- 1Division of Cancer Epidemiology and Genetics, Clinical Genetics Branch, National Cancer Institute, Bethesda, MD, United States
- 2Department of Neurology, Neuromuscular Disease Center, University of Rochester Medical Center, Rochester, NY, United States
- 3John Walton Muscular Dystrophy Research Centre, Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom
- 4National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, London, United Kingdom
- 5Department of Neuropediatrics and Muscle Disorders, Faculty of Medicine, Medical Center, University of Freiburg, Freiburg, Germany
- 6Centro Nacional de Análisis Genómico (CNAG-CRG), Center for Genomic Regulation Barcelona, Institute of Science and Technology (BIST), Barcelona, Spain
- 7Children's Hospital of Eastern Ontario Research Institute, University of Ottawa, Ottawa, ON, Canada
- 8Division of Neurology, Department of Medicine, The Ottawa Hospital, Ottawa, ON, Canada
Introduction: Recent evidence demonstrates that women with myotonic dystrophy type 1 are at increased risk of reproductive organ tumors. However, studies of reproductive cancer risk factors in those patients are lacking.
Methods: Using questionnaires, we collected and analyzed personal history information related to cancer risk factors from women enrolled in a UK and US registry for myotonic dystrophy (dystrophia myotonica; DM) patients.
Results: The survey was completed by 242 DM type 1 (DM1) and 44 DM type 2 (DM2) women enrolled in the UK Registry (N = 124) and the US National Registry (N = 162). The mean age at DM1 diagnosis was 33.8 years (standard deviation, SD = 13.2) and for DM2 was 49.2 (SD = 13.0). Mean age at survey was 48.7 (SD = 12.8) and 59.1 years (SD = 12.8) for DM1 and DM2, respectively. There were no statistically significant differences between DM1 and DM2 regarding menstrual history or fertility-related factors. Yet, women with DM2 were more likely to have used menopausal hormone therapy (HT) than women with DM1 (52.3 vs. 22.1%, p < 0.0001), and more women with DM2 had a hysterectomy (53.5 vs. 29.5%, p < 0.01). These differences were not statistically significant after age adjustment (OR = 2.00, p = 0.08, and OR = 1.40, p = 0.38, respectively). The frequency of self-reported reproductive organ tumors was not significantly different comparing DM1 to DM2 (p = 0.28). However, the data suggested that women with DM2 appear to have a lower risk of malignant tumors compared to those with DM1 (OR = 0.72, p = 0.69).
Discussion: Our study is the first to characterize a wide range of reproductive risk factors in women with DM. We observed no significant differences between DM1 and DM2 in the factors that were evaluated, which suggests that the known excesses of ovarian and endometrial cancer previously reported in women with DM1 cannot be attributed to greater prevalence of standard cancer-related reproductive risk factors. Larger studies evaluating the possible link between reproductive cancer risk factors and risk of tumors in women with DM are needed.
Introduction
The myotonic dystrophies (dystrophia myotonica; DM), are two inherited, multisystem, autosomal dominant disorders that primarily affect skeletal muscle (1). Myotonic dystrophy type 1 (DM1, “Steinert's disease”) is caused by an unstable trinucleotide (CTG) repeat expansion in the 3′-UTR of the dystrophia myotonica-protein kinase (DMPK) gene (2–4). Myotonic dystrophy type 2 (DM2) is caused by an unstable tetranucleotide (CCTG) repeat expansion in the CCHC-type zinc finger nucleic acid binding protein (CNBP) gene (5, 6). Clinical phenotypes of DM1 and DM2 share many features, including progressive muscle weakness, myotonia, and other organ abnormalities, such as, cardiac conduction defects, gastrointestinal alterations, endocrine disturbances (especially insulin resistance), and characteristic cataracts (7–9).
Recent evidence has shown that DM1 patients have an increased risk of both benign and malignant tumors (10–13), including those of the female reproductive organs. A large population-based study used data from the Swedish and Danish population-based patient registries to show that women with DM have ~5- and 8-fold higher risk of ovarian and endometrial cancer than the general population, respectively (13). Studies of the Basque DM1 cohort (14) and from France and the UK (15, 16) observed similar findings. Cross-sectional studies from Italy and the UK reveal that uterine fibroids are the most commonly reported benign tumor in women with DM1 or DM2 (17, 18).
A better understanding of the medical and family history in DM1 and DM2 patients, combined with current standard clinical assessment of female reproductive factors, may shed light on potential risk factors for these cancers and in turn lead to strategies for prevention and early detection. However, studies with information related to female reproductive cancer risk factors in DM are lacking. In this study, we report a wide range of reproductive factors in women with DM1 or DM2, focusing on risk of cancers of the female reproductive system, using data collected from patients enrolled in DM registries from the US and UK.
Materials and Methods
Study Population and Data Collection
This study included 286 women, genetically and/or clinically confirmed as having DM and self-enrolled in the UK DM Registry (19) (https://www.dm-registry.org/uk/; N = 124: DM1 = 120, DM2 = 4) or the US National Registry of DM and Facioscapulohumeral Muscular Dystrophy (20) (US DM registry; https://www.urmc.rochester.edu/neurology/national-registry.aspx; N = 162: DM1 = 122, DM2 = 40), who responded to the reproductive history questionnaire. DM diagnosis was confirmed by the patient-designated healthcare professional in the UK and medical record review in the US (19, 20).
Details describing the methodology of data collection and information obtained have been published previously (18, 21, 22). Briefly, a self-administered questionnaire was sent to DM patients enrolled in the US (via mail) or UK (primarily via email, followed by mail for non-responders) DM registries. The questionnaire requested information on personal history of benign and malignant tumors, familial history of cancer, and known cancer risk factors, including reproductive history. Baseline demographic and DM clinical factor data were obtained from relevant registry databases. Research activities of the US DM registry were approved by the University of Rochester's Institutional Review Board, and those of the UK DM Registry were approved by the UK Research Ethics Service. This report is part of our comprehensive effort aimed at more detailed characterization of the recently-recognized cancer susceptibility in DM patients (18, 21–23).
Collection of Female Reproductive Cancer Risk Factor Information
The questionnaire collected the following information: age at menarche, birth control pill use and duration, consultation for infertility, pregnancy history (number of pregnancies and live-born children), time of last regular menstrual period, menopausal hormone therapy (HT) use and duration, and history of hysterectomy. Information on history of gynecologic tumors was determined from the patient's response to the following questions: “Have you ever been diagnosed with any benign (non-cancerous) tumor?” and “Have you ever been diagnosed with any type of cancer (a malignant growth or invasive tumor)?” If patients responded “yes,” they were asked to specify the tumor type/site and date- or age-at-diagnosis.
Statistical Analysis
We used chi-square or Fisher's exact test to compare the frequency of categorical variables between DM1 and DM2 patients, and t-test for continuous variables. To minimize the possible impact of confounding by age, we used logistic regression models to adjust all comparisons for age at survey. All analyses were conducted using SPSS Version 25.0 (IBM Corp. Armonk, NY) with statistical significance defined as two-sided p < 0.05.
Results
Characteristics of Female DM Patients Participating in the Study
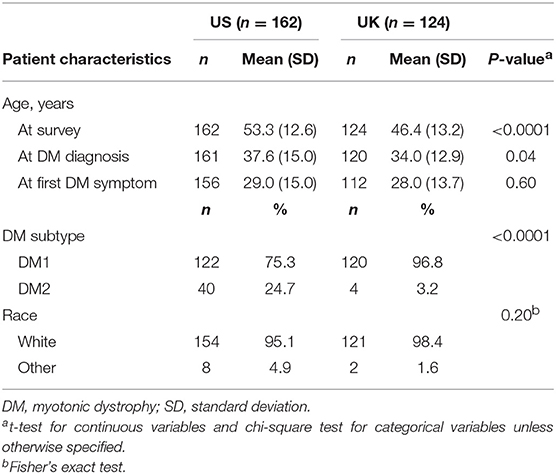
DM patients enrolled from the US DM registry were older at survey and at DM diagnosis (Table 1). In both countries, DM2 patients were older than DM1 patients (mean age at survey = 59.8 vs. 51.2, p = 0.0001 in the US; 52.2 vs. 46.2, p = 0.37 in the UK). As expected, DM2 patients were diagnosed at an older age (mean age at diagnosis = 49.7 vs. 33.7 in the US; 42.3 vs. 33.8 in the UK), and were older at first DM2 symptom than those with DM1 (mean age at first DM symptom = 35.9 vs. 26.8 years in the US; 41.0 vs. 27.8 years in the UK).
Comparison of Female Reproductive Factors Between DM1 and DM2 Patients
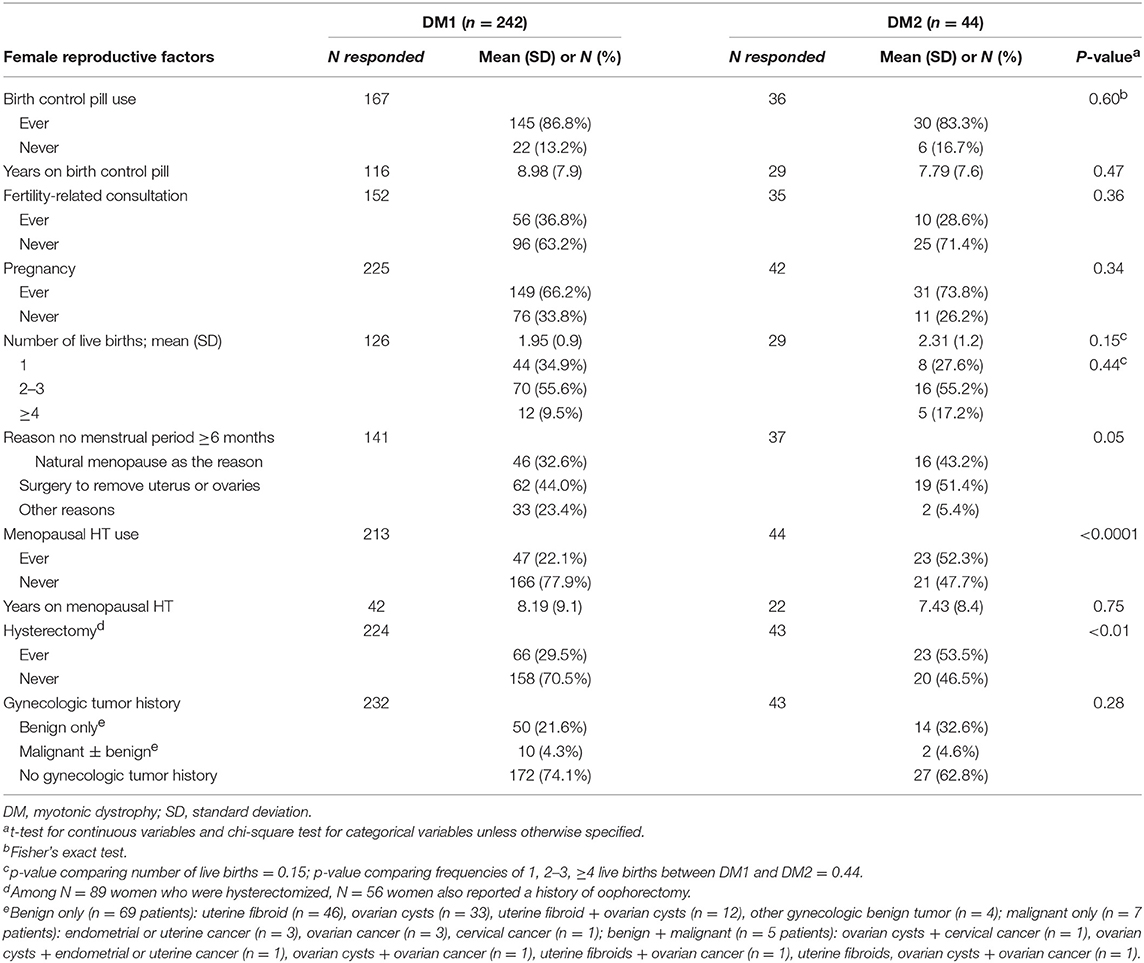
Table 2 summarizes female reproductive factors in patients with DM1 and DM2. The median age at menarche was 13 years for both DM1 and DM2 patients (DM1 range = 9–23, DM2 range = 9–16). DM1 and DM2 patients reported similar use of oral contraceptives, a higher frequency of self-reported menopausal HT use in patients with DM2 was noted (52.3 vs. 22.1%, in DM2 and DM1, respectively, p < 0.0001). Hysterectomy was reported by 33.3% of the patients (n = 89 among 267 responders); 56 of whom also had oophorectomy. Approximately half of those with hysterectomy (48.3%, N = 43) reported a history of gynecologic tumor (DM1 patients = 32, 74.4%, DM2 patients = 11, 25.6%). Surgical menopause (hysterectomy and/or oophorectomy) was the most common reason for cessation of menses (≥6 months) in both DM1 and DM2.
Gynecological tumors were reported by more than one-fourth of the patients. DM1 patients reported 64 benign (in 54 patients), and 10 malignant gynecological tumors (in 10 patients). DM2 patients reported 19 benign (in 15 patients) and 2 malignant gynecological tumors (in 2 patients). Overall, uterine fibroids were the most frequent benign tumor (N = 46 patients, prevalence = 16.7%), followed by ovarian cysts (N = 33 patients, prevalence = 12.0%). For malignant tumor, 6 women (2.2%) reported ovarian cancer, 4 reported endometrial or uterine cancer (1.4%), and 2 reported cervical cancer (0.7%). Among the 12 women with a gynecologic cancer, 5 reported that they also had a benign gynecological tumor.
In age-adjusted analyses, no statistically significant differences between DM1 and DM2 were noted in any of the factors analyzed (Table 3). However, the data suggested that DM2 patients may be more likely to have undergone hysterectomy (OR = 1.40, p = 0.38) or receive menopausal HT (OR = 2.00, p = 0.08), and less likely to have infertility-related consultations (OR = 0.74, p = 0.49), or malignant tumors of the reproductive organs (OR = 0.72, p = 0.69).
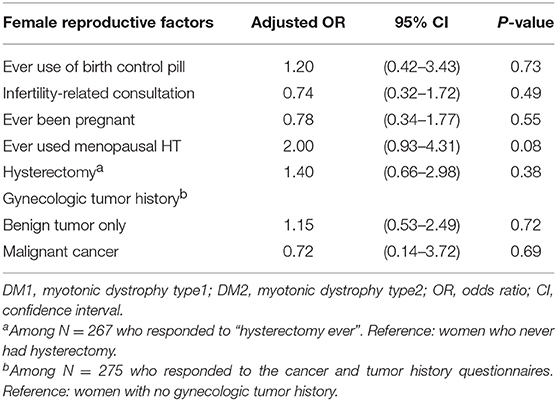
Table 3. Age-adjusted comparison of key female reproductive cancer risk factors between patients with DM2 vs. DM1.
Discussion
In this cross-sectional study of 286 women with DM1 or DM2, we explored differences in reproductive cancer risk factors by DM subtype. Age-adjusted analyses showed no statistically significant differences; however, the data suggested that DM2 patients may be more likely to have undergone hysterectomy and to have used menopausal HT.
In this study, we observed similar ages at menarche for women with DM1 or DM2 (median = 13 years). This was similar to age at menarche reported in the US general population based on data from 2,510 children and adolescents of non-Hispanic whites, blacks, and Mexican Americans in the third National Health and Nutrition Examination Survey (median = 12.4 years) (24). For age at menopause, we did not directly collect this information. However, most patients (DM1 = 91.3%, DM2 = 100%) who reported natural menopause were >50 years old at survey. The median age of natural menopause in the US is 52.6 years (25). On the other hand, 81 patients reported surgical menopause, 21 of which (26%) occurred at or before age 50. One-third of the DM patients in the current study reported a history of hysterectomy. The US prevalence for women 15–44 years of age is 4.0% (26, 27). In the UK, the estimated prevalence of hysterectomy for all ages is approximately 9%, with a peak increase at age 55 and older that reaches 20% (28). Similar to previous studies in DM (17, 18), uterine fibroids were common, affecting 16.7% of the DM patients, followed by ovarian cysts, affecting 12%. The prevalence of uterine fibroids noted in this study is higher than that reported in the general population from the US (6.9%) and UK (4.5%) in a large study of 21,746 women aged 15–49 years from eight countries (29).
The molecular basis underlying tumor development in the female reproductive systems among DM patients has yet to be identified. Ongoing studies seek a better understanding of whether DM disease severity correlates with tumor development. Alsaggaf et al. have provided the first data suggesting that DM1-related cancer susceptibility may be positively correlated with disease severity in adult onset DM1 (16). Upregulation of Wnt-β-catenin signaling (30) and loss of DMPK heterozygosity have been hypothesized as etiologically important (31). Interestingly, a recent gene expression analysis showed microRNA-200c and microRNA-141 tumor suppressor gene expression is sex-dependent in DM1, in which significant downregulation was observed among female DM1 patients (14). Downregulation of miRNA-200c and miRNA141 were implicated in uterine fibroid (32) and endometriosis (33) pathogenesis, respectively. Additionally, dysregulation of miR-200 family has been associated with ovarian cancer (34) and endometrial cancer development (35).
Our current investigation is the first study to characterize a wide range of reproductive risk factors in women with DM. The strengths of our current study include its relatively large sample size, representation of DM patients from two countries, and DM diagnosis having been validated by medical professionals. Our investigation is limited by the self-reported nature of the collected information. Since the study analyzed data from DM patients who were self-enrolled in the DM registries, the results may not be generalizable to the entire DM population. However, our study population did have similar distribution of age-at-diagnosis to the DM general population. Our study focused on DM patients and lacked matched control comparisons, so we used published general population estimates which may not be age comparable to our study population. Our questionnaire lacked information on birth control methods other than pills such as injections. Due to the small number of reported cancers in study participants, we were not able to formally test associations between reproductive factors and risk of specific neoplasms. Also, our reported frequencies may be overestimated in situations of missing information due to patient non-response since denominators were only based on the number of responders.
In conclusion, our study found no significant reproductive cancer factor differences between female DM1 and DM2 subjects, except for a possibility of a higher frequency of hysterectomy and HT use in DM2 patients. The older age of the DM2 patient cohort may have contributed to these observed differences. The observed similarities between DM1 and DM2 cohorts regarding menstrual history or fertility-related factors suggest that the known excesses of ovarian and endometrial cancer seen in DM1 subjects cannot be attributed to greater prevalence of standard gynecologic cancer risk factors. In the future, larger studies evaluating the possible link between reproductive cancer risk factors and risk of tumors in women with DM are needed.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was reviewed and approved by the University of Rochester's Institutional Review Board and activities of the UK DM registry were approved by the UK Research Ethics Service. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SG and MG contributed to the project conceptual design. JH, LW, WM, CM-B, HL, and RM contributed to the data collection and management. CH, YW, and SG contributed to the statistical analysis, data interpretation, and manuscript draft. All authors contributed to the manuscript critical review and approval.
Funding
This study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, and National Institutes of Health. The US National Registry of Myotonic Dystrophy and Facioscapulohumeral Muscular Dystrophy Patients and Family Members was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number P50NS048843 and previously by U54-NS048843.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CNBP, CCHC-type zinc finger nucleic acid binding protein; CTG, Trinucleotide; CCTG, Tetranucleotide; CI, Confidence interval; DM, Myotonic dystrophy; DM1, Myotonic dystrophy type 1; DM2, Myotonic dystrophy type 2; DMPK, dystrophia myotonica-protein kinase; FSHD, US National Registry of DM and Facioscapulohumeral Muscular Dystrophy; HT, Hormone therapy; OR, Odds ratio; SD, Standard deviation; UF, Uterine fibroid.
References
2. Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. (1992) 69:385.
3. Fu YH, Pizzuti A, Fenwick RG Jr, King J, Rajnarayan S, Dunne PW, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. (1992) 255:1256–8. doi: 10.1126/science.1546326
4. Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. (1992) 255:1253–5. doi: 10.1126/science.1546325
5. Ranum LP, Rasmussen PF, Benzow KA, Koob MD, Day JW. Genetic mapping of a second myotonic dystrophy locus. Nat Genet. (1998) 19:196–8. doi: 10.1038/570
6. Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. (2001) 293:864–7. doi: 10.1126/science.1062125
7. Wenninger S, Montagnese F, Schoser B. Core clinical phenotypes in myotonic dystrophies. Front Neurol. (2018) 9:303. doi: 10.3389/fneur.2018.00303
8. Meola G, Cardani R. Myotonic dystrophy type 2: an update on clinical aspects, genetic and pathomolecular mechanism. J Neuromuscul Dis. (2015) 2:S59–71. doi: 10.3233/JND-150088
9. Dalton JC, Ranum LPW, Day JW. Myotonic dystrophy type 2. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews®. Seattle, WA: University of Washington (2006).
10. Alsaggaf R St., George DMM, Zhan M, Pfeiffer RM, Wang Y, Anderson LA, et al. Benign tumors in myotonic dystrophy type I target disease-related cancer sites. Ann Clin Transl Neurol. (2019) 6:1510–8. doi: 10.1002/acn3.50856
11. Emparanza JI, de Munain AL, Greene MH, Matheu A, Fernandez-Torron R, Gadalla SM. Cancer phenotype in myotonic dystrophy patients: results from a meta-analysis. Muscle Nerve. (2018) 58:517–22. doi: 10.1002/mus.26194
12. Wang Y, Pfeiffer RM, Alsaggaf R, Meeraus W, Gage JC, Anderson LA, et al. Risk of skin cancer among patients with myotonic dystrophy type 1 based on primary care physician data from the U.K. Clinical Practice Research Datalink. Int J Cancer. (2018) 142:1174–81. doi: 10.1002/ijc.31143
13. Gadalla SM, Lund M, Pfeiffer RM, Gortz S, Mueller CM, Moxley RT III, et al. Cancer risk among patients with myotonic muscular dystrophy. JAMA. (2011) 306:2480–6. doi: 10.1001/jama.2011.1796
14. Fernandez-Torron R, Garcia-Puga M, Emparanza JI, Maneiro M, Cobo AM, Poza JJ, et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology. (2016) 87:1250–7. doi: 10.1212/WNL.0000000000003124
15. Mohamed S, Pruna L, Kaminsky P. [Increasing risk of tumors in myotonic dystrophy type 1]. Presse Med. (2013) 42:e281–4. doi: 10.1016/j.lpm.2013.01.052
16. Alsaggaf R St., George DMM, Zhan M, Pfeiffer RM, Wang Y, Wagner KR, et al. Cancer risk in myotonic dystrophy type i: evidence of a role for disease severity. JNCI Cancer Spectr. (2018) 2:pky052. doi: 10.1093/jncics/pky052
17. Bianchi ML, Leoncini E, Masciullo M, Modoni A, Gadalla SM, Massa R, et al. Increased risk of tumor in DM1 is not related to exposure to common lifestyle risk factors. J Neurol. (2016) 263:492–8. doi: 10.1007/s00415-015-8006-y
18. Alsaggaf R, Wang Y, Marini-Bettolo C, Wood L, Nikolenko N, Lochmuller H, et al. Benign and malignant tumors in the UK myotonic dystrophy patient registry. Muscle Nerve. (2018) 57:316–20. doi: 10.1002/mus.25736
19. Wood L, Cordts I, Atalaia A, Marini-Bettolo C, Maddison P, Phillips M, et al. The UK myotonic dystrophy patient registry: facilitating and accelerating clinical research. J Neurol. (2017) 264:979–88. doi: 10.1007/s00415-017-8483-2
20. Hilbert JE, Kissel JT, Luebbe EA, Martens WB, McDermott MP, Sanders DB, et al. If you build a rare disease registry, will they enroll and will they use it? Methods and data from the National Registry of Myotonic Dystrophy (DM) and Facioscapulohumeral Muscular Dystrophy (FSHD). Contemp Clin Trials. (2012) 33:302–11. doi: 10.1016/j.cct.2011.11.016
21. Best A, Hilbert JE, Wood L, Martens WB, Nikolenko N, Marini-Bettolo C, et al. Survival patterns and cancer determinants in families with myotonic dystrophy type I. Eur J Neurol. (2018) 25:1326–32. doi: 10.1111/ene.13763
22. Gadalla SM, Hilbert JE, Martens WB, Givens S, Moxley RT III, Greene MH. Pigmentation phenotype, photosensitivity and skin neoplasms in patients with myotonic dystrophy. Eur J Neurol. (2017) 24:713–8. doi: 10.1111/ene.13276
23. Das M, Moxley RT III, Hilbert JE, Martens WB, Letren L, Greene MH, et al. Correlates of tumor development in patients with myotonic dystrophy. J Neurol. (2012) 259:2161–6. doi: 10.1007/s00415-012-6476-8
24. Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, et al. Age at menarche and racial comparisons in US girls. Pediatrics. (2003) 111:110–3. doi: 10.1542/peds.111.1.110
25. Reynolds RF, Obermeyer CM. Age at natural menopause in Spain and the United States: results from the DAMES project. Am J Hum Biol. (2005) 17:331–40. doi: 10.1002/ajhb.20121
27. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. (2005) 23:1–160. doi: 10.1037/e414702008-001
28. Redburn JC, Murphy MF. Hysterectomy prevalence and adjusted cervical and uterine cancer rates in England and Wales. BJOG. (2001) 108:388–95. doi: 10.1016/S0306-5456(00)00098-X
29. Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. (2012) 12:6. doi: 10.1186/1472-6874-12-6
30. Mueller CM, Hilbert JE, Martens W, Thornton CA, Moxley RT III, Greene MH. Hypothesis: neoplasms in myotonic dystrophy. Cancer Causes Control. (2009) 20:2009–20. doi: 10.1007/s10552-009-9395-y
31. Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. (1995) 9:534–46. doi: 10.1101/gad.9.5.534
32. Chuang TD, Panda H, Luo X, Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr Relat Cancer. (2012) 19:541–56. doi: 10.1530/ERC-12-0007
33. Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, et al. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. (2009) 23:265–75. doi: 10.1210/me.2008-0387
34. Choi PW, Ng SW. The functions of microRNA-200 family in ovarian cancer: beyond epithelial-mesenchymal transition. Int J Mol Sci. (2017) 18:1207. doi: 10.3390/ijms18061207
Keywords: myotonic dystrophy, Steinert's disease, female reproductive factors, benign tumor, cancer, endometrial cancer, ovarian cancer
Citation: Higgs C, Hilbert JE, Wood L, Martens WB, Marini-Bettolo C, Nikolenko N, Alsaggaf R, Lochmüller H, Moxley RT, Greene MH, Wang Y and Gadalla SM (2019) Reproductive Cancer Risk Factors in Women With Myotonic Dystrophy (DM): Survey Data From the US and UK DM Registries. Front. Neurol. 10:1071. doi: 10.3389/fneur.2019.01071
Received: 28 June 2019; Accepted: 23 September 2019;
Published: 11 October 2019.
Edited by:
Gabriella Silvestri, Catholic University of the Sacred Heart, ItalyReviewed by:
Maria Laura Ester Bianchi, Azienda Sanitaria Locale - Verbano Cusio Ossola, ItalyTeerin Liewluck, Mayo Clinic, United States
Copyright © 2019 Higgs, Hilbert, Wood, Martens, Marini-Bettolo, Nikolenko, Alsaggaf, Lochmüller, Moxley, Greene, Wang and Gadalla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahinaz M. Gadalla, Z2FkYWxsYXNAbWFpbC5uaWguZ292
†These authors have contributed equally to this work
 Cecilia Higgs
Cecilia Higgs James E. Hilbert2
James E. Hilbert2 Mark H. Greene
Mark H. Greene Youjin Wang
Youjin Wang