- 1LAM-Motion Analysis Laboratory, Neuromotor and Rehabilitation Department, San Sebastiano Hospital, Correggio, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
- 2Department of Rehabilitation & Prevention Engineering, Institute of Applied Medical Engineering, RWTH Aachen University, Aachen, Germany
- 3Shirley Ryan Ability Lab, Single Motor Unit Laboratory, Chicago, IL, United States
- 4Laboratory for Engineering of the Neuromuscular System (LISiN), Department of Electronics and Telecommunications, Politecnico di Torino, Turin, Italy
This article addresses the potential clinical value of techniques based on surface electromyography (sEMG) in rehabilitation medicine with specific focus on neurorehabilitation. Applications in exercise and sport pathophysiology, in movement analysis, in ergonomics and occupational medicine, and in a number of related fields are also considered. The contrast between the extensive scientific literature in these fields and the limited clinical applications is discussed. The “barriers” between research findings and their application are very broad, and are longstanding, cultural, educational, and technical. Cultural barriers relate to the general acceptance and use of the concept of objective measurement in a clinical setting and its role in promoting Evidence Based Medicine. Wide differences between countries exist in appropriate training in the use of such quantitative measurements in general, and in electrical measurements in particular. These differences are manifest in training programs, in degrees granted, and in academic/research career opportunities. Educational barriers are related to the background in mathematics and physics for rehabilitation clinicians, leading to insufficient basic concepts of signal interpretation, as well as to the lack of a common language with rehabilitation engineers. Technical barriers are being overcome progressively, but progress is still impacted by the lack of user-friendly equipment, insufficient market demand, gadget-like devices, relatively high equipment price and a pervasive lack of interest by manufacturers. Despite the recommendations provided by the 20-year old EU project on “Surface EMG for Non-Invasive Assessment of Muscles (SENIAM),” real international standards are still missing and there is minimal international pressure for developing and applying such standards. The need for change in training and teaching is increasingly felt in the academic world, but is much less perceived in the health delivery system and clinical environments. The rapid technological progress in the fields of sensor and measurement technology (including sEMG), assistive devices, and robotic rehabilitation, has not been driven by clinical demands. Our assertion is that the most important and urgent interventions concern enhanced education, more effective technology transfer, and increased academic opportunities for physiotherapists, occupational therapists, and kinesiologists.
Introduction
Quantitative Approaches and Measurements in Neurorehabilitation and Physiotherapy
Prevention of injuries and rehabilitation of movement pathologies are among the branches of clinical practice where the impact of technology is leading to improvement of outcomes and to economic benefits. In times of limited resources, the future perspectives and developments of all rehabilitation-related professions will increasingly depend on the evidence supporting the effectiveness of their preventive and therapeutic interventions. This issue has been discussed in a number of scientific contributions and editorials (1–3).
In the last few decades, impressive developments have taken place in many fields providing powerful quantitative approaches toward instrumentation-based assessments in cardiology (ECG, etc.), neurology (EEG, etc.), and biomechanics (inertial sensors, sEMG). Neuroengineering has made available a wealth of investigational techniques and tools, as well as tutorials and textbooks, for understanding mechanisms, implementing prevention, and measuring performance and results of interventions. The proceedings of the International Conferences on Neurorehabilitation (4–8) provide a view of this progress and tools over the last 7 years. A few examples are the devices for the assessment of force, balance, movement, oxygen consumption, and of course, muscle activity. These tools underwent different degrees of translation to the clinics and to the market. This work focuses on surface EMG (sEMG).
As a “muscle activation measuring tool” sEMG has played a growing and important role in neurorehabilitation over four decades (9–19). Figure 1 shows the increase of international peer-reviewed publications in the sEMG field and Figure 2 shows an example of the development of sEMG technology since 1950. Equally striking developments have taken place in related fields of neurophysiology, signal processing and extraction of physiologically relevant features from sEMG over the last 50 years (23–30). Moreover, the number of clinical situations compatible with objective measurements of muscular activity, for planning treatment and for pre- and post-treatment assessment, is large and rapidly increasing, as described in section Surface EMG Applications.
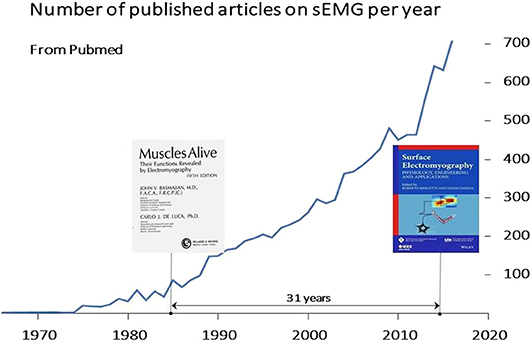
Figure 1. Rate of publication of sEMG articles on international peer-reviewed journals. These articles and more than 20 textbooks (see: https://www.robertomerletti.it/en/emg/material/books/) provide a huge body of knowledge that ranges from technical issues to clinical applications in research labs. A Pubmed search (June 2020, keywords “surface electromyography” OR sEMG) indicated over 5,500 publications (14.8% of the 37,000 publications listed in Pubmed under “neurorehabilitation”). Over 180 review papers are listed by Pubmed in the sEMG field. In most countries, this knowledge is not translated into routine applications for planning treatment, monitoring and assessing outcome in neurorehabilitation.
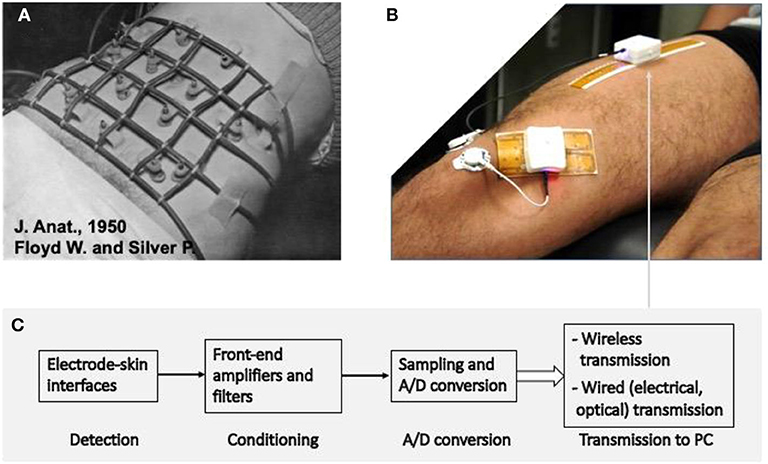
Figure 2. Example of the advances in sEMG detection in the last 70 years. (A) The detection system used by Floyd and Silver in 1950 (20) to monitor abdominal muscles. The electronics used for signal conditioning had the size of a suitcase. (B) Modern system for detection, condition, A/D conversion, and transmission of signals from sEMG electrode arrays. The white box contains the system described in (C) and the rechargeable battery to supply it for a few hours. (C) Schematic diagram of the signal detection, conditioning, conversion, and transmission depicted in (B). Two systems, with different detection grids of 32 electrodes each are applied to the rectus femoris and vastus medialis. Up to four such systems can operate simultaneously and provide images of sEMG activity in four locations (21). (A) Is reprinted, with permission from Floyd and Silver (20).
Despite this large body of knowledge, literature, and collected research works, the clinical acceptance of sEMG advances among physiotherapists (PTs), kinesiologists and medical clinicians remains low (31). This is in contrast with the history of ECG in cardiology (32, 33) and EEG in neurology (34). Apparently, the potential benefits of sEMG in assessing treatment appropriateness and in determining cost saving are not fully demonstrated in neuromuscular rehabilitation, primarily because they have not been investigated (22). Research has been focused on academic achievements rather than on clinical applications.
A number of “barriers” exist limiting the widespread application of sEMG techniques in clinical assessment and in neurorehabilitation. Some barriers are cultural, such as the inappropriate comparison with the diagnostic power of needle EMG (35, 36), or are related to the issue of assessing “function” (with scales and observational descriptions) rather than “impairment” (with measurement of physical quantities), or to the wide-spread diffidence/reluctance with respect to objective measurement, instrumentation, and Evidence Based Practice (EBP) (37–40), or the belief that time spent in assessing results is not productive. There is often a lack of a common language with rehabilitation engineers and many therapists lack the technical background to interpret the sEMG outcomes. Some barriers are technical, like difficulties with the application of sEMG, signal processing and information extraction algorithms which do not directly produce clinically relevant information, or the user-unfriendliness of some equipment. Finally, the cost of the devices, the reimbursement procedures, and the time needed to perform a measurement and obtain a clinically useful information have also to be taken into account.
It is the objective of this work to discuss these barriers and the options for reducing them.
The Role of sEMG for Monitoring Disorders, Planning Treatments, and Assessing Their Effectiveness
Surface EMG can be used in monitoring neuromuscular pathologies, in prevention of work-related disorders and occupational therapy, and in monitoring neuromuscular changes/progress in acute patients (see section Surface EMG Applications). Information on muscle activation during a movement or effort adds to the clinical evaluation and provides a picture of both impairment and functional alteration. As in other branches of medicine, clinical assessment does not always provide the information needed to design a treatment plan. It is crucial to recognize situations in which added value can be expected from instrumental analysis. Some example of questions which can be answered with sEMG, and which have an impact on designing a rehabilitation plan, are presented in section Some Fundamental Questions. Measurement of muscle activation provides information on the motor unit recruitment/derecruitment capability, on fatigue, synergies, co-contractions, etc. as well as evidence of the efficacy of the rehabilitation plan. Section Surface EMG Applications provides examples of applications.
Physiological and Technological Literacy: The Need for Academic Education and Large-Scale Studies
Physiological and technological literacy is a requirement for medical and health-allied professionals.
The measurement of a physiological quantity (e.g., localized myoelectric manifestations of fatigue) is useless if the recipient does not know its meaning, how to use the information contained in the result and how reliable the measurement is. An ECG does not convey much information to a person knowing little about cardiac electrophysiology. Similarly, information about muscle fiber conduction velocity, sEMG patterns, and amplitude or frequency spectrum, etc. do not inform a person knowing little about muscle electrophysiology and basic signal analysis. The insufficient competence of instrument operators in performing and interpreting such measurements leads to the predictable conclusion that measurements do not contain clinically useful information.
In many countries, the lack of this literacy in physiology/technology leads to the education of physiotherapy and occupational therapy graduates, and future teachers, who serve primarily as professional operators with empirical knowledge mainly (41). This is confirmed, in many countries, by the lack of scientific publications by these graduates (42). In addition, in many countries, the unavailability of PhD degrees in physiotherapy or occupational therapy precludes the evolution of a full academic career and the training of qualified teachers and researchers, perpetuating the situation in its current state. One consequence is that scientists able to conduct large, badly needed, multi-center studies to document the validity of the information obtained from sEMG, do not yet exist. The absence of qualified operators results in an abundance of scientific papers focused on muscle electrophysiology and sEMG technology, in contrast with the lack of studies on the clinical applications of sEMG and on the clinical testing of usability and effectiveness of this technology.
Bioelectric Signals: Basic ECG, EEG, and EMG. General Considerations and History
The most important bioelectric signals are generated by the heart (ECG), the brain (EEG), and the muscles (EMG). The technologies for collecting, reading and interpreting these signals were developed 50–60 years ago and reported in many review papers and books (9, 32–34, 43). ECG and EEG are widely accepted for clinical monitoring of heart and brain functions. They are an important part of the training of cardiologists, neurologists and of the associated health operators and technicians. International standards for the detection and interpretation of these signals have been defined decades ago. In contrast, despite the huge amount of international literature from research labs, sEMG suffers from a wide gap between research and clinical applications. This gap is markedly greater in Mediterranean countries than in North-European countries, USA, Canada, and Australia.
Surface EMG is more than a century old. It started with the pioneer work of Piper, Kugelberg, and Denny-Brown (44–46) and progressed in the second half of last century with the fundamental research of Basmajian, Lindstrom, Gydikov, De Luca (9, 23, 24, 43, 47) and the clinical efforts of Kasman, Cram, Kumar, and many others, leading to the systematization of knowledge in sEMG textbooks (10, 11, 18, 19, 48–52). Some of these books are open-access and free for download.
The sEMG signal is the algebraic sum of the motor unit action potentials (MUAP) generated by the active motor units (MU) and detected over the skin. Like any other signal, sEMG provides quantitative information concerning wave-shape, amplitude, power spectral density, etc. Using such information or those derived by visual observation is a clinical choice/decision.
Traditionally, this signal is detected between two electrodes aligned in the direction of the fibers (bipolar or single differential electrode montage). This “conventional” bipolar sEMG provides ready answers to many important questions in rehabilitation. It is simple to apply on multiple muscles, even by non-technical users, and provides reliable information on the general activation of a muscle or muscle groups and on temporal events of muscular activation (53). Conventional bipolar sEMG is applicable in almost all clinically relevant situations, such as in dynamic movements, in isometric contractions, and in patients with severe movement disorders, adults and children.
Advanced sEMG technologies provide a much larger amount of physiological information than the simple bipolar technique. For example, the use of surface electrode arrays, enables the detection of a so called sEMG “image” that is evolving in time like a movie on the skin [see examples in (52)]. This time-varying electrical image provides indirect information on muscle force, on motor unit (MU) recruitment and de-recruitment strategies and discharge rates, on muscle length, on the location of the innervation zones (IZs), myoelectric manifestations of muscle fatigue, and many other phenomena of interest in neurophysiology, neuropathology, neurorehabilitation, ergonomics, aging, sport, and space medicine (14, 54–60). This technique has been applied in research labs and has been ready for large clinical studies for a few decades. A series of open-access tutorials on this topic is being published in the Journal of Electromyography and Kinesiology (61, 62).
The current applications of sEMG mostly concern physiological investigations, monitoring of neurological disorders, planning of treatments, assessment of interventions and control of prostheses and robots (section Surface EMG Applications). For reasons of space, only a few references are provided here for each area of application. A much more extensive description of applications is provided in chapters 10–20 of Merletti and Farina (18).
Surface EMG Applications
General Fields of sEMG Application
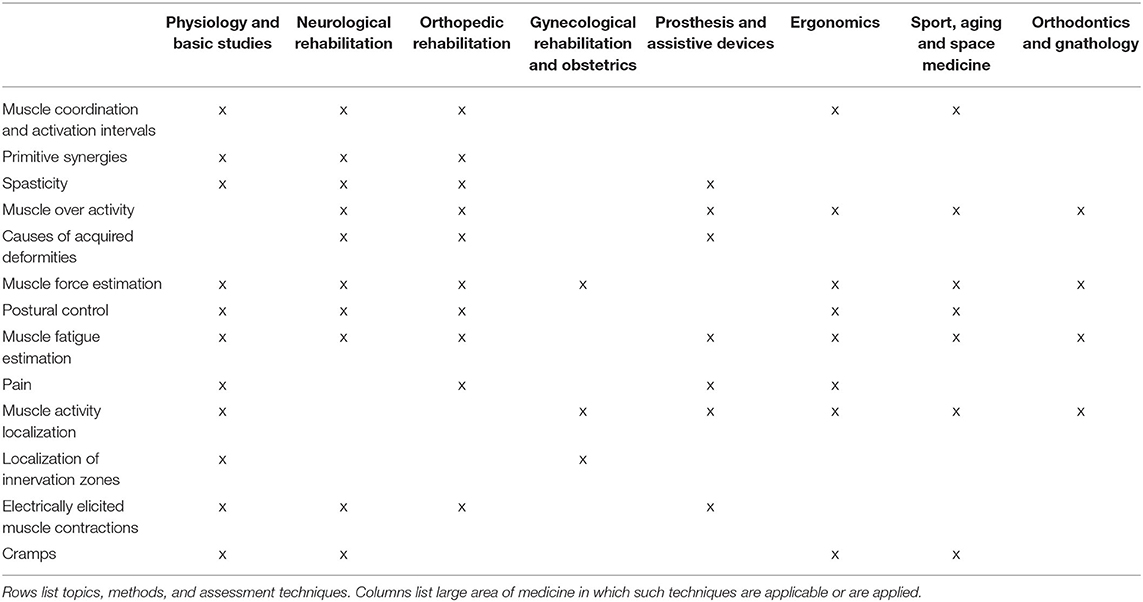
Like other bioelectric signals, sEMG provides a fundamental added value to the assessment of the organ generating it. The information about muscle activation has different forms (amplitude, timing, morphology, spectral features, muscle fiber conduction velocity, displacement of innervation zone, contributing synergies, muscle coordination, control strategy, etc.) and is relevant in many fields ranging from orthopedics and neurorehabilitation, to movement analysis in exercise and sport, from aging to gnathology, from obstetrics to occupational and space medicine (14, 54–60). Each of the specific assessment methods and techniques listed in Table 1 and section Overview of sEMG Applications is transversal to most of these medical applications and rehabilitation fields.
Most of the available literature on sEMG concerns methodological issues and proof of concepts, carried out mostly on healthy subjects. Clinical works on large patient groups are few, as well as case studies and case-series on small samples. This does not mean that the developed techniques have no clinical applications or do not answer clinical questions. Rather, it means that there is a huge gap in translating techniques to the clinical environment (see section Barriers to Widespread Clinical Use of sEMG in Neurorehabilitation). Some articles question the diagnostic and therapeutic value of sEMG (35, 36, 63). Conversely, other studies, indicate the use of sEMG as essential in the decision making of functional surgery and in the assessment of spasticity (22, 52, 64–68).
The availability of evidence to support rehabilitation has been a long-standing challenge. The complexity of designing clinical trials to assess the efficacy of rehabilitative treatments has become a topic in the current literature (69). Since sEMG is not a treatment itself, but an instrumental assessment tool that adds to clinical evaluations, specific studies should be designed to assess its impact on (1) the variation in the choice of the rehabilitative pathway and (2) the incremental efficacy of this variation on functional outcomes and on cost/efficacy indicators. A few efforts in this direction can be found in the literature for instrumental gait analysis (22, 70–73). Similar specific studies on sEMG are needed. To address this gap, research teams should include sEMG experts, clinical rehabilitation professionals, rehabilitation engineers, experts in research methodology, and in Health Technology Assessment.
The sEMG signal is affected by a large number of factors reflecting the pathophysiology of the muscle and of its control strategy. As such, it provides a window into the muscle, the peripheral (PNS), and central nervous system (CNS). These factors range from alteration of the MUAP propagation along the motor unit fibers to the control of force by recruitment/derecruitment and firing rate of the individual motor units. Unraveling the large amount of information contained in the signal is a major technological challenge and an important field of current engineering, physiological and clinical research (26, 27, 50, 61, 62). The use of large wireless electrode arrays is today possible and relatively simple (Figure 2B) so that a fast progress is expected for the next decade. The clinical users of these developments should take a primary role by participating to such progress and orienting it; their education should account for the research instruments of today that will be clinical tools tomorrow (62).
Overview of sEMG Applications
Applications in Physiology and Basic Clinical Studies
Basic and clinical neurophysiology are fields in which sEMG has been extensively applied (12, 17, 74–77). The applications listed below are focused on the pathophysiology of muscles whose knowledge is a pre-requisite for planning clinical interventions and solving clinical problems in the neurorehabilitation field.
Muscle Coordination
It was demonstrated early on that sEMG is suitable for the detection of co-activation of agonist and antagonist muscles, whereby physiological activation patterns could be distinguished from pathological ones (78–80). The clinical relevance of muscular coordination became stronger with the improvement of sEMG techniques (81) and should now be an integral part of any biomechanical analysis of movement (82, 83). The most common use of sEMG to assess muscular coordination is in clinical gait analysis. Here it can be used either in functional diagnosis or in the monitoring of therapeutic outcomes (84, 85). The most prominent fields of application are for neurological impairments like cerebral palsy (CP) and stroke (86), orthopedic impairments, such as back pain (87), anterior cruciate ligament (ACL) injuries (88), and degenerative joint disease (89). However, the interpretation of sEMG signals with respect to muscular coordination requires some caution (90). For this reason, different signal processing methods have been developed in the past to support the interpretation of sEMG signals (91). Currently, the extraction of sEMG primitive synergies is widely used (92, 93). The most recent approaches to categorization take into account biomechanical factors, on which the sEMG signal depends, when determining the physiological or pathological muscular coordination pattern (89, 94).
Extraction of Primitive Synergies
Muscle activation patterns, represented by the sEMG envelopes of a few muscles, can be decomposed into a limited number of “basic” functions or patterns, called “synergies” or “primitives.” These primitive patterns can be combined, with different individual weights, and result in the apparent modular organization of multi-muscle activities across different motor tasks. It has been proposed that the nervous system simplifies muscle control through such modularity, using these basic synergies (primitives) to activate muscles in groups. This discovery has had a huge impact on the analysis of motor control and neurorehabilitation since it implies that the CNS generates forces and movements by optimizing the control strategy of either individual muscles or (more likely) muscle synergies (95–101). Research, largely based on sEMG, is focusing on the alterations of these synergies in stroke and other pathologies.
sEMG-Based Muscle Force Estimation
The net torque at a joint is usually produced by a number of muscles, ligaments and other passive structures, and by external forces (e.g., gravity, closed-kinetic-chain forces, orthosis-produced forces). The force contributed by each individual muscle may fluctuate (as does the sEMG amplitude of the muscle) while the total measured torque may remain constant. The estimation of force sharing among synergic muscles by means of sEMG has been reviewed by Perry 30 years ago (102), and more recently, by many other investigators (103–105) but is not yet satisfactorily solved. It is clinically important to realize that one sEMG channel reflects the activity of one (or part of one, or few) superficial muscles while others (including often non-monitored antagonists) may also contribute to the measured torque at the joint. For this reason, care must be taken in associating changes of sEMG amplitude of one muscle to changes of global torque at a joint. At this time, it is rarely possible to acquire the sEMG signal from all muscles acting on a joint. Figure 3 shows two cases of changing sEMG amplitude in three muscles acting on the elbow during two isometric constant force contractions of the elbow flexors. Brachialis and triceps brachii muscles were not monitored. Although the mechanical contribution of each monitored muscle cannot be estimated, the sEMG amplitude trends suggest that the three contributions are changing in time while the total (measured) torque remains constant. Information of this type should be exploited in sport and rehabilitation medicine to teach or modify the muscle activation patterns.
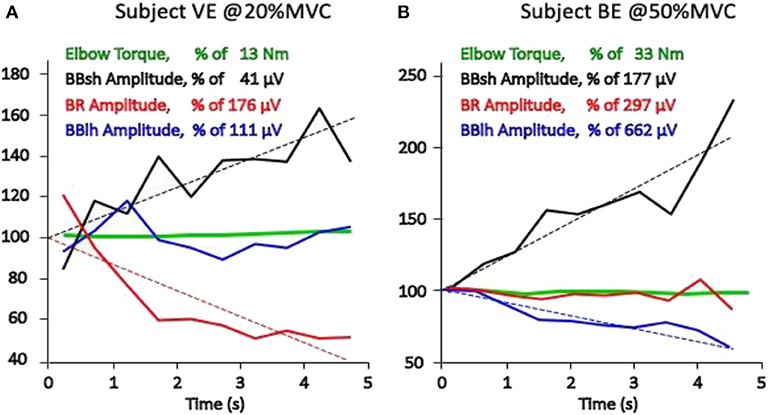
Figure 3. Torque at the elbow and average rectified value (ARV), estimated on epochs of 0.5 s, of the sEMG obtained from three pairs of electrodes placed between the IZ and the tendon endings of the long and short head of the biceps brachii (BBlh, BBsh) and of the brachioradialis (BR) of two healthy subjects (A and B). All values are expressed as percent of the initial value, which is defined here as the intercept of the linear regression of the experimental values (dashed lines). Results from two 5-s isometric constant torque contractions performed at 20% MVC and at 50% MVC are presented. A progressively changing load sharing among the three muscles is evident and different in the two subjects (A and B). Different conclusions would have been reached depending on which single muscle had been monitored (unpublished data).
Myoelectric Manifestations of Muscle Fatigue
The term “muscle fatigue” has many definitions mostly associated with measurements performed during an isometric constant force contraction which is a common and important “bench-test” condition. One definition considers mechanical fatigue as the inability to sustain a given contraction level and is associated with the endurance time (in isometric constant force contractions) or to the inability to perform a task. Another definition refers to “myoelectric manifestations of muscle fatigue” and considers fatigue as the set of changes affecting sEMG features from the very beginning of the contraction. Its main indicator is muscle fiber conduction velocity (CV) which decreases more or less rapidly depending on the level of contraction and is usually measured during isometric constant force contraction. Both types of “fatigue” depend on blood flow and on the stability of the recruited MU pool. Blood flow is blocked, and all MUs are recruited, at contraction levels above about 50% MVC in most muscles (18, 25, 106–110). In this condition many confounding factors are removed (or are constant) so that an acceptable “bench-test” condition is obtained. The reduction of muscle fiber CV causes a compression of the power spectrum of the sEMG toward the lower frequencies and a decrement of the mean and median spectral frequencies that are generally considered as fatigue indicators but that are affected by many additional confounding factors. Measurements of the myoelectric manifestations of muscle fatigue in intermittent or dynamic contractions are very questionable because of many confounding factors (variable blood flow, variable pool of active motor units, etc.) and require considerable competence and caution in defining the specific measurement protocol and the measurement modalities (30, 77, 111).
Muscle Activity Localization
The first use of electrode arrays was described by Gydikov in 1972 (23, 24, 52, 112). Identification of innervation zones using electrode arrays was reported by Masuda et al. in 1985 (113, 114) while the technique of “high density” surface EMG (HDsEMG) was developed 10–15 years later (76, 115–119). The technique is also referred to as sEMG imaging and is used to identify active muscles, the geometry of MUs (e.g., fiber length and orientation), and their innervation zone (IZ). Deep muscles (or deep MUs of a superficial muscle) produce force but their sEMG contributions may be near or below the noise level. Techniques to detect such contributions using HDsEMG are being investigated to obtain a sort of “electromyographic tomography” (120, 121). Figure 4 shows a large grid (128 contacts, 10 mm apart) displaying the regions of activity of the extensors of the fingers of the right hand. Similar maps may be obtained for other muscles or muscle groups, such as the erector spinae, the trapezius, etc. Biofeedback applications, for correcting muscle involvement while performing a task, are potentially valuable.
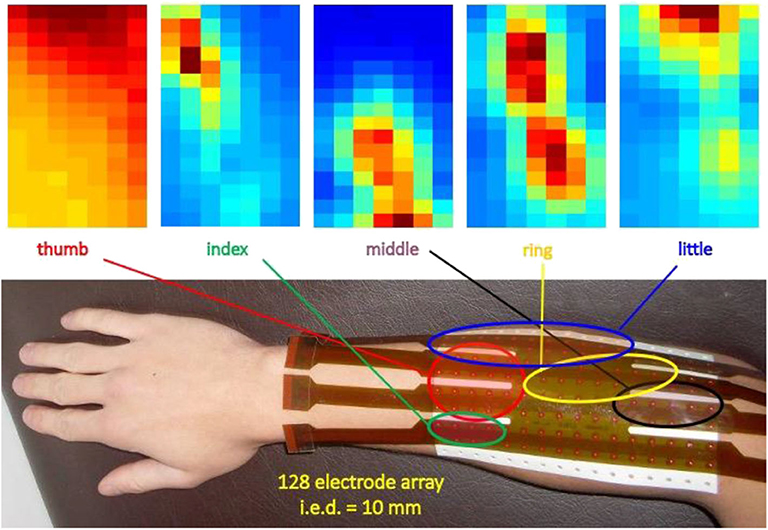
Figure 4. Example of application of a 16 × 8 grid on the dorsal side of the forearm to identify/monitor the regions of activity of the finger extensors. The colors represent the intensity (RMS value) of the longitudinal differential signals (15 × 8 channels). Dark red = strong signal, dark blue = no signal. Interelectrode distance: 10 mm (unpublished data).
Location of Muscle Innervation Zones
A textbook is available with the location of the IZs of 43 muscles (19). Knowledge of the location of the IZs of a muscle is clinically important for (a) proper positioning of a single electrode pair between the IZ and tendon junctions, (b) targeted injection of botulinum toxin (58, 122), and (c) programming surgery in a way that would avoid damage to muscle innervation. The latter application is particularly important for reducing the risk of anal sphincter partial denervation resulting from episiotomy (18, 51, 123–125).
sEMG of Electrically Stimulated Muscles
Neuromuscular electrical stimulation involves the application of electrical stimuli to a nerve, or to the motor point of a superficial skeletal muscle, with the objective of inducing and controlling muscle contractions. The stimulus strength (either current or voltage or pulse width) determines the number of recruited motor units whereas the stimulus frequency determines their synchronized discharge rate. Since all the recruited MUs are activated synchronously, effectively as a single large MU, the sEMG signal is deterministic rather than stochastic and is referred to as M-wave or Compound Motor Action Potential (CMAP). Confounding factors and the effects of variability of CNS control, present in voluntary contractions, are eliminated and myoelectric manifestations of muscle fatigue are easy to measure. The technique provides a powerful bench-test for the quantitative investigation of a muscle's electrical and mechanical properties (126–128). Finally, functional electrical stimulation (FES) devices may be triggered or controlled by residual sEMG activity of partially paralyzed limb muscles (129).
Applications in Neurological Rehabilitation
Support for the Assessment and Treatment of Muscle Spasticity and Overactivity
The most common definition of spasticity goes back to Lance, according to whom spasticity is “…a motor disorder, characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyper-excitability of the stretch reflex as one component of the upper motor neuron syndrome” and does “not include impaired voluntary movement and an abnormal posture” (130). Beside this definition, spasticity is a term that is firmly but not consistently used in the clinical environment and among pathologies (131). A brief history of the term, of its use and ongoing evolution can be found in the paper from Baude et al. (132). Not only is a generally accepted definition of spasticity lacking, but there is also a lack and a need of objective methods for assessing the level of spasticity (133, 134).
Many different studies have shown that sEMG can quantify alterations associated with spasticity, for example through the extraction of sEMG primitive synergies (93). However, these approaches do not distinguish between spasticity, on the one hand, and dystonia, rigidity or voluntary activation, on the other hand. According to the definition of Lance objective assessment of spasticity should be based on the investigation of the tonic stretch reflex. Several studies carried out in the last decades have consequently used sEMG to investigate the muscle response to stretch in the presence of spasticity (135–138). These studies have shown that sEMG provides the easiest and most reliable way of determining the stretch reflex threshold (133). Quantitative assessment of spasticity (139–141) as well as monitoring of treatment are possible (142) by combining sEMG with biomechanical techniques, measuring stretch velocity and torque. More recent approaches use the increased tonic stretch reflex to quantify the occurrence of spasticity during freely performed movements (143–145). Figure 5 shows that in the presence of spasticity, a freely performed extension movement of the elbow leads to an increasing muscular activation with increasing movement velocity. This is in contrast to healthy subjects who use a lower muscular activation when the movement is performed with higher speeds.
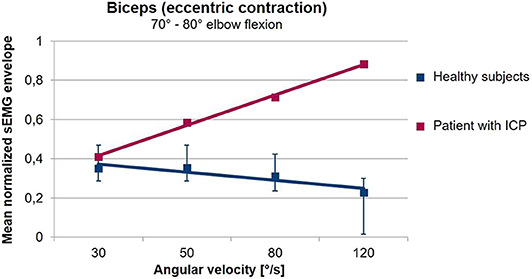
Figure 5. Averaged sEMG envelopes of the Biceps Brachii as a function of the movement velocity during freely performed elbow extension movements. Values are normalized with respect to the 75% of the maximal value of the envelope. The sEMG when the elbow passes an interval from 80 to 70° flexion angle is analyzed. In healthy volunteers (blue), the biceps sEMG envelope decreases with increasing angular velocity. In contrast, in the patient with a spastic movement disorder (red) shown here, muscular activation increases with angular velocity. The gradient of the sEMG envelope–movement velocity relationship is thus a measure for the presence of spasticity during freely performed movements (unpublished data).
Support to the Identification of the Causes of Acquired Deformities and Treatment Selection
Following lesions to the CNS, such as stroke, traumatic brain injury, etc., patients can develop acquired deformities at the lower limb that impair or inhibit walking. These deformities, often termed contractures, are due to a combination of paresis, muscle overactivity, spasticity, along with mechanical barriers, including muscle shortening, increased muscle stiffness and viscosity, and retractions. Other phenomena (e.g., overactivity, spasticity, lack of recruitment) have to be assessed in dynamic conditions, because they may not be detectable during the bedside evaluation, and may be present only during walking, or vice versa (22, 67, 132). The direct assessment of muscle activity with sEMG and indwelling fine-wire EMG for deep muscles allows discriminating between active and passive causes, thus supporting the selection of treatments tailored for each patient (22, 67, 73, 85, 132). For example, in the assessment of the equinovarus foot deformity in stroke survivors, sEMG of the plantar flexors reveals which muscles are overactive during walking (146). This observation supports the clinical decision-making in choosing among focal muscle blockages, non-pharmacological treatments (147), and neuro-orthopedic or functional surgery. It is worth noting that, in stroke patients the triceps surae muscles can be completely silent during swing with equinus (73). Figure 6 presents data from two stroke patients during walking. Both patients have an equinus foot deformity (i.e., limited dorsiflexion) with the same kinematics. They look equal, based on the visual observation of their gait. Yet, on further analysis of sEMG data, the two equinus deformities have completely different causes, and these are outlined by the sEMG traces (and by sEMG only).
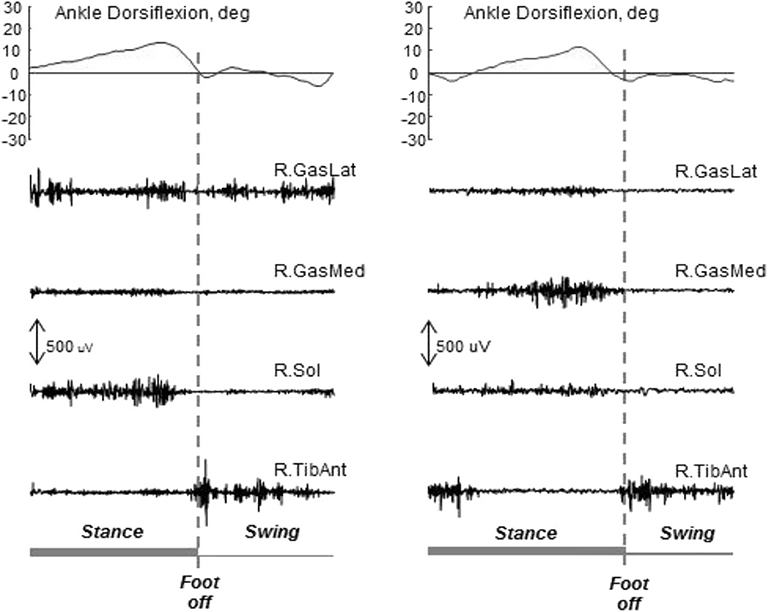
Figure 6. Ankle kinematics and sEMG data from two stroke patients with equinus foot during the swing phase of gait. The same kinematics can be observed, with different underlying mechanisms. In both cases the activity of the tibialis anterior is present at foot off as required to lift the forefoot. On the left, the activity of the gastrocnemius lateralis during the swing phase hinders dorsiflexion. On the right, there is no activity of the triceps surae during the swing phase, and the lack of dorsiflexion is due to the triceps stiffness only. In this situation, sEMG is needed to support the decision-making concerning intervention. Unpublished data acquired in a research project approved by the local Ethics Committee (2017/0123710).
Next, the recruitment of dorsiflexor muscles during walking, regardless of their voluntary activation during tests at the besides, is useful to further tune the surgical plan (e.g., split and transfer of the tibialis anterior tendon) (66, 148). Similarly, sEMG of the quadriceps muscle during walking can be used to support the clinical assessment for selecting the best treatment for stiff-knee gait (22, 65). In stroke survivors, surface EMG can also have a fundamental role in the planning of functional surgery of the upper limb, to support the surgeon's decision about which muscle to lengthen and which muscle insertion to transfer (64). Surface EMG has been used to assess motor function and to support clinical planning of surgical correction of foot deformities in CP children (149). Instrumental gait analysis and sEMG are considered among the fundamental sources of information to drive treatment selection (150).
Surface EMG-derived indices are also used as outcome measures to evaluate the responsiveness to treatments (151). In clinical practice, real-time sEMG can be used by physiotherapists (a) to control if the movement requested to the patient is performed by the proper target muscle(s) or by means of compensatory mechanisms, (b) as a direct measurement of variations consequent to mobilization, verticalization, trunk fixation, in acute neurological patients, (c) to assess the effect of different orthoses on muscle activation, which can vary toward or away from the normal pattern (152).
In conclusion, in patients with either acquired CNS lesions (e.g., stroke, traumatic brain injury, spinal cord injuries) or degenerative diseases (e.g., multiple sclerosis), sEMG can be used (1) to better understand the underlying mechanisms of gait deterioration, especially where multifactorial causes coexists, (2) to support the clinical decision making and/or the rehabilitative pathway, and (3) as a marker of disease progression or intervention effectiveness (153). The same considerations are applicable to many other subsections of the neurorehabilitation field, to exercise physiology, occupational, and sport medicine.
Postural Control
Based on the pioneering work of Joseph and Nightingale (154), we now know that many muscles have a postural role that has been investigated by means of sEMG. These muscles oppose the pull of gravity, react to perturbations and allow us to stand, sit, or maintain a desired posture. They achieve this objective by controlling the stiffness of joints (mainly ankle) and by compensating for gravitational forces in either a continuous or an intermittent way. The standing human body is an intrinsically unstable inverted pendulum that requires continuous micro-adjustments to keep the center of mass and the center of pressure within the space defined by the feet.
This mechanism is altered by age and many pathologies and sEMG provides means to monitor such alterations. The role of sEMG is of paramount importance in helping investigators understand how neural regulation contributes to the prevention of falling, by studying the control of posture and the responses to postural perturbations in healthy and pathological subject (155–160).
Applications in Orthopedic Rehabilitation
The majority of rehabilitative treatments delivered by physiotherapists are related to orthopedic pathologies. In this field, the available sEMG-based indices assessing muscle activation, symmetry and localized fatigue can be used to support the selection of the therapeutic exercises and to monitor their effectiveness over time (161). In patients with low back pain (LBP), sEMG has been used as a tool for functional diagnosis and to assess the effectiveness of treatment (162, 163) and manipulation (164). Two systematic reviews are available on this topic (164, 165), which concluded that sEMG-based parameters of amplitude and localized fatigue are useful tools to monitor the effect of different interventions delivered to relieve LBP.
At the shoulder level, alterations in neuromuscular control of the scapular muscles has been proven in subjects with subacromial pain syndrome based on sEMG data (166). The effect on muscle activation of myofascial treatment techniques, such as dry needling, has been described in women with trapezius myalgia (167). Neck muscle dysfunction has been quantitively analyzed in patients with cervical spine pain (168, 169). It has to be stressed that the acquisition of sEMG data from the shoulder and neck muscles requires specific training and adequate instrumentation.
Lower limb orthopedic pathologies, related to either sport activity or injuries, have been widely investigated with sEMG-based techniques. For example, sEMG has been used to compare the activation of gluteal muscles between healthy and injured runners, to quantify thigh muscle imbalance in subjects with patellofemoral pain (170–172), and to investigate the causes of Achilles tendinopathy (173). Moreover, sEMG can be used to evaluate residual muscle function and abnormalities in patients who underwent a total hip or knee replacement and to tailor the rehabilitation programs (174). Considerable literature on this topic is available, inclusive of reviews and meta-analyses (171, 173, 175).
Applications in the Control of Prosthetic and Assistive Devices
Surface EMG detected from the residual muscles of an amputee has been used for controlling the motors of arm/hand prostheses (myoelectric prostheses) for five decades. This technique is limited to 2–3 basic commands and movements, is not intuitive and requires that the subject learns to associate a specific muscle contraction to the desired output. Recently the technique based on “Targeted Muscle Reinnervation (TMR)” has been tested with success in subjects with amputation at the shoulder level. The residual nerves from the amputated limb are surgically transferred to other, previously denervated, muscles that are not used by the subject. Reinnervation of these muscles takes place within 3–6 months. For example, branches of the median, radial, musculocutaneous, and ulnar nerves may be grafted to specific regions of the serratus or pectoralis muscles. Once reinnervated, these muscles act as biological amplifiers of the neural commands meant for the missing muscles and their sEMG can be detected with electrode arrays and decoded, by pattern recognition processes, for the control of the motors of the prosthesis. The command is therefore intuitive, that is the amputee attempts to move the missing arm and the mechanical arm moves as “desired.” While some problems need to be solved, this technique is highly promising for specific amputees (176–178).
Applications in Pelvic Floor, Obstetrics, and Gynecologic Rehabilitation
Functional Assessment of Pelvic Floor Muscles
Pregnancy and high-impact sport activity are considered as risk factors for pelvic floor dysfunctions, including urinary incontinence. Surface EMG data demonstrated significant test-retest reliability and significant clinical predictive validity for urinary stress and urge incontinence. Pelvic floor muscle sEMG is reliable and consistently predictive of several important clinical status variables, it can be a useful tool in early detection and prophylactic intervention for muscle laxity. Recent advances in sEMG technology make it cost-effective, convenient and easy to learn and administer by trained assisting staff. This technology is a powerful complementary tool for digital assessment of pelvic floor muscles and should be considered for use in gynecologic practice. Prenatal exercise programs, supported by pelvic floor muscle exercises, should be recommended for pregnant women, especially those who are accustomed to higher exercise intensity (179–181). Surface EMG using intravaginal probes is of widespread use as a biofeedback technique as well as for assessing pelvic floor muscles activity in women. Many muscles are involved and the issue of crosstalk during intravaginal sEMG recordings has been reviewed in Flury et al. (182). A gap in knowledge affecting sEMG investigation methods was identified by these authors. Literature addressing the proper electrode location and the crosstalk problem is scarce and often flawed. Conclusions are regularly drawn from an insufficient basis of evidence. Further research and training of operators is required (182).
High density surface EMG (HDsEMG) signals have been used for mapping the activity of the muscles surrounding the vaginal, the urethral and the anal canals (183, 184). Hacad et al. observed that continent and incontinent male patients presented sEMG changes during the first 6 months after radical prostatectomy that could be justified by the denervation/reinnervation of the external urethral sphincter (185).
Prenatal sEMG of the Anal Sphincter to Predict the Impact of Episiotomy
Although very controversial and discouraged, episiotomy is still a widely performed surgery during child delivery. The techniques described above for the location of MU innervation zones (IZ) provide a tool to estimate the risk of partial denervation of the external anal sphincter (EAS) consequent to episiotomy. An intra-anal probe with a circumferential array of 16 electrodes detects the sEMG activity of the EAS during a voluntary contraction. Proper software identifies the location of the IZs of motor units of the EAS. This information can then be used, at the time of delivery, to guide episiotomy (if necessary) to the right or left side to minimize the risk of EAS partial denervation and possible future incontinence. Figures 7A,B show the MUs (and their IZs) detected in one subject at the 34th week of pregnancy and at the 6th week after vaginal delivery with right mediolateral episiotomy. Figures 7C,D show the distribution of IZs identified in 86 cases of episiotomy (all performed on the right side and out of 331 deliveries) around the electrodes of the probe. A statistically significant drop of the number of motor units innervated in the right-ventral (RV) quadrant of the EAS as well as the post-delivery reorganization of the EAS motor units are evident in Figures 7C,D (123, 125). This technique could be used as a biofeedback modality to retrain the muscle as is done with muscles surrounding the vaginal canal.
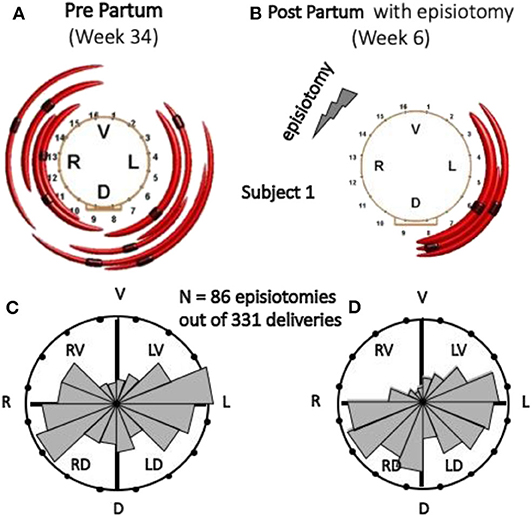
Figure 7. Effect of episiotomy on the EAS innervation pattern. The identified EAS motor units, indicated by red arcs, are not necessarily all the motor units of the EAS. (A,B) Identified MUs and their IZs, 4–6 weeks pre-delivery and 6 weeks post-delivery with right mediolateral episiotomy, in one subject. (C,D) Circular histograms of the number of EAS IZs pre- and post-delivery in 86 cases of right episiotomy (out of 331 deliveries). Both histograms are normalized with respect to the highest bin. The change in the RV quadrant of the EAS is statistically significant (123). V, ventral; L, left; D, dorsal; R, right. (A,B) Reproduced with permission from Cescon et al. (123), (C,D) Reproduced, with permission from Di Vella et al. (186).
Applications in Ergonomics
Surface EMG techniques in ergonomics and occupational medicine for prevention and monitoring of occupational disorders were developed in the 90s (49, 187) and are currently applied for assessing chairs, posture, occupational tasks, fatigue, and risk at work (188–190). As an example, Figure 8 shows maps of sEMG RMS value (one 0.5 s epoch) of the trapezius muscle of a subject typing with and without forearm rest on the desk. Different activation levels of the upper part of the trapezius are evident while the subject is unaware of them. Teaching correct movements/efforts at work and prevention of work-related disorders are largely based on sEMG applications.
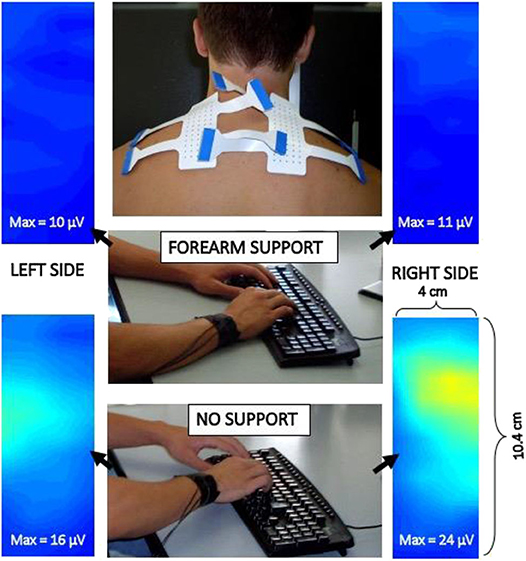
Figure 8. Example of two electrode grids applied to the trapezius muscle to study its activity during typing on a keyboard with and without arm rest on the desk. Images are interpolated and show the sEMG RMS distribution in space (see movies at URL https://www.robertomerletti.it/en/emg/material/videos/f6/ and https://www.robertomerletti.it/en/emg/material/videos/f7/).
Applications in Exercise Physiology, Sports, and Aging
The literature concerning sEMG applications in sports is very extensive and focused on physiology (191, 192) training, prevention of injury, and recovery after injury (in particular the anterior cruciate ligament injury (193). Many sports have been investigated, in particular golf (194), jumping (195), cycling (196), sprinting (197), volleyball (198), but also strength training (199, 200), back pain in rowers (201), patellofemoral pain (170, 202), and aging (59, 203). The distribution of muscle fiber conduction velocity, related to fiber diameter, may provide insight in muscle structure. Mean/median spectral frequencies are affected by too many confounding factors to be used for this purpose but may be useful, in strictly controlled experiments, to monitor muscle fatigue resistance.
Applications in Gnathology
Temporomandibular disorder (TMD) is a term indicating musculoskeletal disorders in the jaw muscles and/or the temporomandibular joint. Surface EMG analysis has been employed to obtain a better understanding of TMD and is useful to elucidate the masticatory muscle function and adaptation in patients with TMD, using indices of dominance, asymmetry, co-ordination and co-operation of temporalis and masseter muscles during mastication. An association exists linking decreased activity to increased severity and asymmetry between affected and non-affected side in unilateral TMD patients (204–208). The ease-of-use of the sEMG assessment of masticatory muscles during static contractions and/or during chewing, has led to the development of standardized examination protocols and output graphs, which are suited for use in clinical routine assessment.
Other Applications
The above list of sEMG application fields is far from comprehensive. Many other fields take advantage of sEMG as a tool for either investigation, clinical assessment or treatment. These fields deal with the joint use of sEMG and ultrasound (209), the study of muscle deterioration in real or simulated (bed rest) microgravity conditions and the assessment of countermeasures (210, 211), the non-invasive detection of fasciculations (212, 213), the study of cramps (56, 214–216), the use of sEMG in rehabilitation games (217, 218).
Barriers to Widespread Clinical Use of sEMG in Neurorehabilitation
Current Situation
Section Surface EMG Applications provided a partial overview of the rapidly growing applications of sEMG in neuro-rehabilitation, movement sciences, occupational and sport medicine, and other fields. Many of the thousands of articles in these fields come from rehabilitation engineering groups, quite a few from movement science laboratories, but relatively few from the clinical world. Despite the extremely large number of publications about signal detection, processing tools, and small clinical studies, publications on routine applications in clinical practice are minimal. In addition, sEMG applications in medium/large clinical studies are rare (22, 64, 67, 73, 219). There is a lack of clinical studies (e.g., observational studies) verifying whether patients exposed to an additional sEMG assessment reach better outcomes than patients undergoing standard assessments do.
Hardware and software for sEMG detection and processing/interpretation has been developed mostly by academic researchers whose objective is to develop scientific innovation, and to publish their findings in respected science journals. In these cases, the research questions are typically technical, rather than clinical. The giant step between publishing a method, or developing a prototype, and the design, manufacturing, and marketing of a device for clinical applications is expensive and can be undertaken by companies only if there is evidence of clinical effectiveness and demand from the market. But a demand from the market implies some awareness of the users and their understanding of the potential use and relevance of the new method/device. If the users do not know why measuring muscle activity may be important, they will not be interested in a device or method to measure it. This brings up the issue of information, education and knowledge-transfer, but also other considerations concerning the potential consequences that making such measurement will imply. For example, who is qualified to decide on the measurement, who performs it, and who will use and interpret the resulting information to make decisions? Which balances will be affected, what will the costs or savings be, and who will gain something from doing it? This is important in private health management systems, where the role of insurance companies conditions the market, as well as in national health systems, where the state (that is the community) covers health cost with tax money.
Most of the scientific breakthroughs produced in academia do not routinely result in a marketable product or procedure. Commercialization of emerging technological innovations is difficult to accomplish; Transferring technological knowledge can also be a time-consuming process resulting often in a market failure. This may be because users are not yet competent or did not contribute to the development of the knowledge. On the other hand, academia-based researchers may also not be competent to assess the needs and boundaries of the clinical procedures. This scenario has been conceptualized as a “valley of death” between research and market. To tackle this general problem, major challenges are being analyzed, at the EU and national levels, and in the USA, involving educated/informed Communities of Practitioners (CoPs) in the process of effective transfer of high-value emerging technologies (220).
In addition to this, recent EU grants required the participation of companies as partners of funded projects.
Some Fundamental Questions
To address the barriers to clinical use of sEMG, we believe it is important, together with the CoPs, to try to answer fundamental primary questions and a set of secondary questions which outline the potential added value that can be provided by sEMG-based assessments. Primary questions concern the pathophysiological status of the neuromuscular system as well as the definition/measurement of key characteristic features (e.g., Is the muscle on or off? Does muscle fatigue occur during a task? Is the control strategy changing during a task?). Secondary questions concern when and why the answer to a primary question is clinically important, who should answer the question and how this person should be trained. The list below includes a few examples of the many primary questions that can be answered by a competent analysis of sEMG:
1. Is the muscle active or not at a given time? When does the muscle turn on and off during a task?
2. Is the muscle relaxed or active or progressively changing its activation level? What level of force is produced?
3. Is muscle activity triggered by muscle lengthening and/or by the velocity of the stretch?
4. What is the level of muscle activation? Is the estimation of force (or force change) of any interest?
5. How are many muscles coordinated and what are the temporal relations between their activations?
6. Is there co-activation of different muscles during a task?
7. Is there a region of a muscle that is more or less active then other regions? Or is there a muscle of a group that is more or less active than other muscles of the same group?
8. What is the strategy adopted for controlling motor unit recruitment and muscle coordination?
9. Where are the innervations zones of the MUs of a muscle located along the muscle?
10. How long are the muscle fibers and how much are they shortening/lengthening during a task?
11. Is conduction velocity of the muscle fibers relevant for the situation at hand? What is its average value? What is its distribution across motor units normal or not?
12. Is muscle fatigue of interest in the situation at hand?
13. Is the number of active motor units of a muscle stable, or changing in time during a task? Are the active motor units rotating, that is are they being de-recruited and replaced by others during performance of a task?
14. What is the fiber size and fatigability of MUs?
The list below includes examples of secondary questions that should be asked and answered in association to each of the primary questions listed above:
1. When and why is the answer to this primary question important?
2. What must be measured to answer the primary question? What instrument should be used?
3. What is the knowledge required to answer this primary question?
4. Who is the competent clinical operator who should perform the measurements required to answer this primary question?
5. What is the knowledge that such operator must have in order to perform the measurement? How can this knowledge be acquired by this operator?
6. Who is the competent clinical operator qualified to interpret the results of the measurement and draw conclusions from them? How can the required competence and expertise be acquired?
The current limited clinical application of sEMG indicates that either type of questions are rarely asked. However, the literature unquestionably indicates that the answers to the primary questions are available and that they are important for understanding the pathophysiology of muscle conditions and motor control strategies. The issue is whether such questions are considered to be relevant, from the clinical viewpoint, by the CoPs, and which barriers prevent answering the relevant ones.
As indicated above and in section Introduction, large studies, and translational efforts from scientific knowledge to clinical application are hindered by a number of barriers a few of which have been previously investigated by other authors and identified as (a) lack of time, (b) lack of skills, (c) misperceptions of EBP (31, 37–40, 221–223). But these barriers go much beyond these issues and much beyond sEMG. They can be roughly grouped in four categories: cultural, educational, technical, and economical.
Cultural Barriers
The cultural barriers limiting the widespread use of sEMG are not specific to this field and are the same that affect many other rehabilitation fields. They are, in general, related to the global approach to measurement in rehabilitation and to the concept of evidence provided by such measurements (39).
The Concepts of Measure and Measurement
In physics, “measurement” is the process of attributing a value to a physical quantity by comparing it to a standard reference quantity called “unit of measurement.” In rehabilitation, “measures” can be measurements of physical quantities (e.g., range of motion, 6-min walking test, etc.), ratings of a specific ability based on an ordinal scale with known levels (e.g., Functional Ambulatory Classification for assessing ambulation, etc.), ratings of multidimensional abilities on item scores that are summed up to obtain a total score (e.g., Barthel Index for assessing independence in the activity of daily life), results of questionnaires or aggregations of tests (pass/fail) or ordinal grades, such as very-poor/poor/sufficient/good/very-good, or 0/1/2/3/4/5 (e.g., for assessing force or resistance to stretch). The exact definition of each level of the scale may change from assessor to assessor. On the one hand, all these tools are useful, easy to be administered and represent a key element for both clinical activities and administrative procedures (e.g., reimbursements). Noteworthy, dichotomous variables are the pillar of epidemiological studies (e.g., exposed/non-exposed vs. dead/alive). On the other hand, they may suffer from metrical issues, from construct validity to sensitivity or reliability, and may lead to huge data-analysis problems when ordinal scores (e.g., 1/2/3/4/5) are treated as numbers and averaged or analyzed with parametric statistics and when the effects of a treatment are computed as numerical difference between scores obtained before and after the treatment. Moreover, electrophysiological variables, such as those describing motor control, cannot be assessed by clinical scales and require instrumental measurements. Some well-known textbooks, such as “Measurement in Neurological Rehabilitation” (224) do not even mention measurement of force/torque, or angular velocity, or sEMG and present only scales and questionnaires.
While physical measurements are the foundation of science and associate the change of a quantity as an effect due to some cause, the classification of the patient's current status or functional ability is the main goal of the “measures” in rehabilitation. Surface EMG amplitude (RMS, ARV) and spectral (MNF, MDF) features or timing during movement or tasks, are measurements of muscles signals that in turn reflect and quantitatively describe pathophysiological events or conditions or recovery level. In some cases, the possibility of turning this numerical information into clinically meaningful categories, based on a-priori knowledge and thresholds, would probably support the use of sEMG based examinations (but not of sEMG itself) in the rehabilitation practice.
Technology and Humanity: Communication Gaps and Lack of a Common Language
It has been properly pointed out that the statement “there can be no evidence in rehabilitation” that is so often heard from medical operators who think that their job is more “humanistic” than “scientific,” challenges the scientific basis of rehabilitation (3). Such thinking would drive physiotherapy and rehabilitation out of the mainstream of science. Rigorous reasoning and measurement-based approaches require a deep understanding of the physiological mechanisms and quantities being measured, of the instruments being used, and of the design of clinical studies in rehabilitation. Tradition and empirical experience alone are bad teachers (3).
Fundamental concepts of mathematics and biomechanics are associated to sEMG measurements and to the need for a language common to clinicians and rehabilitation engineers. Efforts in this direction are under way (e.g., Tutorials and CEDE consensus papers published in the Journal of Electromyography and Kinesiology) (52, 53, 61, 62). Applications of biomechanics and greater interactions between clinicians and rehabilitation engineers cannot take place without a common language that includes the concept of measurement of physical quantities with proper instruments (31, 38, 40). Rehabilitation medicine and physiotherapy are deeply connected with mathematics, physics and biomechanics. The fact that robotics and advanced technologies are entering the rehabilitation field (4, 6–8) is fear-inducing to some clinicians who consider these advances as job-threatening. Medical students and PTs should be taught to see technology as a tool in their hands. Rehabilitation operators demand more “intelligent,” fool-proof, and error-correcting devices to rely on (see section Technical Barriers). For this and other reasons, artificial intelligence, intelligent human-machine interfaces, and self-correcting data acquisition systems, are very important in rehabilitation and must be part of the training of professionals who should use them with proper competence and caution, and never totally rely on them.
Misunderstanding the Purpose of sEMG
Among rehabilitation professionals, there is a tendency to consider sEMG as a therapeutic tool so that the potential benefits of sEMG appear limited to biofeedback applications. In fact, sEMG is much more a monitoring tool, and occasionally, a diagnostic tool. The incorrect view of sEMG as a “therapy” is a barrier to its use.
Educational Barriers
The clinical interpretation of sEMG is based on the timing, amplitude and the morphology (continuous activity, burst-like activity, MUAP shape and firing pattern, etc.) of the signal. Technical aspects related to the type of electrodes, the type of protocol used, the adopted filters, etc., affect the waveform, timing, amplitude, and spectrum of the signal. Also, the modification of the peripheral properties of the muscle and the modification of the central drive have an effect on the morphology of the sEMG signal (225). Although, unlike ECG and EEG, the wrong reading or interpretation of the sEMG tracing may not have dramatic consequences on the patient, it can change therapeutic decisions, surgical options, focal treatment of spasticity, and cost of therapy.
Reading a sEMG recording and linking a pathophysiological and/or biomechanical meaning to its features (that often result from computer processing) requires considerable competences. These are rarely available in the clinical environment. Educational barriers are a bottleneck. Many countries offer a Master in Health Professions (some specifically in physiotherapy). These degrees too often focus on legal, professional and administrative issues and neglect scientific and technical education. Noteworthy, The World Confederation for Physical Therapy (WCPT) advocates that the scope of physical therapist practice is not limited to direct patient/client care, but also includes research (https://www.wcpt.org/policy/ps-descriptionPT). The academic programs in movement sciences often provide a more scientific and research oriented background.
Only a few countries offer a Ph.D. program in physiotherapy and a few more offer a Ph.D. in movement sciences. Where there is no Ph.D., no research fellowships and research positions are available. This precludes the academic career of physiotherapists and has a profound impact on education. A 3-years (or 4-years, as in Belgium and The Netherlands, among other countries) BS program is barely sufficient to train a practitioner, not a contract professor or a clinical researcher able to promote and conduct large-scale studies. In addition, it is unthinkable that a practitioner will acquire this knowledge on his/her own time, in parallel to a heavy burden of clinical work, and publish in qualified journals to achieve an academic status (42). The sEMG field is deeply affected by this situation because of the need for clinical studies that can be carried out only by qualified researchers at the post-graduate level. Moreover, the lack of specific education also prevents the preparation of clinical application guidelines that must become a part of the education of all operators potentially involved in sEMG application.
In countries in which physiotherapy is not a graduate level degree, students are trained to become practitioners rather than clinical researchers. The concept of measuring physical quantities is neglected as well as the fundamentals of physics and biomechanics (from the physical point of view). Moreover, in countries that do not grant a PhD in physiotherapy or movement sciences, teachers of physiotherapy have in general, no or very limited research exposure or international experience. Insufficient continuing education and involvement of teachers in research projects is a barrier to clinical use of all new technologies, and sEMG in particular.
Technical Barriers
Technological evolution led to the development of sEMG hardware that is simple to use and is commercially available. Powerful software can extract sEMG features whose clinical relevance is documented in the available literature. Nevertheless, there is a persistent demand for engineers to build systems that can be easily applied without a high risk of error. Users demand to be technically supported in the interpretation of signals and warned of potential misuse and acquisition error. There is a high demand for artificial expert systems and explanatory components that should be integrated into the sEMG systems and protect the user from errors and misinterpretations. However, no software will correct basic human errors (e.g., electrode misplacement, use of wrong filtering, etc.). This brings up the problem of the degree to which lack of competence can or should be replaced by expert systems, artificial intelligence or automatic devices. This may be a dangerous avenue of research in a field where developers and users have widely different expertise, experience and responsibility. Even if software is subject to the same stringent and reliable regulations as all medical devices, it cannot be fool-proof and cannot replace human expertise and competence. The solution is a more competent operator possibly assisted by a more intelligent machine providing warnings or “suggesting” possible interpretations.
Many researchers made remarkable efforts to (a) introduce sEMG as a tool to integrate biomechanical information for movement analysis, and (b) to provide tutorials and guidelines to clinical operators (29, 61, 108, 226–228). Very important contributions came from the European Project “Surface Electromyography for Non-Invasive Assessment of Muscles (SENIAM)” (226). Additional efforts are under way with the publication of a set of tutorials and consensus papers on the Journal of Electromyography and Kinesiology. These efforts have been designed to increase the competence of sEMG users, but their impact has been limited, suggesting that this may be a necessary but not sufficient step (229).
Economic/Administrative Barriers
The new Medical Device Regulation (MDR) of the European Union requires proof of benefit through clinical studies, based on the Health Technology Assessment (HTA) procedure. This will be difficult to provide for sEMG equipment because of the lack of suitable personnel, creating a vicious circle. In addition, the producers of medical devices will have to maintain competencies in the areas of quality assurance and risk management. This is difficult to ensure, especially for very small companies, and may further hinder the translation of innovative sEMG procedures in the long term. Unresolved reimbursement issues and new regulatory barriers will hinder the development of sEMG systems adapted to clinical needs and their translation into clinical practice.
As mentioned by Duncan and Murray (221) “Whilst the importance of routinely measuring outcomes within the allied health professions is well-recognized, it has largely failed to be delivered in practice. Factors that influence clinicians' ability and desire to undertake routine outcome measurement are bi-directional: they can act as either facilitators or barriers. Routine outcome measurement may only be deliverable if appropriate action is taken at individual therapist, team, and organizational levels of an organization.” The European MDR might be such action.
Should sEMG Measurements Be Fast, Simple, Automatic, and Inexpensive?
In most countries, a physiotherapy session lasts 30–60 min and is usually related to treatment, not to measurement of results. This time constraint is mentioned in many articles reporting results of questionnaires or interviews to physiotherapists (38, 40). In their recent work, Feldner et al. (31) reported that “most clinicians (19 out of 22) relied primarily on clinical observation of functional skills … used palpation, manual muscle testing … their choices were often based on time constraints and reimbursement considerations.” These authors further noticed that “most clinicians (18 out of 22) reported that they received very little training specific to the use of sEMG systems during their professional curricula…” and “…perceived barriers in “convincing” department administration to invest in technology….”
In addition, these authors indicated that “Despite barriers, participants were eager to learn about sEMG, noting that it would not replace but enhance their current clinical methods….” Due to the lack of training, the request is that sEMG equipment and testing should be easy to self-learn, fast and simple to use and inexpensive. The lack of teaching associated to self-teaching by trial and errors or from salespersons only, causes user frustration and is a major barrier to the use of sEMG. This is not so in the case of ECG and EEG (and needle EMG) whose users are provided with proper academic education and training.
Cost of FDA or CE-approved sEMG equipment ranges from about 10 k$ to nearly 40 k$ for wireless systems providing up to 32 channels and processing software. This is in the same order of magnitude of inexpensive to sophisticated ECG, EEG, and needle EMG equipment.
A possible explanation for the differences between ECG, EEG, needle EMG, and sEMG is that the former have a higher diagnostic yield while sEMG only provides information on the functional level, which is associated with prevention, monitoring, assessment, and treatment planning but less to diagnosis. The lower importance attributed to the latter functions with respect to diagnosis is a bias that is hard to overcome and has high social and economic costs.
Coverage by Insurances and National Health Systems
A vicious circle exists between the need to collect more evidence of sEMG effectiveness in assessing results, and the lack of qualified clinical researchers able to do it. This is a clinical activity that should not be left to either the manufactures or to the rehabilitation engineers. It is a clinical activity dealing with, and requiring, studies on patients. Despite the large number of publications on small studies, the evidence does not seem to be sufficient to convince insurance companies or National Health Systems to reimburse the cost of sEMG-based testing for effectiveness of treatments. In North America, sEMG procedures are not routinely reimbursed by insurance, unless they are part of a preoperative protocol, such as used for surgical planning in patients with CP. This is in contrast to diagnostic procedures using intramuscular needles which are done routinely by clinicians for diagnostic reasons.
Research Funding
Evidence supporting the use of sEMG is only partially available because it is limited to small studies. Large studies require substantial funding and competent operators. Competent operators are lacking because of educational barriers. Educational barriers are lower in countries where post-graduate academic degrees are available. Therefore, large-scale studies should be proposed where researchers are available to implement them. Research funding is required to support researchers and pay for equipment and management of large studies.
Conclusions
Hundreds of publications on peer-reviewed journals (Figure 1) provide a consistent body of evidence that the applications of sEMG makes appropriate information available in many medical fields, including the neurorehabilitation and orthopedic areas. Despite these achievements, clinical applications in health delivery institutions remain very limited because of many barriers. Clinicians have ready access to articles, evidence summaries, systematic reviews, and meta-analyses that assess and summarize the state of the art in the field. However, scientific publications are necessary but not sufficient to promote innovation. As indicated in a well-known editorial by A. Jette in 2017 (229) “Publishing our work in journals is essential—but publication of research is not, by itself, sufficient if our goal is to change clinical practice. People follow the lead of other people they know and trust when they decide whether to take up an innovation and change the way they practice!”
In this work, the barriers to a widespread use of sEMG have been classified into four main groups: cultural, educational, technical, and economic. These are strictly linked and interdependent.
Cultural barriers derive from “uneasiness” with technology, from communication gaps, different perceptions and approaches between rehabilitation engineers and clinical operators. These different perceptions hinder technology transfer and generate educational barriers. Overcoming these barriers requires a strongly interdisciplinary educational approach. The lack of a partially overlapping high-level education, involving rehabilitation professionals and engineers, results in different languages, communication gaps, different approaches to common problems, or, in one word, cultural barriers that delay technology transfer. The development of common languages at common institutions would promote the use of sEMG systems and other measurement techniques
To this end it is important to point out and underline the series of open access Tutorials (61, 62) and of Consensus Papers within the “Consensus for experimental design in electromyography (CEDE)” project (53) promoted by the Journal of Electromyography and Kinesiology.
Overcoming educational barriers requires (a) a greater degree of bidirectional osmosis between the clinical and the research environments, (b) funding of translational efforts, (c) use of textbooks and manuals related to the clinical use of sEMG in specific applications prepared by experts (19), (d) design and implementation of large clinical studies. These should rise from (a) simple case-series on the added value provided by sEMG assessment aimed to the selection of a proper treatment, (b) observational studies comparing both pathways and outcomes of cohorts of patients treated in centers with/without sEMG adjunctive assessments, up to RCTs addressing the percentage of modified treatments and the differences in the functional outcomes determined by the use of sEMG-based adjunctive assessments. These activities must be carried out by qualified researchers within post-graduate research programs. This brings up the need for new academic figures merging clinical and physiopathological competences with the capability of understanding and properly using state-of-the art sEMG instrumentation/technology.
The lack of higher academic degrees in physiotherapy and movement sciences prevents (a) the education of qualified researchers able to properly apply the rapidly developing technology and to carry out large clinical studies, and (b) continuing education initiatives in teaching and research to exploit the growing assessment capabilities provided by technology.
Technical barriers are due to (a) sEMG systems considered unfriendly, (b) the lack of familiarity with hardware and signal processing/interpretation techniques, and (c) the demand for fool-proof automatic equipment. The demand for support in the interpretation of signals and automatic warning of potential misuse and acquisition errors cannot be fully satisfied. Automatic expert system are no substitutes for human expertise and competence and may be misleading. No device should be used without knowledge of its performance, limitations and misuse, and without user's critical competence. Education and research should be institutionally planned and provided, like in other fields, at the academic level, by training new figures with a strongly inter- and multi-disciplinary approach. They will, in turn, train a new breed of clinical operators able to manage technology and interact with engineers and manufacturers.
Finally, economical barriers, including cost/benefit analysis, should be seriously considered to identify the most economically rewarding sEMG-based applications, thus turning boundaries into project specifications. This requires fellowships for training researchers and funding for support of large clinical studies whose results will lead to reduction of the economic burden of institutions paying for treatment costs.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are very grateful to Dr. Andrea Merlo, Prof. Dario Farina, and Dr. Nonna Dimitrova for their discussions and comments.
References
1. Ebenbichler G, Kerschan-Schindl K, Brockow T, Resch KL. The future of physical & rehabilitation medicine as a medical specialty in the era of evidence-based medicine. Am J Phys Med Rehabil. (2008) 87:1–3. doi: 10.1097/PHM.0b013e31815e6a49
2. Dieppe P, Chard J, Tallon D, Egger M. Funding clinical research. Lancet. (1999) 353:1626. doi: 10.1016/S0140-6736(05)75755-X
3. Negrini S. Evidence in rehabilitation medicine: between facts and prejudices. Am J Phys Med Rehabil. (2019) 98:88–96. doi: 10.1097/PHM.0000000000001033
4. Pons J, Torricelli D, Pajaro M, editors. Converging Clinical and Engineering Research on Neurorehabilitation. Berlin; Heidelberg: Springer Berlin Heidelberg (2013).
5. Pons J, Torricelli D, editors. Emerging Therapies in Neurorehabilitation. Berlin; Heidelberg: Springer Berlin Heidelberg (2014).
6. Jensen W, Andersen O, Akay M, editors. Replace, Repair, Restore, Relieve–Bridging Clinical and Engineering Solutions in Neurorehabilitation. Springer International Publishing (2014).
7. Masia L, Micera S, Akay M, Pons J, editors. Converging Clinical and Engineering Research on Neurorehabilitation III. Springer International Publishing (2019).
8. Ibáñez J, González-Vargas J, Azorín J, Akay M, Pons J, editors. Converging Clinical and Engineering Research on Neurorehabilitation II. Springer International Publishing (2017).
9. Basmajian J, De Luca C. Muscles Alive: Their Functions Revealed by Electromyography. Baltimore, MD: William and Wilkins (1985).
10. Cram J, Kasman G, Holtz J. Introduction to Surface Electromyography. Gaithersburg, MD: Aspen Publisher Inc. (1998).
12. Zwarts MJ, Drost G, Stegeman DF. Recent progress in the diagnostic use of surface EMG for neurological diseases. J Electromyogr Kinesiol. (2000) 10:287–91. doi: 10.1016/S1050-6411(00)00020-1
13. Hogrel J-Y. Clinical applications of surface electromyography in neuromuscular disorders. Neurophysiol Clin Neurophysiol. (2005) 35:59–71. doi: 10.1016/j.neucli.2005.03.001
14. Drost G, Stegeman DF, van Engelen BGM, Zwarts MJ. Clinical applications of high-density surface EMG: a systematic review. J Electromyogr Kinesiol. (2006) 16:586–602. doi: 10.1016/j.jelekin.2006.09.005
15. Klein CS, Li S, Hu X, Li X. Editorial: electromyography (EMG) techniques for the assessment and rehabilitation of motor impairment following stroke. Front Neurol. (2018) 9:1122. doi: 10.3389/fneur.2018.01122
16. Farina D, Jensen W, Akay M, editors. Introduction to Neural Engineering for Motor Rehabilitation. Hoboken, NJ: John Wiley & Sons, Inc. (2013).
17. Zwarts MJ, Stegeman DF. Multichannel surface EMG: basic aspects and clinical utility. Muscle Nerve. (2003) 28:1–17. doi: 10.1002/mus.10358
18. Merletti R, Farina D, editors. Surface Electromyography: Physiology, Engineering, and Application. Hoboken, NJ: IEEE Press; John Wiley & Sons, Inc. (2016).
20. Floyd W, Silver P. Electromyographic study of patterns of activity of the anterior abdominal wall muscles in man. J Anat. (1950) 84:132–45.
21. Cerone GL, Botter A, Gazzoni M. A modular, smart, and wearable system for high density sEMG detection. IEEE Trans Neural Syst Rehabil Eng. (2019) 66:3371–80. doi: 10.1109/TBME.2019.2904398
22. Merlo A, Campanini I. Impact of instrumental analysis of stiff knee gait on treatment appropriateness and associated costs in stroke patients. Gait Posture. (2019) 72:195–201. doi: 10.1016/j.gaitpost.2019.06.009
23. Gydikov A, Kosarov D. Extraterritorial potential field of impulses from separate motor units in human muscles. Electromyogr Clin Neurophysiol. (1972) 12:283–305.
24. Gydikov A, Kosarov D. Volume conduction of the potentials from separate motor units in human muscle. Electromyogr Clin Neurophysiol. (1972) 12:127–47.
25. De Luca CJ. Myoelectrical manifestations of localized muscular fatigue in humans. Crit Rev Biomed Eng. (1984) 11:251–79.
26. Naik G, editor. Computational Intelligence in Electromyography Analysis–A Perspective on Current Applications and Future Challenges. London: Intech Open (2012).
27. Naik G, editor. Applications, Challenges, and Advancements in Electromyography Signal Processing. Hershey, PA: IGI Global (2014).
28. Farina D, Negro F, Muceli S, Enoka RM. Principles of motor unit physiology evolve with advances in technology. Physiology. (2016) 31:83–94. doi: 10.1152/physiol.00040.2015
29. De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. (1997) 13:135–63. doi: 10.1123/jab.13.2.135
30. Dimitrova NA, Dimitrov GV. Interpretation of EMG changes with fatigue: facts, pitfalls, and fallacies. J Electromyogr Kinesiol. (2003) 13:13–36. doi: 10.1016/S1050-6411(02)00083-4
31. Feldner HA, Howell D, Kelly VE, McCoy SW, Steele KM. “Look, your muscles are firing!”: A qualitative study of clinician perspectives on the use of surface electromyography in neurorehabilitation. Arch Phys Med Rehabil. (2019) 100:663–75. doi: 10.1016/j.apmr.2018.09.120
32. Caceres CA. Present status of computer interpretation of the electrocardiogram: a 20 year overview. Am J Cardiol. (1978) 41:121–5. doi: 10.1016/0002-9149(78)90145-5
33. Surawicz B, Uhley H, Borun R, Laks M, Crevasse L, Rosen K, et al. Task force I: standardization of terminology and interpretation. Am J Cardiol. (1978) 41:130–45. doi: 10.1016/0002-9149(78)90147-9
34. Gevins A, Schaffer R. A critical review of electroencephalographic (EEG) correlates of higher cortical. Crit Rev Bioeng. (1980) 4:113–64.
35. Pullman SL, Goodin DS, Marquinez AI, Tabbal S, Rubin M. Clinical utility of surface EMG: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. (2000) 55:171–7. doi: 10.1212/WNL.55.2.171
36. Meekins GD, So Y, Quan D, Vavricek J. American Association of Neuromuscular & Electrodiagnostic Medicine evidenced-based review: use of surface electromyography in the diagnosis and study of neuromuscular disorders. Muscle Nerve. (2008) 38:1219–24. doi: 10.1002/mus.21055
37. Scurlock-Evans L, Upton P, Upton D. Evidence-based practice in physiotherapy: a systematic review of barriers, enablers and interventions. Physiotherapy. (2014) 100:208–19. doi: 10.1016/j.physio.2014.03.001
38. Braun T, Rieckmann A, Weber F, Grüneberg C. Current use of measurement instruments by physiotherapists working in Germany: a cross-sectional online survey. BMC Health Serv Res. (2018) 18:1–16. doi: 10.1186/s12913-018-3563-2
39. Snöljung Å, Mattsson K, Gustafsson LK. The diverging perception among physiotherapists of how to work with the concept of evidence: a phenomenographic analysis. J Eval Clin Pract. (2014) 20:759–66. doi: 10.1111/jep.12167
40. Swinkels RAHM, Van Peppen RPPS, Wittink H, Custers JWH, Beurskens AJHM. Current use and barriers and facilitators for implementation of standardised measures in physical therapy in the Netherlands. BMC Musculoskelet Disord. (2011) 12:106. doi: 10.1186/1471-2474-12-106
41. Hömberg V. Evidence based medicine in neurological rehabilitation–a critical review. Acta Neurochirur Suppl. (2005) 93:3–14. doi: 10.1007/3-211-27577-0_1
42. Vercelli S, Ravizzotti E, Paci M. Are they publishing? A descriptive cross-sectional profile and bibliometric analysis of the journal publication productivity of Italian physiotherapists. Arch Physiother. (2018) 8:4–9. doi: 10.1186/s40945-017-0042-8
43. Lindstrom LH, Magnusson RI. Interpretation of myoelectric power spectra: a model and its applications. Proc IEEE. (1977) 65:653–62. doi: 10.1109/PROC.1977.10544
45. Kugelberg E. Electromyogram in muscular disorders. J Neurol Neurosur Psychiat. (1947) 60:140–9. doi: 10.1136/jnnp.10.3.122
46. Denny Brown D. Interpretation of the electromyogram. Arch Neurol Psychiatry. (1949) 61:99–128. doi: 10.1001/archneurpsyc.1949.02310080003001
47. Basmajian J. Biofeedback Principles and Practices for Clinicians. Hamilton, ON: McMaster University; William and Wilkins (1967).
48. Kasman G, Cram J, Wolf S. Clinical Applications in Surface Electromyography. Gaithersburg, MD: Aspen Publisher Inc. (1998).
50. Mitchell D. Surface electromyography: Fundamentals, Computational Techniques, and Clinical Applications. Hauppauge, NY: Nova Science.
51. Merletti R. Pelvic floor EMG: principles, technique and applications (Ch. 7). In: Riva D., Minini G. editors. Childbirth Related Pelvic Floor Dysfunctions. Milano: Springer (2016).
52. Merletti R. Available online at: https:/www.robertomerletti.it (accessed August, 2020).
53. Besomi M, Hodges P, Van Dieën J, Carson R, Clancy E, Disselhorst-Klug C, et al. Consensus for experimental design in electromyography (CEDE) project: electrode selection matrix. J Electromyogr Kinesiol. (2019) 48:128–44. doi: 10.1016/j.jelekin.2019.07.008
54. Huppertz HJ, Disselhorst-Klug C, Silny J, Rau G, Heimann G. Diagnostic yield of noninvasive high spatial resolution electromyography in neuromuscular diseases. Muscle Nerve. (1997) 20:1360–70.
55. Disselhorst-Klug C, Rau G, Schmeer A, Silny J. Non-invasive detection of the single motor unit action potential by averaging the spatial potential distribution triggered on a spatially filtered motor unit action potential. J Electromyogr Kinesiol. (1999) 9:67–72. doi: 10.1016/S1050-6411(98)00026-1
56. Roeleveld K, Van Engelen BGM, Stegeman DF. Possible mechanisms of muscle cramp from temporal and spatial surface EMG characteristics. J Appl Physiol. (2000) 88:1698–706. doi: 10.1152/jappl.2000.88.5.1698
57. Kallenberg LAC, Schulte E, Disselhorst-Klug C, Hermens HJ. Myoelectric manifestations of fatigue at low contraction levels in subjects with and without chronic pain. J Electromyogr Kinesiol. (2007) 17:264–74. doi: 10.1016/j.jelekin.2006.04.004
58. Lapatki BG, Van Dijk JP, Van de Warrenburg BPC, Zwarts MJ. Botulinum toxin has an increased effect when targeted toward the muscle's endplate zone: a high-density surface EMG guided study. Clin Neurophysiol. (2011) 122:1611–6. doi: 10.1016/j.clinph.2010.11.018
59. Boccia G, Dardanello D, Rosso V, Pizzigalli L, Rainoldi A. The application of sEMG in aging: a mini review. Gerontology. (2015) 61:477–84. doi: 10.1159/000368655
60. Wiedemann LG, Ward S, Lim E, Wilson NC, Hogan A, Holobar A, et al. High-density electromyographic data during isometric contractions of the ankle joint in children with cerebral palsy pre and post BoNT-A treatment. Data Brief. (2019) 24:103840. doi: 10.1016/j.dib.2019.103840
61. Merletti R, Muceli S. Tutorial. Surface EMG detection in space and time: best practices. J Electromyogr Kinesiol. (2019) 49:102363. doi: 10.1016/j.jelekin.2019.102363
62. Del Vecchio A, Holobar A, Falla D, Felici F, Enoka RM, Farina D. Tutorial: analysis of motor unit discharge characteristics from high-density surface EMG signals. J Electromyogr Kinesiol. (2020) 53:102426. doi: 10.1016/j.jelekin.2020.102426
63. Klasser GD, Okeson JP. The clinical usefulness of surface electromyography in the diagnosis and treatment of temporomandibular disorders. J Am Dent Assoc. (2006) 137:763–71. doi: 10.14219/jada.archive.2006.0288
64. Keenan MA, Fuller DA, Whyte J, Mayer N, Esquenazi A, Fidler-Sheppard R. The influence of dynamic polyelectromyography in formulating a surgical plan in treatment of spastic elbow flexion deformity. Arch Phys Med Rehabil. (2003) 84:291–6. doi: 10.1053/apmr.2003.50099
65. Mazzoli D, Giannotti E, Manca M, Longhi M, Prati P, Cosma M, et al. Electromyographic activity of the vastus intermedius muscle in patients with stiff-knee gait after stroke. A retrospective observational study. Gait Posture. (2018) 60:273–8. doi: 10.1016/j.gaitpost.2017.07.002
66. Giannotti E, Merlo A, Zerbinati P, Longhi M, Prati P, Masiero S, et al. Early rehabilitation treatment combined with equinovarus foot deformity surgical correction in stroke ptients: safety and changes in gait parameters. Eur J Phys Rehabil Med. (2016) 52:296–303.
67. Roche N, Bonnyaud C, Reynaud V, Bensmail D, Pradon D, Esquenazi A. Motion analysis for the evaluation of muscle overactivity: a point of view. Ann Phys Rehabil Med. (2019) 62:442–52. doi: 10.1016/j.rehab.2019.06.004
68. Bar-On L, Aertbeliën E, Wambacq H, Severijns D, Lambrecht K, Dan B, et al. A clinical measurement to quantify spasticity in children with cerebral palsy by integration of multidimensional signals. Gait Posture. (2013) 38:141–7. doi: 10.1016/j.gaitpost.2012.11.003
69. Negrini S, Meyer T, Arienti C, Malmivaara A, Frontera WR. In search of solutions for evidence generation in rehabilitation: the second cochrane rehabilitation methodology meeting. Am J Phys Med Rehabil. (2020) 99:181–2. doi: 10.1097/PHM.0000000000001374
70. Ferrarin M, Rabuffetti M, Bacchini M, Casiraghi A, Castagna A, Pizzi A, et al. Does gait analysis change clinical decision-making in poststroke patients? Results from a pragmatic prospective observational study. Eur J Phys Rehabil Med. (2015) 51:171–84.
71. Wren TAL, Gorton GE, Õunpuu S, Tucker CA. Efficacy of clinical gait analysis: a systematic review. Gait Posture. (2011) 34:149–53. doi: 10.1016/j.gaitpost.2011.03.027
72. Wren TAL, Elihu KJ, Mansour S, Rethlefsen SA, Ryan DD, Smith ML, et al. Differences in implementation of gait analysis recommendations based on affiliation with a gait laboratory. Gait Posture. (2013) 37:206–9. doi: 10.1016/j.gaitpost.2012.07.008
73. Campanini I, Damiano B, Merlo A. Lacking foot dorsiflexion during swing after stroke. Paresis, spasticity or muscle shortening? Gait Posture. (2019) 74:6. doi: 10.1016/j.gaitpost.2019.07.450
74. Kamen G, Caldwell GE. Physiology and Interpretation of the electromyogram. J Clin Neurophysiol. (1996) 13:366–84. doi: 10.1097/00004691-199609000-00002
75. Stegeman DF, Zwarts MJ, Anders C, Hashimoto T. Chapter 20: multi-channel surface EMG in clinical neurophysiology. Suppl Clin Neurophysiol. (2000) 53:155–62. doi: 10.1016/S1567-424X(09)70151-5
76. Drost G, Blok J, Stegeman D, Van Dijk J, van Engelen B, Zwarts M. Propagation disturbance of motor unit action potentials during transient paresis in generalized myotonia: a high-density surface EMG study. Brain. (2001) 124:352–60. doi: 10.1093/brain/124.2.352
77. Zwarts MJ, Bleijenberg G, van Engelen BGM. Clinical neurophysiology of fatigue. Clin Neurophysiol. (2008) 119:2–10. doi: 10.1016/j.clinph.2007.09.126
78. Golla F, Hettwer J. A study of the electromyograms of voluntary movement. Brain. (1924) 47:57–69. doi: 10.1093/brain/47.1.57
79. Bosma JF, Gellhorn E. Electromyographic studies of muscular coordination on stimulation of motor cortex. J Neurophysiol. (1946) 9:263–74. doi: 10.1152/jn.1946.9.4.263
80. Miles M, Mortensen OA, Sullivan WE. Electromyography during normal voluntary movements. Anat Rec. (1947) 98:209–18. doi: 10.1002/ar.1090980209
81. Baumann F, Behr O. Elektromyographische Untersuchungen der Hüftmuskulatur nach Arthrodese. Arch Orthop Unfallchir. (1969) 66:1–17. doi: 10.1007/BF00419113
82. Kleissen RFM, Buurke JH, Harlaar J, Zilvold G. Electromyography in the biomechanical analysis of human movement and its clinical application. Gait Posture. (1998) 8:143–58. doi: 10.1016/S0966-6362(98)00025-3
83. Benedetti MG, Ebenbichler G, Roy SH. Clinician's view: dynamic EMG. IEEE Eng Med Biol Mag. (2001) 20:33–7. doi: 10.1109/51.982273
84. Rechtien JJ, Gelblum JB, Haig AJ, Gitter AJ. Technology assessment: dynamic electromyography in gait and motion analysis. Muscle Nerve. (1996) 19:396–402.
85. Frigo C, Crenna P. Multichannel SEMG in clinical gait analysis: a review and state-of-the-art. Clin Biomech. (2009) 24:236–45. doi: 10.1016/j.clinbiomech.2008.07.012
86. Rosa MCN, Marques A, Demain S, Metcalf CD, Rodrigues J. Methodologies to assess muscle co-contraction during gait in people with neurological impairment–a systematic literature review. J Electromyogr Kinesiol. (2014) 24:179–91. doi: 10.1016/j.jelekin.2013.11.003
87. Becker S, Bergamo F, Schnake KJ, Schreyer S, Rembitzki I, Disselhorst-Klug C. The relationship between functionality and erector spinae activity in patients with specific low back pain during dynamic and static movements. Gait Posture. (2018) 66:208–13. doi: 10.1016/j.gaitpost.2018.08.042
88. Nawasreh ZH, Marmon AR, Logerstedt D, Snyder-Mackler L. Ehe effect of training on a compliant surface on muscle activation and co-contraction after anterior cruciate ligament Injury. Int J Sports Phys Ther. (2019) 14:3554–63. doi: 10.26603/ijspt20190554
89. Batista JP, Facci LM, Pelegrinelli ARM, von Werder SCFA, Souza de DC, Taglietti M, et al. Joint angle and movement velocity effects on muscle activity of elderly with knee osteoarthritis–categorized and probabilistic analysis. J Electromyogr Kinesiol. (2018) 41:50–9. doi: 10.1016/j.jelekin.2018.04.014
90. Hug F. Can muscle coordination be precisely studied by surface electromyography? J Electromyogr Kinesiol. (2011) 21:1–12. doi: 10.1016/j.jelekin.2010.08.009
91. Schmidt-Rohlfing B, Bergamo F, Williams S, Erli HJ, Rau G, Niethard FU, et al. Interpretation of surface EMGs in children with cerebral palsy: an initial study using a fuzzy expert system. J Orthop Res. (2006) 24:438–47. doi: 10.1002/jor.20043
92. Singh RE, Iqbal K, White G, Hutchinson TE. A systematic review on muscle synergies: from building blocks of motor behavior to a neurorehabilitation tool. Appl Bionics Biomech. (2018) 2018:3615368. doi: 10.1155/2018/3615368
93. Steele KM, Munger ME, Peters KM, Shuman BR, Schwartz MH. Repeatability of electromyography recordings and muscle synergies during gait among children with cerebral palsy. Gait Posture. (2019) 67:290–5. doi: 10.1016/j.gaitpost.2018.10.009
94. von Werder SCFA, Disselhorst-Klug C. The role of biceps brachii and brachioradialis for the control of elbow flexion and extension movements. J Electromyogr Kinesiol. (2016) 28:67–75. doi: 10.1016/j.jelekin.2016.03.004
95. Bizzi E, D'Avella A, Saltiel P, Tresch M. Modular organization of spinal motor systems. Neuroscientist. (2002) 8:437–42. doi: 10.1177/107385802236969
96. Jiang N, Falla D, D'Avella A, Graimann B, Farina D. Myoelectric control in neurorehabilitation. Crit Rev Biomed Eng. (2010) 38:381–91. doi: 10.1615/CritRevBiomedEng.v38.i4.30
97. Dominici N, Ivanenko YP, Cappellini G, D'Avella A, Mondì V, Cicchese M, et al. Locomotor primitives in newborn babies and their development. Science. (2011) 334:997–9. doi: 10.1126/science.1210617
98. Lacquaniti F, Ivanenko YP, D'Avella A, Zelik KE, Zago M. Evolutionary and developmental modules. Front Comput Neurosci. (2013) 7:61. doi: 10.3389/fncom.2013.00061
99. D'Avella A, Lacquaniti F. Control of reaching movements by muscle synergy combinations. Front Comput Neurosci. (2013) 7:42. doi: 10.3389/fncom.2013.00042
100. Borzelli D, Berger DJ, Pai DK, D'Avella A. Effort minimization and synergistic muscle recruitment for three-dimensional force generation. Front Comput Neurosci. (2013) 7:186. doi: 10.3389/fncom.2013.00186
101. D'Avella A. Modularity for motor control and motor learning. Adv Exp Med Biol. (2016) 957:3–19. doi: 10.1007/978-3-319-47313-0_1
102. Perry J, Bekey G. EMG-force relationships in skeletal muscle. Crit Rev Biomed Eng. (1981) 7:1–22.
103. Tsirakos D, Baltzopoulos V, Bartlett R. Tsirakos 1997. Crit Rev Biomed Eng. (1997) 25:371–407. doi: 10.1615/CritRevBiomedEng.v25.i4-5.20
104. Disselhorst-Klug C, Schmitz-Rode T, Rau G. Surface electromyography and muscle force: limits in sEMG-force relationship and new approaches for applications. Clin Biomech. (2009) 24:225–35. doi: 10.1016/j.clinbiomech.2008.08.003
105. Zhang LQ, Wang G, Nuber GW, Press JM, Koh JL. In vivo load sharing among the quadriceps components. J Orthop Res. (2003) 21:565–71. doi: 10.1016/S0736-0266(02)00196-1
106. Gandevia S, Enoka R, McComas A, Stuart D, Thomas C. Fatigue: Neural and Muscular Mechanisms. New York, NY: Plenim Press (1995).
107. Botter A, Lanfranco F, Merletti R, Minetto MA. Myoelectric fatigue profiles of three knee extensor muscles. Int J Sports Med. (2009) 30:408–17. doi: 10.1055/s-0028-1112142
108. Merletti R, Farina D, Gazzoni M, Merlo A, Ossola P, Rainoldi A. Surface electromyography: a window on the muscle, a glimpse on the central nervous system. Eura Medicophys. (2001) 37:57–68.
109. Al-Mulla MR, Sepulveda F, Colley M. A review of non-invasive techniques to detect and predict localised muscle fatigue. Sensors. (2011) 11:3545–94. doi: 10.3390/s110403545
110. González-Izal M, Malanda A, Gorostiaga E, Izquierdo M. Electromyographic models to assess muscle fatigue. J Electromyogr Kinesiol. (2012) 22:501–12. doi: 10.1016/j.jelekin.2012.02.019
111. Jurell KC. Surface EMG and fatigue. Phys Med Rehabil Clin N Am. (1998) 9:933–47, viii–ix. doi: 10.1016/S1047-9651(18)30242-0
112. Gydikov A, Kosarov D. The influence of various factors on the shape of the myopotentials in using monopolar electrodes. Electroencephalogr Clin Neurophysiol. (1973) 13:319–43.
113. Masuda T, Miyano H, Sadoyama T. A surface electrode array for detecting action potential trains of single motor units. Electroencephalogr Clin Neurophysiol. (1985) 60:435–43. doi: 10.1016/0013-4694(85)91018-1
114. Masuda T, Sadoyama T. The propagation of single motor unit action potentials detected by a surface electrode array. Electroencephalogr Clin Neurophysiol. (1986) 63:590–8. doi: 10.1016/0013-4694(86)90146-X
115. Prutchi D. A high-resolution large array (HRLA) surface EMG system. Med Eng Phys. (1995) 17:442–54. doi: 10.1016/1350-4533(94)00016-3
116. Kleine BU, Blok JH, Oostenveld R, Praamstra P, Stegeman DF. Magnetic stimulation-induced modulations of motor unit firings extracted from multi-channel surface EMG. Muscle Nerve. (2000) 23:1005–15. doi: 10.1002/1097-4598(200007)23:7<1005::AID-MUS2>3.0.CO;2-2
117. Blok JH, Van Dijk JP, Drost G, Zwarts MJ, Stegeman DF. A high-density multichannel surface electromyography system for the characterization of single motor units. Rev Sci Instrum. (2002) 73:1887. doi: 10.1063/1.1455134
118. Gazzoni M, Celadon N, Mastrapasqua D, Paleari M, Margaria V, Ariano P. Quantifying forearm muscle activity during wrist and finger movements by means of multi-channel electromyography. PLoS ONE. (2014) 9:e0109943. doi: 10.1371/journal.pone.0109943
119. Rasool G, Afsharipour B, Suresh NL, Rymer WZ. Spatial analysis of multichannel surface EMG in hemiplegic stroke. IEEE Trans Neural Syst Rehabil Eng. (2017) 25:1802–11. doi: 10.1109/TNSRE.2017.2682298
120. Nakajima Y, Keeratihattayakorn S, Yoshinari S, Tadano S. An EMG-CT method using multiple surface electrodes in the forearm. J Electromyogr Kinesiol. (2014) 24:875–80. doi: 10.1016/j.jelekin.2014.07.010
121. Staudenmann D, Taube W. Brachialis muscle activity can be assessed with surface electromyography. J Electrom Kinesiol. (2015) 25:199–204. doi: 10.1016/j.jelekin.2014.11.003
122. Zhang C, Chen Y-T, Liu Y, Zhou P, Li S, Zhang Y. Three dimensional innervation zone imaging in spastic muscles of stroke survivors. J Neural Eng. (2019) 16:034001. doi: 10.1088/1741-2552/ab0fe1
123. Cescon C, Riva D, Začesta V, Drusany-Starič K, Martsidis K, Protsepko O, et al. Effect of vaginal delivery on the external anal sphincter muscle innervation pattern evaluated by multichannel surface EMG: results of the multicentre study TASI-2. Int Urogynecol J. (2014) 25:1491–9. doi: 10.1007/s00192-014-2375-0
124. Enck P, Heiko F, Edoardo D, Mastrangelo F, Mesin L, Merletti R, et al. Retest reliability of surface electromyography on the pelvic floor musclesGrape. Neurourol Urodyn. (2009) 2010:449–57. doi: 10.1002/nau.20648
125. Začesta V, Rezeberga D, Plaudis H, Drusany-Staric K, Cescon C. Could the correct side of mediolateral episiotomy be determined according to anal sphincter EMG? Int Urogynecol J. (2018) 29:1501–7. doi: 10.1007/s00192-018-3560-3
126. Knaflitz M, Merletti R, De Luca CJ. Inference of motor unit recruitment order in voluntary and electrically elicited contractions. J Appl Physiol. (1990) 68:1657–67. doi: 10.1152/jappl.1990.68.4.1657
127. Merletti R, Knaflitz M, DeLuca CJ. Electrically evoked myoelectric signals. Crit Rev Biomed Eng. (1992) 19:293–340.
128. Enoka RM. Activation order of motor axons in electrically evoked contractions. Muscle Nerve. (2002) 25:763–4. doi: 10.1002/mus.10117
129. Jonsdottir J, Thorsen R, Aprile I, Galeri S, Spannocchi G, Beghi E, et al. Arm rehabilitation in post stroke subjects: a randomized controlled trial on the efficacy of myoelectrically driven FES applied in a task-oriented approach. PLoS ONE. (2017) 12:e0188642. doi: 10.1371/journal.pone.0188642
131. Ibuki Aileen B. What is spasticity? The discussion continues. Int J Ther Rehabil. (2007) 14:5. doi: 10.12968/ijtr.2007.14.9.24579
132. Baude M, Nielsen JB, Gracies J-MM. The neurophysiology of deforming spastic paresis: a revised taxonomy. Ann Phys Rehabil Med. (2019) 62:426–30. doi: 10.1016/j.rehab.2018.10.004
133. Biering-Sørensen F, Nielsen JB, Klinge K. Spasticity-assessment: a review. Spinal Cord. (2006) 44:708–22. doi: 10.1038/sj.sc.3101928
134. Aloraini SM, Gäverth J, Yeung E, MacKay-Lyons M. Assessment of spasticity after stroke using clinical measures: a systematic review. Disabil Rehabil. (2015) 37:2313–23. doi: 10.3109/09638288.2015.1014933
135. Gassert R, Dietz V. Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective. J Neuroeng Rehabil. (2018) 15:1–15. doi: 10.1186/s12984-018-0383-x
136. Katz R, Rymer W. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil. (1989) 70:144–55.
137. Levin MF, Solomon JM, Shah A, Blanchette AK, Feldman AG. Activation of elbow extensors during passive stretch of flexors in patients with post-stroke spasticity. Clin Neurophysiol. (2018) 129:2065–74. doi: 10.1016/j.clinph.2018.07.007
138. Solopova IA, Zhvansky DS, Dolinskaya IY, Keshishian ES, Selionov VA, Sylos-Labini F, et al. Muscle responses to passive joint movements in infants during the first year of life. Front Physiol. (2019) 10:1158. doi: 10.3389/fphys.2019.01158
139. Jobin A, Levin MF. Regulation of stretch reflex threshold in elbow flexors in children with cerebral palsy: a new measure of spasticity. Dev Med Child Neurol. (2000) 42:S0012162200001018. doi: 10.1017/S0012162200001018
140. Pisano F, Miscio G, Del Conte C, Pianca D, Candeloro E, Colombo R. Quantitative measures of spasticity in post-stroke patients. Clin Neurophysiol. (2000) 111:1015–22. doi: 10.1016/S1388-2457(00)00289-3
141. Calota A, Feldman AG, Levin MF. Spasticity measurement based on tonic stretch reflex threshold in stroke using a portable device. Clin Neurophysiol. (2008) 119:2329–37. doi: 10.1016/j.clinph.2008.07.215
142. Lee HM, Chen JJJ, Wu YN, Wang YL, Huang SC, Piotrkiewicz M. Time course analysis of the effects of botulinum toxin type A on elbow spasticity based on biomechanic and electromyographic parameters. Arch Phys Med Rehabil. (2008) 89:692–9. doi: 10.1016/j.apmr.2007.08.166
143. Beattie C, Gormley M, Wervey R, Wendorf H. An electromyographic protocol that distinguishes spasticity from dystonia. J Pediatr Rehabil Med. (2016) 9:125–32. doi: 10.3233/PRM-160373
144. Tirosh O, Rutz E. Quantifying the velocity-dependent muscle response during gait of children with cerebral palsy. J Electromyogr Kinesiol. (2019) 48:76–83. doi: 10.1016/j.jelekin.2019.06.007
145. Lassek A, von Werder SF, Werner C, Disselhorst-Klug C. Introduction of a procedure to objectively quantify spastic movement impairment during freely performed voluntary movements. J Electromyogr Kinesiol. (2019) 48:44–52. doi: 10.1016/j.jelekin.2019.06.001
146. Boulay C, Pomero V, Viehweger E, Glard Y, Castanier E, Authier G, et al. Dynamic equinus with hindfoot valgus in children with hemiplegia. Gait Posture. (2012) 36:108–12. doi: 10.1016/j.gaitpost.2012.01.015
147. Naro A, Leo A, Russo M, Casella C, Buda A, Crespantini A, et al. Breakthroughs in the spasticity management: are non-pharmacological treatments the future? J Clin Neurosci. (2017) 39:16–27. doi: 10.1016/j.jocn.2017.02.044
148. Giannotti E, Merlo A, Zerbinati P, Prati P, Masiero S, Mazzoli D. Safety and long-term effects on gait of hemiplegic patients in equinovarus foot deformity surgical correction followed by immediate rehabilitation: a prospective observational study. Eur J Phys Rehabil Med. (2019) 55:169–75. doi: 10.23736/S1973-9087.18.05290-5
149. Vinti M, Bayle N, Merlo A, Authier G, Pesenti S, Jouve JL, et al. Muscle shortening and spastic cocontraction in gastrocnemius medialis and peroneus longus in very young hemiparetic children. Biomed Res Int. (2018) 2018:2328601. doi: 10.1155/2018/2328601
150. Davids JR. The foot and ankle in cerebral palsy. Orthop Clin North Am. (2010) 41:579–93. doi: 10.1016/j.ocl.2010.06.002
151. Gómez-Pérez C, Font-Llagunes JM, Martori JC, Vidal Samsó J. Gait parameters in children with bilateral spastic cerebral palsy: a systematic review of randomized controlled trials. Dev Med Child Neurol. (2019) 61:770–82. doi: 10.1111/dmcn.14108
152. Boudarham J, Pradon D, Roche N, Bensmail D, Zory R. Effects of a dynamic-ankle-foot orthosis (Liberté®) on kinematics and electromyographic activity during gait in hemiplegic patients with spastic foot equinus. Neurorehabilitation. (2014) 35:369–79. doi: 10.3233/NRE-141128
153. Cofré Lizama LE, Khan F, Lee PVS, Galea MP. The use of laboratory gait analysis for understanding gait deterioration in people with multiple sclerosis. Mult Scler. (2016) 22:1768–76. doi: 10.1177/1352458516658137
154. Joseph BJ, Nightingale A. Electromyography o fmuscle of posture: leg muscles in males. J Physiol. (1952) 117:484–91. doi: 10.1113/jphysiol.1952.sp004762
155. Preuss R, Fung J. Musculature and biomechanics of the trunk in the maintenance of upright posture. J Electromyogr Kinesiol. (2008) 18:815–28. doi: 10.1016/j.jelekin.2007.03.003
156. Hodson-Tole EF, Loram ID, Vieira TMM. Myoelectric activity along human gastrocnemius medialis: different spatial distributions of postural and electrically elicited surface potentials. J Electromyogr Kinesiol. (2013) 23:43–50. doi: 10.1016/j.jelekin.2012.08.003
157. Tanabe H, Fujii K, Kouzaki M. Intermittent muscle activity in the feedback loop of postural control system during natural quiet standing. Sci Rep. (2017) 7:1–21. doi: 10.1038/s41598-017-10015-8
158. Russo A, Aranceta-Garza A, D'Emanuele S, Serafino F, Merletti R. HDSEMG activity of the lumbar erector spinae in violin players comparison of two chairs. Med Probl Perform Art. (2019) 34:205–14. doi: 10.21091/mppa.2019.4034
159. Vigotsky AD, Halperin I, Lehman GJ, Trajano GS, Vieira TM. Interpreting signal amplitudes in surface electromyography studies in sport and rehabilitation sciences. Front Physiol. (2018) 8:985. doi: 10.3389/fphys.2017.00985
160. Dos Anjos F, Pinto T, Gazzoni M, Vieira T. The spatial distribution of ankle muscles activity discriminates aged from young subjects during standing. Front Hum Neurosci. (2017) 11:190. doi: 10.3389/fnhum.2017.00190
161. Crow J, Pizzari T, Buttifant D. Muscle onset can be improved by therapeutic exercise: a systematic review. Phys Ther Sport. (2011) 12:199–209. doi: 10.1016/j.ptsp.2010.12.002
162. Hu Y, Kwok JW, Tse JYH, Luk KDK. Time-varying surface electromyography topography as a prognostic tool for chronic low back pain rehabilitation. Spine J. (2014) 14:1049–56. doi: 10.1016/j.spinee.2013.11.060
163. Czaprowski D, Kolwicz A, Afeltowicz A, Pawłowska P, Oleksy L. Reliability of measurements of the extension-flexion ratio with surface EMG. J Back Musculoskelet Rehabil. (2015) 28:827–32. doi: 10.3233/BMR-150590
164. Lehman G. Kinesiological research: the use of surface electromyography for assessing the effects of spinal manipulation. J Electromyogr Kinesiol. (2012) 22:692–6. doi: 10.1016/j.jelekin.2012.02.010
165. Bandpei MAM, Rahmani N, Majdoleslam B, Abdollahi I, Ali SS, Ahmad A. Reliability of surface electromyography in the assessment of paraspinal muscle fatigue: an updated systematic review. J Manipulative Physiol Ther. (2014) 37:510–21. doi: 10.1016/j.jmpt.2014.05.006
166. Michener LA, Sharma S, Cools AM, Timmons MK. Relative scapular muscle activity ratios are altered in subacromial pain syndrome. J Shoulder Elb Surg. (2016) 25:1861–7. doi: 10.1016/j.jse.2016.04.010
167. De Meulemeester K, Calders P, Dewitte V, Barbe T, Danneels L, Cagnie B. Surface electromyographic activity of the upper trapezius before and after a single dry needling session in female office workers with trapezius myalgia. Am J Phys Med Rehabil. (2017) 96:861–8. doi: 10.1097/PHM.0000000000000761
168. O'leary S, Falla D, Elliott JM, Jull G. Muscle dysfunction in cervical spine pain: Implications for assessment and management. J Orthop Sports Phys Ther. (2009) 39:324–33. doi: 10.2519/jospt.2009.2872
169. Falla D, Bilenkij G, Jull G. Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine. (2004) 29:1436–40. doi: 10.1097/01.BRS.0000128759.02487.BF
170. Cavazzuti L, Merlo A, Orlandi F, Campanini I. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Gait Posture. (2010) 32:290–5. doi: 10.1016/j.gaitpost.2010.06.025
171. Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. (2013) 47:207–14. doi: 10.1136/bjsports-2012-090953
172. Esculier JF, Roy JS, Bouyer LJ. Lower limb control and strength in runners with and without patellofemoral pain syndrome. Gait Posture. (2015) 41:813–9. doi: 10.1016/j.gaitpost.2015.02.020
173. Ogbonmwan I, Kumar BD, Paton B. New lower-limb gait biomechanical characteristics in individuals with Achilles tendinopathy: a systematic review update. Gait Posture. (2018) 62:146–56. doi: 10.1016/j.gaitpost.2018.03.010
174. Benedetti MG, Catani F, Bilotta TW, Marcacci M, Mariani E, Giannini S. Muscle activation pattern and gait biomechanics after total knee replacement. Clin Biomech. (2003) 18:871–6. doi: 10.1016/S0268-0033(03)00146-3
175. Semciw A, Neate R, Pizzari T. Running related gluteus medius function in health and injury: a systematic review with meta-analysis. J Electromyogr Kinesiol. (2016) 30:98–110. doi: 10.1016/j.jelekin.2016.06.005
176. Kuiken TA, Li G, Lock BA, Lipschutz RD, Miller LA, Stubblefield KA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. J Am Med Assoc. (2009) 301:619–28. doi: 10.1001/jama.2009.116
177. Farina D, Jiang N, Rehbaum H, Holobar A, Graimann B, Dietl H, et al. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: emerging avenues and challenges. IEEE Trans Neural Syst Rehabil Eng. (2014) 22:797–809. doi: 10.1109/TNSRE.2014.2305111
178. Kuiken TA, Barlow AK, Hargrove LJ, Dumanian GA. Targeted muscle reinnervation for the upper and lower extremity. Tech Orthop. (2017) 32:109–16. doi: 10.1097/BTO.0000000000000194
179. Glazer HI, Romanzi L, Polaneczky M. Pelvic floor muscle surface electromyography. Reliability and clinical predictive validity. J Reprod Med. (1999) 44:779–82.
180. Resende APM, Petricelli CD, Bernardes BT, Alexandre SM, Nakamura MU, Zanetti MRD. Electromyographic evaluation of pelvic floor muscles in pregnant and nonpregnant women. Int Urogynecol J. (2012) 23:1041–5. doi: 10.1007/s00192-012-1702-6
181. Szumilewicz A, Dornowski M, Piernicka M, Worska A, Kuchta A, Kortas J, et al. High-low impact exercise program including pelvic floor muscle exercices improves pelvic floor muscle function in healthy pregnant women–a randomized control trial. Front Physiol. (2020) 9:1867. doi: 10.3389/fphys.2018.01867
182. Flury N, Koenig I, Radlinger L. Crosstalk considerations in studies evaluating pelvic floor muscles using surface electromyography in women: a scoping review. Arch Gynecol Obstet. (2017) 295:799–809. doi: 10.1007/s00404-017-4300-5
183. Heesakkers JPFA, Gerretsen RRR. Urinary incontinence: sphincter functioning from a urological perspective. Digestion. (2004) 69:93–101. doi: 10.1159/000077875
184. Peng Y, He J, Khavari R, Boone TB, Zhang Y. Functional mapping of the pelvic floor and sphincter muscles from high-density surface EMG recordings. Int Urogynecol J. (2016) 27:1689–96. doi: 10.1007/s00192-016-3026-4
185. Hacad CR, Glazer HI, Zambon JPC, Burti JS, Almeida FG. Is there any change in pelvic floor electromyography during the first 6 months after radical retropubic prostatectomy? Appl Psychophysiol Biofeedback. (2015) 40:9–15. doi: 10.1007/s10484-015-9271-3
186. Di Vella G, Riva D, Merletti R. External anal sphincter incontinence by episiotomy and prevention of iatrogenic injury. Riv Ital Med Legale. (2015) 2:473–89.
187. Soderberg GL, Minor SD, Nelson RM. A comparison of motor unit behaviour in young and aged subjects. Age Ageing. (1991) 20:8–15. doi: 10.1093/ageing/20.1.8
188. Callegari B, de Resende MM, da Silva Filho M. Hand rest and wrist support are effective in preventing fatigue during prolonged typing. J Hand Ther. (2018) 31:42–51. doi: 10.1016/j.jht.2016.11.008
189. Cattarello P, Vinelli S, D'Emanuele S, Gazzoni M, Merletti R. Comparison of chairs based on HDsEMG of back muscles, biomechanical and comfort indices, for violin and viola players: a short-term study. J Electromyogr Kinesiol. (2018) 42:92–103. doi: 10.1016/j.jelekin.2018.06.013
190. Ranavolo A, Chini G, Silvetti A, Mari S, Serrao M, Draicchio F. Myoelectric manifestation of muscle fatigue in repetitive work detected by means of miniaturized sEMG sensors. Int J Occup Saf Ergon. (2018) 24:464–74. doi: 10.1080/10803548.2017.1357867
191. Felici F. Neuromuscular responses to exercise investigated through surface EMG. J Electromyogr Kinesiol. (2006) 16:578–85. doi: 10.1016/j.jelekin.2006.08.002
192. Farina D. Interpretation of the surface electromyogram in dynamic contractions. Exerc Sport Sci Rev. (2006) 34:121–7. doi: 10.1249/00003677-200607000-00006
193. Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. (2006) 34:490–8. doi: 10.1177/0363546505282619
194. Sorbie GG, Grace FM, Gu Y, Baker JS, Ugbolue UC. Electromyographic analyses of the erector spinae muscles during golf swings using four different clubs. J Sports Sci. (2018) 36:717–23. doi: 10.1080/02640414.2017.1334956
195. Voigt M, Simonsen EB, Dyhre-Poulsen P, Klausen K. Mechanical and muscular factors influencing the performance in maximal vertical jumping after different prestretch loads. J Biomech. (1995) 28:293–307. doi: 10.1016/0021-9290(94)00062-9
196. Hautier CA, Arsac LM, Deghdegh K, Souquet J, Belli A, Lacour JR. Influence of fatigue on EMG/force ratio and cocontraction in cycling. Med Sci Sports Exerc. (2000) 32:839–43. doi: 10.1097/00005768-200004000-00017
197. Howard RM, Conway R, Harrison AJ. Muscle activity in sprinting: a review. Sport Biomech. (2018) 17:1–17. doi: 10.1080/14763141.2016.1252790
198. Masci I, Vannozzi G, Gizzi L, Bellotti P, Felici F. Neuromechanical evidence of improved neuromuscular control around knee joint in volleyball players. Eur J Appl Physiol. (2010) 108:443–50. doi: 10.1007/s00421-009-1226-z
199. Del Vecchio A, Casolo A, Negro F, Scorcelletti M, Bazzucchi I, Enoka R, et al. The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol. (2019) 597:1873–87. doi: 10.1113/JP277250
200. Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, et al. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol. (1998) 84:1341–9. doi: 10.1152/jappl.1998.84.4.1341
201. Martinez-Valdes E, Wilson F, Fleming N, McDonnell SJ, Horgan A, Falla D. Rowers with a recent history of low back pain engage different regions of the lumbar erector spinae during rowing. J Sci Med Sport. (2019) 22:1206–12. doi: 10.1016/j.jsams.2019.07.007
202. Gallina A, Wakeling JM, Hodges PW, Hunt MA, Jayne Garland S. Regional vastus medialis and vastus lateralis activation in females with patellofemoral pain. Med Sci Sports Exerc. (2019) 51:411–20. doi: 10.1249/MSS.0000000000001810
203. Merletti R, Farina D, Gazzoni M, Schieroni M. Effect of age on muscle functions investigated with surface electromyography. Muscle Nerve. (2002) 25:65–76. doi: 10.1002/mus.10014
204. Piancino MG, Falla D, Merlo A, Vallelonga T, De Biase C, Dalessandri D, et al. Effects of therapy on masseter activity and chewing kinematics in patients with unilateral posterior crossbite. Arch Oral Biol. (2016) 67:61–7. doi: 10.1016/j.archoralbio.2016.03.013
205. Pitta NC, Nitsch GS, Machado MB, de Oliveira AS. Activation time analysis and electromyographic fatigue in patients with temporomandibular disorders during clenching. J Electromyogr Kinesiol. (2015) 25:653–7. doi: 10.1016/j.jelekin.2015.04.010
206. Mapelli A, Zanandréa Machado BC, Giglio LD, Sforza C, De Felício CM. Reorganization of muscle activity in patients with chronic temporomandibular disorders. Arch Oral Biol. (2016) 72:164–71. doi: 10.1016/j.archoralbio.2016.08.022
207. Castroflorio T, Falla D, Wang K, Svensson P, Farina D. Effect of experimental jaw-muscle pain on the spatial distribution of surface EMG activity of the human masseter muscle during tooth clenching. J Oral Rehabil. (2012) 39:81–92. doi: 10.1111/j.1365-2842.2011.02246.x
208. Wozniak K, Piatkowska D, Lipsaki M, Mehr K. Surface electromyography in orthodontics–a literature review. Med Sci Monit. (2013) 19:416–23. doi: 10.12659/MSM.883927
209. Botter A, Beltrandi M, Cerone GL, Gazzoni M, Vieira TMM. Development and testing of acoustically-matched hydrogel-based electrodes for simultaneous EMG-ultrasound detection. Med Eng Phys. (2019) 64:74–9. doi: 10.1016/j.medengphy.2018.12.002
210. Kawano F. The mechanisms underlying neuromuscular changes in microgravity environment. Biol Sci Space. (2004) 18:104–5.
211. Cescon C, Gazzoni M. Short term bed-rest reduces conduction velocity of individual motor units in leg muscles. J Electromyogr Kinesiol. (2010) 20:860–7. doi: 10.1016/j.jelekin.2010.03.008
212. Drost G, Kleine BU, Stegeman DF, Van Engelen BGM, Zwarts MJ. Fasciculation potentials in high-density surface EMG. J Clin Neurophysiol. (2007) 24:301–7. doi: 10.1097/WNP.0b013e31803bba04
213. Kleine BU, Stegeman DF, Schelhaas HJ, Zwarts MJ. Firing pattern of fasciculations in ALS: evidence for axonal and neuronal origin. Neurology. (2008) 70:353–9. doi: 10.1212/01.wnl.0000300559.14806.2a
214. Minetto M, Botter A, De Grandis D, Merletti R. Time and frequency domain analysis of surface myoelectric signals during electrically-elicited cramps. Neurophysiol Clin. (2009) 39:15–25. doi: 10.1016/j.neucli.2008.11.002
215. Minetto M, Holobar A, Botter A, Ravenni R, Farina D. Mechanisms of cramp contractions: peripheral or central generation? J Physiol. (2011) 589:5759–73. doi: 10.1113/jphysiol.2011.212332
216. Minetto M, Holobar A, Botter A, Farina D. Origin and development of muscle cramps. Exerc Sport Sci Rev. (2013) 41:3–10. doi: 10.1097/JES.0b013e3182724817
217. Liu L, Chen X, Lu Z, Cao S, Wu D, Zhang X. Development of an EMG-ACC-based upper limb rehabilitation training system. IEEE Trans Neural Syst Rehabil Eng. (2017) 25:244–53. doi: 10.1109/TNSRE.2016.2560906
218. Donoso Brown EV, Dudgeon BJ, Gutman K, Moritz CT, McCoy SW. Understanding upper extremity home programs and the use of gaming technology for persons after stroke. Disabil Health J. (2015) 8:507–13. doi: 10.1016/j.dhjo.2015.03.007
219. Fuller DA, Keenan MAE, Esquenazi A, Whyte J, Mayer NH, Fidler-Sheppard R. The impact of instrumented gait analysis on surgical planning: treatment of spastic equinovarus deformity of the foot and ankle. Foot Ankle Int. (2002) 23:738–43. doi: 10.1177/107110070202300810
220. Páez-Avilés C, Juanola-Feliu E, Tahirbegi IB, Mir M, Samitier J. CoPs in innovation and technology transfer of medical devices. In: Proceedings of ISPIM Conferences. Budapest (2015). p. 1–16. Available online at: http://search.ebscohost.com/login.aspx?direct=true&db=bth&AN=110480625&site=ehost-live
221. Duncan EAS, Murray J. The barriers and facilitators to routine outcome measurement by allied health professionals in practice: a systematic review. BMC Health Serv Res. (2012) 12:96. doi: 10.1186/1472-6963-12-96
222. Stevens JGA, Beurskens AJMH. Implementation of measurement instruments in physical therapist practice: development of a tailored strategy. Phys Ther. (2010) 90:953–61. doi: 10.2522/ptj.20090105
223. Veras M, Kairy D, Paquet N. What is evidence-based physiotherapy? Physiotherapy. (2016) 68:95–6. doi: 10.3138/ptc.68.2.GEE
224. Wade D. Measurements in Neurological Rehabilitaiton. Oxford: Oxford Medical Publications (1992).
225. Merlo A, Campanini I. Applications in movement and gait analysis (Ch. 16). In: Merletti R, Farina D, editors. Surface Electromyography: Physiology, Engineering, and Applications. Hoboken, NJ: IEEE Press; John Wiley & Sons, Inc. (2016). p. 440–59. doi: 10.1002/9781119082934.ch16
226. Hermens H, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, et al. European Recommendations for Surface Electromyography. RRD Publisher (1999). Available online at: www.seniam.org
227. Merlo A, Campanini I. Technical aspects of surface electromyography for clinicians. Open Rehabil J. (2010) 3:98–109. doi: 10.2174/1874943701003010098
228. Kumagai K, Yamada M. The clinical use of multichannel surface electromyography. Pediatr Int. (1991) 33:228–37. doi: 10.1111/j.1442-200X.1991.tb01548.x
Keywords: surface electromyography, sEMG, rehabilitation, clinical applications, motion analysis, education, physiotherapy, movement sciences
Citation: Campanini I, Disselhorst-Klug C, Rymer WZ and Merletti R (2020) Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers Limiting Its Use. Front. Neurol. 11:934. doi: 10.3389/fneur.2020.00934
Received: 28 April 2020; Accepted: 20 July 2020;
Published: 02 September 2020.
Edited by:
Maurizio Ferrarin, Fondazione Don Carlo Gnocchi Onlus (IRCCS), ItalyReviewed by:
Jaap Van Dieen, Vrije Universiteit Amsterdam, NetherlandsRoberto Gatti, Humanitas University, Italy
Copyright © 2020 Campanini, Disselhorst-Klug, Rymer and Merletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Merletti, cm9iZXJ0by5tZXJsZXR0aSYjeDAwMDQwO2Zvcm1lcmZhY3VsdHkucG9saXRvLml0
 Isabella Campanini
Isabella Campanini Catherine Disselhorst-Klug
Catherine Disselhorst-Klug William Z. Rymer
William Z. Rymer Roberto Merletti
Roberto Merletti