
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 20 July 2018
Sec. Neurodegeneration
Volume 12 - 2018 | https://doi.org/10.3389/fnins.2018.00488
This article is part of the Research Topic Network Spread Models of Neurodegenerative Diseases View all 10 articles
Background: Early detection and intervention for post-stroke dysphagia could reduce the incidence of pulmonary complications and mortality. The aims of this study were to investigate the benefits of swallowing therapy in swallowing function and brain neuro-plasticity and to explore the relationship between swallowing function recovery and neuroplasticity after swallowing therapy in cerebral and brainstem stroke patients with dysphagia.
Methods: We collected 17 subacute stroke patients with dysphagia (11 cerebral stroke patients with a median age of 76 years and 6 brainstem stroke patients with a median age of 70 years). Each patient received swallowing therapies during hospitalization. For each patient, functional oral intake scale (FOIS), functional dysphagia scale (FDS) and 8-point penetration-aspiration scale (PAS) in videofluoroscopy swallowing study (VFSS), and brain functional magnetic resonance imaging (fMRI) were evaluated before and after treatment.
Results: FOIS (p = 0.003 in hemispheric group and p = 0.039 in brainstem group) and FDS (p = 0.006 in hemispheric group and p = 0.028 in brainstem group) were both significantly improved after treatment in hemispheric and brainstem stroke patients. In hemispheric stroke patients, changes in FOIS were related to changes of functional brain connectivity in the ventral default mode network (vDMN) of the precuneus in brain functional MRI (fMRI). In brainstem stroke patients, changes in FOIS were related to changes of functional brain connectivity in the left sensorimotor network (LSMN) of the left postcentral region characterized by brain fMRI.
Conclusion: Both hemispheric and brainstem stroke patients with different swallowing difficulties showed improvements after swallowing training. For these two dysphagic stroke groups with corresponding etiologies, swallowing therapy could contribute to different functional neuroplasticity.
Dysphagia is a common disorder after stroke (Paciaroni et al., 2004). Approximately 25~45% of patients show difficulties in swallowing after acute stroke while dysphagia is associated with a high risk of aspiration pneumonia, malnutrition, and mortality after acute stroke (Barer, 1989; Johnson et al., 1993; Martino et al., 2005; Walter et al., 2007; Falsetti et al., 2009). Previous studies have reported that 15% of stroke patients depend on tube feeding in order to maintain their nutritional requirements 6 months after stroke (Croghan et al., 1994; Dennis et al., 2005; Smithard et al., 2007). Consequently, the early detection and management of post-stroke dysphagia could reduce the incidence of subsequent co-morbidities and help to reduce the length of duration of hospitalization (Dziewas et al., 2004; Brotherton and Judd, 2007; Maeshima et al., 2014). Therefore, early swallowing therapy for dysphagia is very important in preventing further pulmonary complications and mortality after stroke (Doggett et al., 2001; Foley et al., 2008).
Brainstem or hemispheric lesions after stroke can lead to motor or sensory deficits in swallowing function. Brainstem stroke may also impair the sensory input or motor function of the mouth, tongue, cheek, pharynx, larynx, vocal fold, or cricopharyngeal muscles during the pharyngeal phase of swallowing (Horner et al., 1991; Moon et al., 2012). Hemispheric stroke may also impede the motor control and coordination of bolus preparation, mastication, or pharyngeal peristalsis during the oral and pharyngeal phases during swallowing. Therefore, different types of stroke may cause swallowing impairments in different ways in stroke patients with dysphagia (Robbins et al., 1993; Moon et al., 2012; Kim et al., 2014).
Several swallowing therapies have been utilized in the management of post-stroke dysphagia, including thermal stimulation, postural compensation, food consistency modifications, oropharyngeal exercises, swallowing maneuver and electrical stimulation (Carnaby et al., 2006). Recently, some investigators have used neuromuscular electrical stimulation for dysphagia patients after stroke and found this mode of treatment to be beneficial in the treatment of dysphagia (Lee et al., 2014; Poorjavad et al., 2014; Byeon and Koh, 2016). In earlier studies, some researchers reported that these therapeutic techniques might provide positive effects on neuroplasticity for stroke patients with dysphagia. Voluntary swallowing is regulated by complex sensorimotor cortical and subcortical network while the swallowing reflex is controlled by swallowing centers in the brainstem (Soros et al., 2009). However, the actual mechanism underlying swallowing function in the brain has yet to be elucidated. In stroke patients, damage to both of the associated hemispheres or brainstem result in swallowing disorders (Hamdy et al., 1996, 1997, 1998a,b; Li et al., 2009, 2014).
Previous studies have used clinical observations, EEG, magnetoencephalography (MEG) or transcranial magnetic stimulation (TMS) to explore the cerebral areas associated with swallowing dysfunction (Robbins et al., 1993; Hamdy et al., 1997; Luan et al., 2013; Jestrovic et al., 2016). However, over recent years, functional magnetic resonance imaging (fMRI) has become a more popular tool with which to evaluate cerebral cortical function during volitional and reflexive swallowing in humans (Hamdy et al., 1999; Mosier K. et al., 1999; Mosier K. M. et al., 1999; Kern et al., 2001; Martin et al., 2001; Malandraki et al., 2011; Dehaghani et al., 2016). Li et al. used fMRI to investigate cerebral cortical activation during swallowing with tasks in acute dysphagic stroke patients involving of unilateral hemisphere (Li et al., 2009). On the basis of these results, they suggested that fMRI is a useful method to investigate the spatial localization of changes in the neuroactivity of the bilateral hemispheres during swallowing tasks which may be related to the functional recovery of dysphagia (Li et al., 2009). The strongest activations were noted in the sensorimotor cortices, insula and cingulated gyrus of the intact hemisphere. Furthermore, they reported decreased connectivity in bilateral swallowing associated brain network while applying rs-fMRI study to explore the alternations of functional and structural connectivity in stroke patients with dysphagia (Li et al., 2014). Another fMRI study investigated recovered swallowing in dysphagic stroke patients and also showed overall decreased fMRI-activation in the associated swallowing network, but increased activities in the contralesional primary somatosensory cortex (S1) (Mihai et al., 2016).
Diffusion Tensor Imaging (DTI) is commonly used to study normal white matter anatomy and structural connectivity. This technique might have the potential to also assess plastic changes in the white matter in response to intense therapy in stroke patients recovering from a motor deficit. However, some of the DTI-derived absolute measures have shown pre-vs. post-therapy changes that were in the order of variability seen in normal subjects. Furthermore, significant remodeling of the ipsilesional and contralesional corticospinal tract was observed in chronic stroke patients undergoing an intense therapy program (Betzler et al., 2009). Consequently, in order to assess the sensitivity of this technique to detect possible structural changes as a function of therapy, it is necessary to carry out an assessment of the observed variance in DTI-derived measurements (Betzler et al., 2009).
To the best of our knowledge, there are limited published literature pertaining to the relationship between the changes in swallowing function and functional connectivity maps of fMRI, and in hemispheric and brainstem stroke patients following swallowing therapies. The aims of the present study were to investigate the benefits of swallowing therapy for swallowing function and brain neuro-plasticity and to explore the relationship between swallowing function and fMRI findings following swallowing therapy in hemispheric and brainstem stroke patients with dysphagia.
Between January 2011 and July 2013, we collected 31 acute stroke patients with dysphagia who met the inclusion criteria from the inpatient rehabilitation unit at one medical center. (clinical trial No.: NCT03048916) Stroke was diagnosed by the attending neurologist according to the patient's neurological insults and findings from brain computed tomography (CT) or magnetic resonance imaging (MRI) findings. The inclusion criteria for the participants were recent hemispheric or brainstem stroke (with a duration of less than 3 months since stroke), dysphagia reported by a physician who had assessed choking or coughing during swallowing, or patients were failed to complete the 100 ml water test during bedside swallowing assessment (Chen et al., 2016). We used functional oral intake scale (FOIS) to present patient's swallowing performance, which may reflex the severity of swallowing; patients were included in the study if their FOIS was equal to or less than 4. The exclusion criteria in the study were as follows: impaired communication ability due to aphasia; cognition impairment; a history of other neurological deficits leading to dysphagia; use of an electrically sensitive biomedical device (such as a cardiac pacemaker or a metal clip in the brain). Only seventeen stroke patients with dysphagia underwent detailed clinical assessments, videofluoroscopic swallowing studies (VFSS), and fMRI examinations before and after swallowing therapy. The study protocol was reviewed and approved by the Institutional Review Broad in our hospital. Informed written consent was obtained from each participant.
For each patient, the following clinical characteristics were recorded during admission into the rehabilitation unit: age; gender; swallowing therapy type; duration since stroke onset; the National Institute of Health Stroke Scale (NIHHS); affected hemisphere and lesion location. Finally, we recruited 11 patients with hemispheric stroke into the hemispheric group and 6 patients with brainstem stroke into the brainstem group; all of these patients underwent the entire evaluation and treatment process.
During hospitalization, these subacute stroke patients with dysphagia received traditional swallowing therapy or a combined therapy including both traditional swallowing therapies and neuromuscular electrical stimulation (NMES) therapy. Every patient was treated 3 times per week (60 min per session). Total ten treatment sessions with traditional or combined therapy were performed for each patient. The traditional swallowing therapy was performed by one experienced speech-language therapist, including oropharyngeal exercises, thermal stimulation, food consistency modifications, compensatory techniques, and swallowing maneuver based on VFSS findings and clinical presentations. NMES therapy was administered by one licensed physiatrist who used the VitalStim device (Chattanooga Group, Hixson, TN, USA) with a dual channel and two bipolar electrodes for each channel (700 μs pulse width, 80 Hz, and 0-25 mA wave-amplitude). The two sets of electrodes were placed on the patient's anterior neck area (Freed et al., 2001). Wave amplitude setting was dependent upon the patient's level of tolerance. The physiatrist gradually increased the amplitude until the patient felt a tingling sensation on the anterior neck and a muscle contraction. The current intensity was determined and maintained during one NMES treatment session based on each patient's tolerance level. The patients all underwent traditional swallowing training and NMES by the same physiatrist while receiving the combined therapies.
The functional oral intake scale (FOIS) for dysphagia was evaluated before and after intervention by a speech-language therapist who was blinded to all study procedures. The FOIS (Crary et al., 2005) is widely used for clinically assessing oral intake in stroke patients. Seven swallowing functional levels were defined according to their oral intake conditions ranging from nothing by mouth (level 1) to total oral diet with no restriction (level 7). The VFSS was performed by another speech-language therapist who was also blinded to the study interventions. The 8-point penetration-aspiration scale (PAS) (Rosenbek et al., 1996) and functional dysphagia scale (FDS) (Han et al., 2001) were assessed according to VFSS findings. The 8-point PAS indicated the severity of aspiration while swallowing. Aspiration was defined as any materials entering the larynx below the vocal folds. The 8-point PAS scale ranged from a score of 1 (normal swallowing without material entering the airway) to a score of 8 (severe airway compromise with material entering the airway and passing below the vocal folds). The FDS includes 11 items relating to oral and pharyngeal function and was investigated during the VFSS according to lip closure, bolus formation, residual matter in the oral cavity, oral transit time, triggering of pharyngeal swallowing, laryngeal elevation and epiglottis closure, nasal penetration, triggering, residue in valleculae and pyriform sinus, pharyngeal coating, and pharyngeal transit time. For each patient, the achievable score is 100 points depended upon the results of the VFSS. A higher FDS score indicated a worse swallowing condition in the VFSS. Another experienced and blinded speech-language therapist interpreted and scored the 8-point PAS and FDS before and after interventions.
Functional imaging data were acquired using a 3.0 T GE Signa MRI scanner (Milwaukee, WI, USA). Resting-state images from 300 contiguous echo planar imaging whole brain functional scans were acquired (TR: 2 s; TE: 30 ms; FOV: 240 mm; flip angle: 80°; matrix size: 64 × 64; thickness: 4 mm). During the resting experiment, the scanner room was darkened and the participants were instructed to relax, with their eyes closed, without falling asleep. A three-dimensional (3D) high-resolution T1-weighted anatomical image was also acquired using an inversion recovery fast spoiled gradient-recalled echo pulse sequence (TR: 9.5 ms; TE: 3.9 ms; TI: 450 ms; flip angle: 20°; field of view: 256 mm; matrix size: 512 × 512).
Rs-fMRI data were preprocessed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) and Data Processing Assistant for rs-fMRI (DPARSF) and in-house software for Matlab (The MathWorks Inc. Natick MA, USA).
In the first step, the first 5 volumes were discarded to reach a steady-state magnetization and allow the participants to adapt to the scanning noise. We excluded any data involving a head motion of more than 2.0 mm maximum displacement in any of the x, y, or z directions, or 2.0° of any angular motion throughout the course of the scan. Data were also visually inspected for movement-related artifacts. The standard Montreal Neurological Institute template provided by SPM was further used for normalization with re-sampling to 3 mm cubic voxels and a Gaussian kernel of 6 mm (full width at half maximum) for spatial smoothing. The waveform of each voxel was finally used to remove linear trends of the time courses and for temporal band-pass filtering (0.01–0.08 Hz) to reduce high-frequency physiological noise.
To investigate rs-fMRI networks, we performed independent component analyses (ICA) using group ICA for fMRI toolbox (GIFT; http://mialab.mrn.org/software/gift). This toolbox supports a group ICA approach, which first concatenates the individual data across time, followed by computation of the subject-specific components and time courses. Data dimensionality (the number of components) was estimated using the minimum description length criteria tool in GIFT (Calhoun et al., 2001), which suggested that 30 was the optimal number of independent components (ICs). The dimensions of the functional data were then reduced using principal component analysis and the ICs were then estimated using the Infomax algorithm (Bell and Sejnowski, 1995). Stable estimation was achieved by rerunning the ICA analyses 20 times using the ICASSO (Himberg et al., 2004; Chenji et al., 2016) toolbox implemented in GIFT. Twenty-four ICs with an ICASSO stability index of <0.9 were then estimated in our patient sample and the spatial maps of the components were converted into z-value maps (Stevens et al., 2009). Further analysis of ICs were selected based on the largest spatial correlation (Calhoun et al., 2001) with specific rs-fMRI network templates reported in previous studies (Shirer et al., 2012), which correspond to known anatomical and functional segmentation. Group statistical maps of subject IC patterns representing rs-fMRI networks were entered into one- and two-sample random effects analyses in SPM8.
SPSS package (version 17.0, Chicago, IL, USA) was used to run statistical analysis for group differences in demography, clinical characteristics, and VFSS findings. The Mann-Whitney test was used to analyse data relating to age, the duration since stroke, and NIHSS, and while Fisher's exact test was used to analyse gender, swallowing therapy types, and the affected hemisphere between the brain and brainstem groups. Within-group and between-group comparisons of FDS, 8-point PAS, and FOIS were performed with the Wilcoxon signed-rank test and Mann-Whitney test. The significance threshold of group difference was p < 0.05, FDR-corrected for multiple comparisons. Functional connectivity (FC) between-group two sample t tests were masked with a within-group mask threshold at p < 0.05, and corrected for multiple comparisons using a combination of an uncorrected height threshold of p < 0.05 with a minimum cluster size. Cluster size was determined over 1000 Monte Carlo simulations using the AlphaSim program distributed with the REST software tool (http://resting-fmri.sourceforge.net/). Anatomical labeling was defined by the Anatomical Automatic Labeling atlas (AAL) (Tzourio-Mazoyer et al., 2002). In voxel based statistical analysis, some significant clusters between groups were found. To evaluate their interactions with the clinical symptoms, the connectivity value was extracted from those significant clusters for further correlation analysis. The correlation between changes in clinical parameters and the alteration of functional connectivity was investigated using Spearman‘s rank correlation.
We initially collected 31 acute stroke patients with dysphagia but excluded 14 patients who showed poor cooperation during the VFSS/ fMRI study or swallowing training. Twenty-three out of 31 stroke patients received full-course swallowing training during hospital stay. Five of those 23 participants were not suitable to receive fMRI assessments and one of 23 patients could not completely take all 3 kinds of food in VFSS. In this study, we did not find any exacerbations of the medical conditions including aspiration or pneumonia during swallowing training and no harm related to all procedures for each participant. Consequently, a total of 17 patients completed all of the procedures and interventions required by this study.
Of the 17 patients included in the study, there were 11 patients with hemispheric stroke assigned to the hemispheric group and 6 patients with brainstem stroke assigned to the brainstem group. The demographic and clinical characteristics of these participants are shown in Table 1. In the hemispheric group (2 women and 9 men; median age: 76 years), 7 patients received combined therapy and 4 patients received traditional swallowing therapy. In the brainstem group (1 woman and 5 men; median age: 70 years), 4 patients received combined therapy and 2 patients received traditional swallowing therapy. There were no significant differences in terms of age, swallowing therapy type, duration since stroke onset, NIHSS, and affected hemisphere. Within-group comparison (Table 2) showed significant differences in terms of thin-liquid FDS (p = 0.012), total FDS scores (p = 0.006), FOIS (p = 0.003) in the hemispheric group after treatment, and significant differences in soft diet FDS (p = 0.043), thin-liquid FDS (p = 0.046), total FDS score (p = 0.028), PAS (p = 0.041), and FOIS (p = 0.039) in the brainstem group after treatment. Between-group comparison showed significant differences in thick-liquid FDS (p = 0.049) and FOIS (p = 0.039) after treatment. There was also a significant difference between the 2 groups of patients after intervention in terms of their relative changes on the 8-point PAS (p = 0.028) (Table 3).
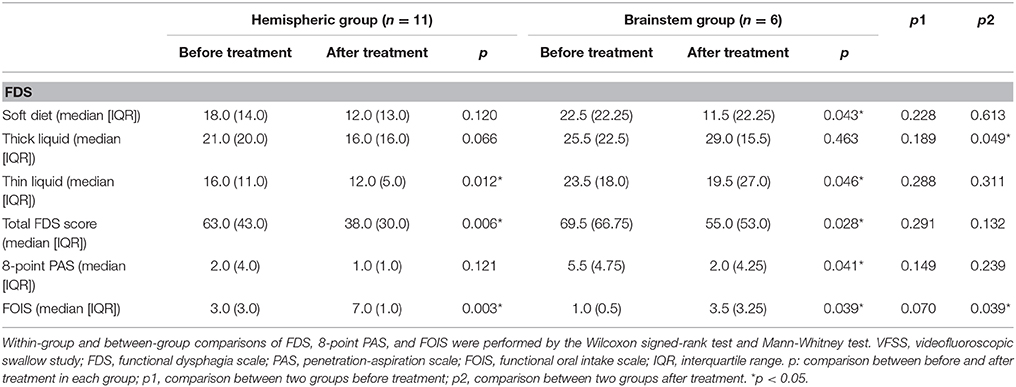
Table 2. A comparison of findings in the VFSS and clinical oral intake conditions within and between hemispheric and brainstem groups.
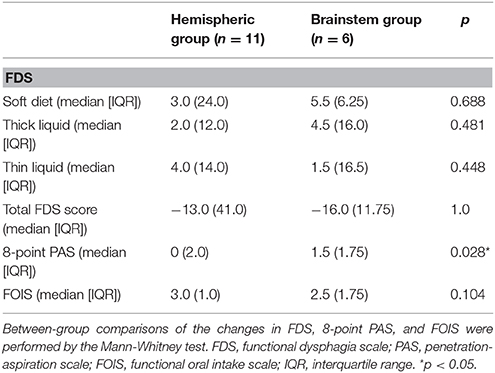
Table 3. A comparison of the changes in clinical outcome measures between the hemispheric and brainstem groups.
Rs-fMRI networks were identified by using 14 templates in all ICA components for each of the 17 subjects. Correlation coefficients between the spatial templates and ICs of ICA analysis were as follows: visuospatial network, VSN (IC01), 0.292; posterior salience network, pSN (IC03), 0.474; left executive control network, LECN (IC04), 0.469; primary visual network, PVN (IC07), 0.398; right sensorimotor network, RSMN (IC09), 0.284; dorsal default mode network, dDMN (IC10), 0.471; ventral default mode network, vDMN (IC15), 0.263; high visual network, HVN (IC16), 0.330; language network, LGN (IC17), 0.330; auditory network, AN (IC21), 0.414; left sensorimotor network, LSMN (IC22), 0.264; precuneus network, PCCN (IC23), 0.368; cerebellar sensorimotor network, CSMN (IC24), 0.272; anterior salience network, aSN (IC26), 0.313; and right executive control network, RECN (IC28), 0.492. The results of the one-sample t-test (P < 0.05, FDR corrected) for patients with hemispheric and brainstem stroke were determined using SPM toolbox and are shown in Figure 1.
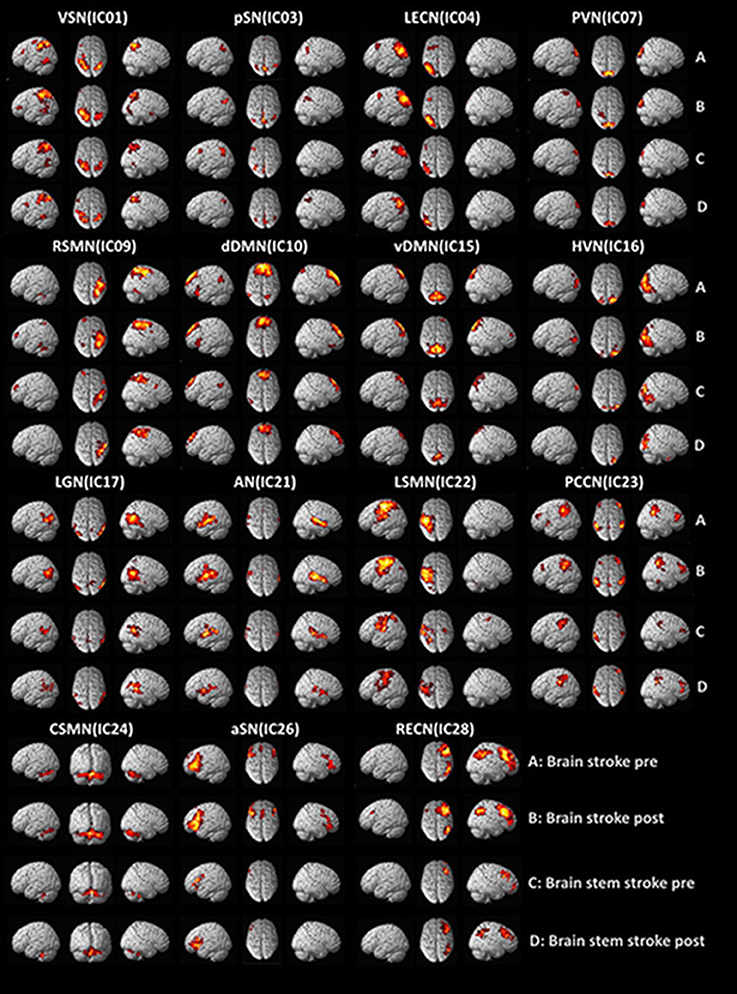
Figure 1. Functional MRI findings in stroke patients with dysphagia before and after swallowing training. VSN, visuospatial network; pSN, posterior salience network; LECN, left executive control network; PVN, primary visual network; RSMN, right sensorimotor network; dDMN, dorsal default mode network; vDMN, ventral default mode network; HVN, high visual network; LGN, language network; AN, auditory network; LSMN, left sensorimotor network; PCCN, precuneus network; CSMN, cerebellar sensorimotor network; aSN, anterior salience network; RECN, right executive control network.
We compared FC between patients with hemispheric and brainstem stroke in a voxel-wise manner. Significance differences arising from two-sample t-tests (between the two groups) and paired t-tests (between the two states) of spatial map regions are presented in Table 4.
The hemispheric group with post-intervention exhibited significantly increased intra-network functional connectivity in the left superior parietal lobule of the VSN, the right middle frontal cortex of dDMN, and the right cerebellum of SMN.
The brainstem group with post-intervention exhibited significantly increased intra-network functional connectivity in the left cerebellum and right calcarine of the PVN. In contrast, the brainstem group with post-intervention exhibited significantly reduced intra-network functional connectivity in the right postcentral cortex of the SMN, left superior parietal lobule of the vDMN, left precuneus of the vDMN, right fusiform of the HVN, left middle temporal lobe of the AN, left postcentral cortex of the SMN and left inferior parietal of the PCCN.
Brain rs-fMRI showed that in hemispheric stroke patients, changes in the FOIS were related to changes in the functional connectivity of the ventral default mode network of the left precuneus (Table 5 and Figure 2A). However, in brainstem stroke patients, changes in the FOIS were related changes in the functional connectivity of the left sensorimotor network of the left postcentral region. (Table 5 and Figure 2B).
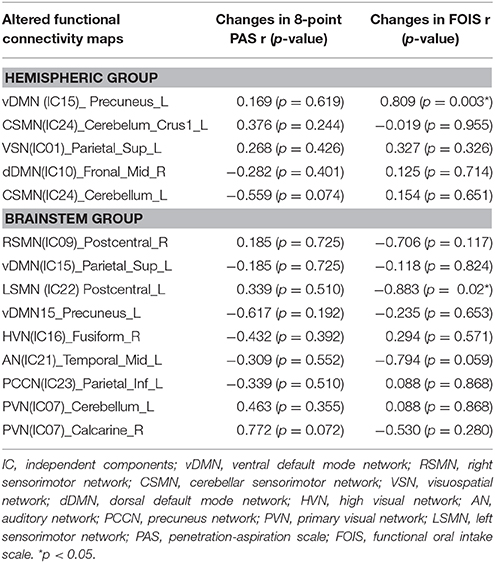
Table 5. Correlation between the changes of clinical parameters and altered functional connectivity maps after treatment in hemispheric and brainstem groups.
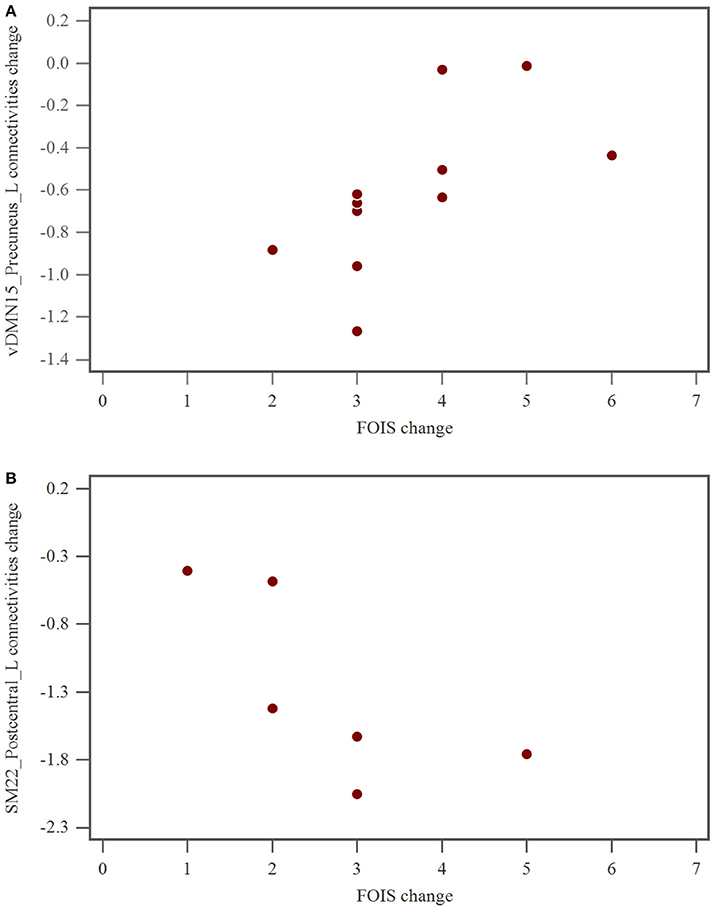
Figure 2. (A) The relationship of FOIS change and vDMN15_Precuneus_L connectivity alteration after swallowing therapy in hemispheric group. FOIS, functional oral intake scale; vDMN, ventral default mode network. (B) The relationship of FOIS change and SM22_Postcentral_L connectivities alteration after swallowing therapy in brainstem group. FOIS, functional oral intake scale; SM, sensorimotor network.
To the best of our knowledge, this represents the first research study to explore the relationship between brain connectivity network changes in rs-fMRI and clinical swallowing functional recovery following swallowing therapy in subacute hemispheric and brainstem stroke patients with oropharyngeal dysphagia. Our findings revealed that swallowing therapy led to a significant improvement in total FDS, FOIS scores in patients with hemispheric and brainstem stroke, and in 8-point PAS score in patients with brainstem stroke. Our research also showed that in patients with hemispheric stroke, the reduced brain connectivity of the ventral default mode network of the left precuneus was related to improvements in FOIS after swallowing therapy. In patients with brainstem stroke, the reduced left sensorimotor connectivity of the left postcentral network was associated with improvements in FOIS after swallowing therapy.
Both hemispheric and brainstem stroke patients with oropharyngeal dysphagia received swallowing therapy and achieved clinical benefits in terms of their swallowing function. In addition, brainstem stroke impaired the movements of the larynx, pharynx, vocal fold, or cricopharyngeal muscles during the pharyngeal phase of swallowing (Martin and Sessle, 1993; Carnaby et al., 2006; Chenji et al., 2016). In the present study, we observed a worse 8-point PAS score (based on VFSS findings) before intervention in patients with brainstem stroke. Therefore, this particular cohort of patients showed significant improvement after training; this was due to the patients developing better muscle coordination during the pharyngeal phase and reducing the severity of their aspiration. These mechanisms also reduced further pulmonary complications.
In earlier studies, associated cortical and sub-cortical regions involving in swallowing had been demonstrated (Teismann et al., 2009, 2011; Luan et al., 2013; Kober et al., 2015) in stroke patients with dysphagia. Li et al. (2009) reported that disrupted functional brain networks related to swallowing motor control in acute stroke patients with dysphagia according to the findings of rs-fMRI study. Some researchers investigated brain imaging using fMRI for acute and chronic stroke patients with dysphagia and they proposed that recovered swallowing were associated with increase of cerebral activation in the contralateral cortex of the intact hemisphere and ipsilateral anterior cerebellum (Li et al., 2009; Mihai et al., 2016). Another research study (Chenji et al., 2016) showed that increased levels of DMN connectivity in patients with greater disability and that this connectivity decreased when performing goal-oriented tasks; consequently, DMN connectivity could be viewed as a baseline functional network. We considered that better swallowing function could lead to reduced levels of DMN connectivity while swallowing. In our present study, we found similar results (Li et al., 2009; Chenji et al., 2016) in that our patients with hemispheric stroke showed improved swallowing function following therapy and that this was associated with reduced brain function network connectivity.
In normal subjects, Babaei et al. (2013) and Malandraki et al. (2009) used brain fMRI to demonstrate increased activation in the cingulate, insula, sensorimotor cortex, prefrontal and parietal cortices, cerebellum, and thalamus during swallowing. Furthermore, Vahdat et al. (2011) observed that alterations in the functional connectivity of the sensorimotor network may be related to motor learning and the importance of the role of somatosensory region in swallowing had been gradually discovered (Babaei et al., 2013; Dehaghani et al., 2016; Mihai et al., 2016). In the present study, stroke patients received a variety of treatments to improve their swallowing, including motor functional training, thermal stimulation, postural compensation, oropharyngeal exercise, compensatory techniques, and swallowing maneuver. We also observed some correlations between clinical swallowing improvements and the SMN network after swallowing therapy in patients suffering from brainstem stroke.
There were several limitations to our study that should be considered when interpreting our conclusions. Firstly, only a small number of patients participated in the study. Secondly, the detailed techniques involved with swallowing interventions were not recorded and compared between hemispheric and brain stem stroke patients. Thirdly, we did not apply longer swallowing interventions for moderate to severe oropharyngeal dysphagia in stroke patients or follow-up the long-term effect of swallowing therapy on swallowing function and neuroplasticity in stroke patients with oropharyngeal dysphagia. In addition, the number of males was 4–5 times as females were enrolled in this study, and the results might be biased by the gender ratio. Finally, we did not recruit stroke patients with oropharyngeal dysphagia in the control group to account for their spontaneous neural recovery, which could have contributed to the alternations in brain fMRI.
In summary, there was both significant recovery of swallowing function after swallowing therapy for subacute stroke patients with different oropharyngeal dysphagia. The clinical improvements in FOIS were associated with default mode network in hemispheric stroke and with sensorimotor network in brainstem stroke characterized by fMRI, respectively. For these two dysphagic stroke groups with corresponding etiologies, swallowing therapy could contribute to different functional neuroplasticity, which might serve as an indicator for further prognostic evaluation.
Institutional Review Board in Chang-Gung Memorial Hospital approved the study protocol, and all of the participants or their guardians provided written informed consent. All of the participants or their guardians provided written informed consent.
Y-CH and W-CL designed the study, participated in data collection, data analysis and interpretation, writing and revising manuscript and final approval of manuscript. T-WH participated in patient enrollment, data collection, data analysis, and data interpretation. H-CH conducted the data statistical analysis, data interpretation, and manuscript drafting. C-PL participated in patient assessment, data analysis and data interpretation. All authors reviewed the manuscript, contributed to its revision, and approved the final version submitted.
This work was supported by funds from the National Science Council (NMRPG896021-3:NSC 99-2314-B-182A-021- 1090 MY3). This study was supported by grants from the Taiwan National Science Council.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Babaei, A., Ward, B. D., Siwiec, R. M., Ahmad, S., Kern, M., Nencka, A., et al. (2013). Functional connectivity of the cortical swallowing network in humans. Neuroimage 76, 33–44. doi: 10.1016/j.neuroimage.2013.01.037
Barer, D. H. (1989). The natural history and functional consequences of dysphagia after hemispheric stroke. J. Neurol. Neurosurg. Psychiatr. 52, 236–241.
Bell, A. J., and Sejnowski, T. J. (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 7, 1129–1159.
Betzler, F., Lindenberg, R., Zhu, L., and Schlaug, G. (2009). Differentiating normal variability from structural plasticity: DTI studies in stroke patients undergoing therapy. NeuroImage 47(Suppl 1), S39–S41. doi: 10.1016/S1053-8119(09)70471-9
Brotherton, A. M., and Judd, P. A. (2007). Quality of life in adult enteral tube feeding patients. J. Hum. Nutr. Diet 20, 513–522. doi: 10.1111/j.1365-277X.2007.00827.x
Byeon, H., and Koh, H. W. (2016). Comparison of treatment effect of neuromuscular electrical stimulation and thermal-tactile stimulation on patients with sub-acute dysphagia caused by stroke. J. Phys. Ther. Sci. 28, 1809–1812. doi: 10.1589/jpts.28.1809
Calhoun, V., Adali, T., Pearlson, G., and Pekar, J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 14, 140–151. doi: 10.1002/hbm.1048
Carnaby, G., Hankey, G. J., and Pizzi, J. (2006). Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurol. 5, 31–37. doi: 10.1016/S1474-4422(05)70252-0
Chen, P. C., Chuang, C. H., Leong, C. P., Guo, S. E., and Hsin, Y. J. (2016). Systematic review and meta-analysis of the diagnostic accuracy of the water swallow test for screening aspiration in stroke patients. J. Adv. Nurs. 72, 2575–2586. doi: 10.1111/jan.13013
Chenji, S., Jha, S., Lee, D., Brown, M., Seres, P., Mah, D., et al. (2016). Investigating default mode and sensorimotor network connectivity in amyotrophic lateral sclerosis. PLoS ONE 11:e0157443. doi: 10.1371/journal.pone.0157443
Crary, M. A., Mann, G. D., and Groher, M. E. (2005). Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 86, 1516–1520. doi: 10.1016/j.apmr.2004.11.049
Croghan, J. E., Burke, E. M., Caplan, S., and Denman, S. (1994). Pilot study of 12-month outcomes of nursing home patients with aspiration on videofluoroscopy. Dysphagia 9, 141–146. doi: 10.1007/BF00341256
Dehaghani, S. E., Yadegari, F., Asgari, A., Chitsaz, A., and Karami, M. (2016). Brain regions involved in swallowing: Evidence from stroke patients in a cross-sectional study. J. Res. Med. Sci. 21:45. doi: 10.4103/1735-1995.183997
Dennis, M. S., Lewis, S. C., Warlow, C., and Collaboration, F. T. (2005). Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet 365, 764–772. doi: 10.1016/S0140-6736(05)17983-5
Doggett, D. L., Tappe, K. A., Mitchell, M. D., Chapell, R., Coates, V., and Turkelson, C. M. (2001). Prevention of pneumonia in elderly stroke patients by systematic diagnosis and treatment of dysphagia: an evidence-based comprehensive analysis of the literature. Dysphagia 16, 279–295. doi: 10.1007/s00455-001-0087-3
Dziewas, R., Ritter, M., Schilling, M., Konrad, C., Oelenberg, S., Nabavi, D. G., et al. (2004). Pneumonia in acute stroke patients fed by nasogastric tube. J. Neurol. Neurosurg. Psychiatr. 75, 852–856. doi: 10.1136/jnnp.2003.019075
Falsetti, P., Acciai, C., Palilla, R., Bosi, M., Carpinteri, F., Zingarelli, A., et al. (2009). Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J. Stroke Cerebrovasc. Dis. 18, 329–335. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.009
Foley, N., Teasell, R., Salter, K., Kruger, E., and Martino, R. (2008). Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing 37, 258–264. doi: 10.1093/ageing/afn064
Freed, M. L., Freed, L., Chatburn, R. L., and Christian, M. (2001). Electrical stimulation for swallowing disorders caused by stroke. Respir. Care 46, 466–474.
Hamdy, S., Aziz, Q., Rothwell, J. C., Singh, K. D., Barlow, J., Hughes, D. G., et al. (1996). The cortical topography of human swallowing musculature in health and disease. Nat. Med. 2, 1217–1224. doi: 10.1038/nm1196-1217
Hamdy, S., Aziz, Q., Rothwell, J. C., Crone, R., Hughes, D., Tallis, R. C., et al. (1997). Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet 350, 686–692. doi: 10.1016/S0140-6736(97)02068-0
Hamdy, S., Aziz, Q., Rothwell, J. C., Power, M., Singh, K. D., Nicholson, D. A., et al. (1998a). Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 115, 1104–1112. doi: 10.1016/S0016-5085(98)70081-2
Hamdy, S., Mikulis, D. J., Crawley, A., Xue, S., Lau, H., Henry, S., et al. (1999). Cortical activation during human volitional swallowing: an event-related fMRI study. Am. J. Physiol. 277(1 Pt 1), G219–G225. doi: 10.1152/ajpgi.1999.277.1.G219
Hamdy, S., Rothwell, J. C., Aziz, Q., Singh, K. D., and Thompson, D. G. (1998b). Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat. Neurosci. 1, 64–68. doi: 10.1038/264
Han, T. R., Paik, N. J., and Park, J. W. (2001). Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch. Phys. Med. Rehabil. 82, 677–682. doi: 10.1053/apmr.2001.21939
Himberg, J., Hyvärinen, A., and Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22, 1214–1222. doi: 10.1016/j.neuroimage.2004.03.027
Horner, J., Buoyer, F. G., Alberts, M. J., and Helms, M. J. (1991). Dysphagia following brain-stem stroke. Clinical correlates and outcome. Arch. Neurol. 48, 1170–1173. doi: 10.1001/archneur.1991.00530230078026
Jestrovic, I., Coyle, J. L., Perera, S., and Sejdic, E. (2016). Functional connectivity patterns of normal human swallowing: difference among various viscosity swallows in normal and chin-tuck head positions. Brain Res. 1652, 158–169. doi: 10.1016/j.brainres.2016.09.041
Johnson, E. R., McKenzie, S. W., and Sievers, A. (1993). Aspiration pneumonia in stroke. Arch. Phys. Med. Rehabil. 74, 973–976.
Kern, M. K., Jaradeh, S., Arndorfer, R. C., and Shaker, R. (2001). Cerebral cortical representation of reflexive and volitional swallowing in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G354–G360. doi: 10.1152/ajpgi.2001.280.3.G354
Kim, S. Y., Kim, T. U., Hyun, J. K., and Lee, S. J. (2014). Differences in videofluoroscopic swallowing study (VFSS) findings according to the vascular territory involved in stroke. Dysphagia 29, 444–449. doi: 10.1007/s00455-014-9525-x
Kober, S. E., Bauernfeind, G., Woller, C., Sampl, M., Grieshofer, P., Neuper, C., et al. (2015). Hemodynamic signal changes accompanying execution and imagery of swallowing in patients with dysphagia: a multiple single-case near-infrared spectroscopy study. Front. Neurol. 6:151. doi: 10.3389/fneur.2015.00151
Lee, K. W., Kim, S. B., Lee, J. H., Lee, S. J., Ri, J. W., and Park, J. G. (2014). The effect of early neuromuscular electrical stimulation therapy in acute/subacute ischemic stroke patients with Dysphagia. Ann. Rehabil. Med. 38, 153–159. doi: 10.5535/arm.2014.38.2.153
Li, S., Luo, C., Yu, B., Yan, B., Gong, Q., He, C., et al. (2009). Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J. Neurol. Neurosurg. Psychiatr. 80, 1320–1329. doi: 10.1136/jnnp.2009.176214
Li, S., Ma, Z., Tu, S., Zhou, M., Chen, S., Guo, Z., et al. (2014). Altered resting-state functional and white matter tract connectivity in stroke patients with dysphagia. Neurorehabil. Neural Repair 28, 260–272. doi: 10.1177/1545968313508227
Luan, B., Soros, P., and Sejdic, E. (2013). A study of brain networks associated with swallowing using graph-theoretical approaches. PLoS ONE 8:e73577. doi: 10.1371/journal.pone.0073577
Maeshima, S., Osawa, A., Hayashi, T., and Tanahashi, N. (2014). Elderly age, bilateral lesions, and severe neurological deficit are correlated with stroke-associated pneumonia. J. Stroke Cerebrovasc. Dis. 23, 484–489. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.004
Malandraki, G. A., Johnson, S., and Robbins, J. (2011). Functional MRI of swallowing: from neurophysiology to neuroplasticity. Head Neck. 33 (Suppl 1), S14–S20. doi: 10.1002/hed.21903
Malandraki, G. A., Sutton, B. P., Perlman, A. L., Karampinos, D. C., and Conway, C. (2009). Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum. Brain Mapp. 30, 3209–3226. doi: 10.1002/hbm.20743
Martin, R. E., Goodyear, B. G., Gati, J. S., and Menon, R. S. (2001). Cerebral cortical representation of automatic and volitional swallowing in humans. J. Neurophysiol. 85, 938–950. doi: 10.1152/jn.2001.85.2.938
Martin, R. E., and Sessle, B. J. (1993). The role of the cerebral cortex in swallowing. Dysphagia 8, 195–202.
Martino, R., Foley, N., Bhogal, S., Diamant, N., Speechley, M., and Teasell, R. (2005). Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 36, 2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb
Mihai, P. G., Otto, M., Domin, M., Platz, T., Hamdy, S., and Lotze, M. (2016). Brain imaging correlates of recovered swallowing after dysphagic stroke: a fMRI and DWI study. Neuroimage Clin. 12, 1013–1021. doi: 10.1016/j.nicl.2016.05.006
Moon, H. I., Pyun, S. B., and Kwon, H. K. (2012). Correlation between location of brain lesion and cognitive function and findings of videofluoroscopic swallowing study. Ann. Rehabil. Med. 36, 347–355. doi: 10.5535/arm.2012.36.3.347
Mosier, K. M., Liu, W. C., Maldjian, J. A., Shah, R., and Modi, B. (1999). Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR Am. J. Neuroradiol. 20, 1520–1526.
Mosier, K., Patel, R., Liu, W. C., Kalnin, A., Maldjian, J., and Baredes, S. (1999). Cortical representation of swallowing in normal adults: functional implications. Laryngoscope 109, 1417–1423. doi: 10.1097/00005537-199909000-00011
Paciaroni, M., Mazzotta, G., Corea, F., Caso, V., Venti, M., Milia, P., et al. (2004). Dysphagia following stroke. Eur. Neurol. 51, 162–167. doi: 10.1159/000077663
Poorjavad, M., Talebian Moghadam, S., Nakhostin Ansari, N., and Daemi, M. (2014). Surface electrical stimulation for treating swallowing disorders after stroke: a review of the stimulation intensity levels and the electrode placements. Stroke Res. Treat. 2014:918057. doi: 10.1155/2014/918057
Robbins, J., Levine, R. L., Maser, A., Rosenbek, J. C., and Kempster, G. B. (1993). Swallowing after unilateral stroke of the cerebral cortex. Arch. Phys. Med. Rehabil. 74, 1295–1300. doi: 10.1016/0003-9993(93)90082-L
Rosenbek, J. C., Robbins, J. A., Roecker, E. B., Coyle, J. L., and Wood, J. L. (1996). A penetration-aspiration scale. Dysphagia 11, 93–98. doi: 10.1007/BF00417897
Shirer, W., Ryali, S., Rykhlevskaia, E., Menon, V., and Greicius, M. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex 22, 158–165. doi: 10.1093/cercor/bhr099
Smithard, D. G., Smeeton, N. C., and Wolfe, C. D. (2007). Long-term outcome after stroke: does dysphagia matter? Age Ageing 36, 90–94. doi: 10.1093/ageing/afl149
Soros, P., Inamoto, Y., and Martin, R. E. (2009). Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum. Brain Mapp. 30, 2426–2439. doi: 10.1002/hbm.20680
Stevens, M. C., Kiehl, K. A., Pearlson, G. D., and Calhoun, V. D. (2009). Brain network dynamics during error commission. Hum. Brain Mapp. 30, 24–37. doi: 10.1002/hbm.20478
Teismann, I. K., Steinstrater, O., Warnecke, T., Suntrup, S., Ringelstein, E. B., Pantev, C., et al. (2009). Tactile thermal oral stimulation increases the cortical representation of swallowing. BMC Neurosci. 10:71. doi: 10.1186/1471-2202-10-71
Teismann, I. K., Suntrup, S., Warnecke, T., Steinsträter, O., Fischer, M., Flöel, A., et al. (2011). Cortical swallowing processing in early subacute stroke. BMC Neurol. 11:34. doi: 10.1186/1471-2377-11-34
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
Vahdat, S., Darainy, M., Milner, T. E., and Ostry, D. J. (2011). Functionally specific changes in resting-state sensorimotor networks after motor learning. J. Neurosci. 31, 16907–16915. doi: 10.1523/JNEUROSCI.2737-11.2011
Keywords: dysphagia, stroke, swallowing therapy, videofluoroscopy, magnetic resonance imaging
Citation: Huang Y-C, Hsu T-W, Leong C-P, Hsieh H-C and Lin W-C (2018) Clinical Effects and Differences in Neural Function Connectivity Revealed by MRI in Subacute Hemispheric and Brainstem Infarction Patients With Dysphagia After Swallowing Therapy. Front. Neurosci. 12:488. doi: 10.3389/fnins.2018.00488
Received: 20 July 2017; Accepted: 29 June 2018;
Published: 20 July 2018.
Edited by:
Yasser Iturria Medina, Montreal Neurological Institute and Hospital, McGill University, CanadaReviewed by:
Karmele López-de-Ipiña, University of the Basque Country (UPV/EHU), SpainCopyright © 2018 Huang, Hsu, Leong, Hsieh and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Che Lin, u64lin@yahoo.com.tw
†These authors have contributed equally to this work and first author.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.