- Department of Radiology, Renmin Hospital, Wuhan, China
Objective: To explore the correlation between diabetic cognitive impairment (DCI) and diabetic retinopathy (DR) through examining the cognitive function and the metabolism of the cerebrum in Type 2 diabetes mellitus (T2DM) by 1H-MRS.
Methods: Fifty-three patients with T2DM were enrolled for this study. According to the fundus examination, the patients were divided into the DR group (n = 26) and the T2DM without DR group (T2DM group, n = 27). Thirty healthy adults were selected as a control group (HC group, n = 30). Cognitive function was measured by Montreal Cognitive Assessment (MoCA). The peak areas of N-acetylaspartate (NAA), Cho-line (Cho), Creatine (Cr), and Myo-inositol (mI) as well as their ratios were detected by proton magnetic resonance spectroscopy (1H-MRS). The difference analysis between the three groups was performed by one-way ANOVA. When p < 0.05, LSD-t was applied. A partial correlation analysis (with age as a covariate) was used to analyze the correlation between metabolites in the DR group and MoCA scores. Among all T2DM patients, Chi-square test age, gender, education level, BMI, SBP, DBP, FPG, HbA1c, TC, TG, HDL-C, LDL-C, DR, and DCI correlation were measured. Differences were statistically significant while P < 0.05.
Results: 1. The scores of MoCA in the DR group or in the T2DM group were significantly less than those in the HC group (F = 3.54, P < 0.05), and the scores of MoCA in the DR group were significantly less than those in the other groups (F = 3.61, P < 0.05). 2. There were significant differences for NAA in the bilateral hippocampus in DR patients, T2DM patients, and healthy controls (P < 0.05). 3. The NAA/Cr was significantly positively correlated with the score of MoCA in DR patients' left hippocampus (r = 0.781, P < 0.01). 4. Chi-square analysis found that there was a correlation between DR and DCI (x2 = 4.6, df = 1, p = 0.032, plt: 0.05). There was no correlation between other influencing factors and DCI (P > 0.05).
Conclusion: DCI is closely correlated with the DR in patients with T2DM. Hippocampal brain metabolism may have some changes in two sides of NAA in patients with DR, 1H-MRS may provide effective imaging strategies and methods for the early diagnosis of brain damage and quantitative assessment cognitive function in T2DM.
Type 2 diabetes mellitus (T2DM) is a comprehensive metabolic disease caused by insufficient insulin secretion or insulin resistance and is associated with a variety of systemic complications (1). The incidence of T2DM in China has reached 9.7% (2). T2DM has similar pathological changes with Alzheimer's disease (AD), namely axonal degeneration, neuronal loss, and extensive fibrosis of the meninges, while the hippocampus undergoes one of the early changes in T2DM brain structure (3), with varying degrees of cognitive impairment. The Montreal Cognitive Assessment Scale (MoCA) is able to assess cognitive impairment, including naming abilities, visual space, and executive ability. Studies have shown that diabetic patients with diabetic cognitive impairment (DCI) have a higher risk of cardiovascular accidents and death than diabetic patients without cognitive impairment (4). Studies (5) found that diabetic retinopathy (DR) may be closely related to DCI and provide indirect evidence of cognitive dysfunction in diabetes, but its clinical symptoms are diverse and unstable, and there is still a lack of prevention. The gold standard for prevention is often easily underestimated or ignored. 1H-MRS (proton magnetic resonance spectroscopy) is a powerful tool for early detection and quantitative assessment of brain microstructural and functional changes in T2DM patients and brings the unique advantages of virtual biopsy. The correlation between cognitive impairment and DR is explored through examining the cognitive function and the metabolism of the cerebrum in T2DM by 1H-MRS and MoCA.
Objects and Methods
Objects
This study was approved by the patients and the ethics committee. Subjects were recruited from January 2016 to December 2018 in our hospital, according to the T2DM diagnostic criteria promulgated by the American Diabetes Association (ADA) in 2010. To avoid as much as possible the course of diabetes, the drugs taken, the way of treatment and the complications of diabetes and other neurological diseases. The course of diabetes was more than 15 years but <30 years. The patients were not using insulin and had standardized oral medication. There were differences in diabetic retinopathy, and none had any other diabetic complications or microangiopathies. Fundus examination was used to diagnose the presence or absence of DR lesions, microaneurysms, and/or small hemorrhage as it is the earliest and most accurate characteristics of DR lesions, and 53 patients with T2DM were enrolled for this study. According to the fundus examination, the patients were divided into the DR group (n = 26) and the T2DM without DR group (T2DM group, n = 27). HC (healthy control group) group inclusion criteria were as follows: no abnormal glucose metabolism, and other conditions were matched with DR and T2DM. Thirty healthy adults were selected as the control group (HC group, n = 30).
Exclusion criteria were organized into three groups: (1) patients who could not complete the MoCA test; (2) patients with a history of mental and neurological diseases; (3) those with organic diseases of the nervous system; (4) patients who were dependent on smoking, alcoholism and psychotropic substances; (5) impaired glucose tolerance or fasting glucose, and ketoacidosis; and (6) patients with contraindications with MRI. There were differences in diabetic retinopathy, they all had no other diabetic complications, and there were no significant differences in gender, age, education, height, and weight (P > 0.05); there were statistical differences among three groups of GLU and HbA1c (P < 0.05) (Table 1).
Cognitive function was measured by MoCA. The peak areas of N-acetylaspartate (NAA), Cho-line (Cho), Creatine (Cr), and Myo-inositol (mI), and their ratios, were detected by 1H-MRS.
Methods
Cognitive Function Test and Fundus Examination
The MoCA assessment was performed by a neurology professional and an ophthalmologist performed the fundus examination. This study used an internationally recognized version developed in November 2004. The scale was developed by Nasreddine et al. (6) with reference to the Mini-mental State Examination and based on clinical experience. It is an assessment tool for rapid screening of cognitive dysfunction. It includes 11 inspection projects in 8 cognitive areas, including concentration, executive function, memory, language, visual structural skills, abstract thinking, calculation, and positioning. It possesses high sensitivity, covers important cognitive areas, has a short test time and is suitable for clinical settings.
Here, n indicates the number of samples, and the table shows that Zα, Zβis 1.96 and 1.28, respectively. p1 and p2 represent the prevalence of the intervention group and the control group, with representing the average of p1 and p2, and representing the average of (1–p1) and (1–p2).
The incidence of T2DM in China is as high as 9.7% (2). According to the above formula, we get n is equal to 6.38, and the total number of diabetic patients enrolled is 53, so the sample size is reasonable and the result is credible.
Equipment and Methods
This study used a GE (Discovery 750w) 3.0T superconducting magnetic resonance imaging scanner and matching head coil (8 channels), and quality testing was employed before each scan to ensure the stability of the machine signal. All objects underwent a T2WI scan in advance, and two professional imaging physicians evaluated the images to rule out brain lesions. A high-resolution 3D-T1 multi-planar image was obtained by 3D-T1 MPRAGE for subsequent scan positioning. The following conditions were present: hippocampus 1H-MRS, STEAM (Stimulated Echo Acquisition Method) mode, point probe Probe-SV35 sequence scan (7), TR 1700 ms, TE 30 ms, FA 90°, ST 15 mm, FOV 16 cm × 16 cm, MS 320 × 320, VS 10 mm × 10 mm × 15 mm. The scan time is usually 6 min 53 s. Coastal horse long axis, according to the axial ROI, supplemented by sagittal, oblique coronal plane, try to avoid air cavity, skull, cerebrospinal fluid, and fat. The bilateral ROI was the same and the whole hippocampus was included as much as possible. The scan was manually corrected and the value was read at the workstation.
GE (Discovery 750w) 3.0T workstation spectroscopy software was used for phase and baseline calibration of the measured line, signal averaging, metabolite identification and spectral line fitting, automatic reading of the bilateral hippocampus, the area under the peak curve of NAA, Cho, Cr, and mI and to calculate the ratio of NAA/Cr, Cho/Cr, and mI/Cr.
Statistical Method
All data were tested and conformed to a normal distribution by SPSS21.0 software package. The statistical analysis of the analytes obtained by 1H-MRS was performed. The data are represented as the mean ± standard deviation. The difference analysis between the three groups was performed by one-way ANOVA. When p < 0.05, LSD-t was applied. A partial correlation analysis (with age as a covariate) was used to analyze the correlation between metabolites in the DR group and MoCA scores. Among all T2DM patients, Chi-square test was used on age, gender, education level, BMI, SBP, DBP, FPG, HbA1c, TC, TG, HDL-C, LDL-C, DR, and DCI correlation. Differences were statistically significant while P < 0.05.
Results
MoCA
One-way ANOVA statistical analysis showed that the MoCA scores in the DR group were significantly less than those in the other groups (P < 0.05), and the MoCA scores in the T2DM group were significantly less than those in the HC group (P < 0.05).
Comparison of Bilateral Hippocampal 1H-MRS Detection Values Between DR Group, T2DM Group, and HC Group
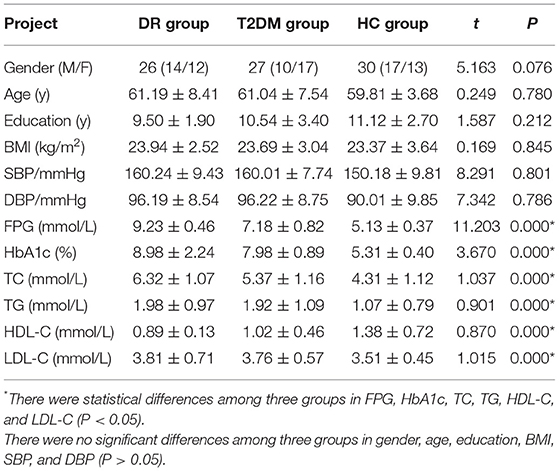
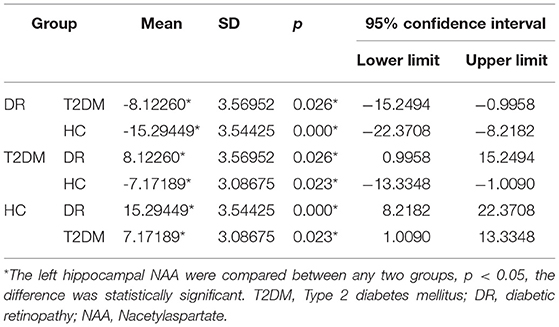
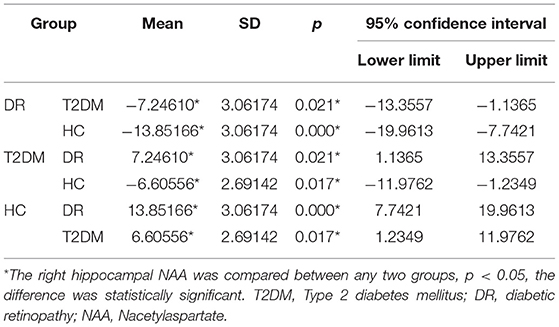
There were statistically significant differences in NAA on the bilateral hippocampus between the DR group, the T2DM group and the HC group (Fleft = 10.052, p < 0.05; Fright = 10.316, p < 0.05). Further examination with LSD-t showed that the DR group was significantly lower than the T2DM and HC groups (p < 0.05) (Figures 1, 2, Tables 2, 3); the differences of the other indicators were not statistically significant (P > 0.05).
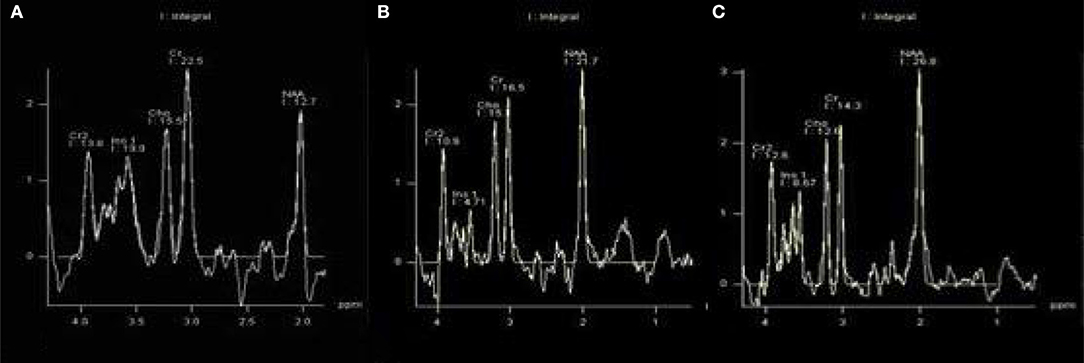
Figure 1. There were statistically significant differences in NAA on bilateral hippocampus. (A) DR group, (B) T2DM group, (C) HC group.
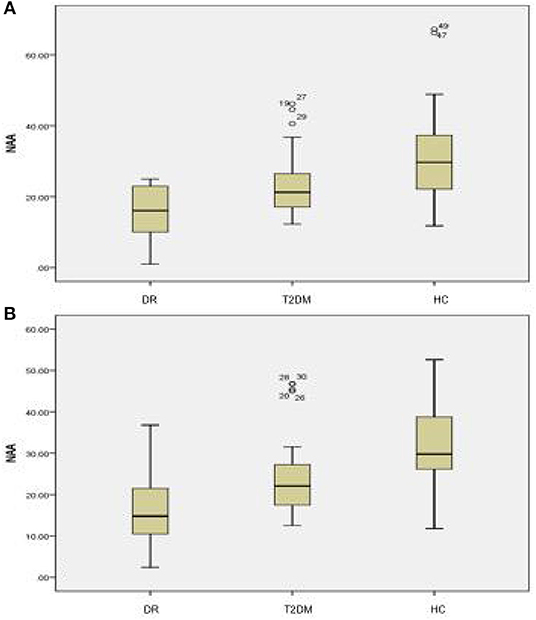
Figure 2. There were statistically significant differences in NAA on the bilateral hippocampus among the DR group, the T2DM group and the HC group. (A) Left hippocampal, (B) right hippocampal.
Correlation Between 1H-MRS and MoCA Scores in DR Group
The NAA/Cr is significantly positively correlated with the MoCA score in DR patients on the left hippocampus (r = 0.774, P < 0.01). There were no correlations among the other detection values of the two hippocampus detection values and the MoCA scores (P > 0.05) (Table 4).
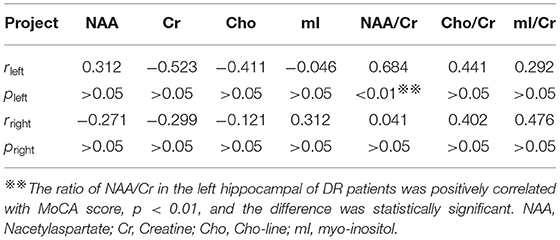
Table 4. Correlation between the detection values of bilateral hippocampus and MoCA score in DR group.
Among all T2DM patients, Chi-square tested age, gender, education level, BMI, SBP, DBP, FPG, HbA1c, TC, TG, HDL-C, LDL-C, DR, and DCI for correlation. Chi-square analysis found that there was a correlation between DR and DCI (x2 = 4.6, df = 1, p = 0.032, plt: 0.05). There was no correlation between other influencing factors and DCI (P > 0.05).
Discussion
With changes in modern lifestyles, the incidence of T2DM is increasing year by year (8). The incidence of cognitive dysfunction caused by T2DM is 10.8–17.5% (9), and its occurrence is related to hippocampus and amygdala atrophy (10). In addition, T2DM not only causes metabolic disorders but also involves multiple systems. DR is one of the more common lesions, and studies have shown that diabetic retinopathy is closely related to DCI (11). The occurrence of these two diseases is parallel (12, 13): (1) mass accumulation of glycosylation end products; (2) the protein kinase C (PKC) pathway is activated at high glucose; (3) oxidative stress: there is an excessive amount of reactive oxygen species, resulting in vascular cell damage, diabetic brain damage, or DR; and (4) DR is also a neurovascular disease, which, like diabetic brain injury, can be specifically reflected by the neuronal marker NAA in the 1H-MRS.
The literature reports that there is early brain tissue damage in patients with diabetic retinopathy, and 1H-MRS can detect this change early (12, 13). In recent years, 1H-MRS has been reported in the diagnosis of cognitive impairment-related diseases, but there are few reports on the relationship between DCI caused by T2DM and DR. Based on the above problems, the research team—based on the previous animal experiments—explored the correlation between DCI and DR through examining the cognitive function and the metabolism of the cerebrum in T2DM by 1H-MRS.
Studies have shown that individual factors such as education level and age group have different degrees of impact on MoCA scores (14). In this study, because the subjects were matched as much as possible in addition to the diabetes itself, the credibility of DCI judgment was higher, and these objects mainly showed memory loss, which is similar to previous reports (15). This indicates that T2DM is a risk factor for developing mild cognitive impairment. The MoCA scores were significantly different between the DR group and the HC group, the T2DM group and the HC group, and the DR group and the T2DM group, which may suggest differences in cognitive function among the three groups, which in turn may prompt diagnosis and early warning. Early clinical support in terms of dysfunction should be provided.
T2DM can play a significant role in the occurrence and/or development of AD either directly or as a cofactor (16). Clinical manifestations such as white matter lesions, cognitive dysfunction, etc., represent the various types of diabetic brain injury (17). Studies have shown that T2DM can cause cognitive decline through changes in hippocampal formation, neurophysiological activity and neurotransmitters (18), and hippocampal formation is one of the first brain structures to be altered (19). Its possible pathological mechanisms are similar to AD at the molecular level, including insulin resistance, metabolic mechanism damage, beta-amyloid (Aβ) formation, oxidative stress, and the presence of advanced glycation end products (AGEs), neuronal apoptosis. van Eldren et al. (19) and most scholars believe that the most important pathological feature of AD is the activation of astrocytes induced by Aβ deposition, which triggers the associated inflammatory response and oxidative stress (17). Although the damage of these nervous systems can be manifested by a variety of examination methods, MRS is uniquely and non-invasively embodied by its unique imaging method, that is, the specific and sensitive expression of the neuronal marker NAA (13). The results of this study showed that the NAA value of the bilateral hippocampal DR group was lower than that of the T2DM group and the HC group, and the NAA value of the bilateral hippocampal T2DM group was lower than that of the HC group (P < 0.05). Similar to the results of some studies (20, 21), the above changes suggest that DM can cause neurological damage. Studies have confirmed that NAA reduction is associated with neuronal or axonal loss (22) and is independently associated with the development of T2DM (23). Increased anaerobic glycolysis in the brain is accompanied by elevated blood sugar. When the accumulation of lactic acid increases, acidosis can occur, which in turn destroys the blood-brain barrier. The damage of nerve cells and glial cells is also aggravated by brain edema caused by the process (24). In addition, mitochondrial damage and cell death, DNA fragment breaks and increased cerebral ischemic damage are also caused by persistently elevated hyperglycemia, the mechanism of which is associated with excessive Ca2+ channel opening (21). Since Cr is a marker of creatine phosphate, it plays a buffer role in energy metabolism, and its changes in the body are relatively stable. Therefore, the main influences of NAA and NAA/Cr are NAA, and NAA is less sensitive and direct, but it may be weaker than NAA/Cr. There were no statistical differences in the rest of the indicators in this study, similar to previous studies (25).
The results of this study indicate that NAA/Cr, which represents neurons in DR patients, is significantly associated with MoCA scores that reflect cognitive function. Some studies found that there is consistency between the change of MoCA score and 1H-MRS, which is partially consistent with the results we obtained (26, 27). It was shown that in the DR patient group, there was synaptic functional remodeling in the hippocampus, which was confirmed in an animal model (28). T2DM spatial learning memory defects (29) are also related. In this study, it was found that anaerobic glycolysis caused by hyperglycemia and excessive opening of Ca2+ channels may damage the blood-brain barrier and cause damage to nerve cells and glial cells (30). This damaging change may be specific to NAA or NAA/Cr. In addition, there is insulin receptor resistance in the left hippocampus (31), and the hippocampus is the pancreatic exocrine structure of human insulin, which can produce a small amount of insulin. As an important neuro-influence factor, insulin maintains nerve function, and nerve cell structure. It has an important role in integrity and post-injury regeneration, which means that the damage and degeneration of the left hippocampal neurons in this study are particularly significant (20). There were no statistical differences in the rest of the indicators in this study, similar to previous studies (30). In summary, NAA/Cr can be considered as a biochemical indicator for evaluating cognitive function.
This study shows that DR was the main risk factor for DCI, similar to the results of the study (31). Some Chinese scholars believe that cognitive dysfunction in patients with type 2 diabetes is associated with diabetic microvascular complications (32). Studies have pointed out that in the early stage of diabetic retinopathy, the pathological changes of the nerve fiber layer precede the retinal microangiopathy (33). The mechanism is that the swelling of the nerve cells causes compression of the surrounding microvessels and stenosis (34). Diabetic microvascular disease caused by diabetes may also cause abnormalities in the metabolism of retinal neurons and glial cells, which may cause degeneration of retinal nerve tissue (35). The typical manifestation is a decrease in retinal ganglion cells and a thinning of the retinal nerve fiber layer (34), causing changes in vision. T2DM microangiopathy is very common in patients with diabetic cerebrovascular disease, mainly caused by changes in brain microvascular structure, which increases arteriovenous short circuiting, resulting in reduced transfer of essential nutrients to nerve tissue, insufficient nutrition, decreased perfusion pressure, and reduced cerebral blood (36, 37). It makes brain tissue more susceptible to hypoxic damage. DR with microvascular disease and brain with cognitive decline have many similarities in microvascular structures, such as capillary basement membrane thickening, lumen narrowing, and increased vascular permeability. Both DCI and DR belong to diabetic microangiopathy. Due to their common pathogenesis: AGEs, activation of polyol pathway, oxidative stress, PKC, inflammatory factors, hemodynamic changes, etc., the occurrence and development of both Parallelism, but due to the genetic susceptibility of the two, the difference in the involvement of cytokines, some differences in diagnosis, etc., the non-parallelism of the two, so the severity of DR and DCI have a certain correlation, but not completely related (36, 37).The 5-year incidence of DR is 8%, and the 10-year incidence is 43%; although, DCI is usually seen in diabetic patients for more than 10 years, the incidence of both increases with the duration of diabetes (36, 37). DR and DCI are not only diabetic microangiopathy but also neuropathy, which is sensitive to hypoxia, ischemia, and metabolic disorders. Therefore, diabetic patients may suffer from both diseases after a period of illness. The difference is that the optic nerve, as part of the peripheral nervous system, is relatively more sensitive to hyperglycemia, leading to abnormal hemodynamics, impaired optic nerve nutrient metabolism, and possibly DR earlier than DCI. In addition to the similar pathological mechanisms of DR, DCI also has a large amount of amyloid deposits, which occurs slightly later than DR, but overall, the longer the duration of diabetes, the greater the likelihood of DR and DCI.
Limitation
DR and DCI with the regularity and correlation of T2DM specific disease course changes, we will improve the group expansion study in the next step.
Conclusion
Diabetic cognitive impairment is closely correlated with the DR in patients with T2DM. Hippocampal brain metabolism may have some changes in two sides of NAA in patients with DR, and 1H-MRS may provide effective imaging strategies and methods for the early diagnosis of brain damage and quantitative assessment of cognitive function in T2DM.
Data Availability Statement
All datasets generated for this study are included in the manuscript/supplementary files.
Ethics Statement
This study was carried out in accordance with the recommendations of the Declaration of Helsinki, Renmin Hospital of Wuhan University Clinical Research Ethics Committee. The protocol was approved by the Renmin Hospital of Wuhan University Clinical Research Ethics Committee. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China 81871332.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
T2DM, Type 2 diabetes mellitus; DCI, diabetic cognitive impairment; DR, diabetic retinopathy; 1H-MRS, proton magnetic resonance spectroscopy; HC group, healthy control group; MoCA, Montreal Cognitive Assessment; NAA, Nacetylaspartate; Cr, Creatine; Cho, Cho-line; mI, myo-inositol; ANOVA, Analysis of Variance.
References
2. Liu ZQ, Liu AP, Wang PY. Epidemiological investigation of prevalence of diabetes in China. Chin J Mult Organ Dis Eld. (2015) 14:547–50. doi: 10.11915/j.issn.1671-5403.2015.07.125
3. Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. (2007) 50:711–9. doi: 10.1007/s00125-007-0602-7
4. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. (2010) 362:1090–101. doi: 10.1056/NEJMoa0908292
5. Demir N, Akkoyunlu G, Yargicoglu P, Agar A, Tanriöver G, Demir R. Fiber structure of optic nerve in cadmium-exposed diabetic rats: an ultrastructural study. Int J Neurosci. (2003) 113:323–37. doi: 10.1080/00207450390162128
6. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
7. Barnhart CD, Yang D, Lein PJ. Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS ONE. (2015) 10:e0124521. doi: 10.1371/journal.pone.0124521
8. Nathan DM, DCCT/Edic Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. (2014) 37:9–16. doi: 10.2337/dc13-2112
9. Tong J, Geng H, Zhang Z, Zhu X, Meng Q, Sun X, et al. Brain metabolite alterations demonstrated by proton magnetic resonance spectroscopy in diabetic patients with retinopathy. Magn Reson Imaging. (2014) 32:1037–42. doi: 10.1016/j.mri.2014.04.020
10. Zhang J, Liu Z, Li Z, Wang Y, Chen Y, Li X, et al. Disrupted white matter network and cognitive decline in type 2 diabetes patients. J Alzheimers Dis. (2016) 53:185–95. doi: 10.3233/JAD-160111
11. Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with Type l diabetes: effects of micro—and macrovascular complications. Diabetologia. (2003) 46:940–8. doi: 10.1007/s00125-003-1128-2
12. Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. (2010) 304:649–56. doi: 10.1001/jama.2010.1111
13. TongJia BanBo. Evaluation of the Brain Damage in Patients with Diabetic Retinopathty Using Magnetic Resonance Spectroscopy and Diffusion Weighted Imaging. Shandong: Shandong University (2014).
14. Datusalia AK, Sharma SS. NF-kappa B inhibition resolves cognitive deficits in experimental type 2 diabetes mellitus through CREB and Glutamate/GABA neurotransmitters pathway. Curr Neurovasc Res. (2016) 13:22–32. doi: 10.2174/1567202612666151030104810
15. Tang Y, Yu C, Wu J, Chen H, Zeng Y, Wang X, et al. Lychee seed extract protects against neuronal injury and improves cognitive function in rats with type II diabetes mellitus with cognitive impairment. Int J Mol Med. (2018) 41:251–63. doi: 10.3892/ijmm.2017.3245
16. Mushtaq G, Khan JA, Kamal MA. Biological mechanisms linking Alzheimer's disease and type-2 diabetes mellitus. CNS Neurol Disord Drug Targets. (2014) 13:1192–201. doi: 10.2174/1871527313666140917114537
17. van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. (2010) 75:997–1002. doi: 10.1212/WNL.0b013e3181f25f06
18. Kimura N. Diabetes mellitus induces Alzheimer's disease pathology: histopathological evidence from animal models. Int J Mol Sci. (2016) 17:503–11. doi: 10.3390/ijms17040503
19. van Elderen SG, Brandts A, Westenberg JJ, van der Grond J, Tamsma JT, van Buchem MA, et al. Aortic stiffness is associated with cardiac function and cerebral small vessel disease in patients with type 1 diabetes mellitus: assessment by magnetic resonance imaging. Eur Radiol. (2010) 20:1132–8. doi: 10.1007/s00330-009-1655-4
20. Ma XC, Wang GZ, Wang YC, Fang Q, Wang KL. Clinical analysis of hydrogen proton spectroscopy in hippocampus of type 2 diabetic patients. Modern J Integr Tradit Chinese Western Med. (2010) 32:45–8.
21. Brundel M, van den Berg E, Reijmer YD, de Bresser J, Kappelle LJ, Biessels GJ, et al. Cerebral ha-emodynamics, cognition and brain volumes in patients with type 2 diabetes. J Diabetes Complications. (2012) 26:205–9. doi: 10.1016/j.jdiacomp.2012.03.021
22. Sinha S, Ekka M, Sharma U, P R, Pandey RM, Jagannathan NR. Assessment of changes in brain metabolites in Indian patients with type-2 diabetes mellitus using proton magnetic resonance spectroscopy. BMC Res Notes. (2014) 41:1–7. doi: 10.1186/1756-0500-7-41
23. Choi MH, Kim HS, Gim SY, Kim WR, Mun KR, Tack GR, et al. Differences in cognitive ability and hippocampal volume between Alzheimer's disease, amnestic mild cognitive impairment, and healthy control groups, and their correlation. Neurosci Lett. (2016) 620:115–20. doi: 10.1016/j.neulet.2016.03.044
24. Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. (2014) 2:246–55. doi: 10.1016/S2213-8587(13)70088-3
25. Brundel M, Kappelle LJ, Biessels GJ. Brain imaging in type 2 diabetes. Eur Neuropsychopharmacol. (2014) 24:1967–81. doi: 10.1016/j.euroneuro.2014.01.023
26. Li W, Huang E. An update on type 2 diabetes mellitus as a risk factor for dementia. J Alzheimers Dis. (2016) 53:393–402. doi: 10.3233/JAD-160114
27. Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer's disease like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav. (2012) 101:567–74. doi: 10.1016/j.pbb.2012.03.002
28. Zheng H, Zheng Y, Zhao L, Chen M, Bai G, Hu Y, et al. Cognitive decline in type 2 diabetic db/db mice may be associated with brain region-specific metabolic disorders. Biochim Biophys Acta. (2017) 1863:266–73. doi: 10.1016/j.bbadis.2016.11.003
29. Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. (2014) 13:148. doi: 10.1186/s12933-014-0148-1
30. Chu X, Zhao Y, Liu F, Mi Y, Shen J, Wang X, et al. Rapidly raise blood sugar will aggravate brain damage after severe hypoglycemia in rats. Cell Biochem Biophys. (2014) 69:131–9. doi: 10.1007/s12013-013-9779-1
31. Wang WT, Lee P, Yeh HW, Smirnova IV, Choi IY. Effects of acute and chronic hyperglycemia on the neurochemical profiles in the rat brain with streptozotocin induced diabetes detected using in vivo 1H MR spectroscopy at 9.4 T. Neurochem. (2012) 121:407–17. doi: 10.1111/j.1471-4159.2012.07698.x
32. Zuo LJ, Xu JM, Ji JL, Jiang KD, Zhao JC, Yu YP. Cognitive function of type 2 diabetics with micro-angiopathy. Chinese J Clin Psychol. (2001) 9:103–5.
33. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 1:45–8. doi: 10.2337/dc11-1909
34. Li J, Zhang S, Zhang L, Wang R, Wang M. Effents of L-3-n-Butylphthalide on cognitive dysfunction and NR2B expression in hippocampus of streptozotocin (STZ) -induced diabetic rats. Cell Biochem Biophys. (2014) 71:315–22. doi: 10.1007/s12013-014-0200-5
35. Wang Y, Bai C, Guan H, Chen R, Wang X, Wang B, et al. Subchronic exposure to arsenic induces apoptosis in the hippocampus of the mouse brains through the Bc12/Bax pathway. J Occup Health. (2015) 57:212–21. doi: 10.1539/joh.14-0226-OA
36. Domínguez RO, Pagano MA, Marschoff ER, González SE, Repetto MG, Serra JA. Alzheimer disease and cognitive impairment associated with diabetes mellitus type 2: associations and a hypothesis. Neurologia. (2014) 29:567–72. doi: 10.1016/j.nrl.2013.05.006
Keywords: T2DM, diabetic brain damage, diabetic retinopathy, hippocampus, 1H-MRS, MoCA
Citation: Lu X, Gong W, Wen Z, Hu L, Peng Z and Zha Y (2019) Correlation Between Diabetic Cognitive Impairment and Diabetic Retinopathy in Patients With T2DM by 1H-MRS. Front. Neurol. 10:1068. doi: 10.3389/fneur.2019.01068
Received: 14 April 2019; Accepted: 23 September 2019;
Published: 12 November 2019.
Edited by:
Franca Rosa Guerini, Fondazione Don Carlo Gnocchi Onlus (IRCCS), ItalyReviewed by:
Shaun Sabico, King Saud University, Saudi ArabiaRajiv Raman, Sankara Nethralaya, India
Copyright © 2019 Lu, Gong, Wen, Hu, Peng and Zha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunfei Zha, bW9uYV82NjY2NjYmI3gwMDA0MDsxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xuefang Lu†
Xuefang Lu† Yunfei Zha
Yunfei Zha