- 1Rare and Complex Epilepsy Unit, Department of Neuroscience, Bambino Gesù Children's Hospital, IRCCS, Member of European Reference Network EpiCARE, Rome, Italy
- 2Neurosurgery Unit, Department of Neuroscience, Bambino Gesù Children's Hospital, IRCCS, Rome, Italy
- 3Department of Neuroscience, Bambino Gesù Children's Hospital, IRCCS, Member of European Reference Network EpiCARE, Rome, Italy
Refractory and super-refractory status epilepticus (RSE, SRSE) are severe conditions that can have long-term neurological consequences with high morbidity and mortality rates. The usefulness of vagus nerve-stimulation (VNS) implantation during RSE has been documented by anecdotal cases and in systematic reviews; however, the use of VNS in RSE has not been widely adopted. We successfully implanted VNS in two patients with genetic epilepsy admitted to hospital for SRSE; detailed descriptions of the clinical findings and VNS parameters are provided. Our patients were implanted 25 and 58 days after status epilepticus (SE) onset, and a stable remission of SE was observed from the seventh and tenth day after VNS implantation, respectively, without change in anti-seizure medication. We used a fast ramp-up of stimulation without evident side effects. Our results support the consideration of VNS implantation as a safe and effective adjunctive treatment for SRSE.
Introduction
Refractory and super-refractory status epilepticus (RSE, SRSE) are severe conditions that can have long-term consequences, including alteration of neuronal networks, neuronal injury, and high morbidity and mortality rates (1, 2). Conventional anti-seizure medications (ASMs) are ineffective, so off-label treatments are often administered (1).
Although vagus nerve stimulation (VNS) has been documented to reduce the occurrence and recurrence of status epilepticus (SE), debate continues on its value during RSE/SRSE. Single case reports and small case series of VNS implantation in RSE were included in a systematic review, where VNS implantation was associated with cessation in 76% of generalized and 26% of focal RSE (3). Also, VNS implantation has been reported to be associated with cessation of RSE/SRSE in 74% (28/38) of cases collected through a more recent systematic review of the literature (4). Also recently, effective VNS implantation during SRSE was reported in one patient with Lafora disease (5).
This report describes the electro-clinical findings and long-term outcome in two patients with genetic epilepsy who were successfully implanted with VNS during SRSE (an overview of clinical findings and VNS parameters is provided in Table 1). The publication of patient data from this investigational approach was approved by the local Ethics Committee.
Case #1
This was a female patient who was aged 16 years at the time of our observations. She had an unremarkable medical history until she experienced her first focal tonic seizure at the age of 8. Brain magnetic resonance (MR) at onset was normal, and electroencephalogram (EEG) showed bilateral temporal and occipital epileptiform abnormalities. She started valproate and clobazam. She was seizure-free for 4 years. At the age of 12, asymmetrical tonic-clonic seizures recurred despite conventional ASMs (lacosamide, primidone, and clonazepam). Seizures occurred weekly from ages 12 to 16, with some intermittent reduction in seizure frequency when a new ASM was added.
She came to our Department of Neuroscience with frequent myoclonic jerks involving the left side of the body, turning to super refractory myoclonic status. She was admitted to the Intensive Care Unit (ICU), and barbiturate coma was induced. First- and second-line treatments for SE and anesthetics were all ineffective. EEG recordings were consistently characterized by continuous epileptiform discharges over the right fronto-central area with bilateral diffusion (Figure 1A). Brain MR showed a hyperintensity on fluid-attenuated inversion recovery (FLAIR) sequences involving the right frontal region (Figure 1C). Metabolic workup was negative. Epilepsy genetic panel revealed a pathogenic heterozygous genetic variant in ADCK3.
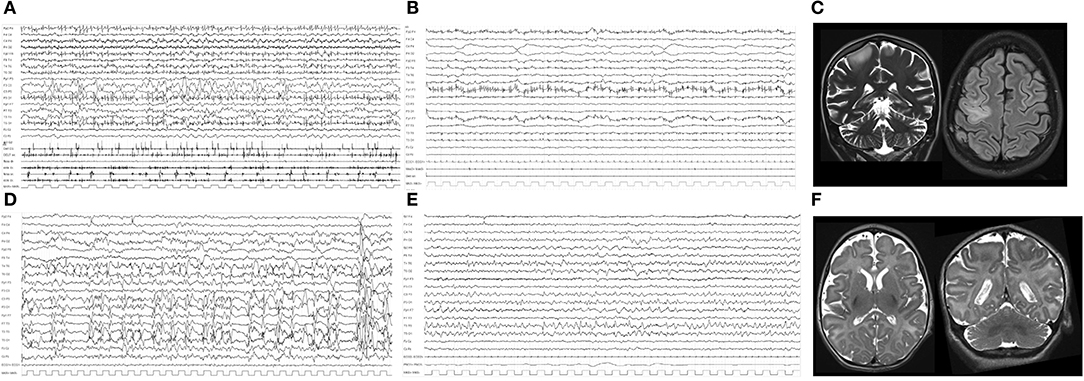
Figure 1. (A) Polygraphic video-EEG recording of Case #1, showing repetitive and continuous myoclonic jerks with an EEG counterpart characterized by diffuse repetitive spikes and multiple artifacts related to continuous myoclonic jerks of the face muscles. (B) Polygraphic recording after VNS implantation and after remission of myoclonic SE, showing low-voltage activity and rare myoclonic jerks on the EMG channels. (C) Coronal T2 and axial FLAIR MRI of Case #1 during RSE, showing mild cerebral atrophy, cerebellar atrophy, and hyperintensity over the right frontal region. (D) EEG during SE showing continuous epileptiform abnormalities over the left hemisphere and multiple repetitive spikes of Case #2. (E) EEG from 15 days after VNS implantation: epileptiform abnormalities were significantly reduced; some spikes over left temporal regions are evident. (F) Axial and coronal T2 brain MR showing cerebral atrophy and a simplification of cortical gyri, more evident over bilateral frontal and central regions of Case #2.
Twenty-five days after admission, VNS (Demipulse 103 Cyberonics) was implanted without changes in ASM regimen (see Table 1). SRSE remitted after 7 days and did not recur (Figure 1B). Midazolam and phenobarbital doses were progressively reduced until they were stopped at 10 and 15 days, respectively, after VNS implantation. After VNS implantation, this patient presented daily myoclonic seizures that did not interfere with vital parameters. The patient died due to the underlying disease (dilated cardiomyopathy with a progressive reduction of ejection fraction until cardiac death) 5 months after VNS implantation at 17 years old.
Case #2
This was the first daughter of healthy unrelated parents. Pregnancy and delivery were uneventful. Focal to bilateral tonic-clonic seizures associated with apnea and cyanosis lasting 30 s started at the age of 3.5 months. Interictal EEG showed slow and multifocal epileptiform abnormalities. Ictal EEG revealed diffuse low-voltage fast activity. Seizures were resistant to multiple ASMs (pyridoxin, phenobarbital, carbamazepine, phenytoin, clonazepam, and topiramate). From onset, she continued to present focal to bilateral tonic-clonic seizures every 4–8 days. She had profound intellectual disability, acquired microcephaly, and hypotonia with tetraplegia. Brain MR showed bilateral frontal simplification of cortical gyri at the age of 2 months, together with progressive diffuse cerebral atrophy at the age of 8 months (Figure 1F). Extended genetic epilepsy panels detected a de novo pathogenetic variant in BRAT1 gene.
When aged 6 months, a refractory convulsive SE occurred (see Table 1). During this status, ASM and anesthetics were administered without success. On the 58th day after SRSE onset, VNS (Demipulse 103 Cyberonics) was implanted. SE remitted after 10 days and did not recur (Figures 1D,E). After VNS implantation, this patient presented weekly focal seizures that did not interfere with vital parameters. The patient died due to pediatric acute respiratory distress syndrome (P-ARDS) at the age of 3.
Discussion
The usefulness of VNS implantation during RSE has been documented by anecdotal cases and in systematic reviews (3–6). Evidence supporting its efficacy is low (level IV), however, and the risk from reporting bias is high (4). We successfully implanted VNS into two patients with documented genetic epilepsy and SRSE. Previous evidence shows the median duration of RSE/SRSE pre- and post-VNS implantation to be 18 (range: 3–1,680) and 8 days (range 3–84), respectively (4). Consistent with previous reports, our patients were implanted 25 (#1) and 58 (#2) days after SE onset and, without ASM changes, a stable remission of SE was observed after the seventh (#1) and tenth (#2) day from VNS implantation. Significant positive effect has been reported in patients with both focal and generalized SE: cessation of SE was reported after a range of 3–14 days in generalized SE and between 15 and 60 days in focal SE (3). Death might occur during RSE, so aggressive treatment is often suggested. In one systematic review, four deaths (11%) during vagal stimulation for RSE/SRSE were reported, and all cases were considered unrelated to VNS implantation (4). In another systematic review of VNS, two out of the 28 patients reported died specifically during SE (3). Finally, in a patient recently described with Lafora disease, death occurred 9 months after implantation due to a tracheostomy complication (5). Our patients died due to the underlying disease (dilated cardiomyopathy with a progressive reduction of ejection fraction until cardiac death) in Case #1 and due to pediatric respiratory complications (P-ARDS) in Case #2.
The anti-seizure effect with VNS is, at least in part, time-dependent. However, the precise mechanism of the VNS anti-seizure effect, either acute or chronic, has not been fully elucidated. The Noda epileptic rat (NER) is a genetic epilepsy model that exhibits spontaneous generalized tonic-clonic seizure (GTC), approximately once every 30 h, and frequent dialeptic seizure (DS). Acute VNS in the NER significantly reduced the frequency of GTC and the duration of DS; chronic VNS decreased the frequency and duration of DS, but not GTC frequency, in a time-dependent manner. The brainstem and midline thalamus of NER were activated after acute and chronic VNS (7). VNS was also effective in the epileptic baboon, a naturally occurring animal model for genetic generalized epilepsy (GGE) (8).
VNS effectiveness in genetic epilepsies has previously been documented in various case series. In CHD2 genetic epilepsy, VNS was associated with a good outcome, though secondary hemophagocytic lymph histiocytosis (SHLH) after a VNS wound infection was also reported (9). VNS was reported to be effective in patients carrying CDKL5 genetic variants. One year after VNS insertion, 9/12 patients reported improved health-related quality of life (HRQoL) and 9/11 patients had improvements in mood, school achievement, and concentration (10). Similarly, HRQoL was improved following VNS by other authors in a CDKL5 patient, and those authors concluded that adjunctive VNS therapy may widen the scope of treatment choices available to these patients, though they also acknowledged that the efficacy of VNS therapy for patients with intractable epilepsy associated with a genetic anomaly remains not fully established (11). Similar results have since been reported in genetic epilepsy with febrile seizures plus (GEFS+) and Dravet syndrome patients. In both of these, VNS seemed to exert a beneficial role in both seizure reduction and cognitive functions (12, 13).
Acute implantation of VNS during SRSE seemed to be effective in stopping SE in previous reports. Four cases have recently been reported with patients presenting different conditions: hemimegaloencephaly, non-ketotic hyperglycinemia, migrating focal seizures of infancy, and microdeletion of 1q43q44 causing microcephalia, corpus callosum agenesia, and epilepsy. All four conditions were genetically determined, and the authors reported cessation of SE in three out of the four cases (14).
Genetic testing has become more widespread, and more genetic mutations in patients with epilepsy are being identified; hence, previous reports of patients with RSE treated with VNS may have represented carriers of an unidentified, unconfirmed genetic mutation. We recognize that our patients are not unique in having genetic epilepsy, SRSE, and VNS; however, our experience does add to the data on confirmed genetic epilepsy.
The side-effect profile associated with VNS includes pharyngeal dysesthesias, change in voice, dysphagia, brady-arrhythmias, and asystole (15). We used a fast ramp-up of stimulations without evident side effects, even when patients were sedated, and this may have biased evaluation of adverse events.
For both of our patients, SE was super refractory—all medications, including anesthetics, were ineffective, as was the ketogenic diet tried in one case. We promptly considered alternative treatments; however, traditional surgery was not possible considering the genetic etiology of the epilepsies. VNS implantation for both patients was proposed by neurologists during epilepsy surgery meetings. The decision was driven by the general condition of the patients, which worsened more rapidly in Case #1 (implantation after 25 days from SE onset) than in Case #2 (implantation after 58 days from SE onset). Corpus callosotomy was another possible palliative surgical approach, which seems to be effective in refractory generalized epilepsy (16), though VNS was considered as the prior option within the treatment paradigm. (17, 18).
Multiple hypotheses exist regarding VNS in the treatment of epileptic seizures, including neurotransmitter modification, increase in the activity of the nucleus tractus solitarius, and a direct effect on neuronal desynchronization (19–21). The only hypothesis so far for explaining the efficacy during SE relies on single-photon emission computed tomography (SPECT) studies that show a normalization of cortical GABAA receptor density (21). To date, despite encouraging evidence, the use of VNS has not been widely adopted for RSE.
We cannot know, either in the presented cases or in those previously reported, whether SE might have ceased independently of VNS implantation. However, we can speculate that both of our patients, who were experiencing SRSE without any reduction in the burden of epilepsy after several medications, were successfully treated with acute VNS. It is also worthwhile to underline that the majority of studies available are heterogeneous and retrospective, as well as that the use of VNS in RSE remains experimental.
Our results further suggest that VNS implantation should be considered as a safe and effective adjunctive treatment of RSE/SRSE when standard ASMs have failed and surgery is not possible. We acknowledge limitations in the present report, as well as in previously published case series reporting the effectiveness of VNS in RSE and SRSE, particularly the open-label approach and the limited number of cases published. Prospective studies should be designed to better understand the effectiveness of VNS in patients with SE.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
Written informed consent was obtained from the patient for the publication of this case report.
Author Contributions
NS and AF drafted the manuscript. NP and MT followed up the patients and reviewed the final draft of the manuscript. CC and GC drafted the figure, performed the EEGs and reviewed the final draft of the manuscript. AD and CM operated the patients, followed-up the VNS parameters and contributed to drafting the manuscript and approved the final draft of the manuscript. LP and FV coordinated the group and reviewed the final draft of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus - report of the ilae task force on classification of status epilepticus. Epilepsia. (2015) 56:1515–23. doi: 10.1111/epi.13121
2. Kantanen A-M, Reinikainen M, Parviainen I, Ruokonen E, Ala-Peijari M, Bäcklund T, et al. Incidence and mortality of super-refractory status epilepticus in adults. Epilepsy Behav. (2015) 49:131–4. doi: 10.1016/j.yebeh.2015.04.065
3. Zeiler FA, Zeiler KJ, Teitelbaum J, Gillman LM, West M. VNS for refractory status epilepticus. Epilepsy Res. (2015) 112:100–13. doi: 10.1016/j.eplepsyres.2015.02.014
4. Dibué-Adjei M, Brigo F, Yamamoto T, Vonck K, Trinka E. Vagus nerve stimulation in refractory and super-refractory status epilepticus – A systematic review. Brain Stimul. (2019) 12:1101–10. doi: 10.1016/j.brs.2019.05.011
5. Mostacci B, Bisulli F, Muccioli L, Minardi I, Bandini M, Licchetta L, et al. Super refractory status epilepticus in Lafora disease interrupted by vagus nerve stimulation: A case report. Brain Stimul. (2019) 12:1605–7. doi: 10.1016/j.brs.2019.08.008
6. San-juan D, Dávila-Rodríguez DO, Jiménez CR, González MS, Carranza SM, Hernández Mendoza JR, et al. Neuromodulation techniques for status epilepticus: A review. Brain Stimul. (2019) 12:835–44. doi: 10.1016/j.brs.2019.04.005
7. Szabó CÁ, Salinas FS, Papanastassiou AM, Begnaud J, Ravan M, Eggleston KS, et al. High-frequency burst vagal nerve simulation therapy in a natural primate model of genetic generalized epilepsy. Epilepsy Res. (2017) 138:46–52. doi: 10.1016/j.eplepsyres.2017.10.010
8. Parisi C, Candela-Cantó S, Serrano M, Catala A, Aparicio J, Hinojosa J. Life-threatening secondary hemophagocytic lymphohistiocytosis following vagal nerve stimulator infection in a child with CHD2 myoclonic encephalopathy: a case report. Child's Nerv Syst. (2020) doi: 10.1007/s00381-020-04558-x. [Epub ahead of print].
9. Katagiri M, Iida K, Ishihara K, Nair D, Harada K, Kagawa K, et al. Anti-seizure effect and neuronal activity change in the genetic-epileptic model rat with acute and chronic vagus nerve stimulation. Epilepsy Res. (2019) 155:106159. doi: 10.1016/j.eplepsyres.2019.106159
10. Amin S, Majumdar A, Mallick AA, Patel J, Scatchard R, Partridge CA, et al. Caregiver's perception of epilepsy treatment, quality of life and comorbidities in an international cohort of CDKL5 patients. Hippokratia. (2017) 21:130–5.
11. Baba S, Sugawara Y, Moriyama K, Inaji M, Maehara T, Yamamoto T, et al. Amelioration of intractable epilepsy by adjunct vagus nerve stimulation therapy in a girl with a CDKL5 mutation. Brain Dev. (2017) 39:341–4. doi: 10.1016/j.braindev.2016.10.007
12. Hanaya R, Niantiarno FH, Kashida Y, Hosoyama H, Maruyama S, Sugata S, et al. Vagus nerve stimulation for generalized epilepsy with febrile seizures plus (GEFS +) accompanying seizures with impaired consciousness. Epilepsy Behav Case Reports. (2017) 7:16–9. doi: 10.1016/j.ebcr.2016.11.001
13. Dlouhy BJ, Miller B, Jeong A, Bertrand ME, Limbrick DD, Smyth MD. Palliative epilepsy surgery in Dravet syndrome—case series and review of the literature. Child's Nerv Syst. (2016) 32:1703–8. doi: 10.1007/s00381-016-3201-4
14. Grioni D, Landi A, Fiori L, Pietro SF. Does emergent implantation of a vagal nerve stimulator stop refractory status epilepticus in children? Seizure. (2018) 61:94–97. doi: 10.1016/j.seizure.2018.08.008
15. González HFJ, Yengo-Kahn A, Englot DJ. Vagus nerve stimulation for the treatment of epilepsy. Neurosurg Clin N Am. (2019) 30:219–30. doi: 10.1016/j.nec.2018.12.005
16. Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: a systematic review. Epilepsia. (2016) 57:1053–1068. doi: 10.1111/epi.13408
17. Rosenfeld WE, Roberts DW. Tonic and atonic seizures: What's next - VNS or callosotomy? Epilepsia. (2009) 50:25–30. doi: 10.1111/j.1528-1167.2009.02232.x
18. Rolston JD, Englot DJ, Wang DD, Garcia PA, Chang EF. Corpus callosotomy versus vagus nerve stimulation for atonic seizures and drop attacks: a systematic review. Epilepsy Behav. (2015) 51:13–7. doi: 10.1016/j.yebeh.2015.06.001
19. Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. (2004) 118:79–88. doi: 10.1037/0735-7044.118.1.79
20. Ravan M. Investigating the correlation between short-term effectiveness of VNS therapy in reducing the severity of seizures and long-term responsiveness. Epilepsy Res. (2017) 133:46–53. doi: 10.1016/j.eplepsyres.2017.04.008
Keywords: epilepsy, status epilepticus, vagal nerve stimulation (VNS), genetic epilepsies, treatment
Citation: Specchio N, Ferretti A, Pietrafusa N, Trivisano M, Calabrese C, Carfì Pavia G, De Benedictis A, Marras CE, de Palma L and Vigevano F (2020) Refractory Status Epilepticus in Genetic Epilepsy—Is Vagus Nerve Stimulation an Option? Front. Neurol. 11:443. doi: 10.3389/fneur.2020.00443
Received: 16 January 2020; Accepted: 27 April 2020;
Published: 12 June 2020.
Edited by:
Jose F. Tellez-Zenteno, University of Saskatchewan, CanadaReviewed by:
Emilio Russo, University of Catanzaro, ItalyFilippo Sean Giorgi, University of Pisa, Italy
John D. Rolston, The University of Utah, United States
Copyright © 2020 Specchio, Ferretti, Pietrafusa, Trivisano, Calabrese, Carfì Pavia, De Benedictis, Marras, de Palma and Vigevano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Specchio, bmljb2xhLnNwZWNjaGlvQG9wYmcubmV0
 Nicola Specchio
Nicola Specchio Alessandro Ferretti
Alessandro Ferretti Nicola Pietrafusa1
Nicola Pietrafusa1 Marina Trivisano
Marina Trivisano Federico Vigevano
Federico Vigevano