
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol. , 31 July 2020
Sec. Stroke
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00786
 Anna Kufner1,2,3
Anna Kufner1,2,3 Martin Ebinger1,4
Martin Ebinger1,4 Gert Jan Luijckx5
Gert Jan Luijckx5 Matthias Endres1,2,3,6,7,8
Matthias Endres1,2,3,6,7,8 Bob Siegerink1,2* on behalf of Dutch String-of-Pearls Stroke Study Group
Bob Siegerink1,2* on behalf of Dutch String-of-Pearls Stroke Study GroupBackground: The smoking-thrombolysis paradox refers to a better outcome in smokers who suffer from acute ischemic stroke (AIS) following treatment with thrombolysis. However, studies on this subject have yielded contradictory results and an interaction analysis of exposure to smoking and thrombolysis in a large, multicenter database is lacking.
Methods: Consecutive AIS patients admitted within 12 h of symptom onset between 2009 and 2014 from the prospective, multicenter stroke registry (Dutch String-of-Pearls Stroke Study) were included for this analysis. We performed a generalized linear model for functional outcome 3 months post-stroke depending on risk of the exposure variables (smoking yes/no, thrombolysis yes/no). The following confounders were adjusted for: age, smoking, hypertension, atrial fibrillation, diabetes mellitus, stroke severity, and stroke etiology.
Results: Out of 468 patients, 30.6% (N = 143) were smokers and median baseline NIHSS was 3 (interquartile range 1–6). Smoking alone had a crude and adjusted relative risk (RR) of 0.99 (95% CI 0.89–1.10) and 0.96 (95% CI 0.86–1.01) for good outcome (modified Rankin Score ≤ 2), respectively. A combination of exposure variables (smoking and thrombolysis) did not change the results significantly [crude RR 0.87 (95% CI 0.74–1.03], adjusted RR 1.1 (95%CI 0.90–1.30)]. Smoking alone had an adjusted RR of 1.2 (95% CI 0.6–2.7) for recanalization following thrombolysis (N = 88).
Conclusions: In patients with mild to moderate AIS admitted within 12 h of symptom onset, smoking did not modify treatment effect of thrombolysis.
The so-called smoking-paradox of an improved outcome following thrombolysis was first described in smokers with myocardial infarction (1, 2). This phenomenon has resurfaced as a topic of interest in the scientific community as a handful of recent studies have reported similar observations in acute ischemic stroke patients treated with recombinant tissue plasminogen activator (r-tPA) as well as endovascular therapies (3–6).
The mechanism underlying the pathophysiology of the smoking-thrombolysis paradox remains unclear. Some attribute the observed phenomenon to a systematic lack of adjustment of confounding factors i.e., lower clinical risk profiles of smokers due to younger age and fewer comorbidities (7, 8). Others argue that there is substantial evidence supporting an alteration of clot dynamics (9, 10) caused by smoke exposure leading to enhanced tPA efficacy in patients with this risk factor.
As of yet, the studies on this subject have yielded contradictory results (3–8, 11, 12). Most likely, there is a cumulative effect of younger age, lower clinical risk profiles, and more aggressive treatment effect that account for the smoking-thrombolysis paradox. However, a large comprehensive interaction analysis of exposure to smoking and treatment with thrombolysis is still lacking. Therefore, we set out to determine the measures of interaction of smoking status (current smokers vs. non-smokers) and treatment (thrombolysis vs. no thrombolysis) and their attributable risk in terms of functional recovery 3 month post-stroke and recanalization rates in a large, national multicenter stroke registry.
All data comes from the Dutch String-of-Pearls Stroke Study—a prospective, multicenter cohort study in which patients with recent stroke presenting within 1-month after symptom onset are eligible for enrollment following informed consent (13). Local ethics committees of all participating hospitals approved the study and all patients provided written informed consent. Research with this data was performed in accordance with the Medical Research Involving Human Subjects Act and codes developed by the Dutch Federation of Medical Scientific Societies.
Consecutive acute ischemic stroke patients between October 2009 and October 2014 were included for this analysis. Major inclusion criteria for the current study include the following: ischemic stroke determined via CT or MRI, known time of symptom onset, admission within 12 h of symptom onset, and documented smoking status at the time of the event. Patients were excluded from the analysis if they received endovascular therapies or if endovascular treatment status was unknown or undocumented. A flow diagram shows the patient selection criteria for this sub-study (Figure 1).
We performed regression analyses for binary endpoints by generalized linear model (glm) using a modified log-Poisson regression model with a robust error variance to reduce risk of overestimation (14). These models were used to estimate relative risks for good functional outcome (mRS ≤ 2), excellent functional outcome (mRS dichotomized at ≤ 1), and mortality 3 months post-stroke depending on risk of the exposure variables (smoking yes/no, thrombolysis yes/no). The two aforementioned exposure variables create four patient groups with combined exposures of smoking and treatment (i.e., –/–, −/+, +/–, and +/+). Crude and adjusted relative risk (RRs) were calculated for the four patient groups based on the exposures. Models were adjusted for age, hypertension, diabetes mellitus, atrial fibrillation, stroke severity (NIHSS), and stroke etiology.
Four hundred sixty-eight patients were included in the final analysis; 30.6% were smokers. For a detailed description of basic demographics and baseline parameters of the entire study group, as well as sub-groups based on smoking-status, refer to Table 1. Compared to non-smokers, smokers had lower rates of hypertension, and atrial fibrillation. Stroke etiology differed significantly between groups; smokers presented with higher rates of large-artery atherosclerotic strokes compared to non-smokers who presented with higher rates of cardioembolic stroke. Infarct localization and stroke severity did not differ among groups (Table 1).
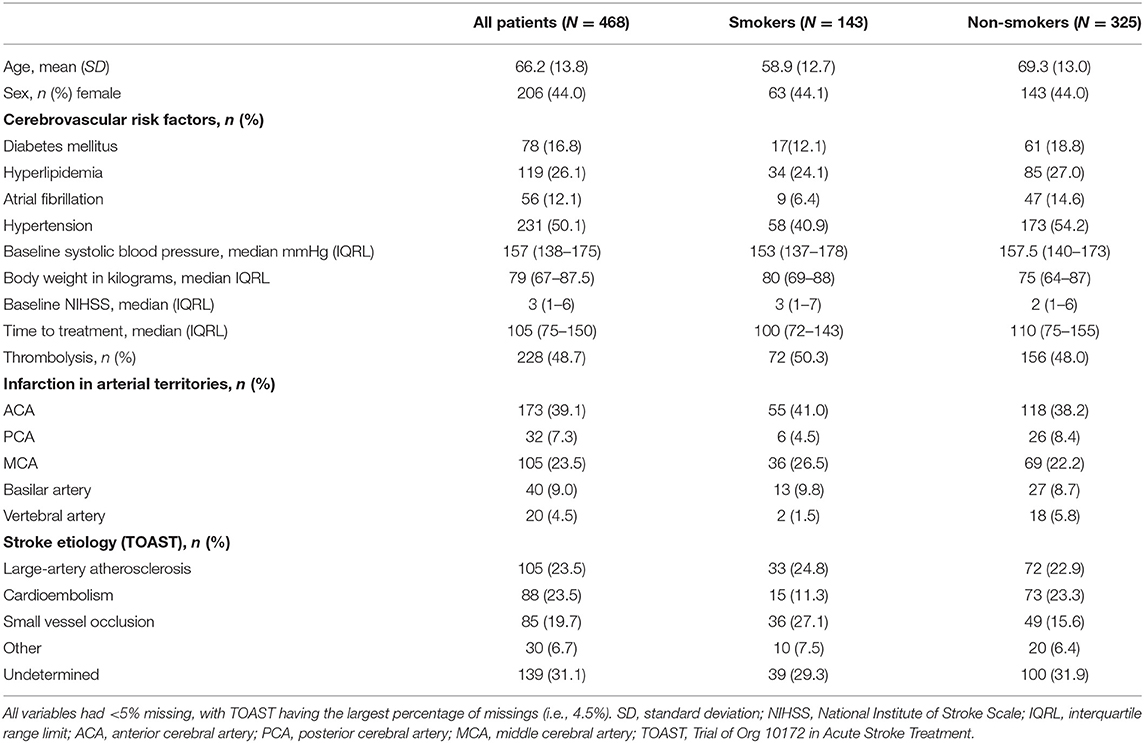
Table 1. Basic demographics and baseline clinical parameters of the entire cohort, and sub-groups based on smoking status.
In the entire cohort (N = 468), comparing smokers and non-smokers revealed no significant differences in terms of good outcome of mRS ≤ 2 (70.6 vs. 72.9%), excellent outcome of mRS ≤ 1 (46.2 vs. 55.4%), or mortality (4.2 vs. 4.9%). In sub-group analysis including only patients treated with thrombolysis (N = 228; Table 2), results were similar; we observed no differences in univariate analysis between smokers and non-smokers for any of the primary outcome endpoints. Overall rate of hemorrhagic transformation following thrombolysis was 3.1%; rates did not differ significantly between groups.
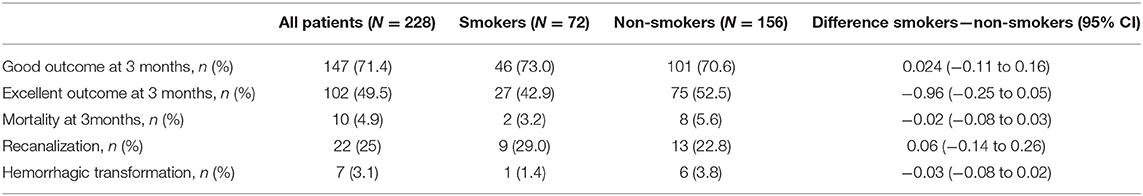
Table 2. Outcome parameters assessed 3 months post-stroke (good outcome [mRS ≤ 2], excellent outcome [mRS ≤ 1], and mortality), as well as rates of recanalization and hemorrhagic transformation reported in all patients who received intravenous thrombolysis (N = 228) and based on smoking status.
Smoking status had a crude and adjusted RR of 0.99 (95% CI 0.89–1.11) and 0.96 (95% CI 0.86–1.01) for a good outcome, respectively. In a sub-group analysis including only patients with documented recanalization status (N = 88), smoking had a crude RR of 1.3 (95% CI 0.6–2.7), and an adjusted RR (including age, time-to-treatment, stroke etiology) of 1.2 (95% CI 0.6–2.7) for recanalization. Results from the combined exposure analyses (i.e., Smoking + thrombolysis) for a good outcome (mRS ≤ 2), excellent outcome (mRS ≤ 1), and mortality 3 months following the index event are presented in Table 3. In short, no clear interaction could be observed.
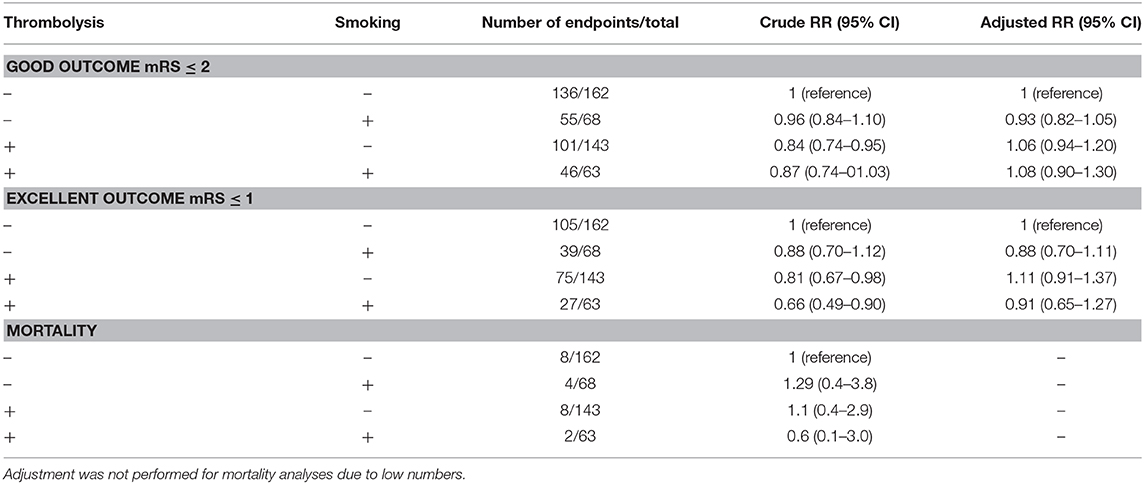
Table 3. Relative risks (RR) for a good outcome (modified Rankin Score [mRS] ≤ 2), excellent outcome (mRS ≤ 1), and mortality 3 months post-stroke, presenting crude, and adjusted values (adjusted for age, presence of hypertension, diabetes mellitus, atrial fibrillation, National Institute of Stroke Scale on admission, stroke etiology categories).
We observed no biological interaction of smoking and intravenous thrombolysis in terms of functional recovery 3 months post-stroke in this multicenter cohort of mild to moderate ischemic stroke patients admitted within 12 h of symptom onset.
In all patients, and in sub-group analysis including only those treated with intravenous thrombolysis, there was no difference in functional outcome between smokers and non-smokers in univariate analysis (Table 2). Interestingly, both crude and adjusted analyses suggest that smoking alone has no effect on long-term functional recovery post-stroke despite lower clinical risk profiles of these patients, although the precision of these analyses is limited. In our combined exposure analyses, smoking alone had a crude and adjusted RR of 0.88 for an excellent outcome. The seemingly adverse effect of tPA-administration alone on functional recovery disappeared when confounding factors such as stroke severity were considered (crude RR of 0.84 and adjusted RR of 1.06 compared to crude RR of 0.81 and adjusted RR 1.11 for good and excellent outcome, respectively), which can be explained by confounding by indication. A combination of tPA-administration and smoking did not lead to an improved functional outcome (Table 3). These results are an indicator that smoking status negatively influences outcome, regardless of treatment with thrombolysis in this cohort.
Similar to previous reports (3–5, 8), ~31% of patients were smokers. As expected, patients with this risk factor had fewer cerebrovascular comorbidities [hypertension (3, 4, 11) and AF (11)], and more frequently suffered from large-artery atherosclerotic strokes (4, 11) (Table 1). Smoking led to increased recanalization rates in patients treated with thrombolysis in this cohort [adjusted RR 1.2 (95% CI 0.6–2.7)]. However, due to small numbers an additive interaction analysis for smoking and thrombolysis for recanalization could not be performed and results should be interpreted with caution.
This cohort included predominately minor strokes (median baseline NIHSS: 3 IQR 1–6). Assuming smoking may modify treatment effect of thrombolysis by increasing recanalization rates of large vessel occlusions, any biological interaction of smoking, and tPA may have been missed in this analysis. Previous studies that found an enhanced treatment efficacy in smokers included patients with more severe strokes and higher rates of proven vessel occlusion (3–6). Therefore, a similar analysis in an independent cohort of patients with large vessel occlusion is warranted to further shed light on a possible increased treatment efficacy of thrombolysis in smokers.
An important limitation of this study is the exclusion of a large number of patients with undocumented time to treatment. This is most likely due to the fact that this stroke registry consists of subacute stroke patients with onset within 30 days, PSI where time to treatment has less clinical relevancy. This certainly led to a selection of patients which limits extrapolation of our results into other populations, notably to those without known stroke onset. An additional limitation of this analysis is missing information relevant to our research question (i.e., vessel occlusion size and methods applied for assessment of recanalization) as well as missing data on pre-treatment glucose levels and rates of symptomatic intracerebral hemorrhage which may have influenced functional outcome in these patients (15). These limitations are inherent to our study design, as data was retrospectively analyzed from a stroke registry not primarily designed to address our research aim.
In conclusion, smoking did not modify treatment effect of thrombolysis in acute ischemic stroke patients in this cohort. However, a large, multicenter cohort analysis including patients with proven vessel occlusion and more severe strokes is warranted to further investigate a potential biological interaction between smoking and intravenous and intra-arterial thrombolysis.
The data analyzed in this study was subject to the following licenses/restrictions: data will be made available upon reasonable request. Requests to access these datasets should be directed to Ym9iLnNpZWdlcmlua0BjaGFyaXRlLmRl.
The studies involving human participants were reviewed and approved by the String-of-Pearls Institute has provided a regulatory framework in which ethical and legal rules and guidelines have been described (http://www.string-of-pearls.org/). The patients/participants provided their written informed consent to participate in this study.
AK, MEb, MEn, and BS conceived and designed the project. AK and BS performed the data analysis. AK wrote the first draft of the manuscript. All authors interpreted the data, reviewed and edited the manuscript, and approved the final version of the manuscript.
AK was a participant in the BIH-Charité Junior Clinical Scientist Program funded by the Charité–Universitätsmedizin Berlin and the Berlin Institute of Health. MEn received funding from Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy—EXC-2049-−390688087 and from Bundesministerium für Bildung und Forschung (BMBF; German Ministry for Education and Research) for the Center for Stroke Research Berlin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Grines CL, Topol EJ, O'Neill WW, George BS, Kereiakes D, Phillips HR, et al. Effect of cigarette smoking on outcome after thrombolytic therapy for myocardial infarction. Circulation. (1995) 91:298–303. doi: 10.1161/01.CIR.91.2.298
2. Barbash GI, White HD, Modan M, Diaz R, Hampton JR, Heikkila J, et al. Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction. Experience gleaned from the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Circulation. (1993) 87:53–8. doi: 10.1161/01.CIR.87.1.53
3. Kufner A, Nolte CH, Galinovic I, Brunecker P, Kufner GM, Endres M, et al. Smoking-thrombolysis paradox: recanalization and reperfusion rates after intravenous tissue plasminogen activator in smokers with ischemic stroke. Stroke. (2013) 44:407–13. doi: 10.1161/STROKEAHA.112.662148
4. Kvistad CE, Oeygarden H, Logallo N, Thomassen L, Waje-Andreassen U, Naess H. Is smoking associated with favourable outcome in tPA-treated stroke patients? Acta Neurol Scand. (2014) 130:299–304. doi: 10.1111/ane.12225
5. Meseguer E, Labreuche J, Gonzalez-Valcarcel J, Sirimarco G, Guidoux C, Cabrejo L, et al. The smoking paradox: impact of smoking on recanalization in the setting of intra-arterial thrombolysis. Cerebrovasc Dis Extra. (2014) 4:84–91. doi: 10.1159/000357218
6. von Martial R, Gralla J, Mordasini P, El Koussy M, Bellwald S, Volbers B, et al. Impact of smoking on stroke outcome after endovascular treatment. PLoS ONE. (2018) 13:e0194652. doi: 10.1371/journal.pone.0194652
7. Aune E, Roislien J, Mathisen M, Thelle DS, Otterstad JE. The “smoker's paradox” in patients with acute coronary syndrome: a systematic review. BMC Med. (2011) 9:97. doi: 10.1186/1741-7015-9-97
8. Ali SF, Smith EE, Reeves MJ, Zhao X, Xian Y, Hernandez AF, et al. Smoking paradox in patients hospitalized with coronary artery disease or acute ischemic stroke. Circ Cardiovasc Qual Outcomes. (2015) 8(6 Suppl. 3):S73–80. doi: 10.1161/CIRCOUTCOMES.114.001244
9. Barua RS, Sy F, Srikanth S, Huang G, Javed U, Buhari C, et al. Effects of cigarette smoke exposure on clot dynamics and fibrin structure: an ex vivo investigation. Arter Thromb Vasc Biol. (2010) 30:75–9. doi: 10.1161/ATVBAHA.109.195024
10. Meade TW, Imeson J, Stirling Y. Effects of changes in smoking and other characteristics on clotting factors and the risk of ischaemic heart disease. Lancet. (1987) 330:986–8. doi: 10.1016/S0140-6736(87)92556-6
11. Lee J-H, Lee JY, Ahn SH, Jang MU, Oh MS, Kim C-H, et al. Smoking is not a good prognostic factor following first-ever acute ischemic stroke. J Stroke. (2015) 17:177–91. doi: 10.5853/jos.2015.17.2.177
12. Schlemm L, Kufner A, Boutitie F, Nave AH, Gerloff C, Thomalla G, et al. Current smoking does not modify the treatment effect of intravenous thrombolysis in acute ischemic stroke patients—a post-hoc analysis of the WAKE-UP trial. Front Neurol. (2019) 10:1239. doi: 10.3389/fneur.2019.01239
13. Nederkoorn PJ, van Dijk EJ, Koudstaal PJ, Luijckx GJ, van Oostenbrugge RJ, Visser MC, et al. The Dutch String-of-Pearls Stroke Study: protocol of a large prospective multicenter genetic cohort study. Int J Stroke. (2015) 10:120–2. doi: 10.1111/ijs.12359
14. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. (2004) 159:702–6. doi: 10.1093/aje/kwh090
15. Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: Safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. (2012) 43:1524–31. doi: 10.1161/STROKEAHA.111.644815
Keywords: stroke, smoking, thrombolysis (tPA), ischemic stroke, cerebrovascular risk factors
Citation: Kufner A, Ebinger M, Luijckx GJ, Endres M and Siegerink B (2020) Smoking Does Not Alter Treatment Effect of Intravenous Thrombolysis in Mild to Moderate Acute Ischemic Stroke—A Dutch String-of-Pearls Institute (PSI) Stroke Study. Front. Neurol. 11:786. doi: 10.3389/fneur.2020.00786
Received: 15 April 2020; Accepted: 25 June 2020;
Published: 31 July 2020.
Edited by:
Aristeidis H. Katsanos, McMaster University, CanadaReviewed by:
Craig S. Anderson, University of New South Wales, AustraliaCopyright © 2020 Kufner, Ebinger, Luijckx, Endres and Siegerink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bob Siegerink, Ym9iLnNpZWdlcmlua0BjaGFyaXRlLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.