
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 25 August 2020
Sec. Movement Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00874
This article is part of the Research Topic Managing Parkinson's Disease with a Multidisciplinary Perspective View all 13 articles
Background: Essential tremor (ET) is manifested as an isolated syndrome of bilateral upper limb action tremor. Parkinson's disease (PD) is the second most common neurodegenerative disease, with typical motor symptoms of bradykinesia, rigidity, and resting tremor. ET-PD describes the new-onset of PD in ET patients. Recently, numerous studies on epidemiology, genetics, pathology, clinical features, and neuroimaging studies are challenging the idea that ET is an isolated disease, suggesting that patients with ET have the tendency to develop PD.
Methods: In this review article, we collected recent findings that reveal prodromal markers of PD in patients with ET.
Results: Substantia nigra hyperechogenicity serves as a prodromal marker for predicting the development of PD in patients with ET and provides a reference for therapeutic strategies. Additional potential markers include other neuroimaging, clinical features, heart rate, and genetics, whereas others lack sufficient evidence.
Conclusion: In consideration of the limited research of PD in patients with ET, we are still far from revealing the prodromal markers. However, from the existing follow-up studies on ET patients, Substantia nigra hyperechogenicity may enable further exploration of the relationship between ET and PD and the search for pathogenesis-based therapies.
Essential tremor (ET) is defined as an isolated postural and/or kinetic tremor syndrome of the bilateral upper limbs, with or without tremor in other locations (e.g., head, voice, or lower limbs). The traditional idea proposes that no other neurological signs such as dystonia, ataxia, or parkinsonism are present (1–3), although ET is sometimes considered a diagnostic of exclusion (4). With accumulated studies on ET, researchers today recognize the concept of ET plus (3), which refers to tremor with the characteristics of ET and additional neurological signs such as impaired tandem gait, questionable dystonic posturing, memory impairment, or other mild neurologic signs. However, these additional neurological signs are of unknown significance and are insufficient to make an additional syndrome classification or diagnosis. Dividing ET into two categories (ET and ET plus) is helpful in determining pathophysiological characteristics and therapeutic strategies.
Parkinson's disease (PD) is the second most common neurodegenerative disease, characterized by the degeneration of dopaminergic neurons in the substantia nigra (SN) and reduced dopamine levels in the midbrain (5–8). The pathological hallmark of PD is the presence of Lewy bodies, and the classical motor symptoms of PD are bradykinesia, rigidity and resting tremor, and numerous non-motor symptoms including constipation, hyposmia/anosmia, rapid eye movement sleep behavior disorder (RBD), and so on (9).
ET-PD referred to in this article means new onset of PD in patients previously diagnosed with ET. Essential tremor has been traditionally viewed as a “benign” disease; however, accumulated data from the studies of clinical characteristics, epidemiology, neuroimaging, genetics, and pathology support a poorer prognosis than originally believed (10–12). Evidence indicates that patients with ET are four times more likely to develop PD than those without baseline ET (13). Additionally, nigrostriatal degeneration developed before the onset of motor symptoms of parkinsonism (14, 15). Sensitive and effective prodromal markers predicting which ET patients will subsequently develop PD are needed to further understand the biological nature of ET, which will provide a pathology-based categorization, diagnosis, prognosis, and eventual treatment of ET.
Transcranial sonography (TCS) can show the structure of the brain parenchyma and reveal the lesions of the SN. It is anecdotally viewed as a non-invasive tool that can be utilized to detect the intensity of the SN and measure the ratio of the hyperechoic area to midbrain area (S/M ratio). The SN hyperechogenicity refers to the high intensity and large area (>0.8–0.2 cm2) of the echo in the SN shown by transcranial ultrasound.
As early as 1995 (16), scientists first described the mysterious relationship between SN hyperechogenicity and PD, which provided a new perspective and direction for research. It was verified that SN hyperechogenicity as reflected by TCS, seemed to be a prodromal marker for PD (17–20). In 2007, research including 164 healthy Taiwanese, 40 early-onset PD patients, and 40 late-onset PD patients focused on the area of SN hyperechogenicity and the S/M ratio (21). The results indicated that the S/M ratio is a more sensitive marker than SN hyperechogenicity in diagnosing PD. Based on the investigation that tremor-dominant PD (TDPD) patients had significantly higher nigral R2* relaxation rate values in magnetic resonance imaging (MRI) (34.1 ± 5.7) than those with tremor in dystonia (30.0 ± 3.9), ET (30.6 ± 4.8), and controls (30.0 ± 2.8). This and other research confirm that increased iron content in the SN is significantly associated with PD. Afterward, combining iron metabolism and the features of TCS (22, 23), researchers presumed that the change of the echogenicity in the SN may be attributed to oxidative stress and the injury of neurons caused by the fluctuation of iron content. In a prospective multicenter study, researchers also demonstrated that the elderly with SN hyperechogenicity had a higher risk of developing PD (24).
Transcranial sonography measurement of the midbrain is a sensitive and specific preliminary screening method to identify certain population with higher risk of developing PD. A small trial also supported its use at predicting ET patients who will develop PD, but this needs to be replicated in larger trials (25). After performing TCS of the SN and clinical examination in 80 PD patients, 30 ET patients, and 80 age- and gender-matched controls, researchers realized that SN hyperechogenicity may be associated with an increased risk developing PD later in life or might be due to the damage of areas near the nucleus ruber in ET patients (26). Subsequently, Sprenger et al. (27) conducted a prospective cohort study in 70 ET patients, which evaluated demographics, TCS, Bain tremor scale, and Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale. After an average of 6.16 years' follow-up, they identified nine ET-PD patients, seven of whom had SN hyperechogenicity greater than baseline. Statistical analysis showed that the relative risk of developing PD in patients with ET who had hyperechogenicity at baseline vs. those without hyperechogenicity was 7.00 (27). Moreover, a recent longitudinal study demonstrated that after 3 years' follow-up, 9 of 59 ET patients developed clinical features meeting diagnostic criteria for probable PD (ET-PD), and this group had a significantly greater SN hyperechogenicity at baseline from healthy controls (28).
Several studies have consistently demonstrated that SN hyperechogenicity on TCS is a prodromal marker for development of PD in ET patients (29, 30). It serves as a visualization tool for the diagnosis and prognosis of ET patients. Different characteristics of the echo are associated with not only the diagnosis of PD but also the therapeutic response (31). Hence, in addition to differential diagnosis between PD and other movement disorders (32), SN hyperechogenicity can also serve as a prodromal marker for predicting the development of PD in patients with ET and provide a guidance for therapeutic strategy (Table 1).
Brain structural and functional neuroimaging methods might show abnormalities in ET patients. Voxel-based morphometry (VBM) is commonly used to learn about gray matter and white matter size. Benito-Leon et al. (33) found significant white matter changes in right cerebellum, left medulla, right parietal lobe, and right limbic lobe, as well as gray matter alteration in bilateral cerebellum, bilateral parietal lobe, right frontal lobe, and right insula in ET patients compared with 20 age- and gender-matched healthy controls. Lin et al. (34) compared VBM in 10 ET patients with 10 PD patients and revealed that PD and ET caused specific patterns of brain volume alterations in the examined brains. The brain volume of the ET group was significantly smaller in the thalamus and the middle temporal gyrus and larger of the gray matter in the middle frontal gyrus, the middle temporal gyrus, and the cerebellum posterior lobe than that of the PD group (34). These studies suggested comprehensive changes in the gray and white matter of brain in ET patients.
Diffusion tensor imaging (DTI) derived mean diffusivity (MD) and fractional anisotropy. It exhibits great fidelity in showing brain microstructure and connections. A DTI-based study performed in 67 ET patients (29 ET with and 38 without resting tremor) and 39 age-matched healthy controls indicated that MD was significantly higher in the cerebellar gray matter in the ET group. It demonstrates that ET patients have cerebellum microstructural changes, and some other networks alterations may exist in the development of ET (35).
After enrolling 15 ET patients with resting tremor and 15 TDPD patients, Cherubini et al. (36) did a combination of VBM and DTI to distinguish these two groups with a ground truth of Dopamine transporter 123I-FP-CIT-single-photon emission tomography (DAT-SPECT), and they found the combination shows 100% accuracy in differentiating these two groups.
Proton MR spectroscopy (1H-MRS) is a non-invasive, quantitative technique that reflects the neurometabolic alterations in vivo. There are many biochemical markers including NAA/Cr (a neural density marker), Glx/Cr (an intracellular neurotransmitter marker), and Cho/Cr (a membrane marker). In 2002, researchers measured 16 ET patients and 11 controls with MRS and found that cortical NAA/Cr in the cerebellar was reduced in ET cases, and the value was inversely proportional to arm tremor severity (37). With the application of a 3-T scanner, Barbagallo et al. (38) found a statistically significant increase in Glx and Glx/Cr values in 16 ET patients in both thalami compared to 14 healthy controls, and the tremor severity was directly proportional to these two values. These results suggested that increased thalamic glutamatergic transmission and cerebellum play a role in the pathogenesis in ET. In addition to these imaging techniques (Table 2), MRI imaging focused on brain iron deposition, and functional MRI and other neuroimaging methods also indicated the details of the involved brain networks in ET and provided some help to differentiate mixed tremor, ET, and PD (39–41). Even though some of the research did not explore prodromal markers of PD in ET patients from a longitudinal-study perspective, it still reflects the relationship between the two disorders, provides methods in differential diagnosis, and promotes the knowledge of behind pathophysiologic mechanisms.
Identifying clinical characteristics that increase the risk of developing PD in ET patients may help in the development of potential disease-modifying therapies. Essential tremor classically presents as a bilateral but asymmetric kinetic and postural tremor of the upper limbs, head, voice, or a combination. Parkinson disease is generally manifested as asymmetric symptoms of bradykinesia, rigidity, resting tremor, and later postural instability. Many studies have summarized the similarities and differences of the two diseases based on clinical characteristics (i.e., motor and non-motor symptoms) (30, 42, 43), but there are still few prodromal markers with respect to the clinical characteristics of ET-PD patients, and many of them are still in the conjecture stage (Table 3).
An epidemiological survey showed that tremor of the upper limbs was presented in more than 95% of the ET patients, followed by the head (>30%), voice (>20%), tongue (20%), face and/or jaw (10%), lower limbs (10%) and trunk (5%) (61, 62). Essential tremor and PD tremor are mainly identified based on aspects of location, frequency, and form. However, few studies have identified risk markers based on tremor characteristics in ET patients who have the potential to develop PD.
The location of tremor in the two groups was most common in upper limbs, whereas the prevalence of resting tremor in ET-PD patients was higher than that in patients with isolated ET, and the tremor distribution was more limited in ET-PD (p < 0.05) (30). Another study involving 53 ET-PD patients and 150 ET patients noted a biased distribution toward male (67.9% male) of ET-PD, which is identical to that of PD (67.9% male) (44). The latency from the onset of ET to develop into PD ranged from short duration (<5 years, in 38.5%) to long duration (>20 years), with a mean duration of 14 ± 15 years. In ET-PD patients, the side of dominant initial ET severity usually coincides with that of dominant PD severity (p < 0.05). The initial cardinal sign was resting tremor in the vast majority of PD patients, which indicated that resting tremor may be an omen of PD (44). This evidence implied that circumscribed resting tremor may be a prodromal marker for ET patients to develop PD. However, some studies also pointed out that resting tremor (in the arms but not the legs) can occur as a late feature in ET patients (63), so it is worth considering the role of circumscribed resting tremor in the course of predicting PD in ET patients. Additionally, data from a retrospective observation of 13 patients initially diagnosed as ET based on tremor characteristics, alcohol responsiveness, and family history who met the PD criteria after a variable long latent period suggest that late-onset asymmetrical postural tremor may be a signal for developing PD in the long term even if there is no resting tremor (45).
In addition to upper limbs, tremors of the head, voice, and jaw also exist in ET with long disease duration (46). The incidence of jaw tremor ranges from 7.5 to 18% (47, 64, 65) in ET patients. Essential tremor patients with jaw tremor have more severe clinical symptoms and more widely distributed tremor than patients without it. Essential tremor patients with head tremor may be also regarded as a more severe clinical subtype (66). This manifestation combined with the hypothesis that the evolution from ET to PD is caused by the spread of Lewy bodies in the cerebellothalamocortical circuit leads us to speculate that there may be some relationship between jaw tremor and subsequent PD. Additional longitudinal studies are needed to assess whether jaw tremor in ET is a prodromal marker for subsequent PD.
In addition to the research on prodromal markers based on the position, range, and form of the tremor (67–70), a recent study investigated the motor feature: the Tremor Stability Index (TSI) (48). The TSI can be obtained by kinematics measurement of tremor activity and applied to the analysis of tremor characteristics. After testing a cohort comprising 16 rest tremor recordings in TDPD and 20 postural tremor recordings in ET, researchers found a difference in TSI between these two groups [mean 0.7 ± 0.175 (SEM) in TDPD, 1.9 ± 0.134 in ET, t(34) = −5.481, p < 0.001]. This suggests electrophysiological methods may be helpful to evaluate the nature of the tremor and to explore the underlying central oscillator circuits from peripheral tremor. We can also examine additional parameters to evaluate the prognosis of ET patients.
Non-motor symptoms are increasingly recognized as an important part of ET. Essential tremor patients show a variety of non-motor symptoms, such as cognitive decline, mood disorder, and hearing loss (71–78). However, studies on non-motor symptoms as possible prodromal factors for developing PD in ET patients are mostly lacking.
There has been increasing interest in olfactory dysfunction because it was identified as a prodromal feature of PD. Just as in PD patients, olfactory decline (hyposmia or anosmia) becomes more evident as the disease progresses in ET patients (49, 79); however, there are also studies that show no olfactory loss in ET. In 2008, Louis et al. (50) found that higher blood harmane concentration was correlated with olfactory decline in 83 ET cases (62.5%, p < 0.001). As a cerebellar toxin, harmane reflects the relationship between olfactory decline and a part of pathophysiology in the cerebellum. In 2016, a large European multicenter study using the 16-item Sniffin' Sticks test (SS-16) found that olfactory performance was lower in PD patients compared with atypical parkinsonism and non-PD patients in all cohorts (each p < 0.001), and a set of eight smell reduction olfactory tests can be used as a rapid detection tool for PD (51). This calls for olfactory testing of ET patients and longitudinal studies to explore whether hyposmia can be used as a prodromal marker for the development of PD in ET patients.
It is still controversial whether cognition declines in ET. As early as 2001, Gasparini et al. (52) carried out a preliminary study focusing on performance on frontal lobe tasks of 27 ET patients, 15 PD patients, and 15 healthy controls, and they found significant impairments both in attention and conceptual thinking tasks in ET patients, but with no statistical difference between ET patients and PD patients. Consistent with this research, many subsequent studies identified cognitive impairments in ET patients (80, 81). In 2019, researchers enrolled 23 ET patients and 23 healthy controls to evaluate topological properties of brain function network with the aid of resting-state functional MRI, which revealed that changes took place in many regions including hippocampus in ET patients (82). By employing a structured neuropsychological battery to access cognition in 40 ET patients and 40 healthy controls, Prasad et al. (83) revealed that ET patients with cognitive impairment have significant volumetric abnormalities of specific brain regions including several hippocampal subfields.
Recently, by evaluating the hippocampal subregions of PD with cognitive decline and PD without cognitive decline, Xu et al. (84) found that the hippocampal CA2/3, CA4, and DG subfields appeared sensitive in groups of PD with cognitive decline longitudinally, and the volume loss of CA2/3 and CA4-DG correlated with the degree of cognitive impairment. With the assistance of quantitative susceptibility mapping, Thomas et al. (85) tracked cognitive changes in PD and identified lower Montreal Cognitive Assessment (MoCA) scores in the hippocampus and thalamus. Compared with PD patients, ET-PD patients have poorer cognitive performance. After enrolling 30 ET-PD patients and 53 age-matched PD patients, researchers found that both the cursory total score of Mini-Mental State Examination (p = 0.001) and the Telephone Interview for Cognitive Status (p < 0.001) were lower in ET-PD than in PD, as well as the subscores related to orientation (p < 0.001), language (p < 0.001), and working memory (p = 0.001) (53). It has also been demonstrated that hippocampal microstructural damage is related to subclinical memory impairment in ET patients (57). Considering that cognitive disorders with involvement of the hippocampus occurred both in ET and PD patients, and ET-PD patients have poorer cognitive performance than PD patients, the role of cognitive impairment in predicting the development of PD in ET patients is worth exploring in future research.
Numerous studies have indicated that sleep disturbance, especially RBD, may be an early prodromal marker for developing PD in ET patients (58–60, 86). One study using the RBD Screening Questionnaire (RBDSQ) revealed that ~0.5% in the general population suffered from RBD (43.5%) (87). Another study in which 92 ET patients were assessed on the Scales for Outcomes in Parkinson's Disease–Autonomic questionnaire to evaluate autonomic symptoms and the RBDSQ to assess the RBD symptoms with ≥5 as a cut-off value for probable RBD (pRBD), 26.4% of the ET patients had pRBD and 98.1% of them reported at least one autonomic symptom (88). This association suggested that a subgroup of ET patients with pRBD may be at higher risk of PD progression. Salsone et al. (89) proposed that the presence of RBD in ET could identify a specific clinical phenotype and demonstrated significantly reduced scores on memory of ET patients with RBD compared to those without RBD, indicating RBD in ET patients is associated with cognitive impairment. Because RBD is a risk marker of PD in healthy people, its role in prediction of PD in ET patients deserves further longitudinal study.
Recent autonomic symptom questionnaire-based analyses and sympathetic skin response–based studies have shown a variety of autonomic dysfunctions associated with ET, especially in the fields of cardiovascular and urogenital diseases (90–92).
Heart rate variability (HRV) evaluates cardiac autonomic nervous regulation function based on the measurements of beat-to-beat RR variability, including time domains and frequency domains analysis. By measuring the components of HRV analysis in the frequency domain during a 12-h daytime and verifying by DAT-SPECT and cardiac MIBG uptake of 10 ET patients, 10 PD patients, and 10 age-sex-matched controls, investigators found that low-frequency components of HRV analysis helped differentiate between ET and PD (93). In another case-control study enrolling 23 ET patients, 27 TDPD patients, and 23 healthy controls, researchers found that HRV was significantly lower in the TDPD group, and the low-frequency component was the best diagnostic marker (AUC = 0.87) for differentiating ET and PD (94). Another study based on sympathoneural imaging in four family members indicated that overexpression of normal α-synuclein and cardiac sympathetic denervation had an impact on parkinsonism (95).
Heart rate variability analysis as a non-invasive tool and a reflection of autonomic nervous function plays an important role in differentiating ET and TDPD at the early stage of disease (94). However, the results of HRV can be affected by many factors, including age, gender, cardiovascular risk factors, and medications. Even deep brain stimulation can produce certain effect on cardiac electrophysiological activity (96, 97). The analysis of HRV can reflect autonomic nerve regulation of the heart and provide information about sympathetic nerve function status.
A recent clinical study with PD showed that failure to increase total peripheral resistance with cardiac denervation under orthostatic stress was associated with systolic blood pressure reduction leading to orthostatic hypotension (98). Another study that enrolled 75 elderly patients with ET and 25 age-matched controls found no difference between the two groups in orthostatic vital signs, ambulatory 24-h blood pressure monitoring and 24-h Holter monitoring values (99). Therefore, more studies are needed to explore the role of HRV in the development of ET-PD.
Elucidating the genetic background of ET and PD is crucial for understanding the pathogenesis and improving diagnostic and therapeutic strategies. Research has shown that the risk of ET is significantly increased in PD relatives (100, 101); similarly, studies have found that the risk of PD is increased in first-degree relatives of ET patients (102), which indicate that although the two diseases are mostly distinct in their etiology and symptoms, there may be potential genetic pleiotropy between them.
By comparing and analyzing the clinical characteristics of 25 patients with ET-PD and a control group, Ryu et al. (69) found that ET-PD patients had an obvious family history of tremor of first-degree relatives. Abundant genetic studies have revealed that the family history of tremor may be a prodromal marker for PD in ET patients (103).
While a number of studies have failed to identify the causative genes (104–111), several risk genes appear to have an overlapping role in ET and PD (112). For instance, association studies have found that leucine-rich repeat and immunoglobulin containing 1 gene is involved in the pathogenesis of ET and PD (113–118) and serves as a potential therapeutic target in these two disorders. HTRA2, which encodes a serine protease, plays a role in the pathogenesis of genetic type ET (119, 120), and its homozygous allele is involved in the pathogenesis of PD (121), providing genetic evidence of a link between the two disorders, although an Asian study did not find a role for this gene in ET-PD (122, 123). Moreover, using polymerase chain reaction, research has shown that the intermediate copy number of C9ORF72 repeats increases the risk of PD and ET-PD (124). However, a recent study focusing on the genotype excluded 56 samples from analysis as they had genotyping call rates <0.90. There were no variants significantly deviated from Hardy–Weinberg equilibrium (all had p > 0.01) (125). Generally, the role of genetic risk factors in neurodegenerative diseases needs to be further explored in larger studies, including genes that can serve as prodromal markers for the development of PD in ET.
Electrophysiological parameters of tremors have the potential to become prodromal markers as technology becomes more clinic-friendly. A study found that the concordance rate between clinical and electrophysiological methods in diagnosing of ET was 94.4% (51 of 54) (126), indicating that electrophysiological methods may be of value in quantifying preclinical patients. One study utilizing an electrophysiological approach to access children and adolescents found an interesting phenomenon: the mean tremor frequency with arms extended was different between children (5.3 Hz) and adolescents (9.0 Hz) (127). This finding suggests that the pathogenesis may be different between children and adolescents. Previous studies have shown an increased R2 recovery of the blink reflex in PD and increased R2 recovery component of the blink reflex (R2-BRrc) in ET associated with resting tremor while normal in ET patients (128). A 2015 study revealed that the probability of the auditory startle reaction was significantly lower in ET-PD patients, whereas it was similar in both healthy subjects and ET or PD patients (P < 0.001) (129). Another study that enrolled 19 ET-PD patients and 85 controls (i.e., 48 ET patients and 37 PD patients) found that electrophysiological parameters (i.e., a synchronous resting tremor pattern and the abnormal blink-recovery cycle) were the most accurate biomarkers in distinguishing ET-PD patients from ET or PD patients (130), which calls for longitudinal studies to evaluate whether it can be used as a prodromal marker for the development of PD in ET. Additionally, Crowell et al. (131) utilized an electrocorticography study to address movement disorders such as PD, primary dystonia, and ET with abnormalities in synchronized oscillatory activity. With the development of artificial intelligence and big data, smart phones and sports bracelets can sense and record the characteristics of various parameters of tremor and then enter them into big data analyses (132, 133). Electrophysiological approaches may be new and effective prodromal markers for predicting the development of PD in ET patients.
Studies of behavioral factors in ET and PD are indispensable. For instance, alcohol consumption and dairy intake may be risk factors for PD (134). A study using a population-based, case-control design analyzed ever smokers and never smokers and revealed that ever smokers had less than half the risk of ET (odds ratio = 0.58, 95% confidence interval = 0.40–0.84, p = 0.004). The amount of smoking played a subtle role in the development (135). Another study that examined body mass index and waist circumference of PD patients found a possible interaction between anthropometry, sex, and smoking and PD risk (136). Similarly, new prodromal markers are expected to be found when large-scale statistical analyses of patient lifestyle factors are completed.
Identification of prodromal markers for the development of PD from ET represents one of the most urgent unmet needs in neurology. This relationship is garnering an increasing amount of interest among researchers. Evidence based on clinical characteristics, epidemiology, neuroimaging, genetics, pathology, and many other aspects has demonstrated numerous associations between these two conditions. Essential tremor patients have an increased risk of developing PD, especially those with SN hyperechogenicity in TCS which is directly demonstrated by a longitudinal study or a prospective cohort study among ET patients. Similarly, ET patients with specific tremor characteristics or non-motor symptoms, HRV, and certain genetic variations are likely to have a higher risk of developing of PD afterward (Figure 1) with the evidence derived from retrospective study, cross-sectional study or prospective study focusing on the characteristics of ET, PD, ET-PD or the differential diagnosis (Table 4). However, given the lack of research directly evaluating PD prodromal markers in ET, we are far from understanding the relationship, and large longitudinal studies of clinical characteristics, genetics, and pathology are needed.
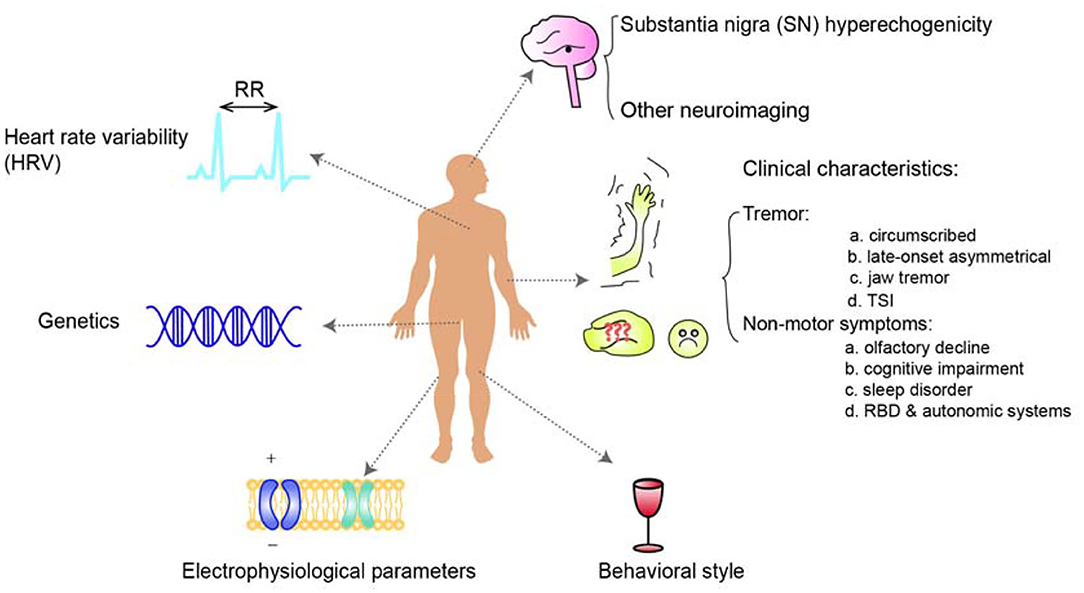
Figure 1. An overview of the risk markers of Parkinson's disease in patients with essential tremor. Essential tremor patients have an increased risk of the development of PD, especially those with SN hyperechogenicity in TCS. Similarly, ET patients with specific tremor or non-motor symptoms, additional HRV, genetic risk, or the other risk markers such as Tremor Stability Index are undergoing a higher risk of the development of PD afterward.
Y-CW provided fund support, revised the manuscript, and designed the project ideas. X-XW, YF, and XL searched for literature and wrote the manuscript. X-YZ, DT, and WO revised the paper. All authors contributed to the article and approved the submitted version.
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81671251 and 81971185).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ET, essential tremor; PD, Parkinson's disease; RBD, rapid eye movement sleep behavior disorder; SN, substantia nigra; TCS, Transcranial sonography; TSI, Tremor Stability Index; RBDSQ, RBD screening questionnaire; pRBD, probable RBD; SS-16, 16-item Sniffin' Sticks test; HRV, Heart rate variability; TDPD, tremor-dominant PD.
2. Espay AJ, Lang AE, Erro R, Merola A, Fasano A, Berardelli A, et al. Essential pitfalls in “essential” tremor. Mov Disord. (2017) 32:325–31. doi: 10.1002/mds.26919
3. Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. (2018) 33:75–87. doi: 10.1002/mds.27121
4. Fasano A, Lang AE, Espay AJ. What is “Essential” about essential tremor? A diagnostic placeholder. Mov Disord. (2018) 33:58–61. doi: 10.1002/mds.27288
5. Kalia LV, Lang AE. Parkinson's disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
6. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. (2016) 15:1257–72. doi: 10.1016/S1474-4422(16)30230–7
7. de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. (2006) 5:525–35. doi: 10.1016/S1474-4422(06)70471-9
8. Collaborators GBDN. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:459–480. doi: 10.1016/S1474-4422(18)30499-X
9. Wolters E. Non-motor extranigral signs and symptoms in Parkinson's disease. Parkinsonism Relat Disord. (2009) 15(Suppl. 3):S6–12. doi: 10.1016/S1353-8020(09)70770-9
10. Algarni M, Fasano A. The overlap between essential tremor and Parkinson disease. Parkinsonism Relat Disord. (2018) 46(Suppl. 1):S101–4. doi: 10.1016/j.parkreldis.2017.07.006
11. Louis ED, Benito-Leon J, Faust PL. Essential tremor seems to be a risk factor for Parkinson's disease. Parkinsonism Relat Disord. (2016) 26:82–3. doi: 10.1016/j.parkreldis.2016.02.026
12. Louis ED, Benito-Leon J, Faust PL. Essential tremor is a risk factor for Parkinson's disease. Parkinsonism Relat Disord. (2016) 24:143–4. doi: 10.1016/j.parkreldis.2016.01.009
13. Benito-Leon J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain Study G. Risk of incident Parkinson's disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry. (2009) 80:423–5. doi: 10.1136/jnnp.2008.147223
14. Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. (1991) 114:2283–301. doi: 10.1093/brain/114.5.2283
15. Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. (2013) 136:2419–31. doi: 10.1093/brain/awt192
16. Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K. Degeneration of substantia nigra in chronic Parkinson's disease visualized by transcranial color-coded real-time sonography. Neurology. (1995) 45:182–4. doi: 10.1212/WNL.45.1.182
17. Pilotto A, Yilmaz R, Berg D. Developments in the role of transcranial sonography for the differential diagnosis of parkinsonism. Curr Neurol Neurosci Rep. (2015) 15:43. doi: 10.1007/s11910-015-0566-9
18. Walter U, Klein C, Hilker R, Benecke R, Pramstaller PP, Dressler D. Brain parenchyma sonography detects preclinical parkinsonism. Mov Disord. (2004) 19:1445–9. doi: 10.1002/mds.20232
19. Berg D, Siefker C, Ruprecht-Dorfler P, Becker G. Relationship of substantia nigra echogenicity and motor function in elderly subjects. Neurology. (2001) 56:13–7. doi: 10.1212/WNL.56.1.13
20. Berg D, Seppi K, Behnke S, Liepelt I, Schweitzer K, Stockner H, et al. Enlarged substantia nigra hyperechogenicity and risk for Parkinson disease: a 37-month 3-center study of 1847 older persons. Arch Neurol. (2011) 68:932–7. doi: 10.1001/archneurol.2011.141
21. Huang YW, Jeng JS, Tsai CF, Chen LL, Wu RM. Transcranial imaging of substantia nigra hyperechogenicity in a Taiwanese cohort of Parkinson's disease. Mov Disord. (2007) 22:550–5. doi: 10.1002/mds.21372
22. Yu SY, Cao CJ, Zuo LJ, Chen ZJ, Lian TH, Wang F, et al. Clinical features and dysfunctions of iron metabolism in Parkinson disease patients with hyper echogenicity in substantia nigra: a cross-sectional study. BMC Neurol. (2018) 18:9. doi: 10.1186/s12883-018-1016-5
23. Homayoon N, Pirpamer L, Franthal S, Katschnig-Winter P, Kogl M, Seiler S, et al. Nigral iron deposition in common tremor disorders. Mov Disord. (2019) 34:129–32. doi: 10.1002/mds.27549
24. Berg D, Behnke S, Seppi K, Godau J, Lerche S, Mahlknecht P, et al. Enlarged hyperechogenic substantia nigra as a risk marker for Parkinson's disease. Mov Disord. (2013) 28:216–9. doi: 10.1002/mds.25192
25. Stockner H, Sojer M, K KS, Mueller J, Wenning GK, Schmidauer C, et al. Midbrain sonography in patients with essential tremor. Mov Disord. (2007) 22:414–7. doi: 10.1002/mds.21344
26. Budisic M, Trkanjec Z, Bosnjak J, Lovrencic-Huzjan A, Vukovic V, Demarin V. Distinguishing Parkinson's disease and essential tremor with transcranial sonography. Acta Neurol Scand. (2009) 119:17–21. doi: 10.1111/j.1600-0404.2008.01056.x
27. Sprenger FS, Wurster I, Seppi K, Stockner H, Scherfler C, Sojer M, et al. Substantia nigra hyperechogenicity and Parkinson's disease risk in patients with essential tremor. Mov Disord. (2016) 31:579–83. doi: 10.1002/mds.26515
28. Cardaioli G, Ripandelli F, Paolini Paoletti F, Nigro P, Simoni S, Brahimi E, et al. Substantia nigra hyperechogenicity in essential tremor and Parkinson disease: a longitudinal study. Eur J Neurol. (2019) 26:1370–1376. doi: 10.1111/ene.13988
29. Stockner H, Wurster I. Transcranial sonography in essential tremor. Int Rev Neurobiol. (2010) 90:189–97. doi: 10.1016/S0074-7742(10)90014-7
30. Lauckaite K, Rastenyte D, Surkiene D, Vaidelyte B, Dambrauskaite G, Sakalauskas A, et al. Ultrasonographic (TCS) and clinical findings in overlapping phenotype of essential tremor and Parkinson's disease (ET-PD). BMC Neurol. (2014) 14:54. doi: 10.1186/1471-2377-14-54
31. Alonso-Canovas A, Lopez-Sendon Moreno JL, Buisan J, Sainz de la Maza S, Costa-Frossard L, Garcia-Ribas G, et al. Does normal substantia nigra echogenicity make a difference in Parkinson's disease diagnosis? A real clinical practice follow-up study. J Neurol. (2018) 265:2363–9. doi: 10.1007/s00415-018-9006-5
32. Grippe TC, Allam N, Brandao PRP, Pereira DA, Cardoso FEC, Aguilar ACR, et al. Is transcranial sonography useful for diagnosing Parkinson's disease in clinical practice? Arq Neuropsiquiatr. (2018) 76:459–66. doi: 10.1590/0004-282x20180067
33. Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Louis ED. Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. J Neurol Sci. (2009) 287:138–42. doi: 10.1016/j.jns.2009.08.037
34. Lin CH, Chen CM, Lu MK, Tsai CH, Chiou JC, Liao JR, et al. VBM reveals brain volume differences between Parkinson's disease and essential tremor patients. Front Hum Neurosci. (2013) 7:247. doi: 10.3389/fnhum.2013.00247
35. Novellino F, Nicoletti G, Cherubini A, Caligiuri ME, Nistico R, Salsone M, et al. Cerebellar involvement in essential tremor with and without resting tremor: a Diffusion Tensor Imaging study. Parkinsonism Relat Disord. (2016) 27:61–6. doi: 10.1016/j.parkreldis.2016.03.022
36. Cherubini A, Nistico R, Novellino F, Salsone M, Nigro S, Donzuso G, et al. Magnetic resonance support vector machine discriminates essential tremor with rest tremor from tremor-dominant Parkinson disease. Mov Disord. (2014) 29:1216–9. doi: 10.1002/mds.25869
37. Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. (2002) 333:17–20. doi: 10.1016/S0304-3940(02)00966-7
38. Barbagallo G, Arabia G, Novellino F, Nistico R, Salsone M, Morelli M, et al. Increased glutamate + glutamine levels in the thalamus of patients with essential tremor: A preliminary proton MR spectroscopic study. Parkinsonism Relat Disord. (2018) 47:57–63. doi: 10.1016/j.parkreldis.2017.11.345
39. Novellino F, Cherubini A, Chiriaco C, Morelli M, Salsone M, Arabia G, et al. Brain iron deposition in essential tremor: a quantitative 3-Tesla magnetic resonance imaging study. Mov Disord. (2013) 28:196–200. doi: 10.1002/mds.25263
40. Novellino F, Arabia G, Bagnato A, Cascini GL, Salsone M, Nicoletti G, et al. Combined use of DAT-SPECT and cardiac MIBG scintigraphy in mixed tremors. Mov Disord. (2009) 24:2242–8. doi: 10.1002/mds.22771
41. Passamonti L, Novellino F, Cerasa A, Chiriaco C, Rocca F, Matina MS, et al. Altered cortical-cerebellar circuits during verbal working memory in essential tremor. Brain. (2011) 134:2274–86. doi: 10.1093/brain/awr164
43. Thenganatt MA, Jankovic J. The relationship between essential tremor and Parkinson's disease. Parkinsonism Relat Disord. (2016) 22(Suppl. 1):S162–5. doi: 10.1016/j.parkreldis.2015.09.032
44. Minen MT, Louis ED. Emergence of Parkinson's disease in essential tremor: a study of the clinical correlates in 53 patients. Mov Disord. (2008) 23:1602–5. doi: 10.1002/mds.22161
45. Chaudhuri KR, Buxton-Thomas M, Dhawan V, Peng R, Meilak C, Brooks DJ. Long duration asymmetrical postural tremor is likely to predict development of Parkinson's disease and not essential tremor: clinical follow up study of 13 cases. J Neurol Neurosurg Psychiatry. (2005) 76:115–7. doi: 10.1136/jnnp.2004.046292
46. Lenka A, Bhalsing KS, Jhunjhunwala KR, Chandran V, Pal PK. Are patients with limb and head tremor a clinically distinct subtype of essential tremor? Can J Neurol Sci. (2015) 42:181–6. doi: 10.1017/cjn.2015.23
47. Louis ED, Rios E, Applegate LM, Hernandez NC, Andrews HF. Jaw tremor: prevalence and clinical correlates in three essential tremor case samples. Mov Disord. (2006) 21:1872–8. doi: 10.1002/mds.21069
48. di Biase L, Brittain JS, Shah SA, Pedrosa DJ, Cagnan H, Mathy A, et al. Tremor stability index: a new tool for differential diagnosis in tremor syndromes. Brain. (2017) 140:1977–86. doi: 10.1093/brain/awx104
49. Shah M, Muhammed N, Findley LJ, Hawkes CH. Olfactory tests in the diagnosis of essential tremor. Parkinsonism Relat Disord. (2008) 14:563–8. doi: 10.1016/j.parkreldis.2007.12.006
50. Louis ED, Rios E, Pellegrino KM, Jiang W, Factor-Litvak P, Zheng W. Higher blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations correlate with lower olfactory scores in essential tremor. Neurotoxicology. (2008) 29:460–5. doi: 10.1016/j.neuro.2008.02.013
51. Mahlknecht P, Pechlaner R, Boesveldt S, Volc D, Pinter B, Reiter E, et al. Optimizing odor identification testing as quick and accurate diagnostic tool for Parkinson's disease. Mov Disord. (2016) 31:1408–13. doi: 10.1002/mds.26637
52. Gasparini M, Bonifati V, Fabrizio E, Fabbrini G, Brusa L, Lenzi GL, et al. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol. (2001) 248:399–402. doi: 10.1007/s004150170181
53. Louis ED, Rohl B, Collins K, Cosentino S. Poorer cognitive performance in patients with essential tremor-Parkinson's disease vs. patients with Parkinson's disease. Front Neurol. (2015) 6:106. doi: 10.3389/fneur.2015.00106
54. Fyfe I. Movement disorders: comparison of cognitive impairment in Parkinson disease and essential tremor. Nat Rev Neurol. (2017) 13:260. doi: 10.1038/nrneurol.2017.40
55. Sanchez-Ferro A, Benito-Leon J, Louis ED, Contador I, Hernandez-Gallego J, Puertas-Martin V, et al. Cognition in non-demented Parkinson's disease vs essential tremor: a population-based study. Acta Neurol Scand. (2017) 136:393–400. doi: 10.1111/ane.12752
56. Liang KJ, Carlson ES. Resistance, vulnerability and resilience: a review of the cognitive cerebellum in aging and neurodegenerative diseases. Neurobiol Learn Mem. (2019) 170:106981. doi: 10.1016/j.nlm.2019.01.004
57. Novellino F, Vasta R, Sacca V, Nistico R, Morelli M, Arabia G, et al. Hippocampal impairment in patients with Essential Tremor. Parkinsonism Relat Disord. (2020) 72:56–61. doi: 10.1016/j.parkreldis.2020.02.006
58. Rohl B, Collins K, Morgan S, Cosentino S, Huey ED, Louis ED. Daytime sleepiness and nighttime sleep quality across the full spectrum of cognitive presentations in essential tremor. J Neurol Sci. (2016) 371:24–31. doi: 10.1016/j.jns.2016.10.006
59. Nodel MR, Tsenteradze SL, Poluektov MG. REM-sleep behavior disorder and sleepwalking in a patient with Parkinson's disease and essential tremor. Zh Nevrol Psikhiatr Im S S Korsakova. (2017) 117:88–94. doi: 10.17116/jnevro201711712188-94
60. Driver-Dunckley ED, Adler CH. Movement disorders and sleep. Neurol Clin. (2012) 30:1345–58. doi: 10.1016/j.ncl.2012.08.019
61. Elble RJ. Diagnostic criteria for essential tremor and differential diagnosis. Neurology. (2000) 54:S2–6.
62. Whaley NR, Putzke JD, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: phenotypic expression in a clinical cohort. Parkinsonism Relat Disord. (2007) 13:333–9. doi: 10.1016/j.parkreldis.2006.12.004
63. Louis ED. Twelve clinical pearls to help distinguish essential tremor from other tremors. Expert Rev Neurother. (2014) 14:1057–65. doi: 10.1586/14737175.2014.936389
64. Erro R, Stamelou M, Saifee TA, Ganos C, Antelmi E, Balint B, et al. Facial tremor in dystonia. Parkinsonism Relat Disord. (2014) 20:924–5. doi: 10.1016/j.parkreldis.2014.04.029
66. Peng J, Wang L, Li N, Li J, Duan L, Peng R. Distinct non-motor features of essential tremor with head tremor patients. Acta Neurol Scand. (2020) 142:74–82. doi: 10.1111/ane.13242
67. Lee MS, Kim YD, Im JH, Kim HJ, Rinne JO, Bhatia KP. 123I-IPT brain SPECT study in essential tremor and Parkinson's disease. Neurology. (1999) 52:1422–6. doi: 10.1212/WNL.52.7.1422
68. Cohen O, Pullman S, Jurewicz E, Watner D, Louis ED. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. (2003) 60:405–10. doi: 10.1001/archneur.60.3.405
69. Ryu DW, Lee SH, Oh YS, An JY, Park JW, Song IU, et al. Clinical characteristics of Parkinson's disease developed from essential tremor. J Parkinsons Dis. (2017) 7:369–76. doi: 10.3233/JPD-160992
70. Brittain JS, Cagnan H, Mehta AR, Saifee TA, Edwards MJ, Brown P. Distinguishing the central drive to tremor in Parkinson's disease and essential tremor. J Neurosci. (2015) 35:795–806. doi: 10.1523/JNEUROSCI.3768–14.2015
71. Ghika A, Kyrozis A, Potagas C, Louis ED. Motor and non-motor features: differences between patients with isolated essential tremor and patients with both essential tremor and Parkinson's disease. Tremor Other Hyperkinet Mov. (2015) 5:335. doi: 10.5334/tohm.248
72. Teive HA. Essential tremor: phenotypes. Parkinsonism Relat Disord. (2012) 18(Suppl. 1):S140–2. doi: 10.1016/S1353-8020(11)70044-X
73. Chandran V, Pal PK. Essential tremor: beyond the motor features. Parkinsonism Relat Disord. (2012) 18:407–13. doi: 10.1016/j.parkreldis.2011.12.003
74. Park IS, Oh YS, Lee KS, Yang DW, Song IU, Park JW. Subtype of mild cognitive impairment in elderly patients with essential tremor. Alzheimer Dis Assoc Disord. (2015) 29:141–5. doi: 10.1097/WAD.0000000000000054
75. Kim JS, Song IU, Shim YS, Park JW, Yoo JY, Kim YI, et al. Cognitive impairment in essential tremor without dementia. J Clin Neurol. (2009) 5:81–4. doi: 10.3988/jcn.2009.5.2.81
76. Bermejo-Pareja F. Essential tremor–a neurodegenerative disorder associated with cognitive defects? Nat Rev Neurol. (2011) 7:273–82. doi: 10.1038/nrneurol.2011.44
77. Smeltere L, Kuznecovs V, Erts R. Depression and social phobia in essential tremor and Parkinson's disease. Brain Behav. (2017) 7:e00781. doi: 10.1002/brb3.781
78. Mameli F, Tomasini E, Scelzo E, Fumagalli M, Ferrucci R, Bertolasi L, et al. Lies tell the truth about cognitive dysfunction in essential tremor: an experimental deception study with the guilty knowledge task. J Neurol Neurosurg Psychiatry. (2013) 84:1008–13. doi: 10.1136/jnnp-2012-304674
79. Liberini P, Parola S, Spano PF, Antonini L. Olfaction in Parkinson's disease: methods of assessment and clinical relevance. J Neurol. (2000) 247:88–96. doi: 10.1007/PL00007803
80. Benito-Leon J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain Study G. Population-based case-control study of cognitive function in essential tremor. Neurology. (2006) 66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec
81. Bermejo-Pareja F, Louis ED, Benito-Leon J, Neurological Disorders in Central Spain Study G. Risk of incident dementia in essential tremor: a population-based study. Mov Disord. (2007) 22:1573–80. doi: 10.1002/mds.21553
82. Benito-Leon J, Sanz-Morales E, Melero H, Louis ED, Romero JP, Rocon E, et al. Graph theory analysis of resting-state functional magnetic resonance imaging in essential tremor. Hum Brain Mapp. (2019) 40:4686–702. doi: 10.1002/hbm.24730
83. Prasad S, Shah A, Bhalsing KS, Kumar KJ, Saini J, Ingalhalikar M, et al. Abnormal hippocampal subfields are associated with cognitive impairment in essential tremor. J Neural Transm. (2019) 126:597–606. doi: 10.1007/s00702-019-01992-3
84. Xu R, Hu X, Jiang X, Zhang Y, Wang J, Zeng X. Longitudinal volume changes of hippocampal subfields and cognitive decline in Parkinson's disease. Quant Imaging Med Surg. (2020) 10:220–32. doi: 10.21037/qims.2019.10.17
85. Thomas GEC, Leyland LA, Schrag AE, Lees AJ, Acosta-Cabronero J, Weil RS. Brain iron deposition is linked with cognitive severity in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2020) 91:418–425. doi: 10.1136/jnnp-2019–322042
86. Kim JS, Oh YS, Kim YI, Koo JS, Yang DW, Lee KS. Transcranial sonography (TCS) in Parkinson's disease (PD) and essential tremor (ET) in relation with putative premotor symptoms of PD. Arch Gerontol Geriatr. (2012) 54:e436–9. doi: 10.1016/j.archger.2012.01.001
87. Lacerte A, Chouinard S, Jodoin N, Bernard G, Rouleau GA, Panisset M. Increased prevalence of non-motor symptoms in essential tremor. Tremor Other Hyperkinet Mov. (2014) 4:162. doi: 10.5334/tohm.176
88. Barbosa R, Mendonca M, Ladeira F, Miguel R, Bugalho P. Probable REM-sleep behavior disorder and dysautonomic symptoms in essential tremor. Tremor Other Hyperkinet Mov. (2017) 7:522. doi: 10.5334/tohm.350
89. Salsone M, Arabia G, Manfredini L, Quattrone A, Chiriaco C, Vescio B, et al. REM-sleep behavior disorder in patients with essential tremor: what is its clinical significance? Front Neurol. (2019) 10:315. doi: 10.3389/fneur.2019.00315
90. Lee SM, Kim M, Lee HM, Kwon KY, Koh SB. Nonmotor symptoms in essential tremor: Comparison with Parkinson's disease and normal control. J Neurol Sci. (2015) 349:168–73. doi: 10.1016/j.jns.2015.01.012
91. Habipoglu Y, Alpua M, Bilkay C, Turkel Y, Dag E. Autonomic dysfunction in patients with essential tremor. Neurol Sci. (2017) 38:265–9. doi: 10.1007/s10072-016–2754-z
92. Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. (2015) 14:625–39. doi: 10.1016/S1474-4422(15)00007-1
93. Salsone M, Nistico R, Vescio B, Novellino F, Morelli M, Lupo A, et al. Heart rate variability in patients with essential tremor: a cross sectional study. Parkinsonism Relat Disord. (2016) 33:134–7. doi: 10.1016/j.parkreldis.2016.09.027
94. Yoon JH, Kim MS, Lee SM, Kim HJ, Hong JM. Heart rate variability to differentiate essential tremor from early-stage tremor-dominant Parkinson's disease. J Neurol Sci. (2016) 368:55–8. doi: 10.1016/j.jns.2016.06.059
95. Singleton A, Gwinn-Hardy K, Sharabi Y, Li ST, Holmes C, Dendi R, et al. Association between cardiac denervation and parkinsonism caused by alpha-synuclein gene triplication. Brain. (2004) 127:768–72. doi: 10.1093/brain/awh081
96. Basiago A, Binder DK. Effects of deep brain stimulation on autonomic function. Brain Sci. (2016) 6:33. doi: 10.3390/brainsci6030033
97. Guinand A, Noble S, Frei A, Renard J, Tramer MR, Burri H. Extra-cardiac stimulators: what do cardiologists need to know? Europace. (2016) 18:1299–307. doi: 10.1093/europace/euv453
98. Nakamura T, Hirayama M, Hara T, Mizutani Y, Suzuki J, Watanabe H, et al. Role of cardiac sympathetic nerves in preventing orthostatic hypotension in Parkinson's disease. Parkinsonism Relat Disord. (2014) 20:409–14. doi: 10.1016/j.parkreldis.2014.01.003
99. Kim JS, Oh YS, Park HE, Lee SH, Park JW, Song IU, et al. Cardiovascular autonomic dysfunctions in elderly patients with essential tremor: comparison with healthy controls. Neurol Sci. (2016) 37:711–6. doi: 10.1007/s10072-015-2465-x
100. Rocca WA, Bower JH, Ahlskog JE, Elbaz A, Grossardt BR, McDonnell SK, et al. Increased risk of essential tremor in first-degree relatives of patients with Parkinson's disease. Mov Disord. (2007) 22:1607–14. doi: 10.1002/mds.21584
101. Jankovic J, Beach J, Schwartz K, Contant C. Tremor and longevity in relatives of patients with Parkinson's disease, essential tremor, and control subjects. Neurology. (1995) 45:645–8. doi: 10.1212/WNL.45.4.645
102. Zorzon M, Capus L, Pellegrino A, Cazzato G, Zivadinov R. Familial and environmental risk factors in Parkinson's disease: a case-control study in north-east Italy. Acta Neurol Scand. (2002) 105:77–82. doi: 10.1034/j.1600-0404.2002.1o040.x
103. Muller SH, Girard SL, Hopfner F, Merner ND, Bourassa CV, Lorenz D, et al. Genome-wide association study in essential tremor identifies three new loci. Brain. (2016) 139:3163–9. doi: 10.1093/brain/aww242
104. Ross JP, Mohtashami S, Leveille E, Johnson AM, Xiong L, Dion PA, et al. Association study of essential tremor genetic loci in Parkinson's disease. Neurobiol Aging. (2018) 66:178 e113–5. doi: 10.1016/j.neurobiolaging.2018.01.001
105. Paus S, Gadow F, Kaut O, Knapp M, Klein C, Klockgether T, et al. Tremor in Parkinson's disease is not associated with the DRD3 Ser9Gly polymorphism. Parkinsonism Relat Disord. (2010) 16:381–3. doi: 10.1016/j.parkreldis.2010.03.006
106. Keeling BH, Vilarino-Guell C, Ross OA, Wszolek ZK, Uitti RJ, Farrer MJ. DRD3 Ser9Gly and HS1BP3 Ala265Gly are not associated with Parkinson disease. Neurosci Lett. (2009) 461:74–5. doi: 10.1016/j.neulet.2009.05.084
107. Chen H, Song Z, Yuan L, Xiong W, Yang Z, Gong L, et al. Genetic analysis of PITX3 variants in patients with essential tremor. Acta Neurol Scand. (2017) 135:373–6. doi: 10.1111/ane.12608
108. Yuan L, Song Z, Deng X, Zheng W, Yang Z, Yang Y, et al. Genetic analysis of FGF20 variants in Chinese Han patients with essential tremor. Neurosci Lett. (2016) 620:159–62. doi: 10.1016/j.neulet.2016.03.055
109. Gao C, Chen YM, Sun Q, He YC, Huang P, Wang T, et al. Mutation analysis of CHCHD2 gene in Chinese Han familial essential tremor patients and familial Parkinson's disease patients. Neurobiol Aging. (2017) 49:218 e219–11. doi: 10.1016/j.neurobiolaging.2016.10.001
110. Wu H, Lu X, Cen Z, Xie F, Zheng X, Chen Y, et al. Genetic analysis of the CHCHD2 gene in Chinese patients with familial essential tremor. Neurosci Lett. (2016) 634:104–6. doi: 10.1016/j.neulet.2016.10.005
111. Chen H, Yuan L, Song Z, Deng X, Yang Z, Gong L, et al. Genetic analysis of LRRK1 and LRRK2 variants in essential tremor patients. Genet Test Mol Biomarkers. (2018) 22:398–402. doi: 10.1089/gtmb.2017.0277
112. Zhang Y, Zhao Y, Zhou X, Yi M, Li K, Zhou X, et al. Relationship between GWAS-linked three new loci in essential tremor and risk of Parkinson's disease in Chinese population. Parkinsonism Relat Disord. (2017) 43:124–6. doi: 10.1016/j.parkreldis.2017.08.014
113. Vilarino-Guell C, Wider C, Ross OA, Jasinska-Myga B, Kachergus J, Cobb SA, et al. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics. (2010) 11:401–8. doi: 10.1007/s10048-010-0241-x
114. Annesi F, De Marco EV, Rocca FE, Nicoletti A, Pugliese P, Nicoletti G, et al. Association study between the LINGO1 gene and Parkinson's disease in the Italian population. Parkinsonism Relat Disord. (2011) 17:638–41. doi: 10.1016/j.parkreldis.2011.06.020
115. Wu YW, Rong TY, Li HH, Xiao Q, Fei QZ, Tan EK, et al. Analysis of Lingo1 variant in sporadic and familial essential tremor among Asians. Acta Neurol Scand. (2011) 124:264–8. doi: 10.1111/j.1600–0404.2010.01466.x
116. Guo Y, Jankovic J, Song Z, Yang H, Zheng W, Le W, et al. LINGO1 rs9652490 variant in Parkinson disease patients. Neurosci Lett. (2011) 487:174–6. doi: 10.1016/j.neulet.2010.10.016
117. Lorenzo-Betancor O, Samaranch L, Garcia-Martin E, Cervantes S, Agundez JA, Jimenez-Jimenez FJ, et al. LINGO1 gene analysis in Parkinson's disease phenotypes. Mov Disord. (2011) 26:722–7. doi: 10.1002/mds.23452
118. Deng H, Gu S, Jankovic J. LINGO1 variants in essential tremor and Parkinson's disease. Acta Neurol Scand. (2012) 125:1–7. doi: 10.1111/j.1600-0404.2011.01516.x
119. Unal Gulsuner H, Gulsuner S, Mercan FN, Onat OE, Walsh T, Shahin H, et al. Mitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson disease. Proc Natl Acad Sci USA. (2014) 111:18285–90. doi: 10.1073/pnas.1419581111
120. Tian JY, Guo JF, Wang L, Sun QY, Yao LY, Luo LZ, et al. Mutation analysis of LRRK2, SCNA, UCHL1, HtrA2 and GIGYF2 genes in Chinese patients with autosomal dorminant Parkinson's disease. Neurosci Lett. (2012) 516:207–11. doi: 10.1016/j.neulet.2012.03.086
121. Fu K, Wang Y, Guo D, Wang G, Ren H. Familial Parkinson's disease-associated L166P mutant DJ-1 is cleaved by mitochondrial serine protease Omi/HtrA2. Neurosci Bull. (2017) 33:685–94. doi: 10.1007/s12264-017–0196-0
122. Chao YX, Ng EY, Foo JN, Liu J, Zhao Y, Tan EK. Mitochondrial serine protease HTRA2 gene mutation in Asians with coexistent essential tremor and Parkinson disease. Neurogenetics. (2015) 16:241–2. doi: 10.1007/s10048-015-0443-3
123. He YC, Huang P, Li QQ, Sun Q, Li DH, Wang T, et al. Mutation analysis of HTRA2 gene in Chinese familial essential tremor and familial Parkinson's disease. Parkinsons Dis. (2017) 2017:3217474. doi: 10.1155/2017/3217474
124. Nuytemans K, Bademci G, Kohli MM, Beecham GW, Wang L, Young JI, et al. C9ORF72 intermediate repeat copies are a significant risk factor for Parkinson disease. Ann Hum Genet. (2013) 77:351–63. doi: 10.1111/ahg.12033
125. Lorenzo-Betancor O, Garcia-Martin E, Cervantes S, Agundez JA, Jimenez-Jimenez FJ, Alonso-Navarro H, et al. Lack of association of LINGO1 rs9652490 and rs11856808 SNPs with familial essential tremor. Eur J Neurol. (2011) 18:1085–9. doi: 10.1111/j.1468-1331.2010.03251.x
126. Louis ED, Pullman SL. Comparison of clinical vs. electrophysiological methods of diagnosing of essential tremor. Mov Disord. (2001) 16:668–73. doi: 10.1002/mds.1144
127. Fusco C, Valls-Sole J, Iturriaga C, Colomer J, Fernandez-Alvarez E. Electrophysiological approach to the study of essential tremor in children and adolescents. Dev Med Child Neurol. (2003) 45:624–7. doi: 10.1017/S0012162203001130
128. Nistico R, Pirritano D, Novellino F, Salsone M, Morelli M, Valentino P, et al. Blink reflex recovery cycle in patients with essential tremor associated with resting tremor. Neurology. (2012) 79:1490–5. doi: 10.1212/WNL.0b013e31826d5f83
129. Yavuz D, Gunduz A, Ertan S, Apaydin H, Sifoglu A, Kiziltan G, et al. Specific brainstem and cortico-spinal reflex abnormalities in coexisting essential tremor and Parkinson's disease (ET-PD). Neurophysiol Clin. (2015) 45:143–9. doi: 10.1016/j.neucli.2015.01.001
130. Arabia G, Lupo A, Manfredini LI, Vescio B, Nistico R, Barbagallo G, et al. Clinical, electrophysiological, and imaging study in essential tremor-Parkinson's disease syndrome. Parkinsonism Relat Disord. (2018) 56:20–6. doi: 10.1016/j.parkreldis.2018.06.005
131. Crowell AL, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Shimamoto S, Lim DA, et al. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain. (2012) 135:615–30. doi: 10.1093/brain/awr332
132. Stamford JA, Schmidt PN, Friedl KE. What engineering technology could do for quality of life in Parkinson's disease: a review of current needs and opportunities. IEEE J Biomed Health Inform. (2015) 19:1862–72. doi: 10.1109/JBHI.2015.2464354
133. Daneault JF. Could wearable and mobile technology improve the management of essential tremor? Front Neurol. (2018) 9:257. doi: 10.3389/fneur.2018.00257
134. Nam GE, Kim SM, Han K, Kim NH, Chung HS, Kim JW, et al. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med. (2018) 15:e1002640. doi: 10.1371/journal.pmed.1002640
135. Benito-Leon J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain Study G. Population-based case-control study of cigarette smoking and essential tremor. Mov Disord. (2008) 23:246–52. doi: 10.1002/mds.21810
Keywords: essential tremor, Parkinson's disease, ET-PD, prodromal markers, substantia nigra hyperechogenicity
Citation: Wang X-X, Feng Y, Li X, Zhu X-Y, Truong D, Ondo WG and Wu Y-C (2020) Prodromal Markers of Parkinson's Disease in Patients With Essential Tremor. Front. Neurol. 11:874. doi: 10.3389/fneur.2020.00874
Received: 02 February 2020; Accepted: 09 July 2020;
Published: 25 August 2020.
Edited by:
Adolfo Ramirez-Zamora, University of Florida Health, United StatesReviewed by:
Fabiana Novellino, National Research Council (CNR), ItalyCopyright © 2020 Wang, Feng, Li, Zhu, Truong, Ondo and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Cheng Wu, eXVuY2h3QG1lZG1haWwuY29tLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.