- 1Department of Neurology, People's Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Neurology, People Hospital of Beijing Daxing District, Beijing, China
- 3Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
Background: In recent years, the phenomenon of coexisting systemic autoimmune diseases (ADs) in patients with autoimmune encephalitis (AE) has been increasingly found, while its clinical significance remains unexplored. This study aimed to investigate the types and potential clinical associations of autoimmune comorbidities in patients with antibody-positive AE.
Methods: A retrospective cohort study of patients with antibody-positive AE was conducted from 2011 to 2018. The demographics, clinical characteristics, and follow-up data were reviewed.
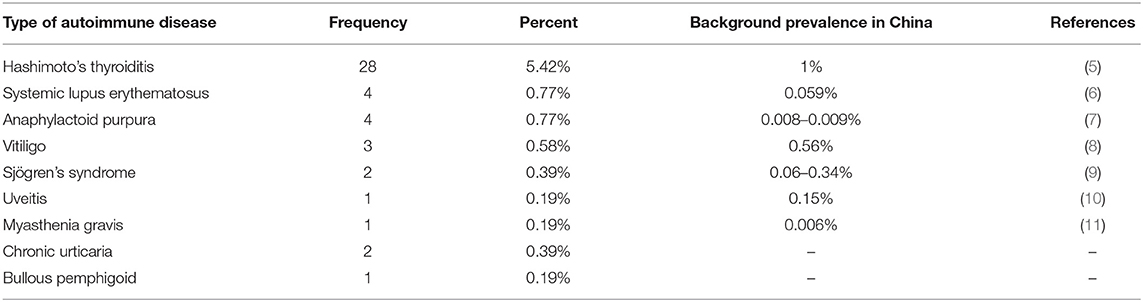
Results: We enrolled 517 patients, among whom 45 were affected by one or more types of ADs, including Hashimoto's thyroiditis (HT) (n = 28), systemic lupus erythematosus (SLE) (n = 3), anaphylactoid purpura (n = 3), vitiligo (n = 3), Sjögren's syndrome (SS) (n = 2), chronic urticaria (n = 2), bullous pemphigoid (n = 1), uveitis (n = 1), myasthenia gravis (MG) (n = 1), and the coexistence of SLE and anaphylactoid purpura (n = 1). The proportion of patients with coexisting ADs was higher in those with anti–leucine-rich glioma-inactivated 1 (LGI1) encephalitis than in those with anti–N-methyl-d-aspartate receptor (NMDAR) encephalitis (13/111 vs. 16/307) (P = 0.021). In anti-NMDAR and anti-LGI1 encephalitis patients, there were no significant differences in the age at onset, sex ratio, proportion of patients with tumors, disease severity, or recurrence between the groups with and without ADs.
Conclusions: One or more types of ADs developed in AE patients, and patients with anti-LGI1 encephalitis had a higher frequency of autoimmune comorbidities than those with anti-NMDAR encephalitis. And we found that autoimmune comorbidities did not affect the clinical course of AE.
Introduction
Since the discovery of anti–N-methyl-d-aspartate receptor (NMDAR) encephalitis in 2007, a series of types of autoimmune encephalitis (AE) mediated by autoantibodies against neuronal surface receptors and synaptic proteins have been discovered (1). The most common type of AE is anti-NMDAR encephalitis, followed by anti–leucine-rich glioma-inactivated 1 (LGI1) encephalitis and anti–gamma aminobutyric acid B receptor (GABABR) encephalitis. Dubey et al. (2) reported that the incidence and prevalence of AE were comparable to infectious encephalitis. With the increase in the number of cases with confirmed AE, the phenomenon of the coexistence of AE and other systemic autoimmune diseases (ADs) has attracted scholars' attention, although its clinical significance remains unexplored. ADs have a complex, multifactorial basis, and both genetics and environmental factors contribute to the development of ADs. Specific autoantibodies and autoreactive T cells seem to be shared characteristics of AE and other ADs, while the potential pathogenesis of the coexistence remains to be further explored.
To the best of our knowledge, except for some case reports, there has been no study concentrating on autoimmune comorbidities in patients with AE. Our study recruited 517 antibody-positive AE patients, of whom 45 had autoimmune comorbidities. The main aim of this study was to investigate the possible clinical significance of the coexistence of AE and other ADs.
Methods
Patients
We eventually recruited 517 patients with AE who were admitted to Peking Union Medical College Hospital and the People's Hospital of Zhengzhou University from 2011 to 2018. All patients met the diagnostic criteria of definite AE (3), with positivity for autoantibodies and clinical manifestations and auxiliary examination results indicative of AE. Diagnoses of Hashimoto's thyroiditis (HT) were established by a combination of the presence of anti-thyroid antibodies (mainly antibodies to thyroid peroxidase and thyroglobulin), appearance on thyroid sonogram, and clinical features (4). Diagnoses of other ADs were based on the clinical manifestations, specific antibodies, biopsy results (if required), and reference to the diagnostic criteria of ADs by the corresponding specialists. This study was approved by the Ethics Review Committees of Peking Union Medical College Hospital and the People's Hospital of Zhengzhou University. All participants provided informed consent.
Detection of Anti-neuronal Antibodies
All serum and cerebrospinal fluid (CSF) antibodies were measured using indirect immunofluorescence test (IIFT) kits purchased from EUROIMMUN AG (Lübeck, Germany) and used according to the manufacturer's instructions.
Data Collection
Clinical data were collected from the recruited patients, including age, sex, the presence of coexisting ADs, clinical characteristics, tumors, the frequency of relapses, and the recurrence interval. Relapse was identified if there was a new symptom of AE or a worsening of preexisting symptoms after at least 2 months of stabilization or improvement.
Statistical Analysis
The SPSS 21.0 statistical software package was used to perform statistical analysis. Continuous data are presented as the mean ± standard deviation, and categorical variables are presented as proportions and ratios. Independent sample t-test and chi-square test were used to compare the differences between the two groups as appropriate. A P-value (two-sided) < 0.05 was considered statistically significant.
Results
Clinical Characteristics and Types of AE of Recruited Patients
This study comprised 517 AE patients (249 females and 268 males). The clinical characteristics of the recruited patients are listed in Table 1. The types of AE consisted of anti-NMDAR encephalitis (n = 307), anti-LGI1 encephalitis (n = 111), anti-GABABR encephalitis (n = 52), anti–contactin-associated protein-like 2 (CASPR2) encephalitis (n = 13), anti–α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid 2-receptor (AMPA2-R) encephalitis (n = 6), anti–α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid 1-receptor (AMPA1-R) encephalitis (n = 1), anti-IgLON5 encephalopathy (n = 3), anti–glutamic acid decarboxylase (GAD) encephalitis (n = 9), anti–myelin oligodendrocyte glycoprotein (MOG) antibody syndrome (n = 2), and AE with multiple autoantibodies, including the coexistence of anti-CASPR2 and anti-LGI1 antibodies (n = 7), anti-NMDAR and anti-GABABR antibodies (n = 2), anti-NMDAR and anti-CASPR2 antibodies (n = 1), anti-NMDAR and anti-GAD antibodies (n = 1), anti-NMDAR and anti-aquaporin-4 (AQP4) antibodies (n = 1), and anti-LGI1 and anti-GAD antibodies (n = 1).
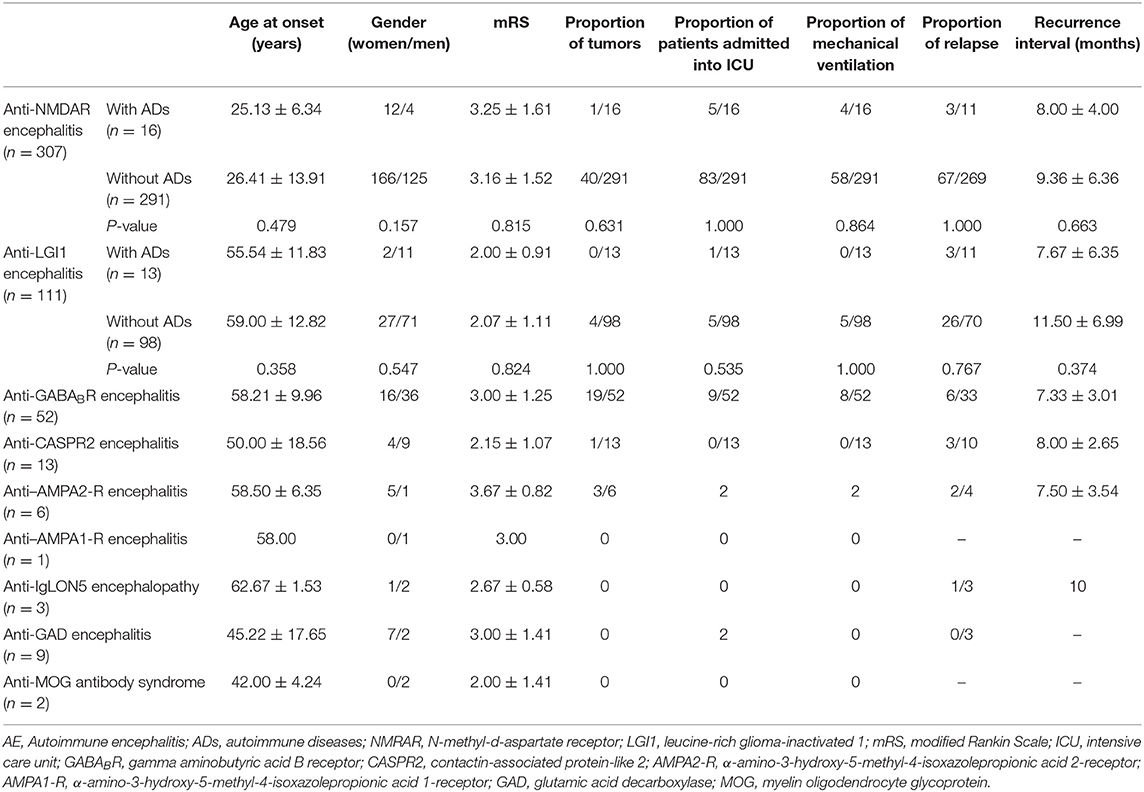
Table 1. Clinical characteristics of AE patients and comparison of the clinical characteristics between the groups with and without coexisting ADs in anti-NMDAR and anti-LGI1 encephalitis patients.
The Types of ADs in Patients With Different Types of AE and the Percentages of Concomitant ADs in our Recruited Patients
Among the 307 anti-NMDAR encephalitis patients, 16 patients had ADs, including HT (n = 11), systemic lupus erythematosus (SLE) (n = 2), chronic urticaria (n = 2), and anaphylactoid purpura (n = 1). Among the 111 anti-LGI1 encephalitis patients, 13 patients had ADs, including HT (n = 6), vitiligo (n = 2), anaphylactoid purpura (n = 1), SLE (n = 1), the coexistence of SLE and anaphylactoid purpura (n = 1), Sjögren's syndrome (SS) (n = 1), and uveitis (n = 1). The proportion of patients with coexisting ADs was higher in those with anti-LGI1 encephalitis than in those with anti-NMDAR encephalitis (13/111 vs. 16/307) (P = 0.021).
Among the 52 anti-GABABR encephalitis patients, 3 patients had HT, and 1 patient had SS. Among the 13 anti-CASPR2 encephalitis patients, 1 patient had HT, and 1 patient had bullous pemphigoid. Among the six anti–AMPA2-R encephalitis patients, one patient had myasthenia gravis (MG), and one patient had HT. Among the three anti-IgLON5 encephalopathy patients, one patient had vitiligo. Among the nine anti-GAD encephalitis patients, five patients had HT. Among the two patients with anti-MOG antibody syndrome, one patient had HT, and one patient had anaphylactoid purpura.
The percentages of concomitant ADs in our recruited patients and the background prevalence of some ADs in China are shown in Table 2 (5–11). The percentages of some concomitant ADs in our recruited patients are higher than the background prevalence in China.
Twenty-four patients had confirmed diagnoses of ADs before the onset of AE (the time interval between the onset of ADs and AE ranged from 2 months to 12 years), while 20 patients were diagnosed with AE and ADs simultaneously during hospitalization in our hospital; for 1 patient, the diagnosis of ADs was made 1 year after the onset of AE.
Comparison of the Clinical Characteristics of Anti-NMDAR Encephalitis and Anti-LGI1 Encephalitis Patients Between the Groups With and Without Coexisting ADs
As shown in Table 1, there were no significant differences in the age at onset, sex ratio, proportion of patients with tumors, disease severity, proportion of patients who relapsed, or recurrence interval between the two groups.
Discussion
This study aimed to explore the phenomenon of the coexistence of antibody-positive AE and other ADs. Previous studies have shown that one AD increases the chance of an additional AD (12), and we found that the percentages of some ADs in our recruited patients were higher than the background prevalence in China.
We also found that patients with anti-LGI1 encephalitis were more prone to having autoimmune comorbidities than patients with anti-NMDAR encephalitis (P = 0.021). Interestingly, previous studies showed that anti-LGI1 encephalitis was highly associated with several human leukocyte antigen (HLA) class II alleles, whereas anti-NMDAR encephalitis was not (13–15). Recently, Shu et al. (16) found that anti-NMDAR encephalitis was associated with the HLA class II allele DRB1*16:02, although the carrier frequency of this allele was rather low (≤ 30%). Compared with anti-NMDAR encephalitis, anti-LGI1 encephalitis seems to show a stronger genetic predisposition mediated by HLA class II alleles. In epidemiological and genetic studies, associations between HLA class II alleles and many ADs (such as HT and SLE) have been found (17, 18). Perhaps in anti-LGI1 encephalitis patients, the susceptibility genes played an important role in the formation of the autoimmune milieu, which was conducive to the coexistence of ADs.
In this study, the most frequent autoimmune comorbidity in patients with antibody-positive AE was HT (5.42%). We hypothesized that this finding may be related to the high prevalence of HT in the population (the prevalence of HT in our regions was approximately 1%). Tuzun et al. (19) showed that patients with anti-thyroid antibodies were inclined to develop AE. In encephalopathy, the presence of anti-thyroid antibodies is often taken as evidence of Hashimoto's encephalitis (HE). According to the diagnostic criteria for AE (3), when anti-thyroid and anti-neuronal antibodies exist simultaneously, antibody-positive AE should be diagnosed as the priority. Hence, before diagnosing HE, anti-neuronal antibodies should be detected to avoid misdiagnosis.
In this study, several AE patients had coexisting autoimmune skin or mucosal lesions, such as vitiligo, bullous pemphigoid, etc. Both the skin and the nervous system originate from the ectoderm, and some studies have found that the NMDA and LGI1 receptors can be expressed outside the nervous system (e.g., in human epidermal melanocytes and sebaceous glands in the skin) (20, 21). Skin lesions may lead to the exposure of autoantigens, which induce the production of autoantibodies in the case of the loss of immune tolerance.
AE can coexist with non-organ-specific ADs, such as SLE and SS. A previous study (22) showed that autoantibodies to the NMDAR NR1 subunit could be detected in patients with SLE, especially neuropsychiatric SLE (NPSLE). However, this detection method was ELISA instead of cell-based assay, and ELISA may have a risk of false positivity. Further studies are required to explore whether lupus antibodies could cross-react with the NMDAR NR1 subunit. As AE and NPSLE have similar clinical features, the detection of specific antibodies seems to be vital.
In this study, six patients with anti–AMPA2-R encephalitis were recruited, and three of them had thymoma. As a central lymphoid organ, the thymus provides an inductive environment for the development of T cells and induces the central tolerance (23). Thus, the presence of thymoma is often accompanied by ADs. One anti–AMPA2-R encephalitis patient with coexisting thymoma achieved clinical remission after thymectomy and immunosuppressive therapy. However, 1 year later, this patient manifested clinical symptoms of MG. Previous studies have shown that the mutilation of the autoimmune regulator (AIRE) gene was associated with thymoma-related MG (24, 25). Hence, that patient may have mutilation of the AIRE gene, which resulted in a disturbance of the homeostasis in the immune system.
In this study, there were no significant differences in disease severity or relapse between the two groups, indicating that the presence of ADs did not affect the progression or clinical outcomes of AE. Thus, the treatment strategies for AE patients are not affected by the presence of ADs.
Conclusions
In summary, this was the first study to concentrate on the coexistence of AE and other ADs. Our study indicated that patients with anti-LGI1 encephalitis are more prone to having autoimmune comorbidities than patients with anti-NMDAR encephalitis. In addition, the presence of ADs does not affect the clinical course of AE.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Peking Union Medical College Hospital Ethics Review Committees and People's Hospital of Zhengzhou University Ethics Review Committees. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
HG and JZhang participated in the design of this study. JZhao, CW, XX, YZ, and HR collected clinical data. JZhao, CW, ZR, and GL analyzed the data. JZhao and CW draft the manuscript. All the authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADs, autoimmune diseases; AE, autoimmune encephalitis; HT, Hashimoto's thyroiditis; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome; MG, myasthenia gravis; NMRAR, N-methyl-d-aspartate receptor; LGI1, leucine-rich glioma-inactivated 1; GABABR, gamma aminobutyric acid B receptor; CSF, cerebrospinal fluid; IIFT, indirect immunofluorescence test; CASPR2, contactin-associated protein-like 2; AMPA2-R, α-amino-3-hydroxy-5- methyl-4-isoxazolepropionic acid 2-receptor; AMPA1-R, α-amino-3-hydroxy-5- methyl-4-isoxazolepropionic acid 1-receptor; GAD, glutamic acid decarboxylase; MOG, myelin oligodendrocyte glycoprotein; AQP4, aquaporin-4; HLA, human leukocyte antigen; HE, Hashimoto's encephalitis; NPSLE, neuropsychiatric systemic lupus erythematosus; AIRE, autoimmune regulator; mRS, modified Rankin Scale; ICU, intensive care unit.
References
1. Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. (2007) 61:25–36. doi: 10.1002/ana.21050
2. Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. (2018) 83:166–77. doi: 10.1002/ana.25131
3. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
4. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. (2014) 13:391–7. doi: 10.1016/j.autrev.2014.01.007
5. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. (2006) 354:2783–93. doi: 10.1056/NEJMoa054022
6. Mok CC. Management of systemic lupus erythematosus in Chinese patients. Expert Rev Clin Immunol. (2007) 3:925–35. doi: 10.1586/1744666X.3.6.925
7. Xu Y, Wang JJ, Liu FF, Wu Y, Wu YF, Samadli S, et al. Predisposing factors of childhood Henoch-Schonlein purpura in Anhui province, China. J Investig Med. (2019) 67:771–8. doi: 10.1136/jim-2018-000906
8. Wang X, Du J, Wang T, Zhou C, Shen Y, Ding X, et al. Prevalence and Clinical Profile of Vitiligo in China: a community-based study in six cities. Acta Dermato Venereologica. (2013) 93:62–5. doi: 10.2340/00015555-1397
9. Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Ann Rheumatic Dis. (2015) 74:1983–9. doi: 10.1136/annrheumdis-2014-205375
10. Hsu YR, Huang JC, Tao Y, Kaburaki T, Lee CS, Lin TC, et al. Noninfectious uveitis in the Asia-Pacific region. Eye. (2019) 33:66–77. doi: 10.1038/s41433-018-0223-z
11. Yu YL, Hawkins BR, Ip MS, Wong V, Woo E. Myasthenia gravis in Hong Kong Chinese. 1. Epidemiology and adult disease. Acta Neurol Scand. (1992) 86:113–9. doi: 10.1111/j.1600-0404.1992.tb05050.x
12. Zhernakova A, Withoff S, Wijmenga C. Clinical implications of shared genetics and pathogenesis in autoimmune diseases. Nat Rev Endocrinol. (2013) 9:646–59. doi: 10.1038/nrendo.2013.161
13. Kim TJ, Lee ST, Moon J, Sunwoo JS, Byun JI, Lim JA, et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann Neurol. (2017) 81:183–92. doi: 10.1002/ana.24860
14. van Sonderen A, Roelen DL, Stoop JA, Verdijk RM, Haasnoot GW, Thijs RD, et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann Neurol. (2017) 81:193–8. doi: 10.1002/ana.24858
15. Mueller SH, Färber A, Prüss H, Melzer N, Golombeck KS, Kümpfel T, et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann Neurol. (2018) 83:863–9. doi: 10.1002/ana.25216
16. Shu Y, Qiu W, Zheng J, Sun X, Yin J, Yang X, et al. HLA class II allele DRB1*16:02 is associated with anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. (2019) 90:652–8. doi: 10.1136/jnnp-2018-319714
17. Zeitlin AA, Heward JM, Newby PR, Carr-Smith JD, Franklyn JA, Gough SC, et al. Analysis of HLA class II genes in Hashimoto's thyroiditis reveals differences compared to Graves' disease. Genes Immun. (2008) 9:358–63. doi: 10.1038/gene.2008.26
18. Paradowska-Gorycka A, Stypinska B, Olesinska M, Felis-Giemza A, Manczak M, Czuszynska Z, et al. Association of HLA-DRB1 alleles with susceptibility to mixed connective tissue disease in Polish patients. HLA. (2016) 87:13–8. doi: 10.1111/tan.12698
19. Tuzun E, Erdag E, Durmus H, Brenner T, Turkoglu R, Kurtuncu M, et al. Autoantibodies to neuronal surface antigens in thyroid antibody-positive and -negative limbic encephalitis. Neurol India. (2011) 59:47–50. doi: 10.4103/0028-3886.76857
20. Head K, Gong S, Joseph S, Wang C, Burkhardt T, Rossi MR, et al. Defining the expression pattern of the LGI1 gene in BAC transgenic mice. Mamm Genome. (2007) 18:328–37. doi: 10.1007/s00335-007-9024-6
21. Yin H, Zhu C, Ren H, Yang X, Peng B, Cui L, et al. Resection of melanocytic nevi as a potential treatment of anti-NMDAR encephalitis patients without tumor: report of three cases. Neurol Sci. (2018) 39:165–7. doi: 10.1007/s10072-017-3173-5
22. Ogawa E, Nagai T, Sakuma Y, Arinuma Y, Hirohata S. Association of antibodies to the NR1 subunit of N-methyl-D-aspartate receptors with neuropsychiatric systemic lupus erythematosus. Mod Rheumatol. (2016) 26:377–83. doi: 10.3109/14397595.2015.1083163
23. Miller JF. Revisiting thymus function. Front Immunol. (2014) 5:411. doi: 10.3389/fimmu.2014.00411
24. Strobel P, Chuang WY, Chuvpilo S, Zettl A, Katzenberger T, Kalbacher H, et al. Common cellular and diverse genetic basis of thymoma-associated myasthenia gravis: role of MHC class II and AIRE genes and genetic polymorphisms. Ann N Y Acad Sci. (2008) 1132:143–56. doi: 10.1196/annals.1405.018
Keywords: autoantibodies, encephalitis, autoimmune encephalitis, autoimmune diseases, comorbidities
Citation: Zhao J, Wang C, Xu X, Zhang Y, Ren H, Ren Z, Li G, Zhang J and Guan H (2019) Coexistence of Autoimmune Encephalitis and Other Systemic Autoimmune Diseases. Front. Neurol. 10:1142. doi: 10.3389/fneur.2019.01142
Received: 22 June 2019; Accepted: 11 October 2019;
Published: 31 October 2019.
Edited by:
Fabienne Brilot, University of Sydney, AustraliaReviewed by:
Alessandro Antonelli, University of Pisa, ItalyShekeeb S. Mohammad, University of Sydney, Australia
Copyright © 2019 Zhao, Wang, Xu, Zhang, Ren, Ren, Li, Zhang and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiewen Zhang, emhhbmdqaWV3ZW45OTAwQDEyNi5jb20=; Hongzhi Guan, Z3Vhbmh6QDI2My5uZXQ=
†These authors have contributed equally to this work
 Jing Zhao
Jing Zhao Cancan Wang2†
Cancan Wang2† Jiewen Zhang
Jiewen Zhang Hongzhi Guan
Hongzhi Guan