- 1Department of Neurology, Yale School of Medicine, Yale University, New Haven, CT, United States
- 2Department of Chronic Disease Epidemiology, Yale School of Public Health, Yale University, New Haven, CT, United States
- 3Center for Neuroepidemiology and Clinical Neurological Research, Yale School of Medicine, Yale University, New Haven, CT, United States
Introduction: The diagnosis of essential tremor (ET) remains a clinical one, and diagnostic errors are common. We aimed to (1) determine precisely how frequently ET diagnoses are misapplied (i.e., what percentage of patients who have been assigned an “ET” diagnosis actually have another movement disorder), (2) determine which other movement disorders are most often misclassified as “ET,” and (3) examine the clinical features that were most associated with diagnostic errors.
Methods: One hundred four consecutive patients were included who met the following criteria: (1) initial outpatient evaluation by one of the authors (EDL) between January 2015 and December 2019 and (2) pre-evaluation diagnosis of ET. Data on an extensive number of clinical features were extracted from the electronic medical record.
Results: Forty-seven (45.2%) patients received a post-evaluation diagnosis of ET, 29 (27.9%) of dystonia, and 28 (26.9%) of other diagnoses including Parkinson's disease (PD) [6 (5.8%)]. Factors associated with an alternative post-evaluation diagnosis other than ET were pre-evaluation diagnosis made by a non-neurologist, shorter tremor duration, irregular tremor, abnormal limb postures, among others.
Discussion: Diagnosing ET remains a challenge, with the diagnosis being over-applied and being used as a “waste basket.” More than one-half of the patients who were referred to our clinic with an intake diagnosis of ET were given an alternative post-evaluation diagnosis. While PD was reported to be the most frequently missed diagnosis in a past study, dystonia was most commonly missed in our study. Several clinical features can help to differentiate ET from other tremor disorders.
Introduction
Essential tremor (ET) is one of the most common movement disorders, with a prevalence of 4.6% among persons aged 65 and older (1). The diagnosis remains a clinical one, and diagnostic errors are quite common, with frequent misclassification with respect to other movement disorders, especially Parkinson's disease (PD), dystonia, and enhanced physiologic tremor (2–4). In particular, earlier and mild stages of these diseases remain a diagnostic challenge, as clinical phenomenology is often subtler in these settings. While a variety of ancillary tests are used to support the diagnosis of PD, including 123I-FP-CIT (DaTSCAN) single-photon emission computerized tomography (5) and substantia nigra neuromelanin magnetic resonance imaging (6), there are still no specific biomarkers or imaging procedures to diagnose ET.
An earlier literature suggested that as many as 37–50% of “ET” cases have other neurological disorders, with PD being among the most common (2, 3). With the advent of greater societal awareness of PD and the widespread availability of DaTSCAN, however, it is quite probable that this is less often the case. Nevertheless, this topic has not been revisited in the past 15–20 years (2, 3), with no studies during the intervening period. Hence, there is a need for updated and renewed knowledge, with a fresh look at this topic. Our a priori hypothesis was that diagnostic errors with respect to ET would remain common, likely more than 25%, but that misdiagnosis with PD would be less of an issue than reported in earlier studies.
The aims of this study were to (1) determine how frequently “ET” diagnoses are misapplied (i.e., what percentage of patients who have been assigned an “ET” diagnosis actually have another movement disorder), (2) determine which other movement disorders were most often misclassified as “ET,” and (3) examine the clinical features that were most associated with diagnostic errors.
These results have a number of potential clinical implications. One of these is that misdiagnosis is the beginning of errors in treatment, and it is important for clinicians to be aware of and try to prevent such errors. Another clinical implication is that many of these illnesses in question (i.e., ET, dystonia, PD) have a familial component, so that arriving at the correct diagnosis has broader implications for offspring and other family members of patients, who themselves deserve to know, correctly, for which disease they may be at increased risk. Finally, issues related to the diagnosis of ET and its association with other movement disorders are increasingly at the forefront of the scientific dialogue on ET. We believe these data will contribute additional information, which will be of value in addressing this challenge.
Methods
Study Setting and Data Abstraction
The study setting was a clinical practice at an academic medical center, which served the local community and also served as a referral center. Through our university computerized clinical interface, we searched and identified all consecutive patients who fulfilled each of the following inclusion criteria: (1) initial outpatient evaluation by one of the authors (EDL), (2) pre-evaluation diagnosis of ET, (3) initial evaluation by EDL occurred between January 2015 (initiation of the author's practice at this medical center) and December 2019 (present). One of the authors (CJA) retrospectively extracted data from the patients' electronic medical records. The data included the post-evaluation diagnosis [assigned by EDL using diagnostic criteria from the International Parkinson and Movement Disorder Society (MDS) (MDS) consensus statement (7)].
We carefully pre-specified variables of interest and extracted these from the initial evaluation by EDL. See Table 1 for a detailed explanation of each clinical variable of interest.

Table 1. Explanation and hypothesis for the historical and clinical characteristics of the included patients.
Data Analysis
There were 104 patients. As a first analytic step, we pooled all 47 patients who had been assigned a post-evaluation diagnosis of “not-ET” by EDL into one group in order to evaluate which details of the history and the clinical examination were most associated with the non-ET diagnosis. Given that dystonia was the most commonly missed alternative diagnosis in our study population, we then conducted a separate analysis to evaluate which details of the history and the clinical examination were most associated with the dystonia diagnosis. Statistical analyses were performed using SPSS Version 24.0 (Tables 1–4). Student t-tests were used to analyze continuous variables and χ2 as well as Fisher exact tests were used for comparison of categorical variables. Non-parametric tests (e.g., Mann–Whitney test) were used when appropriate. Binary logistic regression analyses were performed in order to assess the relationship between each clinical characteristic and diagnosis (Tables 3, 4). Each binary logistic regression analysis yielded an odds ratio (OR) and 95% confidence interval (CI) (Tables 3, 4). We also repeated each of these logistic regression models, including gender as a covariate, but this did not appreciably change the results; hence, those data are not reported.
Results
Demographic Characteristics
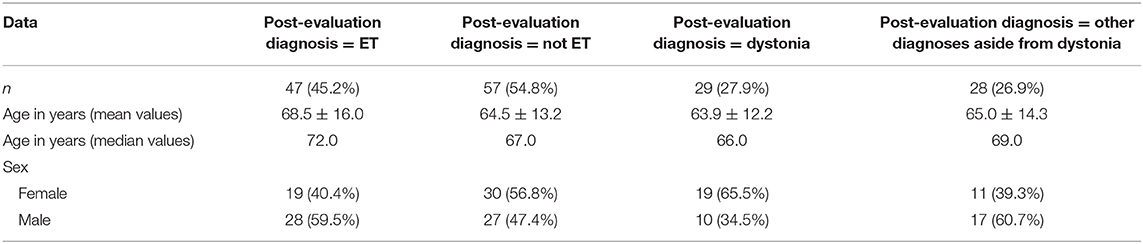
We identified 104 consecutive patients. Their demographic characteristics are shown (Table 2).
Diagnostic Validity
Of the 104 patients, 47 (45.2%) were diagnosed with ET, 29 (27.9%) with dystonia, and 28 (26.9%) with other diagnoses including PD (6 or 5.8%), ET-PD (4 or 3.9%), ET and dystonia (3 or 2.9%), functional tremor (2 or 1.9%), primary writing tremor (2 or 1.9%), parkinsonian syndrome (1 or 1.0%), multiple system atrophy (1 or 1.0%), myoclonus dystonia (1 or 1.0%), enhanced physiologic tremor (1 or 1.0%), and uncertain diagnoses (7 or 6.7%).
Clinical Characteristics of 47 Patients With a Post-evaluation Diagnosis of Essential Tremor vs. 57 Patients With All Other Post-evaluation Diagnoses
Patients who received a post-evaluation diagnosis of “not ET” differed in a number of respects from patients who received a post-evaluation diagnoses of ET (Tables 2, 3). Patients who received a post-evaluation diagnosis of ET were statistically similar regarding their sex distribution (p = 0.21) and were of similar age compared to their counterparts who received a post-evaluation diagnosis of “not ET” (p = 0.064). Patients who received a post-evaluation diagnosis of “not ET” were 3.8 times more likely to have previously been diagnosed by a non-neurologist than were patients who received a post-evaluation diagnosis of ET (Table 3). Patients with a post-evaluation diagnosis of “not ET” were 4.6 times more likely to have had tremor of short duration (i.e., <5 years) than were patients who received a post-evaluation diagnoses of ET (Table 3).
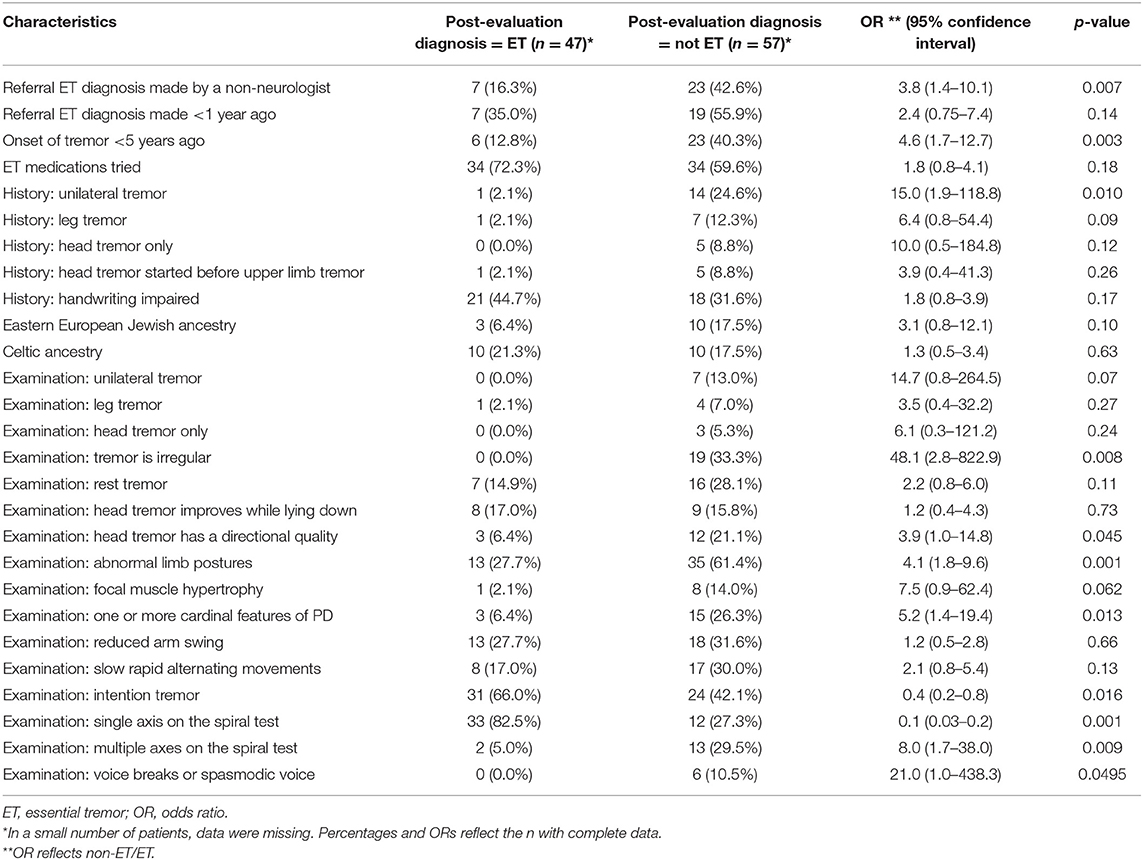
Table 3. Additional clinical characteristics of 47 patients with a post-evaluation diagnosis of ET versus 57 patients with all other post-evaluation diagnoses.
By history, patients who were assigned a post-evaluation of “not ET” were more likely to have unilateral tremor (OR = 15.0). The presence of a leg tremor (OR = 6.4) or isolated head tremor (OR = 10.0) also seemed to be more common among “non-ET” patients; but despite the large ORs, in each of these comparisons, p-values were not statistically significant (p = 0.09 and p = 0.12) (Table 3).
On examination, a unilateral tremor seemed to be more common in patients who received a post-evaluation diagnosis of “not ET”; however, the difference did not reach a statistically significant level (OR = 14.7, p = 0.07) (Table 3). If the observed head tremor was of an irregular rhythmicity, it was far more likely not be classified as ET after evaluation in our clinic (OR = 48.1) (Table 3). The presence of typical dystonic features such as abnormal limb postures (OR = 4.1) was associated with post-evaluation diagnoses of “not ET” (Table 3). PD was the second most common alternative diagnosis [six cases (10.5%)], and it was 5.2 times more likely that the post-evaluation diagnosis was not ET if at least one of the cardinal features of PD was present on examination (Table 3). An intention tremor on examination was less likely to be found in patients who received a post-evaluation diagnosis of “not ET” (OR = 0.4), as was a single axis on the spiral test (OR = 0.1), whereas multiple axes on the spiral test were significantly associated with a post-evaluation diagnosis of “not ET” (OR = 8.0) (Table 3).
Clinical Characteristics of 47 Patients With a Post-evaluation Diagnosis of Essential Tremor vs. 29 Patients With a Post-evaluation Diagnosis of Dystonia
When compared with patients with a post-evaluation diagnosis of dystonia, a larger proportion of patients with a post-evaluation diagnosis of ET were male (p = 0.026). The two groups were also different in age (p = 0.046) (Table 2).
Patients who had been previously diagnosed by a non-neurologist seemed to be more likely to have a post-evaluation diagnosis of dystonia (OR = 2.7) even though this did not reach statistical significance (p = 0.08) (Table 4). By history, it was significantly more likely that a patient would be eventually diagnosed with dystonia if he or she reported the presence of an isolated head tremor (OR = 21.3) (Table 4). With regard to family history and ancestry, patients with a post-evaluation diagnosis of dystonia were more likely to have been of Eastern European Jewish ancestry than were those with a post-evaluation diagnosis of ET (OR = 5.6) (Table 4).
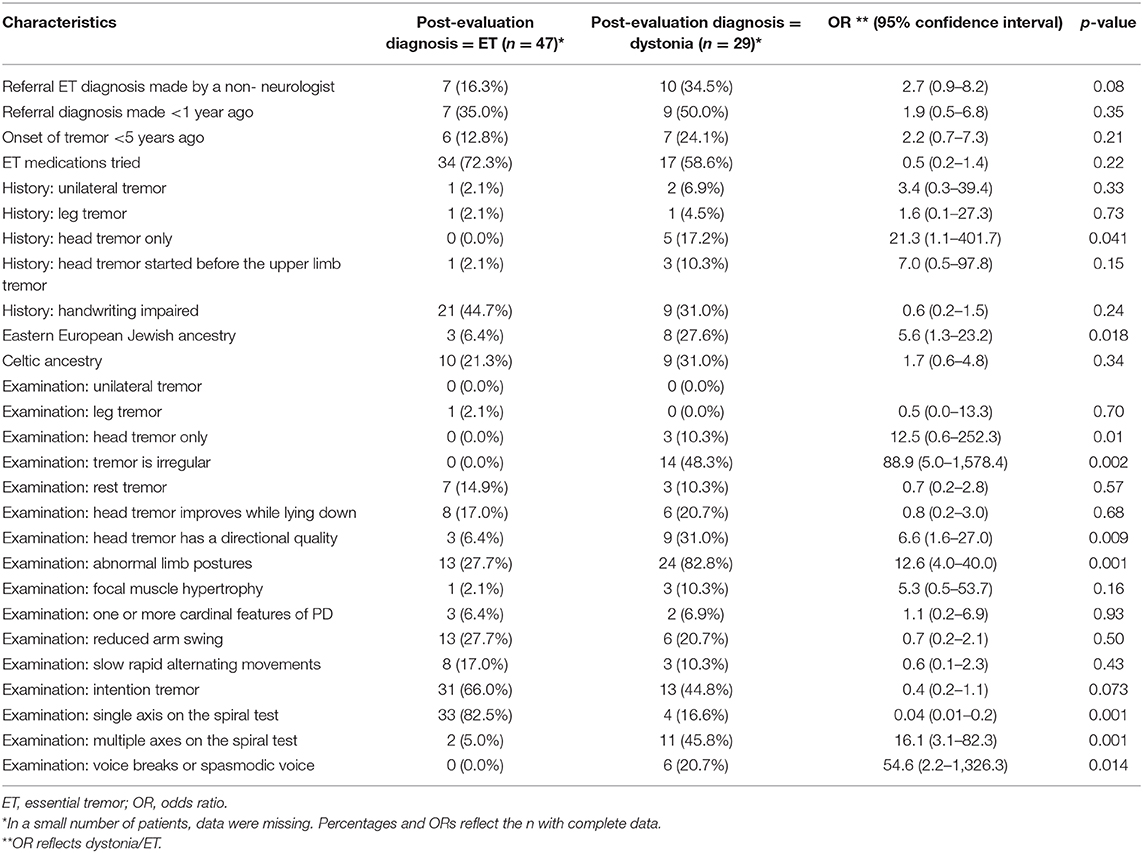
Table 4. Additional clinical characteristics of 47 patients with a post-evaluation diagnosis of ET vs. 29 patients with a post-evaluation diagnosis of dystonia.
On examination, an irregular rhythmicity was strongly associated with a post-evaluation diagnosis of dystonia (OR = 88.9, p = 0.002) as was the presence of an isolated head tremor (OR = 12.5, p = 0.01) (Table 4). When present, it did not make a difference whether the head tremor improved or resolved when lying down; however, when the head tremor had a directional quality, it was 6.6 times more likely to be diagnosed as a dystonic tremor (Table 4). Similarly, the presence of abnormal upper limb postures such as spooning of the hand or hyperflexion of the thumb was strongly associated with a post-evaluation diagnosis of dystonia (OR = 12.6, p = 0.001) (Table 4).
The majority of patients did not have a voice tremor [81/104 (77.9%)]; however, when a voice tremor was present and had a spasmodic quality or if there were voice breaks, the patient was 54.6 times more likely to have a post-evaluation diagnosis of dystonia (Table 4). Patients with a post-evaluation diagnosis of dystonia were 16.1 times more likely to have multiple axes on their spiral test (Table 4).
Discussion
Our study indicates that diagnosing ET remains a challenge. Indeed, the diagnosis is over-applied and used as somewhat of a “waste basket” for a variety of tremor disorders. More than one-half of the patients who were referred to our movement disorders clinic with an intake diagnosis of ET were given an alternative diagnosis at the end of their encounter. Dystonia was the most commonly previously missed diagnosis followed by PD. Interestingly, a previous study with a similar methodology (3) reported that PD was the most commonly missed diagnosis—in that study, 15% of the cases had pure PD and 7% had PD with ET. It is possible that nowadays PD is more accurately diagnosed as a result of greater societal awareness and new ancillary tests, as noted above. Nevertheless, in another prior study with a different methodological approach (2), it was already estimated that dystonia was the most frequent differential diagnosis of ET, such as in our current study.
Our data also illustrate that an ET diagnosis is more valid when assigned by a neurologist and when the tremor has been present for a longer period of time (more than 5 years). The latter point seems to confirm the diagnostic challenges in tremor patients when the symptom onset is recent and accompanying clinical features, which might help to ascertain the correct diagnosis, are subtle or not yet present.
Patients with ET can during the course of their illness develop signs of dystonia and parkinsonism, and some may even develop a secondary PD (22, 23). This is generally a later phenomenon. We report a discrepancy between intake and post-evaluation diagnosis of ET. It is possible that some of the intake diagnoses were assigned many years prior to seeing us, and that dystonic features and parkinsonism postdated those assessments. Indeed, in more than one-half of our patients with discrepant diagnoses, the latency from initial diagnosis to our evaluation was 20 or more years. This could explain some of the discrepancy we see between intake and post-evaluation diagnoses. However, a sizable proportion of our patients had clear dystonia on examination, primary writing tremor, functional tremor, or myoclonus, and this was their post-evaluation diagnosis; we believe that the intake diagnosis in those was simply incorrect due to a failure on the part of the clinician to recognize their clinical features.
Both dystonia and ET are purely clinical diagnoses as there is no specific ancillary test. While the spiral test can help distinguish between the two conditions (21), this test has only modest diagnostic validity. Certain clinical features of the tremor such as its amplitude or the presence of spooning and other abnormal postures (20, 24) can also help in the differentiation between ET and dystonia. One of the main reasons why dystonia is frequently misdiagnosed as ET may be that ET is such a common disease [with an estimated 4% prevalence of ET among individuals older than 60 years (1) and an estimated prevalence of dystonia of approximately 0.4% (25)]. According to our data, several historical and clinical details were more likely to be associated with dystonia and might be useful to establish the correct diagnosis. Features that indicated a likely diagnosis of dystonia were isolated head tremor, Eastern European Jewish ancestry [see (26)], lack of rhythmicity, directionality of the tremor, abnormal postures [see (24, 27)], voice breaks or a spasmodic voice, and multiple axes on the spiral test compared to one single axis in ET patients (20, 21). Of note, while the presence of an intention tremor in patients with true ET helped to differentiate them from patients with any non-ET diagnosis, this was not the case when comparing ET to dystonia patients, which is in line with recent evidence that implies the existence of a cerebellar dysfunction in dystonia (28).
There are certain limitations to our study. First, the patients were evaluated at a tertiary referral center, which might indicate that their cases were more complex and therefore the probability of a misdiagnosis higher than for patients with a diagnosis of ET for whom it was thought to be unnecessary to refer them to a higher level of care. However, the study setting was a clinical practice at an academic medical center, which in addition to being a tertiary referral center also served the local community, so this critical comment is not totally valid. Second, all of our patients were evaluated by solely one provider (EDL), so it is difficult to generalize these results to all settings and all practitioners. Nonetheless, this study design resulted in a high degree of final diagnostic validity as the provider has a long-standing interest and deep knowledge of ET and its diagnostic nuances. Hence, this design feature was a relative strength of the study rather than a limitation. Third, the number of patients we included in our study was small, which is why some of the differences, though accompanied by high ORs, did not reach statistical significance. Despite this, in Tables 3, 4, 20 comparisons were statistically significant. This represents a full 37% of all 54 comparisons, and another six (11%) had p-values that were between 0.05 and 0.10. Larger samples would even further facilitate statistical testing and help in evaluating the role of chance in comparisons that were bordering on significant.
Nonetheless, our study provides important evidence that ET is still frequently overdiagnosed and that the correct diagnosis of alternative movement disorders is oftentimes missed, particularly when the initial ET diagnosis was established by someone who is not a neurologist and when the tremor onset is recent. This observation is not of purely academic interest given that the treatment for ET, dystonia, PD, or other tremor disorders is very different. The importance of the aforementioned point becomes evident when taking into consideration that there was no difference between our patients with a true diagnosis of ET or a non-ET diagnosis regarding the question of whether they had been tried on ET medications before being evaluated in our clinic. This means not only that did they not receive the appropriate therapy for their condition but also that they were started on a treatment that could have induced side effects without providing a potential symptomatic benefit.
Ultimately, raising the awareness of specific clinical features and ancillary tests that can be useful in establishing the correct diagnosis of a tremor condition would be an important step to increase the accuracy of ET and non-ET diagnoses and to shorten the time period between tremor onset and initiation of adequate therapy if necessary.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Yale IRBs– Yale University Institutional Review Boards. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. (2010) 25:534–41. doi: 10.1002/mds.22838
2. Schrag A, Munchau A, Bhatia KP, Quinn NP, Marsden CD. Essential tremor: an overdiagnosed condition? J Neurol. (2000) 247:955–9. doi: 10.1007/s004150070053
3. Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. (2006) 63:1100–4. doi: 10.1001/archneur.63.8.1100
4. Benito-Leon J, Louis ED. Clinical update: diagnosis and treatment of essential tremor. Lancet. (2007) 369:1152–4. doi: 10.1016/S0140-6736(07)60544-3
5. Benamer TS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord. (2000) 15:503–10. doi: 10.1002/1531-8257(200005)15:3<503::AID-MDS1013>3.0.CO;2-V
6. Reimão S, Pita Lobo P, Neutel D, Guedes LC, Coelho M, Rosa MM, et al. Substantia nigra neuromelanin-MR imaging differentiates essential tremor from Parkinson's disease. Mov Disord. (2015) 30:953–9. doi: 10.1002/mds.26182
7. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad hoc Scientific Committee. Mov Disord. (1998) 13(Suppl. 3):2–23. doi: 10.1002/mds.870131303
8. Rajalingam R, Breen DP, Chen R, Fox S, Kalia LV, Munhoz RP, et al. The clinical significance of lower limb tremors. Parkinsonism Relat Disord. (2019) 65:165–71. doi: 10.1016/j.parkreldis.2019.06.007
9. Poston KL, Rios E, Louis ED. Action tremor of the legs in essential tremor: prevalence, clinical correlates, and comparison with age-matched controls. Parkinsonism Relat Disord. (2009) 15:602–5. doi: 10.1016/j.parkreldis.2008.11.006
10. Louis ED, Dogu O. Isolated head tremor: part of the clinical spectrum of essential tremor? Data from population-based and clinic-based case samples. Mov Disord. (2009) 24:2281–5. doi: 10.1002/mds.22777
11. Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. (1991) 41:(Pt 1):234–8. doi: 10.1212/WNL.41.2_Part_1.234
12. Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. (1991) 41:1088–91. doi: 10.1212/WNL.41.7.1088
13. Williams L, McGovern E, Kimmich O, Molloy A, Beiser I, Butler JS, et al. Epidemiological, clinical and genetic aspects of adult onset isolated focal dystonia in Ireland. Eur J Neurol. (2017) 24:73–81. doi: 10.1111/ene.13133
14. Phibbs F, Fang JY, Cooper MK, Charles DP, Davis TL, Hedera P. Prevalence of unilateral tremor in autosomal dominant essential tremor. Mov Disord. (2009) 24:108–11. doi: 10.1002/mds.22113
15. Louis ED. Diagnosis and management of tremor. Continuum. (2016). 22:1143–58. doi: 10.1212/CON.0000000000000346
16. Louis ED, Hernandez N, Michalec M. Prevalence and correlates of rest tremor in essential tremor: cross-sectional survey of 831 patients across four distinct cohorts. Eur J Neurol. (2015) 22:927–32. doi: 10.1111/ene.12683
17. Agnew A, Frucht SJ, Louis ED. Supine head tremor: a clinical comparison of essential tremor and spasmodic torticollis patients. J Neurol Neurosurg Psychiatr. (2012) 83:179–81. doi: 10.1136/jnnp-2011-300823
18. Louis ED. Twelve clinical pearls to help distinguish essential tremor from other tremors. Expert Rev Neurother. (2014) 14:1057–65. doi: 10.1586/14737175.2014.936389
19. Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: prevalence and association with disease duration. Mov Disord. (2009) 24:626–7. doi: 10.1002/mds.22370
20. Louis ED, Yu Q, Floyd AG, Moskowitz C, Pullman SL. Axis is a feature of handwritten spirals in essential tremor. Mov Disord. (2006) 21:1294–5. doi: 10.1002/mds.20915
21. Michalec M, Hernandez N, Clark LN, Louis ED. The spiral axis as a clinical tool to distinguish essential tremor from dystonia cases. Parkinsonism Relat Disord. (2014) 20:541–4. doi: 10.1016/j.parkreldis.2014.01.021
22. Pandey S, Bhattad S. Questionable dystonia in essential tremor plus: a video-based assessment of 19 patients. Mov Disord Clin Pract. (2019) 6:722–3. doi: 10.1002/mdc3.12838
23. Rajput AH, Rajput EF, Bocking SM, Auer RN, Rajput A. Parkinsonism in essential tremor cases: a clinicopathological study. Mov Disord. (2019) 34:1031–40. doi: 10.1002/mds.27729
24. Kim CY, Louis ED. “Spooning”: a subtle sign of limb dystonia. Tremor Other Hyperkinet Mov. (2018) 8:607. doi: 10.7916/D8B00NRV
25. Ortiz R, Scheperjans F, Mertsalmi T, Pekkonen E. The prevalence of adult-onset isolated dystonia in Finland 2007-2016. PLoS ONE. (2018) 13:e0207729. doi: 10.1371/journal.pone.0207729
26. Inzelberg R, Hassin-Baer S, Jankovic J. Genetic movement disorders in patients of Jewish ancestry. JAMA Neurol. (2014) 71:1567–72. doi: 10.1001/jamaneurol.2014.1364
27. Louis ED. Essential tremor: a nuanced approach to the clinical features. Pract Neurol. (2019) 9:389–98. doi: 10.1136/practneurol-2018-002183
Keywords: essential tremor, dystonia, Parkinson's disease, differential diagnosis, clinical
Citation: Amlang CJ, Trujillo Diaz D and Louis ED (2020) Essential Tremor as a “Waste Basket” Diagnosis: Diagnosing Essential Tremor Remains a Challenge. Front. Neurol. 11:172. doi: 10.3389/fneur.2020.00172
Received: 22 October 2019; Accepted: 24 February 2020;
Published: 25 March 2020.
Edited by:
Alain Kaelin-Lang, Neurocenter of Southern Switzerland, SwitzerlandReviewed by:
Matteo Bologna, Sapienza University of Rome, ItalyGiovanni Rizzo, University of Bologna, Italy
Copyright © 2020 Amlang, Trujillo Diaz and Louis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elan D. Louis, ZWxhbi5sb3Vpc0B5YWxlLmVkdQ==
 Christian J. Amlang
Christian J. Amlang Daniel Trujillo Diaz
Daniel Trujillo Diaz Elan D. Louis
Elan D. Louis