
In summary, the current work identified proof-of-concept relationships between RSFC of the brain, passive mobile smartphone sensing features (unlock duration and circadian similarity of stillness and number of location visited), web-based self-reported surveys of depressive symptoms (PHQ-8) and mobile smartphone based ecological momentary assessments of depressive symptoms (PHQ-4). The results observed here extend previous work which relates the amount of time spent using a phone is with depressive symptoms. Further, these symptoms, both before and after time-of-scanning (PHQ-8 and PHQ-2/4, respectively), show a relationship with connectivity between areas implicated in depression, reward and processing of valenced self-relevant material. Importantly, these initial results predominantly replicate across the two separate cohorts and similar results are observed across three passive sensing features, increasing the applicability and scope of the findings herein. Although the current results do not elucidate causality in the relationship between phone usage metrics, depression and brain connectivity, future work should aim to do so, especially given recent changes to public policy, with professional groups such as the American Academy of Pediatrics providing suggesting screen-time limits and policy and investor groups calling on media device makes such as Apple and other phone makers. Previous research was extended, with results that replicate across multiple MRI scanners and cohorts all while combining data from a while variety of sources. The analyses done here are by no means comprehensive and we hope that the findings of this study and future research methods proposed herein are useful to a wide-range of researchers. Ultimately continuation and extensions of this research has the potential to provide important insights into mental health, as well as inform psychological treatments and other interventions.
Author Contributions
JH, PH, WK, TH, and AC designed the study. Data collection was performed by JH, CR, RL, Ad, EM, and EH. All analyses were conduced by JH. All authors provided manuscript feedback and guidance.
Funding
This work was supported by NIMH nos. 5R01MH059282-12 and 5R01DA022582-10.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge Yaroslav O. Halchenko for assistance with MRI data conversion and BIDS organization, Terry Sackett for assistance with MRI data collection and the editor and reviewers for their helpful feedback. Pre-print available for reference (Huckins et al., 2018).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2019.00248/full#supplementary-material
Footnotes
References
Arroll, B., Goodyear-Smith, F., Crengle, S., Gunn, J., Kerse, N., Fishman, T., et al. (2010). Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann. Fam. Med. 8, 348–353. doi: 10.1370/afm.1139
Atlas, L. Y., Bolger, N., Lindquist, M. A., and Wager, T. D. (2010). Brain mediators of predictive cue effects on perceived pain. J. Neurosci. 30, 12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010
Ben-Zeev, D., Scherer, E. A., Wang, R., Xie, H., and Campbell, A. T. (2015). Next-generation psychiatric assessment: using smartphone sensors to monitor behavior and mental health. Psychiatr. Rehabil. J. 38, 218–226. doi: 10.1037/prj0000130
Berman, M. G., Yourganov, G., Askren, M. K., Ayduk, O., Casey, B. J., Gotlib, I. H., et al. (2013). Dimensionality of brain networks linked to life-long individual differences in self-control. Nat. Commun. 4:1373. doi: 10.1038/ncomms2374
Birn, R. M., Molloy, E. K., Patriat, R., Parker, T., Meier, T. B., Kirk, G. R., et al. (2013). The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage 83, 550–558. doi: 10.1016/j.neuroimage.2013.05.099
Burns, M. N., Begale, M., Duffecy, J., Gergle, D., Karr, C. J., Giangrande, E., et al. (2011). Harnessing context sensing to develop a mobile intervention for depression. J. Med. Internet Res. 13:e55. doi: 10.2196/jmir.1838
Cameron, I. M., Crawford, J. R., Lawton, K., and Reid, I. C. (2008). Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Brit. J. Gen. Pract. 58, 32–36. doi: 10.3399/bjgp08X263794
Canzian, L., and Musolesi, M. (2015). “Trajectories of depression,” in Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing – UbiComp ’15 (New York, NY: ACM Press), 1293–1304. doi: 10.1145/2750858.2805845
Cheng, M.-Y., Honda, T., Li, J., and Peng, H. (2014). Nonparametric independence screening and structure identification for ultra-high dimensional longitudinal data. Ann. Stat. 42, 1819–1849. doi: 10.1214/14-AOS1236
Choi, E. Y., Yeo, B. T. T., and Buckner, R. L. (2012). The organization of the human striatum estimated by intrinsic functional connectivity. J. Neurophysiol. 108, 2242–2263. doi: 10.1152/jn.00270.2012
Chu, W., Li, R., and Reimherr, M. (2016). Feature screening for time-varying coefficient models with ultrahigh-dimensional longitudinal data. Ann. Appl. Stat. 10, 596–617. doi: 10.1214/16-AOAS912
Cox, R. W., Chen, G., Glen, D. R., Reynolds, R. C., and Taylor, P. A. (2017). fMRI clustering and false-positive rates. Proc. Nat. Acad. Sci. U.S.A. 114, E3370–E3371. doi: 10.1073/pnas.1614961114
Dale, A. M., Fischl, B., and Sereno, M. I. (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. doi: 10.1006/nimg.1998.0395
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Drysdale, A. T., Grosenick, L., Downar, J., Dunlop, K., Mansouri, F., Meng, Y., et al. (2016). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23, 28–38. doi: 10.1038/nm.4246
Eisenberg, D., Hunt, J., and Speer, N. (2013). Mental health in American colleges and universities: variation across student subgroups and across campuses. J. Nerv. Ment. Dis. 201, 60–67. doi: 10.1097/NMD.0b013e31827ab077
Foti, N. J., Hughes, J. M., and Rockmore, D. N. (2011). Nonparametric sparsification of complex multiscale networks. PLoS One 6:e16431. doi: 10.1371/journal.pone.0016431
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S., and Turner, R. (1996). Movement-related effects in fMRI time-series. Magn. Reson. Med. 35, 346–355. doi: 10.1002/mrm.1910350312
Goldsmith, J., Crainiceanu, C. M., Caffo, B., and Reich, D. (2012). Longitudinal penalized functional regression for cognitive outcomes on neuronal tract measurements. J. R. Stat. Soc. Ser. C 61, 453–469. doi: 10.1111/j.1467-9876.2011.01031.x
Gordon, E. M., Laumann, T. O., Adeyemo, B., Gilmore, A. W., Nelson, S. M., Dosenbach, N. U. F., et al. (2017). Individual-specific features of brain systems identified with resting state functional correlations. NeuroImage 146, 918–939. doi: 10.1016/j.neuroimage.2016.08.032
Gordon, E. M., Laumann, T. O., Adeyemo, B., Huckins, J. F., Kelley, W. M., and Petersen, S. E. (2016). Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex 26, 288–303. doi: 10.1093/cercor/bhu239
Gorgolewski, K. J., Auer, T., Calhoun, V. D., Craddock, R. C., Das, S., Duff, E. P., et al. (2016). The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci. Data 3:160044. doi: 10.1038/sdata.2016.44
Gratton, C., Laumann, T. O., Nielsen, A. N., Greene, D. J., Gordon, E. M., Gilmore, A. W., et al. (2018). Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron 98:439-452.e. doi: 10.1016/j.neuron.2018.03.035
Greenberg, P. E., Kessler, R. C., Birnbaum, H. G., Leong, S. A., Lowe, S. W., Berglund, P. A., et al. (2003). The economic burden of depression in the United States: how did it change between 1990 and 2000? J. Clin. Psychiatry 64, 1465–1475. doi: 10.4088/JCP.v64n1211
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–437. doi: 10.1016/j.biopsych.2006.09.020
Guntupalli, J. S., Hanke, M., Halchenko, Y. O., Connolly, A. C., Ramadge, P. J., and Haxby, J. V. (2016). A model of representational spaces in human cortex. Cereb. Cortex 26, 2919–2934. doi: 10.1093/cercor/bhw068
Halchenko, Y., Hanke, M., Poldrack, B., Debanjum, Alteva, G., Gors, J., et al. (2017). datalad/datalad 0.9.1. Available at: https://zenodo.org/record/1000098#.XH8o4skzaM8
Hansen, R. A., Dusetzina, S. B., Ellis, A. R., Stürmer, T., Farley, J. F., and Gaynes, B. N. (2012). Risk of adverse events in treatment-resistant depression: propensity-score-matched comparison of antidepressant augment and switch strategies. Gen. Hosp. Psychiatry 34, 192–200. doi: 10.1016/j.genhosppsych.2011.10.001
Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., and Conde, J. G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381. doi: 10.1016/j.jbi.2008.08.010
Holtzheimer, P. E. (2012). Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry 69, 150–158. doi: 10.1001/archgenpsychiatry.2011.1456
Horwitz, A. V., Wakefield, J. C., and Lorenzo-Luaces, L. (2016). “History of depression,” in The Oxford Handbook of Mood Disorders, eds R. J. DeRubeis and D. R. Strunk (Oxford: Oxford University Press).
Huckins, J. F., Adeyemo, B., Miezin, F. M., Power, J. D., Gordon, E. M., Laumann, T. O., et al. (2019). Reward-related regions form a preferentially coupled system at rest. Hum. Brain Mapp. 40, 361–376. doi: 10.1002/hbm.24377
Huckins, J. F., daSilva, A. W., Wang, R., Wang, W., Hedlund, E. L., Murphy, E. I., et al. (2018). Fusing mobile phone sensing and brain imaging to assess depression in college students: a proof-of-concept study. bioRxiv [Preprint]. doi: 10.1101/276568
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751. doi: 10.1176/appi.ajp.2010.09091379
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D., and Pizzagalli, D. A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611. doi: 10.1001/jamapsychiatry.2015.0071
Khubchandani, J., Brey, R., Kotecki, J., Kleinfelder, J., and Anderson, J. (2016). The psychometric properties of PHQ-4 depression and anxiety screening scale among college students. Arch. Psychiatric Nurs. 30, 457–462. doi: 10.1016/j.apnu.2016.01.014
Kroenke, K., Spitzer, R. L., and Williams, J. B. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Int. Med. 16, 606–613.
Kroenke, K., Spitzer, R. L., Williams, J. B. W., and Löwe, B. (2009a). An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 50, 613–621. doi: 10.1176/appi.psy.50.6.613
Kroenke, K., Strine, T. W., Spitzer, R. L., Williams, J. B. W., Berry, J. T., and Mokdad, A. H. (2009b). The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 114, 163–173. doi: 10.1016/j.jad.2008.06.026
Kross, E., Verduyn, P., Demiralp, E., Park, J., Lee, D. S., Lin, N., et al. (2013). Facebook use predicts declines in subjective well-being in young adults. PLoS One 8:e69841. doi: 10.1371/journal.pone.0069841
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., and Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157. doi: 10.1038/35084005
Lomb, N. R. (1976). Least-squares frequency analysis of unequally spaced data. Astrophys. Space Sci. 39, 447–462. doi: 10.1007/BF00648343
Lyall, L. M., Wyse, C. A., Graham, N., Ferguson, A., Lyall, D. M., Cullen, B., et al. (2018). Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry 5, 507–514. doi: 10.1016/S2215-0366(18)30139-1
Marcus, D. S., Fotenos, A. F., Csernansky, J. G., Morris, J. C., and Buckner, R. L. (2010). Open access series of imaging studies: longitudinal MRI data in nondemented and demented older adults. J. Cogn. Neurosci. 22, 2677–2684. doi: 10.1162/jocn.2009.21407
Matar, B. J., and Jaalouk, D. (2017). Depression, anxiety, and smartphone addiction in university students- a cross sectional study. PLoS One 12:e0182239. doi: 10.1371/journal.pone.0182239
Mathias, A., Grond, F., Guardans, R., Seese, D., Canela, M., and Diebner, H. H., (2004). Algorithms for spectral analysis of irregularly sampled time series. J. Stat. Softw. 11, 1–26. doi: 10.18637/jss.v011.i02
Mayberg, H. S., Lozano, A. M., Voon, V., McNeely, H. E., Seminowicz, D., Hamani, C., et al. (2005). Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660. doi: 10.1016/j.neuron.2005.02.014
Mehrotra, A., Tsapeli, F., Hendley, R., and Musolesi, M. (2017). MyTraces: Investigating correlation and causation between users’ emotional states and mobile phone interaction. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 1, 1–21. doi: 10.1145/3130948
Moran, J. M., Macrae, C. N., Heatherton, T. F., Wyland, C. L., and Kelley, W. M. (2006). Neuroanatomical evidence for distinct cognitive and affective components of self. J. Cogn. Neurosci. 18, 1586–1594. doi: 10.1162/jocn.2006.18.9.1586
Nielsen.com (2016). Millennials are Top Smartphone Users. Available at: https://www.nielsen.com/us/en/insights/news/2016/millennials-are-top-smartphone-users.html
Patriat, R., Molloy, E. K., Meier, T. B., Kirk, G. R., Nair, V. A., Meyerand, M. E., et al. (2013). The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. NeuroImage 78, 463–473. doi: 10.1016/j.neuroimage.2013.04.013
Poldrack, R. A., Laumann, T. O., Koyejo, O., Gregory, B., Hover, A., Chen, M.-Y., et al. (2015). Long-term neural and physiological phenotyping of a single human. Nat. Commun. 6:8885. doi: 10.1038/ncomms9885
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., and Petersen, S. E., (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. doi: 10.1016/j.neuroimage.2011.10.018
Power, J. D., Cohen, A. L., Nelson, S. M., Wig, G. S., Barnes, K. A., Church, J. A., et al. (2011). Functional network organization of the human brain. Neuron 72, 665–678. doi: 10.1016/j.neuron.2011.09.006
Power, J. D., Mitra, A., Laumann, T. O., Snyder, A. Z., Schlaggar, B. L., and Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84, 320–341. doi: 10.1016/j.neuroimage.2013.08.048
Richiardi, J., Altmann, A., Milazzo, A.-C., Chang, C., Chakravarty, M. M., Banaschewski, T., et al. (2015). Correlated gene expression supports synchronous activity in brain networks. Science 348, 1241–1244. doi: 10.1126/science.1255905
Rogers, P. J., and Lloyd, H. M. (1994). Nutrition and mental performance. Proc. Nutr. Soc. 53, 443–456. doi: 10.1079/PNS19940049
Rosenman, R., Tennekoon, V., and Hill, L. G. (2011). Measuring bias in self-reported data. Int. J. Behav. Healthc. Res. 2:320–332. doi: 10.1504/IJBHR.2011.043414
Rosvall, M., and Bergstrom, C. T. (2008). Maps of random walks on complex networks reveal community structure. Proc. Nat. Acad. Sci. U. S. A. 105, 1118–1123. doi: 10.1073/pnas.0706851105
Saeb, S., Zhang, M., Karr, C. J., Schueller, S. M., Corden, M. E., Kording, K. P., et al. (2015). Mobile phone sensor correlates of depressive symptom severity in daily-life behavior: an exploratory study. J. Med. Internet Res. 17:e175. doi: 10.2196/jmir.4273
Serrano, M. A., Boguñá, M., and Vespignani, A. (2009). Extracting the multiscale backbone of complex weighted networks. Proc. Nat. Acad. Sci. U. S. A. 106, 6483–6488. doi: 10.1073/pnas.0808904106
Shehzad, Z., Kelly, A. M. C., Reiss, P. T., Gee, D. G., Gotimer, K., Uddin, L. Q., et al. (2009). The resting brain: unconstrained yet reliable. Cereb. Cortex 19, 2209–2229. doi: 10.1093/cercor/bhn256
Shiffman, S., Stone, A. A., and Hufford, M. R. (2008). Ecological momentary assessment. Ann. Rev. Clin. Psychol. 4, 1–32.
Shrout, P. E., and Fleiss, J. L. (1979). Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 86, 420–428. doi: 10.1037/0033-2909.86.2.420
Sinclair, B., Hansell, N. K., Blokland, G. A. M., Martin, N. G., Thompson, P. M., Breakspear, M., et al. (2015). Heritability of the network architecture of intrinsic brain functional connectivity. NeuroImage 121, 243–252. doi: 10.1016/j.neuroimage.2015.07.048
Smith, A. (2017). Record Shares of Americans Now Own Smartphones, have Home Broadband. Available at: http://www.pewresearch.org/fact-tank/2017/01/12/evolution-of-technology/
Somerville, L. H., Heatherton, T. F., and Kelley, W. M. (2006). Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat. Neurosci. 9, 1007–1008. doi: 10.1038/nn1728
Stuart, E. A. (2010). Matching methods for causal inference: a review and a look forward. Stat. Sci. 25, 1–21. doi: 10.1214/09-STS313
Talairach, J., and Tournoux, P. (1988). Co-Planar Stereotaxis Atlas of the Human Brain. New York, N.Y: Thieme Medical Publishers Inc.
Twenge, J. M., Joiner, T. E., Rogers, M. L., and Martin, G. N. (2018). Increases in depressive symptoms, suicide-related outcomes, and suicide rates among U.S. adolescents after 2010 and links to increased new media screen time. Clin. Psychol. Sci. 6, 3–17. doi: 10.1177/2167702617723376
Ustün, T. B., Ayuso-Mateos, J. L., Chatterji, S., Mathers, C., and Murray, C. J. L. (2004). Global burden of depressive disorders in the year 2000. Brit. J. Psychiatry? J. Ment. Sci. 184, 386–392.
Van Essen, D. C., Glasser, M. F., Dierker, D. L., Harwell, J., and Coalson, T. (2012). Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex 22, 2241–2262. doi: 10.1093/cercor/bhr291
Volkow, N. D., Koob, G. F., Croyle, R. T., Bianchi, D. W., Gordon, J. A., Koroshetz, W. J., et al. (2017). The conception of the ABCD study: from substance use to a broad NIH collaboration. Devel. Cogn. Neurosci. 32, 4–7. doi: 10.1016/j.dcn.2017.10.002
Wager, T. D., Atlas, L. Y., Lindquist, M. A., Roy, M., Woo, C.-W., and Kross, E. (2013). An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397. doi: 10.1056/NEJMoa1204471
Wang, L., Zhou, J., and Qu, A. (2012). Penalized generalized estimating equations for high-dimensional longitudinal data analysis. Biometrics 68, 353–360. doi: 10.1111/j.1541-0420.2011.01678.x
Wang, R., Chen, F., Chen, Z., Li, T., Harari, G., Tignor, S., et al. (2014). “StudentLife,” in Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing – UbiComp ’14 Adjunct, (New York, NY: ACM Press), 3–14. doi: 10.1145/2632048.2632054
Wang, R., Wang, W., daSilva, A., Huckins, J. F., Kelley, W. M., Heatherton, T. F., et al. (2018). Tracking depression dynamics in college students using mobile phone and wearable sensing. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2, 1–26. doi: 10.1145/3191775
Wang, R., Wang, W., DaSilva, A., Huckins, J. F., Kelley, W. M., Heatherton, T. F., et al. (n.d.). Tracking depression dynamics in college students using mobile phone and wearable sensing. Proc. ACM Interact. Mob.Wearable Ubiquitous Technol. 2:43.
World Health Organization (2018). WHO Depression Fact Sheet. Available at: https://www.who.int/news-room/fact-sheets/detail/depression.
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C., and Wager, T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670. doi: 10.1038/nmeth.1635
Yeo, B. T. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. doi: 10.1152/jn.00338.2011
Zipunnikov, V., Greven, S., Shou, H., Caffo, B. S., Reich, D. S., and Crainiceanu, C. M. (2014). Longitudinal high-dimensional principal components analysis with application to diffusion tensor imaging of multiple sclerosis. Ann. Appl. Stat. 8, 2175–2202. doi: 10.1214/14-AOAS748
Keywords: depression, mental health, smartphone, screen time, fMRI, resting-state, circadian rhythm
Citation: Huckins JF, daSilva AW, Wang R, Wang W, Hedlund EL, Murphy EI, Lopez RB, Rogers C, Holtzheimer PE, Kelley WM, Heatherton TF, Wagner DD, Haxby JV and Campbell AT (2019) Fusing Mobile Phone Sensing and Brain Imaging to Assess Depression in College Students. Front. Neurosci. 13:248. doi: 10.3389/fnins.2019.00248
Received: 16 September 2018; Accepted: 04 March 2019;
Published: 21 March 2019.
Edited by:
Rüdiger Christoph Pryss, Ulm University, GermanyReviewed by:
Brent Winslow, Design Interactive, United StatesElouise Koops, University Medical Center Groningen, Netherlands
Copyright © 2019 Huckins, daSilva, Wang, Wang, Hedlund, Murphy, Lopez, Rogers, Holtzheimer, Kelley, Heatherton, Wagner, Haxby and Campbell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy F. Huckins, amVyZW15LmYuaHVja2luc0BkYXJ0bW91dGguZWR1
 Jeremy F. Huckins
Jeremy F. Huckins Alex W. daSilva1
Alex W. daSilva1 Richard B. Lopez
Richard B. Lopez Paul E. Holtzheimer
Paul E. Holtzheimer Todd F. Heatherton
Todd F. Heatherton Dylan D. Wagner
Dylan D. Wagner James V. Haxby
James V. Haxby